Abstract
Neuroglobin (NGB), distributed mainly in central and peripheral nervous systems, is a nerve globin with neuroprotective effects against oxidative stress resulting from hypoxia and ischemia. Recent studies have indicated that the expression of NGB is related to neurodegenerative disorders and cancers, but the molecular mechanisms for its transcriptional regulation and protection are not well defined. Here, we report that the expression of NGB in glioma is grade related and is negatively regulated by PPARγ. Specific PPARγ agonist reduces the expression of NGB, while its inhibitor enhances the expression. Moreover, NGB participates in regulating the phosphorylation of AKT in glioma cells, which may contribute to the glioma progression where accumulating oxidative pressure presents. Overexpression of NGB could protect glioma cells against 4-HNE induced cell death, and partially reverse PPARγ’s pro-apoptotic and anti-proliferative abilities. These results display an important role of NGB in glioma progression and a mechanism for its transcriptional regulation, and suggest that the treatment on glioma through PPARγ agonist appears to be triggered by the modulation of NGB.
Highlights
-
•
The expression of NGB is grade related in glioma progression.
-
•
The expression of NGB is negatively regulated by PPARγ.
-
•
NGB regulates the phosphorylation of AKT in glioma cells.
-
•
NGB protects glioma cells from oxidative stress and functions against PPARγ’s pro-apoptotic and anti-proliferative abilities.
Graphical abstract
1. Introduction
Reactive oxygen species (ROS) are continuously generated in cells largely by the mitochondria during energy metabolism process [1]. It is reported that ROS, in particular hydrogen peroxide, have physiological roles as signaling molecules involved in cell growth, differentiation and death [2]. However, when the ROS production overwhelms the clearance conducted by the endogenous antioxidant systems, excessive ROS accumulation may cause progressive oxidative damages to lipids, proteins and DNAs, and may trigger cell death [3], [4]. These damages are collectively defined as oxidative stress, which has been implicated in a wide variety of human diseases, such as neurodegenerative disorders, cardiovascular diseases and cancers [5], [6]. Whereas, multiple researches have indicated that cancer cells maintain much higher ROS levels than normal cells, which are critical for its high proliferation rate, accelerated metabolism and tumor progression [7], [8], [9]. Therefore, it is fundamentally important for cancer cells to balance the ROS levels with further oxidative damage that cells can bear. However, how cancer cells deal with such draconic oxidative pressure is complex and remains incompletely understood.
Gliomas are the most common primary tumors in central nervous system, which make up about 30% of all brain and central nervous system tumors and 80% of all malignant brain tumors [10]. Gliomas can be graded from I to IV according to the World Health Organization (WHO), or divided into low grade (WHO grade I and II) and high grade (WHO grade III and IV), which are determined by the pathologic evaluation of the tumors [11]. Despite the frequency of gliomas, the molecular mechanism and etiology for this thorny tumor are not well defined, making its treatment a challenge. Multiple molecules have been reported with potentiality in glioma treatment, and peroxisome proliferator-activated receptors (PPARs) family member – PPARγ – is one of them. PPARs are ligand-activated transcription factors regulating various genes involved in lipid metabolism and energy homeostasis, and its three subtypes, PPARα, PPARδ and PPARγ, have been identified [12], [13]. PPARγ is widely involved in different cancers, and its agonists, including thiazolidinediones (TDZs) and nonthiazolidinediones, are emerging to block the motility and invasiveness of glioma cells. Grommes et al. have indicated that micromolar doses of pioglitazone (30 μM) counteract C6 rat glioma cell invasiveness in vivo [14]. Eyupoglu et al. have found that troglitazone treatment could effectively block glioma progression and brain invasion with an in vitro glioma invasion model [15]. However, the mechanisms evoked by PPARγ agonists in glioma treatment are not fully understood.
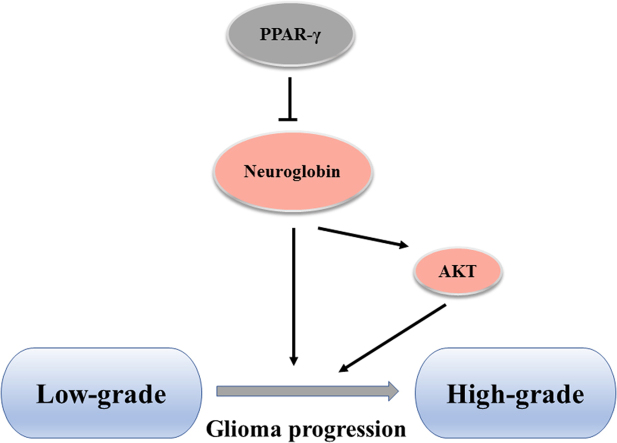
Neuroglobin (NGB) is a recently identified protein, distributed mainly in central and peripheral nervous systems [16]. It's one of the vertebrate globin family members, together with hemoglobin, myoglobin and cytoglobin [17]. The expression of NGB increases in vitro cells subjected to ischemia and hypoxia, and protects cells from these insults [18], [19], [20]. Meanwhile, multiple researches have shown that NGB could protect rats and mice against experimental stroke in vivo, while the expression of NGB has been found increased in the peri-infarct region and essentially absent from the infarct core in human stroke patients [21], [22]. These works show the protective effects of NGB against oxidative stress results from ischemia and hypoxia. Moreover, increasing evidences have shown the close relationship between NGB and cancers. Fiocchetti et al. reported that NGB could promote estrogen receptor α-positive cancer cells survive under oxidative stress [23], but zhang et al. indicated that NGB functions as a tumor suppressor in Hepatocellular Carcinoma [24]. Therefore, more work are needed to investigate the role NGB plays in cancers. Here, we report that the expression of NGB, modulated by PPARγ, is required for glioma to resist oxidative pressure during its progression. We find that the expression of NGB is directly correlated with the grade of the glioma, and is inversely correlated with PPARγ expression. Activation of PPARγ results in suppressed NGB expression, while inhibition of it enhances the latter. Overexpression of NGB protects against oxidative stress resulting from PPARγ agonists treatment and 4-HNE insult, indicating the important role of enhanced NGB expression in glioma progression. Our work reveals a mechanism for the regulation of NGB expression, and demonstrates that NGB is an important part of the antioxidant defense required for glioma progression.
