Abstract
Objective
Since Graves' disease (GD) is resistant to antithyroid drugs (ATDs), an accurate quantitative thyroid function measurement is required for the prediction of early responses to ATD. Quantitative parameters derived from the novel technology, single-photon emission computed tomography/computed tomography (SPECT/CT), were investigated for the prediction of achievement of euthyroidism after methimazole (MMI) treatment in GD.
Materials and Methods
A total of 36 GD patients (10 males, 26 females; mean age, 45.3 ± 13.8 years) were enrolled for this study, from April 2015 to January 2016. They underwent quantitative thyroid SPECT/CT 20 minutes post-injection of 99mTc-pertechnetate (5 mCi). Association between the time to biochemical euthyroidism after MMI treatment and %uptake, standardized uptake value (SUV), functional thyroid mass (SUVmean × thyroid volume) from the SPECT/CT, and clinical/biochemical variables, were investigated.
Results
GD patients had a significantly greater %uptake (6.9 ± 6.4%) than historical control euthyroid patients (n = 20, 0.8 ± 0.5%, p < 0.001) from the same quantitative SPECT/CT protocol. Euthyroidism was achieved in 14 patients at 156 ± 62 days post-MMI treatment, but 22 patients had still not achieved euthyroidism by the last follow-up time-point (208 ± 80 days). In the univariate Cox regression analysis, the initial MMI dose (p = 0.014), %uptake (p = 0.015), and functional thyroid mass (p = 0.016) were significant predictors of euthyroidism in response to MMI treatment. However, only %uptake remained significant in a multivariate Cox regression analysis (p = 0.034). A %uptake cutoff of 5.0% dichotomized the faster responding versus the slower responding GD patients (p = 0.006).
Conclusion
A novel parameter of thyroid %uptake from quantitative SPECT/CT is a predictive indicator of an early response to MMI in GD patients.
Keywords: Graves' disease, Single-photon emission computed tomography, Computed tomography, Euthyroidism, Methimazole
INTRODUCTION
Graves' disease (GD) is an autoimmune thyroid disorder, 550mediated by the thyroid-stimulating hormone receptor antibody (TSHR-Ab), and is the most common cause of hyperthyroidism (1,2). There are three treatments for GD: antithyroid drugs (ATDs), radioactive iodine (RAI), and surgery (1,3,4). ATDs are used as the first line treatment in many countries, while RAI therapy is preferred in the United States. Surgery is rarely advocated as the first-line treatment. The complete removal of the thyroid tissue by RAI or thyroidectomy is a GD treatment option, but side effects such as permanent hypothyroidism or surgical injury to adjacent organs are major drawbacks of this protocol (1,3,4).
Antithyroid drugs are easy to administer, and they are readily titratable which is advantageous with regard to achieving euthyroidism. However, maintaining the remission state via ATDs alone is very difficult, and an optimal ATD treatment regimen is yet to be established. As a result, despite prolonged treatment for 1–2 years, the relapse rate is approximately 50–70%, and therefore more sophisticated risk stratification is required (5,6,7).
For predicting responses to ATD treatment, age, sex, goiter size, degree of hyperthyroidism, and TSHR-Ab are reportedly useful, as indicated by several well-designed large-scale studies. In one study (6), young (aged < 40 years) male GD patients were poorly responsive to ATD treatment. Another study showed that large goiter size and high thyroid hormone levels prior to the treatment were reportedly associated with poor responses to ATD treatment (8). Elevated TSHR-Ab levels at the end of ATD treatment (9,10) or at the time of initial diagnosis (5) are also predictive of a poor outcome. However, no quantitative parameter has been suggested for the prediction of ATD responses, thus the “optimal” treatment decision for GD patients is currently based on patients' preferences or physicians' empirical determinations (4,7).
In the current study, we investigated 99mTc-pertechnetate thyroid uptake as an objective quantitative measure for the prediction of ATD responses in GD patients. This study was motivated by the recent successful application of single-photon emission computed tomography/computed tomography (SPECT/CT) for the quantitation of %uptake and standardized uptake values (SUV) in a variety of functional thyroid diseases, including GD (11). In the current study, we hypothesized that in GD patients, the parameters of quantitative SPECT/CT may be useful for the prediction of early responses to methimazole (MMI), the most commonly used ATD (2,12).
