Abstract
Pigeons typically do not show evidence for symmetry in two-alternative matching-to-sample but do demonstrate this emergent relation in successive (go/no-go) matching-to-sample. Because the sample and comparison stimuli are presented in the same spatial location (viz., on one key) during successive matching training and testing, this may be one reason why pigeons pass tests for symmetry in this paradigm. To evaluate this, one group of pigeons received successive matching training with hue-sample stimuli on the center key and form-comparison stimuli on the left key of a three-key chamber. A control group was trained with all stimuli appearing on the same (left) key. Training also involved concurrent hue- and form-identity successive matching with the same spatial location arrangement as each group’s respective hue–form task. Later, nonreinforced form–hue (symmetry) probes structured in the same way as the baseline trials were given. Of the six birds in each group, five trained with different locations and two trained with constant location responded more to the reverse of baseline positive hue–form combinations than to negative ones in testing. Results confirm the prediction from Urcuioli’s (2008) theory that symmetry should emerge even with varying spatial locations, as long as functional stimuli are held constant.
Keywords: stimulus equivalence, symmetry, successive matching, key peck, pigeons
Emergent relations between stimuli are typically examined within the context of a matching-to-sample (MTS) procedure with at least two stimuli each in two or more experimenter-defined classes. If a linear series training protocol is used, the subject learns to match B comparisons to A samples and C comparisons to B samples. These explicitly trained conditional discriminations sometimes yield a variety of novel, untrained conditional discriminations indicative of stimulus equivalence. As defined by Sidman and Tailby (1982), stimulus equivalence requires that stimuli become interchangeable with one another through reflexivity, matching each stimulus to itself (A⟶A, B⟶B, and C⟶C); symmetry, reversing the baseline A⟶B and B⟶C relations by matching former comparisons to former samples (B⟶A and C⟶B); and transitivity, matching a comparison from one baseline relation to a sample from another baseline relation (A⟶C).
Unlike humans, pigeons and other nonhuman animals typically do not show evidence for symmetry when using two-alternative matching-to-sample procedures (see Lionello-DeNolf, 2009 for a review). An early example in which pigeons did not demonstrate symmetrical responding was reported by Hogan and Zentall (1977) who tested for backward associations (symmetry) after symbolic matching-to-sample training. Pigeons learned to match blue and yellow comparison stimuli to green and red sample stimuli, respectively, in Experiment 1, and red and green comparisons to circle and cross samples, respectively, in Experiment 2. Afterwards, half of the birds were given “positive” transfer tests in which the reverse of the reinforced sample–comparison combinations from training were reinforced, and the remaining birds were given “negative” transfer tests in which the reverse of the nonreinforced sample–comparison combinations were now reinforced. For example, if the blue comparison was matched to the green sample in baseline, then responding to the green comparison when the sample was blue was reinforced for the positive transfer group. Conversely, responding to the red comparison when the sample was blue was reinforced for the negative transfer group. The positive transfer group did not show better transfer early in testing or acquire the transfer task faster than the negative transfer group in either experiment.
A common aspect of training and testing with n-alternative matching-to-sample procedures is that sample and comparison stimuli have fixed but different spatial locations in training which are then switched in testing. Thus, the A samples appearing on the center key in training become A comparisons appearing on the side keys in testing and, likewise, the B comparisons appearing on the side keys in training become B samples appearing on the center key in testing. If the location of the stimuli matters, then the A and the B stimuli are not properly defined by just their nominal properties. In other words, A-on-the-center is different from A-on-the-side and B-on-the-side is different from B-on-the-center (Lionello & Urcuioli, 1998). In symmetry testing in which B stimuli appear on the center key and A stimuli appear on the side keys, the pigeon has never seen these stimuli before so chance performance on symmetry tests would be expected and, in fact, occurs. Alternatively, the subject may have learned to respond to the various sample–comparison baseline combinations as individual configurations (e.g., Iversen, Sidman, & Carrigan, 1986; Sidman, 1992). Thus, a configuration with center-A1 sample, left-B1 comparison, and right-B2 comparison may occasion a left-key response. A different configuration with center-A1 sample, left-B2 comparison, and right-B1 comparison may occasion a right-key response. On symmetry tests in which B1 or B2 is the sample stimulus appearing on the center key with A1 and A2 comparison stimuli appearing on the side keys, the resulting configurations represent four never-before-seen combinations (from left to right keys: A1B1A2, A1B2A2, A2B1A1, and A2B2A1), so responding should be at chance. In either case, the change in stimulus spatial location from training to testing would preclude the possibility of observing symmetry (Lionello & Urcuioli, 1998).
Interestingly, Lionello-DeNolf and Urcuioli (2002) did not find evidence for symmetry with pigeons in any of four experiments designed to minimize or eliminate the effects of location as a part of the functional stimulus. For instance, a hue sample could appear on the left or the right key while the form comparison stimuli would appear on the center key and on whichever side key the sample did not appear. Later, pigeons were tested for B⟶A symmetry with center-key form sample stimuli and side-key hue comparison stimuli. In addition, they received novel-location A⟶B tests in order to see if they could maintain their baseline accuracies when the A samples now appeared on the center key. Despite high accuracy on the novel-location baseline trials, no evidence for symmetry was found even when precautions were taken to ensure that all prerequisite successive (sample) and simultaneous (comparison) discriminations were in place prior to symmetry tests. Multiple-exemplar training with other symmetrical discriminations also failed to yield accurate responding on tests for symmetry (although see Velasco, Huziwara, Machado, & Tomanari, 2010).
