Abstract
Conjugation with glucuronic acid is a prevalent metabolic pathway of orally administrated curcumin, the bioactive diphenol of the spice turmeric. The major in vitro degradation reaction of curcumin is autoxidative transformation resulting in oxygenation and cyclization of the heptadienedione chain to form cyclopentadione derivatives. Here we show that curcumin-glucuronide is much more stable than curcumin, degrading about two orders of magnitude slower. Horseradish peroxidase-catalyzed oxidation of curcumin-glucuronide occurred at about 80% of the rate with curcumin, achieving efficient transformation. Using LC-MS and NMR analyses the major products of oxidative transformation were identified as glucuronidated bicyclopentadione diastereomers. Cleavage into vanillin-glucuronide accounted for about 10% of the products. Myeloperoxidase and lactoperoxidase oxidized curcumin-glucuronide whereas tyrosinase and xanthine oxidase were not active. Phorbol ester-activated primary human leukocytes showed increased oxidative transformation of curcumin-glucuronide which was inhibited by the peroxidase inhibitor sodium azide. These studies provide evidence that the glucuronide of curcumin is not an inert product and may undergo further enzymatic and non-enzymatic metabolism. Oxidative transformation by leukocyte myeloperoxidase may represent a novel metabolic pathway of curcumin and its glucuronide conjugate.
Keywords: Curcumin, autoxidation, peroxidase, glucuronide, bicyclopentadione, myeloperoxidase
Graphical abstract
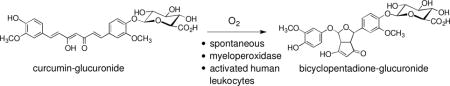
1 Introduction
Conjugation with glucuronic acid or sulfate is the major pathway of phase II metabolism of curcumin following oral administration (1,2). Concurrent with conjugation is the stepwise reduction of the double bonds of the heptadienedione chain of curcumin to yield di-, tetra-, hexa-, and octahydrocurcumin (3). Rapid metabolism by conjugation and reduction together with poor intestinal absorption result in low plasma levels of free curcumin in man, even when large oral doses (8–12 g) are ingested (4). The near absence of free curcumin in human and animal plasma makes it difficult to rationalize its biological and therapeutic effects (5).
An attractive hypothesis, therefore, is to invoke metabolites of curcumin as mediators of its biological effects. Such has been suggested for the glucuronic acid conjugate (Fig. 1A) (6), for the reduced metabolites, especially tetra- and hexahydrocurcumin (7), for the degradation products formed via chain cleavage (vanillin, ferulic acid, and feruloylmethane) (8,9), and, lastly, for its oxidative metabolites, the formation of which has been recognized only recently (10–12). Few studies have tested the biological effects of curcumin-glucuronide in cell culture based assays, and these implied that the glucuronide is biologically inert or less active in the growth inhibition of cancer cells and suppression of inflammatory markers and cytokines (13,14). The reduced metabolites appear to be able to recapitulate some but not all biological activities of curcumin (15–18). For example, tetrahydrocurcumin was active in azoxymethane-induced colon carcinogenesis (15–18) but less active in the inhibition of COX-2 in human colonic epithelial cells (15–18). The cleavage products vanillin and ferulic acid show greater overlap with the activities of curcumin (9,19) – the relevance of these observations, however, has been contested when the initial finding of Wang and co-workers that vanillin and ferulic acid are minor degradation products of curcumin was confirmed, and the mechanism of the degradation reaction was elucidated (20–22). The major pathway of non-enzymatic degradation of curcumin at physiological pH is an oxidative transformation leading to dioxygenated bicyclopentadione (BCP) diastereomers (Fig. 1B) (21). Oxidative transformation of curcumin occurs spontaneously as autoxidation and can also be catalyzed by the peroxidase activity of cyclooxygenase-2 (10).
Fig. 1.

(A) Structure of curcumin-glucuronide. (B) Oxidative transformation of curcumin to its bicyclopentadione derivative. The transformation occurs as a spontaneous autoxidation as well as catalyzed by peroxidases and proceeds with the incorporation of O2 followed by an exchange of water (10,21).
We sought to determine whether curcumin-glucuronide undergoes non-enzymatic and enzymatic oxidative transformation. We describe conditions for oxidative transformation, identify products formed, and tested different peroxidases for in vitro catalysis. Oxidative transformation catalyzed by activated human leukocytes indicated that this may represent a novel metabolic pathway of curcumin.
2 Materials and Methods
2.1 Materials
Curcumin was synthesized from vanillin and acetylacetone as described (23). The isotopic standards d6-curcumin and d6-bicyclopentadione were synthesized as described (24). Horseradish peroxidase (P8250; Type-II, 5 kU/ml; 25.9 mg/ml), myeloperoxidase (M6908), lactoperoxidase (61328), and tyrosinase (T3824) were purchased from Sigma (St. Louis, MO), xanthine oxidase (682151) was from EMD Millipore (Billerica, CA). β-Glucuronidase was from MP Biomedicals (Santa Ana, CA). Chemicals were purchased from Sigma (St. Louis, MO) or Thermo Fisher Scientific (Waltham, MA) and used at highest grade available.
