SUMMARY
A patient with recurrent multifocal glioblastoma received chimeric antigen receptor (CAR)–engineered T cells targeting the tumor-associated antigen interleukin-13 receptor alpha 2 (IL13Rα2). Multiple infusions of CAR T cells were administered over 220 days through two intracranial delivery routes — infusions into the resected tumor cavity followed by infusions into the ventricular system. Intracranial infusions of IL13Rα2-targeted CAR T cells were not associated with any toxic effects of grade 3 or higher. After CAR T-cell treatment, regression of all intracranial and spinal tumors was observed, along with corresponding increases in levels of cytokines and immune cells in the cerebrospinal fluid. This clinical response continued for 7.5 months after the initiation of CAR T-cell therapy.
Glioblastoma, an aggressive primary brain tumor, is among the most lethal of human cancers. We present evidence of the potential therapeutic benefit of adoptive T-cell therapy against glioblastoma with the use of CAR-engineered T cells targeting IL13Rα2, a glioma-associated antigen linked to a reduced rate of survival.1–3 The clinical potential of CAR T-cell therapy has been most convincingly shown by the successful use of CD19-specific CAR T cells against refractory B-cell cancers.4–6 However, extension of the use of CAR therapy beyond hematologic cancers and the efficacy of this therapy against solid tumors remain to be established.7,8
Our previous clinical study evaluating intracranial administration of CD8 T cells expressing a first-generation IL13Rα2-targeted CAR in patients with glioblastoma showed transient antiglioma responses with no high-grade therapy-related side effects.9,10 Building on these initial results, we modified the IL13Rα2-targeted CAR T cells to improve antitumor potency and T-cell persistence by incorporating 4-1BB (CD137) costimulation and a mutated IgG4-Fc linker to reduce off-target Fc-receptor interactions11 into the CAR (IL13BBζ) and by genetically engineering enriched central memory T cells.12,13 To evaluate the safety and therapeutic potential of IL13BBζ–CAR T-cell therapy for the treatment of high-grade glioma, we initiated a clinical trial and report here our clinical experience with one patient.
CASE REPORT
A 50-year-old man presented with glioblastoma in the right temporal lobe, with an unmethylated O6-methylguanine–DNA methyltransferase (MGMT) promoter, a nonmutated IDH1 R132H, and an IL13Rα2 H score of 100 (with no staining in 30% of cells, weak-intensity staining in 30%, moderate-intensity staining in 20%, and high-intensity staining in 10%) (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The H score, a method of quantitating immunohistochemical results, is based on the following formula: (3 × the percentage of strongly staining cells)+(2×the percentage of moderately staining cells) + (1 × the percentage of weakly staining cells), resulting in a range of 0 to 300.
The patient received standard-of-care therapy consisting of tumor resection, radiation therapy, and temozolomide.14 Six months after the diagnosis, magnetic resonance imaging (MRI) and positron-emission tomography–computed tomography (PET-CT) of the brain showed evidence of disease recurrence (Fig. S2A in the Supplementary Appendix). The patient was then enrolled in this clinical study of IL13Rα2-targeted CAR T cells (Fig. S2A in the Supplementary Appendix).
While the IL13BBζ–CAR T cells were being manufactured, the patient participated in an investigational clinical trial (ClinicalTrials.gov number, NCT01975701) at a different institution (Fig. S2A in the Supplementary Appendix). However, the disease progressed rapidly during treatment, with the development of multifocal leptomeningeal glioblastoma involving both cerebral hemispheres (Figs. S3 and S4 in the Supplementary Appendix).
The patient then began to receive treatment in our clinical study and underwent resection of three of five progressing intracranial tumors (Figs. S4 and S5 in the Supplementary Appendix), including the largest tumor in the right temporal–occipital region (tumor 1) and two tumors in the right frontal lobe (tumors 2 and 3). Two smaller tumors in the left temporal lobe (tumors 4 and 5) were not surgically removed.
IL13BBζ–CAR T cells were administered according to dose schedule 1 (an initial infusion of 2×106 CAR+ T cells followed by five infusions of 10×106 CAR+ T cells) (Table S1 in the Supplementary Appendix), and the patient received weekly intracavitary infusions of IL13BBζ–CAR T cells into the resected cavity of tumor 1 through a catheter device. Treatment was paused for assessment of safety and disease after the third and sixth infusions (Fig. 1, and Fig. S2A in the Supplementary Appendix).