By contrast, when trained and tested on go/no-go procedures in which the sample and comparison stimuli appear in the same spatial location, pigeons have shown evidence for symmetry. For example, Frank and Wasserman (2005) trained pigeons on symbolic (A⟶B) successive matching with color clipart stimuli (A1-snail, A2-flower, B1-butterfly, and B2-plant). Some pigeons also received intermixed A⟶A identity and B⟶B identity training trials to guarantee that each stimulus was seen both as a sample and as a comparison prior to the symmetry test which consisted of infrequent, nonreinforced B⟶A probe trials. Three of the four pigeons responded more on probe trials that reversed the reinforced sample–comparison combinations from training (B1⟶A1 and B2⟶A2) than on probe trials that reversed the nonreinforced sample–comparison baseline combinations (B1⟶A2 and B2⟶A1). Apparently, when spatial location is held constant and all prerequisite sample and comparison discriminations are made, pigeons will respond in accord with symmetry.
Urcuioli (2008, Experiment 3) replicated this important finding with pigeons trained concurrently on hue–form symbolic, hue identity, and form identity successive matching. Here, too, all stimuli (red, green, triangle, and horizontal lines) appeared on the center key, with “positive” (reinforced) sample–comparison combinations ending in food and “negative” (nonreinforced) sample–comparison combinations ending without food. The B⟶A symmetry test trials were the reverse of the symbolic (A⟶B) baseline trials and ended without food regardless of comparison responding. Several birds responded more to the comparison stimuli on symmetry probes that were the reverse of the positive symbolic baseline combinations rather than to the comparison stimuli on probes that were the reverse of the negative symbolic baseline combinations. These results stood in stark contrast to those of Experiments 1A, 1B, and 2 of Urcuioli (2008) which found no evidence for symmetry in pigeons when two-alternative MTS was used for training and testing, once again implicating the adverse impact of stimulus location.
In conjunction with another observed emergent relation called “antisymmetry” (cf. Urcuioli, 2008, Experiment 4), the results of these experiments led Urcuioli to propose a theory of pigeons’ stimulus-class formation to account for the conditions under which symmetry would and would not emerge. According to the theory, the functional stimuli in successive matching are the nominal or physical attributes of the stimuli plus their ordinal or temporal positions within a matching trial. Thus, a red sample stimulus should be designated R1 (red at Time 1), whereas a red comparison stimulus should be designated as R2 (red at Time 2). Although not explicitly stated in Urcuioli (2008), stimulus location, too, is presumed to be a component of the functional stimuli (see Sweeney & Urcuioli, 2010, Footnote 1, and Urcuioli & Swisher, 2012b, p. 291). Thus, a red sample and red comparison that each appear in the same location – e.g., the center key – should properly be designated as c-R1 and c-R2, respectively, a point to which we will return shortly.
Another consequential property of the go/no-go procedure is that, regardless of the subject’s performance, only half of the trials will end in food (the “go” trials). The “no-go” trials on which comparison responding is nonreinforced continue to be presented with the same frequency as the “go” trials throughout training. This continual juxtaposition of reinforced and nonreinforced sample–comparison combinations is hypothesized to separate the stimuli into different stimulus classes which are assumed to consist of the elements from positive (reinforced) baseline relations. Thus, if red–triangle (R⟶T) and green–horizontal (G⟶H) sample–comparison combinations are reinforced as well as red–red (R⟶R), green–green (G⟶G), triangle–triangle (T⟶T), and horizontal–horizontal (H⟶H) combinations, and the alternative combinations (R⟶H, G⟶T, R⟶G, G⟶R, T⟶H, and H⟶T) are, not, six stimulus classes should form—each containing a reinforced sample- and comparison-stimulus combination: [R1, T2], [G1, H2], [R1, R2], [G1, G2], [T1, T2], and [H1, H2].
Lastly, Urcuioli (2008) assumes that elements common to more than one class cause class merger. Thus, continuing with the example, R1 is common to [R1, T2] and [R1, R2], so those classes will merge; G1 is common to [G1, H2] and [G1, G2] causing those classes to merge; and the common T2 and H2 elements cause the [R1, T2] and [T1, T2] classes and the [G1, H2] and [H1, H2] classes, respectively, to merge. The net result is two four-member stimulus classes, [R1, T2, T1, R2] and [G1, H2, H1, G2], from which symmetry can be derived. In other words, reinforcing the R⟶T (R1⟶T2) and G⟶H (G1⟶H2) symbolic relations should yield higher rates of comparison responding on their symmetrical counterparts T⟶R (T1⟶R2) and H⟶G (H1⟶G2) Stated another way, because T1 and R2 belong to the same class as R1 and T2, the subject should preferentially respond to R2 (rather than G2) after T1. Similarly, because H1 and G2 belong to the same class as G1 and H2, the subject should respond more to G2 after H1 (rather than to R2 after H1).
Although these assumptions readily capture symmetry and other emergent effects seen following successive matching training in which stimulus location is held constant (Sweeney & Urcuioli, 2010; Urcuioli & Swisher, 2012a, 2012b), they leave unanswered the question of whether or not such effects would be observed if sample and comparison locations differed, as in the n-alternative paradigm. On the one hand, the absence of symmetry after training on two-alternative symbolic MTS (Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Urcuioli, 2008, Experiments 1A, 1B, and 2) would seem to suggest that presenting the sample and comparison stimuli in successive matching in different locations would be a “deal breaker”, so to speak—that is, that symmetry would not emerge under these conditions. On the other hand, assuming that spatial location, like temporal/ordinal position, is part of the functional matching stimuli (see, for example, Urcuioli, Jones, & Lionello-DeNolf, 2013), it is possible to create a set of successive matching contingencies involving different sample and comparison locations that, theoretically, should yield symmetry. This experiment was designed to test this prediction.
