Abstract
Objective
To identify post-operative computed tomography (CT) findings associated with delayed flap failures following head and neck cancer surgery.
Materials and Methods
We retrospectively reviewed 60 patients who underwent flap reconstruction after head and neck cancer surgery and post-operative (3–14 days) contrast-enhanced CT scans for suspected complications. Patients were divided into two groups: delayed flap failure patients (patients required flap revision) (n = 18) and flap success patients (n = 42). Clinical data (age, sex, T-stage, type of flap, and time interval between reconstruction surgery and CT) and post-operative CT findings of flap status (maximum dimension of the flap, intra- or peri-flap fluid collection and intra- or peri-flap air collection, fat infiltration within the flap, fistula to adjacent aerodigestive tract or skin, and enhanced vascular pedicle) were assessed and compared between the two groups.
Results
CT findings showed that the following flap anomalies were observed more frequently in the delayed flap failure group than in the flap success group: intra- or peri-flap fluid collection > 4 cm (61.1% vs. 23.8%, p < 0.05), intra- or peri-flap air collection > 2 cm (61.1% vs. 2.4%, p < 0.001), and fistula to adjacent aerodigestive tract or skin (44.4% vs. 0%, p < 0.001). The maximum dimension of the flap, fat infiltration within the flap, and enhanced vascular pedicle were not associated with delayed flap failures.
Conclusion
A large amount of fluid or air collection and fistula are the CT findings that were associated with delayed flap failures in patients with suspected post-operative complications after head and neck cancer surgery.
Keywords: Head and neck cancer, Post-operative CT, Flap reconstruction, Surgical flaps, Reconstructive surgical procedures, Flap failure, Post-operative period
INTRODUCTION
With current technical advancements in microsurgery, flap reconstruction has become a common procedure that can provide the benefit of functional and esthetic restoration to the surgical defect following radical resection of head and neck cancer (1). However, various complications such as impaired perfusion, infection, and wound breakdown result in a flap failure and the need for further surgery.
Several pre-operative, intra-operative, and post-operative clinical factors that might increase the risk of flap failure have been suggested. These include patient age, nutritional state, body mass index, diabetes mellitus, bleeding tendency, smoking, previous radiation therapy, location and stage of primary tumor, type of flap reconstruction, duration of anesthesia, and serum C-reactive protein level (1,2,3,4,5,6,7,8,9,10).
Post-operative computed tomography (CT) scans are generally used for the detection of residual tumor, complications, and status of the flap. To our knowledge, however, no study to date has evaluated post-operative CT findings that might predict flap failures.
The purpose of this study was therefore to assess the potential benefit of a post-operative CT scan for predicting delayed flap failures after head and neck cancer surgery.
MATERIALS AND METHODS
Approval for this study was obtained from the Institutional Review Board, and informed consent was waived because routine diagnostic data was analyzed retrospectively.
