Abstract
Atherosclerosis is one of the most important cardiovascular diseases that involve vessels through the development of fatty streaks and plaques. Plant-based compounds can help treat or prevent atherosclerosis through affecting the involved factors. The main purpose of this review article is to investigate and introduce medicinal plants and their potential activities regarding antioxidant properties, effective on lipids level and development of plaque, atherosclerosis, and progression of atherosclerosis as well as the development of cardiovascular disease and ischemia. To search for the relevant articles indexed in Information Sciences Institute, PubMed, Scientific Information Database, IranMedex, and Scopus between 1980 and 2013, with further emphasis on those indexed from 2004 to 2015, we used these search terms: atherosclerosis, antioxidant, cholesterol, inflammation, and the medicinal plants below. Then, the articles with inclusion criteria were used in the final analysis of the findings. Plant-based active compounds, including phenols, flavonoids, and antioxidants, can be effective on atherosclerosis predisposing factors and hence in preventing this disease and associated harmful complications, especially through reducing cholesterol, preventing increase in free radicals, and ultimately decreasing vascular plaque and vascular resistance. Hence, medicinal plants can contribute to treating atherosclerosis and preventing its progression through reducing cholesterolemia, free radicals, inflammation, vascular resistance, and certain enzymes. They, alone or in combination with hypocholesterolemic drugs, can therefore be useful for patients with hyperlipidemia and its complications.
Keywords: Antioxidant, atherosclerosis, hypercholesterolemia
INTRODUCTION
Atherosclerosis is the most prevalent and important cardiac disease which involves heart and brain. This disease progresses slowly and is the most important cause of mortality that begins from childhood and leads to clinical manifestations in adulthood.[1]
Epidemiological studies have demonstrated a marked association between certain factors and development of atherosclerosis. These factors are referred as coronary artery disease (CAD) risk factors and include hypercholesterolemia, oxidative stress associated with increased free radicals in the blood, smoking, hypertension, diabetes, age, male gender, hyperhomocysteinemia, inflammatory factors, family history, previous cardiac ischemia in the first degree relatives (in men under 55 years and in women under 65 years), atherogenic diet, increased lipoprotein A, and CAD[2] as well as physical inactivity, obesity, increased blood clotting, alcohol, and psychiatric factors (aggression, hostility, and anger).[3] A prospective study demonstrated that atherosclerotic plaque is more prevalent in thoracic aorta artery, carotid artery, and femoral artery compared to people without CAD. Accordingly, the obstruction rate of thoracic aorta artery, carotid artery, and femoral artery is considered a determinative factor in people with atherosclerosis (noncoronary arteries). In that study, the sensitivity and specificity of carotid atherosclerosis were reported 72% and 53%, respectively, for coronary artery obstruction.[4,5]
In the recent decades, in most countries, alternative therapies, particularly phytotherapy and dietary supplements, have been increasingly investigated for the treatment of diseases. In this article, medicinal plants and their potential activities are investigated and introduced regarding antioxidant properties, effects on lipids level and development of plaque, atherosclerosis, and progression of atherosclerosis as well as the development of cardiovascular disease and ischemia.
MATERIALS AND METHODS
To search for relevant articles indexed in Information Sciences Institute, PubMed, Scientific Information Database, IranMedex, and Scopus from 1980 to 2015, with further emphasis on those indexed between 2004 and 2015, these search terms were used: atherosclerosis, antioxidant, cholesterol, inflammation, and the following medicinal plants. After the retrieved articles had been examined, eligible and relevant articles were included in the analysis.
RESULTS
Pathogenesis of atherosclerosis
Pathogenesis of atherosclerosis starts with epicardial coronary arteries and damage to vascular endothelium. Endothelium is responsible for muscle tone and intravenous thrombosis and may be damaged by some conditions such as hypertension, hypercholesterolemia, and smoking. The symptoms of atherosclerosis increase with increase in low-density lipoprotein (LDL) reserves (LDL, high cholesterol) in the blood and its accumulation and reserve start in the inner lining of vessels. LDL accumulation causes stimulation and adhesion of monocytes to endothelium and finally facilitates their differentiation to macrophages. Then, the macrophages are activated to digest fats which lead alongside lipids phagocytosis, to the development of fatty streak in coronary lining.
Then, macrophages lead to increased oxidation of LDL and development of plaques. Development of plaques and proliferation of fibroblasts and smooth muscle cells are associated with the development of fibrinolytic lesion or fat-rich fibrous cap that cause a 90% decrease in vessels, stenosis and hence decrease in blood flow.[6,7] Evolution and progression of atherosclerosis are associated with the involvement of T-lymphocytes and development of acute coronary syndrome.[6] With increased accumulation of lipoproteins in macrophages and addition of necrosis process, lipid core forms a connective tissue, composed of collagen fibers, elastic fibers, proteoglycans, and fibrous capsule, around plaques.[8] A sensitive plaque with a nucleus full of lipid and fibrous cap causes pressure to and rupture of the vessel and plaque, collagen and lipid enter bloodstream thrombogenically and develop venous thrombosis. Calcification of the vessels wall due to atherosclerosis has been consistently considered a risk factor for rupture of atheromatous plaques.[9,10] The severity of plaque rupture and the extent of thrombosis are clinically associated with a wide spectrum of acute coronary syndromes consisting of unstable angina, non-Q-wave myocardial infarction (MI), and Q-wave MI. The risk factors for this disease may be inherited, nutritional, metabolic, hemodynamic, inflammatory, and infectious. Besides that, other potential risk factors are under investigation.[11]
In the recent studies, the role of new factors such as apolipoprotein B (apoB), nitrate, nitrite, fibrinogen, and factor 7, alongside conventional factors, has been addressed in the development of atherosclerosis.[12]
Güldiken et al.'s study demonstrated that increase in apoB and certain lipoproteins such as LDL, very LDL, and lipoprotein A plays a significant role in stimulating inflammatory cycle.[13] Increased oxidative stress leads to increased production of reactive oxygen species (ROS) and hence inflammation and atherosclerosis. Oxidized LDL (OxLDL) causes absorption of monocytes and T-cells, change in macrophages, and migration to endothelial inner lining. OxLDL is then absorbed by collecting receptors and results in the development of foam cells and fatty streaks and hence arterial stiffness.[14]
ApoA serves as an antiatherogenic component through increasing antioxidant activity, prevents oxidation, and exerts anti-inflammatory activity, which inhibits variations in LDL and prevents function of lipoxygenase. A study indicated that apoA causes decrease in vascular lipids and macrophages and prevents atherosclerosis.[15]
Fibrinogen is a circulating glycoprotein that acts in the steps of coagulation response to tissue and vascular injury. The amount of plasma fibrinogen is associated with the risk of acquiring CAD.[15] In addition to playing thrombotic role, fibrinogen causes proliferation, vasoconstriction in the damaged sites of vessels wall, stimulation of platelets accumulation, and regulation of cell adhesion.[16]
Factor 7 is a coagulation protein that contributes greatly to thrombosis. Studies have demonstrated that factor 7 is associated with inflammatory parameters such as interleukin 6 and C-reactive protein in patients with hypercholesterolemia which represents a pathophysiological association between them in patients with atherosclerosis. Moreover, the components of coagulation system (fibrinogen and factor 7) and fibrinolytic factors (t-PA and PAI-1) have been reported to be associated with atherosclerosis.[17]
Because of the expression of endothelial nitric oxide synthase (eNOS) in vessels wall, the amount of eNOS decreases in advanced atherosclerosis, which may be explained by decreased translation or increased instability of eNOS mRNA. Endothelium-dependent dilation occurs in atherosclerotic vessels even before changes occur in vessels structure, and represents decreased eNOS. The eNOS has been demonstrated to become disturbed under certain pathological conditions such as severe hypercholesterolemia, and peroxynitrite is produced instead of nitric oxide.[18]
The use of high-fat foods can affect other risk factors such as homeostasis, markers of inflammation, and endothelial functions. A study demonstrated that following consumption of high-fat food, the plasma concentrations of inflammatory cytokines, and adhesion molecules increased.[19] Endothelial function is disturbed, and atherosclerosis and inflammation progress further.[20]
Atherosclerosis, acute coronary syndromes, and brain ischemia or ischemia can be prevented by inhibiting coagulation and inflammatory activities and plaques.