One group of pigeons learned hue–form (AB), hue–hue (AA), and form–form (BB) successive matching in which the samples always appeared on the center key and the comparisons always appeared on the left side key. For this group, then, location varied as a function of whether the stimulus was a sample or a comparison. A second, control group learned the same three successive matching tasks but with location held constant. Specifically, for this latter group, samples and comparisons both appeared on the left key and the expectation was that they would exhibit symmetry (BA matching), just as Frank and Wasserman (2005) and Urcuioli (2008, Experiment 3) found under their constant-location conditions.
For the main group of interest, Figures 1 and 2 provide a visual, theoretical depiction of why symmetry should be observed after training with center-key (c) samples and left-key (l) comparisons. As previously described, the elements from the positive baseline relations form six 2-member stimulus classes shown in Figure 1. When the triangle-on-the-left comparison is matched to the red-on-the-center sample and the horizontal-on-the-left comparison is matched to the green-on-the-center sample, the two resulting classes can be represented as: [c-R1, l-T2] and [c-G1, l-H2]. Likewise, matching the red-on-the-left comparison to the red-on-the-center sample and the green-on-the-left comparison to the green-on-the-center sample should yield [c-R1, l-R2] and [c-G1, l-G2] classes. Finally, classes [c-T1, l-T2] and [c-H1, l-H2] should arise from the reinforced form identity baseline relations.
Fig. 1.
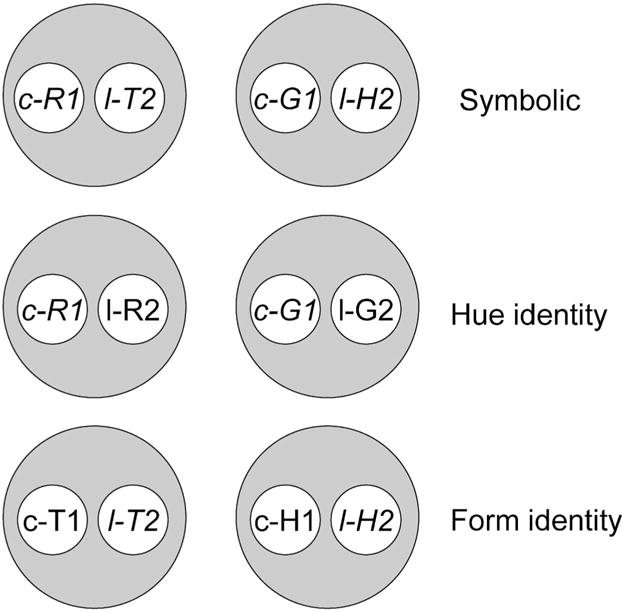
The six stimulus classes hypothesized to result from hue–form symbolic, hue identity, and form identity successive matching training (Group DL). Italics denote common elements (i.e., those appearing in more than one class). R = red, G = green, T = triangle, H = horizontal, c = stimulus appearing on center key, l = stimulus appearing on left key, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus).
Fig. 2.
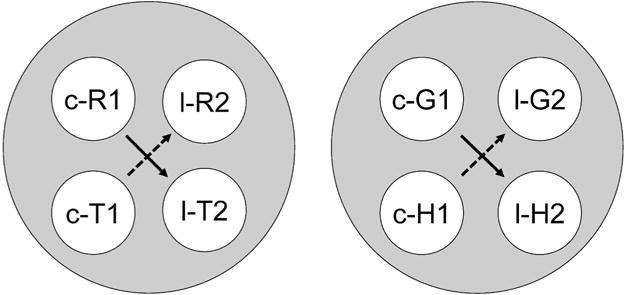
The two 4-member stimulus classes hypothesized to result from class merger via common elements after training on hue–form symbolic, hue identity, and form identity successive matching. Solid and dashed arrows denote explicitly trained and predicted emergent symbolic relations, respectively. R = red, G = green, T = triangle, H = horizontal, c = stimulus appearing on center key, l = stimulus appearing on left key, 1 = first ordinal position (sample stimulus), 2 = second ordinal position (comparison stimulus).
The italicized stimuli in Figure 1 indicate those stimuli common to more than one class. For example, the center–red sample is common to one of the symbolic and one of the hue identity classes. Consequently, the [c-R1, l-T2] class should merge with the [c-R1, l-R2]. Likewise, the left–triangle comparison is common to one of the symbolic and one of the form identity classes, [c-R1, l-T2] and [c-T1, l-T2], respectively. The net result of these mergers is the four-member class [c-R1, l-R2, c-T1, l-T2] shown in Figure 2. The same common elements/class merger assumptions yield the other four-member class: [c-G1, l-G2, c-H1, l-H2]. The prediction of symmetry (c-T1⟶l-R2 and c-H1⟶l-G2) via the symbolic baseline relations (c-R1⟶l-T2 and c-G1⟶l-H2) is shown by the dashed arrows in Figure 2. The subject should match the red-on-the-left comparison to the triangle-on-the-center sample and the green-on-the-left comparison to the horizontal-on-the-center sample, even though the stimuli in symbolic training were red-on-the-center, triangle-on-the-left, green-on-the-center, and horizontal-on-the-left. In short, this group, too, should demonstrate symmetry according to Urcuioli’s (2008) theory despite different sample and comparison stimulus locations.