Patients
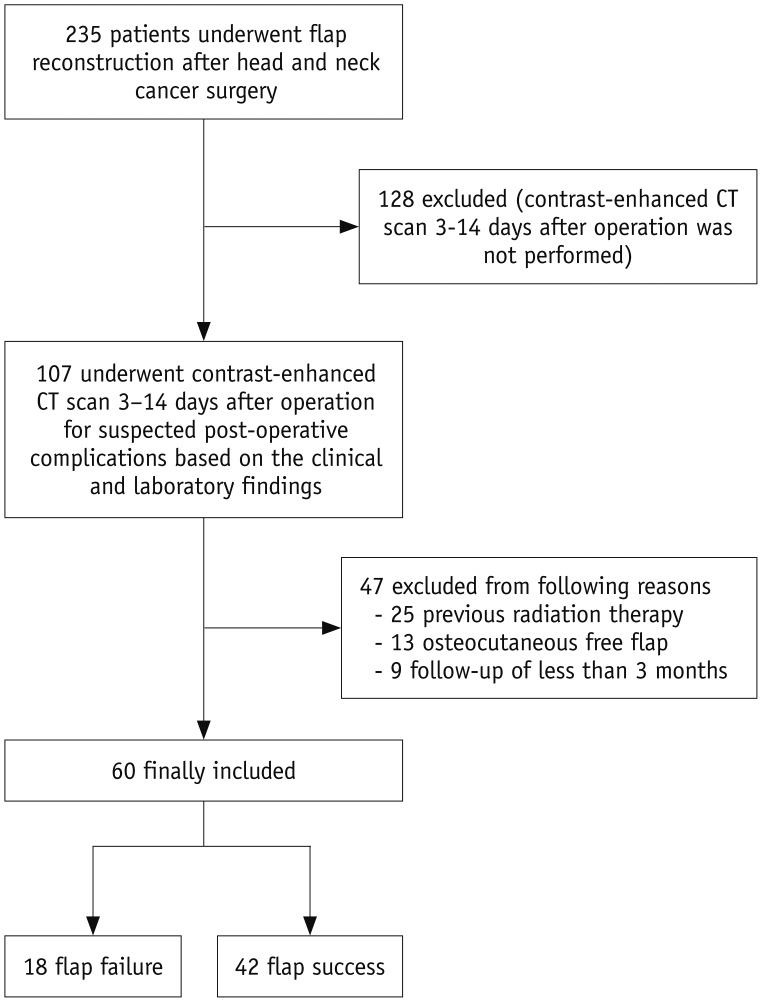
From January 2010 to June 2015, 235 patients underwent flap reconstruction after head and neck cancer surgery in our institution. Among these, 9 patients (3.8%) underwent flap revision within 3 days after surgery due to immediate flap failures. Flap re-operation on these patients was performed by surgeons based only on clinical findings without the requirement of imaging. A total of 107 patients underwent contrast-enhanced CT scans 3–14 days after surgery to identify suspected post-operative complications based on clinical and laboratory findings. Patients with a history of radiation therapy (n = 25), osteocutaneous free flap (n = 13), and less than 3 months follow-up (n = 9), were excluded from the study. The remaining 60 patients comprised the final group for this study (Fig. 1).
Fig. 1. Flowchart showing process of showing inclusion of patients in study.
Patients were divided into two groups: 18 patients with delayed flap failure (14 men, 4 women; mean age, 55.8 ± 11.5 years; age range, 34–75 years) and 42 patients with flap success (33 men, 9 women; mean age, 57.4 ± 10.6 years; age range, 30–72 years) groups. Delayed flap failure was defined as a non-viable flap that required subsequent flap revision surgery at > 3 days after flap reconstruction. Cases in which reoperations were performed to treat other post-operative complications such as infection, seroma, hematoma, abscess, or wound dehiscence were not considered as flap failures. Flap success was defined as a flap still present without flap revision surgery after 3-months follow-up.
Sites of primary head and neck malignancies were the tongue (n = 17), hypopharynx (n = 15), tonsil (n = 7), buccal mucosa (n = 5) and others (n = 16). All patients were surgically treated with radical resection of cancer and neck dissection, followed by flap reconstruction for surgical defects under the same anesthetic. The types of flap were the radial forearm flap in 48 patients (80.0%), anterolateral thigh flap in 9 patients (15.0%), and pectoralis major myocutaneous flap in 3 patients (5.0%). All cancer surgeries and flap reconstructions were performed by the same surgical team which included otolaryngology–head and neck surgeons and plastic surgeons.