Etiology
According to some studies, increased antioxidant compounds, decreased blood pressure, decreased cholesterol, and CAD can cause decrease in arterial stiffness and also decrease in the development of atherosclerotic plaques in laboratory animals.[21,22] For example, a study demonstrated that inhibited oxidative stress and decreased lipid peroxidation due to consumption of cranberry was an important cause of decrease in the risk of acquiring atherosclerosis.[23]
Inhibition of oxidative stress and decrease in lipid peroxidation due to use of Anethum graveolens extract were important causes of decreased incidence and treatment of atherosclerosis. Moreover, the antiglycemic property of A. graveolens was demonstrated in this study. Since glycemia is associated with elevated oxidative stress, the antiglycemic property of A. graveolens can represent the supportive effect of this plant.[24]
Diagnosis
The diagnosis of atherosclerosis is made by measuring total cholesterol, LDL, high-density lipoprotein (HDL), triglyceride, blood pressure, and body mass index (BMI), history of smoking, MI, severity of angina, and measuring stenosis by coronary angiography.[25]
Treatment
In the light of the growing prevalence of cardiovascular diseases and reduction in age at development of these diseases, it is necessary to find the drugs and medicinal plants that are routinely used in diet, are capable of controlling and preventing the development of atherosclerotic plaques, and can decrease risk factors for cardiovascular diseases. Regarding cardiovascular diseases, there are many medicinal plants that have a high potential to exert antioxidant effect, and these antioxidant agents play a significant role in fighting free radicals.[26]
The positive effect of vegetarian diet has been shown in slowing down the growth of coronary atherosclerotic lesions and preventing and recovering them as well as the clinically useful effects of this diet in reducing angina, total cholesterol, LDL, and BMI in the patients. The main purpose of this study was to study and introduce these food ingredients and plant-based compounds, with potential antioxidant properties, that are able to prevent formation of free radicals and to reduce lipids level, plaques, atherosclerosis, cardiovascular diseases, and ischemia.
Effective factors on atherosclerosis
A wide variety of factors are effective on atherosclerosis, the most important of them are food fibers, exercise, vitamins, acids, and herbal remedies.
Food fibers are complex carbohydrate, plant-based polymers that are composed of simple sugars and generally classified according to their water solubility. Soluble fibers are composed of gel-forming materials, including pectin, gum, and mucilage, that are easily decomposed by bacteria in the colon. Insoluble fibers include structural fibers or certain networks such as lignin, cellulose, and hemicellulose which are excreted without change. Several in vitro and clinical studies have found the effect of food fibers in decreasing cholesterolemia and in treating cardiovascular disease and atherosclerosis.[27,28]
Exercise
Moderate and cumulative exercise can reduce the atherosclerotic lesions caused by atherogenic diet in thoracic aorta which is associated with decreased plasma and aortic concentrations of F2a, homocysteine, and 8-iso prostaglandin in the animals fed with standard diet. Exercise can exert reinforcing and antioxidant defense activities and has biological activity of nitric oxide.
The restricted effect of exercise on plasma and aortic concentrations of F2a, homocysteine, and F2a 8-isoprostaglandin in the animals on an atherogenic diet, which was associated with nonsignificant decrease in them, can be partly due to variations in the animals’ ability to adapt to cumulative oxidative stress induced by exercise and cholesterol. Total F2a can be considered a more reliable indicator of oxidative stress and lipids oxidation than malondialdehyde (MDA).[29]
Iron, ferritin
The association between plasma ferritin level and CAD is under investigation. Iron overload in cardiac muscle cells starts the first cardiac damage due to iron. Production of hydroxyl radicals is another adverse effect of iron which causes cardiac cells damage. This adverse effect is intensified considerably in case of iron overload. These changes lead to different clinical manifestations such as restrictive cardiomyopathy, pericarditis, and sometimes thoracic angina in the presence of normal coronary arteries. Besides that, conduction disorders and different arrhythmias are seen in these patients. Iron reserves are practically associated with atherosclerosis.[30]
Homocysteine
Homocysteine is a sulfur-containing amino acid which is produced by intracellular demethylation of methionine. Homocysteine has been addressed as an independent and adjustable risk factor for atherosclerosis. Homocysteine, as one of the glutathione precursors, may be released into the blood to fight exercise-induced oxidative stress and hence serve as a useful antioxidant in vascular diseases and atherosclerosis.[31]
Vitamin C (ascorbic acid)
Vitamin C or ascorbic acid is a water-soluble antioxidant which causes other antioxidants, such as Vitamin E and urate, to enter cycle again in addition to scavenging ROS free radicals. It is therefore likely that the use of these two antioxidants prevent the complications of vascular disease and atherosclerosis.[32]
Vitamin E (tocopherol)
A study on seminiferous tubules demonstrated that because of being fat-soluble, Vitamin E or alpha-tocopherol can prevent destructive effects of ROS on sperm parameters.[33] Because tocopherol molecule, as a volatile antioxidant of the chain, can inhibit two lipid peroxyl radicals and hence two potential reactions of peroxidation chain.[34] It can prevent the complications of cardiovascular disease and atherosclerosis.
Acids
Reducing acids can have anti-food effects through binding to the minerals in foods, such as iron and zinc, and preventing gastrointestinal absorption of these minerals. These acids include oxalic acid found in cocoa, spinach, turnip, and rhubarb; phytic acid in barley, grains, corn, and vegetables; and tannins in tea, bean, and cabbage.[35]
In the light of many benefits of the foods containing these acids, as rich sources of antioxidants, it is necessary to use them to treat and prevent cardiovascular disease. However, these acids should be used alongside meat and zinc- and iron-containing diets less often. Calcium deficiency and iron deficiency in diet is due to using meat and high absorption of phytic acid which is because of using grains.
Uric acid
Uric acid can be considered a supportive agent against oxidative stress. Serum uric acid exerts antioxidant properties and serves to scavenge and trap free radicals in human serum. Uric acid is involved with peroxynitrite to develop stable nitric oxide and hence vascular dilation increases and peroxynitrite-induced oxidative stress decreases.[36]
Bacteria
Certain bacteria such as Helicobacter pylori and Chlamydia pneumonia and certain viruses such as cytomegalovirus and herpes virus contribute to the development of atherosclerosis and atherosclerosis through involvement in the cell membrane, oxidation of the host cell membrane, production of oxygen free radicals and damage to the cell membrane, and occasionally induction of apoptosis and increase in oxidation of lipids and cholesterol in the atherosclerotic plaque.[37]
Medicinal plants
Quercus
The antioxidant activity and cardiovascular effects of Quercus robur have been revealed previously. The plants like Q. robur which have high antioxidant activity prevent LDL oxidation, hence preventing atherosclerosis.[38]
Tannins (50%–70%), and in less amounts, gallic acid and ellagic acid are the main constituents of Quercus infectoria.[39] This plant is used for its Mann which is a sweat substance which is released from its leaves. D-mannose is one of these which is used against bladder infections.[40]
Medicago sativa Linn
Medicago sativa contains flavonoids, various amino acids such as proline, hydrocarbons such as sucrose and fructose, polyols such as mannitol, ions such as sodium, potassium, and calcium, and organic acids such as malate and citrate.[41] Studies have indicated that M. sativa seeds have hypocholesterolemic effects and can prevent associated diseases.[42]
Gundelia tournefortii
Gundelia tournefortii has anti-atherosclerotic effects because of having antioxidant properties, decreasing cholesterol through inhibiting its synthesis, and inhibiting cholesterol oxidation. Through modulation of LDL, lipids, and apos, which is the main property of G. tournefortii, this plant can fight risk factors for development and progression of atherosclerosis. This is directly associated with the presence of coumarins, alkaloids, flavonoids, and terpenoids, repeatedly reported by previous studies. For example, a study found that the protective and antioxidant properties of G. tournefortii are due mainly to Vitamin E and alpha-tocopherol.[43]
Through its antioxidant effects, G. tournefortii can inhibit oxidation. It is an appropriate plant to modulate and improve diet. The compounds found in G. tournefortii leaf, such as cinarin and luteolin, may contribute to decreasing synthesis and levels of cholesterolemia.[44]
Pulicaria gnaphalodes
Pulicaria gnaphalodes can play a significant role in keeping foods and maintaining humans’ health because of containing phenolic compounds and exerting antioxidant and antiradical compounds. P. gnaphalodes leaf has such properties and contains many types of flavonoid compounds, tannins, and anthocyanins. These compounds exert antioxidant properties against risk factors for development and progression of atherosclerosis.[45]
Valeriana officinalis
Hydroalcoholic Valeriana officinalis root extract mitigates physiological responses to anxiety such as blood pressure and pulse rate. Vascular endothelium is the biggest organ of the body's endocrine system and plays an active role in controlling vascular tone. Nitric oxide is the main cause of endothelium-dependent vascular dilation of L-arginine pathway. Given the significance of disturbed levels of nitric oxide during atherosclerosis, the most prevalent cardiovascular disease, L-NAME which is the inhibitor of this pathway was used.