Method
Subjects
Twelve White Carneau retired breeders obtained from the Double “T” Farm (Glenwood, IA) were used in the present experiment. They were all experimentally naïve, approximately 1–2 years old, and were housed individually in a colony room on a 14 h–10 h light-dark cycle (lights on at 07:00). Upon arrival in the laboratory, free-feeding weights were established by providing free access to Purina ProGrains over a period of 6–30 days. Pigeons were gradually reduced to their 80% ad-lib weights before participation in the experiment, and these weights were maintained by providing food only during the experimental sessions. Pigeons were fed in their home cages 1 day/week and any time they did not maintain their 80% ad-lib weight on the food obtained in the experimental chambers. Water and grit were freely available in the home cages. Pigeons were randomly divided into two groups of six (Groups DL and SL) before participation in the experiment, and three birds per group were run in each of two chambers.
Apparatus
Two pigeon operant chambers consisting of Model PIP-016 three-key panels inside Model SEC-002 enclosures (BRS/LVE, Laurel MD) were used. The center and left keys were active during the experiment. A stimulus projector mounted behind each key could display a solid, inverted white triangle on a black background, three white horizontal lines on a black background, three small white circular dots oriented 45°counterclockwise from vertical on a black background, and red and green homogeneous fields (BRS/LVE Pattern 692). The house light (GE No. 1829 bulb) was located 7.6 cm above the 2.5-cm-diameter center key and its light was directed toward the ceiling by a metal housing partially covering the bulb. A 5.8 cm × 5.8 cm opening directly below the center key provided access to a rear-mounted food hopper which, when raised, was illuminated by a miniature bulb (ESB-28). A continuously running blower fan attached to the outside of each chamber provided ventilation and masking noise. IBM-compatible computers controlled and recorded all experimental events.
Procedure
Preliminary training
Each pigeon learned to approach and eat quickly from a lit, raised hopper. Pigeons were then trained to peck three circular white dots on the center key by the method of successive approximations. Next, a single peck to three horizontal white lines and an inverted triangle on the left key was required for food in one 60-trial session and then to red and green hues on the center key in another 60-trial session. Pigeons in Group DL also had two 60-trial sessions pecking hues and forms on the center key. Each form or hue stimulus appeared an equal number of times in these sessions with successive trials separated by a 10-s intertrial interval (ITI). The house light remained on throughout each session, and reinforcement consisted of 3-s access to grain.
All pigeons then had 10 sessions of increasing fixed-interval (FI) schedules of reinforcement for pecking red and green hues and for pecking triangle and horizontal-lines on the left key. The two hues and two forms appeared equally often within their five consecutive 60-trial sessions, respectively. The first (hue or form) session started with FI 2 s, then the FI value was increased to 3 s, and the last three sessions were with FI 5 s. For the last FI 5 s session, the probability of reinforcement on any given trial was 50%. Because the Group DL pigeons would also see stimuli on the center key during successive matching training (see below), they received five additional 60-trial sessions with red and green center-key hues, and five with horizontal and triangle center-key forms, with corresponding, increasing FI values. The ITI for these sessions was 15 s with the house light off during the first 14 s. The house light came on in the last 1 s of the ITI and remained on throughout the trial until the end of reinforcement. Within a session, the reinforcement duration was constant, but across sessions, reinforcement could vary between 2 and 6 s to maintain each pigeon’s 80% ad-lib weight.
Successive matching acquisition
All pigeons were concurrently trained with symbolic (hue–form), hue identity (hue–hue), and form identity (form–form) successive matching trial types (Table 1) albeit with samples and comparisons appearing either in the same location (Group SL) or in different locations (Group DL). For group DL, the sample stimuli appeared on the center key while the comparison stimuli appeared on the left key. For group SL, the sample and comparison stimuli all appeared on the left key. The reinforcement contingencies for half of the birds in each group were matching triangle (T2) comparisons to red (R1) samples (R1⟶T2) and horizontal (H2) comparisons to green (G1) samples (G1⟶H2) for food, whereas the contingencies for the other half were matching horizontal (H2) comparisons to red (R1) samples (R1⟶H2) and triangle (T2) comparisons to green (G1) samples (G1⟶T2) for food. IfR1⟶T2 and G1⟶H2 were the positive (reinforced) sample–comparison combinations, responding to the remaining, negative combinations (R1⟶H2 and G1⟶T2) ended in extinction. Similarly, if R1⟶H2 and G1⟶T2 were the positive sample–comparison combinations, R1⟶T2 and G1⟶H2 were the negative (nonreinforced) combinations.
Table 1.
Successive Matching Training Contingencies for Groups SL and DL
| Hue-Form (AB) Matching | Hue-Hue (AA) Identity | Form-Form (BB) Identity |
|---|---|---|
| Group SL | ||
| l-R ⟶ l-T - FI 5 s | l-R ⟶ l-R - FI 5 s | l-T ⟶ l-T - FI 5 s |
| l-R ⟶ l-H - EXT | l-R ⟶ l-G - EXT | l-T ⟶ l-H - EXT |
| l-G ⟶ l-T - EXT | l-G ⟶ l-R - EXT | l-H ⟶ l-T - EXT |
| l-G ⟶ l-H - FI 5 s | l-G ⟶ l-G - FI 5 s | l-H ⟶ l-H - FI 5 s |
|
| ||
| Hue-Form (AB) Matching | Hue-Hue (AA) Identity | Form-Form (BB) Identity |
|
| ||
| Group DL | ||
| c-R ⟶ l-T - FI 5 s | c-R ⟶ l-R - FI 5 s | c-T ⟶ l-T - FI 5 s |
| c-R ⟶ l-H - EXT | c-R ⟶ l-G - EXT | c-T ⟶ l-H - EXT |
| c-G ⟶ l-T - EXT | c-G ⟶ l-R - EXT | c-H ⟶ l-T - EXT |
| c-G ⟶ l-H - FI 5 s | c-G ⟶ l-G - FI 5 s | c-H ⟶ l-H - FI 5 s |
Note R = red, G = green, T = triangle, H = horizontal, c = stimulus presented on center key, l = stimulus presented on left key, FI = fixed interval schedule, EXT = nonreinforced. The first stimulus in the trial sequence (the sample) is shown to the left of the arrows, and the second stimulus (the comparison) is shown to the right. Counterbalancing of the hue-form matching contingencies has been omitted.