2.2 Synthesis of curcumin-glucuronide
Curcumin-glucuronide was synthesized following a protocol by Moon et al. (25). Curcumin (100 mg, 0.27 mmol) and acetobromo-α-D-glucuronic acid methyl ester (500 mg, 1.26 mmol) were dissolved in 5 ml dimethylformamide. K2CO3 (100 mg, 0.72 mmol) was added, and the solution was stirred for 2 h at room temperature. Then 30 ml cooled H2O were added and the solution was acidified using formic acid. Acidification resulted in the formation of a precipitate which was collected by centrifugation (4°C, 5000 rpm, 20 min). The precipitate was washed with 5 ml 0.2% formic acid and dissolved in 10 ml MeOH/CHCl3 (1:1, by vol.). The solvent was evaporated and the residue was dissolved in 5 ml dry MeOH. In order to remove the acetyl moieties 150 μl NaOH (28% in MeOH) were added, and the solution was stirred for 30 min at 4°C. The methyl ester was hydrolyzed by treatment with 5 ml H2O for 30 min at room temperature. Then 2 N HCl (370 μl) and a few drops of formic acid were added to acidy the solution. The solution was filtered, and the solvent evaporated. The product was purified by RP-HPLC (Econosil C18 column, 250 mm × 10 mm) eluted with a solvent of MeCN/H2 (45/55) with 0.01% acetic acid at a flow rate of 4 ml/min.
2.3 Transformation of curcumin-glucuronide
Curcumin-glucuronide (25 μM) was added to 500 μl 20 mM Na-phosphate buffer pH 7.5. The reaction was monitored in a UV/Vis spectrophotometer by repetitive scanning from 700 to 220 nm every 1 or 2 min or by following the disappearance of the chromophore at 430 nm in the time drive mode. To some reactions K3Fe(CN)6 (15 μM final; from a 5 mM stock solution in water) or horseradish peroxidase (0.01 U/ml) and H2O2 (40 μM) were added. For the initial chromatographic analyses, an HRP-oxidized samples was hydrolyzed with β-glucuronidase, conducted at pH 4 for one hour at 37°.
Lactoperoxidase (0.444 U) was added to curcumin-glucuronide (40 μM) and H2O2 (40 μM) in 500 μl 100 mM K-phosphate buffer pH 6 containing 0.25% BSA. Myeloperoxidase (0.64 U) was added to curcumin-glucuronide (40 μM) and H2O2 (20 μM) in 500 μl 50 mM K-phosphate buffer pH 7 with 0.25% BSA. Tyrosinase (1162 U) was added to curcumin-glucuronide (40 μM) in 500 μl 50 mM K-phosphate buffer pH 6.5 with 0.25% BSA. Xanthine oxidase (0.2 U) was added to curcumin-glucuronide (40 μM) in 500 μl 50 mM K-phosphate buffer pH 7.5 with 0.25% BSA. The enzymatic reactions were monitored in a UV/Vis spectrophotometer by repetitive scanning from 700 to 220 nm every 1 or 2 min.
A large scale incubation of curcumin-glucuronide (1 mg) with 50 μM K3Fe(CN)6 was conducted in 40 ml of buffer for 20 min. Products were extracted using a Waters HLB cartridge and products were eluted with MeOH. Samples were analyzed by HPLC using a Waters T3 column (250 mm × 4.6 mm). Products were eluted using a linear gradient of 20% to 80% MeCN in H2O containing 0.05% acetic acid within 20 min at a flow rate of 1 ml/min.
2.4 Leukocyte isolation and incubations
The study was approved by the Vanderbilt University Medical Center IRB. Normal healthy volunteers were enrolled and gave written informed consent. Blood was drawn (45 ml) from a forearm vein into a syringe that contained 10 ml of a 6% dextran solution and 4.5 ml of sodium citrate. The syringe was kept upright for 60 min to allow red cells to settle. The upper layer containing leukocytes was removed, centrifuged, and the pellet was washed with PBS. Remaining red blood cells were lysed by incubation with H2O for 30 s. Tonicity was restored by adding 10x PBS. Leukocytes were washed, centrifuged, and diluted with PBS. Incubations were performed with 6 × 106 cells per sample. Some of the leukocytes were treated with 200 nM phorbol-12-myristate-13-acetate (PMA), 0.5 mM H2O2 or 100 μM sodium azide. After 30 min at 37°, 50 μM of curcumin or curcumin-glucuronide were added and the reaction proceeded for additional 30 min. The reaction was stopped by the addition of 100 mM HCl and the samples were kept on ice for 10 min. Samples were extracted using Waters HLB cartridges and analyzed by LC-MS.
2.5 LC-MS analyses
LC-MS analyses were performed on a TSQ Vantage Triple Quadrupole instrument using electrospray ionization. Mass spectra were acquired at a rate of 2 s/scan in positive ion mode. The instrument settings were optimized by direct infusion of a solution of BCP in acetonitrile/water 50:50 (v/v). Samples were introduced into the instrument using a Waters Symmetry Shield C18 1.7 μm column (2.1 × 50 mm) eluted with a linear gradient of 15 to 85% acetonitrile in 0.1% formic acid over 3 min followed by isocratic elution with 95% acetonitrile in 0.1% formic acid for 2 min at a flow rate of 400 μl/min. Metabolites were analyzed by selected reaction monitoring (SRM) using the transitions: m/z 369 → 177 for curcumin, m/z 375 → 180 for d6-curcumin, m/z 401 → 249 for bicyclopentadione, m/z 407 → 252 for d6-bicyclopentadione, m/z 545 → 369 for curcumin-glucuronide, m/z 551 → 375 for d6-curcumin-glucuronide and m/z 577 → 249 for bicyclopentadione-glucuronide.