2. Material and methods
2.1. Subjects
The study was approved by the Ethics Committee of Shuangnan Hospital, Chengdu and the Committee of Sichuan Agricultural University. We ensured that all research subjects in the study have gave informed consent. Thirty-nine patients with brain glioma admitted by the Department of Neurosurgery, Shuangnan Hospital of Chengdu from June 2010 to October 2014 were randomly selected. There were 21 male and 18 female patients, aged between 15 and 73 years. All patients received cranial MRI scan, and the glioma was confirmed by pathological examination after surgery. Their gliomas were dissected and divided into two pieces, with one rapidly frozen in liquid nitrogen and preserved at −80 °C pending for western blot, qRT-PCR, and NADPH/NADP assay, and the other one fixed in paraformaldehyde (4%) was used for further immuno-histochemical staining. Before the western blot, qRT-PCR, and NADPH/NADP assay were performed, each of the frozen samples was further divided into three pieces pending for these assays.
2.2. Immuno-histochemical staining
Part of the tissue was fixed in 4% paraformaldehyde before paraffin sections were performed. Then, immune-histochemical staining was performed with standard methods and the primary antibodies of NGB (ab37258, Abcam, MA, USA; 1:100) and PPARγ (ab45036, Abcam, MA, USA; 1:500). Positive signals were visualized using colorimetric detection with diaminobenzidine (DAB), and the hematoxylin indicated the nucleus. Finally, the images were photographed with a microscope (BX43, OLYMPUS).
2.3. Immunofluorescence staining
Parts of the tissue was fixed in 4% PFA and kept in 30% sucrose at 4 °C before frozen sectioning was performed. Standard Immunofluorescence staining was carried out to examine the expression of NGB in different grade of glioma using anti-NGB antibody (ab37258, Abcam, MA, USA; 1:100) as primary antibody, and the Goat anti-Mouse IgG antibody (Alexa Fluor 488, Invitrogen, A-11029, MA, USA, 1:1000) was used as a secondary antibody. DAPI indicates the nucleus and the images were photographed with a fluorescent microscope (BX63, OLYMPUS) with excitation at 488 nm.
2.4. Quantitative realtime PCR
RNA extraction and real-time PCR were performed as previously reported. Briefly, total RNA was extracted from the tissues using Trizol reagent (Invitrogen, Waltham, MA, USA). RNA was subjected to reverse transcription with reverse transcriptase according to the manufacturer's instructions (Fermentas, Waltham, MA, USA). Quantitative real-time PCR was performed using the Bio-Rad CFX96 system, and the relative gene expression was normalized to internal control Actin. Primer sequences for SYBR Green probes of NGB are: Forward: 5′-ggc acc gtc ctg ttt gcc ag-3′ and Reverse: 5′-cga gca tca cct tcc tga tg-3′; β-Actin: Forward: 5′-tcc ttc ctg ggc atg gag t-3′ and Reverse: 5′-aaa gcc atg cca atc tca tc-3′.
2.5. Western blotting
Standard Western blotting procedures were carried out as previously reported [25] with the following antibodies: anti-4-Hydroxy-2-Nonenal (4-HNE) antibody (ab48506, Abcam, MA, USA; 1:1000) to detect lipid peroxidation, anti-PTEN (ab32199, Abcam, MA, USA; 1:500) and anti-AKT (ab8805, Abcam, MA, USA; 1:1000), anti-NGB antibody (ab37258, Abcam, MA, USA; 1:500), anti-PPARγ (ab45036, Abcam, MA, USA; 1:1000), anti-Phospho-Akt (Ser473) antibody (mAb #4060, Cell signaling, Danvers, MA; 1:1000), anti- Phospho-Akt (T308) (mAb #13038, Cell signaling, Danvers, MA; 1:1000), anti-β-actin (BA0410, Boster, Wuhan, China; 1:1000), anti-GAPDH (A00227, Boster, Wuhan, China; 1:1000). Briefly, samples from each patient were lysed and solubilized in PBS buffer containing 1% triton X-100, 1% SDS, Protease inhibitor cocktail III (BioVision) and Phosphatase Inhibitor Cocktail IV (BioVision). Ultrasonication was performed, and the total protein was collected after refrigerated centrifugation at 4 °C. Protein concentrations were determined by Bradford assay using BSA as a standard. 15–20 μg of protein from each sample was separated on a 12.5% Tris-HCl denaturing gel by SDS-PAGE, transferred to nitrocellulose membranes for 1 h at 300 V, 300 mA, blocked with 5% non-fat milk, incubated with primary antibodies overnight at 4 °C and followed by a series of 3 washes in PBS. Membranes were then incubated with a HRP-conjugated secondary antibody for 1 h at room temperature followed by 5 washes in PBS. HRP detection was performed using the Super Signal West Pico Chemiluminescent Substrate (PIERCE) and membranes were exposed to standard FUJI film using an automated film developer.
2.6. Cell culture
U87 cells were routinely cultured in DMEM (Gibco, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS) in an incubator under an atmosphere of 5% CO2 at 37 °C. Cells were plated in 6-well cell plates, 24 h after the PPARγ agonist troglitazone and pioglitazone (Sigma-Aldrich) supplement, or 12 h after the PPARγ inhibitor GW9662 (Sigma-Aldrich) treatment, cells were collected for genes and proteins expressions detection. To investigate the protection of NGB, 50 µM 4-Hydroxy-2-nonenal (4-HNE, Cat#2083, BioVision) or pioglitazone were added 24 h after the NGB transfection in 96-well cell plate, and cell viability was assayed with CCK-8 kit 12 h after the insults.
