Abstract
Classical thinking in endocrine physiology squeezes our diagnostic handling into a simple negative feedback mechanism with a controller and a controlled variable. In the case of the thyroid this is reduced to TSH and fT3 and fT4, respectively. The setting of this tight notion has no free space for any additions. In this paper we want to challenge this model of limited application by proposing a construct based on a systems approach departing from two basic considerations. In first place since the majority of cases of thyroid disease develop and appear during life it has to be considered as an acquired condition. In the second place, our experience with the reversibility of morphological changes makes the autoimmune theory inconsistent.
While medical complexity can expand into the era of OMICS as well as into one where manipulations with the use of knock-outs and -ins are common in science, we have preferred to maintain a simple and practical approach. We will describe the interactions of iron, magnesium, zinc, selenium and coenzyme Q10 with the thyroid axis. The discourse will be then brought into the context of ovarian function, i.e. steroid hormone production. Finally the same elemental players will be presented in relation to the basic mitochondrial machinery that supports the endocrine.
We propose that an intact mitochondrial function can guard the normal endocrine function of both the thyroid as well as of the ovarian axis. The basic elements required for this function appear to be magnesium and iron. In the case of the thyroid, magnesium-ATP acts in iodine uptake and the heme protein peroxidase in thyroid hormone synthesis. A similar biochemical process is found in steroid synthesis with cholesterol uptake being the initial energy-dependent step and later the heme protein ferredoxin 1 which is required for steroid synthesis. Magnesium plays a central role in determining the clinical picture associated with thyroid disease and is also involved in maintaining fertility. With the aid of 3D sonography patients needing selenium and/or coenzyme Q10 can be easily identified. By this we firmly believe that physicians should know more about basic biochemistry and the way it fits into mitochondrial function in order to understand metabolism. Contemplating only TSH is highly reductionistic.
Outline
-
•
Author's profiles and motivation for this analysis
-
•
The philosophical alternatives in science and medicine
-
•
Reductionism vs. systems approach in clinical thyroid disease guidelines
-
•
The entry into complexity: the involvement of the musculoskeletal system
-
•
Integrating East and West: teachings from Chinese Medicine and from evidence based medicine (EBM)
-
•
Can a mathematical model represent complexity in the daily thyroid practice?
-
•
How effective is thyroxine treatment?
-
•
Resolving the situation of residual symptoms in treated patients with thyroid disease
-
•
Importance of iron, zinc and magnesium in relation to thyroid function
-
•
Putting together new concepts related to thyroid function for a systems approach
-
•
Expanding our model into general aspects of medicine
1. Author's profiles, motivation and contribution for this evaluation
We have been involved in clinical practice dealing with endocrinology since many years, in fact decades, i.e. RM since 1976 and HM since 1986. Our individual areas of work have been thyroid disease and reproductive medicine including assisted reproduction techniques, respectively. In 1995 we made a short joint excursion into the field of ovarian autoimmunity [1]. Our initial publications were derived from academic medical and scientific work at the University of Ulm, Germany, and later at the Medical School of the University of Innsbruck, Austria. Since 2002 we have continued to do research in both fields on the basis of private academic initiative. Our daily work is currently done by including additional techniques such as principles of locomotion biomechanics, Applied Kinesiology, TCM, acupuncture, herbal therapy, manual medicine, and natural medicine from the Shuar people of Ecuador.
In times of advancement of science the simple world of ligand and receptor interaction [2], [3] has evolved into an age of OMICS [4]. This information has widened our horizon but at the same times it can overwhelm our perception and simple facts might disappear. With this in mind, we will attempt here to link modern biochemical knowledge into basic thyroid and ovarian physiology and present it in a simple way according to the thesis of elementological biology of Nishimuta, i.e. the interactions between chemistry and biology [5].
2. The philosophical alternatives in science and medicine
Table 1.
Reductionism vs. systems approach.
| The limits of reductionism in medicine: could systems biology offer an alternative? [6] | The clinical applications of a systems approach [7] |
|---|---|
| Since Descartes and the Renaissance, science, including medicine, has taken a distinct path in its analytical evaluation of the natural world. This approach can be described as one of “divide and conquer,” and it is rooted in the assumption that complex problems are solvable by dividing them into smaller, simpler, and thus more tractable units. | Reductionism, as a guiding principle, is tremendously helpful and useful. The problem with reductionism stems not from its use but from the wrongful assumption that it is the only solution. Reductionism becomes less effective when the act of dividing a problem into its parts leads to loss of important information about the whole. |
The reductionism found in Evidence Based Medicine (EBM) has been criticized as lacking realism. In order to overcome this, different perspectives should work additively to include practical choice options [9], i.e. connecting the parts. In the words of Henry H.Q. Heng [10]: “Clinical therapies must be individualized, balancing the parts of the system and the response of the patient as a whole”. Whatever approach is taken the researcher and the clinician has to be aware of possible falsification of data or of concepts as has been described by Popper [11].
Looking back at the development of evidence based medicine one can consider it to be a direct consequence of the condition that affected Archibald Cochrane personally. He had to go through a tortuous path of medical examinations in order to have a diagnosis and cure of his own illness [12], [13]. It is therefore not surprising that Cochrane discussed the general scientific problem of testing a hypothesis as to whether a certain treatment alters the natural history of a disease for the better (page 20 in [14]). This was stated in his monograph entitled: “Effectiveness and Efficiency. Random Reflections on Health Services” [14].
In the following sections we will review data related to clinical work in the field of thyroid diseases. We will then proceed to develop a systems approach based on the concepts contained in the WOMED model of benign thyroid disease [15], [16]. Finally we will attempt to integrate the functions of single elements such as iron, magnesium, coenzyme Q10 (CoQ10), selenium and zinc into a frame for mitochondrial function.
3. Reductionism in thyroid disease studies
In 2002 and 2003 two studies based purely on epidemiological laboratory examinations of serum TSH values were published. The final intention was to consider TSH as the master parameter that could describe thyroid function as a reductionist entity. Their results were not similar. We will look at the circumstances surrounding these studies.
The first publication by Hollowel et al. [17] reported the 95% reference limits for this analyte as being 4.12 (3.94–4.45) mIU/l. Hollowel's publication was based on the data collection of the NHANES III investigation which was conducted at the end of the past century (between 1988 and 1994) [17]. The NHANES study included 17,353 persons after a selection process called oversampling [18]. Oversampling was declared as the driving method (references [12], [13] in [17]). Current documentation to oversampling states: “NHANES typically samples larger numbers of certain subgroups who are of particular public health interest. Oversampling is done to increase the reliability and precision of estimates of health status indicators for these population subgroups”. (http://www.cdc.gov/nchs/tutorials/nhanes/FAQs.htm). Thus, in plain terms one can understand that oversampling is similar to counting the same data more than once. Hollowel, however, failed to explain or identify the subgroups that were created by oversampling. The potential bias produced by such an artificial - perhaps obscure – approach has never been discussed. In general terms allocation bias will distort the image of any study population [19]. The second publication on TSH values was made by Baloch and co-workers and had the title: “Laboratory medicine practice guidelines”. They suggested a theoretical upper reference value for TSH of 2.5 mIU/l [20]. We have recently commented on the aftermath of the suggestions made by Baloch and also by Abalovich [21] and shown that they are invalid [22]. In other words, lowering the upper reference value for TSH to 2.5 mIU/l was no panacea.
4. The entry into complexity: the involvement of the musculoskeletal system
In 2007 we described changes in the musculoskeletal system in patients with thyroid associated ophthalmopathy [23]. The main findings were a deviation of the body axis occurring frequently after an ankle sprain. The physiological negative correlation between calcium and magnesium was altered. Using radionuclide imaging techniques Hartman Kainz et al. demonstrated accumulation of a 99mTc-labelled octreotide tracer in the musculoskeletal system of these patients [24]. Putting both pieces of evidence together we proposed that these patients had a process of low level inflammation in the muscles. A common denominator in these patients was an altered circular rotation of the ankle. This situation appeared to be a consequence of a supination flexion lesion which has happened many years before. Additional changes in the musculoskeletal system include stiffness of the shank muscles and idiopathic moving toes, i.e. involuntary movement of the toes while the contralateral foot is rotating [23]. Biochemical evaluations showed that these alterations are primarily related to lower magnesium levels in blood. Patients with thyroid disease also presented selenium [25] and CoQ10 deficiencies [16].
5. Integrating East and West: teachings from Chinese Medicine and evidence based medicine (EBM)
Fig. 2.
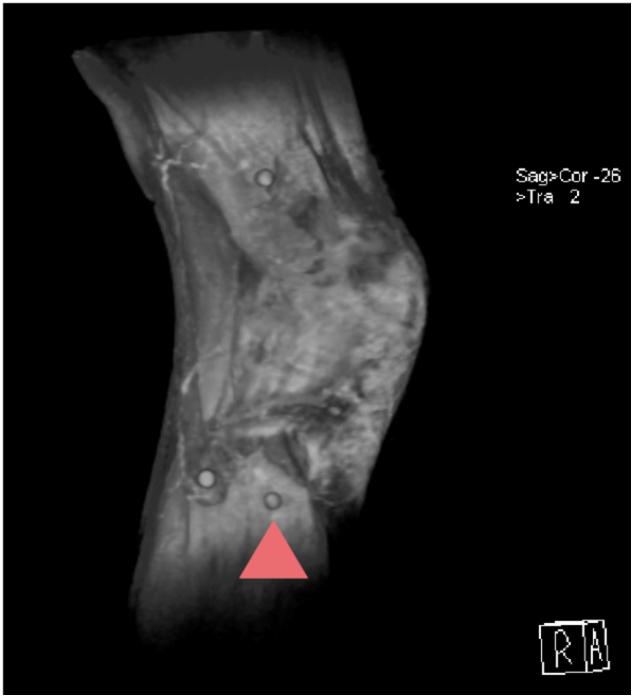
Magnetic resonance Imaging of the right knee of a healthy subject. The arrow on a round marker shows the location of acupoint stomach 36 or Zu San Li. Technical details of the imaging procedure have been described before [34], [35].
Fig. 3.