2.6 NMR analysis
Samples were dissolved in 150 μl of d4-methanol in a 3 mm-sample tube and analyzed using a Bruker AV-II 600 MHz spectrometer equipped with a cryoprobe. Chemical shifts are reported relative to residual methanol (δ = 3.30 ppm).
2.7 Statistical analysis
The statistical difference between groups in figure 6A was determined using Mann-Whitney test. Overall differences in BCP-glucuronide levels among treatments (Fig. 6B) were analyzed using one-way ANOVA. If a significant overall treatment difference was found, paired comparisons between treatment groups were performed using paired t test with Bonferroni correction as a post hoc test. Data p values less than 0.05 and 0.01 are indicated in figures by one or two asterisks, respectively.
Fig. 6.
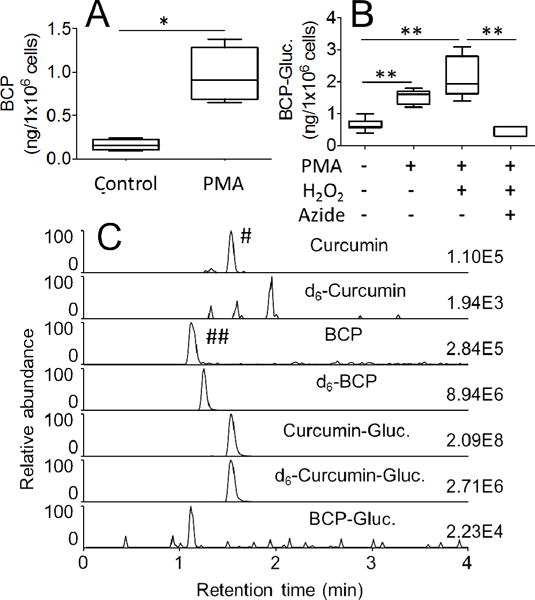
Oxidative transformation of curcumin and curcumin-glucuronide by leukocytes isolated from human blood. (A) Leukocytes were pretreated with phorbol ester (PMA) or vehicle (control) for 30 min, and oxidation of curcumin to bicyclopentadione was quantified by LC-MS. (B) Leukocytes were pretreated with vehicle or phorbol ester (PMA) in the presence or absence of H2O2 (40 μM) and sodium azide (100 μM), and oxidation of curcumin-glucuronide to bicyclopentadione-glucuronide was quantified by LC-MS. (C) SRM-ion traces for the analysis of curcumin, d6-curcumin, bicyclopentadione (BCP), d6-bicyclopentadione (d6-BCP), curcumin-glucuronide (Curcumin-Gluc.), d6-curcumin-glucuronide (d6-Curcumin-Gluc.) and bicyclopentadione-glucuronide (BCP-Gluc.) are shown. Formation of the two peaks marked with # and ## is explained in the Results section. * and ** represent statistically significant differences between incubations with p<0.05 and p<0.01, respectively (n = 3). Whiskers in boxplot represent minimum and maximum values.
3 Results
3.1 Stability of curcumin-glucuronide
The stability of curcumin-glucuronide was tested by incubation in 50 mM phosphate buffer pH 7.5. Incubation of curcumin-glucuronide for 10 min showed little change of the chromophore as determined by repetitive scanning in a UV/Vis spectrophotometer (Fig. 2A). Calculation of the rate of degradation of curcumin-glucuronide required longer reaction times. Thus, curcumin-glucuronide was incubated in buffer at room temperature for 48 h, and remaining curcumin-glucuronide was quantified by RP-HPLC relative to the starting amount. Curcumin-glucuronide was decreased to 55 ± 12% (n = 4) within 48 h reaction time, equivalent to a rate of degradation of 0.48 μM/h. Curcumin, in contrast, degraded about 250-times faster (10).
Fig. 2.

Transformation of curcumin-glucuronide. Curcumin-glucuronide (25 μM) was added to 500 μl 20 mM Na-phosphate buffer pH 7.5, and the sample was scanned repetitively in 1 min intervals for 10 min (A). In (B) horseradish peroxidase (HRP; 0.01 U/ml) was added after the first scan, and 40 μM H2O2 were added after the third scan. The sample was scanned every 1 min for the next 7 min. (C) Potassium ferricyanide K3Fe(CN)6 (15 μM) was added to induce oxidative transformation of curcumin-glucuronide. The hashed arrows indicate decrease and increase of the absorbance during the reaction.
When curcumin-glucuronide was incubated in DMEM cell culture medium in the presence of 10% FBS for 17 hours, the rate of degradation at 37°C was 2.2-times faster than at room temperature.
3.2 Oxidative transformation of curcumin-glucuronide
Addition of horseradish peroxidase (HRP) and H2O2 resulted in rapid transformation of curcumin-glucuronide. The initial rate of the HRP-catalyzed oxidation of curcumin-glucuronide (33.3 ± 3.5 μM/min) was comparable to that of curcumin (40.6 ± 1.8 μM/min). The decrease of the chromophore of curcumin-glucuronide at 430 nm was accompanied by an increase at ≈260 nm, indicating the formation of a spiroepoxide intermediate (Fig. 2B). Formation of a spiroepoxide suggested that the reaction proceeded analogous to the oxidative transformation of curcumin (21). When curcumin-glucuronide was transformed using the oxidizing agent potassium ferricyanide (K3Fe(CN)6) a similar decrease at 430 nm and increase at ≈260 nm were observed (Fig. 2C).
