Abstract
This study investigated the effects of temperature on bitter taste in humans. The experiments were conducted within the context of current understanding of the neurobiology of bitter taste and recent evidence of stimulus-dependent effects of temperature on sweet taste. In the first experiment, the bitterness of caffeine and quinine sampled with the tongue tip was assessed at 4 different temperatures (10°, 21°, 30°, and 37 °C) following pre-exposure to the same solution or to water for 0, 3, or 10 s. The results showed that initial bitterness (0-s pre-exposure) followed an inverted U-shaped function of temperature for both stimuli, but the differences across temperature were statistically significant only for quinine. Conversely, temperature significantly affected adaptation to the bitterness of quinine but not caffeine. A second experiment used the same procedure to test 2 additional stimuli, naringin and denatonium benzoate. Temperature significantly affected the initial bitterness of both stimuli but had no effect on adaptation to either stimulus. These results confirm that like sweet taste, temperature affects bitter taste sensitivity and adaptation in stimulus-dependent ways. However, the thermal effect on quinine adaptation, which increased with warming, was opposite to what had been found previously for adaptation to sweetness. The implications of these results are discussed in relation to findings from prior studies of temperature and bitter taste in humans and the possible neurobiological mechanisms of gustatory thermal sensitivity.
Key words: adaptation, bitter, human, taste, temperature
Introduction
Multiple lines of evidence indicate that the effects of temperature on taste arise primarily from the thermal sensitivity of peripheral neural mechanisms: psychophysical studies have revealed differential effects of temperature within and between taste modalities (Frankmann and Green 1987; Green and Frankmann 1987, 1988; Bajec et al. 2012; Green and Nachtigal 2012; Green and Nachtigal 2015); electrophysiological studies have shown that the response of the chorda tympani nerve can be modulated by temperature (Sato and Yamashita 1965; Yamashita et al. 1970; Nakamura and Kurihara 1987; Nakamura and Kurihara 1991; Lundy and Contreras 1997; Lu et al. 2016); and cellular studies have shown both that the cation channel ENaC, which is involved in salt taste transduction, is sensitive to cold (Askwith et al. 2001), and that the nonselective sodium channel TRPM5, which is a critical step in the transduction pathway of G-protein coupled taste receptors (Pérez et al. 2003; Liman 2007; Zhang et al. 2007; Huang and Roper 2010), is sensitive to heat (Talavera et al. 2005).
The latter study, which found the response of TRPM5 as expressed in HEK293 cells varies directly with temperature, is germane to the neurobiology of thermal sensitivity in bitter taste. Because bitter-tasting compounds activate the T2R family of type C G-protein coupled receptors (C-GPCRs) (Chandrashekar et al. 2000; Pérez et al. 2003; Behrens and Meyerhof 2006; Meyerhof et al. 2010; Roura et al. 2015), bitterness would be expected to vary directly with temperature and independently of stimulus. Prior psychophysical and electrophysiological data have not always shown this relationship. Whereas most human psychophysical studies have found that bitter taste is attenuated by colder temperatures (Paulus and Reisch 1980; Green and Frankmann 1987; Haraguchi et al. 2011), McBurney (1973) found that the detection threshold for quinine followed a u-shaped function of temperature with a minimum between 20° and 30°C, which is in good agreement with the reported effect of temperature on the response to quinine of the chorda tympani nerve in rodents and cats (Nagaki et al. 1964; Yamashita et al. 1964; Yamashita and Sato 1965). However, Moskowitz (1973) found no effect of temperature on the psychophysical function for the bitterness of quinine, and most recently, Bajec et al. (2012) reported that a cold solution led to slightly but significantly higher peak ratings of the bitterness propylthiouracil (PROP).
A primary aim of the present study was to revisit the effect of temperature on bitter taste in humans in the context of the TRPM5 hypothesis and current understanding of hT2R bitter taste receptors and their ligands (Kuhn et al. 2004; Pronin et al. 2004; Meyerhof et al. 2010). Another aim was to investigate the effect of temperature on bitter taste adaptation. Recent studies from this laboratory have shown that temperature can affect both the initial taste intensity and the rate of taste adaptation in sweet taste (Green and Nachtigal 2015), whereas it affects only the initial intensity of umami taste (Green et al. 2016). Because both sweet and umami taste are mediated by C-GPCRs, these results imply that whereas the TRPM5 hypothesis of thermal sensitivity of taste may be adequate to explain the effect of temperature on umami taste, it cannot explain the dual thermal effects on sweet taste. Bitter taste offered another test of the hypothesis in human taste and the opportunity to establish whether inconsistencies among previous psychophysical studies might depend in part on the stimuli that were chosen for testing, and whether temperature effects depend on which hT2R receptors are stimulated.
The study comprised 2 experiments: In experiment 1, we measured the effects of temperature on initial sensitivity and adaptation to the bitterness of caffeine and quinine. These 2 stimuli were chosen because a much earlier study from this laboratory found an effect of temperature on the bitterness of caffeine (Green and Frankmann 1987), whereas a more recent study indicated that adaptation to the bitterness of quinine was not temperature-sensitive (Green and Nachtigal 2012). After these stimuli yielded somewhat different results, 2 additional bitter stimuli were tested in experiment 2: denatonium benzoate, which is an agonist of some of the same hT2R receptors as caffeine and quinine, and naringin, for which no hT2R receptor has so far been identified.
Methods
Experiment 1
Subjects
A total of 43 individuals (24 F and 19 M, 18–45 years of age) were recruited using flyers posted around the Yale University campus. Thirty-three subjects (18F, 15M) qualified for and completed the study. Of the 10 subjects who did not complete the study, 2 failed to understand the scale in the training session and did not return, 7 did not perceive either bitter stimulus in the sensitivity screening task (see below), and 1 did not return for the second of 2 testing sessions. The study protocol complies with the Declaration of Helsinki for Medical Research involving Human Subjects and was approved by the Human Investigations Committee of the Yale University IRB. Each subject provided informed consent and was compensated for their participation. All subjects were fluent English-speakers, self-reported healthy nonsmokers who had no known taste or smell disorders or deficiencies, were not pregnant, and had no lip, cheek, or tongue piercings. Subjects were asked to refrain from eating or drinking foods or beverages for at least 1 h prior to their scheduled session and to avoid hot/spicy food for 24 h before their session.