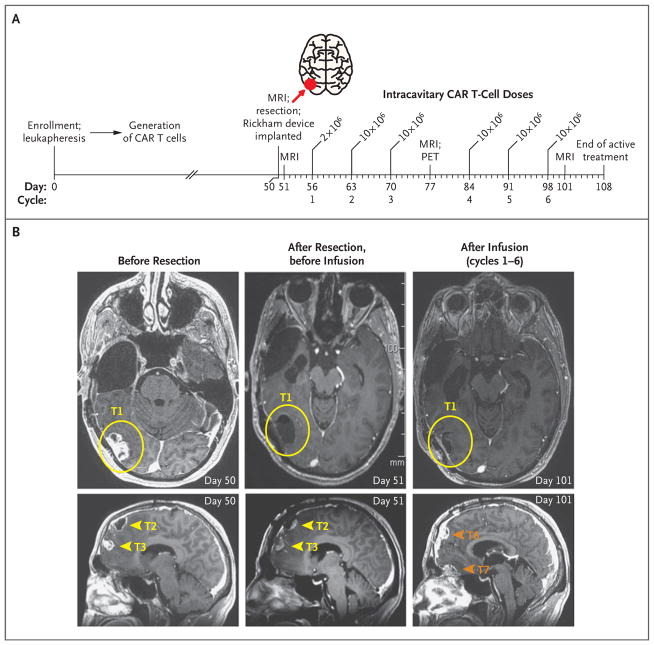
Figure 1. Local Tumor Control after Intracavitary Delivery of IL13BBζ–Chimeric Antigen Receptor (CAR) T Cells.
Panel A shows an overview of the intracavitary administration of six cycles of IL13BBζ–CAR T cells according to dose schedule 1 (see Table S1 in the Supplementary Appendix). CAR T cells were delivered through a Rickham catheter device into the right temporal–occipital region (tumor 1; red arrow) from day 56 to day 98 after enrollment, with 1 week of rest after cycles 3 and 6 for evaluation of safety and disease. MRI denotes magnetic resonance imaging, and PET positron-emission tomography. Panel B shows axial MRI (T1-weighted with gadolinium enhancement) of the brain highlighting the site of the resected tumor at which the catheter was placed for delivery of CAR T cells (tumor 1 [T1]; yellow circles), as well as the resected-only tumor sites in the frontal lobe (tumors 2 and 3 [T2 and T3]; yellow arrowheads) and the sites of tumors that developed during the intracavitary treatment period (tumors 6 and 7 [T6 and T7]; orange arrowheads). The CAR T-cell injection site (T1) remained stable without evidence of disease recurrence, whereas other disease foci, including T6 and T7, which were adjacent to resected T2 and T3 but distant from the CAR T-cell injection site, continued to progress.
Although the local treated site (tumor 1) remained stable during this treatment phase, with no evidence of disease progression, two new lesions (tumors 6 and 7) appeared near the previously resected frontal-lobe tumors (tumors 2 and 3), and the nonresected tumors (tumors 4 and 5) continued to progress. Therefore, on the basis of the rationale that delivery of cells into the cerebrospinal fluid would improve their trafficking to sites of multifocal disease, a second catheter device was placed in the right lateral ventricle. This allowed the patient to receive 10 additional intraventricular treatment cycles at 1- to 3-week intervals, with a 6-week break between the fifth and sixth infusions (Fig. 2). We report clinical outcomes through 298 days after enrollment.