2.7. NADPH/NADP ratio assay
The NADPH/NADP ratio assay was performed on 39 glioma extracts using the NADP/NADPH assay kit (Cat#K347, BioVision) according to the manufacturer's instructions. Briefly, ~20 mg samples were extracted in 400 µL of the recommended extraction buffer, and 50 µL were processed following instructions for each duplicate. OD450 measurements were made on a plate-reader (Thermo, Waltham, MA, USA) at 25 °C, and the data was converted to nmol/sample using a standard curve and values were used for ratio as previously reported [26].
2.8. Cell viability assay
Cell viability was measured by Cell Counting Kit-8 (CCK-8) system (Dojindo, CK04-11, Minato-ku, Tokyo, Japan) according to the manufacturer's instructions. Briefly, CCK-8 solution (10 µL per 100 µL of medium in each well) was added, and the plates were then incubated at 37 °C for 1 h. The absorbance of each well was read at 450 nm using a microplate reader (Thermo, Waltham, MA, USA).
2.9. ROS level analysis
U87 Cells were plated in 6-well cell plates, and they were transfected with Vehicle (GFP) and GFP-NGB plasmid using Lipofectamine 2000 (Invitrogen). Twenty-four hours after the transfection, pioglitazone (50 µM) was supplemented for another 24 h. Then, fresh culture medium with ROS-sensitive fluorescent probes (Ethidium, Invitrogen) was added, and incubated for 30 min at 37 °C. Finally, the cells were collected and intracellular ROS was detected by fluorescence-activated cell sorting FACS of ~10,000 GFP positive cells.
2.10. Statistical analysis
Data represent the means and SEM. One-way ANOVA and post hoc tests were performed for all statistical significance analysis using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). * P<0.05, ** P<0.01, *** P<0.001.
3. Results
3.1. Patients information
As shown in Table 1, thirty-nine patients with tumor were enrolled in this study, including 21 males and 18 females. Age are between 15 and 73 years, with the mean age of 42.5. Every patient received a cranial MRI scan before surgery. The results revealed that most of the lesions located in the frontal, parietal and temporal lobe, while some others located in basal ganglia, lateral ventricles and thalamus. All these lesions were confirmed as glioma by pathological examination after operation, and were graded from I to IV. There were 2 cases in grade I, 16 in grade II, 7 in grade III, and 14 in grade IV, and all of these cases were divided into Low grade (grade I and II, 18 cases) and High grade (grade III and IV, 21cases).
Table 1.
General information and clinical diagnosis of patients with glioma.
| No. | Gender | Age | Lesion area | WHO grade | Low or High grade |
|---|---|---|---|---|---|
| 1 | Male | 39 | Frontal and Temporal Lobe | 2 | Low |
| 2 | Male | 27 | Temporal Lobe | 2 | Low |
| 3 | Female | 43 | Lateral Ventricles | 2 | Low |
| 4 | Female | 17 | Frontal and Temporal Lobe | 2 | Low |
| 5 | Male | 22 | Parietal Lobe | 2 | Low |
| 6 | Male | 38 | Temporal Lobe (right) | 1 | Low |
| 7 | Male | 49 | Frontal and Temporal Lobe | 2 | Low |
| 8 | Female | 42 | Basal Ganglia, Insula | 4 | High |
| 9 | Male | 54 | Frontal Lobe | 2 | Low |
| 10 | Male | 30 | Frontal and Parietal Lobe | 2 | Low |
| 11 | Male | 73 | Temporal Lobe | 2 | Low |
| 12 | Male | 48 | Frontal and Temporal Lobe | 2 | Low |
| 13 | Male | 53 | Basal Ganglia | 3 | High |
| 14 | Female | 48 | Basal Ganglia | 3 | High |
| 15 | Male | 33 | Parietal and Temporal Lobe | 4 | High |
| 16 | Female | 45 | Frontal, Parietal and Temporal Lobe | 4 | High |
| 17 | Female | 38 | Parietal and Frontal Lobe | 2 | Low |
| 18 | Female | 55 | Frontal and Temporal Lobe | 3 | High |
| 19 | Male | 40 | Frontal, Parietal and Temporal Lobe | 4 | High |
| 20 | Female | 42 | Frontal, Parietal and Temporal Lobe | 4 | High |
| 21 | Male | 42 | Frontal, Parietal and Temporal Lobe | 3 | High |
| 22 | Male | 27 | Frontal and Temporal Lobe | 4 | High |
| 23 | Female | 64 | Frontal Lobe, Lateral Ventricles | 4 | High |
| 24 | Male | 39 | Parietal Lobe | 2 | Low |
| 25 | Female | 56 | Basal Ganglia, Insula | 4 | High |
| 26 | Female | 41 | Frontal, Parietal and Temporal Lobe | 4 | High |
| 27 | Female | 72 | Lateral Ventricles | 2 | Low |