Stimuli
The test stimuli were quinine (QHCl) (Acros Organics) and caffeine (Sigma-Aldrich), each delivered in 5 concentrations in 1/4 log steps: 0.01, 0.018, 0.032, 0.056, and 0.10 mM QHCl, and 5.6, 10, 18, 32, and 56 mM caffeine. All solutions were prepared weekly in 250 mL aliquots with deionized water (dH2O) and stored in airtight 250 mL flasks.
Session 1: training, screening, and practice
All recruited subjects attended a practice session to learn to use the general version of the Labeled Magnitude Scale (gLMS; Green et al. 1993; Green et al. 1996; Bartoshuk et al. 2003) to rate sensation intensity and to be screened for sensitivity to the test stimuli. A session began with the experimenter reading instructions about how to use the gLMS, after which subjects were asked to rate the intensities of 15 imagined sensations (e.g. the sweetness of milk, pain of biting your tongue, weight of a feather in your hand) that were also read to them by the experimenter. This procedure enabled the experimenter to determine if subjects grasped the concept of the gLMS and were using it as instructed (i.e., making their ratings in the context of the strongest imaginable sensation of any kind). Subjects were then given practice rating actual sensations of various kinds (e.g. the coolness of a penny placed in the hand, the touch sensation from a cotton swab, the brightness of the ceiling light) before rating several taste stimuli (0.56 M sucrose, 18 mM citric acid, 0.32 M NaCl, 0.18 mM QHCl, 100 mM MPG, and 5 binary mixtures). Subjects sipped and expectorated each taste stimulus sample before rating the intensity of sweetness, saltiness, sourness, bitterness, and umami on the gLMS.
Following successful completion of the training and practice portion of the session, subjects were screened to confirm that they could perceive at least 1 of the 5 concentrations of the 2 test stimuli. This was important because of the large individual differences in sensitivity to bitter taste (Yokomukai et al. 1993; Roura et al. 2015). The stimuli had concentrations that ranged from 0.01 to 0.10 mM for quinine and 5.6–56 mM for caffeine in 1/4 log steps. The samples were presented in weigh boats (≈7.5 mL volumes) at room temperature (21 °C) and were arranged in a line on the countertop in an ascending concentration series, beginning with a dH2O blank. Subjects dipped the tongue tip into the samples sequentially for 3 s each (timed by the experimenter) until they perceived a “clearly identifiable bitter taste” in one of the samples. Only subjects who perceived bitterness from at least one of the stimuli qualified for the study, and the stimuli used had concentrations 1/4 log step higher than the sample in which the subject first tasted bitterness. A total of 25 subjects qualified for testing with caffeine and 25 qualified for testing with QHCl (17 subjects were sensitive to both stimuli).
Test session procedure
There were 2 testing sessions, 1 for caffeine and 1 for quinine, the order of which was counterbalanced across subjects. Each test session comprised 20 trials, and subjects sampled solutions at 4 temperatures (10°, 21°, 30°, and 37 °C) in 3 different conditions: 1) exposure to the test stimulus only for 3-s; 2) 3- or 10-s pre-exposures to the test stimulus followed by the 3-s test stimulus; and 3) 3- or 10-s pre-exposures to dH2O followed by the 3-s test stimulus. Condition 1 quantified the initial effect of temperature on bitterness; Condition 2 measured bitter taste adaptation across temperatures; and Condition 3 controlled for the effects of temperature alone, independent of stimulus adaptation. Testing conditions and stimuli were presented in 4 different pseudorandom orders that were also counterbalanced across subjects.
As in the practice session, stimuli were sampled by dipping the tongue tip into weigh boats containing 7.5 mL of solution. The experimenter timed the duration of exposure and verbally signaled the subject when it was time to remove the tongue from the solution and either dip it immediately into the second weigh boat (Conditions 2 and 3) or to begin making her/his rating. Bitterness ratings were made as quickly as possible with the tongue still extended outside the mouth. In making their ratings, subjects were instructed to ignore as best they could sensations of temperature, and they were told that they might not perceive bitterness on every trial. There was a 1-min inter-trial interval during which subjects rinsed with dH2O at least 3 times to remove any lingering bitter taste.
Experiment 2
Subjects
A total of 67 subjects (40 F, 27 M) were recruited using postings around the Yale campus and online websites (Facebook and Craigslist), and 38 subjects (25F, 13M) completed the experiment. Of those who did not complete the experiment, 10 subjects failed to use the scale correctly in the training session and were not invited to return for the test sessions, 14 did not perceive either bitter stimulus in the sensitivity screening task, and 5 did not return for the second test session. The criteria to participate were the same as experiment 1. The study protocol complies with the Declaration of Helsinki for Medical Research involving Human Subjects and was approved by the Human Investigations Committee of the Yale University IRB. Each subject provided informed consent and was compensated for their participation.
Stimuli
The test stimuli were naringin (Sigma-Aldrich) and denatonium benzoate (Fluka BioChemika). As in experiment 1, there were 5 concentrations of each stimulus in 1/4 log steps: 0.056, 0.10, 0.18, 0.32, and 0.56 mM naringin, and 0.018, 0.032, 0.056, 0.10, and 0.18 µM denatonium benzoate. The same practice stimuli were used as in experiment 1, and all solutions were prepared weekly in 250 mL volumes with dH2O and stored in airtight flasks.
Practice session and testing procedure
Subjects completed the same training, practice, and bitter taste sensitivity screening as in experiment 1. Screening concentrations used ranged from 0.018 to 0.18 µM for denatonium benzoate and 0.056 to 0.56 mM for naringin. A total of 25 subjects qualified for testing with naringin and 30 with denatonium (17 subjects were sensitive to both stimuli). The experimental conditions and psychophysical procedures were the same as before, with the exception that replicate ratings were collected for each stimulus and condition, resulting in 4 testing sessions.