MATERIALS AND METHODS
Patients
The study was approved by the Institutional Review Board, and the requirement for informed consent was waived. Patients were identified via Seoul National University Bundang Hospital's Clinical Data Warehouse program. Inclusion criteria were patients initially diagnosed with thyrotoxicosis, hyperthyroidism, or GD, who underwent thyroid SPECT/CT from April 2015 to January 2016 at the hospital. Of the initially identified 137 patients, 54 were confirmed as GD cases due to elevated levels of free T4, T3, and TSHR-Ab in conjunction with suppressed thyroid-stimulating hormone (TSH). In addition, initial 99mTc-pertechnetate uptake was diffuse and homogeneously increased, as evident via scintigraphy. Remaining patients were diagnosed as cases of thyroiditis, toxic nodular disease, or other conditions. Of the 54 GD patients, those with short follow-up times (less than 2 months after MMI treatment) (n = 3), initial use of ATDs other than MMI (n = 2), low initial MMI doses (≤ 5 mg of MMI, n = 11), thyroid hormone checked using a non-radioimmunoassay (RIA) (n = 1), or incomplete follow-up data (n = 1) were excluded. After applying these exclusion criteria, 36 patients (10 male, 26 female; mean age, 45.3 ± 13.8 years) were finally enrolled in our study. An expert endocrinologist with 15 years of clinical experience, determined the final diagnosis of GD and prescribed MMI as initial treatment. The characteristics of the patients are summarized in Table 1. Of the 36 patients included, 10 (27.8%) had a history of previous treatment for GD for 13.5 ± 6.1 years (range 5–23 years) before the current study, including 9 who were treated with MMI, and 1 who was pregnant at the time of treatment and was thus treated with propylthiouracil. Thyroid hormone levels indicated the typical hyperthyroidism pattern of elevated thyroid hormones (T3 and/or free T4), positive TSHR-Ab, and suppressed TSH. Thyroid hormone levels were rechecked 1 month after MMI treatment, and every 2–3 months thereafter. The initial daily dose of MMI was 19.4 ± 8.4 mg (range 10–30 mg). The dose was gradually reduced to the maintenance level (≤ 5 mg MMI per day). In accordance with the typical prescription schedule, 1 month after the initiation of MMI treatment, the MMI dose was reduced to 10–20 mg/day, depending on the improvement of the patients' symptoms and thyroid hormone levels. The reduced MMI dose was maintained until free T4 levels were normalized. Subsequently, the MMI dose was further reduced to maintenance dose levels in conjunction with normalized levels of free T4 and T3 (2,5,12,13). In typical cases of a good responder, biochemical euthyroidism (normalized levels of free T4, T3, and TSH) was achieved under the maintenance dose of MMI at later time-points.
Table 1. Patient Characteristics.
| GD (n = 36) | |
|---|---|
| Age (years) | 45.3 ± 13.8 |
| Sex (M:F) | 10:26 |
| Previous treatment history due to GD (yes:no) | 10:26 |
| T3 (ng/dL) | 262.8 ± 118.8 |
| Free T4 (ng/dL) | 3.22 ± 2.72 |
| T3/free T4 ratio | 91.4 ± 24.4 |
| TSH (µIU/mL) | 0.05 ± 0.01 |
| TSHR-Ab (IU/L) | 17.7 ± 24.8 |
| Initial MMI dose (mg/day) | 19.4 ± 8.4 |
T3 (79–200 ng/dL), free T4 (0.89–1.79 ng/dL), TSH (0.3–4.0 µIU/mL), and TSHR-Ab (0–1.0 IU/L). GD = Graves' disease, MMI = methimazole, TSH = thyroid-stimulating hormone, TSHR-Ab = thyroid-stimulating hormone receptor antibody
The study was approved by an Institutional Review Board and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Outcome
For the assessment of early response to MMI, the achievement of biochemical euthyroidism (within a normal range of free T4, T3, and TSH) was set as the outcome (2).
SPECT/CT Acquisition
All patients underwent quantitative thyroid SPECT/CT 3.7 ± 9.4 days before the initiation of MMI administration. A quantitative SPECT/CT scanner (NMCT/670, GE Healthcare, Pittsburgh, PA, USA) was used throughout the study. The thyroid SPECT/CT protocol utilized is described in a previous study (11). In brief, a typical quantitative SPECT/CT study was performed. First, the SPECT/CT scanner was calibrated to a dose calibrator (CRC-15R, Capintec, Inc., Ramsey, NJ, USA) and the system sensitivity (the conversion factor for radioactivity from counts/sec) was determined as 10176 counts/sec per mCi, which was used for the calculation of the quantitative parameters. Second, the SPECT images were reconstructed using CT-based attenuation correction, dual-energy window-based scatter correction, and resolution recovery (Volumetrix MI™, GE Healthcare). Lastly, the quantitative parameters were obtained using a dedicated software (Q.metrix™, GE Healthcare).
The required measurements for quantitative parameters were: the activity of 99mTc-pertechnetate in the syringe before injection (typically 5 mCi) and the measurement time, the remnant 99mTc-pertechnetate activity in the syringe after injection and the measurement time, the activity injection time, the image acquisition start time, and the system sensitivity (10176 counts/sec per mCi).
The SPECT images were acquired 20 minutes after 99mTc-pertechnetate injection, at the time when the 99mTc-pertechnetate retention was stable, and the %uptake correlated with the thyroid function (14). The SPECT acquisition parameters were: head-first supine position, 1-minute-long continuous scanning mode without body contour option, counter-clockwise rotation, peak energy at 140 KeV with a 20% window (126–154 KeV), and a scatter energy of 120 KeV with a 10% window (115–125 KeV). The acquired data were reconstructed using an ordered-subset expectation maximization algorithm, with 2 iterations and 10 subsets. A post-reconstruction filter (Butterworth with a frequency of 0.48 and an order of 10) was applied. With a zoom factor of 1.5, the slice thickness and image matrix of the SPECT images were 2.95 mm and 128 × 128, respectively.