This extract has been reported to exert relaxant effects in the presence of L-NAME, which are not exerted through endothelial cells but are exerted directly on smooth muscles of the vessels wall. They can, therefore, serve to treat the diseases causing endothelial dysfunction such as atherosclerosis, hypercholesterolemia, diabetes, and hypertension. Accordingly, hypotension, following administration of these extracts, is due to decreased resistance of peripheral arteries. In other words, this extract's effect is exerted through relaxing smooth muscle cells of vessels wall.[46]
Eremurus persicus
Berberine, present in Eremurus persicus, can reduce lipogenesis and exert inhibitory effects on lipid and oxidation. E. persicus can be used as a supplementary antioxidant to prevent or treat certain diseases such as diabetes, liver diseases, and atherosclerosis.[47] A study demonstrated that the activity of antioxidant enzymes such as catalase and superoxide dismutase was higher in the livers of the rats on an E. persicus-containing diet than the control group, representative of the inhibitory effect of E. persicus on lipids peroxidation through increasing antioxidant enzymes. E. persicus causes regulation of sugar hemostasis through decreasing production of sugar, oxidative stress, and vascular diseases.[48]
Sesamum indicum Linn
The compounds of Sesamum indicum decrease development of atherosclerotic lesions, the levels of plasma triglyceride and cholesterol, and LDL-cholesterol (LDL-C).[49] Antioxidant and anti-inflammatory properties of S. indicum and the positive effect of this medicinal plant on lipoprotein can potentially contribute to the process of development of atherosclerotic plaques.[50] Lignan, sesamolinol, and sesamol found in S. indicum essential oil, exert inhibitory effects on membrane lipid peroxidation, microsomal peroxidation, ADP-Fe3+/NADH-induced peroxidation, and Cu ions-induced LDL oxidation.[51] Sesamolinol and sesamin are the most abundantly found lignans in S. indicum with antioxidant effects. Sesamin with antioxidant effect, present in S. indicum lignan, is hydrophilic and exerts a highly potent antioxidant effect.[52]
Allium ampeloprasum
Allium ampeloprasum increases some enzymes such as superoxide dismutase, catalase, peroxidase, and glutathione peroxidase. The plants from family Alliaceae can prevent development and progression of neurological and cardiovascular complications of diabetes because of containing a potent inhibitor of aldose reductase, i.e., isoliquiritigenin. This compound can produce prostaglandin I2 which exerts dilatory effects on the vessels and aorta in diabetes conditions.[53]
A. ampeloprasum has considerable antioxidant effects because of containing several flavonoids with antioxidant and protective properties including sulfur-containing compounds such as diallyl disulfide, ajuin, and allicin. The antioxidant properties of A. ampeloprasum have been reported to be even greater than other plants from the family Alliaceae.[54]
Allium latifolium
Cysteine sulfoxides and sulfoxide acids of Allium latifolium have antidiabetic and antioxidant effects.[55] Antioxidant agents with lipid peroxidation-reducing property are abundantly found in A. latifolium. As well, the flavonoids of this plant cause decrease in oxidative stress and prevent hypertension and atherosclerosis.[56]
Origanum majorana spp.
Origanum majorana is one of the most important plant resources of phenolic antioxidants. Origanum vulgare has high levels of dietary antioxidants and O. majorana has moderate levels of dietary antioxidants. The antioxidant effects of O. majorana essential oil are dose-dependent and a little lower than those of ascorbic acid or butylated hydroxytoluene. A study attributed this effect to high concentration of phenolic compounds such as carvacrol and thymol methyl ether in the O. majorana essential oil.[57] These compounds cause equilibrium between free radicals-producing systems and scavenging systems, leading to oxidative stress, and prevent development of foam cells in atherosclerotic vessels.
Morus nigra fruit
Morus nigra fruit is a rich source of anthocyanins. Anthocyanins are glycosylated derivatives of polyhydroxy and polymethoxy from cathinone 2 of phenyl benzopyrylium, i.e., cathinone flavylium. Aglycone is the main constituent of anthocyanins which is composed of binary bonds. Many studies have demonstrated antioxidant activities and health benefits of anthocyanins found in different fruits and vegetables. Decreasing angiogenic index and the levels of triglyceride and free fatty acids is another pharmacological property of anthocyanins. Because of exerting anticancer, antioxidant, anti-angiogenic, anti-atherosclerotic, and anti-inflammatory properties, anthocyanins help the body keep healthy, which is a remarkable issue.[58]
Linum usitatissimum
Linum usitatissimum contains phenolic compounds, protein, carotenoid, anthocyanin, flavonoid, estrogen, Vitamin E, Vitamin C, proline, and fiber. L. usitatissimum seed is an appropriate source of unsaturated fatty acids, mainly including omega-6 and omega-3, at 0.3/1 ratio, alpha-linolenic acid, lignans, dietary and protein fibers, minerals, and vitamins. Linolenic acid, found in fatty acids of L. usitatissimum essential oil, exerts useful effects in decreasing cholesterolemia, LDLT, atherosclerosis and associated heart disease, and atherosclerosis.[59]
Portulaca oleracea Linn
Study of Portulaca oleracea effect on hepatocyte oxidation system and nonenzymatic glycosylation of hemoglobin and red blood cells demonstrated that this plant has an acceptable antioxidant property. The antioxidant effects of P. oleracea can be due to the presence of omega fatty acids such as alpha-linolenic acid, flavonoids, coumarins, alkaloids, 3-monoterpenes, and betalain. Betalain exerts antioxidant and anti-atherosclerotic effects and prevents different diseases, including cardiovascular and liver diseases, through inhibiting free radicals.[60]
Silybum marianum Linn
Silybum marianum is a medicinal plant with hepatoprotective effects and has several compounds, such as flavonoids, with antioxidant, cell membrane-stabilizing, and blood glutathione-reducing properties, that have exerted optimal in vitro effects on several diseases including hypolipidemia, atherosclerosis, and vascular disease.[61]
Ziziphus jujuba
Ziziphus jujuba has antidiabetic effects. Z. jujube is likely to have antioxidant properties especially in two systems - hemolysis of red blood cells and nonenzymatic glycosylation. Besides that, red blood cells are exposed to large amounts of oxygen and are appropriate sites for development of free radicals under pathological conditions, which leads to destruction of free radicals. Z. jujube can be examined for prevention of this condition.[62]
Bunium persicum Boiss and Zataria multiflora
Bunium persicum and Zataria multiflora (at 0.6% and 0.1% concentrations, respectively) can be considered to have antioxidant activity equal to a synthetic antioxidant, butylated hydroxyanisole (BHA), at 0.2% concentration in soybean oil. The antioxidant activity of the studied essential oils was likely to be related to the monoterpene compounds present in them. This study can be an introduction to use of B. persicum and Z. multiflora in foods and for treatment and prevention of cardiovascular diseases and atherosclerosis.[63]
Camellia sinensis
Camellia sinensis seed essential oil has a potent antioxidant property which can help keep sunflower oil more efficiently at 5% concentration. This therapeutic effect of C. sinensis, with different concentrations of thymol and carvacrol in sunflower oil, can slow down oxidation and is useful to treat and prevent cardiovascular diseases and atherosclerosis.[64]
Hypericum perforatum Linn
Hypericum perforatum has certain flavonoids such as flavonol, flavones, bioflavonoids, and catechins, phenolic compounds, essential oils, acids, volatile oils, carotenoids, beta-sitosterols, and phytosterols. These compounds can exert efficient antioxidant effects and destroy free radicals. H. perforatum is used to treat cardiovascular diseases and atherosclerosis and helps decrease atherosclerotic lesions.[65]
Amaranthus caudatus Linn
Amaranthus caudatus contains high amounts of protein, beta-sterol, phytosterol, minerals, vitamins, and unsaturated fatty acids. Different types of A. caudatus have tocotrienol, tocopherol, and scovaline which contribute to cholesterol biosynthesis. Beta-carotene and ascorbic acid have antioxidant activity and cause destruction of free radicals. Flavonols and anthocyanins play a significant role in regulating and decreasing different risk factors for cardiovascular diseases including decreasing platelets accumulation and increasing antioxidant activity and markers of inflammation. A. caudatus is used to treat allergy, liver diseases, and cardiovascular diseases.[66]
Kelussia odoratissima
Kelussia odoratissima is a herbaceous, edible plant with large amounts of polyphenols with antioxidant property. K. odoratissima can decrease oxidative stress and protect the body's metabolic tissues such as liver against chemical damage. Oral use of K. odoratissima can decrease total cholesterol, cholesterol, LDL, and triglyceride.