Results
Experiment 1
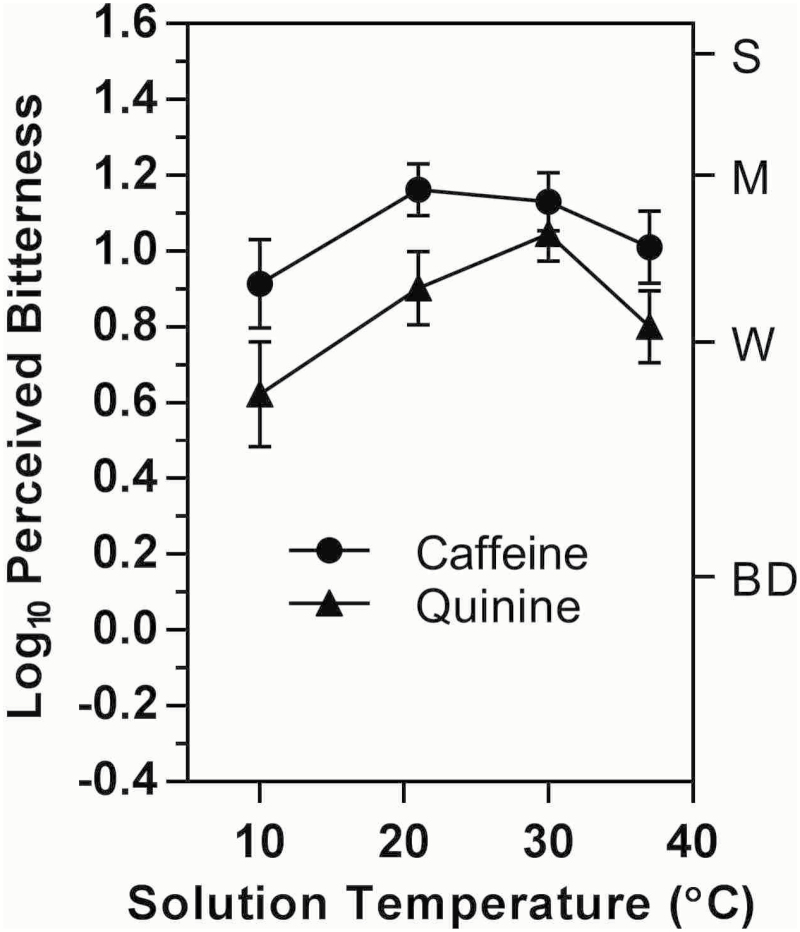
The initial bitter taste intensities of caffeine and QHCl are shown in Figure 1 as a function of solution temperature. Both stimuli exhibited trends toward lower bitterness at the coolest and warmest temperatures; however, repeated-measures analyses of variance indicated the effect was statistically significant for quinine (F 3,72 = 4.38, P < 0.01) but fell just short of significance for caffeine (F 3,72 = 2.33, P = 0.08). The bitterness of QHCl was significantly higher at 30° than at 10 °C (Tukey HSD, P < 0.005), where it was 2.34 times stronger than at 10° (∆log10 = +0.34).
Figure 1.
Shown are log10 mean ratings of bitterness intensity for 3-s exposures to caffeine or quinine as a function of solution temperature. Letters on the right y-axis represent semantic labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong. Vertical bars indicate the standard error of the means (SEMs).
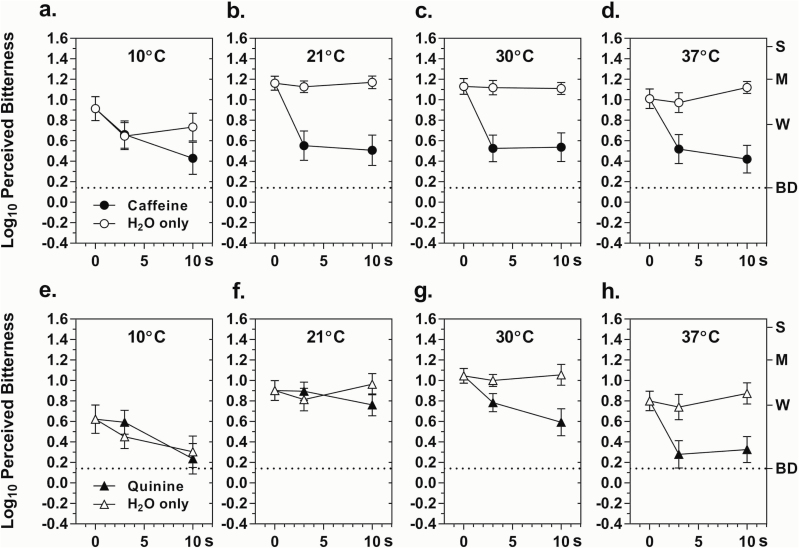
Figure 2 shows that the effects of temperature on the rate and amount of adaptation to bitterness differed greatly for the 2 stimuli: above 10 °C adaptation to caffeine was rapid and independent of temperature, whereas adaption to QHCl was directly related to temperature between 21° and 37°. Repeated-measures ANOVAs on the data over this temperature range indicated that for caffeine there were main effects of Condition (F 1,24 = 42.29, P < 0.00001) and Time (F 2,48 = 19.42, P < 0.00001), and a significant Condition × Time interaction (F 2,48 = 21.06, P < 0.000001), but no significant effect of temperature. The same analysis for QHCl showed that in addition to main effects of Condition (F 1,24 = 19.19, P < 0.0005) and Time (F 2,48 = 8.75, P < 0.001) and a Condition × Time interaction (F 2,48 = 12.9, P < 0.00005), there was also a main effect of Temperature (F 2,48 = 18.55, P < 0.00001) and Condition × Temperature (F 2,48 = 6.30, P < 0.005) and Condition × Temperature × Time (F 4,94 = 2.76, P < 0.05) interactions, confirming that adaptation to QHCl was temperature dependent. At 37 °C, adaptation was similar for QHCl and caffeine (Figure 2d and h), with reductions in bitterness after the 10-s pre-exposure of 71% (∆log10 = −0.54) and 80% (∆log10 = −0.70), respectively. The somewhat smaller difference for QHCl appears attributable to a floor effect, as bitterness ratings had already fallen to near “barely detectable” after the 3-s pre-exposure.