Computed tomography images were acquired using a spiral CT mode with the following acquisition parameters: tube voltage 120 kVp, tube current 180 mA, beam collimation 20 mm (16 × 1.25), table speed 37 mm/second, tube rotation time 0.5 second, and pitch 0.938:1. The CT images were reconstructed into 2.5 mm thick transaxial slices, incorporating an image matrix of 512 × 512, via an adaptive statistical iterative reconstruction algorithm (ASiR™, GE Healthcare).
Quantitative Parameters of Thyroid SPECT/CT
Using dedicated software (Q.metrix™, GE Healthcare), 3-dimensional volumes-of-interest (VOIs) were generated via multiple 2-dimensional regions-of-interest derived from transaxial CT images (Fig. 1). The quantitative parameters were obtained directly from the VOIs via the Q.Metrix™ software, using the following equations:
Fig. 1. Acquisition of thyroidal 3-dimensional VOI from multiple 2-dimensional ROIs.
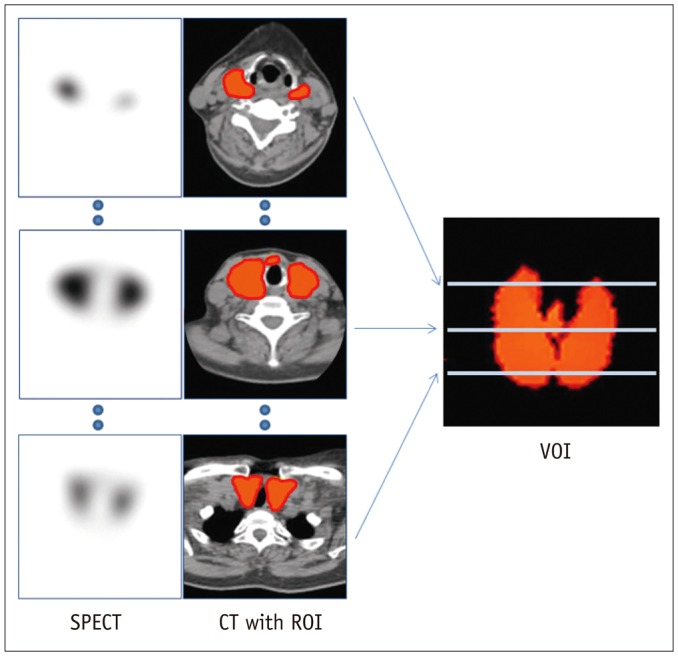
On transaxial CT images, ROIs were manually drawn along contour of thyroid. CT = computed tomography, ROI = region-of-interest, SPECT = single-photon emission computed tomography, VOI = volume-of-interest
The voxel volume in the current study protocol was 3.2 × 10-3 mL.
In addition to the SPECT quantitative parameters (%uptake, SUVmax, and SUVmean), we also ascertained the thyroid volume via the CT images. Functional thyroid mass (g) was then defined as SUVmean (g/mL) × CT-derived thyroid volume (mL), which is equivalent to metabolic tumor volume (SUVmean × tumor volume) of 18F-fluorodeoxyglucose PET in oncology (15,16).
Laboratory Tests for Thyroid Hormones and Autoantibody
In all patients, thyroid hormones and autoantibodies were measured using the following RIA kits: free T4, IMMUNOTECH (Prague, Czech Republic); T3, BRAHMS (Hennigsdorf, Germany); TSH, Immunoradiometric Assay kit, DiaSorin (Saluggia, Italy); and TSHR-Ab, BRAHMS.
Statistical Analysis
The MedCalc (version 12.4.0.0, MedCalc Software Bvda, Ostenel, Belgium) statistical software package was used for all statistical analyses. For group comparisons, the Mann-Whitney U test (a non-parametric test) or the chi-square test were used. For the determination of predictors of early responses to MMI, univariate and multivariate Cox regression analyses were performed. Kaplan-Meier curves were obtained, and the probability difference was tested using the log-rank test. p values of < 0.05 were considered statistically significant.