| 28 | Male | 42 | Basal Ganglia, Insula | 4 | High |
| 29 | Female | 53 | Frontal and Temporal Lobe | 2 | Low |
| 30 | Female | 28 | Frontal, Parietal and Temporal Lobe | 1 | Low |
| 31 | Male | 26 | Frontal, Parietal, Temporal Lobe and Basal Ganglia | 4 | High |
| 32 | Male | 15 | Thalamus | 3 | High |
| 33 | Female | 31 | Frontal, Parietal and Temporal Lobe | 4 | High |
| 34 | Female | 66 | Frontal, Parietal and Temporal Lobe | 3 | High |
| 35 | Female | 45 | Frontal and Temporal Lobe | 4 | High |
| 36 | Male | 54 | Frontal and Temporal Lobe | 3 | High |
| 37 | Male | 52 | Lateral Ventricles | 2 | Low |
| 38 | Female | 45 | Frontal and Temporal Lobe | 2 | Low |
| 39 | Male | 25 | Frontal Lobe, Lateral Ventricles | 4 | High |
3.2. Tumor grade related expression of NGB is negatively correlated with that of PPARγ
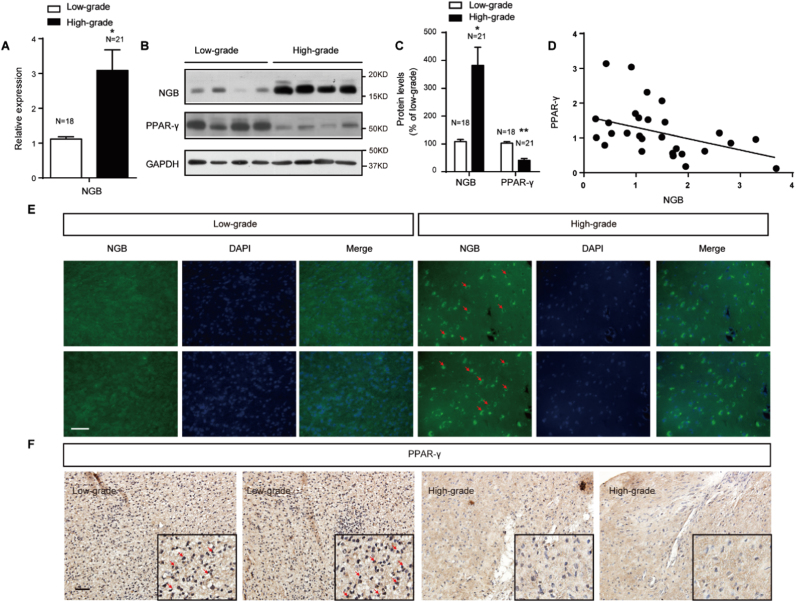
Literatures have reported the expression of NGB in lots of tumors [27], [28], [29]. To investigate the expression of NGB in glioma, 18 low-grade and 21 high grade glioma samples were examined. We have found that the expression of NGB presented in glioma, and the mRNA level of NGB were much higher in high-grade gliomas than that in low-grade ones (Fig. 1A). Western blots and immunofluorescence staining revealed the same findings of NGB protein levels in glioma (Fig. 1B, C and E). Positive correlation between NGB expression and the grade of glioma suggests an important role of NGB in glioma progression. PPARγ, which is widely expressed in multiple cancers, functions as a tumor suppressor [30]. In glioma, western blots and immune-histochemical staining displayed suppressed expression of PPARγ in high-grade gliomas (Fig. 1B, C and F), and the expressions of NGB and PPARγ were inversely related (Fig. 1D, E and F). These findings reveal the completely contrary expressions of NGB and PPARγ related to the grade of glioma.
Fig. 1.
Tumor grade related expression of NGB is negatively correlated with that of PPARγ. (A) qRT-PCR shows a robust expression of NGB mRNA in high-grade gliomas (N=21) compared with low-grade gliomas (N=18). Error bars indicate SEM. *p<0.05. (B and C) Representative images of western blots and quantification show higher NGB and less PPARγ protein levels in high-grade gliomas (N=21) than those in low-grade gliomas (N=18). Error bars indicate SEM. *p<0.05, **p<0.01. (D) Scatter dots distribution shows the change trend of NGB/β-actin in different grade gliomas with PPARγ/β-actin. (E) Immunofluorescence staining with NGB antibody shows an increase in NGB signaling (green, indicated by arrows) in high-grade gliomas; Bar, 50 mm. (F) Immuno-histochemical staining shows the expression pattern of PPARγ in different grade of gliomas. Bar, 100 µm. Dark brown indicates PPARγ. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.3. PPARγ modulates the expression of NGB in vitro
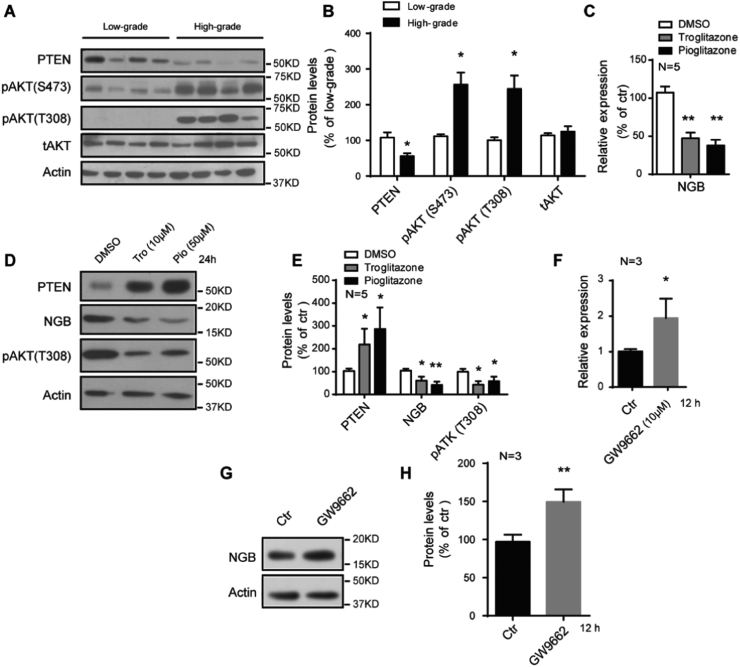
PPARγ functions as a regulator of genes involved in multiple processes. PTEN, the downstream target of PPARγ, is upregulated after PPARγ activation, resulting in dephosphorylation of phosphatidylinositol 3,4,5-triphosphate (PIP3) and AKT [31]. We have noticed decreased PTEN protein levels in high-grade gliomas, where the PPARγ was suppressed, compared with low-grade gliomas (Fig. 2A and B). Consistent with this, the phosphorylation of AKT (S473 and T308) exhibited a robust increase in high-grade gliomas (Fig. 2A and B), contributing to the proliferation and survival of glioma cells [32]. To investigate whether PPARγ modulates the expression of NGB, in vitro experiments were performed. We found that the mRNA and protein levels of NGB were suppressed after the treatment of typically PPARγ agonist, troglitazone and pioglitazone, accompanied by increased PTEN expression and decreased phosphorylation of AKT (Fig. 2C, D and E). Moreover, the expression of NGB exhibited robust increase under the insult of PPARγ inhibitor GW9662 (Fig. 2F, G and H). Previous reports have shown that PPARγ could negatively regulate gene expression after its activation [33], [34], and our results display the same regulation effect of NGB expression through PPARγ activation.