Figure 2.
Log10 mean ratings of bitterness intensity perceived for 3-s post-exposures to caffeine (a–d) or quinine (e–h) at temperatures of 10°–37 °C following 0-, 3-, or 10-s pre-exposures to caffeine or H2O (only) at the same temperatures. The data points at 0-s for each temperature are the same as those in Figure 1. Letters on the right y-axis represent semantic labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong. Vertical bars indicate SEMs.
Separate analyses of the data for the 10 °C solutions confirmed there were main effects of Time for both caffeine (F 2,48 = 5.32, P < 0.01) and QHCl (F 2,48 = 5.56, P < 0.01), but no main effect of Condition. There was also no Condition x Time interaction, the latter falling just short of statistical significance (F 2,48 = 2.97, P = 0.06). These results indicate the sensitivity to caffeine and QHCl decreased as exposure to cold dH2O continued, resulting in no difference between the control and adaptation conditions.
Experiment 2
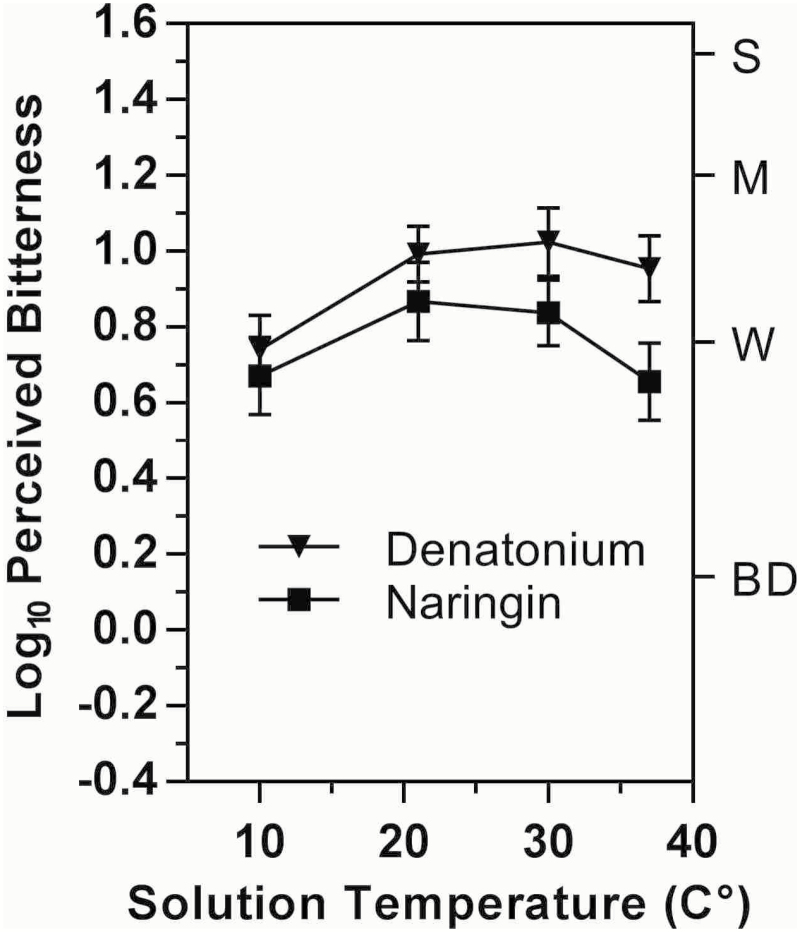
Temperature significantly affected the initial bitter taste of both stimuli (main effects of Temperature: denatonium, F 3,87 = 8.66, P < 0.00005; naringin, F 3,72 = 3.1, P < 0.05). As is apparent in Figure 3, the effect of temperature for denatonium was driven by a reduction in bitterness for the 10 °C solution compared to the 3 warmer temperatures (Tukey HSD, P’s < 0.005), where bitterness varied little between 21° and 37°. In contrast, the bitterness of naringin followed an inverted-U shaped function of temperature that was similar to the effects on the bitterness of caffeine and QHCl, although Tukey HSD tests found no significant difference between specific pairs of temperatures.
Figure 3.
Log10 mean ratings of bitterness intensity for 3-s exposures to naringin or denatonium benzoate as a function of solution temperature. Letters on the right y-axis represent semantic labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong. Vertical bars indicate the standard error of the means (SEMs).
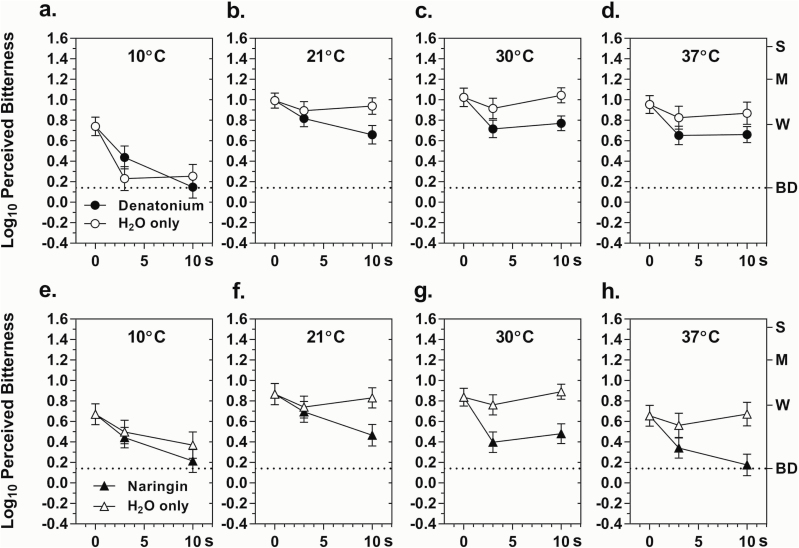
Figure 4 contains the data on adaptation to the bitterness of naringin and denatonium. As was true for caffeine and QHCl, exposure to 10 °C dH2O alone reduced bitter taste over time independent of stimulus adaptation. Separate analyses of the data for the 10 °C solutions showed significant main effects of Time for both naringin (F 2,58 = 8.11, P < 0.005) and denatonium (F 2,58 = 20.91, P < 0.000001) and a significant Condition × Time interaction for denatonium (F 2,58 = 6.58, P < 0.005), which was driven by a faster decline in bitterness in the dH2O control condition compared to the adaptation condition.