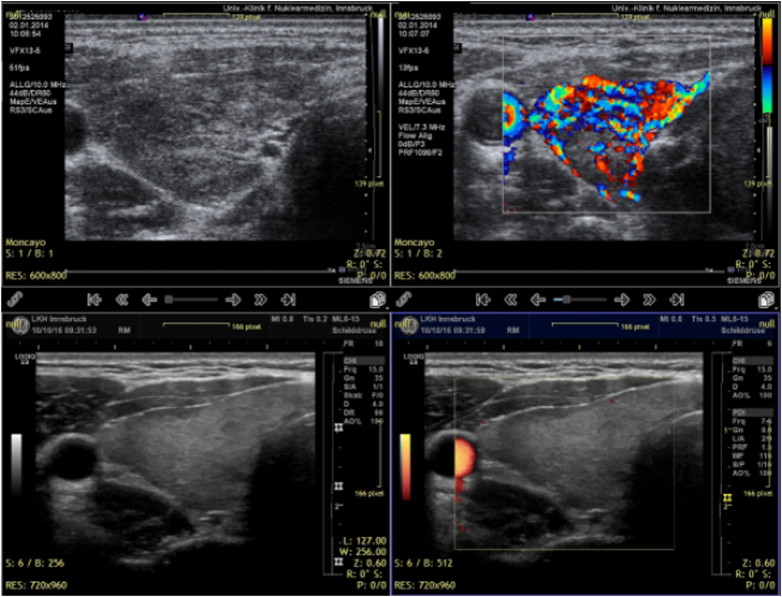
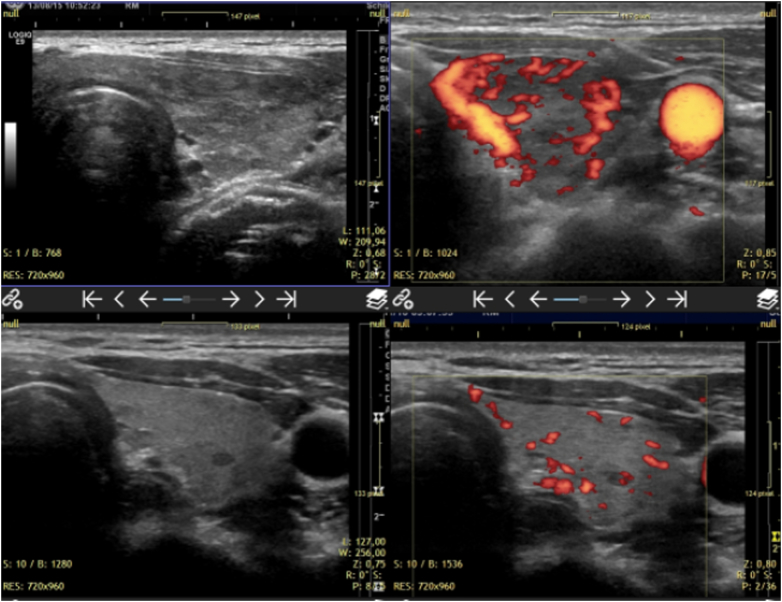
Two years of follow up in a case of hyperthyroidism. The initial images are on the top row. They show markedly diminished echogenicity together with hyperperfusion. The lower row shows the current images with recovered echogenicity and normal perfusion. Two different machines were used in this evaluation, Siemens Antares (upper row) and GE Logiq E9 (lower row).
How can these concepts fit into the RCT-driven cosmos of evidence based medicine? One proposal has been presented by Ana Fernandez [42]: “EBM needs to move forward and perceive health and healthcare as a complex interaction, i.e. an interconnected, non-linear phenomenon that may be better analyzed using a variety of complexity science techniques”. The use of manual medicine or acupuncture has not been included in existing guidelines for thyroid diseases. However, when treating the individual patient the clinical manual examination allows us to choose an adequate approach with manual therapy and acupuncture. Sizer et al. [43], when talking about orthopedic manual physical therapy point out the following: “… the clinician–patient team can make appropriate care decisions that may or may not match what the ‘best evidence’ may recommend” (page 117 in [43]). The authors concluded their article with the following statement: “Moreover, while a chosen alternative may not be fully supported by presently available evidence and could be considered an outlier, that outlier of today may be the basis for developing future evidence. Thus, choosing that alternative based on sound clinical reasoning places the clinicians in a position to navigate future clinical science discoveries” (page 118 in [43]). Mazzocchi has discussed the topic on how to be holistic, i.e. how to reach a concept of systems biology [44]. Even looking at practice guidelines, the inclusion of personal experience is not a disregarded issue [45]. Personal experience is the foundation of observational studies.
6. Can a mathematical model represent complexity seen in the daily thyroid practice?
The journal “Frontiers in Endocrinology” includes a Research Topic section dealing with regulatory aspects of thyroid function. The original statement about this section described its aim as: “a comprehensive overview on state-of-the-art methodology and recent results from the emerging new world of thyroidology, which tries to rationalize a scenario of previously unknown complexity.” The first article of this section by Hoermann et al. presented a mathematical model that claimed to describe the homeostatic relationship between TSH and thyroid hormones [46]. This mathematical model of thyroid function was original developed by J.W. Dietrich [47] and has been published with slight variations in several Journals dedicated to Endocrinology [46], [48], [49], [50], [51], [52], [53]. Unfortunately the graphical depiction of the model suffers from a severe omission by leaving out any consideration of iodine metabolism which is the basic component of thyroid hormones (Fig. 1 in [47]). Another important omission is that of leaving out components of the deiodinase system such as selenium [54], [55] and flavoproteins [56], [57], [58]. This same fundamental omission, however, can also be found in another model of thyroid function [59]. As a result, only the measurable hormone variables, i.e. TSH and thyroid hormones, have been used for a limited reductionist approach. Although Hoermann et al. emphasize the fact that: “a disturbingly high proportion of patients remains unsatisfied with the treatment they receive“(page 2 in [52]) the authors reached a conclusion that states: “Possible long-term consequences of the observed biochemical alterations such as the altered FT3–FT4 ratio are also presently unknown “(page 8 in [52]). This conclusion does not differ greatly from the general statement made by James E.A. McIntosh and Rosalind P. McIntosh in 1980 in their chapter on Concepts of Feedback when talking about mathematical models: “Therefore it may be difficult to identify and include in a model all relevant components and interactions, stimuli and responses, or even to know what stimuli are appropriate to the activation of a feedback system” [60].
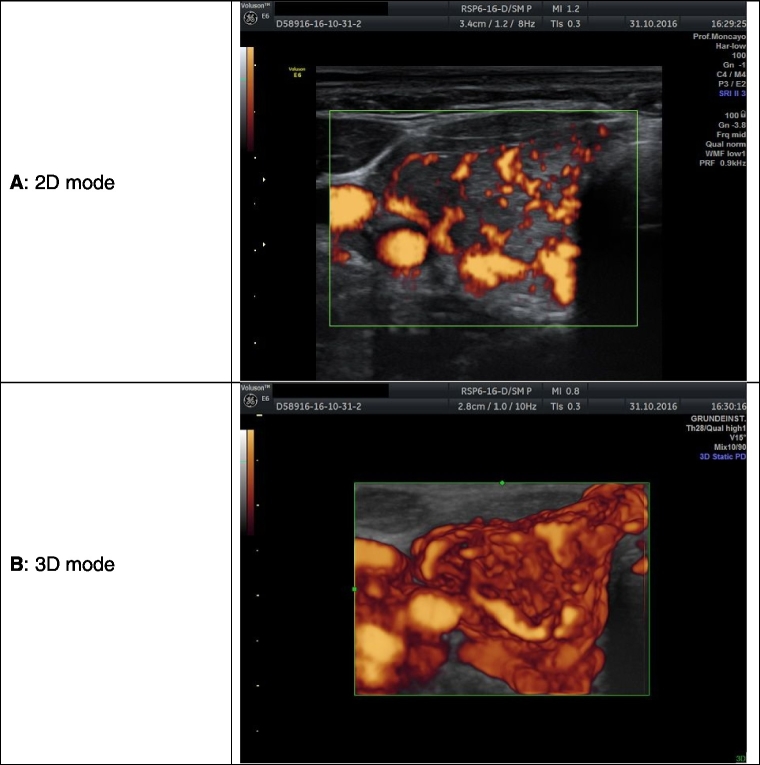
Fig. 1.
Imaging of the thyroid in power Doppler mode in a case of newly diagnosed hyperthyroidism. The 2D image allows the examiner to recognize dispersed areas that correspond to vessels. The 3D image shows the complex continuous structure of vessels. This picture is associated with low CoQ10 values.
7. How effective is thyroxine treatment? Also known as resolving the situation of residual symptoms in treated patients with thyroid disease
In spite of all laboratory work trying to define normalcy on the basis of a single TSH level, it often fails to tell that patients are free of complaints. Many publications refer to this situation of “residual symptoms” in apparently well treated hypothyroid patients. This situation is of relevance for each patient.
7.1. A short account of the situation or residual symptoms
In 2001 the results of the Basel Thyroid Study carried at the end of the 1990s were published [61]. The authors reported an improvement of clinical symptoms as measured by 2 scoring systems, i.e. Billewicz [62] and Zulewski [63]. In a later paper the same authors described the lack of association between TSH levels and the degree of clinical symptoms found in hypothyroidism [64]. The authors stated that TSH is a poor measure in the evaluation of the severity of thyroid failure and as such it should not be taken as the indicator for starting a therapy with thyroxine. Recently Winther et al. demonstrated that tiredness remained as the cardinal impairment in hypothyroidism in spite of therapy [65]. An Editorial in “Thyroid” written by Peter Andreas Kopp in 2014 [66], pointed out the important drawback found in the situation of residual complaints, i.e. patients remain symptomatic despite having normal thyroid hormone levels. Similar to the opinion of Kopp, Mary H. Samuels recently stated the following: “It is a common clinical observation that some otherwise healthy patients with hypothyroidism continue to complain of fatigue, poor mood, inability to concentrate, and vague cognitive difficulties (often described as “brain fog”) despite normal TSH levels” [67]. Similar comments have been made by other authors in the past [68], [69]. The condition of well-being has been also matter of investigation in relation to treatment with thyroid hormones. Walsh et al. [70] presented an evaluation of changing thyroxine dosage in the treatment of women with primary hypothyroidism. They found no significant treatment effects on their instruments aiming at measuring well-being and symptoms. They concluded that the target TSH range should not differ from the general laboratory range. Unfortunately none of the previous publications has provided an explanation for the condition.
A different therapeutic modality to be used in primary overt hypothyroidism called biopsychosocial approach, which was based on the use of a neuro-emotional-technique, was proposed in 2010 by Benjamin T. Brown [71]. They argued that this biopsychosocial model would be superior to the usual biomedical management of thyroid disease. In 2015 the study was finished and the final conclusion was that there was no clinical benefit using this approach [72]. Again a solution to resolve residual symptoms was not successful.
7.2. Our own experience
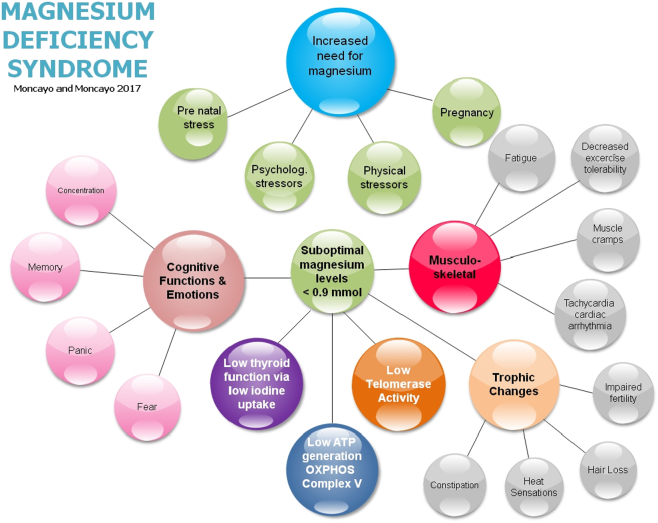
The initial motivation for our investigations was the complaint from many patients that they were unhappy with their treatment either for hypo- or for hyperthyroidism. Some patients who had had a total thyroidectomy still talked about the thyroid being responsible for their condition. The most common symptom we have observed was fatigue. The next most common ones were exhaustibility, muscle cramps, muscle aches, irregular heartbeat, nervousness, perspiration, irritability, depressive mood, poor concentration, and poor memory, vertigo sensation and cephalea. This complex clinical picture could be misinterpreted as a psychosomatic disease. Dwelling deeper into this clinical situation we were able to identify a common denominator behind the “so-called” thyroid disease complex of symptoms, namely magnesium deficiency [15]. We have found that magnesium deficiency was related to psychological stressors, to physical stressors due to alterations in the musculoskeletal system, to muscular injuries, to infection, to the post-partum period [16] and physiologically to early pregnancy after IVF [73].