Image Acquisition and Data Achievement
The mean interval between flap surgery and a post-operative contrast-enhanced head and neck CT scan was 7.4 ± 2.1 days (range 3 to 14 days). CT scans were conducted with a 16-slice multidetector computed tomography (MDCT) scanner (MX8000-IDT, Philips Medical Systems, Best, the Netherlands) (n = 4) or a 256-MDCT scanner (Brilliance iCT, Philips Medical Systems) (n = 56). The technical parameters were as follows: pitch, 0.61–1.5; gantry rotation time, 50–270 ms; collimation, 4 x 1.5–6.4 x 0.625 mm; 120 kV; 132–200 mAs; matrix, 512 x 512; and slice thickness, 3 mm. Images were obtained from the level of the maxillary sinus to the tracheal bifurcation (mean coverage, 250 mm) 60 seconds after intravenous injection of 100 mL of iomeprol 350 mg/mL (Iomeron 350, Bracco, Milan, Italy). Axial, coronal, and sagittal images were reconstructed.
All CT scans were retrospectively evaluated by two independent board-certified radiologists (with 15 and 10 years post-training experience, respectively, in interpreting head and neck images) who were blinded to the final outcome of the flap tissue. If there were disagreements, the reviewers discussed and reached final decisions by consensus.
The following CT findings were assessed: maximum dimension of the flap (≤ 5 cm or > 5 cm), maximum dimension of intra- or peri-flap fluid collection (≤ 4 cm or > 4 cm), maximum dimension of intra- or peri-flap air collection (≤ 2 cm or > 2 cm), fat infiltration within the flap (present or absent), fistula to adjacent aerodigestive tract or skin (present or absent), and enhanced vascular pedicle (present or absent). A fistula was recorded if there was a definite communication between the flap and adjacent tissue. An enhancement of a vascular pedicle was recorded if there was a paired linear enhancing structure from the entry point of the flap.
Statistical Analysis
Post-operative CT findings described in the section above were compared between the two groups (delayed flap failure vs. flap success). Statistical analyses were performed with the Student's t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the chi-square test for categorical variables. In all comparisons, p values < 0.05 were considered to indicate statistically significant differences. The interobserver agreement between both readers for each variable was determined using kappa statistics. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) software (version 19.0 for Windows, SPSS Inc., Chicago, IL, USA).
RESULTS
Eighteen patients were assigned to the “delayed flap failure” group, and 42 patients were assigned to the “flap success” group according to the criteria previously described, with a rate of delayed flap failure of 30.0% (18/60). Patients in the delayed flap failure group underwent flap transposition (n = 8) and a second flap surgery (n = 10) at 5.7 ± 4.8 days (range: 0–16 days) after the CT scan. No mortality was observed in patients who experienced flap failure and reoperation.
Demographics and clinical characteristics of patients are shown in Table 1. A smaller proportion of the patients in the delayed flap failure group underwent reconstruction by a radial forearm flap, compared to patients in the flap success group (66.7% vs. 85.7%, p < 0.05). In contrast, no significant differences were observed between the two groups in terms of age and gender of patients, T-stage of primary tumor, and time interval between operation and CT.
Table 1. Demographics and Clinical Findings of Patients with Flap Failure and Flap Success after Head and Neck Cancer Surgery.
| Flap Failure (n = 18) | Flap Success (n = 42) | P | |
|---|---|---|---|
| Mean age (years)* | 55.8 ± 11.5 | 57.4 ± 10.6 | 0.6040 |
| Sex (%) | 0.7844 | ||
| Men | 14 (77.8) | 33 (78.6) | |
| Women | 4 (22.2) | 9 (21.4) | |
| T-stage (%) | 0.1717 | ||
| T1 | 0 (0) | 3 (7.1) | |
| T2 | 4 (22.2) | 17 (40.5) | |
| T3 | 4 (22.2) | 10 (23.8) | |
| T4 | 10 (55.6) | 12 (28.6) | |
| Type of flap (%) | < 0.05 | ||
| Radial forearm flap | 12 (66.7) | 36 (85.7) | |
| Pectoralis major flap | 3 (16.7) | 0 (0) | |
| Anterolateral thigh flap | 3 (16.7) | 6 (14.3) | |
| Time interval between operation and CT (days)* | 7.4 ± 2.8 | 7.5 ± 1.8 | 0.9913 |
*Mean ± standard deviation
The CT findings are summarized in Table 2. The following findings were significantly more frequent in the flap failure group than in the flap success group: intra- or peri-flap fluid collection > 4 cm (61.1% vs. 23.8%, p < 0.05); intra- or peri-flap air collection > 2 cm (61.1% vs. 2.4%, p < 0.001); and fistula to adjacent aerodigestive tract or skin (44.4% vs. 0%, p < 0.001) (Figs. 2, 3). There was no significant difference between the two groups in the maximum dimension of the flap, infiltration in fat within the flap, and enhanced vascular pedicle (Fig. 4).