Figure 4.
Log10 mean ratings of bitterness intensity perceived for 3-s post-exposures to naringin (a–d) or denatonium benzoate (e–h) for temperatures of 10°–37 °C following 0-, 3-, or 10-s pre-exposures to denatonium, narigin, or H2O (only) at the same temperatures. The data points at 0-s are the same as those in Figure 3. Letters on the right y-axis represent semantic labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong. Vertical bars indicate SEMs.
ANOVAs on the data for the 3 highest temperatures revealed the same statistically significant effects for both stimuli: Condition (naringin, F 1,24 = 19.35, P < 0.0005; denatonium, F 1,29 = 9.66, P < 0.005), Temperature (naringin, F 2,48 = 9.35, P < 0.0005; denatonium, F 2,58 = 3.22, P < 0.05), and Time (naringin, F 2,48 = 9.90, P < 0.0005; denatonium, F 2,48 = 16.67, P < 0.00005), as well as a Condition × Temperature interaction (naringin, F 2,48 = 14.51, P < 0.00005; denatonium, F 2,58 = 7.72, P < 0.005). Like caffeine in experiment 1, adaptation was statistically independent of temperature above 10 °C, i.e., there was no Condition × Temperature × Time interaction for either stimulus (naringin, F 4,96 = 1.15, P = 0.34; denatonium F 4,116 = 0.59, P = 0.67). Adaptation to denatonium tended to be less than adaptation to naringin, particularly at 37 °C (Tukey HSD, P < 0.05), where after the 10-s pre-exposure, bitterness declined by 38% (∆log10 = −0.21) for denatonium (Figure 4h) compared to 68% (∆log10 = −0.49) for naringin (Figure 4d), which reduced the bitterness of naringin to “barely detectable”. However, in an ANOVA conducted on the subset of data from the 17 subjects who were tested with both naringin and denatonium, the difference in adaptation between conditions at 37 °C fell just short of statistical significance (Condition × Stimulus, F 1,32 = 3.49, P = 0.07).
Discussion
Effects of temperature on initial bitter taste intensity
For the 4 stimuli tested, bitterness followed an inverted u-shaped function of temperature. This result is consistent with McBurney’s (1973) data on the effect of solution temperature on the detection threshold for quinine, which showed that the highest sensitivity was between 20° and 30 °C. However, the downturn in bitterness at 37 °C was smallest for denatonium, where bitterness remained significantly higher than at 10°. A recent study in this laboratory also found that the effects of temperature on sweet taste intensity (Green and Nachtigal 2015) were stimulus dependent. In that study cooling also reduced the sweetness of all stimuli, albeit to varying degrees, while the sweetness of some stimuli did not decline at warmer temperatures. It was speculated that the differences across sweet stimuli might result from differential effects of temperature on the ability of some agonists to bind to and excite hT1R2-hT1R3, or possibly to a second sweet taste transduction pathway (Ohkuri et al. 2009; Glendinning et al. 2015) that is less sensitive to temperature. Both possibilities must also be considered for bitter taste. No published data are available that directly address whether moderate temperatures can affect agonist binding or receptor conformation in C-GCPR taste receptors. However, such effects are plausible based on evidence that greater structural flexibility in CGRP proteins, which is required for ligand binding and allostery, is associated with greater thermal instability (Vihinen 1987; Yuan et al. 2005). It is also not yet certain that hT2R receptors are the sole transduction pathway for bitter taste in humans (Zubare-Samuelov et al. 2003; Oliveira-Maia et al. 2009). This remains an open question in part because cognate hT2R receptors have not been identified for several bitter substances that have been tested, including naringin (Meyerhof et al. 2010).
It is noteworthy in this regard that the bitterness of naringin followed an inverted u-shaped function very similar to QHCl and caffeine, which have been demonstrated in vitro to be agonists of 9 and 5 hT2R receptors, respectively (Meyerhof et al. 2010). A markedly different result for naringin would have supported the possibility that its bitterness is not mediated exclusively by hT2Rs. Instead denatonium, which activates at least 8 hT2Rs, produced results for initial bitterness that were the least similar to the other stimuli. A possible explanation for this result is denatonium’s extremely high affinity. Denatonium was perceived as bitter at concentrations approximately 1 × 10–3 lower than QHCl and 1 × 10–5 lower than caffeine. High affinities might render agonists less vulnerable to possible thermal effects on the rate of receptor binding and/or dissociation.
Regarding the source of the inverted u-shaped function of temperature, the thermal sensitivity of TRPM5 as measured in vitro is not consistent with lower bitterness intensity at warm temperatures. As noted earlier, when expressed in HEK293 cells, activation of TRPM5 is a monotonically increasing function of temperature between 15° and 35 °C (Talavera et al. 2005). Together with the evidence that some sweet taste stimuli are also perceived as less sweet at warmer temperatures (Green and Nachtigal 2015), the present results suggest the thermal sensitivity of TRPM5 may contribute to the lower sensitivity of human sweet taste and bitter taste at cold temperatures, but it is unlikely to be the only temperature-sensitive step in the transduction cascade of gustatory C-GPCRs. The tendency for bitterness and sweetness to decrease in sensitivity at temperatures above 30 °C must result from a disruptive effect of heat at a different stage of taste processing. Recent studies in mice have found that the effect of temperature on the response of the chorda tympani nerve (Breza et al. 2006; Lu et al. 2016) and NTS units (Wilson and Lemon 2013) follows a monotonic rather inverted u-shape function up to 37 °C. Those data introduce the possibility that temperature has a different effect on the peripheral gustatory systems of mice and humans, or that the downturn in sensitivity we observed is caused by an adaptation mechanism located more central in the taste pathway than the NTS. However, a central neural mechanism would be expected to produce more consistent results across stimuli than those found in both the present study and the recent study of thermal effects on sweet taste (Green and Nachtigal 2015).