Fig. 2.
PPARγ negatively regulates the expression of NGB. (A and B) Representative images of western blots and quantification show decreased PTEN and increased phosphorylation of AKT in high-grade gliomas (N=21) than those in low-grade gliomas (N=18). Error bars indicate SEM. *p<0.05, **p<0.01. (C) qRT-PCR shows a suppressed expression of NGB mRNA after troglitazone and pioglitazone treatment in U87 cells. Error bars indicate SEM. *p<0.05, N=5. (D and E) Representative images of western blots and quantification show repressed NGB protein levels, accompanied by increased PTEN and decreased phosphorylation of AKT after troglitazone and pioglitazone treatment in U87 cells. Error bars indicate SEM. *p<0.05, **p<0.01, N=5. (F) qRT-PCR shows an enhanced expression of NGB mRNA after GW9662 treatment in U87 cells. Error bars indicate SEM. *p<0.05, N=3. (G and H) Representative images of western blots and quantification show increased NGB protein levels after GW9662 treatment in U87 cells. Error bars indicate SEM. **p<0.01, N=3.
3.4. NGB regulates the phosphorylation of AKT in glioma cells
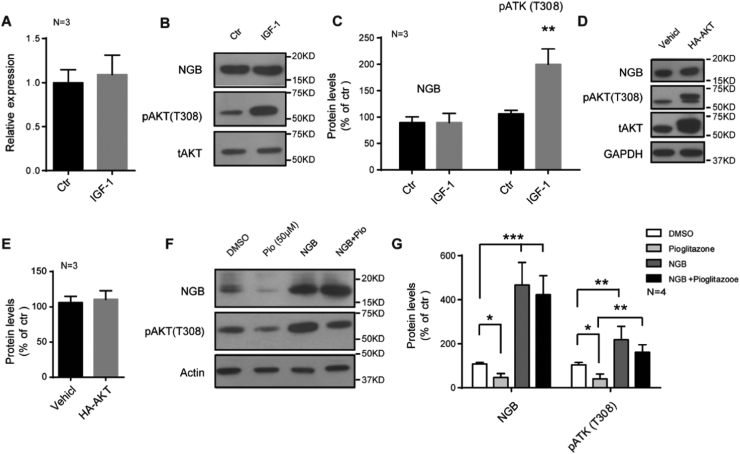
Recent studies have shown that AKT/PKB is able to regulate cell survival by monitoring the activities of transcriptional factors, such as FOXO family, CREB and YAP, which are responsible for pro- and anti-apoptotic genes [35]. To figure out whether the increased NGB expression is due to enhanced phosphorylation of AKT, we stimulated AKT pathway through IGF-1 treatment, which resulted in an enhanced phosphorylation of AKT, but neither the mRNA nor protein levels of NGB increased under this stimulation in U87 cells (Fig. 3A, B and C). Furthermore, we transfected HA-AKT plasmid into U87 cells, but no changes of NGB protein levels were observed (Fig. 3D and E). Researchers have reported that NGB could attenuate tau hyper-phosphorylation and promote neurite outgrowth through the regulation of AKT phosphorylation [36], [37]. We have noticed an increased phosphorylation of AKT in U87 cells, which were transfected with NGB plasmids, suggesting that the activation of AKT pathway in glioma may partly result from robust enhanced expression of NGB (Fig. 3F and G, Fig. 1B). Meanwhile, the expression of NGB could partly reverse the inhibition of AKT activity induced by pioglitazone treatment (Fig. 3F and G). All these indicate that NGB could regulate the phosphorylation of AKT in glioma cells, thus the enhanced expression of NGB may contribute to the activation of AKT pathway in glioma and promote glioma progression.
Fig. 3.
NGB regulates the phosphorylation of AKT in glioma cells. (A) qRT-PCR shows no changes of NGB mRNA levels after IGF-1 (100 ng/ml) treatment for 6 h in U87 cells. Error bars indicate SEM. N=3. (B and C) Representative images of western blots and quantification show no changes of NGB protein levels after IGF-1 (100 ng/ml) treatment for 6 h in U87 cells. Error bars indicate SEM, **p<0.01. N=3. (D and E) Representative images of western blots and quantification show no changes of NGB protein levels after the overexpression of HA-AKT in U87 cells. Error bars indicate SEM. N=3. (F and G) Representative images of western blots and quantification show an increased phosphorylation of AKT in NGB transfected U87 cells, and the expression of NGB could reverse the inhibition of AKT activity induced by pioglitazone treatment. Error bars indicate SEM. *p<0.05, **p<0.01, ***p<0.01. N=4.