Table 2. Post-Operative Contrast-Enhanced CT Findings of Flap Failure and Flap Success after Head and Neck Cancer Surgery.
| CT Findings | Flap Failure (n = 18) | Flap Success (n = 42) | P | Kappa-Value |
|---|---|---|---|---|
| Maximum dimension of flap (%) | 0.534 | 0.7991 | ||
| ≤ 5 cm | 8/32 (25) | 24/32 (75) | ||
| > 5 cm | 10/28 (36) | 18/28 (64) | ||
| Maximum dimension of intra- or peri-flap fluid collection (%) | < 0.05 | 0.8322 | ||
| ≤ 4 cm | 7/39 (18) | 32/39 (82) | ||
| > 4 cm | 11/21 (52) | 10/21 (48) | ||
| Maximum dimension of intra- or peri-flap air collection (%) | < 0.001 | 0.8993 | ||
| ≤ 2 cm | 7/48 (14.6) | 41/48 (85.4) | ||
| > 2 cm | 11/12 (91.7) | 1/12 (8.3) | ||
| Infiltration in fat within flap (%) | 0.7651 | 0.6993 | ||
| Present | 11/40 (27.5) | 29/40 (72.5) | ||
| Absent | 7/20 (35.0) | 13/20 (65.0) | ||
| Fistula to adjacent aerodigestive tract or skin (%) | < 0.001 | 0.8315 | ||
| Present | 8/8 (100) | 0/8 (0) | ||
| Absent | 10/52 (19.2) | 42/52 (80.8) | ||
| Enhanced vascular pedicle (%) | 0.9510 | 0.8322 | ||
| Present | 12/42 (28.6) | 30/42 (71.4) | ||
| Absent | 6/18 (33.3) | 12/18 (66.7) |
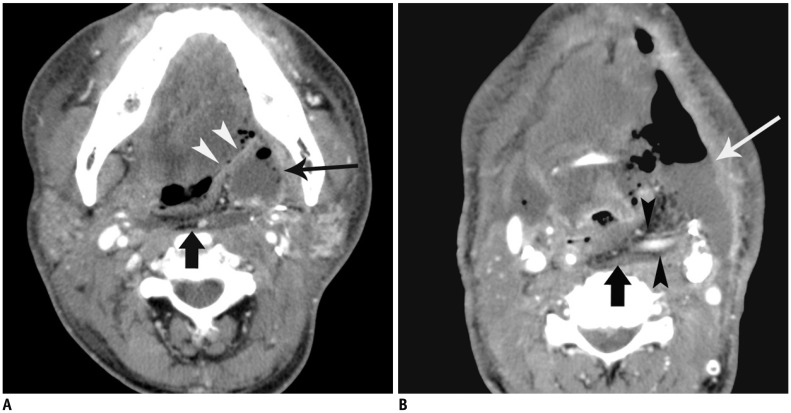
Fig. 2. 59-year-old man with flap failure.
Patient underwent reconstruction by radial forearm flap after resection of oropharyngeal cancer, and second flap surgery after 15 post-operative days due to flap failure.