It is interesting to consider whether an inverted u-shaped function of temperature has adaptive value, given that it tends to reduce the bitter and sweet signals of potentially dangerous and nutritive ingesta. However, prior to the advent of cooking, foods and liquids at body temperature (37 °C) or above were rarely if ever encountered in the natural environment. When cooking first began remains controversial (Wrangham 2009), but even if it arose sufficiently early in human history to potentially influence the evolution of taste, its effect would likely have been small because consumption of hot food was under voluntary control and therefore imposed no environmental threat. Also, recent evidence from this laboratory showed that sensitivity to a cool sucrose solution can be rescued by brief (3-s) exposure to a 37 °C solution (Green and Nachtigal 2012). This finding implies the blunting effect of cooling on taste is at least partially counteracted as solutions are warmed by the mouth during ingestion.
Effects of temperature over time
The present data show that temperature can affect bitter taste over time in at least 2 ways: 1) via a direct, progressive effect of cold on sensitivity below 21 °C, and 2) by modulating adaptation at temperatures ≥21°. Significant direct effects of cold were observed for all stimuli at 10 °C, where with the exception of caffeine, ratings of bitterness fell to “barely detectable” after only a 10-s exposure to cold dH2O. Reducing bitterness to such low levels precluded measurement of bitter taste adaptation. A similar progressive effect of cooling at 10° and below was obtained in 2 recent studies of sweet and umami taste (Green and Nachtigal 2015; Green et al. 2016). Because bitterness, sweetness and umami are all mediated by C-GPCRs, the thermal sensitivity of TRPM5 (Talavera et al. 2005; Talavera et al. 2007) must again be considered the possible source of these direct thermal effects. However, the monotonic effect of temperature on the activation of TRPM5 (Talavera et al. 2005) suggests that smaller progressive effects of cooling should also occur at 21° and 30°, but no trace of this can be seen at either temperature (Figures 2 and 4).
The clearest effect of temperature over time above 10 °C occurred for QHCl, for which bitterness did not adapt at 21° but adapted in increasing amounts at 30° and 37°. In contrast, caffeine adapted rapidly and significantly at all 3 temperatures, with bitterness at 21 °C reduced by 75.5% (∆log10 = −0.61) after only a 3-s pre-exposure to the stimulus. Lesser amounts of adaptation were found for naringin and denatonium, particularly at 21 °C, where it was minimal after a 3-s pre-exposure, and there was no effect of solution temperature on adaptation overall. Because the mechanism of receptor adaptation for T2Rs has not yet been determined, it is difficult to speculate why exposure to a 21 °C solution retarded adaptation to QHCl and not caffeine, or why the amount of adaptation tended to differ across stimuli even at warm temperatures. However, a possible explanation for the absence of adaptation to QHCl at 21 °C comes from the fact that quinine activates 2 hT2Rs (hT2R40, hT2R44) in vitro that none of the other 3 stimuli activate (Meyerhof et al. 2010). If adaptation of one or both of these 2 receptors is slower at 21 °C, the sensitivity to quinine could be sustained over time. Alternatively, it has been proposed that as an ionic molecule, quinine may activate an additional transduction pathway (Frank et al. 1983; Frank et al. 2004), which may be resistant to adaptation at 21°. This explanation is complicated by the fact that the bitterness of denatonium, which is also an ionic stimulus, adapted at 21 °C, though less so than caffeine. The very high affinity of denatonium to hT2Rs must also be considered again here, as it is conceivable adaptation is forestalled as denatonium remains bound to receptors longer than lower affinity agonists. This speculation is consistent with the finding that after 10-s pre-exposures to the stimulus at 37 °C, bitterness fell nearly to “barely detectable” for all stimuli except denatonium. However, data from prior studies of adaptation to sweeteners have not shown a consistent relationship between affinity and rate of adaptation (Bornstein et al. 1993; Schiffman et al. 1994).
The increase in adaptation for QHCl with temperature points to a fundamental difference in the effect of temperature on adaptation to bitter and sweet stimuli: whereas sweet taste adaptation tends to increase at cooler temperatures (Green and Nachtigal 2015), bitter taste adaptation is unchanged at cool temperatures, or in the case of QHCl, increases at warm temperatures. This difference suggests the mechanisms of adaptation in hT2Rs and the hT1R2-hT1R3 sweet taste receptor may not be exactly the same. Unfortunately, speculation about the source of this difference is hampered by a lack of understanding of the mechanism of gustatory adaptation. The present results suggest that studies of how temperature affects the phasic response of the chorda tympani (CT) nerve to bitter stimuli would be particularly useful for investigating the peripheral effects of temperature on both initial sensitivity and adaptation. For example, although a study by Danilova and Hellekant (2003) did not investigate temperature as a factor in the response of the CT in mice to bitter stimuli, their data shows the phasic response to quinine at 33 °C has a time course similar to adaptation to bitterness in the present study.
Relation to previous psychophysical studies
The absence of adaptation to QHCl at 21 °C and the rapid adaptation of QHCl at 37° may explain some of the discrepancies among prior studies of the effect of temperature on bitter taste. The finding that no adaptation occurred at 21 °C replicates an earlier result from our laboratory in which QSO4 bitterness also failed to adapt significantly on the tongue tip (Green and Nachtigal 2012). In that study, the effect of temperature on adaptation was compared for quinine and sucrose at 21 °C only, and the result was taken as evidence that adaptation to bitterness is less temperature-dependent than adaptation to sweetness. The present finding that QHCl adapts significantly at temperatures above 30 °C shows that this assumption was incorrect. To determine whether quinine is unique in this regard will require a larger scale survey of bitter stimuli.