RESULTS
SPECT/CT Quantitative Parameters
In all 36 patients, the quantitative parameters derived from SPECT/CT were in the upper normal range. The %uptake, SUVmax, and SUVmean were 6.9 ± 6.4%, 221.1 ± 136.7, and 87.4 ± 56.0, respectively. In a previous report using the same quantitative SPECT/CT protocol, the mean ± standard deviation of the %uptake in 20 euthyroid patients was 0.8 ± 0.5% (11), a narrower range with lower levels than those of previous studies (0.5–7.0%) using thyroid uptake systems or planar scintigraphy (14,17,18). The lower %uptake determined via SPECT/CT was attributed to the effect of exclusion of 99mTc-pertechnetate activity from the salivary glands or the oral cavity saliva, which was not possible using the 2-dimensional thyroid uptake measurements (11). The reference ranges for SUVmax and SUVmean in the historical control euthyroid patients were 45.56 ± 30.74 and 33.51 ± 23.54, respectively (11). The differences between the quantitative parameters in the GD patients in the current study and the same parameters in euthyroid patients in the previous study (11) were statistically significant (p < 0.001, p < 0.001, and p < 0.001 for %uptake, SUVmax, and SUVmean, respectively). The CT-measured thyroid volume was 43.7 ± 24.0 mL, which was comparable to the reported thyroid volume in GD patients measured by ultrasonography (39.7 ± 5.6 mL) (19), and greater than the thyroid volume of healthy volunteers (10.7 ± 4.6 mL) (20). The functional thyroid mass, calculated as thyroid volume multiplied by SUVmean, was 4111.4 ± 4878.9 g.
Euthyroidism Achievement
The mean follow-up period of all 36 patients in the current study was 187 ± 76 days (range 85–335 days) from the initiation of MMI treatment. A biochemical euthyroid state (within the normal ranges of free T4, T3, and TSH) was achieved in 14 patients at 156 ± 62 days (range 85–330 days) post-initial MMI treatment. Biochemical euthyroid state was not achieved in 22 patients till the last follow-up time-point (mean follow-up duration 207 ± 80 days, range 89–335 days). The 14 patients who achieved a euthyroid state had significantly smaller mean thyroid volume (or goiter size) (p = 0.016), and lower mean initial MMI dose (p = 0.039) than the 22 patients still in the hyperthyroid state (Table 2). Regarding the quantitative SPECT/CT parameters, the 14 euthyroidal patients had significantly lower mean %uptake (p = 0.027) and functional thyroid mass (p = 0.021) than the 22 hyperthyroid patients (Table 2). In a univariate Cox regression analysis for achieving a euthyroid state, the initial MMI dose (p = 0.014, exp(β) = 0.9043, 95% confidence interval = 0.8348–0.9795), %uptake (p = 0.015, exp(β) = 0.8027, 95% confidence interval = 0.6734–0.9567), and functional thyroid mass (p = 0.016, exp(β) = 0.9996, 95% confidence interval = 0.9993–0.9999) were significant variables, but the other variables investigated were not significant (Table 3). In stepwise (entering conditions with p < 0.05 and removing conditions with p > 0.01) multivariate Cox regression analysis, only %uptake remained a significant predictor of early MMI response: lower %uptake was associated with faster achievement of euthyroidism (p = 0.034, exp(β) = 0.8199, 95% confidence interval = 0.6834–0.9837). Initial MMI dose remained in the model, but the p value was greater than 0.05 and the 95% confidence interval of exp(β) contained 1.0. Functional thyroid mass was dropped from the model (Table 3).