A. Contrast-enhanced CT scan 9 days after operation shows fat-containing flap (arrow) in retropharyngeal space and peri-flap fluid collection (long arrow) in left parapharyngeal space. Fistula from oropharyngeal wall to left submandibular space (arrowheads) is noted. B. Contrast-enhanced CT scan obtained at more caudal level than (A) shows fat-containing flap (arrow) and large peri-flap fluid collection with air collection (long arrow) in left submandibular space. Note enhanced vascular pedicles (arrowheads) of flap.
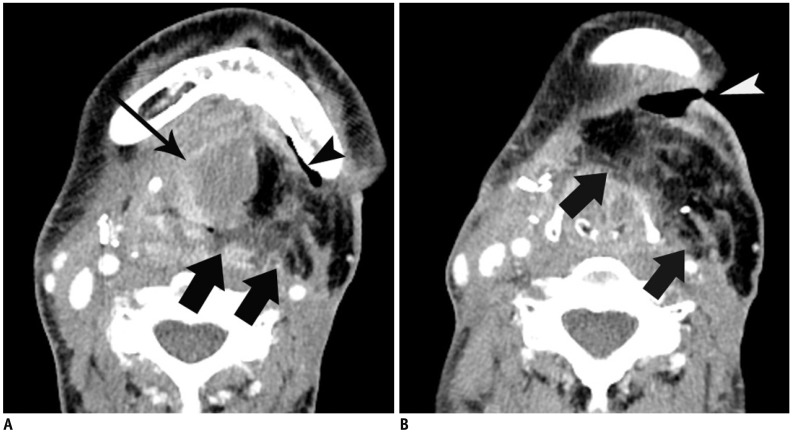
Fig. 3. 35-year-old woman with flap failure.
Patient underwent reconstruction by anterolateral thigh flap after resection of tongue cancer, and flap revision surgery after 20 post-operative days due to flap failure.
A. Contrast-enhanced CT scan 6 days after operation shows fat-containing flap (arrows) with intra-flap fluid collection (long arrow) in left submandibular space and air collection (arrowhead) in left submandibular space. B. Contrast-enhanced CT scan obtained at more caudal level than (A) shows fat-containing flap (arrows) with fat infiltration. Cutaneous fistula (arrowhead) is also noted in left submandibular area.
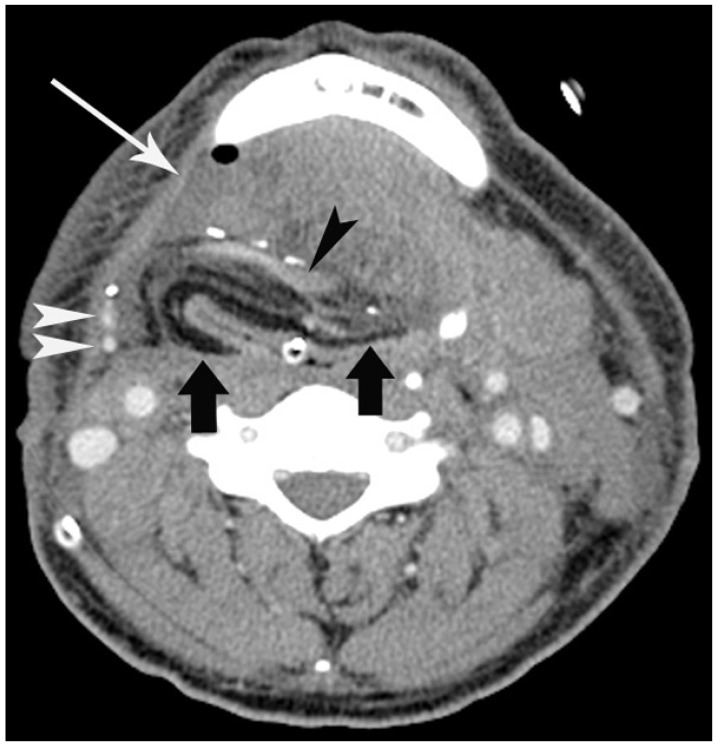
Fig. 4. 55-year-old man with flap success.