3.3 Identification of the transformation products
The products of the HRP/H2O2-catalyzed transformation of curcumin-glucuronide were analyzed using RP-HPLC with diode array detection. The chromatogram showed three major products eluting as a single peak at 7.5 min (1-gluc) and a double peak (2-gluc and 3-gluc) at 7.9/8.1 min retention time, respectively (Fig. 3A). LC-ESI-MS analysis in the positive ion mode gave a molecular ion of m/z 577 for all three products, indicating an increase of 32 mass units compared to the curcumin-glucuronide substrate (m/z 545). This was compatible with the incorporation of two atoms of oxygen during the transformation.
Fig. 3.
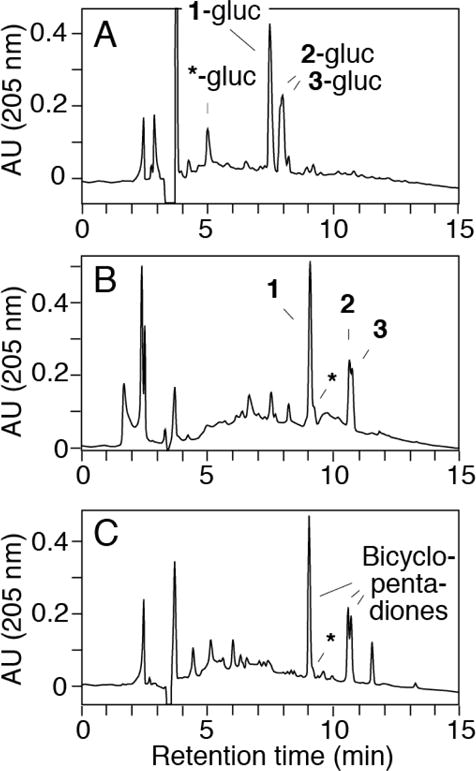
RP-HPLC analysis of the transformation of curcumin-glucuronide by horseradish peroxidase (HRP) and H2O2. (A) Analysis of products from the reaction of curcumin-glucuronide (25 μM) with HRP (0.01 U/ml) and H2O2 (40 μM). (B) The sample was reanalyzed after hydrolysis with β-glucuronidase (pH 4, 1 h at 37°C). (C) Curcumin (30 μM) was reacted with HRP and H2O2. The asterisk indicates the elution of vanillin as the glucuronic acid conjugate (in A) or the free compound (in B and C). Samples were analyzed using a Waters Atlantis T3 5 μm column (250 × 4.6 mm) eluted with a gradient of 20% to 80% acetonitrile in water (0.05% acetic acid) within 20 min at a flow rate of 1 ml/min. Product elution was monitored using a diode array UV detector, and chromatograms recorded at 205 nm are shown.
The reaction mixture was treated with β-glucuronidase to remove the glucuronic acid moiety from the products. The aglycons 1, 2, and 3 eluted later than their glucuronide conjugates and matched retention times and UV/Vis spectra of the BCP diastereomers formed by autoxidation of curcumin (Fig. 3B, C) (21). In LC-MS analyses products 1, 2, and 3 showed the same MS1 and MS2 spectra as the BCP diastereomers (not shown). These data indicated that 1, 2, and 3 were identical to the BCP diastereomers formed from curcumin, and that 1-gluc, 2-gluc, and 3-gluc were the corresponding phenolic glucuronic acid conjugates.
The glucuronic acid conjugate of vanillin was detected as a minor product of the HRP/H2O2 catalyzed transformation of curcumin-glucuronide. Vanillin-glucuronide (marked with an asterisk in Fig. 3A) showed a characteristic UV spectrum with maxima at 230, 279, and 309 nm in the RP-HPLC solvent. Following β-glucuronidase treatment, the peak of vanillin-glucuronide disappeared, and liberated vanillin eluted immediately after the main BCP diastereomer (Fig. 3B) (11). Formation of vanillin was confirmed using LC-MS analyses in the MS1 and MS2 modes. The amount of vanillin-glucuronide formed was about 10% of the three BCP isomers (1-gluc, 2-gluc, and 3-gluc) as estimated from the UV 205 nm absorbance in the RP-HPLC analyses (Fig. 3A).
3.4 NMR analysis of BCP-glucuronide
We used NMR analysis to determine which of the phenolic rings the glucuronic acid moiety of the BCP was attached to. BCP-glucuronide was isolated from a large-scale transformation of curcumin-glucuronide using K3Fe(CN)6. The 1H-NMR signals for the aromatic hydrogens H2″, H5″, and H6″ of 1-gluc were shifted by 0.14, 0.44, and 0.15 ppm, respectively, relative to the unconjugated BCP whereas the corresponding signals H2′, H5′, and H6′ showed little to no differences in chemical shift (Fig. 4). Furthermore, H1‴′ (of the glucuronic acid moiety) showed a cross-peak to C-4″ of the adjacent aromatic ring in a heteronuclear multiple bond correlation experiment. Thus, the glucuronic acid moiety is located at the aromatic ring attached to C-7 of the BCP. The homo- and heteronuclear NMR analyses independently confirmed the structural identification of 1-gluc as a glucuronidated BCP isomer.
Fig. 4.