Given the oxidative stress-causing role of polysaccharides, flavonoids, glycoproteins, polypeptides, steroids, alkaloids, and pectin, found in medicinal plants, the potential hypoglycemic and hypolipidemic effects of some of the medicinal plants used to treat diabetes, such as aerial parts of Albizia odoratissima, can be partly explained in terms of preventing biochemical variations in the blood. Besides that, caffeic acid, present in A. odoratissima, has antioxidant properties because of having o-quinol.[67]
Camellia sinensis
There are growing evidence on the significant contribution of dyslipidemia, oxidative stress, and inflammatory factors in pathogenesis of diabetes and development of its complications. In patients with diabetes mellitus, different factors such as increased formation of oxygen-free radicals cause an increase in incidence of atherosclerosis and cardiovascular diseases through increasing glycemia and intensifying lipid peroxidation. In this regard, cardiovascular problems due to diseases, especially metabolic disorders such as diabetes mellitus, hyperlipidemia, and obesity, affect a high proportion of population especially older people.[68]
This imposes a heavy burden on health care system. The main compounds of 20% fraction of C. sinensis are caffeine, epicatechin gallate, quercetin, and kaempferol. Administration of C. sinensis has optimal effects on incidence and progression of diabetes complications.[69] In addition to exerting antioxidant and cardioprotective properties, this plant can inhibit angiotensin I-converting enzyme and hence decrease arterial blood pressure.[70]
Allium sativum Linn
Allium sativum causes inhibition of cyclooxygenase and lipoxygenase and prevents accumulation of thrombocytes.[71] In addition, large amounts of flavonoids, such as kaempferol with antioxidant, antidiabetic, and cardiac system- and bloodstream-protective properties, have been demonstrated to exist in this plant. Administration of total A. sativum extract causes decrease in the levels of lipid peroxidation markers, such as MDA and superoxide and hydroxyl radicals, tissue-protective enzymes with antioxidative property, arterial blood pressure, and atherosclerosis.[72]
Morus nigra
L-butanol of M. nigra leaf decreased arteries atherosclerotic plaques in hypercholesterolemic rabbits. L-butanol of M. nigra leaf decreased serum lipids concentration and atheromatous thickness of arteries intima in hypercholesterolemic rabbits.[73]
Olea europaea
Olea europaea powder has antioxidant property and prevents LDL oxidation. Previous studies found that O. europaea leaf contains a compound, referred to as oleuropein, that prevents LDL oxidation, and because LDL oxidation is the main stage of development of atherosclerotic plaques, oleuropein is considered an important agent to treat atherosclerosis.[74]
Punica granatum
Measuring antioxidant property of Punica granatum kernel essential oil demonstrated that its antioxidant property is approximately equal to that of a commercial antioxidant agent, BHA. The amount of phenolic compounds in P. granatum kernel essential oil was reported to be 0.015%. These potent antioxidant compounds are also useful to treat atherosclerosis and vascular diseases.[75]
Mentha piperita Linn
Mentha piperita extract contains large amounts of phenolic and flavonoid compounds and exhibits acceptable antioxidant activity.[76]
Rosmarinus officinalis
Rosmarinus officinalis has been demonstrated to have high antioxidant activity that is directly associated with phenolic content of this plant.[77]
Trigonella foenum-graecum Linn
Fat-free Trigonella foenum-graecum seed, alongside diet, total cholesterol, and triglyceride, decreases insulin-dependent diabetes and LDL considerably.[78]
Coriandrum sativum
Coriandrum sativum has hypocholesterolemic and antioxidant properties that can be due to phenolic and carotenoid compounds present in this plant. Anti-radical property of C. sativum has been reported to be 80%. Phenolic compounds serve as iron donators and are likely to neutralize unpleasant reactions induced by free radicals in the body. Antioxidants can, therefore, cause decrease in the risk of acquiring cardiovascular diseases, atherosclerosis, and MI.[79]
Trachyspermum copticum Linn
Diphenyl picryl hydrazyl is a stable and nitrogen-containing free radical that is widely used to test free radicals-scavenging. According to some studies, the antioxidant property of diphenyl picryl hydrazyl of Trachyspermum copticum was found to be greater than that of C. sativum and to cause decrease in the risk of acquiring cardiovascular diseases, atherosclerosis, and MI.[80]
Nigella sativa
Plant-based extracts, especially Nigella sativa seed extract, contains phytosterols consisting of beta-sitosterol, stigmasterol, and campesterol. N. sativa compounds cause changes in the levels of lipoproteins appropriate for markers of inflammation and plasma antioxidant properties. The presence of phytosterols in N. sativa causes decrease in development of atherosclerotic lesions. Moreover, N. sativa causes prevention of LDL oxidation.[81]
Studies demonstrated that thymoquinone and its metabolites, i.e., dihydrothymoquinone, prevent lipids nonenzymatic peroxidation in mice liver. This compound ameliorates free radicals-induced renal injuries in rats and mouse[82] and induces antioxidant and hence antitoxic effects through increasing oxidants-scavenging system.[83]
Vitis sylvestris
Vitis sylvestris is an acidic extract and ancient product which is used as seasoning and has many pharmaceutical uses. V. sylvestris contains certain flavonoids such as catechin and anthocyanins.[84] A study on V. sylvestris acute effects on some of the risk factors for atherosclerosis demonstrated that acute (daily) use of V. sylvestris caused decrease in glucose, fibrinogen, OxLDL, and MDA and ameliorated the severity of atherosclerotic plaque. This is probably due to the effect of flavonoids on inflammatory reactions and arachidonic acid metabolism, decreased production of free radicals and peroxidation of lipids, and increased HDL concentration.
V. sylvestris ameliorates the destructive effects of high-cholesterol diet through decreasing the levels of lipids profile risk factors and increasing antioxidant capacity.[85] Moreover, a study demonstrated antioxidative effects of flavonols and their glycosides such as quercetin, myrcene, and kaempferol on free radicals.[86] Moreover, polyphenols are adequately absorbed and therefore are potentially able to exert their biological effects such as antioxidant capacity-increasing.[87]
Glycyrrhiza glabra
Glycyrrhiza glabra root contains some flavonoids of types of flavone and chalcone for which pharmacological studies have reported antibacterial, antitumor, and antioxidant activities.[88] Glycyrrhizin is considered the sweet substance of G. glabra and if pure, it is seen as a crystalized and white powder. Glycyrrhizin concentration is 1%–6% in the dried root and exerts hypolipidemic effects. Glycyrrhizin and its nonsugar constituent, glycyrrhetic acid, are the main compounds of G. glabra and are used to treat hyperlipidemia.[89]
The presence of some compounds with potent antioxidant activity in G. glabra extract and its hypolipidemic property has caused this plant to be known as an effective agent to prevent progression of atherosclerosis, especially in people at high risk (with hypercholesterolemia). G. glabra extract caused a significant decrease in the lesions of vessels wall compared to the control group with hypercholesterolemia. The only lesions were foam cells developed in intima. G. glabra extract contains certain flavonoids such as hispaglabridin A, hispaglabridin B, and formononetin that affect the metabolism of arachidonic acid and cause decrease in production of free radicals.
They can also exert antiplatelet, anti-inflammatory, and antioxidant properties.[90] Isoprenyl, chalcone, formononetin, glabridin, and isoliquiritigenin are the antioxidant compounds isolated from G. glabra root. Among these compounds, glabridin has the highest concentration in the extract.[91] Through preventing activity of LDL NADPH oxidase, glabridin prevents cholesterol oxidation.[92]
Gundelia tournefortii Linn
Polyphenolic content and consequently antioxidant property of G. tournefortii seed is greater than other aerial parts. The effect of G. tournefortii is considerable in modulating lipids and apos as the most important risk factors for development and progression of atherosclerosis. Besides that, OxLDL is considered an important factor for atherosclerosis pathogenesis and one of the pioneering agents in development of atherosclerosis. G. tournefortii effect is, therefore, considerable in decreasing these factors.[93]
ApoB has been recently known to be a risk factor for cardiovascular disease and its amount represents the particles leading to atherogenic; therefore, decrease in apoB amount leads to decrease in the risk factors for cardiovascular disease.[94]
Glycine max M glycine is considered a protein-rich food in Asia and a cholesterol-free meat in the US traditional cuisine. Several studies have demonstrated that S. marianum decreases cholesterolemia by approximately 10%. The use of S. marianum decreases cholesterolemia and improves LDL/HDL ratio. Average daily consumption of 47 g S. marianum decreases cholesterolemia by approximately 9%, LDL by approximately 13%, and triglyceride by approximately 10%. The effects of S. marianum were less marked on HDL. Evidence indicates that the isoflavones existing in S. marianum are the main factors effective in decreasing cholesterolemia.