The reinforcement contingencies for the concurrently trained hue identity and form identity tasks were the same for both groups. Trials with R1⟶R2, G1⟶ G2, T1⟶T2, and H1⟶H2 always ended in reinforcement for responding to the comparison stimuli, but trials with R1⟶G2, G1⟶R2, T1⟶H2, and H1⟶T2 were never followed by food.
Sessions consisted of 96 trials equally divided among the three tasks (hue–form, hue–hue, and form–form). For each task, eight trials of each of the four possible sample–comparison combinations occurred equally often. Trials within a session were randomized with the constraint that no sample–comparison combination could occur more than three consecutive times. When the sample was displayed, the first peck started an FI 5-s interval, and the last peck after 5 s had elapsed turned off the sample and initiated the 1-s delay. After the 1-s delay, a comparison stimulus appeared either on the same key (Group SL) or a different key (Group DL). On reinforced trials, the first comparison–stimulus peck started a 5-s interval which ended in food presentation contingent on the first peck after the interval. On nonreinforced trials, the comparison stimulus went off after 5 s regardless of responding. The house light turned off after food was provided on reinforced trials or when the comparison stimulus went off on nonreinforced trials. A 15-s ITI preceded the next trial, and the house light remained off for the first 14 s of the ITI. In the last second of the ITI, the house light was turned on before the next trial’s sample stimulus was presented.
The criterion for successive matching acquisition was five out of six consecutive sessions with discrimination ratios (DRs) at or above .80 for hue–form, hue–hue, and form–form matching. DRs were calculated by dividing the total number of pecks to the comparison stimuli on the reinforced trials of each task by the total number of comparison pecks on both reinforced and nonreinforced trials of that task. Only pecks to the comparison stimuli within the first 5 s of comparison onset were used to calculate DRs. After meeting criterion, pigeons received at least 10 overtraining sessions that ended when performances again met the criterion.
Successive matching testing
After overtraining, testing for emergent form–hue (BA) symmetrical relations began. Eight test sessions were scheduled in blocks of two sessions separated by a sufficient number of baseline sessions (minimum = five sessions) to meet the previously mentioned performance criterion. Test sessions consisted of 104 trials with 96 baseline trials arranged as previously stated and eight nonreinforced probe trials: two of each form–hue combination (T1⟶R2, H1⟶R2, T1⟶G2, and H1⟶G2). The comparison stimulus went off automatically after 5 s on these probe trials. The probe trials were presented randomly within a test session with the constraints that at least one of all of the 12 combinations of baseline trials were presented first and that individual probe trials were separated by at least six baseline trials. The dependent variable was the number of comparison pecks occurring on the reverse of the reinforced or “positive” hue–form baseline trials and the reverse of the nonreinforced or “negative” hue–form baseline trials.
Analysis of variance (ANOVA) was used to analyze individual subject test data averaged across all eight test sessions (unless otherwise noted). Type I error rate was set at .05 (Rodger, 1975) on a per-decision basis.
Results
Acquisition and Baseline Performance
All of the pigeons met the .80 DR criterion for the three baseline tasks (hue identity, form identity, and hue–form matching). For Group SL, the average numbers of sessions to criterion were 15.3 for the hue identity task, 20.8 for the form identity task, and 15.2 sessions for the hue–form matching task. The numerical difference was not statistically significant, F(2, 15) = 1.42. For Group DL, the corresponding averages were 58.2, 57.2, and 69.7, respectively. The numerical difference was not statistically significant, F(2, 15) = .07. Group DL’s sessions-to-criterion were numerically higher than for Group SL, but this was due to two pigeons (DL3 and DL4) that required about 100 more sessions to meet criterion than the remaining pigeons in the group. Consequently, the overall between-group difference in sessions-to-criterion was not statistically significant for hue identity, form identity, or hue–form matching trial types, Fs(1, 10) = 2.53, 2.23, and 3.40, respectively. For the last five sessions of overtraining, the average DRs across the three tasks for Group SL were identical for hue identity (.91), form identity (.91), and hue–form matching tasks (.91), F(2, 15) = .04. The corresponding DRs for Group DL were .92, .90, and .91, respectively, F(2, 15) = .13.
During the test sessions, most of the baseline DRs were at or above .80. Only 35 out of 288 baseline DRs (3 baseline tasks × 12 pigeons × 8 test sessions) fell below .80 and, of those, only 7 fell below .70.
Test Performances
Figures 3 and 4 show the comparison pecks/sec for Group DL and Group SL, respectively, on the baseline (open circles) and the nonreinforced form–hue probe (filled circles) trials. (Due to experimenter error, pigeon SL1’s baseline hue–form contingencies were switched following tests 5 and 6, so only its first six test sessions are included in Figure 4). The baseline data are the average number of pecks from four randomly selected “positive” (reinforced) and four randomly selected “negative” (nonreinforced) hue–form trials (two R⟶T, R⟶H, G⟶T, and G⟶ H trials) from each test session. As is evident from the figures, all pigeons maintained their baseline discriminations in testing by pecking at much higher rates to the positive comparison stimuli than to the negative comparison stimuli.