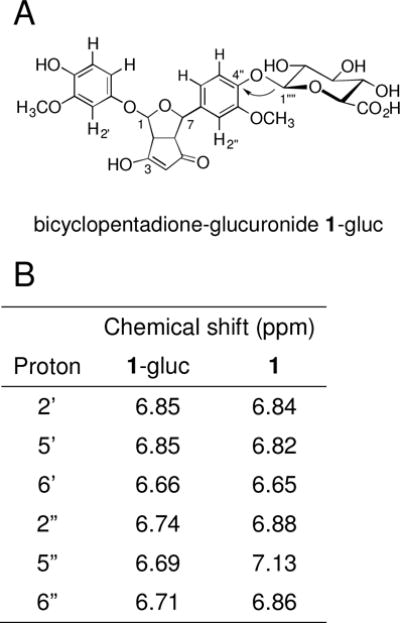
Structure of bicyclopentadione-glucuronide 1-gluc. (A) The arrow indicates the heteronuclear H,C multiple bond correlation observed between H1‴′ of the glucuronic acid and C4″ of the aromatic ring. (B) Chemical shifts (ppm; in CD3OD, 600 MHz) of the aromatic hydrogen atoms of 1-gluc (BCP-glucuronide) and 1 (BCP).
3.5 Enzymatic transformation of curcumin-glucuronide
Since curcumin-glucuronide was oxidized by HRP, we analyzed whether it was a substrate for other peroxidases, namely, myeloperoxidase, lactoperoxidase, and tyrosinase, as well as for xanthine oxidase (26). The enzymatic transformation of curcumin-glucuronide was compared to curcumin as substrate. Because curcumin readily undergoes spontaneous oxidative transformation in the absence of catalytic enzyme, the enzymatic reactions were conducted in the presence of 0.25% BSA which was sufficient to inhibit autoxidation of curcumin.
Repetitive scanning using a UV/Vis spectrophotometer showed a decrease of the curcumin-glucuronide chromophore at 430 nm with lactoperoxidase (0.44 U) and myeloperoxidase (0.64 U) (Fig. 5A,B). Both enzymes transformed curcumin-glucuronide with similar efficiency and showed the characteristic increase at ≈260 nm indicating formation of a spiroepoxide intermediate (21). Tyrosinase did not react with curcumin-glucuronide even when used at much higher enzyme concentration (1168 U) (Fig. 5C). The decrease in absorbance at 430 nm in Fig. 5C was attributed to the addition of aliquots of enzyme that induced a raise in the baseline at lower wavelength that was not indicative of enzymatic transformation. Xanthine oxidase (0.04 and 0.4 U) showed likewise no continuous decrease of the absorbance at 430 nm indicating no reaction with curcumin-glucuronide (Fig. 5D). Product analysis using RP-HPLC and LC-MS confirmed formation of BCP-glucuronides as the major products of the reactions of myeloperoxidase and lactoperoxidase with curcumin-glucuronide (Fig. 5E, F). Curcumin was likewise a substrate for myeloperoxidase and lactoperoxidase and was not transformed by tyrosinase or xanthine oxidase (data not shown).
Fig. 5.
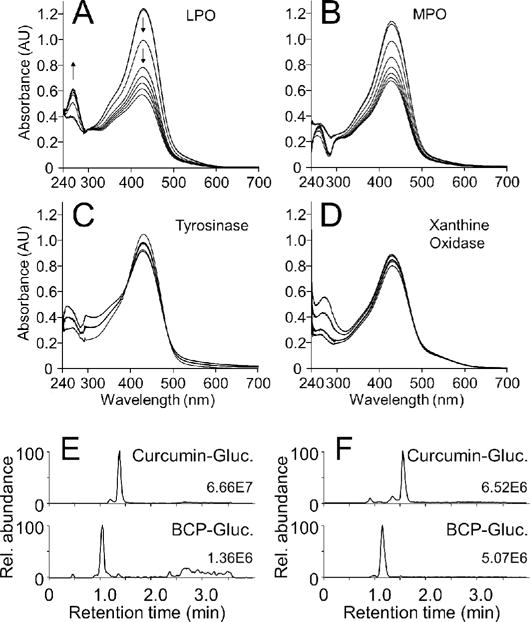
Enzymatic transformation of curcumin-glucuronide. Curcumin-glucuronide (40 μM) was reacted with (A) lactoperoxidase (LPO), (B) myeloperoxidase (MPO), (C) tyrosinase, and (D) xanthine oxidase, each in the presence (A, B) or absence (C, D) of H2O2 (20 μM). Samples were scanned following the addition of enzyme and H2O2 in 2-min intervals for the next 20 min. In (E) and (F) the samples from myeloperoxidase- and lactoperoxidase-catalyzed transformation of curcumin-glucuronide were analyzed using LC-MS in the positive ion mode. Ion traces for curcumin-glucuronide (m/z 545; upper) and bicyclopentadione-glucuronide (BCP-Gluc.; m/z 577; lower) are shown.
3.6 Transformation of curcumin and its glucuronide by human leukocytes
Freshly isolated human leukocytes were treated with PMA to activate myeloperoxidase prior to incubation with curcumin (27). BCP as the major stable oxidation product of curcumin was quantified by LC-MS using d6-BCP as internal, hexadeuterated standard (24). Stimulation of the leukocytes with PMA resulted in a 5-fold increase of the levels of BCP compared to unstimulated cells (Fig. 6A). Formation of BCP in the absence of PMA was possibly due to both enzymatic as well as non-enzymatic oxidation of curcumin.
With curcumin-glucuronide PMA-stimulation resulted in a 3- to 4-fold increase in formation of BCP-glucuronide in comparison to unstimulated leukocytes (Fig. 6B). Additional supplementation of the cells with H2O2 as a peroxidase co-substrate did not increase the amount of BCP-glucuronide formed, indicating that the cells were operating at a maximum capacity for oxidative metabolism of curcumin-glucuronide. When the cells were pre-treated with the heme protein inhibitor sodium azide (28), formation of BCP-glucuronide was reduced to the levels of unstimulated cells. The levels of curcumin-glucuronide (around 500 ng/106 cells) were unchanged by the treatments. Hydrolysis of curcumin-glucuronide to curcumin was below 0.1% and not induced by PMA stimulation (data not shown).