The use of different psychophysical methods must of course be considered when comparing results across studies. Tongue-tip stimulation in the present study was intended to provide better control of stimulus duration and solution temperature than is achievable with sip-and-spit procedures, in which exposure to different gustatory areas varies over time as solutions undergo warming (or cooling) in the mouth (Green 1986). Warming or cooling of residual stimulus after expectoration can also confuse judgments of taste intensity. In addition, use of a separate, 3-s post-exposure stimulus after the adapting stimulus enabled subjects to rate the bitterness of a temporally discrete, and thus more perceptually independent taste sensation. This is opposite to what occurs in whole-mouth, time-intensity (TI) procedures. Although the TI method more closely mimics normal tasting, it is less able to provide data on how sensory and physical factors such as adaptation and temperature interact. For example, a recent study (Bajec et al. 2012) used a TI procedure to track the bitterness of warm and cold solutions both before and after expectoration over a total of 112 s. The results showed that for quinine, a cold (5 °C) solution produced slightly higher peak bitterness ratings than did a warm (37 °C) solution. However, the data did not reveal that cool and cold temperatures can suppress quinine’s initial bitterness and retard its adaptation, with the result that warm solutions may be perceived at first to taste more bitter than cold solutions before beginning to adapt after as little as 3 s. In contrast, cold quinine solutions might initially be perceived as less bitter but also adapt less rapidly, resulting in little or no effect of solution temperature over time.
At first glance the results for caffeine appear to disagree with a much earlier study by Green and Frankmann (1987) that measured the contributions of solution temperature and tongue temperature to thermal effects on taste intensity. The results of that study, which used whole-mouth stimulation, indicated that cooling the tongue to 28° or 20 °C significantly reduced the bitterness of caffeine, whereas the present data (Figure 2b and c) show no difference in bitterness between 30° and 21 °C. However, Green and Frankmann also found that unless the tongue was first cooled to 20 °C, a 20° caffeine solution was no less bitter than a 36° solution. This outcome led to the conclusion that for bitter (and sweet) taste, tongue temperature is more important than solution temperature. This is consistent with the present finding that initial bitterness was significantly reduced for the 10 °C solution, which would more effectively lower the surface temperature of the tongue than would temperatures ≥21°. Complicating this interpretation somewhat is the evidence that unlike the other 3 stimuli, caffeine bitterness did not decline further after 10-s compared to 3-s exposures to 10 °C water. However, because this inconsistency is based on just a single data point, further study will be necessary to determine whether caffeine bitterness is in fact less sensitive to tongue cooling.
The present results are also in accord with the absence of an effect of temperature on quinine (QSO4) bitterness reported by Moskowitz (1973). The lowest temperature tested in that study was 25 °C, which falls between temperatures (21° and 30°) which in the present study yielded the highest bitterness ratings for QHCl.
Despite the general concordance between the present findings and those from studies that used whole-mouth procedures, limiting stimulation to the tongue tip leaves open the possibility that temperature has different effects in the posterior oral cavity. However, we could find no published evidence that expression of T2Rs varies across gustatory regions, and human psychophysical studies have found the suprathreshold perception of quinine in humans differs little (Green and Schullery 2003; Green and George 2004) if at all (Green and Hayes 2003) between the front and back of the tongue.
Summary and conclusions
The data presented here show that similar to sweet taste, temperature can affect the perception of bitter taste in at least 2 stimulus-dependent ways: by modulating initial sensitivity and/or bitterness adaptation. The initial and progressive suppression of bitterness at 10 °C might be accounted for by the thermal sensitivity of TRPM5, but the monotonic property of the TRPM5 response to heat in vitro is inconsistent with the inverted u-shaped functions of temperature found here and in other studies. The effect of temperature on adaptation was limited to the bitterness of quinine, for which adaptation varied directly with temperature between 21° and 37 °C. This result is opposite to a recent finding for sweet taste adaptation and suggests the mechanisms of adaptation in hT2R bitter taste receptors and hT1R2-hT1R3 sweet taste receptors are not identical. Overall, these new findings help to explain inconsistencies in the literature on the effects of temperature on bitter taste and offer new information germane to the thermal sensitivity of bitter taste transduction and adaptation in humans.
Funding
This research was supported in part by a grant from the National Institutes of Health, RO1-DC05002.
Acknowledgement
The authors thank the anonymous reviewers for their thoughtful comments and suggestions which led to significant improvements in the manuscript.