If we consider some of these situations to represent a stress condition it is important to point out that that anxiety and hypothalamic-pituitary-adrenal (HPA) axis dysregulation are the consequence of primary magnesium deficiency as has been shown in mice [74]. Some studies have shown a relation between psychosocial stress and lower levels of magnesium in humans [75], [76]. Recently Harbeck and coworkers described that on biochemical terms TSH can be taken as an early and sensitive predictor of stress while at the same time there were no changes seen in cortisol, glucose or norepinephrine levels [77]. These data prompted us to reconsider the original stress model published by Hans Selye in 1936 where he described the effect of acute nocuous agents under experimental situations [78]. In a second letter in 1938 he postulated that adaptation of an organism depends upon a “special hitherto unrecognized type of energy” [79]. Looking back at his own research Selye spoke then of “adaptation energy” in 1950 [80]. This short account shows the connections of stress to energy, a connection which we will address in the following sections.
7.3. The therapeutic approach
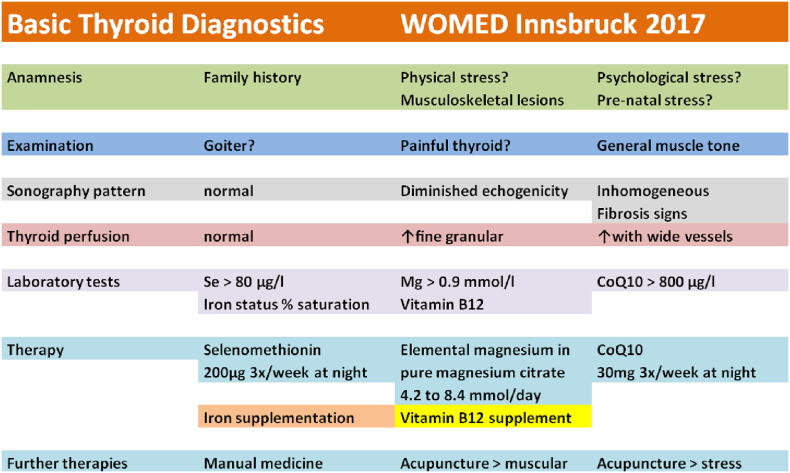
Our definition of low magnesium levels was based on the comparison with serum values found in control subjects who did not present any physical or psychological stressors. In a previous investigation we have shown how these stressors relate to low magnesium levels [15]. The blood levels of magnesium in these subjects had a mean level 0.95 ± 0.08 mmol/l. In cases of physical or psychological stress these levels are lower. Subjects with magnesium deficiency can be supplemented with pure magnesium citrate. The preparation we use contains 1.4 mmol of elemental magnesium per capsule. The initial dose is 1.4 mmol 3 to 4 times per day during 3 months. If this dose is not effective it can be increased to 6 capsules per day, i.e. 8.4 mmol of elemental magnesium. In pregnant women we use an initial dose of 6 times 1.4 mmol. Ideally blood controls should document an increase of magnesium levels to a level of at least 0.9 mmol/l.
In cases of CoQ10 deficiency, supplementation with 30 mg daily can be carried out [16]. If the selenium concentration in blood is < 80 μg/l, selenomethionin 200 μ/day, 3 times per week, is added to the supplementation. Follow up of supplementation can be supported by sonography with the power Doppler mode. Besides correcting deficiency conditions, the changes found in the musculoskeletal system require an individual manual and acupuncture therapy in order to recover body alignment.
Our emphasis on magnesium as being the vulnerable variable in the system is based on the following considerations. Stress situations are related to magnesium deficiency [74]; intra-cellular selenium is related to magnesium levels (Fig. 2 in [16]); selenium and CoQ10 levels are correlated [81] and CoQ10 levels are diminished in selenium deficiency [82]. We consider a magnesium level of 0.9 mmol/l as adequate to meet physiological needs [16]. The following ultrasound images will demonstrate the beneficial effect of our combined therapeutic approach for thyroid affections.
In situations where fibrosis of the thyroid is not found in the ultrasound examination, the changes in morphology and perfusion are reversible under this tailored treatment, i.e. the thyroid gland can regain a normal appearance [83]. Our experience shows that an age < 35 years is a biological turning point for achieving these results. This fact contradicts the dogmatic model of autoimmunity. We have replaced this belief with a model of an acquired deficiency of mitochondrial function affecting Complex V of the oxidative phosphorylation and being primarily induced by magnesium deficiency [16]. Using the outlined supplementation strategy, changes in thyroid morphology and perfusion will revert to normal.
Fig. 4.
Two years of follow up in a case of hyperthyroidism. Vascular structures are depicted using 2D power Doppler mode. The improvement of thyroid texture and perfusion can be seen by comparing the upper and the lower row of images. All images were done using a GE LOGIQ E9 ultrasound machine in power Doppler modus.
Single cases that still present symptoms like anxiety and fear require sometimes special acupuncture techniques that help to resolve previous stressing situations [84]. The importance of psychological stress has to be considered and treated in all cases of thyroid disease.
Fig. 5.
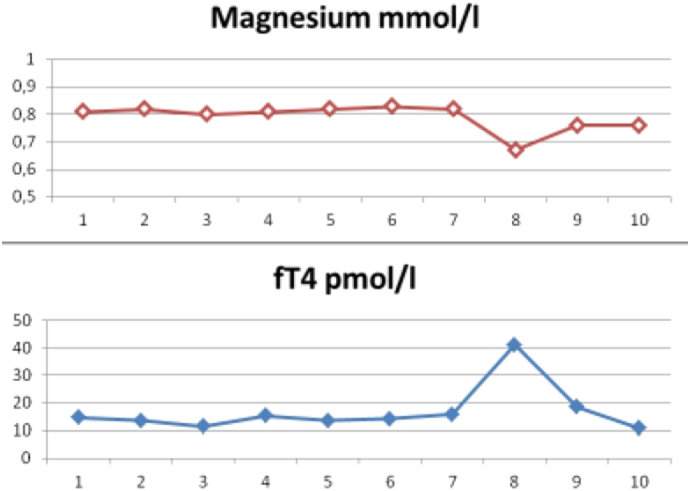
Time relation between fT4 and magnesium levels observed during 10 follow-up time points. At time point 7 increased stress at work and an infection preceded a relapse of hyperthyroidism. At this time magnesium levels showed a concomitant drop from 0.82 to 0.67 mmol/l. Following supplementation with 8.4 mmol elemental magnesium fT4 normalized and magnesium rose to 0.76 mmol/l.
8. Minerals and elements at the metabolic crossroads between thyroid and mitochondria
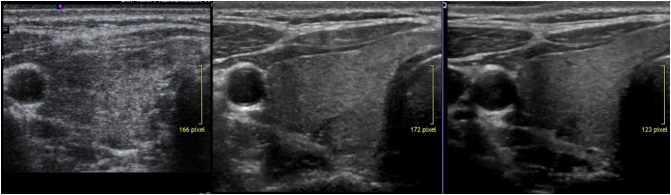
Fig. 6.
Shows a simple example of B mode sonography of the thyroid during follow up. The initial diffuse hypoechogenic pattern disappeared after 3 years of specific supplementation.
8.1. Iron
The 2015 Global Burden of Disease Study revealed that iron deficiency is at place 4 among the top eight causes of chronic diseases affecting > 10% of the population [89]. Experimental data has shown that iron deficiency reduces the activity of thyroid peroxidase [90]. The kinetics of thyroid hormones [91] can also be impaired in iron deficiency. On the other hand iron supplementation can lead to reduction of goiter size [92]. Iron deficiency in fetal and neonatal periods can reduce the levels of thyroid hormone-responsive genes mRNA in the hippocampus and cerebral cortex of the neonatal rat [93]. Furthermore iron deficiency in pregnant rats can produce hypothyroxinemia [94].
Fig. 7.
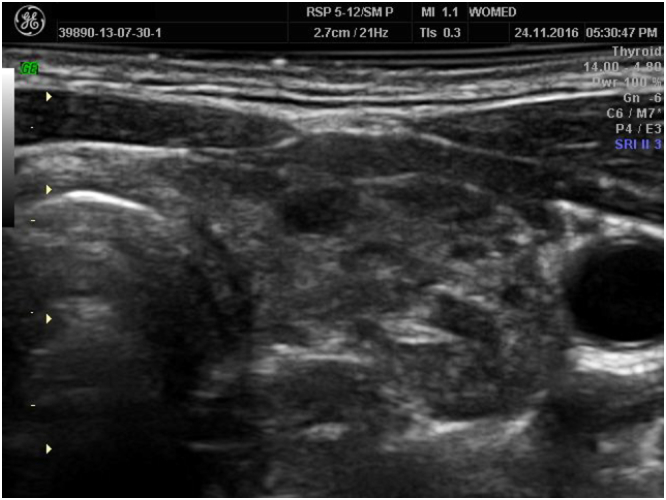
Shows fibrotic changes of the thyroid. In this condition recovery of thyroid morphology is not achievable.
The central role that iron has in thyroid economy can be explained by one good reason: thyroid peroxidase is a hemeprotein. Heme is produced in mitochondria and the path into these organelles requires a coordinated action between intestinal uptake or iron, iron transport and entry into the cytoplasm and into the mitochondria [105]. Besides iron other nutritional requirements for heme synthesis include: vitamin B6, riboflavin, biotin, copper and zinc. Starting from succinyl-CoA porphyrins are synthetized to be converted to heme [106]. Thyroid can also influence iron metabolism by modulating the interaction between the ferritin mRNA iron responsive element and iron regulatory proteins [107].
Besides heme, mitochondria also synthetize iron-sulfur proteins [108] which are related to iron sensing and intracellular iron delivery [109]. Hemes are integral components of the respiratory complexes II, III, and IV that perform multi-electron transport and catalysis [110]. One group of these iron-sulfur proteins is mammalian adrenodoxin or ferredoxin 1 (Fdx1) which is essential for steroid hormone synthesis. A second ferroxin, Fdx2, participates in the synthesis of heme A and of iron-sulfur proteins [111]. Cellular iron is also a regulator of mitochondrial biogenesis [112]. On the other hand the biogenesis of iron-sulfur clusters requires GTP, NAD and ATP [113].
In a direct tissue analysis study Melenovsky et al. [114] have shown that heart failure appears to be related to myocardial iron deficiency which in turn affects aconitase and citrate synthase [114]. Oxidative damage can also affect the aconitase Fe-S cluster [115]. The inactive form of aconitase has a 3Fe-4S cluster and one more iron atom is needed to produce the activated form leading to a 4Fe-4S cluster. This occurs via iron regulatory proteins [116], [117]. More information on the homeodynamics of iron in relation to ROS stress and aconitase has been presented by Bresgen and Eckl [118]. These observations appear relevant to thyroid disease because in both organs H2O2 constitutes the source of oxidative challenge. This noxious situation requires regulation through glutathione peroxidase, a selenoprotein [119], [120], [121].