Table 2. Achievement of Euthyroidism.
| Euthyroid (n = 14) | Hyperthyroid (n = 22) | P | |
|---|---|---|---|
| Age (years) | 43.6 ± 13.7 | 46.3 ± 14.0 | 0.338 |
| Sex (M:F) | 5:9 | 5:17 | 0.641 |
| Family history | 1:13 | 3:19 | 0.952 |
| Previous treatment history due to GD (yes:no) | 5:9 | 5:17 | 0.641 |
| Goiter size (mL)* | 33.8 ± 15.5 | 50.0 ± 26.5 | 0.016 |
| T3 (ng/dL) | 215.1 ± 67.6 | 293.1 ± 134.9 | 0.108 |
| Free T4 (ng/dL) | 2.50 ± 0.96 | 3.68 ± 3.35 | 0.056 |
| T3/free T4 ratio | 87.6 ± 13.6 | 93.9 ± 29.3 | 0.399 |
| TSH (µIU/mL) | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.764 |
| TSHR-Ab (IU/L) | 16.0 ± 31.5 | 18.8 ± 20.2 | 0.060 |
| Initial MMI dose (mg/day)* | 15.7 ± 7.6 | 21.8 ± 8.2 | 0.039 |
| %uptake* | 3.92 ± 2.83 | 8.84 ± 7.27 | 0.027 |
| SUVmean (g/mL) | 71.7 ± 50.6 | 97.4 ± 58.2 | 0.270 |
| SUVmax (g/mL) | 184.4 ± 123.2 | 244.4 ± 142.5 | 0.218 |
| Functional thyroid mass (g)* | 2166.2 ± 1513.5 | 5349.3 ± 5841.8 | 0.021 |
*Significant variables. GD = Graves' disease, MMI = methimazole, SUV = standardized uptake valuse, TSH = thyroid-stimulating hormone, TSHR-Ab = thyroid-stimulating hormone receptor antibody
Table 3. Cox's Regression Analysis in Achieving Euthyroidism.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| P | Exp(β) | 95% CI | P | Exp(β) | 95% CI | |
| Age (years) | 0.797 | 1.0055 | 0.9644–1.0483 | |||
| Sex (M:F) | 0.869 | 1.0990 | 0.3595–3.3604 | |||
| Family history | 0.981 | 0.9760 | 0.1273–7.4829 | |||
| Previous treatment history due to GD | 0.287 | 1.8363 | 0.6035–5.5875 | |||
| Goiter size (mL) | 0.058 | 0.9479 | 0.8971–1.0015 | |||
| T3 (ng/dL) | 0.071 | 0.9932 | 0.9860–1.0005 | |||
| Free T4 (ng/dL) | 0.290 | 0.7570 | 0.4530–1.2650 | |||
| T3/free T4 ratio | 0.121 | 0.9827 | 0.9615–1.0045 | |||
| TSHR-Ab (IU/L) | 0.652 | 0.9940 | 0.9686–1.0201 | |||
| Initial MMI dose (mg/day)* | 0.014 | 0.9043 | 0.8348–0.9795 | 0.077 | 0.9207 | 0.8406–1.0085 |
| SUVmean (g/mL) | 0.103 | 0.9911 | 0.9806–1.0018 | |||
| SUVmax (g/mL) | 0.120 | 0.9965 | 0.9922–1.0009 | |||
| %uptake* | 0.015 | 0.8027 | 0.6734–0.9567 | 0.034 | 0.8199 | 0.6834–0.9837 |
| Functional thyroid mass (g)* | 0.016 | 0.9996 | 0.9993–0.9999 | Dropped | ||
*Significant variables. CI = confidence interval, GD = Graves' disease, MMI = methimazole, SUV = standardized uptake valuse, TSHR-Ab = thyroid-stimulating hormone receptor antibody
Using a %uptake cutoff of 5.0%, 18 patients with lower %uptake (2.6 ± 1.2%) reached a euthyroid state significantly faster than 18 patients with higher %uptake (11.3 ± 6.5%) (p = 0.006 by log-rank test) (Fig. 2). At the follow-up time-point of 6 months from the initial MMI treatment, 55.6% (10/18) of the patients with lower %uptake (less than 5.0%) achieved euthyroidism, whereas only 11.1% (2/18) of the patients with higher %uptake (greater than 5.0%) achieved euthyroidism (Fig. 2). Representative cases are shown in Figure 3.
Fig. 2. Kaplan-Meier curve difference for achieving euthyroidism using %uptake cutoff of 5.0%.
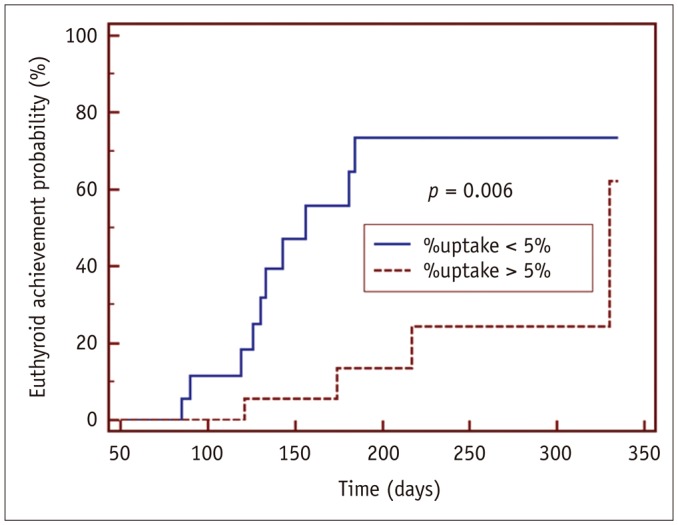
Patients with rapid responses (n = 18) had lower mean %uptake (2.6 ± 1.2%) than those with slow responses (n = 18, 11.3 ± 6.5%). Time to euthyroidism differed significantly between two groups (p = 0.006, log-rank test).
Fig. 3. Representative images.