3.5. NGB protects glioma cells from oxidative stress and functions against PPARγ’s pro-apoptotic and anti-proliferative abilities
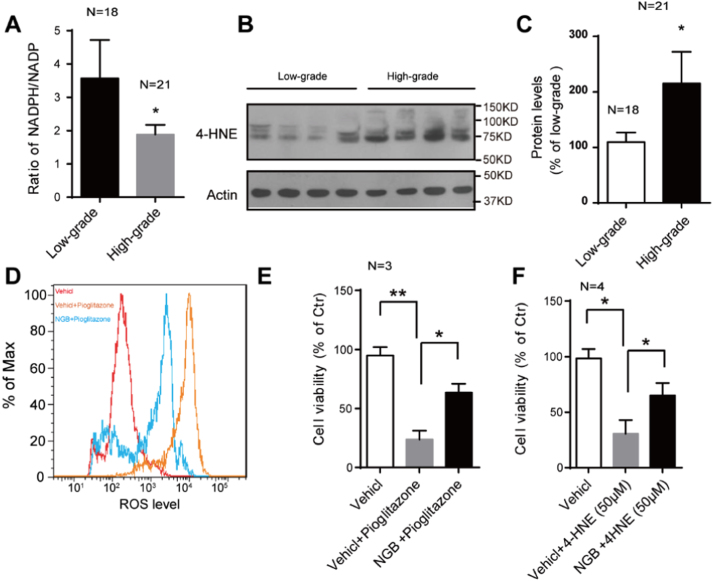
High metabolic rate of cancer cells results in generating more ROS, which have been validated in multiple tumors [9], [38]. We have found a suppression in the ratio of NADPH/NADP in high-grade gliomas, indicating oxidative stress resulting from redox imbalance (Fig. 4A). 4-Hydoxy-2-nonenal (4-HNE) is an end product formed by the reaction of ROS with polyunsaturated fatty acids during oxidative stress, the accumulation of which will cause apoptosis [39], [40]. Larger amounts of 4-HNE accumulation were detected in high-grade gliomas than the low-grade ones (Fig. 4B and C), but they didn’t trigger cell death. This phenotype may be attributed to the protection from the enhanced NGB expression, because we have noticed that the NGB overexpression could protect U87 cells from 4-HNE induced cell death (Fig. 4F). Previous works have shown that PPARγ agonist could induce glioma cells death and repress the tumor progression [14], [15]. Our work indicated that NGB overexpression could resist this process through mitigating the ROS levels in U87 cells treated with pioglitazone (Fig. 3D and E). These findings display the protection effect of NGB involved in oxidative stress and its antagonistic effect against PPARγ’s pro-apoptotic and anti-proliferative abilities.
Fig. 4.
NGB protects glioma cells from oxidative stress. (A) Quantification shows the ratio of NADPH/NADP in low-grade gliomas (N=18) and the high-grade ones (N=21). Decreased ratio is indicative of oxidative stress state. Error bars indicate SEM, *p<0.05. (B and C) Representative images of western blots and quantification show increased 4-HNE accumulation in high-grade gliomas (N=21) than that in low-grade ones (n=18). Error bars indicate SEM. *p<0.05, **p<0.01. (D) Pioglitazone treatment causes an increase in ROS levels in U87 cells, while NGB overexpression mitigates this ROS accumulation. (E) Quantification shows overexpression of NGB functions against PPARγ’s pro-apoptotic and anti-proliferative abilities in pioglitazone treated U87 cells. Error bars indicate SEM. *p<0.05, **p<0.01. N=3. (F) Quantification shows overexpression of NGB could protect U87 cells against 4-HNE induced U87 cell death. Error bars indicate SEM. *p<0.05. N=4.
4. Discussion
ROS, a double-edged sword for cancer cells, are critical for high metabolic rate maintenance, survival, and cellular migration, but toxic to cell integrity. Therefore, antioxidant mechanisms responded for cellular redox balance are much more robust in cancer cells rather than those in normal cells. Glutathione (GSH), distributed among all living organisms, exhibits detoxification effects of xenobiotics and some endogenous oxidative compounds either spontaneously or enzymatically with Glutathione peroxidase (GSH-Px) and Glutathione S-transferase (GSTs) [41]. Elevated GSH levels are observed in various types of tumors, and this makes the neoplastic tissues more resistant to chemotherapy [42], [43]. Superoxide dismutases (SODs), important antioxidant enzymes responsible for the elimination of superoxide radical, are highly expressed in ovarian cancer [44], colorectal cancer [45] and lung cancer [46]. NGB is a hypoxic and ischemic induced protein, which may function as antioxidant to scavenge reactive oxygen (ROS) and nitrogen species (RNS) [21], [47]. We have shown that the expression of NGB in glioma is grade-related, reflecting its important biological role in glioma progression. Accumulating oxidative pressure and lipid oxidation increase the risk of apoptosis, while the enhanced NGB expression may protect against these insults in glioma progression. We have found the imbalance of redox signals, accompanied with increase 4-HNE accumulation, develops more severe in high-grade gliomas, where apoptosis has not been triggered. Meanwhile, in vitro study indicates that NGB overexpression protects glioma cells from 4-HNE induced cell death. Furthermore, we have noticed that the expression of NGB may participate in regulating AKT phosphorylation in glioma cells, suggesting that the protection effects of NGB may function through either itself or AKT related pathways.