Looking beyond iron itself, Semba et al. found an association between low selenium levels with anemia in older women [122]. In addition, Christensen et al. [123] described the role of selenium in regulating the expression of genes for proteins related to iron metabolism in rat liver cells. Iron deficiency leads to decreased expression of the selenoprotein glutathione peroxidase [124] by which thyroid function can be compromised. Iron has an indirect effect on OXPHOS by increasing respiration and downregulating glucose utilization. The opposite occurs in iron depletion [117].
Localization of iron in ovarian cells has been documented in the intercellular space of enlarged cells [125]. Iron seems to be relevant in the process of steroidogenesis as it is part of frataxin [126] which is related to ferredoxins. Transferrin has been found in granulosa cells [127]. Using a proteomics approach, Wu et al. [128] have documented an increase in transferrin following controlled ovarian hyper-stimulation. Chavarro et al. [129] described a positive effect of iron consumption on fertility. Angelucci et al. have found an inflammation-like profile in human follicular fluid following assisted reproductive technology intervention which included a high concentration of transferrin [130]. A similar finding has been reported for studies done with follicular fluid of mares [131]. On the other hand, low levels of transferrin can be found in older women [132].
8.2. Zinc (Zn)
There are only few clinical accounts relating Zn to thyroid function. In 1979 Hartoma et al. described the association between low levels of zinc and decreased biochemical indices of metabolism including low levels of thyroid hormones [133]. Low basal metabolic rate and lower levels of thyroid hormones were also described by Wada and King [134]. An epidemiological study in Germany in 1997 showed no correlation between zinc levels and thyroid function [135]. Zn deficiency can down regulate the activity of the TRH degrading enzyme in the hypothalamus and pituitary [136]. This results in increased levels of TSH and PRL. In cases of hypothyroidism and severe alopecia zinc levels can be found to be reduced [137]. In patients with Down syndrome, supplementing with Zn sulfate can improve thyroid function [138]. Under experimental conditions a combined state of deficiency of iodine, selenium and Zn can alter significantly the morphology of the thyroid gland [139].
Low levels of Zn have been described as being related to uncoupling of mitochondrial function acting at the level of cytochrome b and c [140] interfering also with ubiquinone [141]. Further interactions of Zn in the body come from its role in signal transduction arising from mobile reactive Zn. Zinc regulation is related to Zn-finger proteins, metallothioneins, Zn importers and Zn transporters [142], [143]. Zinc can influence cardiac function, and other organs such as secretory glands, i.e. pancreas, prostate, and mammary glands [144].
8.3. Magnesium
Magnesium plays a central role in energy balance as well as in a process described as cellular timekeeping [145]. The daily energetic processes of the organism are related to the circadian control of NAD (+) bioavailability [146]. In addition to NAD, intracellular magnesium regulation appears to underlie a similar mechanism [147].
In our original publication of the WOMED model of thyroid disease we have already discussed the relation of magnesium to changes in perfusion of the thyroid [16]. We also reviewed the significance of magnesium in all processes that require energy which is delivered by magnesium-ATP. Magnesium-ATP is produced in Complex V of the oxidative phosphorylation (OXPHOS) chain of molecules [148], [149]. A central role of magnesium in thyroid economy can be found in relation to iodine uptake [150]. This essential event, however, is generally ignored in publications that describe the metabolism of iodine [151]. Experimental data have shown that high doses of magnesium increase the activity of the thyroid [152]. Magnesium deficiency can influence bioavailability and tissue distribution of selenium which then appears diminished [153]. This description is similar to our clinical observations [16].
Magnesium deficiency during pregnancy affects the size and function of the placenta [154]. Stanton and Lowenstein described the relation between pregnancy, menopause and magnesium levels [155]. Magnesium levels are lower in pregnant women as well as in women taking oral contraceptives as compared to controls; in the menopause magnesium levels were found to be higher. Magnesium loss due to stress [156] could contribute to magnesium deficiency during pregnancy. We have described this mechanism as part of the complex known as pre-natal maternal stress [157].
There is a series of negative effects on fertility and pregnancy that can be assigned to magnesium deficiency or to alteration of the regulating channel. Similar to thyroid economy, steroid hormone synthesis is also an active process that requires energy [158]. Usually such biochemical steps are generally taken for granted, and the relation to energy dependency is forgotten. Magnesium and its cell transport mechanisms, i.e. the transient receptor potential cation channel subfamily M member 7 (TRPM7) [159], [160], have known important relations to reproduction processes. Initiation of embryo development is influenced by TRPM7 channels [161], [162], [163], [164]. On the other hand magnesium deficiency has deleterious effects on fetal outcome [165]. TRPM is functionally expressed in human endometrial cells during the luteal phase [166]. Looking at magnesium supplementation per se one finds a positive effect of magnesium on pregnancy outcome [167].
Fig. 8.
The magnesium deficiency syndrome 2017 by Moncayo and Moncayo.
8.4. Riboflavin and flavoproteins
An element that receives little attention in the context of thyroid hormone effects is vitamin B12, i.e. riboflavin. It plays a role in the process of thyroid hormone deiodination in the form of flavoproteins [56], [57], [169]. Flavoproteins in the form of the nicotinamide adenine dinucleotide phosphate (NADPH) are involved in the function of the membrane–bound thyroid oxidase located at the apical pole of thyroid cells which are required for the generation of H2O2 [170], [171], [172]. H2O2 is indispensable for thyroid hormone synthesis.
In steroid hormone synthesis, placenta mitochondria need the presence of NADH in order to have a functioning cholesterol side-chain cleavage enzyme system [173].
8.5. Selenium and coenzyme Q10 (CoQ10)
We consider that the main place for selenium in body functions is in its protective property in the form of selenoproteins [174]. The main groups involved are glutathione peroxidase and the deiodinases [175]. Thioredoxin reductase plays an important role in the response to iodine excess [176]. As mentioned before thyroid damage can occur in situations of iodine excess and selenium deficiency [177]. Under experimental conditions a combined iodine and selenium deficiency can alter the tissue distribution pattern of trace elements [178]. Sustained physical and psychological stress in men has been shown to induce lower levels of zinc, iron, and selenium [179]. Both the structure, i.e. loss of cristae, and the electron transport function of mitochondria can be altered in selenium deficiency [180]. These actions on mitochondrial biogenesis are complemented by thyroid hormones [181]. Besides the known action of deiodinases as selenoproteins [182], a new interesting observation has been contributed by Leoni et al. [183] who showed a positive influence of selenium on the expression of TSH-induced sodium-iodide symporter (NIS) activity. Finally it has to be stressed that selenoprotein synthesis is also an active process which requires ATP (e.g. Fig. 1 in [184]). In our model of the endocrine we associate energy supply via ATP with magnesium.
Recently data has appeared as to the negative disruptor effect on mitochondrial function which can occur through high fructose diet. Rats subjected to this diet presented high levels of ROS, reduced glutathione content, and reduced aconitase activity. The administration of glycyrrhizin can attenuate these alterations [185].
In our own studies we have seen the association of diminished concentrations of CoQ10 with hyperperfusion of the thyroid [16]. Another important association is that of low levels of CoQ10 following amiodarone administration [186]. Extensive information on the CoQ10 can be found in the publication by Wang and Hekimi [187]. Thyroid disease is not yet included.
CoQ10 is a component of the mitochondria and is considered as one of the small connecting molecules together with cytochrome c [188]. There is an interaction between CoQ10 and the 3Fe-4S cluster for effective electron transfer in complex II [189]. Mitofusins appear to be related to CoQ10 synthesis since knock-outs Mfn2 models results in low concentrations [190]. Mfn2 is important for the mitochondrial-endoplasmic reticulum tethering [191], [192].
CoQ10 restores oocyte mitochondrial function and fertility during reproductive aging [193]. The obesity related mitochondrial experimental changes in mouse oocytes can be prevented and rescued with CoQ10. Some of the underlying changes were lower levels of ATP and citrate [194].
In pregnant Holstein heifers, selenium supplementation can increase progesterone levels [195]. A similar effect can be seen during the menstrual cycle [196]. Selenium supplementation during pregnancy can have positive effects on glucose metabolism [197].
Vadhanavikit and Ganther [82], [198] have described an interrelation between selenium and CoQ10 in the sense that CoQ10 levels can decrease in situations of selenium deficiency. In humans, Alehagen et al. [199] have shown that combined supplementation with selenium and CoQ10 effectively reduces cardiovascular mortality in situations of selenium deficiency.
9. A new player: sterol regulatory element binding proteins - SREBPs
In 1993 a nuclear protein that binds to the sterol regulatory element of LDL receptor promoter was described [200], [201]. These proteins now called SERBPs have been found to be involved in more functions than just lipid and cholesterol metabolism [202], [203], [204], [205] as they can regulate the ATP citrate-lyase gene [206]. More interesting is the fact that they can regulate some of the elements involved in thyroid function such as the NIS gene in the thyroid [207] as well as mammary epithelial cells [208], the thyroid peroxidase [209], and the thyroglobulin gene [210]. Besides these relations to thyroid function there are also interaction between the SERBPs and steroidogenesis. Following the activation of cAMP and protein kinase A via LH stimulation, there is the addition interaction of the cholesterol-sensing SCAP-SREBP2 path which will coordinate steroidogenesis [211]. The connection between thyroid economy and lipids is given by the regulatory action of thyroid hormone on the SREBP-2 gene [212]. Under experimental conditions high dose selenium can up-regulate the activity of SERBP1 [213]. These data bring new light into the tuning of the endocrine.
10. Conclusions
We describe a systems approach model of thyroid disease which is based on clinical observations and satisfactory clinical experience resulting in benefit for the patients. We must caution that some of the concepts brought here are an extrapolation of basic research, i.e. there is yet no clinical confirmation. Still these ideas should motivate other researchers to conceive further research activities and to improve prevailing simplistic models of thyroid function (Fig. 1 in [214]).
Fig. 9.
Basic thyroid diagnostics.
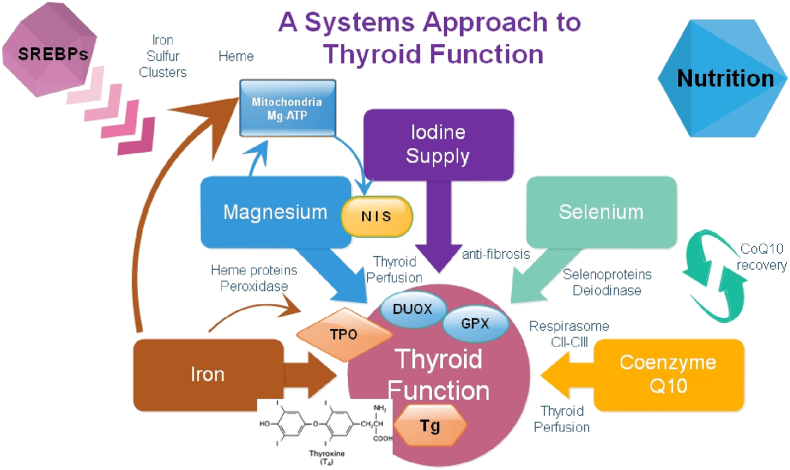
Fig. 10.
Integrated systems approach to thyroid function.