Fig. 3.
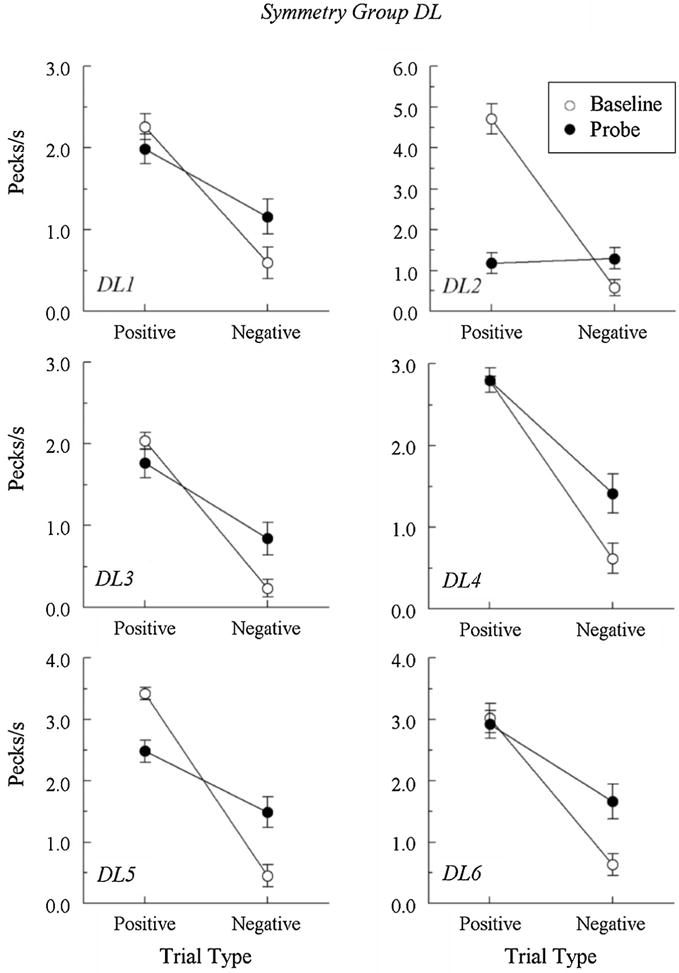
Comparison-response rates in pecks/sec (± 1 SEM) on the hue–form matching baseline trials (open circles) and the nonreinforced symmetry (form–hue) probe trials (filled circles) averaged over the eight test sessions for each Group DL pigeon. Positive = reinforced hue–form baseline trials and nonreinforced test trials where the samples and comparisons of the reinforced baseline trials were reversed. Negative = nonreinforced hue–form baseline trials and test trials where the samples and comparisons of the baseline trials were reversed.
Fig. 4.
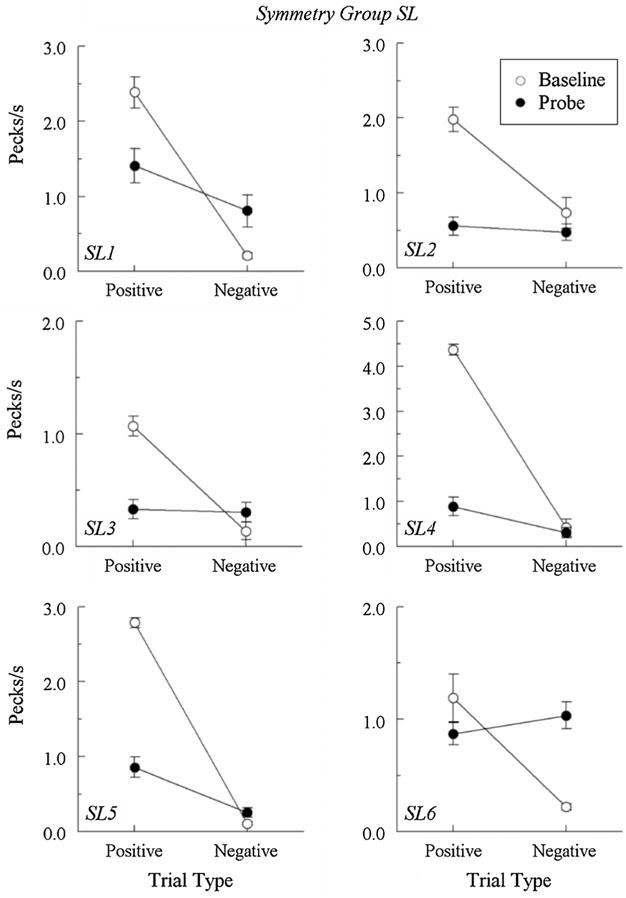
Comparison-response rates in pecks/sec (± 1 SEM) on the hue–form matching baseline trials (open circles) and the nonreinforced symmetry (form–hue) probe trials (filled circles) averaged over the eight test sessions for each Group SL pigeon. Data shown for pigeon SL1 includes only six test sessions (see text for details). Positive = reinforced hue–form baseline trials and nonreinforced test trials where the samples and comparisons of the reinforced baseline trials were reversed. Negative = nonreinforced hue–form baseline trials and test trials where the samples and comparisons of the baseline trials were reversed.
The symmetry probe data are the average number of pecks from the four trials that were the reverse of the reinforced (“positive”) baseline hue–form combinations and the four trials that were the reverse of the nonreinforced (“negative”) baseline hue–form combinations from each test session. Five pigeons in Group DL (DL1, DL3, DL4, DL5, and DL6) all responded more to the reverse of the positive baseline relations than to the reverse of the negative baseline relations; these differences were statistically significant, Fs(1, 62) = 8.64, 12.38, 24.24, 10.43, and 12.06, respectively.
