Abstract
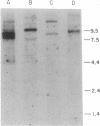
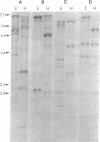
We describe the isolation and characterization of several rat brain cDNAs that are homologous to the alpha 1 subunit of heart and skeletal muscle dihydropyridine-sensitive Ca channels. Northern blot analysis of 32 cDNAs shows that they can be grouped into four distinct classes (A, B, C, and D), each corresponding to a distinct hybridization pattern of brain mRNAs. Southern blot and DNA sequencing suggest that each class of cDNA represents a distinct gene or gene family. In the regions sequenced, the rat brain class C and D gene products share approximately 75% amino acid identity with the rabbit skeletal muscle Ca channel. In addition, the class C polypeptide is almost identical to the rabbit cardiac Ca channel (97% identity). In contrast, the rat brain class A and B cDNAs are more distantly related to dihydropyridine-sensitive Ca channels (47-64% amino acid identity) and to the brain class C and D genes (51-55% amino acid identity). To examine the functional significance of the isolated brain cDNAs, hybrid depletion experiments were performed in Xenopus oocytes. Antisense oligonucleotides against class A and B cDNAs each partially inhibited, and a class C oligonucleotide almost fully inhibited, the expression of Ba current in rat brain mRNA injected oocytes; but none of the oligonucleotides affected the expression of voltage-gated Na or K conductances. The clone characterization and sequencing results demonstrate that a number of distinct, yet related, voltage-gated Ca-channel genes are expressed in the brain. The antisense oligonucleotide experiments specifically show that one or several of the Ca-channel classes are related to the Ca channels observed in rat brain mRNA injected oocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Auld V. J., Goldin A. L., Krafte D. S., Marshall J., Dunn J. M., Catterall W. A., Lester H. A., Davidson N., Dunn R. J. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988 Aug;1(6):449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. P., Leung A. T., Sharp A. H. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends Neurosci. 1988 Oct;11(10):425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Noda M., Flockerzi V., Takahashi H., Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988 Feb 8;228(1):187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]