Abbreviations used: SREBPs: sterol regulatory element binding proteins; Mg-ATP: magnesium ATP, NIS: natrium iodide symporter, TPO: thyroid peroxidase, DUOX: dual oxidases, GPX: glutathione peroxidases, Tg: thyroglobulin. CII–CIII: complex II and III of the mitochondrial respiratory chain.
Transparency Document
Transparency document.
Footnotes
The Transparency document associated with this article can be found in the online version.
References
- 1.Moncayo R., Moncayo H.E. R.G. Landes; Austin, Texas: 1995. Ovarian Autoimmunity: Clinical and Experimental Data. [Google Scholar]
- 2.Starling E.H. Croonian lecture: on the chemical correlation of the functions of the body I. Lancet. 1905;166:339–341. [Google Scholar]
- 3.Michaelis L., Menten M. Die Kinetik der Invertinwirkung. Biochem. Z. 1913;49:333–369. [Google Scholar]
- 4.Provart N.J., McCourt P. Systems approaches to understanding cell signaling and gene regulation. Curr. Opin. Plant Biol. 2004;7:605–609. doi: 10.1016/j.pbi.2004.07.001. (PM:15337105) [DOI] [PubMed] [Google Scholar]
- 5.Nishimuta M. The concept of intracellular-, extracellular- and bone-minerals. Biofactors. 2000;12:35–38. doi: 10.1002/biof.5520120106. (PM:11216502) [DOI] [PubMed] [Google Scholar]
- 6.Ahn A.C. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030208. (PM:16681415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn A.C. The clinical applications of a systems approach. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030209. (PM:16683861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn A.C. Applying principles from complex systems to studying the efficacy of CAM therapies. J. Altern. Complement. Med. 2010;16:1015–1022. doi: 10.1089/acm.2009.0593. (PM:20715978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgers J.S. Criticism of evidence-based medicine: from reductionism to realism in the application of guidelines. Ned. Tijdschr. Geneeskd. 2015;159 (PM:25650035) [PubMed] [Google Scholar]
- 10.Heng H.H. The conflict between complex systems and reductionism. JAMA. 2008;300:1580–1581. doi: 10.1001/jama.300.13.1580. (PM:18827215) [DOI] [PubMed] [Google Scholar]
- 11.Popper K.R. J.C.B. Mohr; Tübingen: 1984. Logik Der Forschung. [Google Scholar]
- 12.Hill G.B. Archie Cochrane and his legacy. An internal challenge to physicians' autonomy? J. Clin. Epidemiol. 2000;53:1189–1192. doi: 10.1016/s0895-4356(00)00253-5. (PM:11146263) [DOI] [PubMed] [Google Scholar]
- 13.Stavrou A. Archibald Cochrane (1909–1988): the father of evidence-based medicine. Interact. Cardiovasc. Thorac. Surg. 2014;18:121–124. doi: 10.1093/icvts/ivt451. (PM:24140816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochrane A.L. Nuffield Provincial Hospitals Trust; Nuffield: 1972. Effectiveness and Efficiency: Random Reflections on Health Services. [Google Scholar]
- 15.Moncayo R., Moncayo H. Exploring the aspect of psychosomatics in hypothyroidism: the WOMED model of body-mind interactions based on musculoskeletal changes, psychological stressors, and low levels of magnesium. Woman - Psychosomatic Gynaecol. Obstet. 2014;1:1–11. [Google Scholar]
- 16.Moncayo R., Moncayo H. The WOMED model of benign thyroid disease: acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. Biochim. Biophys. Acta Rev. Biomembr. Clin. 2015;3:44–64. doi: 10.1016/j.bbacli.2014.11.002. (PM:26675817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollowell J.G. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J. Clin. Endocrinol. Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. (PM:11836274) [DOI] [PubMed] [Google Scholar]
- 18.Lee P.H. Resampling methods improve the predictive power of modeling in class-imbalanced datasets. Int. J. Environ. Res. Public Health. 2014;11:9776–9789. doi: 10.3390/ijerph110909776. (PM:25238271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paludan-Müller A. Mechanisms and direction of allocation bias in randomised clinical trials. BMC Med. Res. Methodol. 2016;16:133. doi: 10.1186/s12874-016-0235-y. (PM:27717321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baloch Z. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. (PM:12625976) [DOI] [PubMed] [Google Scholar]
- 21.Abalovich M. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2007;92(8 Suppl):s1–s47. doi: 10.1210/jc.2007-0141. (PM:17948378) [DOI] [PubMed] [Google Scholar]
- 22.Moncayo R., Moncayo H. A post-publication analysis of the idealized upper reference value of 2.5 mIU/L for TSH: time to support the thyroid axis with magnesium and iron especially in the setting of reproduction medicine. Biochim. Biophys. Acta Rev. Biomembr. 2017 doi: 10.1016/j.bbacli.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moncayo R., Moncayo H. A musculoskeletal model of low grade connective tissue inflammation in patients with thyroid associated ophthalmopathy (TAO): the WOMED concept of lateral tension and its general implications in disease. BMC Musculoskelet. Disord. 2007;8:17. doi: 10.1186/1471-2474-8-17. (PM:17319961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kainz H. Image fusion analysis of (99m)Tc-HYNIC-octreotide scintigraphy and CT/MRI in patients with thyroid-associated orbitopathy: the importance of the lacrimal gland. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1155–1159. doi: 10.1007/s00259-003-1207-0. (PM:12811420) [DOI] [PubMed] [Google Scholar]
- 25.Moncayo R. The role of selenium, vitamin C, and zinc in benign thyroid diseases and of Se in malignant thyroid diseases: low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr. Disord. 2008;8:2. doi: 10.1186/1472-6823-8-2. (PM:18221503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pairleitner H. Three-dimensional power Doppler sonography: imaging and quantifying blood flow and vascularization. Ultrasound Obstet. Gynecol. 1999;14:139–143. doi: 10.1046/j.1469-0705.1999.14020139.x. (PM:10492874) [DOI] [PubMed] [Google Scholar]
- 27.Moncayo R., Moncayo H. Advanced 3D sonography of the thyroid: focus on vascularity. In: Thoirs K., editor. Sonography. Intech; Rijeka, Croatia: 2012. pp. 273–292. (vol. http://www.intechopen.com/articles/show/title/thyroid-sonography-in-3d-with-emphasis-on-perfusion) [Google Scholar]
- 28.Contempre B. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta) Mol. Cell. Endocrinol. 1996;124:7–15. doi: 10.1016/s0303-7207(96)03921-4. (PM:9027319) [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto K., Birch S. Paradigm Publications; Brookline: 1986. Extraordinary Vessels. [Google Scholar]
- 30.Ross J. Churchill Livingstone; 1995. Acupuncture Point Combinations: The Key to Clinical Success. [Google Scholar]
- 31.Kirschbaum B. Medizinisch Literarische Verlagsgesellschaft mbH; Uelzen: 2000. Die 8 Außerordentlichen Gefäße in Der Traditionellen Chinesischen Medizin. [Google Scholar]
- 32.Deadman P., Al-Khafaji M., Baker K. Journal of Chinese Medicine Publications; Hove: 2001. A Manual of Acupuncture. [Google Scholar]
- 33.Maciocia G. Churchill Livinstone; 2006. The Channels of Acupuncture: Clinical Use of the Secondary Channels and Eight Extraordinary Vessels. [Google Scholar]
- 34.Moncayo R. In-vivo visualisation of the anatomical structures related to the acupuncture points Dai mai and Shen mai by MRI: a single-case pilot study. BMC Med. Imaging. 2007;7:4. doi: 10.1186/1471-2342-7-4. (PM:17359521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moncayo R. 3D-MRI rendering of the anatomical structures related to acupuncture points of the Dai mai, Yin qiao mai and Yang qiao mai meridians within the context of the WOMED concept of lateral tension: implications for musculoskeletal disease. BMC Musculoskelet. Disord. 2007;8:33. doi: 10.1186/1471-2474-8-33. (PM:17425796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis A., Wiseman N., Boss K. Paradigm Publications; Brookline: 1989. Grasping the Wind. [Google Scholar]
- 37.Wu S.Y. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: mechanosensitive TRPV1 as an "acupuncture-responding channel". BMC Complement. Altern. Med. 2014;14:96. doi: 10.1186/1472-6882-14-96. (PM:24612851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W., Ahn A.C. Subcutaneous fascial bands—a qualitative and morphometric analysis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023987. (PM:21931632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamata S. Structure of the rat subcutaneous connective tissue in relation to its sliding mechanism. Arch. Histol. Cytol. 2003;66:273–279. doi: 10.1679/aohc.66.273. (PM:14527168) [DOI] [PubMed] [Google Scholar]
- 40.Guimberteau J.C. Introduction to the knowledge of subcutaneous sliding system in humans. Ann. Chir. Plast. Esthet. 2005;50:19–34. doi: 10.1016/j.anplas.2004.10.012. (PM:15695007) [DOI] [PubMed] [Google Scholar]
- 41.Bodó E. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633–1642. doi: 10.1210/en.2009-0306. (PM:20176727) [DOI] [PubMed] [Google Scholar]
- 42.Fernandez A. Evidence-based medicine: is it a bridge too far? Health Res. Policy Syst. 2015;13:66. doi: 10.1186/s12961-015-0057-0. (PM:26546273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sizer P.S., Jr. Should evidence or sound clinical reasoning dictate patient care? J. Man. Manip. Ther. 2016;24:117–119. doi: 10.1080/10669817.2016.1185296. (PM:27559281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzocchi F. Complexity and the reductionism-holism debate in systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012;4:413–427. doi: 10.1002/wsbm.1181. (PM:22761024) [DOI] [PubMed] [Google Scholar]
- 45.Ross D.S. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. (PM:27521067) [DOI] [PubMed] [Google Scholar]
- 46.Hoermann R. Homeostatic control of the thyroid-pituitary axis: perspectives for diagnosis and treatment. Front. Endocrinol. 2015;6:177. doi: 10.3389/fendo.2015.00177. (PM:26635726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dietrich J.W. Thyrotropic feedback control: evidence for an additional ultrashort feedback loop from fractal analysis. Cybern. Syst. 2004;35:315–331. [Google Scholar]
- 48.Hoermann R. Complex relationship between free thyroxine and thyrotropin in the regulation of thyroid function. Eur. J. Endocrinol. 2010;162:1123–1129. doi: 10.1530/EJE-10-0106. (PM:20299491) [DOI] [PubMed] [Google Scholar]
- 49.Hoermann R. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment? Eur. J. Endocrinol. 2013;168:271–280. doi: 10.1530/EJE-12-0819. (PM:23184912) [DOI] [PubMed] [Google Scholar]
- 50.Midgley J.E. Physiological states and functional relation between thyrotropin and free thyroxine in thyroid health and disease: in vivo and in silico data suggest a hierarchical model. J. Clin. Pathol. 2013;66:335–342. doi: 10.1136/jclinpath-2012-201213. (PM:23423518) [DOI] [PubMed] [Google Scholar]
- 51.Hoermann R. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin. Endocrinol. 2014;81:907–915. doi: 10.1111/cen.12527. (PM:24953754) [DOI] [PubMed] [Google Scholar]