PPAR gamma has been implicated in multiple senescence and senescence-related diseases such as inflammation [48], obesity [49], diabetes [50], and various cancers [30]. The applications implicated with PPARγ in cancer treatment are widely studied, but the results are paradoxical and the mechanisms underline are not well defined [51]. In glioma, PPARγ activation induced by its agonists effectively blocks the glioma progression [14], and some clinical studies show that PPARγ agonists exhibit synergistic anti-tumor effect in patients with high-grade glioma [52], [53], [54]. Proliferative advantage over normal tissue represents important hallmarks of cancers cells. One possible mechanism by which PPARγ agonists could inhibit glioma cell proliferation is the induction of cell-cycle arrest in G0/G1 phase and reduction of the proportion of cells entering S phase [55], [56]. Another widely described response of glioma cells to PPARγ agonists is the induction of apoptosis, which may be mediated by Bax-dependent mechanisms, as Bax up-regulation could be caused by PPARγ activation [14], [57]. Redox signals are critical to cancer cells, and PPARγ is broadly involved in these processes. Several antioxidant genes (Catalase, MnSOD, GPX, HO-1 etc) are reported to be upregulated after PPARγ activation in normal cells [58], but there exhibits a different situation in glioma cells which were treated with agonists and failed to exhibit an increase in catalase expression and/or activity [25]. Therefore, more work need to be done to understand the exact role of PPARγ in redox balance in glioma. In this report, we have shown that the expression of PPARγ decreased along with the grade of glioma, suggesting its antagonism effect on glioma. PPARγ activation results in excessive ROS accumulation in glioma cells, which finally triggered cell death. Furthermore, our data indicate that the expression of NGB is negatively regulated by PPARγ, which was different from other antioxidant genes. And, NGB overexpression attenuates the toxicity of PPARγ agonists to glioma cells. In general, the expression of PPARγ is repressed during the glioma progression, resulting in enhanced expression of NGB, which further protects glioma cells against accumulating oxidative pressure. These results provide a new perspective to understand the functions of PPARγ and NGB in glioma progression, and identify NGB as a potential target for glioma treatment.
Competing interests
The authors declare that there are no competing interests to disclose.
Acknowledgements
This work was supported by grants from National Key Technology Support Program (2014BAI03B01 to Z.C), and in part by the National Natural Science Foundation of China (31501200 to C.H).
Contributor Information
Zhengli Chen, Email: chzhli75@163.com.
Chao Huang, Email: huangchao@sicau.edu.cn.
References
- 1.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Andersen J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 6.Mimeault M., Batra S.K. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res. Rev. 2009;8:94–112. doi: 10.1016/j.arr.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent A., Nicco C., Chereau C., Goulvestre C., Alexandre J., Alves A., Levy E., Goldwasser F., Panis Y., Soubrane O., Weill B., Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 8.Wu J.K., Chikaraishi D.M. Differential expression of ros oncogene in primary human astrocytomas and astrocytoma cell lines. Cancer Res. 1990;50:3032–3035. [PubMed] [Google Scholar]
- 9.Schumacker P.T. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Goodenberger M.L., Jenkins R.B. Genetics of adult glioma. Cancer Genet. 2012;205:613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Fuller G.N. 4th ed. Vol. 132. Archives of Pathology & Laboratory Medicine; 2008. The WHO Classification of Tumours of the Central Nervous System; p. 906. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuclear Receptors Nomenclature C. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 14.Grommes C., Landreth G.E., Sastre M., Beck M., Feinstein D.L., Jacobs A.H., Schlegel U., Heneka M.T. Inhibition of in vivo glioma growth and invasion by peroxisome proliferator-activated receptor gamma agonist treatment. Mol. Pharmacol. 2006;70:1524–1533. doi: 10.1124/mol.106.022194. [DOI] [PubMed] [Google Scholar]
- 15.Eyupoglu I.Y., Hahnen E., Heckel A., Siebzehnrubl F.A., Buslei R., Fahlbusch R., Blumcke I. Malignant glioma-induced neuronal cell death in an organotypic glioma invasion model. Technical note. J. Neurosurg. 2005;102:738–744. doi: 10.3171/jns.2005.102.4.0738. [DOI] [PubMed] [Google Scholar]
- 16.Emara M., Salloum N., Allalunis-Turner J. Expression and hypoxic up-regulation of neuroglobin in human glioblastoma cells. Mol. Oncol. 2009;3:45–53. doi: 10.1016/j.molonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burmester T., Weich B., Reinhardt S., Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 18.Liu N., Yu Z., Xiang S., Zhao S., Tjarnlund-Wolf A., Xing C., Zhang J., Wang X. Transcriptional regulation mechanisms of hypoxia-induced neuroglobin gene expression. Biochem. J. 2012;443:153–164. doi: 10.1042/BJ20111856. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Kastner R., Haberkamp M., Schmitz C., Hankeln T., Burmester T. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 2006;1103:173–180. doi: 10.1016/j.brainres.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y., Jin K., Mao X.O., Zhu Y., Greenberg D.A. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y., Jin K., Peel A., Mao X.O., Xie L., Greenberg D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin K., Mao Y., Mao X., Xie L., Greenberg D.A. Neuroglobin expression in ischemic stroke. Stroke; J. Cereb. Circ. 2010;41:557–559. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiocchetti M., Nuzzo M.T., Totta P., Acconcia F., Ascenzi P., Marino M. Neuroglobin, a pro-survival player in estrogen receptor alpha-positive cancer cells. Cell Death Dis. 2014;5:e1449. doi: 10.1038/cddis.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Lan S.J., Liu Q.R., Liu J.M., Chen X.Q. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol. Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 25.Khoo N.K., Hebbar S., Zhao W., Moore S.A., Domann F.E., Robbins M.E. Differential activation of catalase expression and activity by PPAR agonists: implications for astrocyte protection in anti-glioma therapy. Redox Biol. 2013;1:70–79. doi: 10.1016/j.redox.