References
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM. 2001. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA. 98(11):6459–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajec MR, Pickering GJ, DeCourville N. 2012. Influence of stimulus temperature on orosensory perception and variation with taste phenotype. Chemosens Percept. 5:243–265. [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. 2003. Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Pref. 14 (2):125–138. [Google Scholar]
- Behrens M, Meyerhof W. 2006. Bitter taste receptors and human bitter taste perception. Cell Mol Life Sci. 63(13):1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein BL, Wiet SG, Pombo M. 1993. Sweetness adaptation of some carbohydrate and high potency sweeteners. J Food Sci. 58:595–598. [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. 2006. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 95(2):674–685. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100(6):703–711. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. 2003. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Bouverat BP, MacKinnon BI, Hettinger TP. 2004. The distinctiveness of ionic and nonionic bitter stimuli. Physiol Behav. 80(4):421–431. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. 1983. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 50(4):941–960. [DOI] [PubMed] [Google Scholar]
- Frankmann SP, Green BG. 1987. Differential-effects of cooling on the intensity of taste. Ann N Y Acad Sci. 510:300–303. [Google Scholar]
- Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. 2015. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol. 309(5):R552–R560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG. 1986. Oral perception of the temperature of liquids. Percept Psychophys. 39(1):19–24. [DOI] [PubMed] [Google Scholar]
- Green BG, Alvarado C, Andrew K, Nachtigal D. 2016. The effect of temperature on umami taste. Chem Senses. 41(6):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21:323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Frankmann SP. 1987. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses. 12:609–619. [Google Scholar]
- Green BG, Frankmann SP. 1988. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav. 43(4):515–519. [DOI] [PubMed] [Google Scholar]
- Green BG, George P. 2004. ‘Thermal taste’ predicts higher responsiveness to chemical taste and flavor. Chem Senses. 29(7):617–628. [DOI] [PubMed] [Google Scholar]
- Green BG, Hayes JE. 2003. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol Behav. 79(4–5):811–821. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D. 2012. Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav. 107(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Nachtigal D. 2015. Temperature affects human sweet taste via at least two mechanisms. Chem Senses. 40(6):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. 1993. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 18 (6): 683–702. [Google Scholar]
- Green BG, Schullery MT. 2003. Stimulation of bitterness by capsaicin and menthol: differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem Senses. 28(1):45–55. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Yoshida M, Hazekawa M, Uchida T. 2011. Synergistic effects of sour taste and low temperature in suppressing the bitterness of Aminoleban® EN. Chem Pharm Bull (Tokyo). 59(5):536–540. [DOI] [PubMed] [Google Scholar]
- Huang YA, Roper SD. 2010. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 588(Pt 13):2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. 2004. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 24(45):10260–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER. 2007. TRPM5 and taste transduction. Handb Exp Pharmacol: 287–298. [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Contreras RJ. 2016. Temperature influences chorda tympani nerve responses to sweet, salty, sour, umami, and bitter stimuli in mice. Chem Senses. 41(9):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr, Contreras RJ. 1997. Temperature and amiloride alter taste nerve responses to Na+, K+, and NH+4 salts in rats. Brain Res. 744(2):309–317. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Collings VB, Glanz LM. 1973. Temperature dependence of human taste responses. Physiol Behav. 11(1):89–94. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. 2010. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 35(2):157–170. [DOI] [PubMed] [Google Scholar]
- Moskowitz HR. 1973. Effect of solution temperature on taste intensity in humans. Physiol Behav. 10(2):289–292. [DOI] [PubMed] [Google Scholar]
- Nagaki J, Yamashita S, Sato M. 1964. Neural response of cat to taste stimuli of varying temperatures. Jpn J Physiol. 14:67–89. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K. 1987. Temperature-dependence of the rat and frog taste nerve responses and its origin. Chem Senses. 12: 520. [Google Scholar]
- Nakamura M, Kurihara K. 1991. Differential temperature dependence of taste nerve responses to various taste stimuli in dogs and rats. Am J Physiol. 261(6 Pt 2):R1402–R1408. [DOI] [PubMed] [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. 2009. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 296(4):R960–R971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan T-HT, Mummalaneni S, Melone P, DeSimone JA, Nicolelis MAL, Simon SA. 2009. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci. 106:1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus K, Reisch AM. 1980. The influence of temperature on the threshold values of primary tastes. Chem Senses. 5:11–21. [Google Scholar]
- Pérez CA, Margolskee RF, Kinnamon SC, Ogura T. 2003. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 33(5-6):541–549. [DOI] [PubMed] [Google Scholar]
- Pronin AN, Tang H, Connor J, Keung W. 2004. Identification of ligands for two human bitter T2R receptors. Chem Senses. 29(7):583–593. [DOI] [PubMed] [Google Scholar]
- Roura E, Aldayyani A, Thavaraj P, Prakash S, Greenway D, Thomas WG, Meyerhof W, Roudnitzky N, Foster SR. 2015. Variability in human bitter taste sensitivity to chemically diverse compounds can be accounted for by differential TAS2R activation. Chem Senses. 40(6):427–435. [DOI] [PubMed] [Google Scholar]
- Sato M, Yamashita S. 1965. The effect of temperature on the taste response of rats. Kumamoto Med J. 18(1):41–43. [PubMed] [Google Scholar]
- Schiffman SS, Pecore SD, Booth BJ, Losee ML, Carr BT, Sattely-Miller E, Graham BG, Warwick ZS. 1994. Adaptation of sweeteners in water and in tannic acid solutions. Physiol Behav. 55(3):547–559. [DOI] [PubMed] [Google Scholar]
- Talavera K, Ninomiya Y, Winkel C, Voets T, Nilius B. 2007. Influence of temperature on taste perception. Cell Mol Life Sci. 64(4):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. 2005. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 438(7070):1022–1025. [DOI] [PubMed] [Google Scholar]
- Vihinen M. 1987. Relationship of protein flexibility to thermostability. Protein Eng. 1(6):477–480. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH. 2013. Modulation of central gustatory coding by temperature. J Neurophysiol. 110(5):1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham RW. 2009. Catching fire: how cooking made us human. New York: Basic Books. [Google Scholar]
- Yamashita S, Ogawa H, Kiyoara T, Sato M. 1970. Modification by temperature change of gustatory impulse discharges in chorda tympani fibres of rats. Jpn J Physiol. 20(3):348–363. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Sato M. 1965. The effects of temperature on gustatory response of rats. J Cell Physiol. 66(1):1–17. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Yamada K, Sato M. 1964. The effect of temperature on neural taste response of cats. Jpn J Physiol. 14:505–514. [DOI] [PubMed] [Google Scholar]
- Yokomukai Y, Cowart BJ, Beauchamp GK. 1993. Individual-differences in sensitivity to bitter-tasting substances. Chem Senses. 18: 669–681. [Google Scholar]
- Yuan Z, Bailey TL, Teasdale RD. 2005. Prediction of protein B-factor profiles. Proteins. 58(4):905–912. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee R, Liman E. 2007. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 27(21):5777–5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubare-Samuelov M, Peri I, Tal M, Tarshish M, Spielman AI, Naim M. 2003. Some sweet and bitter tastants stimulate inhibitory pathway of adenylyl cyclase via melatonin and alpha 2-adrenergic receptors in Xenopus laevis melanophores. Am J Physiol Cell Physiol. 285(5):C1255–C1262. [DOI] [PubMed] [Google Scholar]