The LC-MS analyses conducted in the SRM mode showed that curcumin-glucuronide and BCP-glucuronide were detected not only in the ion chromatograms of their respective transitions, i.e., m/z 545 to m/z 369 and m/z 577 to m/z 249, but also in the ion traces of the corresponding aglycons, i.e., curcumin (m/z 369 to m/z 177) and BCP (m/z 401 to m/z 249). The two corresponding peaks are indicated by # and ##, respectively, in the chromatograms in Fig. 6C. It appeared that the glucuronic acid moiety was lost from the analytes by fragmentation during the ionization process in the MS interface. This fragmentation was unintentional and could not be decreased by lowering the voltage applied for declustering of solvent adducts using in-source fragmentation. Treatment of the samples with β-glucuronidase removed the peaks for curcumin-and BCP-glucuronide as well as the marked peaks, confirming their origin. The signal of BCP resulting from fragmentation of BCP-glucuronide (marked by # in Fig. 6C) was about 10-fold more intense than the signal for BCP-glucuronide itself, and therefore, was considered in the quantitative analysis (Fig. 6B). Because the internal standard does not contain the glucuronide moiety it can not compensate for its loss during in-source fragmentation of BCP-glucuronide. Therefore, BCP-glucuronide was quantified by adding up both peaks, i.e., the area of the signals at m/z 577 to m/z 249 plus m/z 401 to m/z 249. BCP as well as BCP-glucuronide were quantified using d6-BCP internal standard.
In the case of curcumin-glucuronide we assumed that the isotopic internal standard d6-curcumin-glucuronide undergoes similar in-source fragmentation and compensates for the loss. Thus, the corresponding peak in the curcumin trace marked by ## in Fig. 6C was not included in the quantification of curcumin-glucuronide.
4 Discussion
Curcumin-glucuronide is significantly more stable at physiological pH than curcumin. Curcumin degrades in a matter of minutes (10) whereas curcumin-glucuronide degraded by only about half within three days. Our findings on the stability of curcumin-glucuronide are different from results by Pfeiffer and co-workers who found that curcumin and curcumin-glucuronide degrade at about similar rate (6). There is no ready explanation as to why the outcome would be so different between the studies by Pfeiffer and co-workers and ours since the degradation reactions in buffer are straightforward experiments. In our hands, efficient transformation of curcumin-glucuronide required the addition of either a peroxidase and H2O2 or a chemical oxidizing agent like potassium ferricyanide. Both measures induced oxidation at a rate close to that obtained with curcumin under the same conditions. The major transformation products were bicyclopentadione-glucuronide diastereomers.
Oxidative transformation of curcumin-glucuronide to the respective bicyclopentadiones likely follows the same mechanism as described for curcumin (21). Autoxidation of curcumin is initiated by H-abstraction from a phenolic hydroxyl followed by formation of a phenoxyl radical in a sequential proton loss electron transfer (SPLET) process (29,30). Once the initial radical is formed, the transformation of curcumin proceeds as a radical chain reaction resulting in stable incorporation of two oxygen atoms, one of which comes from O2 and one from H2O, and two cyclization reactions to form the final bicyclopentadione product (cf. Figs. 1, 4) (22).
Although curcumin-glucuronide contains a free phenolic hydroxyl that is available for H-abstraction it does not efficiently undergo autoxidation. In this way it behaves similar to 4′-O-methylcurcumin which likewise does not readily autoxidize although one phenolic hydroxyl is available for hydrogen abstraction (10). It appears that both phenolic hydroxyls are required for efficient autoxidation and this points toward an enabling contribution of the second phenolic hydroxyl through a mechanism that is not yet understood. This contribution is reminiscent of the enabling role of the 3-methoxy groups, lack of which results in reduced autoxidation and incomplete transformation of the natural curcuminoids, demethoxy- and bisdemethoxycurcumin, respectively (22,31).
The BCP-glucuronide has the glucuronic acid moiety attached to C-7 of the BCP. The same ring carries the 4′-O-methyl or lacks a methoxy in the BCP derived from asymmetric curcumin analogs, 4′-O-methylcurcumin and demethoxycurcumin, respectively (21,31). This location is consistent with the proposed mechanism of oxidative transformation where H-abstraction occurs at the unmodified methoxyphenol ring (21). This was also manifest in the formation of vanillin-glucuronide (rather than vanillin or both) which was formed as an unexpectedly abundant cleavage product. Chain cleavage of the heptadienedione of curcumin during spontaneous or enzymatic oxidation is well below 1% (11) but was increased to ≈10% from curcumin-glucuronide. It is possible that cleavage is an alternative reaction pathway during oxidative transformation of curcumin and curcumin-glucuronide. A suggested mechanism for the chain cleavage involves a dioxetane intermediate that cleaves to two aldehydic fragments (32–34). The exact mechanism of vanillin formation during the degradation of curcumin, however, has not been elucidated.