In addition, the proteins found in S. marianum are likely to play a more important role than these flavones. In the USA, the Food and Drug Administration has permitted the producers of S. marianum-containing foods to label their products as heart healthy.[95]
The O. europaea leaves have been shown to possess hypotensive, hypolipidemic, vasodilator, as well as antioxidant activities.[96] The leaves are the most potent part of the O. europaea regarding polyphenolics and Oleuropein which have good effects on cardiovascular disease and atherosclerosis.[97]
Vitis vinifera seeds are used for skin disorders and vomiting. It has high level of polyphenolic compounds with antioxidant activity. Recent studies have revealed that V. vinifera seeds possess hypotensive, hypolipidemic, and endothelium protective properties.[98,99]
Theobroma cacao seeds are effective in cardiovascular disease and are potent vasodilator. Hence, they are used as hypotensive remedy. The effects have been attributed to polyphenolics, especially to epigallocatechin. Epigallocatechin causes expression of VCAM-1 and ICAM-1 in apoE gens.[100,101]
A combination of Hordeum vulgare and Avena sativa extracts have been shown to reduce hyperlipidemia and clotting factors. H. vulgare has substantial effect on hyperlipidemia, especially on LDL. It also increases the HDL, hence is a good remedy for hyperlipidemic patients.[102]
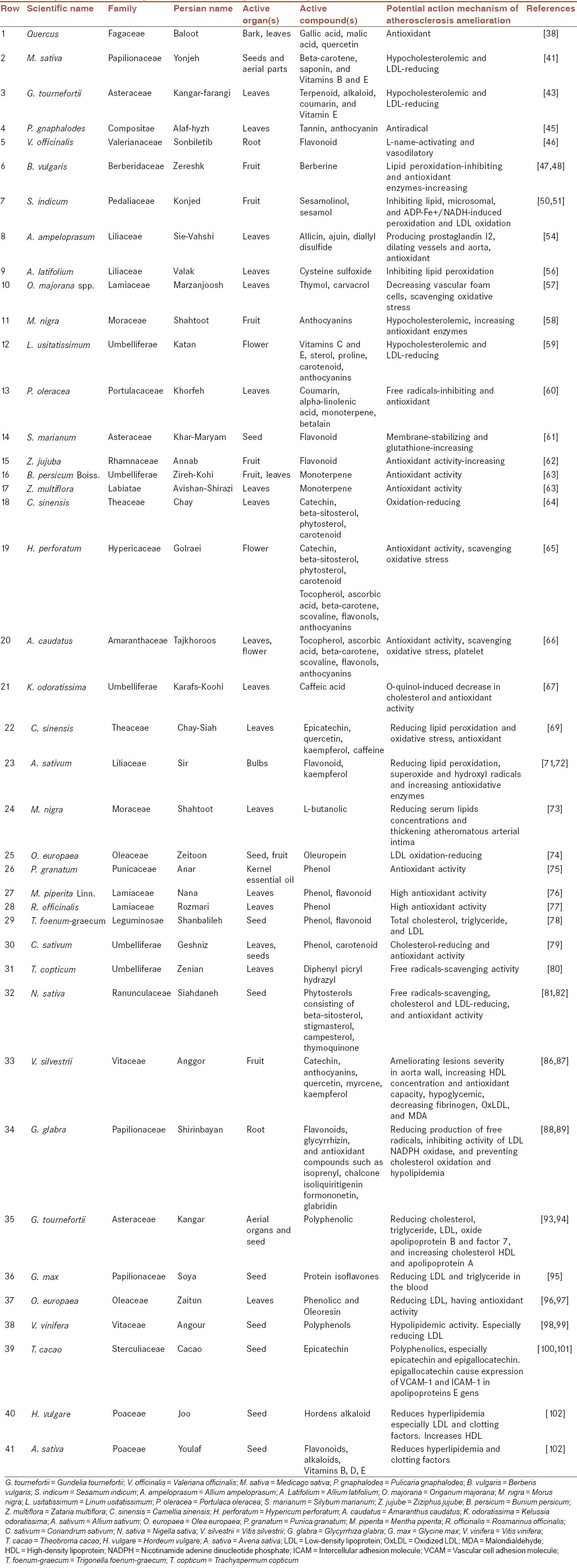
Table 1 enlists some of the medicinal plants, their active organs and compounds, and the potential action mechanism on atherosclerosis. As shown in Table 1, we can examine these plants, including those from the same families, for treating atherosclerosis.
Table 1.
Important medicinal plants and their compounds with potential mechanism actions on atherosclerosis
DISCUSSION
Atherosclerosis is an important cause of mortality. The drugs that are considered to be effective to prevent and treat atherosclerosis are still being challenged. Moreover, many efforts are being made by pharmaceutical industries to develop more efficient drugs. The use of medicinal plants is still being welcome across the world.[1] The following factors have been demonstrated to contribute to development of atherosclerosis: coagulation system, inflammation, hyperlipidemia, and environmental factors. Besides that, the conducted studies on atherosclerosis have demonstrated the active effects of medicinal plants and plant-based compounds in decreasing and ameliorating atherosclerosis with remarkable therapeutic effects in decreasing glucose, fibrinogen, OxLDL, and MDA, decreasing production of free radicals, preventing LDL NADPH oxidase activity and cholesterol oxidation, decreasing hyperlipidemia and high antioxidant activity, producing prostaglandins, and dilating vessels and aorta.[103,104,105,106,107,108,109,110]
Studying and introducing these food ingredients and plant-based compounds with potential antioxidant properties can contribute to preventing formation of free radicals and decreasing lipid levels, plaques, atherosclerosis, cardiovascular disease, and ischemia. Phenolic and polyphenolic compounds, flavonoids, kaempferol, anthocyanin, catechin, quercetin, sterol, carotenoid, caffeic acid, beta-carotene, and gallic acid are the most important active compounds with these properties.[111]
Coagulation system
Studies have confirmed an association of the constituents of coagulation system (fibrinogen and factor 7) and fibrinolytic factors including tissue plasminogen activator and tissue plasminogen inhibitor with atherosclerosis.[38]
Inflammation
Throughout inflammation, macrophages produce some cytokines such as interleukin 1 and tumor necrosis factor which cause increase in leukocytes adhesion and platelet accumulation, and monocytic chemotactic proteins cause increase in leukocytes function inside atheromatous plaques. The free radicals produced from macrophages cause proliferation of smooth muscle cells through oxidizing LDL and hence atherosclerosis becomes symptomatic. This condition is the main complication due to heart attack and stroke. NADPH oxidase, NOS, myeloperoxidase, xanthine oxidase, lipoxygenase, cyclo-oxygenase, and the weakened antioxidant defense system contribute to development of atherosclerosis.[112]
Environmental factors
Coronary arteries involved in atherosclerosis are one of the risk factors for atherosclerosis. Smoking causes vascular atherosclerosis more often than other environmental factors.[113]
Hyperlipidemia
Hyperlipidemia is one of the risk factors for atherosclerosis, diabetes-induced complications, and especially cardiovascular disease. Diabetes is a long-term complication of hyperlipidemia that involves many of the body's organs and is responsible for diabetes-associated morbidity and mortality and is effective in acquiring atherosclerosis.[114]
Studies on atherosclerosis have demonstrated that flavonoids and phenolic compounds present in plants exert several biological effects including antioxidant, free radicals-scavenging, anti-inflammatory, and anticancer. Free radicals-induced oxidization of flavonoids leads to development of less active and more stable radicals and exacerbation of the disease.[115]
According to Grenett et al. study, the polyphenols of catechin, quercetin, and ethanol exert supportive effects against vascular disease, which can be explained by increased fibrinolytic activity and expression of the proteins involved in fibrinolytic system.[116]
Prasad and Kalra study demonstrated the presence of MDA as the final product of peroxidation and the association of oxygen free radicals amount with the risk of atherosclerosis. MDA decreases with antioxidants-induced decrease in atherosclerosis. This is due to the presence of flavonoid compounds in these plants that cause peroxidation of lipids and reinforcement of antioxidant enzymes.[117]
Postprandial hyperlipidemia has been also known to be a risk factor for atherosclerosis. Flavonoid compounds existing in plants cause decrease in free radicals and support against tocopherol, isolation of LDL-α or production of OxLDL-α, isolation of metal ions involved in oxidation reactions, and decrease in the risk of acquiring atherosclerosis.[118]
Natella et al. study demonstrated that LDL oxidation decreases after consumption of procyanidins (grapes kernel) antioxidant in food, and causes decrease in atherosclerosis.[119]
Some plant-based essential oils and extracts can cause increase in the natural and OxLDL tendency to the respective receptors through their antioxidant properties. This can cause optimal effects in treating atherosclerosis and decreasing plasma cholesterol.[120]
Studies have shown an inverse association between consumption of polyphenols-rich foods and cardio-arterial diseases. This finding is due to polyphenols ability to inhibit LDL-C oxidation. Interacting directly with lipoproteins and exerting indirect effects through accumulating in arterial microphages, polyphenols inhibit LDL-C oxidation. This effect is exerted by P. granatum polyphenols, as well. These polyphenols increase the activity of serum paraoxonase, which leads to hydrolysis of lipid peroxides in oxidized lipoproteins and atherosclerotic plaques. Much evidence exists on anti-atherosclerotic activity of paraoxonase.[121]
Aviram et al. demonstrated the effects of P. granatum polyphenols in humans and mice with atherosclerosis and defective production of aposE. According to Aviram et al. study, feeding atherosclerotic mice with polyphenols-rich P. granatum juice caused a significant inhibition of atherosclerosis progression, which was attributed to the protective role of P. granatum polyphenols in LDL-C oxidation.[122]
Studies have demonstrated that internal anger was associated with carotid intima-media thickening and development of atherosclerotic plaques. The association of hostility and anger with CAD can be largely explained by releasing catecholamine and increasing cardiovascular reactivity.[123]
CONCLUSION
The active effects of medicinal plants and synthetic compounds can indicate that medicinal plants and their effects and uses have never been disregarded. The active ingredients of medicinal plants including flavonoids and other phenolic compounds with antioxidant activity can scavenge free radicals and be effective against atherosclerosis. There are still many issues about atherosclerosis which we have limited information on and therefore deserve much further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
MS prepared the first draft; MRK, MB and SA completed it. MRK and SA edited the last version. All read and confirmed it.