- 52.Midgley J.E. Variation in the biochemical response to l-thyroxine therapy and relationship with peripheral thyroid hormone conversion. Endocr. Connect. 2015;4:196–205. doi: 10.1530/EC-150056. (PM:26265111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoermann R. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm. Metab. Res. 2015;47:674–680. doi: 10.1055/s-0034-1398616. (PM:25750078) [DOI] [PubMed] [Google Scholar]
- 54.Beckett G.J. Inter-relationships between selenium and thyroid hormone metabolism in the rat and man. J. Trace Elem. Electrolytes Health Dis. 1991;5:265–267. (PM:1822335) [PubMed] [Google Scholar]
- 55.Behne D., Kyriakopoulos A. Effects of dietary selenium on the tissue concentrations of type I iodothyronine 5′-deiodinase and other selenoproteins. Am. J. Clin. Nutr. 1993;57:310S–312S. doi: 10.1093/ajcn/57.2.310S. (PM:8427210) [DOI] [PubMed] [Google Scholar]
- 56.Goswami A., Rosenberg I.N. Characterization of a flavoprotein iodotyrosine deiodinase from bovine thyroid. Flavin nucleotide binding and oxidation-reduction properties. J. Biol. Chem. 1979;254:12326–12330. (PM:500718) [PubMed] [Google Scholar]
- 57.Rosenberg I.N., Goswami A. Purification and characterization of a flavoprotein from bovine thyroid with iodotyrosine deiodinase activity. J. Biol. Chem. 1979;254:12318–12325. (PM:500717) [PubMed] [Google Scholar]
- 58.Thomas S.R. Crystal structure of iodotyrosine deiodinase, a novel flavoprotein responsible for iodide salvage in thyroid glands. J. Biol. Chem. 2009;284:19659–19667. doi: 10.1074/jbc.M109.013458. (PM:19436071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisenberg M. Extensions, validation, and clinical applications of a feedback control system simulator of the hypothalamo-pituitary-thyroid axis. Thyroid. 2008;18:1071–1085. doi: 10.1089/thy.2007.0388. (PM:18844475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIntosh J.E.A., McIntosh R.P. Springer-Verlag; Berlin: 1980. Mathematical Modeling and Computers in Endocrinology. [Google Scholar]
- 61.Meier C. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study) J. Clin. Endocrinol. Metab. 2001;86:4860–4866. doi: 10.1210/jcem.86.10.7973. (PM:11600554) [DOI] [PubMed] [Google Scholar]
- 62.Billewicz W.Z. Statistical methods applied to the diagnosis of hypothyroidism. Q. J. Med. 1969;38:255–266. (PM:4181088) [PubMed] [Google Scholar]
- 63.Zulewski H. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J. Clin. Endocrinol. Metab. 1997;82:771–776. doi: 10.1210/jcem.82.3.3810. (PM:9062480) [DOI] [PubMed] [Google Scholar]
- 64.Meier C. Serum thyroid stimulating hormone in assessment of severity of tissue hypothyroidism in patients with overt primary thyroid failure: cross sectional survey. BMJ. 2003;326:311–312. doi: 10.1136/bmj.326.7384.311. (PM:12574044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winther K.H. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156925. (PM:27257805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopp P.A. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid. 2014;24:1667–1669. doi: 10.1089/thy.2014.2412. (PM:25495371) [DOI] [PubMed] [Google Scholar]
- 67.Samuels M.H. Effect of thyroid function variations within the laboratory reference range on health status, mood, and cognition in levothyroxine-treated subjects. Thyroid. 2016;26:1173–1184. doi: 10.1089/thy.2016.0141. (PM:27338133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saravanan P. Psychological well-being in patients on 'adequate' doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. (PM:12390330) [DOI] [PubMed] [Google Scholar]
- 69.Wekking E.M. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. (PM:16322379) [DOI] [PubMed] [Google Scholar]
- 70.Walsh J.P. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J. Clin. Endocrinol. Metab. 2006;91:2624–2630. doi: 10.1210/jc.2006-0099. (PM:16670161) [DOI] [PubMed] [Google Scholar]
- 71.Brown B.T. The influence of a biopsychosocial-based treatment approach to primary overt hypothyroidism: a protocol for a pilot study. Trials. 2010;11:106. doi: 10.1186/1745-6215-11-106. (PM:21073760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown B.T. A biopsychosocial approach to primary hypothyroidism: treatment and harms data from a randomized controlled trial. Chiropr. Man. Therap. 2015;23:24. doi: 10.1186/s12998-015-0068-5. (PM:26301086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stuefer S. The role of magnesium and thyroid function in early pregnancy after in-vitro fertilization (IVF): new aspects in endocrine physiology. Biochim. Biophys. Acta Clin. 2015;3:196–204. doi: 10.1016/j.bbacli.2015.02.006. (PM:26675754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sartori S.B. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2012;62:304–312. doi: 10.1016/j.neuropharm.2011.07.027. (PM:21835188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henrotte J.G. Blood and urinary magnesium, zinc, calcium, free fatty acids, and catecholamines in type A and type B subjects. J. Am. Coll. Nutr. 1985;4:165–172. doi: 10.1080/07315724.1985.10720073. (PM:4019939) [DOI] [PubMed] [Google Scholar]
- 76.Grases G. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes. Res. 2006;19:102–106. (PM:16955721) [PubMed] [Google Scholar]
- 77.Harbeck B. No stress after 24-hour on-call shifts? J. Occup. Health. 2015;57:438–447. doi: 10.1539/joh.14-0276-OA. (PM:26119209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 79.Selye H. Adaptation energy. Nature. 1938;141:926. [Google Scholar]
- 80.Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. (PM:15426759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pedersen H.S. High serum coenzyme Q10, positively correlated with age, selenium and cholesterol, in Inuit of Greenland. A pilot study. Biofactors. 1999;9:319–323. doi: 10.1002/biof.5520090230. (PM:10416047) [DOI] [PubMed] [Google Scholar]
- 82.Vadhanavikit S., Ganther H.E. Selenium deficiency and decreased coenzyme Q levels. Mol. Asp. Med. 1994;15(Suppl):s103–s107. doi: 10.1016/0098-2997(94)90019-1. (PM:7752821) [DOI] [PubMed] [Google Scholar]
- 83.Moncayo R., Moncayo H. Proof of concept of the WOMED model of benign thyroid disease: restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. Biochim. Biophys. Acta (BBA) Clin. 2015;3:113–122. doi: 10.1016/j.bbacli.2014.12.005. (PM:26672672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farrell Y.R. Singing Dragon; London: 2016. Psycho-emotional Pain and the Eight Extraordinary Vessels. [Google Scholar]
- 85.Goldenthal M.J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol. Cell. Biochem. 2004;265:97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. (PM:15553939) [DOI] [PubMed] [Google Scholar]
- 86.Goldenthal M.J. Nuclear-mitochondrial cross-talk in cardiomyocyte T3 signaling: a time-course analysis. J. Mol. Cell. Cardiol. 2005;39:319–326. doi: 10.1016/j.yjmcc.2005.03.016. (PM:15893763) [DOI] [PubMed] [Google Scholar]
- 87.Forini F. Mitochondria as key targets of cardioprotection in cardiac ischemic disease: role of thyroid hormone triiodothyronine. Int. J. Mol. Sci. 2015;16:6312–6336. doi: 10.3390/ijms16036312. (PM:25809607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forini F. Low T3 state is correlated with cardiac mitochondrial impairments after ischemia reperfusion injury: evidence from a proteomic approach. Int. J. Mol. Sci. 2015;16:26687–26705. doi: 10.3390/ijms161125973. (PM:26561807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. (PM:27733282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hess S.Y. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J. Nutr. 2002;132:1951–1955. doi: 10.1093/jn/132.7.1951. (PM:12097675) [DOI] [PubMed] [Google Scholar]
- 91.Beard J.L. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J. Nutr. 1998;128:1401–1408. doi: 10.1093/jn/128.8.1401. (PM:9687562) [DOI] [PubMed] [Google Scholar]
- 92.Ordooei M. The effect of iron supplement on children with euthyroid goiter: a randomized placebo-controlled clinical trial. Iran J. Ped. Hematol. Oncol. 2014;4:84–88. (PM:25254085) [PMC free article] [PubMed] [Google Scholar]
- 93.Bastian T.W. Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mRNA levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology. 2012;153:5668–5680. doi: 10.1210/en.2012-1067. (PM:23054056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu X. Iron deficiency without anemia causes maternal hypothyroxinemia in pregnant rats. Nutr. Res. 2014;34:604–612. doi: 10.1016/j.nutres.2014.06.007. (PM:25150119) [DOI] [PubMed] [Google Scholar]
- 95.Beard J.L. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am. J. Clin. Nutr. 1990;52:813–819. doi: 10.1093/ajcn/52.5.813. (PM:2239756) [DOI] [PubMed] [Google Scholar]
- 96.Shakir K.M. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin. Proc. 2000;75:189–192. (PM:10683660) [PubMed] [Google Scholar]
- 97.Harris Rosenzweig P., Volpe S.L. Effect of iron supplementation on thyroid hormone levels and resting metabolic rate in two college female athletes: a case study. Int. J. Sport Nutr. Exerc. Metab. 2000;10:434–443. doi: 10.1123/ijsnem.10.4.434. (PM:11099370) [DOI] [PubMed] [Google Scholar]
- 98.Yu X. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J. Clin. Endocrinol. Metab. 2015;100:1594–1601. doi: 10.1210/jc.2014-3887. (PM:25599388) [DOI] [PubMed] [Google Scholar]
- 99.Veltri F. Prevalence of thyroid autoimmunity and dysfunction in women with iron deficiency during early pregnancy: is it altered? Eur. J. Endocrinol. 2016;175:191–199. doi: 10.1530/EJE-16-0288. (PM:27450694) [DOI] [PubMed] [Google Scholar]
- 100.Zimmermann M.B. Iron deficiency predicts poor maternal thyroid status during pregnancy. J. Clin. Endocrinol. Metab. 2007;92:3436–3440. doi: 10.1210/jc.2007-1082. (PM:17566085) [DOI] [PubMed] [Google Scholar]
- 101.Murray-Kolb L.E., Beard J.L. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin. Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. (PM:17344500) [DOI] [PubMed] [Google Scholar]
- 102.Fretham S.J. The role of iron in learning and memory. Adv. Nutr. 2011;2:112–121. doi: 10.3945/an.110.000190. (PM:22332040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tran P.V. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J. Nutr. 2008;138:2495–2501. doi: 10.3945/jn.108.091553. (PM:19022978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tran P.V. Gestational-neonatal iron deficiency suppresses and iron treatment reactivates IGF signaling in developing rat hippocampus. Am. J. Physiol. Endocrinol. Metab. 2012;302:E316–E324. doi: 10.1152/ajpendo.00369.2011. (PM:22068601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richardson D.R. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. (PM:20495089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Atamna H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res. Rev. 2004;3:303–318. doi: 10.1016/j.arr.2004.02.002. (PM:15231238) [DOI] [PubMed] [Google Scholar]