The phenolic hydroxyl of curcumin-glucuronide served as electron donor for the reduction of H2O2 by myeloperoxidase and lactoperoxidase. Myeloperoxidase has been shown to catalyze the one-electron oxidation of xenobiotics into reactive metabolites (35). For example, bioactivation of the methoxyphenol eugenol has been described in activated human neutrophils (27,36). Oxidative transformation of phenolic compounds by activated leukocytes is taken as an indication that the same reaction can occur in vivo (37). Lactoperoxidase can likewise catalyze one-electron oxidation of phenolic hydroxyl groups (38,39). Both enzymes were active with curcumin and curcumin-glucuronide and formed the corresponding BCP products. Although tyrosinase can oxidize catechols to ortho-quinones (40) it did not react with curcumin-glucuronide or curcumin. Since studies on the interaction of curcumin with cyclooxygenase-2 have shown that inhibition can go hand-in-hand with serving as a substrate (7,10,41), we tested the reaction of curcumin and its glucuronide with xanthine oxidase (42). Curcumin has been suggested as an inhibitor of xanthine oxidase (43) although this finding has been contested (44). In our experiments, neither curcumin nor its glucuronide were converted by xanthine oxidase.
Oxidative transformation of curcumin-glucuronide proceeds in a reaction that involves several highly reactive intermediates that are likely to adduct to cellular protein or glutathione (21). In vivo these metabolites are prone to be scavenged, making it difficult to detect the final BCP product in plasma or urine after oral administration to human or animals. In attempting this goal, we observed increased oxidation of curcumin and curcumin-glucuronide upon activation of neutrophils ex vivo by quantifying the corresponding BCP metabolites. Inhibition of oxidative transformation by activated leukocytes using the peroxidase inhibitor azide indicated that oxidation was an enzymatic process, likely catalyzed by neutrophil myeloperoxidase. The direct detection of oxidized metabolites is an important finding. Generally, reactive oxidative metabolites of xenobiotics and phenols can only be detected as the corresponding glutathione adducts (45,46). Curcumin forms a glutathione adduct through Michael reaction of its α,β- unsaturated carbonyl moiety (47,48). Thus, the detection of a curcumin-glutathione adduct in and of itself does not necessarily indicate that oxidative metabolism has occurred. The BCP, however, requires such oxidation for its formation. In fact, curcumin represents the remarkable case of a molecule capable of forming a reactive metabolite that is quenched by itself with the help of molecular oxygen. Our studies indicate that oxidation by activated neutrophils may represent a novel metabolic pathway of curcumin and curcumin-glucuronide. Future studies should address whether oxidative transformation of curcumin-glucuronide contribute to the biological effects of curcumin.
Acknowledgments
This work was supported by award R01AT006896 from the National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH) and in part by pilot awards from the Vanderbilt Institute in Chemical Biology and the NCI SPORE in GI Cancer (5P50CA095103) to CS. PBL is supported by postdoctoral fellowship award 16POST27250138 from the American Heart Association. ONG acknowledges support by training grants 2T32GM07628 and pre-doctoral fellowship award F31AT007287 from NIH. K.U. acknowledges funding by a Grant-in-Aid for Scientific Research (A) (No. 26252018), and Grant-in-Aid for Scientific Research on Innovative Areas “Oxygen Biology: a new criterion for integrated understanding of life” (No. 26111011) of the Ministry of Education, Sciences, Sports, Technology (MEXT), Japan. T.S. was supported by a grant from the JST PRESTO program. Mass spectrometric analyses were in part performed through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404 Core Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
6 Abbreviations
- BCP
bicyclopentadione
- ESI
electrospray ionization
- HRP
horseradish peroxidase
- LPO
lactoperoxidase
- MPO
myeloperoxidase
- PMA
phorbol-12-myristate-13-acetate
- SRM
selected reaction monitoring
Footnotes
Chemical compounds studied in this article:
Curcumin (PubChem CID: 969516); curcumin-glucuronide (PubChem CID: 92024088); curcumin-d6 (PubChem CID: 53464495)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7 Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 2.Hoehle SI, Pfeiffer E, Metzler M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res. 2007;51:932–938. doi: 10.1002/mnfr.200600283. [DOI] [PubMed] [Google Scholar]
- 3.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica; the fate of foreign compounds in biological systems. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 5.Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. BioFactors. 2013;39:14–20. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007;55:538–544. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 7.Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 8.Appiah-Opong R, Commandeur JN, van Vugt-Lussenburg B, Vermeulen NP. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 2007;235:83–91. doi: 10.1016/j.tox.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends Mol Med. 2012;18:138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J Biol Chem. 2011;286:1114–1124. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol Med. 2012;18:361–363. doi: 10.1016/j.molmed.2012.04.011. author reply 363–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketron AC, Gordon ON, Schneider C, Osheroff N. Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry. 2013;52:221–227. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal A, Sung B, Bhanu Prasad BA, Schuber PT, Jr, Prasad S, Aggarwal BB, Bornmann WG. Curcumin glucuronides: Assessing the proliferative activity against human cell lines. Bioorg Med Chem. 2014;22:435–439. doi: 10.1016/j.bmc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji M, Nakagawa K, Watanabe A, Tsuduki T, Yamada T, Kuwahara S, Kimura F, Miyazawa T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014;151:126–132. doi: 10.1016/j.foodchem.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 16.Lai CS, Wu JC, Yu SF, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55:1819–1828. doi: 10.1002/mnfr.201100290. [DOI] [PubMed] [Google Scholar]