Acknowledgments
We would like to thank Shahrekord University of Medical Sciences for its support.
REFERENCES
- 1.McGill HC, Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72(5 Suppl):1307S–15S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. The pathologenesis of atherosclerosis. In: Braunwald E, editor. Heart Disease. London: WB Saunders; 1994. p. 1105. [Google Scholar]
- 3.John A, Farmer MD, Antonio M, Gotto JR. Dyslipidimia and other risk factors for CAD. In: Braunwald E, editor. Heart Disease. London: WB Saunders; 1997. p. 1126. [Google Scholar]
- 4.Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80:1429–33. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 5.Giral P, Pithois-Merli I, Filitti V, Levenson J, Plainfosse MC, Mainardi C, et al. Risk factors and early extracoronary atherosclerotic plaques detected by three-site ultrasound imaging in hypercholesterolemic men. Prévention Cardio-vasculaire en Médecine du Travail METRA Group. Arch Intern Med. 1991;151:950–6. [PubMed] [Google Scholar]
- 6.Rashidi B, Mohammadi M, Mirzaei F, Badalzadeh R, Reisi P. Amlodipine treatment decreases plasma and carotid artery tissue levels of endothelin-1 in atherosclerotic rabbits. Pathophysiology. 2011;18:137–42. doi: 10.1016/j.pathophys.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–8. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 8.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–30. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 9.Insull W., Jr The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am J Med. 2009;122(1 Suppl):S3–14. doi: 10.1016/j.amjmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Rementer C, Giachelli CM. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365–73. doi: 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oalmann MC, Malcom GT, Toca VT, Guzmán MA, Strong JP. Community pathology of atherosclerosis and coronary heart disease: Post mortem serum cholesterol and extent of coronary atherosclerosis. Am J Epidemiol. 1981;113:396–403. doi: 10.1093/oxfordjournals.aje.a113107. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez F, Santos S. Braunwalds Heart Disease: A Text Book of Cardiovascular Medicine. 7th ed. London: Elsevier Saunders; 2005. pp. 4–17. 939-42, 936-61, 1035-7, 1074-85. [Google Scholar]
- 13.Güldiken S, Demir M, Arýkan E, Tuðrul A. The level of serum high sensitive C-reactive protein in women with hyperthyroidism. Turk J Endocrinol Metab. 2005;3:85–8. [Google Scholar]
- 14.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 15.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: Risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med. 2004;255:188–205. doi: 10.1046/j.1365-2796.2003.01276.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Minno G, Mancini M. Measuring plasma fibrinogen to predict stroke and myocardial infarction. Arteriosclerosis. 1990;10:1–7. doi: 10.1161/01.atv.10.1.1. [DOI] [PubMed] [Google Scholar]
- 17.McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis. 2010;209:18–27. doi: 10.1016/j.atherosclerosis.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, et al. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–55. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 19.Poppitt SD. Postprandial lipaemia, haemostasis, inflammatory response and other emerging risk factors for cardiovascular disease: The influence of fatty meals. Curr Nutr Food Sci. 2005;1:23–34. [Google Scholar]
- 20.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–50. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 21.Zipes DP, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: W. B. Saunders; 2005. [Google Scholar]
- 22.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Poutanen K, Mykkänen H, et al. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: The Sysdimet study. PLoS One. 2011;6:e22646. doi: 10.1371/journal.pone.0022646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahmani M, Mirhoseini M, Shirzad H, Sedighi M, Shahinfard N, Rafieian-Kopaei M. A review on promising natural agents effective on hyperlipidemia. J Evid Based Complementary Altern Med. 2015;20:228–38. doi: 10.1177/2156587214568457. [DOI] [PubMed] [Google Scholar]
- 24.Setorki M, Rafieian-Kopaei M, Merikhi A, Heidarian E, Shahinfard N, Ansari R, et al. Suppressive impact of anethum graveolens consumption on biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Int J Prev Med. 2013;4:889–95. [PMC free article] [PubMed] [Google Scholar]
- 25.Braunwald E, editor . Heart Disease. London: WB Saunders; 1997. The history; p. 12. [Google Scholar]
- 26.Krajcovicová-Kudlácková M, Simoncic R, Béderová A, Ondreicka R, Klvanová J. Selected parameters of lipid metabolism in young vegetarians. Ann Nutr Metab. 1994;38:331–5. doi: 10.1159/000177830. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Kumar D, Rrakash O. Evaluation of antioxidant potential, phenolic and flavonoid contents of Hibiscus tiliaceus flowers. Electronic Journal of Envinonmental Agriculture and Food Chemistry. 2008;7:2863–71. [Google Scholar]
- 28.Hunt R, Fedorak R, Frohlich J, McLennan C, Pavilanis A. Therapeutic role of dietary fibre. Can Fam Physician. 1993;39:897. [PMC free article] [PubMed] [Google Scholar]
- 29.Mohsen A, Davood S, Ramazan F, Fereidoun H, Mustafa M. Effect of aerobic moderate exercise intensity on plasma and aorta homocysteine and 15-F21-isoprostane concentrations in high cholesterol diet-induced atherosclerosis. Physiol Pharmacol. 2007;11:199–207. [Google Scholar]
- 30.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in Eastern Finnish men. Circulation. 1992;86:803–11. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence de Koning AB, Werstuck GH, Zhou J, Austin RC. Hyperhomocysteinemia and its role in the development ofatherosclerosis. Clin Biochem. 2003;36:431–41. doi: 10.1016/s0009-9120(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 32.Aldana L, Tsutsumi V, Craigmill A, Silveira MI, Gonzalez de Mejia E. alpha-Tocopherol modulates liver toxicity of the pyrethroid cypermethrin. Toxicol Lett. 2001;125:107–16. doi: 10.1016/s0378-4274(01)00427-1. [DOI] [PubMed] [Google Scholar]
- 33.John S, Kale M, Rathore N, Bhatnagar D. Protective effect of Vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem. 2001;12:500–4. doi: 10.1016/s0955-2863(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 34.Wolf R, Wolf D, Ruocco V. Vitamin E: The radical protector. J Eur Acad Dermatol Venereol. 1998;10:103–17. [PubMed] [Google Scholar]
- 35.Barry H, John MC. Gutteridge Free Radicals in Biology and Medicine. London: Oxford University Press; 2007. [Google Scholar]
- 36.Skinner KA, White CR, Patel R, Tan S, Barnes S, Kirk M, et al. Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem. 1998;273:24491–7. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- 37.Raygan F, Khorasanifar H, Momen Heravi M, Arj A, Akbari H. The association between acute myocardial infarction and anti helicobacter pylori antibody. Zahedan J Res Med Sci. 2009;11:31–6. [Google Scholar]
- 38.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47:1673–706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikram M, Nowshad F. Constituents of Quercus infectoria. Planta Med. 1977;31:286–7. doi: 10.1055/s-0028-1097531. [DOI] [PubMed] [Google Scholar]
- 40.Antoine AA. An Introduction to Botanical Medicines: History, Science, Uses, and Dangers. Westport, Conn: Praeger Publishers; 2008. pp. 87–95. [Google Scholar]
- 41.Zhang J, Nguyen HT, Blum A. Genetic analysis of osmotic adjustment in crop plants. J Exp Bot. 1999;50:291–302. [Google Scholar]
- 42.Chedraui P, San Miguel G, Hidalgo L, Morocho N, Ross S. Effect of Trifolium pratense-derived isoflavones on the lipid profile of postmenopausal women with increased body mass index. Gynecol Endocrinol. 2008;24:620–4. doi: 10.1080/09513590802288283. [DOI] [PubMed] [Google Scholar]