Patient underwent reconstruction by radial forearm flap after resection of laryngeal cancer. Contrast-enhanced CT scan 7 days after operation shows fat-containing flap (arrows) and small peri-flap fluid collection (long arrow) with small air bubble in right submandibular space. Note enhanced vascular pedicles (black and white arrowheads) of flap.
There was good interobserver agreement for the maximum dimension of the flap and fat infiltration within the flap, and excellent agreement for intra- or peri-flap fluid collection, intra- or peri-flap air collection, fistula to adjacent aerodigestive tract or skin, and enhanced vascular pedicle for all CT findings.
DISCUSSION
Flap reconstruction after head and neck cancer surgery has been shown to improve the patient's quality of life and increase the survival rate (11). With advanced surgical techniques and post-operative care, the rate of flap failures has been steadily reduced. The reported incidence of flap failures in head and neck reconstructions ranges from 2.3% to 4.8% in the last decade (1,2,12). The rate of delayed flap failures in the present study was 30.0%, which was much higher than that of previous studies. The reason for this high rate might be due to differences in the study populations. In this study, all patients underwent contrast-enhanced CT scans for suspected post-operative complications based on clinical and laboratory findings. In addition, flap reconstruction of head and neck cancer defects is complex and may be affected by multiple component variables including tumor staging. This study population consisted of 36 (60.0%) patients with T3 and T4 tumors.
The authors investigated whether delayed flap failure can be predicted by post-operative CT performed 3–14 days after head and neck surgery. Several CT findings in this study were significantly more frequent in cases of delayed flap failure in patients with suspected post-operative complications. First, fistula to adjacent aerodigestive tract or skin was strongly associated with delayed flap failure. All 8 patients with this complication were treated by surgical intervention because of clinical decline in spite of aggressive antibiotic treatment. It is widely known that wound dehiscences or aerodigestive fistulas after head and neck cancer surgery have delayed healing because of secretion and normal flora of the aerodigestive tract. Fistulas detectable on CT scans were highly specific for flap failures in our study and might be a good predictor of delayed flap failures. However, its sensitivity was low, so that it may not be useful for screening for flaps in asymptomatic patients. Second, this study also showed that a large amount of intra- or peri-flap fluid (> 4 cm) and air (> 2 cm) collections seen in post-operative CT scans were significantly associated with delayed flap failures. These CT findings might be related to the presence of infection, dehiscence, or fistulas.
Nevertheless, our study showed that maximum flap dimensions, fat infiltration within the flap, and an enhanced vascular pedicle were not associated with delayed flap failures. One interesting finding of this study was that the absence of enhanced pedicles in contrast-enhanced CT scans cannot be used to predict delayed flap failures.
Arteries and veins within a flap pedicle may be too small or too short to be seen on a routine contrast-enhanced CT scan. In addition, the head and neck area has an excellent blood supply, thus neovascularization of free flaps from the surrounding tissues may prevent flap failures in cases of compromised pedicle vessels (13).
This study has a number of limitations. First, arterial or venous compromise is the most common cause for immediate flap failures and occurs within the first 24–48 hours post-operatively (14,15). Immediate flap failures were not analyzed in this study, because all flap revisions in this time period were performed without CT assistance. Second, a selection bias may have been present for patients in this study because of the retrospective nature of the data collection. CT scans should not be performed routinely after head and neck cancer surgery, but only in cases of clinically suspected complications based on clinical and laboratory findings. Furthermore, the inclusion of a variety of patients and types of flaps may influence the outcome of the flap tissue in this study. Third, because of the relatively small sample size of patients in this study, it was not possible to perform multivariate logistic regression analysis. Finally, because post-operative change or complication is a relatively dynamic process, a single CT scan may not reflect all post-operative changes of flap reconstruction. Moreover, this study did not take into account the influence of drug therapy, aspiration of fluid, tube removal, and oral feeding that might alter CT findings.