- 107.Leedman P.J. Thyroid hormone modulates the interaction between iron regulatory proteins and the ferritin mRNA iron-responsive element. J. Biol. Chem. 1996;271:12017–12023. doi: 10.1074/jbc.271.20.12017. (PM:8662626) [DOI] [PubMed] [Google Scholar]
- 108.Maio N., Rouault T.A. Iron-sulfur cluster biogenesis in mammalian cells: new insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta. 2015;1853:1493–1512. doi: 10.1016/j.bbamcr.2014.09.009. (PM:25245479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lill R. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta. 2012;1823:1491–1508. doi: 10.1016/j.bbamcr.2012.05.009. (PM:22609301) [DOI] [PubMed] [Google Scholar]
- 110.Kim H.J. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta. 2012;1823:1604–1616. doi: 10.1016/j.bbamcr.2012.04.008. (PM:22554985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sheftel A.D. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11775–11780. doi: 10.1073/pnas.1004250107. (PM:20547883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rensvold J.W. Complementary RNA and protein profiling identifies iron as a key regulator of mitochondrial biogenesis. Cell Rep. 2013;3:237–245. doi: 10.1016/j.celrep.2012.11.029. (PM:23318259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pandey A. Fe-S cluster biogenesis in isolated mammalian mitochondria: coordinated use of persulfide sulfur and iron and requirements for GTP, NADH, and ATP. J. Biol. Chem. 2015;290:640–657. doi: 10.1074/jbc.M114.610402. (PM:25398879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melenovsky V. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur. J. Heart Fail. 2016 doi: 10.1002/ejhf.640. (PM:27647766) [DOI] [PubMed] [Google Scholar]
- 115.Gardner P.R. Superoxide-driven aconitase FE-S center cycling. Biosci. Rep. 1997;17:33–42. doi: 10.1023/a:1027383100936. (PM:9171919) [DOI] [PubMed] [Google Scholar]
- 116.Gruer M.J. The aconitase family: three structural variations on a common theme. Trends Biochem. Sci. 1997;22:3–6. doi: 10.1016/s0968-0004(96)10069-4. (PM:9020582) [DOI] [PubMed] [Google Scholar]
- 117.Oexle H. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta. 1999;1413:99–107. doi: 10.1016/s0005-2728(99)00088-2. (PM:10556622) [DOI] [PubMed] [Google Scholar]
- 118.Bresgen N., Eckl P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomol. Ther. 2015;5:808–847. doi: 10.3390/biom5020808. (PM:25970586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rose A.H., Hoffmann P.R. Selenoproteins and cardiovascular stress. Thromb. Haemost. 2015;113:494–504. doi: 10.1160/TH14-07-0603. (PM:25354851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hiraoka Y. Association between function of selenium and heart disease. Nihon Rinsho. 2016;74:1192–1198. (PM:27455811) [PubMed] [Google Scholar]
- 121.Benstoem C. Selenium and its supplementation in cardiovascular disease—what do we know? Nutrients. 2015;7:3094–3118. doi: 10.3390/nu7053094. (PM:25923656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Semba R.D. Low serum selenium is associated with anemia among older women living in the community: the Women's Health and aging studies I and II. Biol. Trace Elem. Res. 2006;112:97–108. doi: 10.1385/BTER:112:2:97. (PM:17805227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Christensen M.J. Selenium regulates expression in rat liver of genes for proteins involved in iron metabolism. Biol. Trace Elem. Res. 2000;74:55–70. doi: 10.1385/BTER:74:1:55. (PM:11049200) [DOI] [PubMed] [Google Scholar]
- 124.Moriarty P.M. Classical selenium-dependent glutathione peroxidase expression is decreased secondary to iron deficiency in rats. J. Nutr. 1995;125:293–301. doi: 10.1093/jn/125.2.293. (PM:7861256) [DOI] [PubMed] [Google Scholar]
- 125.Sato E. Morphodynamics of ovarian follicles during oogenesis in mice. Microsc. Res. Tech. 2006;69:427–435. doi: 10.1002/jemt.20302. (PM:16718657) [DOI] [PubMed] [Google Scholar]
- 126.Palandri A. Frataxin inactivation leads to steroid deficiency in flies and human ovarian cells. Hum. Mol. Genet. 2015;24:2615–2626. doi: 10.1093/hmg/ddv024. (PM:25628335) [DOI] [PubMed] [Google Scholar]
- 127.Briggs D.A. Transferrin in the developing ovarian follicle: evidence for de-novo expression by granulosa cells. Mol. Hum. Reprod. 1999;5:1107–1114. doi: 10.1093/molehr/5.12.1107. (PM:10587364) [DOI] [PubMed] [Google Scholar]
- 128.Wu Y.T. Preliminary proteomic analysis on the alterations in follicular fluid proteins from women undergoing natural cycles or controlled ovarian hyperstimulation. J. Assist. Reprod. Genet. 2015;32:417–427. doi: 10.1007/s10815-014-0419-5. (PM:25595538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chavarro J.E. Iron intake and risk of ovulatory infertility. Obstet. Gynecol. 2006;108:1145–1152. doi: 10.1097/01.AOG.0000238333.37423.ab. (PM:17077236) [DOI] [PubMed] [Google Scholar]
- 130.Angelucci S. Proteome analysis of human follicular fluid. Biochim. Biophys. Acta. 2006;1764:1775–1785. doi: 10.1016/j.bbapap.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 131.Fahiminiya S. Proteomic analysis of mare follicular fluid during late follicle development. Proteome Sci. 2011;9:54. doi: 10.1186/1477-5956-9-54. (PM:21923925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashemitabar M. A proteomic analysis of human follicular fluid: comparison between younger and older women with normal FSH levels. Int. J. Mol. Sci. 2014;15:17518–17540. doi: 10.3390/ijms151017518. (PM:25268621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hartoma T.R. Effect of zinc on some biochemical indices of metabolism. Nutr. Metab. 1979;23:294–300. doi: 10.1159/000176268. (PM:440633) [DOI] [PubMed] [Google Scholar]
- 134.Wada L., King J.C. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. J. Nutr. 1986;116:1045–1053. doi: 10.1093/jn/116.6.1045. (PM:3723200) [DOI] [PubMed] [Google Scholar]
- 135.Hampel R. Serum zinc levels and goitre epidemiology in Germany. Z. Ernahrungswiss. 1997;36:12–15. doi: 10.1007/BF01618894. (PM:9095534) [DOI] [PubMed] [Google Scholar]
- 136.Alvarez-Salas E. Mediobasal hypothalamic and adenohypophyseal TRH-degrading enzyme (PPII) is down-regulated by zinc deficiency. Int. J. Dev. Neurosci. 2015;46:115–124. doi: 10.1016/j.ijdevneu.2015.08.001. (PM:26315400) [DOI] [PubMed] [Google Scholar]
- 137.Betsy A. Zinc deficiency associated with hypothyroidism: an overlooked cause of severe alopecia. Int. J. Trichol. 2013;5:40–42. doi: 10.4103/0974-7753.114714. (PM:23960398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bucci I. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol. Trace Elem. Res. 1999;67:257–268. doi: 10.1007/BF02784425. (PM:10201332) [DOI] [PubMed] [Google Scholar]
- 139.Ruz M. Single and multiple selenium-zinc-iodine deficiencies affect rat thyroid metabolism and ultrastructure. J. Nutr. 1999;129:174–180. doi: 10.1093/jn/129.1.174. (PM:9915896) [DOI] [PubMed] [Google Scholar]
- 140.Skulachev V.P. Inhibition of the respiratory chain by zinc ions. Biochem. Biophys. Res. Commun. 1967;26:1–6. doi: 10.1016/0006-291x(67)90242-2. (PM:4291553) [DOI] [PubMed] [Google Scholar]
- 141.Kleiner D., von J.G. On the inhibition of mitochondrial electron transport by Zn(2 +) ions. FEBS Lett. 1972;20:229–232. doi: 10.1016/0014-5793(72)80802-0. (PM:11946424) [DOI] [PubMed] [Google Scholar]
- 142.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv. Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. (PM:23319127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee S.R. The critical roles of zinc: beyond impact on myocardial signaling. Korean J. Physiol. Pharmacol. 2015;19:389–399. doi: 10.4196/kjpp.2015.19.5.389. (PM:26330751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelleher S.L. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. (PM:22332039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feeney K.A. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. (PM:27074515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peek C.B. Circadian clock NAD + cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. (PM:24051248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dunlap J.C., Loros J.J. Yes, circadian rhythms actually do affect almost everything. Cell Res. 2016;26:759–760. doi: 10.1038/cr.2016.65. (PM:27241553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jonckheere A.I. Mitochondrial ATP synthase: architecture, function and pathology. J. Inherit. Metab. Dis. 2012;35:211–225. doi: 10.1007/s10545-011-9382-9. (PM:21874297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Habersetzer J. Human F1F0 ATP synthase, mitochondrial ultrastructure and OXPHOS impairment: a (super-)complex matter? PLoS One. 2013;8 doi: 10.1371/journal.pone.0075429. (PM:24098383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tyler D.D. Influence of mitochondrial inhibitors on the respiration and energy-dependent uptake of iodide by thyroid slices. Biochem. J. 1968;106:123–133. doi: 10.1042/bj1060123. (PM:4238489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andersen S.L. Iodine status in pregnant and breastfeeding women: a Danish regional investigation. Dan. Med. J. 2015;62 (PM:26050837) [PubMed] [Google Scholar]
- 152.Chandra A.K. Effects of magnesium on cytomorphology and enzyme activities in thyroid of rats. Indian J. Exp. Biol. 2014;52:787–792. (PM:25141541) [PubMed] [Google Scholar]
- 153.Jiménez A. Changes in bioavailability and tissue distribution of selenium caused by magnesium deficiency in rats. J. Am. Coll. Nutr. 1997;16:175–180. doi: 10.1080/07315724.1997.10718669. (PM:9100219) [DOI] [PubMed] [Google Scholar]
- 154.Rosner J.Y. Magnesium deficiency during pregnancy in mice impairs placental size and function. Placenta. 2016;39:87–93. doi: 10.1016/j.placenta.2016.01.009. (PM:26992680) [DOI] [PubMed] [Google Scholar]
- 155.Stanton M.F., Lowenstein F.W. Serum magnesium in women during pregnancy, while taking contraceptives, and after menopause. J. Am. Coll. Nutr. 1987;6:313–319. doi: 10.1080/07315724.1987.10720193. (PM:3611529) [DOI] [PubMed] [Google Scholar]
- 156.Nishimuta M. Stress induced manesiuresis in human. Maguneshumu. 1982;7:123–132. [Google Scholar]
- 157.Moncayo R., Ortner K. Multifactorial determinants of cognition – Thyroid function is not the only one. Biochim. Biophys. Acta Clin. 2015;3:289–298. doi: 10.1016/j.bbacli.2015.04.002. (PM:26672993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stocco D.M. Intramitochondrial cholesterol transfer. Biochim. Biophys. Acta. 2000;1486:184–197. doi: 10.1016/s1388-1981(00)00056-1. (PM:10856721) [DOI] [PubMed] [Google Scholar]
- 159.Schlingmann K.P. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. (PM:17481860) [DOI] [PubMed] [Google Scholar]
- 160.Paravicini T.M. TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 2012;44:1381–1384. doi: 10.1016/j.biocel.2012.05.010. (PM:22634382) [DOI] [PubMed] [Google Scholar]