- 43.Coinu R, Carta S, Urgeghe PP, Mulinacci N, Pinelli P, Franconi F, et al. Dose-effect study on the antioxidant properties of leaves and outer bracts of extracts obtained from Violetto di Toscana artichoke. Food Chem. 2007;101:524–31. [Google Scholar]
- 44.Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek-Gärtner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine. 1994;1:107–15. doi: 10.1016/S0944-7113(11)80027-9. [DOI] [PubMed] [Google Scholar]
- 45.Shariatifar N. Qualitative and quantitative study of Pulicaria gnaphalodes essential oil and plant extract and evaluation of oxidative stability of soya bean oil in the presence of plant essential oil and extracts. [Thesis], Tehran University; 2011. [Google Scholar]
- 46.Cropley M, Cave Z, Ellis J, Middleton RW. Effect of kava and valerian on human physiological and psychological responses to mental stress assessed under laboratory conditions. Phytother Res. 2002;16:23–7. doi: 10.1002/ptr.1002. [DOI] [PubMed] [Google Scholar]
- 47.Hayakawa H, Raij L. Relationship between hypercholesterolaemia, endothelial dysfunction and hypertension. J Hypertens. 1999;17:611–9. doi: 10.1097/00004872-199917050-00004. [DOI] [PubMed] [Google Scholar]
- 48.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 49.Kanda Samy M, Veerendar Channabasappa Y, Bhim Charan M, Tapankumar M. Hepatoprotective and antioxidant role of berberis tinctoria Lesch leaves on paracetamol induced hepatic damage in rats. Iran J Pharmacol Ther. 2005;4:64–9. [Google Scholar]
- 50.Podolsky DK, Isselbacher KJ. Derangements of hepatic metabolism. In: Fauci AS, Braunwald E, Lsseldacher KJ, editors. Harrison's Principles of Internal Medicine. 14th ed. New York: McGraw Hill; 1998. pp. 1667–72. [Google Scholar]
- 51.Bialecka M. The effect of bioflavonoids and lecithin on the course of experimental atherosclerosis in rabbits. Ann Acad Med Stetin. 1997;43:41–56. [PubMed] [Google Scholar]
- 52.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–63. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 53.Ernst E. Plasma fibrinogen – an independent cardiovascular risk factor. J Intern Med. 1990;227:365–72. doi: 10.1111/j.1365-2796.1990.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 54.Wakasugi M, Noguchi T, Inoue M, Tawata M, Shindo H, Onaya T. Effects of aldose reductase inhibitors on prostacyclin (PGI2) synthesis by aortic rings from rats with streptozotocin-induced diabetes. Prostaglandins Leukot Essent Fatty Acids. 1991;44:233–6. doi: 10.1016/0952-3278(91)90022-w. [DOI] [PubMed] [Google Scholar]
- 55.Fritsch RM, Keusgen M. Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (Alliaceae) Phytochemistry. 2006;67:1127–35. doi: 10.1016/j.phytochem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Sheela CG, Kumud K, Augusti KT. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Med. 1995;61:356–7. doi: 10.1055/s-2006-958099. [DOI] [PubMed] [Google Scholar]
- 57.Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol Pharm Bull. 2003;26:1725–9. doi: 10.1248/bpb.26.1725. [DOI] [PubMed] [Google Scholar]
- 58.Rossi A, Serraino I, Dugo P, Di Paola R, Mondello L, Genovese T, et al. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic Res. 2003;37:891–900. doi: 10.1080/1071576031000112690. [DOI] [PubMed] [Google Scholar]
- 59.Brouillard R. Chemical structure of anthocyanins. In: Markakis P, editor. Anthocyanins as Food Colors. New York: Academic Press Inc; 1982. pp. 1–38. [Google Scholar]
- 60.Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Svagera Z, Skottová N, Vána P, Vecera R, Urbánek K, Belejová M, et al. Plasma lipoproteins in transport of silibinin, an antioxidant flavonolignan from Silybum marianum. Phytother Res. 2003;17:524–30. doi: 10.1002/ptr.1187. [DOI] [PubMed] [Google Scholar]
- 62.Jaydari F, Johari H, Taati M, Asadian P, Alirezaei M, Sheikhzadeh F. The effects of fruit extract on catalase activity and lipid peroxidation in the heart and erythrocytes of rats following chronic ethanol consumption. J Vet Res. 2011;3:179–83. [Google Scholar]
- 63.Shahsavari N, Barzegar M, Sahari MA. The effect antioxidant of the essential oils Zataria multiflora Boiss and Bunium persicum Boiss in soybean oil. Plant Foods Hum Nutr. 2008;63:183–8. doi: 10.1007/s11130-008-0091-y. [DOI] [PubMed] [Google Scholar]
- 64.Sahari MA, Ataii D, Hamedi M. Characteristics of tea seed oil in comparison with sunflower and olive oils and its effect as a natural antioxidant. J Am Oil Chem Soc. 2004;81:585–8. [Google Scholar]
- 65.Kabiri N, Asgary S, Rahimi P. Reduction and regression athe rosclerosis lesions by hydroalcoholic extracts of Hypericum perforatum L. in hypercholesterolemic rabbits. Iran J Med Aromat Plants. 2012;27:624–34. [Google Scholar]
- 66.Bhatia AL, Jain M. Amaranthus paniculatus (Linn.) improves learning after radiation stress. J Ethnopharmacol. 2003;85:73–9. doi: 10.1016/s0378-8741(02)00337-9. [DOI] [PubMed] [Google Scholar]
- 67.Asgary S, Naderi G, Dashti G, Paknahad Z. Effect of Amirkabiria odorastissima mozaffarian on the development and progression of fatty streaks in hypercholesterolemic rabbits. Phytother Res. 2004;18:370–2. doi: 10.1002/ptr.1423. [DOI] [PubMed] [Google Scholar]
- 68.Rajesh M, Nagarajan A, Perumal S, Sellamuthu M. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107:1000–7. [Google Scholar]
- 69.Alipoor B, Alipoor S, Delazar A, Ostadrahimi A, Bamdad Mogadam S. Recognition of the most components in the most effective hydromethanol fraction of Iranian black tea on lipid profile, oxidative stress and inflammatory factors in type I diabetic rats. Pharm Sci. 2010;16:90–8. [Google Scholar]
- 70.Tripathi BK, Srivastava AK. Diabetes mellitus: Complications and therapeutics. Med Sci Monit. 2006;12:RA130–47. [PubMed] [Google Scholar]
- 71.Rietz B, Isensee H, Strobach H, Makdessi S, Jacob R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol Cell Biochem. 1993;119:143–50. doi: 10.1007/BF00926865. [DOI] [PubMed] [Google Scholar]
- 72.Sendl A, Elbl G, Steinke B, Redl K, Breu W, Wagner H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992;58:1–7. doi: 10.1055/s-2006-961378. [DOI] [PubMed] [Google Scholar]
- 73.Doi K, Kojima T, Fujimoto Y. Mulberry leaf extract inhibits the oxidative modification of rabbit and human low density lipoprotein. Biol Pharm Bull. 2000;23:1066–71. doi: 10.1248/bpb.23.1066. [DOI] [PubMed] [Google Scholar]
- 74.Visioli F, Galli C. Oleuropein protects low density lipoprotein from oxidation. Life Sci. 1994;55:1965–71. doi: 10.1016/0024-3205(94)00529-x. [DOI] [PubMed] [Google Scholar]
- 75.Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28:193–9. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 76.Swetie R, Raesh CH, Arun S. Antioxidant potential of mint (Mentha spicata L.) in radiationprocessed lamb meat. Food Chem. 2007;100:451–8. [Google Scholar]
- 77.Zhang Y, Yang L, Zu Y, Chen X, Wang F, Liu F. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 2010;118(3):656–62. doi: 10.1016/j.foodchem.2011.02.082. [DOI] [PubMed] [Google Scholar]
- 78.Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990;44:301–6. [PubMed] [Google Scholar]
- 79.Ghorbani A, Rakhshandeh H, Asadpour E, Sadeghnia HR. Effects of Coriandrum sativum extracts on glucose/serum deprivationinduced neuronal cell death. Avicenna J Phytomed. 2012;1:4–9. [Google Scholar]
- 80.Nickavar B, Abolhasani FA. Screening of antioxidant properties of seven Umbelliferae fruits from Iran. Pak J Pharm Sci. 2009;22:30–5. [PubMed] [Google Scholar]
- 81.Young IS, McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 2001;29(Pt 2):358–62. doi: 10.1042/0300-5127:0290358. [DOI] [PubMed] [Google Scholar]
- 82.Badary OA, Abdel-Naim AB, Abdel-Wahab MH, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143:219–26. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 83.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 84.U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Flavonoid Content of Selected Foods. 2008. [Last accessed on 2010 Apr 22]. Available from: http://www.nal.usda.gov/fnic/foodcomp .