In conclusion, a large amount of fluid or air collection and fistula CT findings were associated with delayed flap failures in patients with suspected post-operative complications after head and neck cancer surgery.
References
- 1.le Nobel GJ, Higgins KM, Enepekides DJ. Predictors of complications of free flap reconstruction in head and neck surgery: analysis of 304 free flap reconstruction procedures. Laryngoscope. 2012;122:1014–1019. doi: 10.1002/lary.22454. [DOI] [PubMed] [Google Scholar]
- 2.Shum J, Markiewicz MR, Park E, Bui T, Lubek J, Bell RB, et al. Low prealbumin level is a risk factor for microvascular free flap failure. J Oral Maxillofac Surg. 2014;72:169–177. doi: 10.1016/j.joms.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Kim BD, Ver Halen JP, Grant DW, Kim JY. Anesthesia duration as an independent risk factor for postoperative complications in free flap surgery: a review of 1,305 surgical cases. J Reconstr Microsurg. 2014;30:217–226. doi: 10.1055/s-0033-1358382. [DOI] [PubMed] [Google Scholar]
- 4.Song H, Kim JH, Park MC, Park DH, Lee K, Lee IJ. Usefulness of serum C-reactive protein level for predicting flap complication after performing free microvascular head and neck reconstruction. J Craniofac Surg. 2014;25:1348–1351. doi: 10.1097/SCS.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 5.Bourget A, Chang JT, Wu DB, Chang CJ, Wei FC. Free flap reconstruction in the head and neck region following radiotherapy: a cohort study identifying negative outcome predictors. Plast Reconstr Surg. 2011;127:1901–1908. doi: 10.1097/PRS.0b013e31820cf216. [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103:403–411. doi: 10.1097/00006534-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bozikov K, Arnez ZM. Factors predicting free flap complications in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2006;59:737–742. doi: 10.1016/j.bjps.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Mücke T, Rau A, Weitz J, Ljubic A, Rohleder N, Wolff KD, et al. Influence of irradiation and oncologic surgery on head and neck microsurgical reconstructions. Oral Oncol. 2012;48:367–371. doi: 10.1016/j.oraloncology.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Genden EM, Rinaldo A, Suárez C, Wei WI, Bradley PJ, Ferlito A. Complications of free flap transfers for head and neck reconstruction following cancer resection. Oral Oncol. 2004;40:979–984. doi: 10.1016/j.oraloncology.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Mücke T, Wolff KD, Wagenpfeil S, Mitchell DA, Hölzle F. Immediate microsurgical reconstruction after tumor ablation predicts survival among patients with head and neck carcinoma. Ann Surg Oncol. 2010;17:287–295. doi: 10.1245/s10434-009-0758-0. [DOI] [PubMed] [Google Scholar]
- 12.Frederick JW, Sweeny L, Carroll WR, Peters GE, Rosenthal EL. Outcomes in head and neck reconstruction by surgical site and donor site. Laryngoscope. 2013;123:1612–1617. doi: 10.1002/lary.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher J, Wood MB. Late necrosis of a latissimus dorsi free flap. Plast Reconstr Surg. 1984;74:274–281. doi: 10.1097/00006534-198408000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Wolff KD, Hölzle F, Wysluch A, Mücke T, Kesting M. Incidence and time of intraoperative vascular complications in head and neck microsurgery. Microsurgery. 2008;28:143–146. doi: 10.1002/micr.20468. [DOI] [PubMed] [Google Scholar]
- 15.Clinton MS, Sepka RS, Bristol D, Pederson WC, Barwick WJ, Serafin D, et al. Establishment of normal ranges of laser Doppler blood flow in autologous tissue transplants. Plast Reconstr Surg. 1991;87:299–309. doi: 10.1097/00006534-199102000-00012. [DOI] [PubMed] [Google Scholar]