- 161.Liu W. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev. Biol. 2011;350:348–357. doi: 10.1016/j.ydbio.2010.11.034. (PM:21145885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Komiya Y. Magnesium and embryonic development. Magnes. Res. 2014;27:1–8. doi: 10.1684/mrh.2014.0356. (PM:24721994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Komiya Y., Runnels L.W. TRPM channels and magnesium in early embryonic development. Int. J. Dev. Biol. 2015;59:281–288. doi: 10.1387/ijdb.150196lr. (PM:26679946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carvacho I. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci. Rep. 2016;6:34236. doi: 10.1038/srep34236. (PM:27681336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Almonte R.A. Gestational magnesium deficiency is deleterious to fetal outcome. Biol. Neonate. 1999;76:26–32. doi: 10.1159/000014128. (PM:10364636) [DOI] [PubMed] [Google Scholar]
- 166.De Clercq K. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015;30:1421–1436. doi: 10.1093/humrep/dev068. (PM:25820697) [DOI] [PubMed] [Google Scholar]
- 167.Kovács L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe Frauenheilkd. 1988;48:595–600. (PM:3063587) [PubMed] [Google Scholar]
- 168.Seelig M.S. The requirement of magnesium by the normal adult. Summary and analysis of published data. Am. J. Clin. Nutr. 1964;14:342–390. doi: 10.1093/ajcn/14.6.342. (PM:14168977) [DOI] [PubMed] [Google Scholar]
- 169.Goswami A., Rosenberg I.N. Ferredoxin and ferredoxin reductase activities in bovine thyroid. Possible relationship to iodotyrosine deiodinase. J. Biol. Chem. 1981;256:893–899. (PM:6778876) [PubMed] [Google Scholar]
- 170.Leseney A.M. Biochemical characterization of a Ca2 +/NAD(P)H-dependent H2O2 generator in human thyroid tissue. Biochimie. 1999;81:373–380. doi: 10.1016/s0300-9084(99)80084-4. (PM:10401672) [DOI] [PubMed] [Google Scholar]
- 171.Faggiano A. Functional characterization of human thyroid tissue with immunohistochemistry. Thyroid. 2007;17:203–211. doi: 10.1089/thy.2006.0174. (PM:17381352) [DOI] [PubMed] [Google Scholar]
- 172.Milenkovic M. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J. Endocrinol. 2007;192:615–626. doi: 10.1677/JOE-06-0003. (PM:17332529) [DOI] [PubMed] [Google Scholar]
- 173.Mason J.I., Boyd G.S. The cholesterol side-chain cleavage enzyme system in mitochondria of human term placenta. Eur. J. Biochem. 1971;21:308–321. doi: 10.1111/j.1432-1033.1971.tb01471.x. (PM:4398212) [DOI] [PubMed] [Google Scholar]
- 174.Köhrle J. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. (PM:16174820) [DOI] [PubMed] [Google Scholar]
- 175.Beckett G.J., Arthur J.R. Selenium and endocrine systems. J. Endocrinol. 2005;184:455–465. doi: 10.1677/joe.1.05971. (PM:15749805) [DOI] [PubMed] [Google Scholar]
- 176.Leoni S.G. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol. Endocrinol. 2011;25:1924–1935. doi: 10.1210/me.2011-0038. (PM:21903721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Contempre B. Selenium deficiency aggravates the necrotizing effects of a high iodide dose in iodine deficient rats. Endocrinology. 1993;132:1866–1868. doi: 10.1210/endo.132.4.8462484. (PM:8462484) [DOI] [PubMed] [Google Scholar]
- 178.Giray B. Iodine and/or selenium deficiency alters tissue distribution pattern of other trace elements in rats. Biol. Trace Elem. Res. 2003;95:247–258. doi: 10.1385/BTER:95:3:247. (PM:14665730) [DOI] [PubMed] [Google Scholar]
- 179.Singh A. Biochemical indices of selected trace minerals in men: effect of stress. Am. J. Clin. Nutr. 1991;53:126–131. doi: 10.1093/ajcn/53.1.126. (PM:1984337) [DOI] [PubMed] [Google Scholar]
- 180.Rani P., Lalitha K. Evidence for altered structure and impaired mitochondrial electron transport function in selenium deficiency. Biol. Trace Elem. Res. 1996;51:225–234. doi: 10.1007/BF02784077. (PM:8727670) [DOI] [PubMed] [Google Scholar]
- 181.Izquierdo J.M. Hypothyroidism affects the expression of the beta-F1-ATPase gene and limits mitochondrial proliferation in rat liver at all stages of development. Eur. J. Biochem. 1995;232:344–350. doi: 10.1111/j.1432-1033.1995.344zz.x. (PM:7556180) [DOI] [PubMed] [Google Scholar]
- 182.Shchedrina V.A. Structure-function relationships, physiological roles and evolution of mammalian ER-resident selenoproteins. Antioxid. Redox Signal. 2010;12:839–849. doi: 10.1089/ars.2009.2865. (PM:19747065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Leoni S.G. Selenium increases thyroid-stimulating hormone-induced sodium/iodide symporter expression through thioredoxin/apurinic/apyrimidinic endonuclease 1-dependent regulation of paired box 8 binding activity. Antioxid. Redox Signal. 2016;24:855–866. doi: 10.1089/ars.2014.6228. (PM:26650895) [DOI] [PubMed] [Google Scholar]
- 184.Schweizer U., Fradejas-Villar N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016;30:3669–3681. doi: 10.1096/fj.201600424. (PM:27473727) [DOI] [PubMed] [Google Scholar]
- 185.Sil R., Chakraborti A.S. Oxidative inactivation of liver mitochondria in high fructose diet-induced metabolic syndrome in rats: effect of glycyrrhizin treatment. Phytother. Res. 2016;30:1503–1512. doi: 10.1002/ptr.5654. (PM:27255442) [DOI] [PubMed] [Google Scholar]
- 186.Mancini A. Evaluation of metabolic status in amiodarone-induced thyroid disorders: plasma coenzyme Q10 determination. J. Endocrinol. Investig. 1989;12:511–516. doi: 10.1007/BF03350748. (PM:2592737) [DOI] [PubMed] [Google Scholar]
- 187.Wang Y., Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26:367–378. doi: 10.1016/j.tcb.2015.12.007. (PM:26827090) [DOI] [PubMed] [Google Scholar]
- 188.Lenaz G., Genova M.L. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid. Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. (PM:19739941) [DOI] [PubMed] [Google Scholar]
- 189.Anderson R.F. Electron-transfer pathways in the heme and quinone-binding domain of complex II (succinate dehydrogenase) Biochemistry. 2014;53:1637–1646. doi: 10.1021/bi401630m. (PM:24559074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mourier A. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 2015;208:429–442. doi: 10.1083/jcb.201411100. (PM:25688136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Brito O.M., Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. (PM:19052620) [DOI] [PubMed] [Google Scholar]
- 192.Merkwirth C., Langer T. Mitofusin 2 builds a bridge between ER and mitochondria. Cell. 2008;135:1165–1167. doi: 10.1016/j.cell.2008.12.005. (PM:19109886) [DOI] [PubMed] [Google Scholar]
- 193.Ben-Meir A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. (PM:26111777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boots C.E. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum. Reprod. 2016;31:2090–2097. doi: 10.1093/humrep/dew181. (PM:27432748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kamada H. Effects of selenium supplementation on plasma progesterone concentrations in pregnant heifers. Anim. Sci. J. 2014;85:241–246. doi: 10.1111/asj.12139. (PM:24206213) [DOI] [PubMed] [Google Scholar]
- 196.Cerny K.L. Form of supplemental selenium fed to cycling cows affects systemic concentrations of progesterone but not those of estradiol. Theriogenology. 2016;85:800–806. doi: 10.1016/j.theriogenology.2015.10.022. (PM:26559468) [DOI] [PubMed] [Google Scholar]
- 197.Asemi Z. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: randomized, double-blind, placebo-controlled trial. Nutrition. 2015;31:1235–1242. doi: 10.1016/j.nut.2015.04.014. (PM:26250486) [DOI] [PubMed] [Google Scholar]
- 198.Vadhanavikit S., Ganther H.E. Decreased ubiquinone levels in tissues of rats deficient in selenium. Biochem. Biophys. Res. Commun. 1993;190:921–926. doi: 10.1006/bbrc.1993.1137. (PM:8439341) [DOI] [PubMed] [Google Scholar]
- 199.Alehagen U. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157541. (PM:27367855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang X. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. II. Purification and characterization. J. Biol. Chem. 1993;268:14497–14504. (PM:8314806) [PubMed] [Google Scholar]
- 201.Briggs M.R. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J. Biol. Chem. 1993;268:14490–14496. (PM:8390995) [PubMed] [Google Scholar]
- 202.Brown M.S., Goldstein J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. (PM:9150132) [DOI] [PubMed] [Google Scholar]
- 203.Shimano H. Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism. Vitam. Horm. 2002;65:167–194. doi: 10.1016/s0083-6729(02)65064-2. (PM:12481547) [DOI] [PubMed] [Google Scholar]
- 204.Oberkofler H. Sterol regulatory element binding proteins: relationship of adipose tissue gene expression with obesity in humans. Biochim. Biophys. Acta. 2002;1575:75–81. doi: 10.1016/s0167-4781(02)00279-8. (PM:12020821) [DOI] [PubMed] [Google Scholar]
- 205.Weber L.W. Maintaining cholesterol homeostasis: sterol regulatory element-binding proteins. World J. Gastroenterol. 2004;10:3081–3087. doi: 10.3748/wjg.v10.i21.3081. (PM:15457548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sato R. Transcriptional regulation of the ATP citrate-lyase gene by sterol regulatory element-binding proteins. J. Biol. Chem. 2000;275:12497–12502. doi: 10.1074/jbc.275.17.12497. (PM:10777536) [DOI] [PubMed] [Google Scholar]
- 207.Ringseis R. Sterol regulatory element-binding proteins are regulators of the NIS gene in thyroid cells. Mol. Endocrinol. 2013;27:781–800. doi: 10.1210/me.2012-1269. (PM:23542164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wen G. Sterol regulatory element-binding proteins are regulators of the sodium/iodide symporter in mammary epithelial cells. J. Dairy Sci. 2016;99:1–16. doi: 10.3168/jds.2016-11174. (PM:27614840) [DOI] [PubMed] [Google Scholar]
- 209.Rauer C. Sterol regulatory element-binding proteins are regulators of the rat thyroid peroxidase gene in thyroid cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091265. (PM:24625548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wen G. Sterol regulatory element-binding proteins are transcriptional regulators of the thyroglobulin gene in thyroid cells. Biochim. Biophys. Acta. 2016;1859:994–1003. doi: 10.1016/j.bbagrm.2016.06.004. (PM:27321819) [DOI] [PubMed] [Google Scholar]
- 211.Shimizu-Albergine M. SCAP/SREBP pathway is required for the full steroidogenic response to cyclic AMP. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E5685–E5693. doi: 10.1073/pnas.1611424113. (PM:27601673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shin D.J., Osborne T.F. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2) J. Biol. Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. (PM:12829694) [DOI] [PubMed] [Google Scholar]
- 213.Zhao Z. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J. Nutr. 2016;146:1625–1633. doi: 10.3945/jn.116.229955. (PM:27466604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pesce L., Kopp P. Iodide transport: implications for health and disease. Int. J. Pediatr. Endocrinol. 2014;2014:8. doi: 10.1186/1687-9856-2014-8. (PM:25009573) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.