- 85.Setorki M, Asgary S, Eidi A, Haeri Rohani A. Effects of acute verjuice consumption with a high-cholesterol diet on some biochemical risk factors of atherosclerosis in rabbits. Med Sci Monit. 2010;16:BR124–30. [PubMed] [Google Scholar]
- 86.Hou L, Zhou B, Yang L, Liu ZL. Inhibition of human low density lipoprotein oxidation by flavonols and their glycosides. Chem Phys Lipids. 2004;129:209–19. doi: 10.1016/j.chemphyslip.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Lotito S, Frei B. The increase in human plasma antioxidant capacity alter apple consumption is due to the metabolic effect of fructose on urate, not apple drived antioxidant flavonoids. Biol Med. 2004;37:251–8. doi: 10.1016/j.freeradbiomed.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 88.Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39:1–11. doi: 10.1207/S15327914nc391_1. [DOI] [PubMed] [Google Scholar]
- 89.Lankin VZ, Tikhaze AK, Kukharchuk VV, Konovalova GG, Pisarenko OI, Kaminnyi AI, et al. Antioxidants decreases the intensification of low density lipoprotein in vivo peroxidation during therapy with statins. Mol Cell Biochem. 2003;249:129–40. [PubMed] [Google Scholar]
- 90.Somjen D, Knoll E, Vaya J, Stern N, Tamir S. Estrogen-like activity of licorice root constituents: Glabridin and glabrene, in vascular tissues in vitro and in vivo. J Steroid Biochem Mol Biol. 2004;91:147–55. doi: 10.1016/j.jsbmb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–13. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 92.Rosenblat M, Belinky P, Vaya J, Levy R, Hayek T, Coleman R, et al. Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein. A possible role for protein kinase C. J Biol Chem. 1999;274:13790–9. doi: 10.1074/jbc.274.20.13790. [DOI] [PubMed] [Google Scholar]
- 93.Coruh N, Saghdicoglu Celep AG, Ozgokce F, Iscan M. Antioxidant capacities of Gundelia tournefortii L. extract and inhibition on glutathione-S-transferase activity. Food Chem. 2007;100:1249–53. [Google Scholar]
- 94.Schmidt C, Fagerberg B. ApoB/apoA-I ratio is related to femoral artery plaques in 64-year-old women also in cases with low LDL cholesterol. Atherosclerosis. 2008;196:817–22. doi: 10.1016/j.atherosclerosis.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 96.Dimitriou M, Rallidis LS, Theodoraki EV, Kalafati IP, Kolovou G, Dedoussis GV. Exclusive olive oil consumption has a protective effect on coronary artery disease; overview of the THISEAS study. Public Health Nutr. 2016;19:1081–7. doi: 10.1017/S1368980015002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guinda A. Use of solid residue from the olive industry. Grasas Aceitis. 2006;57:107–15. [Google Scholar]
- 98.Draijer R, de Graaf Y, Slettenaar M, de Groot E, Wright CI. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients. 2015;7:3138–53. doi: 10.3390/nu7053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siasos G, Tousoulis D, Kokkou E, Oikonomou E, Kollia ME, Verveniotis A, et al. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am J Hypertens. 2014;27:38–45. doi: 10.1093/ajh/hpt176. [DOI] [PubMed] [Google Scholar]
- 100.Natsume M, Baba S. Suppressive effects of cacao polyphenols on the development of atherosclerosis in apolipoprotein E-deficient mice. Subcell Biochem. 2014;77:189–98. doi: 10.1007/978-94-007-7920-4_16. [DOI] [PubMed] [Google Scholar]
- 101.Novakovic A, Marinko M, Vranic A, Jankovic G, Milojevic P, Stojanovic I, et al. Mechanisms underlying the vasorelaxation of human internal mammary artery induced by (−)-epicatechin. Eur J Pharmacol. 2015;762:306–12. doi: 10.1016/j.ejphar.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 102.Arshadi A, Talebi E, Tahery E, Kargar J. Effect of barley and oats on high-density lipoprotein cholesterol andtriglycerides in rats. Pars J Med Sci. 2014;12(2):29–35. [Google Scholar]
- 103.Mirhosseini M, Baradaran A, Rafieian-Kopaei M. Anethum graveolens and hyperlipidemia: A randomized clinical trial. J Res Med Sci. 2014;19:758–61. [PMC free article] [PubMed] [Google Scholar]
- 104.Rafieian-Kopaei M, Shahinfard N, Rouhi-Boroujeni H, Gharipour M, Darvishzadeh-Boroujeni P. Effects of Ferulago angulata extract on serum lipids and lipid peroxidation. Evid Based Complement Alternat Med 2014. 2014:680856. doi: 10.1155/2014/680856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asgary S, Sahebkar A, Afshani M, Keshvari M, Haghjooyjavanmard SH, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28(2):193–9. doi: 10.1002/ptr.4977. [Doi: 10.1002/ptr.4977] [DOI] [PubMed] [Google Scholar]
- 106.Khosravi-Boroujeni H, Mohammadifard N, Sarrafzadegan N, Sajjadi F, Maghroun M, Khosravi A, et al. Potato consumption and cardiovascular disease risk factors among Iranian population. Int J Food Sci Nutr. 2012;63:913–20. doi: 10.3109/09637486.2012.690024. [DOI] [PubMed] [Google Scholar]
- 107.Asgary S, Rafieian-Kopaei M, Shamsi F, Najafi S, Sahebkar A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J Complement Integr Med. 2014;11:63–9. doi: 10.1515/jcim-2013-0022. [DOI] [PubMed] [Google Scholar]
- 108.Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N, Sajjadi F, Maghroun M, Asgari S, et al. White rice consumption and CVD risk factors among Iranian population. J Health Popul Nutr. 2013;31:252–61. doi: 10.3329/jhpn.v31i2.16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rafieian-Kopaei M, Asgary S, Adelnia A, Setorki M, Khazaei M, Kazemi S, et al. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J Med Plants Res. 2011;5:2670–6. [Google Scholar]
- 110.Shayganni E, Bahmani M, Asgary S, Rafieian-Kopaei M. Inflammaging and cardiovascular disease: Management by medicinal plants. Phytomedicine. 2016;23:1119–26. doi: 10.1016/j.phymed.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 111.Gensini GF, Comeglio M, Colcila A. Hemostatic factors, atherogenesis and atherosclerosis. Biomed Pharmacother. 1996;50:395–405. [Google Scholar]
- 112.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47(12):1673–706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greager MA, Libby P. Peripheral arterial disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1437–61. [Google Scholar]
- 114.de Wardener HE. Salt reduction and cardiovascular risk: The anatomy of a myth. J Hum Hypertens. 1999;13:1–4. doi: 10.1038/sj.jhh.1000759. [DOI] [PubMed] [Google Scholar]
- 115.Kumar S, Kumar D, Prakash O. Evaluation of antioxidant potential, phenolic and flavonoid contents of Hibiscus tiliaceus flowers. Electronic Journal of Envinonmental Agriculture and Food Chemistry. 2008;7:2863–71. [Google Scholar]
- 116.Grenett HE, Abou-Agag LA, Parks DA, Booyse FM. Ethanol and polyphenols (CAT, QUER) increase expression of fibrinolytic protein mRNAs in vivo in rat aortic endothelium. Biol Res. 2004;37:342. [Google Scholar]
- 117.Prasad K, Kalra J. Oxygen free radicals and hypercholesterolemic atherosclerosis: Effect of Vitamin E. Am Heart J. 1993;125:958–73. doi: 10.1016/0002-8703(93)90102-f. [DOI] [PubMed] [Google Scholar]
- 118.Yan LJ, Droy-Lefaix MT, Packer L. Ginkgo biloba extract (EGb 761) protects human low density lipoproteins against oxidative modification mediated by copper. Biochem Biophys Res Commun. 1995;212:360–6. doi: 10.1006/bbrc.1995.1978. [DOI] [PubMed] [Google Scholar]
- 119.Natella F, Ghiselli A, Guidi A, Ursini F, Scaccini C. Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radic Biol Med. 2001;30:1036–44. doi: 10.1016/s0891-5849(01)00504-4. [DOI] [PubMed] [Google Scholar]
- 120.Madihi Y, Merrikhi A, Baradaran A, Ghobadi S, Shahinfard N, Ansari R, et al. Bioactive components and the effect of hydroalcoholic extract of Vaccinium myrtillus on postprandial atherosclerosis risk factors in rabbits. Pak J Med Sci. 2013;29:384–9. [Google Scholar]
- 121.Durrington PN, Mackness B, Mackness MI. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler Thromb Vasc Biol. 2002;22:1248–50. doi: 10.1161/01.atv.0000027414.34728.1f. [DOI] [PubMed] [Google Scholar]
- 122.Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp Clin Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 123.Chang PP, Ford DE, Meoni LA, Wang NY, Klag MJ. Anger in young men and subsequent premature cardiovascular disease: The precursors study. Arch Intern Med. 2002;162:901–6. doi: 10.1001/archinte.162.8.901. [DOI] [PubMed] [Google Scholar]