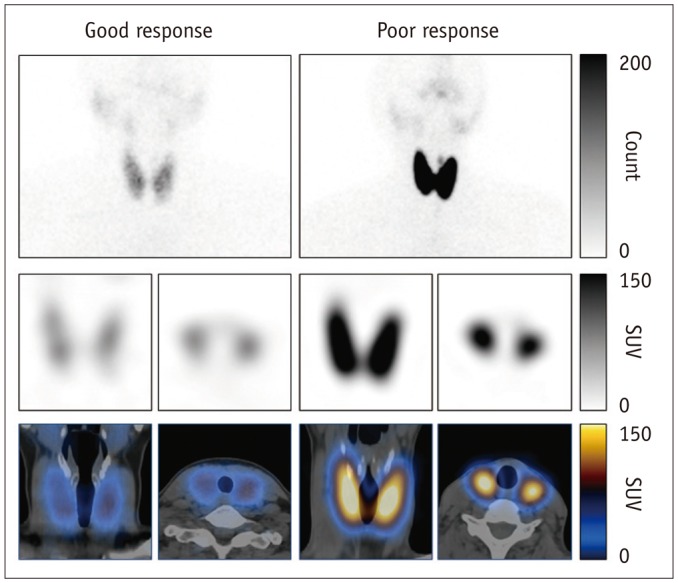
(Good response) 46-year-old female Graves' disease patient showed mildly increased %uptake (2.6%). Notably, euthyroid patients had mean %uptake of 0.8 ± 0.5% as determined via same SPECT/CT protocol (11). Patient's initial MMI dose was 30 mg per day, and she achieved euthyroidism 130 days after initial MMI administration. Values of other variables investigated were SUVmax (98.48), SUVmean (30.03), functional thyroid mass (2330 g), T3 (400 ng/dL), free T4 (5.54 ng/dL), TSH (0.01 µIU/mL), TSHR-Ab (2.13 IU/L), and thyroid volume (77.6 mL). (Poor response) this 38-year-old female Graves' disease patient showed markedly increased %uptake of 12.66%. Initial MMI dose was 30 mg per day, but she had not achieved euthyroidism by last follow-up time-point of 167 days post-MMI administration. Values of other variables investigated were SUVmax (362.43), SUVmean (149.06), functional thyroid mass (6827 g), T3 (461 ng/dL), free T4 (17.74 ng/dL), TSH (0.05 µIU/mL), TSHR-Ab (33.14 IU/L), and thyroid volume (45.8 mL). Upper row: planar scintigraphy, middle row: SPECT (coronal and transaxial images), bottom row: SPECT/CT (coronal and transaxial images). MMI = methimazole, SPECT/CT = single-photon emission computed tomography/computed tomography, SUV = standardized uptake value, TSH = thyroid-stimulating hormone, TSHR-Ab = thyroid-stimulating hormone receptor antibody
DISCUSSION
In the current study, an early response to MMI in GD patients was strongly associated with 99mTc-pertechnetate thyroid uptake, measured using state-of-the-art quantitative SPECT/CT technology. Greater %uptake of 99mTc-pertechnetate in the thyroid was associated with slower achievement of biochemical euthyroidism post-MMI administration. The time to achieving a euthyroid state was effectively predicted using the pre-therapeutic 99mTc-pertechnetate thyroid uptake. Therefore, GD patients who would hypothetically be resistant to ATD, may be quickly shifted to other treatment options such as RAI therapy: this point is clinically important because ATD elicits significant adverse effects, and the required long-term prescription of ATD (up to 2 years) often causes a psycho-economic burden to the GD patients.
The superiority of 99mTc-pertechnetate %uptake compared to other known prognostic parameters of GD is interesting. Age, sex, thyroid volume, thyroid hormone levels, and TSHR-Ab were not significant predictors (Table 3). Only %uptake was a significant predictor of an early response to MMI, indicating the robustness of nuclear functional assessment over other clinical or biochemical variables. In fact, 99mTc-pertechnetate %uptake is reported to be an independent prognostic factor in multivariate analyses in GD patients with RAI therapy (21,22), but to our knowledge the ATD response has not been investigated using 99mTc-pertechnetate %uptake, except the current study.
99mTc-pertechnetate thyroid uptake is an old nuclear medicine technique for the assessment of thyroid function, introduced almost 5 decades ago (18,23). Due to the simplicity of the measurement procedure and its utility for discriminating GD from destructive thyroiditis, 99mTc-pertechnetate thyroid uptake has been widely used in departments of nuclear medicine all over the world (14). However, the technique is not considered the gold standard test for thyroid function due to the lack of a retention mechanism of 99mTc-pertechnetate in thyroid cells (17,24). RAI uptake (RAIU), measured 4–24 hours post-123Iodine or 131Iodine administration, is the current standard test for thyroid function assessment, and RAI therapy for GD is not guided by 99mTc-pertechnetate thyroid uptake but by RAIU (25,26).
Recently developed quantitative SPECT/CT seems to be a game changer when it comes to the measurement of radiotracer uptake (27,28). Accurate measurement of %injected dose (percentage accumulated activity over injected dose), namely the %uptake in the current study, has been achieved for 99mTc-labeled phosphonates (29,30). Quantitative 99mTc-pertechnetate SPECT/CT has been successfully applied to hypothyroid and hyperthyroid patients, and exhibited remarkable accuracy and reliability (11). Therefore, the results of the current study are compatible with the recent utilization of quantitative SPECT/CT as a promising nuclear imaging tool. It is to be noted that the RAIs (123Iodine and 131Iodine) have so far not been investigated using contemporary quantitative SPECT/CT technology. Since RAIU is a well-proven predictor of RAI therapy response in GD, RAIU measurement using quantitative SPECT/CT would be preferable to 99mTc-pertechnetate SPECT/CT for thyroid function assessment (25,31,32,33).
The achievement of euthyroidism post-initial ATD administration may have some limitations as an outcome measure. The remission rate after the cessation of long-term ATD treatment is considered the outcome measure of clinical trials using ATDs (2,7,8,12,34,35). Therefore, this is a preliminary study. However, the achievement of biochemical euthyroidism after MMI treatment is accepted as a primary outcome measure in GD in a large-scale multicenter study (8). The current study results may simply imply that higher the %uptake at disease presentation, more time is required for the patient to reach euthyroidism. However, it has also been reported that rapid interim responses to ATDs were associated with better outcomes in the long run (34,35). In this regard, future long-term prospective studies are warranted.