The remaining pigeon (DL2) responded at roughly equal rates on the probe trials, F(1, 62) = .10. To test whether the different key locations of the samples and comparisons contributed to the absence of differential responding by DL2, it was subsequently retrained with all stimuli appearing on the left key (as in Group SL). Baseline response rates during symmetry testing following retraining were highly differential (positive = 5.11 pecks/s and negative = .70 pecks/s), but probe-trial responding was still nondifferential (positive = 1.05 pecks/s and negative = 1.06 pecks/s), F(1, 62) = .001. Thus, the lack of symmetry in DL2 was independent of the location of the sample and comparison stimuli.
In Group SL, three pigeons pecked at higher rates on the reverse of the positive baseline trials than on the reverse of the negative baseline trials. For pigeons SL4 and SL5, the probe comparison–response rates were significantly different, Fs(1, 62) = 5.86 and 16.32, respectively; for pigeon SL1 they were not, F(1, 46) = 3.65. The remaining three pigeons (SL2, SL3, and SL6) responded nondifferentially on probe trials, pecking at roughly equal rates to the comparison stimuli on both the positive and negative probe trials, Fs(1, 62) = .24, .04, and 1.07, respectively.
Discussion
The major contribution of the present experiment to the nonhuman literature on emergent stimulus–stimulus relations is the demonstration that associative symmetry can be observed in derived-relations tests even though the baseline symbolic relations involve sample and comparison stimuli that appear in different spatial locations. Five of the six Group DL pigeons, for which successive matching training involved center-key samples and left-key comparisons, responded more in testing to the reverse of the reinforced symbolic (AB) sample–comparison combinations than to the reverse of the nonreinforced symbolic combinations. Symmetry (BA) testing likewise involved center-key samples and left-key comparisons. Importantly, prior to testing, pigeons saw each hue and form matching stimulus as both a center-key sample and as a side-key comparison. This was accomplished by training pigeons on hue–hue (AA) and form–form (BB) successive matching concurrently with their symbolic, hue–form baseline (AB) task. Consequently, the shift from baseline training to symmetry testing did not involve a change in the location at which each hue and each form matching stimulus was seen (cf. Hogan & Zentall, 1977; Iversen, Sidman, & Carrigan, 1986; Lionello & Urcuioli, 1998).
However, controlling for stimulus location in this manner is clearly not sufficient to produce symmetry following conditional discrimination learning in pigeons. For example, in Lionello-DeNolf and Urcuioli (2002, Experiment 2), pigeons received training on AB symbolic matching with A sample stimuli appearing on the left or right side keys and B comparison stimuli appearing on the center key and the remaining side key in addition to hue identity (AA) and form identity (BB) matching structured in the same fashion. Thus, each matching stimulus was trained as a sample and as a comparison and was seen in each location prior to BA symmetry testing and AB location testing with center-key samples and side-key comparisons. Despite the fact that most pigeons matched accurately on the AB location test, none showed evidence for BA symmetry.
Similarly, Urcuioli (2008, Experiment 2) trained AB symbolic matching with A sample stimuli appearing on all three keys (left, center, and right) and B comparison stimuli appearing on the remaining two keys along with multiple-location hue identity (AA) and form identity (BB) matching. Thus, all stimuli appeared in all spatial locations as both samples and comparisons. Here, too, none of the pigeons showed symmetry. Clearly, n-alternative matching training with stimuli appearing in each possible spatial (and temporal) location does not yield symmetry.
Urcuioli (2008) hypothesized that one reason for these negative findings is that the n-alternative task is not conducive to pigeons’ stimulus-class formation. Specifically, as pigeons become increasingly accurate in their comparison choices in n-alternative matching, they encounter (by definition) fewer and fewer instances of explicitly nonreinforced sample–comparison combinations. By contrast, go/no-go matching guarantees that independent of the pigeon’s level of discriminative performance, the frequency of each explicitly nonreinforced combination is identical to the frequency of each explicitly reinforced combination. This constant juxtaposition is assumed to facilitate the formation of stimulus classes containing the elements of the reinforced combinations (cf. Urcuioli, 2008).
Importantly, by assuming that the functional matching stimuli include their ordinal position and spatial locations (e.g., Urcuioli & Swisher, 2012b), the present experiment showed that pigeons will show evidence for symmetry despite different sample and comparison locations if the samples and comparisons appearing on the symmetry test are members of the same, hypothesized class. In other words, we acknowledge (as previous data have demonstrated) that a center–red sample (c-R1) is not the same functional stimulus as the left–red sample (l-R1), just as a red sample stimulus (R1) is not the same functional stimulus as a red comparison stimulus (R2). The stimuli whose location must remain constant are those which promote class merger (e.g., c-R1, l-T2, c-G1, and l-H2 in Figure 1). If the spatial locations of these stimuli had not been the same across the baseline relations, class merger could not occur and no emergent relations would have been predicted.
Likewise, had the symmetry tests consisted instead of matching a center–red comparison to a left–triangle sample (l-T1⟶c-R2) and a center–green comparison to a left–horizontal sample (l-H1⟶c-G2), the pigeons should not have responded more to these combinations than to the remaining ones. Note that although probe trials such as these present each nominal stimulus in the same spatial location as in training (e.g., the triangle was seen on the left key both in training and in testing), their temporal location (ordinal position) is different, thus making them functionally different stimuli.