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mugoni V., Postel R., Catanzaro V., De Luca E., Turco E., Digilio G., Silengo L., Murphy M.P., Medana C., Stainier D.Y., Bakkers J., Santoro M.M. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504–518. doi: 10.1016/j.cell.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emara M., Turner A.R., Allalunis-Turner J. Hypoxic regulation of cytoglobin and neuroglobin expression in human normal and tumor tissues. Cancer Cell Int. 2010;10:33. doi: 10.1186/1475-2867-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oleksiewicz U., Daskoulidou N., Liloglou T., Tasopoulou K., Bryan J., Gosney J.R., Field J.K., Xinarianos G. Neuroglobin and myoglobin in non-small cell lung cancer: expression, regulation and prognosis. Lung Cancer. 2011;74:411–418. doi: 10.1016/j.lungcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Fiocchetti M., Cipolletti M., Leone S., Naldini A., Carraro F., Giordano D., Verde C., Ascenzi P., Marino M. Neuroglobin in breast cancer cells: effect of hypoxia and oxidative stress on protein level, localization, and anti-apoptotic function. PloS One. 2016;11:e0154959. doi: 10.1371/journal.pone.0154959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panigrahy D., Kaipainen A., Kieran M.W., Huang S. PPARs: a double-edged sword in cancer therapy? PPAR Res. 2008;2008:350351. doi: 10.1155/2008/350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song M.S., Salmena L., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 32.Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 33.Lovekamp-Swan T., Jetten A.M., Davis B.J. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol. Cell. Endocrinol. 2003;201:133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 34.Remels A.H., Langen R.C., Gosker H.R., Russell A.P., Spaapen F., Voncken J.W., Schrauwen P., Schols A.M. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009;297:E174–E183. doi: 10.1152/ajpendo.90632.2008. [DOI] [PubMed] [Google Scholar]
- 35.Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L., Liu Q.R., Xiong X.X., Liu J.M., Lai X.J., Cheng C., Pan F., Chen Y., Yu S.B., Yu A.C., Chen X.Q. Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol. Neurobiol. 2014;49:149–162. doi: 10.1007/s12035-013-8506-7. [DOI] [PubMed] [Google Scholar]
- 37.Chen L.M., Xiong Y.S., Kong F.L., Qu M., Wang Q., Chen X.Q., Wang J.Z., Zhu L.Q. Neuroglobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. J. Neurochem. 2012;120:157–164. doi: 10.1111/j.1471-4159.2011.07275.x. [DOI] [PubMed] [Google Scholar]
- 38.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 39.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 40.Ji Y., Dai Z., Wu G., Wu Z. 4-Hydroxy-2-nonenal induces apoptosis by activating ERK1/2 signaling and depleting intracellular glutathione in intestinal epithelial cells. Sci. Rep. 2016;6:32929. doi: 10.1038/srep32929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balendiran G.K., Dabur R., Fraser D. The role of glutathione in cancer. Cell Biochem. Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 42.Calvert P., Yao K.S., Hamilton T.C., O'Dwyer P.J. Clinical studies of reversal of drug resistance based on glutathione. Chem. Biol. Interact. 1998;111–112:213–224. doi: 10.1016/s0009-2797(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 43.Estrela J.M., Ortega A., Obrador E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y., Rosen D.G., Zhou Y., Feng L., Yang G., Liu J., Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 45.Satomi A., Murakami S., Hashimoto T., Ishida K., Matsuki M., Sonoda M. Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: correlation with malignant intensity. J. Gastroenterol. 1995;30:177–182. doi: 10.1007/BF02348662. [DOI] [PubMed] [Google Scholar]
- 46.Svensk A.M., Soini Y., Paakko P., Hiravikoski P., Kinnula V.L. Differential expression of superoxide dismutases in lung cancer. Am. J. Clin. Pathol. 2004;122:395–404. doi: 10.1309/A45Q-HB0Q-RRX6-CT9A. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y., Jin K., Mao X.O., Zhu Y., Greenberg D.A. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- 49.Sharma A.M., Staels B. Review: peroxisome proliferator-activated receptor gamma and adipose tissue – understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 50.Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan A., Nair S.A., Pillai M.R. Biology of PPAR gamma in cancer: a critical review on existing lacunae. Curr. Mol. Med. 2007;7:532–540. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]
- 52.Grommes C., Conway D.S., Alshekhlee A., Barnholtz-Sloan J.S. Inverse association of PPARgamma agonists use and high grade glioma development. J. Neuro Oncol. 2010;100:233–239. doi: 10.1007/s11060-010-0185-x. [DOI] [PubMed] [Google Scholar]
- 53.Hau P., Kunz-Schughart L., Bogdahn U., Baumgart U., Hirschmann B., Weimann E., Muhleisen H., Ruemmele P., Steinbrecher A., Reichle A. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas – a phase II study. Oncology. 2007;73:21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- 54.Lichtor T., Spagnolo A., Glick R.P., Feinstein D.L. PPAR-gamma thiazolidinedione agonists and immunotherapy in the treatment of brain tumors. PPAR Res. 2008;2008:547470. doi: 10.1155/2008/547470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zang C., Wachter M., Liu H., Posch M.G., Fenner M.H., Stadelmann C., von Deimling A., Possinger K., Black K.L., Koeffler H.P., Elstner E. Ligands for PPARgamma and RAR cause induction of growth inhibition and apoptosis in human glioblastomas. J. NeuroOncol. 2003;65:107–118. doi: 10.1023/b:neon.0000003728.80052.a8. [DOI] [PubMed] [Google Scholar]
- 56.Liu D.C., Zang C.B., Liu H.Y., Possinger K., Fan S.G., Elstner E. A novel PPAR alpha/gamma dual agonist inhibits cell growth and induces apoptosis in human glioblastoma T98G cells. Acta Pharmacol. Sin. 2004;25:1312–1319. [PubMed] [Google Scholar]
- 57.Zander T., Kraus J.A., Grommes C., Schlegel U., Feinstein D., Klockgether T., Landreth G., Koenigsknecht J., Heneka M.T. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARgamma. J. Neurochem. 2002;81:1052–1060. doi: 10.1046/j.1471-4159.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- 58.Polvani S., Tarocchi M., Galli A. PPARgamma and oxidative stress: con(beta) catenating NRF2 and FOXO. PPAR Res. 2012;2012:641087. doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]