The current study had other limitations. Instead of different ATD treatment strategies, only the titration regimen strategy was evaluated, which started with a low dose of MMI (7). A high-dose ATD therapy with or without L-thyroxine replacement, or a fixed dose approach, may generate different results. Further, since this study was conducted in a country with rich iodine-intake, the results may not be the same in low iodine-intake countries. For example, the achievement of biochemical euthyroidism may have occurred in more patients in countries with lower iodine-intake. The small number of enrolled patients and the retrospective design are also other limitations.
In conclusion, quantitative parameters (%uptake, SUVmax, SUVmean, and functional thyroid mass) from 99mTc-pertechnetate thyroid SPECT/CT were evaluated for prognosis prediction in GD patients. Despite many limitations, it can be concluded that thyroid %uptake determined via quantitative SPECT/CT, is a predictive indicator of an early response to MMI in GD. It remains to be seen whether long-term remission is significantly improved by pre-selecting MMI-resistant GD patients under base of the pre-therapeutic %uptake.
Footnotes
This study was supported in part by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2015R1D1A1A01059146) and by the Seoul National University Bundang Hospital Research Fund (14-2016-012).
References
- 1.Brent GA. Clinical practice. Graves' disease. N Engl J Med. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 3.Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine--a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986–2993. doi: 10.1210/jcem.81.8.8768863. [DOI] [PubMed] [Google Scholar]
- 4.Burch HB, Cooper DS. Management of Graves disease: a review. JAMA. 2015;314:2544–2554. doi: 10.1001/jama.2015.16535. [DOI] [PubMed] [Google Scholar]
- 5.Vitti P, Rago T, Chiovato L, Pallini S, Santini F, Fiore E, et al. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369–375. doi: 10.1089/thy.1997.7.369. [DOI] [PubMed] [Google Scholar]
- 6.Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1038–1042. doi: 10.1210/jcem.85.3.6430. [DOI] [PubMed] [Google Scholar]
- 7.Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol. 2005;153:489–498. doi: 10.1530/eje.1.01993. [DOI] [PubMed] [Google Scholar]
- 8.Benker G, Vitti P, Kahaly G, Raue F, Tegler L, Hirche H, et al. The European Multicenter Study Group. Response to methimazole in Graves' disease. Clin Endocrinol (Oxf) 1995;43:257–263. doi: 10.1111/j.1365-2265.1995.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 9.Madec AM, Laurent MC, Lorcy Y, Le Guerrier AM, Rostagnat-Stefanutti A, Orgiazzi J, et al. Thyroid stimulating antibodies: an aid to the strategy of treatment of Graves' disease? Clin Endocrinol (Oxf) 1984;21:247–255. doi: 10.1111/j.1365-2265.1984.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D, de Nayer P, Bex M Belgian Collaborative Study Group on Graves' Disease. Effects of l-thyroxine administration, TSH-receptor antibodies and smoking on the risk of recurrence in Graves' hyperthyroidism treated with antithyroid drugs: a double-blind prospective randomized study. Eur J Endocrinol. 2001;144:475–483. doi: 10.1530/eje.0.1440475. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Kim JH, Kang YK, Moon JH, So Y, Lee WW. Quantitative single-photon emission computed tomography/computed tomography for technetium pertechnetate thyroid uptake measurement. Medicine (Baltimore) 2016;95:e4170. doi: 10.1097/MD.0000000000004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- 13.Mazza E, Carlini M, Flecchia D, Blatto A, Zuccarini O, Gamba S, et al. Long-term follow-up of patients with hyperthyroidism due to Graves' disease treated with methimazole. Comparison of usual treatment schedule with drug discontinuation vs continuous treatment with low methimazole doses: a retrospective study. J Endocrinol Invest. 2008;31:866–872. doi: 10.1007/BF03346433. [DOI] [PubMed] [Google Scholar]
- 14.Meller J, Becker W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur J Nucl Med Mol Imaging. 2002;29(Suppl 2):S425–S438. doi: 10.1007/s00259-002-0811-8. [DOI] [PubMed] [Google Scholar]
- 15.Chang KJ, Lim I, Park JY, Jo AR, Kong CB, Song WS, et al. The role of (18)F-FDG PET/CT as a prognostic factor in patients with synovial sarcoma. Nucl Med Mol Imaging. 2015;49:33–41. doi: 10.1007/s13139-014-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S, Lee E, Rhee S, Cho J, Choi S, Lee S, et al. Correlation between semi-quantitative (18)F-FDG PET/CT parameters and Ki-67 expression in small cell lung cancer. Nucl Med Mol Imaging. 2016;50:24–30. doi: 10.1007/s13139-015-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimmins JG, Harden RM, Alexander WD. Loss of pertechnetate from the human thyroid. J Nucl Med. 1969;10:637–640. [PubMed] [Google Scholar]