As Figures 1 and 2 indicate, positive elements common across the baseline relations must have the same nominal, spatial, and temporal aspects to promote class merger. With center–red samples and left–triangle comparisons merging the elements into one 4-member class [c-R1, c-T1, l-T2, and l-R2] and center–green samples and left–horizontal comparisons merging the elements into another 4-member class [c-G1, c-H1, l-H2, and l-G2], it became possible for pigeons to then match a left–red comparison to a center–triangle sample (c-T1⟶l-R2) and a left–green comparison to a center–horizontal sample (c-H1⟶l-G2)—that is, to show symmetry (see Figure 2). Indeed, most of the Group DL birds showed this theoretically predicted pattern of results.
For Group SL, the sample and comparison stimuli appeared in the same spatial location, just as in the emergent-relations studies by Urcuioli (2008, Experiments 3 & 4) and others (Frank & Wasserman, 2005; Sweeney & Urcuioli, 2010; Urcuioli & Swisher, 2012a, 2012b). Here, the constant location was the left (rather than the center) key, but in every other way, this group had the same training (AB, AA, and BB matching) as Group DL. When location is constant, theoretical derivations can ignore it, although this does not mean that location is inconsequential. In other words, although symmetry was also predicted for Group SL given the hypothesized classes [l-R1, l-T2, l-T1, l-R2] and [l-G1, l-H2, l-H1, l-G2], it would not have been predicted if all of its probe trials involved center-key samples and comparisons.
Surprisingly, however, only two of the Group SL pigeons demonstrated evidence for symmetry, although an emergent effect may have been obscured for two of the remaining four pigeons (viz., SL2 and SL3) because of their very low overall response rates on the symmetry probes. Alternatively, perhaps stimulus classes did not develop from the baseline relations for these birds, thus precluding symmetry (e.g., Frank & Wasserman, 2005; Sweeney & Urcuioli, 2010; Urcuioli, 2008, Experiment 3; Urcuioli & Swisher, 2012b). In any event, in Urcuioli’s (2008, Experiment 3) go/no-go symmetry study with center-key samples and comparisons, only three out of six pigeons showed this emergent effect when performances were averaged over the first four test sessions, so Group SL’s test results do not seem unusual in this context.
If the stimulus location is held constant in training and testing, Urcuioli’s (2008) theory does not make different predictions about the likelihood of symmetry as a function of whether the matching stimuli appear on the left, the center, or the right key. For example, with samples and comparisons appearing on the right key, the positive elements from the reinforced baseline relations should yield six individual classes that would have some members in common (viz., r-R1, r-G1, r-T2, r-H2). These common members should result in two 4-member classes [r-R1, r-T2, r-T1, r-R2] and [r-G1, r-H2, r-H1, r-G2] via class merger. Consequently, the r-R1⟶r-T2 baseline relation should yield the symmetrical r-T1⟶r-R2 relation, and likewise for relations involving G and H. If future studies should find that symmetry depends on which constant location is used in training and test, this would be a clear theoretical disconfirmation, albeit one we think is highly unlikely. Similarly, the theory does not anticipate a difference in results from same- versus different-location training, providing that such training generates the classes necessary to observe symmetry (cf. Figures 1 & 2).
It is important to consider that the functional stimuli in matching-to-sample consist of what they are, where they appear, and when they appear in order to determine whether or not new stimulus–stimulus relations will emerge. Using the assumptions of Urcuioli’s (2008) theory, we can derive a prediction of symmetry in a situation in which symmetry might not be expected given many past failures to find this effect when stimuli appear in different spatial locations (Lionello-DeNolf, 2009). It may be difficult or even impossible to remove spatial location and temporal position as controlling variables in matching-to-sample procedures, but emergent relations can still arise as long as they are properly taken into account.
Acknowledgments
This research was supported by NICHD Grant R01 HD061322. The authors thank Heloísa Campos, Lisa Macklin, Greg Erhardt, and Blake Polak for their assistance in conducting this research.
References
- Frank AJ, Wasserman EA. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DE, Zentall TR. Backward associations in the pigeon. The American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Iversen IH, Sidman M, Carrigan P. Stimulus definition in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello KM, Urcuioli PJ. Control by sample location in pigeon’s matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM, Urcuioli PJ. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger RS. The number of non-zero, post hoc contrasts from ANOVA and error-rate. I. British Journal of Mathematical and Statistical Psychology. 1975;28:71–78. [Google Scholar]
- Sidman M. Adventitious control by the location of comparison stimuli in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1992;58:173–182. doi: 10.1901/jeab.1992.58-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MM, Urcuioli PJ. A reflexivity effect in pigeons. Journal of the Experimental Analysis of Behavior. 2010;94:267–282. doi: 10.1901/jeab.2010.94-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Associative symmetry, antisymmetry, and a theory of pigeons’ equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Jones BM, Lionello-DeNolf KM. Evidence for response membership in stimulus classes by pigeons. Journal of the Experimental Analysis of Behavior. 2013;99:129–149. doi: 10.1002/jeab.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher M. Emergent identity matching after successive matching training. II: Reflexivity or transitivity? Journal of the Experimental Analysis of Behavior. 2012a;97:5–27. doi: 10.1901/jeab.2012.97-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher M. A replication and extension of the antisymmetry effect in pigeons. Journal of the Experimental Analysis of Behavior. 2012b;98:283–293. doi: 10.1901/jeab.2012.98-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco SM, Huziwara EM, Machado A, Tomanari GY. Associative symmetry by pigeons after few-exemplar training. Journal of the Experimental Analysis of Behavior. 2010;94:283–295. doi: 10.1901/jeab.2010.94-283. [DOI] [PMC free article] [PubMed] [Google Scholar]