- 17.Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55:1646–1654. doi: 10.1002/mnfr.201100454. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi N, Qipshidze N, Munjal C, Vacek JC, Metreveli N, Givvimani S, Tyagi SC. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J Mol Neurosci. 2012;47:128–138. doi: 10.1007/s12031-011-9695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Ji HF. Contribution of degradation products to the anticancer activity of curcumin. Clin Cancer Res. 2009;15:7108. doi: 10.1158/1078-0432.CCR-09-1749. [DOI] [PubMed] [Google Scholar]
- 20.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 21.Gordon ON, Luis PB, Sintim HO, Schneider C. Unraveling curcumin degradation. Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J Biol Chem. 2015;290:4817–4828. doi: 10.1074/jbc.M114.618785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider C, Gordon ON, Edwards RL, Luis PB. Degradation of curcumin: From mechanism to biological implications. J Agric Food Chem. 2015;63:7606–7614. doi: 10.1021/acs.jafc.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabon HJJ. A synthesis of curcumin and related compounds. Recl Trav Chim Pays Bas. 1964;83:379–386. [Google Scholar]
- 24.Gordon ON, Graham LA, Schneider C. Facile synthesis of deuterated and [(14) C]labeled analogs of vanillin and curcumin for use as mechanistic and analytical tools. J Labelled Comp Radiopharm. 2013;56:696–699. doi: 10.1002/jlcr.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 26.Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Thompson D, Constantin-Teodosiu D, Norbeck K, Svensson B, Moldeus P. Metabolic activation of eugenol by myeloperoxidase and polymorphonuclear leukocytes. Chem Res Toxicol. 1989;2:186–192. doi: 10.1021/tx00009a011. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz de Montellano PR, David SK, Ator MA, Tew D. Mechanism-based inactivation of horseradish-peroxidase by sodium-azide - Formation of mesoazidoprotoporphyrin-IX. Biochemistry. 1988;27:5470–5476. doi: 10.1021/bi00415a013. [DOI] [PubMed] [Google Scholar]
- 29.Litwinienko G, Ingold KU. Abnormal solvent effects on hydrogen atom abstraction.2. Resolution of the curcumin antioxidant controversy The role of sequential proton loss electron transfer. J Org Chem. 2004;69:5888–5896. doi: 10.1021/jo049254j. [DOI] [PubMed] [Google Scholar]
- 30.Litwinienko G, Ingold KU. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc Chem Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]
- 31.Gordon ON, Luis PB, Ashley RE, Osheroff N, Schneider C. Oxidative transformation of demethoxy- and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of human topoisomerase IIalpha. Chem Res Toxicol. 2015;28:989–996. doi: 10.1021/acs.chemrestox.5b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares CHL, Bechara EJH. Enzymatic generation of triplet biacetyl. Photochem Photobiol. 1982;36:117–119. [Google Scholar]
- 33.Masuda T, Hidaka K, Shinohara A, Maekawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J Agric Food Chem. 1999;47:71–77. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- 34.Esparan V, Krings U, Struch M, Berger RG. A three-enzyme-system to degrade curcumin to natural vanillin. Molecules. 2015;20:6640–6653. doi: 10.3390/molecules20046640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 36.Thompson D, Norbeck K, Olsson LI, Constantin-Teodosiu D, Van der Zee J, Moldeus P. Peroxidase-catalyzed oxidation of eugenol: formation of a cytotoxic metabolite(s) J Biol Chem. 1989;264:1016–1021. [PubMed] [Google Scholar]
- 37.Lobach AR, Uetrecht J. Involvement of myeloperoxidase and NADPH oxidase in the covalent binding of amodiaquine and clozapine to neutrophils: implications for drug-induced agranulocytosis. Chem Res Toxicol. 2014;27:699–709. doi: 10.1021/tx500019u. [DOI] [PubMed] [Google Scholar]
- 38.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 39.Bafort E, Parisi O, Perraudin JP, Jijakli MH. Mode of action of lactoperoxidase as related to its antimicrobial activity: a review. Enzyme Res. 2014;2014 doi: 10.1155/2014/517164. Article ID 517164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsden CA, Riley PA. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg Med Chem. 2014;22:2388–2395. doi: 10.1016/j.bmc.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 41.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–226. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 42.Nishino T, Okamoto K, Eger BT, Pai EF, Nishino T. Mammalian xanthine oxidoreductase - mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 43.Lin JK, Shih CA. Inhibitory effect of curcumin on xanthine dehydrogenase/oxidase induced by phorbol-12-myristate-13-acetate in NIH3T3 cells. Carcinogenesis. 1994;15:1717–1721. doi: 10.1093/carcin/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 44.Pauff JM, Hille R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod. 2009;72:725–731. doi: 10.1021/np8007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boersma MG, Vervoort J, Szymusiak H, Lemanska K, Tyrakowska B, Cenas N, Segura-Aguilar J, Rietjens IM. Regioselectivity and reversibility of the glutathione conjugation of quercetin quinone methide. Chem Res Toxicol. 2000;13:185–191. doi: 10.1021/tx990161k. [DOI] [PubMed] [Google Scholar]
- 46.Awad HM, Boersma MG, Boeren S, Van Bladeren PJ, Vervoort J, Rietjens IM. Quenching of quercetin quinone/quinone methides by different thiolate scavengers: stability and reversibility of conjugate formation. Chem Res Toxicol. 2003;16:822–831. doi: 10.1021/tx020079g. [DOI] [PubMed] [Google Scholar]
- 47.Mathews S, Rao MNA. Interaction of Curcumin with Glutathione. Int J Pharm. 1991;76:257–259. [Google Scholar]
- 48.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Awasthi YC, Ansari GAS. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem-Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]