- 18.Atkins HL, Richards P. Assessment of thyroid function and anatomy with technetium-99m as pertechnetate. J Nucl Med. 1968;9:7–15. [PubMed] [Google Scholar]
- 19.Lucas KJ. Use of thyroid ultrasound volume in calculating radioactive iodine dose in hyperthyroidism. Thyroid. 2000;10:151–155. doi: 10.1089/thy.2000.10.151. [DOI] [PubMed] [Google Scholar]
- 20.Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf) 1987;26:273–280. doi: 10.1111/j.1365-2265.1987.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 21.Zantut-Wittmann DE, Ramos CD, Santos AO, Lima MM, Panzan AD, Facuri FV, et al. High pre-therapy [99mTc]pertechnetate thyroid uptake, thyroid size and thyrostatic drugs: predictive factors of failure in [131I]iodide therapy in Graves' disease. Nucl Med Commun. 2005;26:957–963. doi: 10.1097/01.mnm.0000183795.59097.42. [DOI] [PubMed] [Google Scholar]
- 22.El-Kareem MA, Derwish WA, Moustafa HM. Response rate and factors affecting the outcome of a fixed dose of RAI-131 therapy in Graves' disease: a 10-year Egyptian experience. Nucl Med Commun. 2014;35:900–907. doi: 10.1097/MNM.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 23.Atkins HL, Fleay RF. Data blending with 99mTc in evaluating thyroid anatomy by scintillation scanning. J Nucl Med. 1968;9:66–73. [PubMed] [Google Scholar]
- 24.Lee WW, Lee B, Kim SJ, Jin J, Moon DH, Lee H. Kinetics of iodide uptake and efflux in various human thyroid cancer cells by expressing sodium iodide symporter gene via a recombinant adenovirus. Oncol Rep. 2003;10:845–849. [PubMed] [Google Scholar]
- 25.Alexander EK, Larsen PR. High dose of (131)I therapy for the treatment of hyperthyroidism caused by Graves' disease. J Clin Endocrinol Metab. 2002;87:1073–1077. doi: 10.1210/jcem.87.3.8333. [DOI] [PubMed] [Google Scholar]
- 26.Silberstein EB, Alavi A, Balon HR, Clarke SE, Divgi C, Gelfand MJ, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med. 2012;53:1633–1651. doi: 10.2967/jnumed.112.105148. [DOI] [PubMed] [Google Scholar]
- 27.Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011;38(Suppl 1):S69–S77. doi: 10.1007/s00259-011-1770-8. [DOI] [PubMed] [Google Scholar]
- 28.Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013;54:83–89. doi: 10.2967/jnumed.112.111476. [DOI] [PubMed] [Google Scholar]
- 29.Cachovan M, Vija AH, Hornegger J, Kuwert T. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013;3:45. doi: 10.1186/2191-219X-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh MS, Lee WW, Kim YK, Yun PY, Kim SE. Maximum standardized uptake value of (99m)Tc hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology. 2016;280:890–896. doi: 10.1148/radiol.2016152294. [DOI] [PubMed] [Google Scholar]
- 31.Walter MA, Christ-Crain M, Eckard B, Schindler C, Nitzsche EU, Müller-Brand J, et al. Radioiodine therapy in hyperthyroidism: inverse correlation of pretherapeutic iodine uptake level and post-therapeutic outcome. Eur J Clin Invest. 2004;34:365–370. doi: 10.1111/j.1365-2362.2004.01349.x. [DOI] [PubMed] [Google Scholar]
- 32.Kristoffersen US, Hesse B, Rasmussen AK, Kjaer A. Radioiodine therapy in hyperthyroid disease: poorer outcome in patients with high 24 hours radioiodine uptake. Clin Physiol Funct Imaging. 2006;26:167–170. doi: 10.1111/j.1475-097X.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 33.Damle N, Bal C, Kumar P, Reddy R, Virkar D. The predictive role of 24h RAIU with respect to the outcome of low fixed dose radioiodine therapy in patients with diffuse toxic goiter. Hormones (Athens) 2012;11:451–457. doi: 10.14310/horm.2002.1377. [DOI] [PubMed] [Google Scholar]
- 34.Glaser NS, Styne DM; Organization of Pediatric Endocrinologists of Northern California Collaborative Graves' Disease Study Group. Predicting the likelihood of remission in children with Graves' disease: a prospective, multicenter study. Pediatrics. 2008;121:e481–e488. doi: 10.1542/peds.2007-1535. [DOI] [PubMed] [Google Scholar]
- 35.Kaguelidou F, Alberti C, Castanet M, Guitteny MA, Czernichow P, Léger J, et al. Predictors of autoimmune hyperthyroidism relapse in children after discontinuation of antithyroid drug treatment. J Clin Endocrinol Metab. 2008;93:3817–3826. doi: 10.1210/jc.2008-0842. [DOI] [PubMed] [Google Scholar]