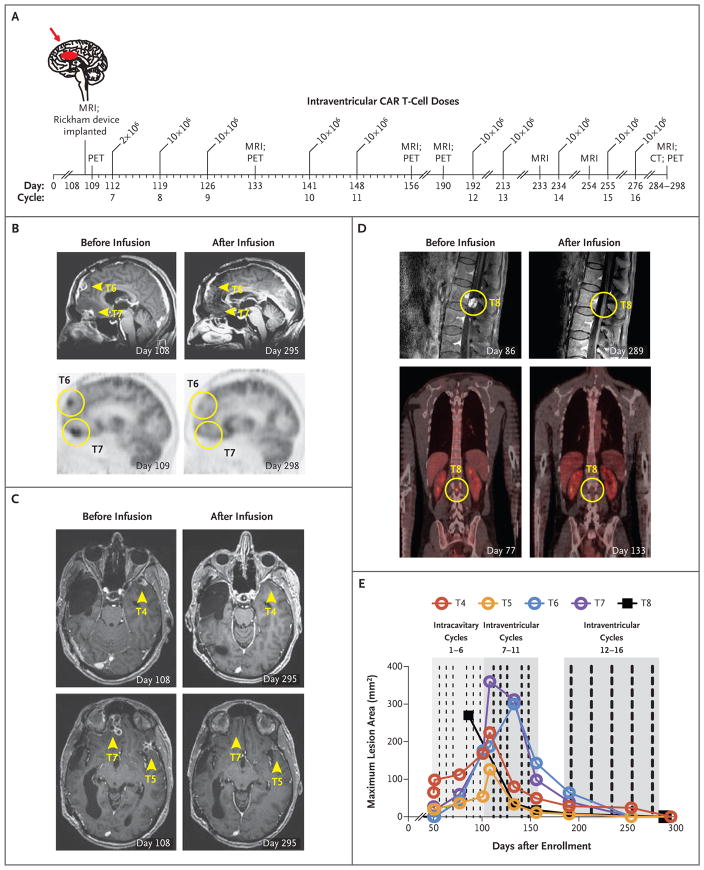
Figure 2. Regression of Recurrent Multifocal Glioblastoma, Including Spinal Metastases, after Intraventricular Delivery of IL13Rα2-Targeted CAR T Cells.
Panel A shows an overview of intraventricular delivery (through a Rickham catheter device) of 10 cycles of IL13BBζ–CAR T cells into the right lateral ventricle (red arrow) from day 112 to day 298 after enrollment. The first five weekly intraventricular infusions were interrupted for 1 week for assessment of safety and disease after the third infusion (cycle 9), a break of 6 weeks occurred during manufacture of the second product (between cycle 11 and cycle 12), and the remaining five intraventricular infusions were administered once every 3 weeks. CT denotes computed tomography. Panels B through D show MRI with gadolinium enhancement and 18F-fluorodeoxyglucose PET images before infusions of IL13BBζ–CAR T cells and after cycle 16 of intraventricular therapy. Panel B shows sagittal MRI (top) and PET images (bottom) of the brain (tumors 6 and 7 [T6 and T7]; yellow arrowheads and circles). Panel C shows axial MRI of the brain (tumors 4, 5, and 7 [T4, T5, and T7]; yellow arrowheads). Panel D shows sagittal MRI (top) and coronal PET-CT (bottom) images of the spine (tumor 8 [T8]; yellow circles). Panel E shows the maximum lesion area for nonresected tumors 4 through 8 (T4 through T8) with their respective decreases over time. These decreases indicate regression of all intracranial lesions (tumors 4 through 7 [T4 through T7]) and spinal lesions (T8) after intraventricular therapy.
METHODS
STUDY DESIGN
In this phase 1 study for recurrent malignant glioma, intracavitary infusions of CAR T cells targeting IL13Rα2 were administered as described in the Supplemental Methods section in the Supplementary Appendix. Intraventricular infusions were then provided according to a compassionate-use protocol. Both protocols were approved by the institutional review board at the City of Hope, and the patient provided written informed consent. The protocols are available at NEJM.org. There was no commercial support for this study.
MANUFACTURE OF CELL PRODUCTS
Enriched central memory T cells were lentivirally transduced with an interleukin-13 (E13Y-mutated) ligand-based CAR2,15 containing a 4-1BB costimulatory domain (IL13BBζ), and truncated CD19 (CD19t) was used as a marker for transduction (Fig. S6A and the Supplemental Methods section in the Supplementary Appendix). Details regarding the construction and manufacturing of the CAR T cells, product infusion, and additional procedures are included in the Supplemental Methods section in the Supplementary Appendix.
To obtain sufficient material for 16 infusions, we manufactured two CAR T-cell products; the first was used for cycles 1 through 11, and the second was used for cycles 12 through 16. Both products (Fig. S6B and S6C in the Supplementary Appendix) were phenotypically similar and predominantly CD4+ (74% and 90%, respectively); their CD19t expression (64% and 81%) was used to calculate CAR T-cell dosing.
RESULTS
SAFETY AND ADVERSE-EVENT PROFILE
Intracavitary infusions (cycles 1 through 6) and intraventricular infusions (cycles 7 through 16) of IL13BBζ–CAR T cells were delivered at a maximum dose of 10×106 CAR+ T cells. These infusions were not associated with any toxic effects of grade 3 or higher (Table S2 in the Supplementary Appendix).
Grade 1 or 2 events that were at least possibly attributable to therapy were observed within 72 hours after the T-cell infusions. These events included headaches, generalized fatigue, myalgia, and olfactory auras. Concomitant medications that may have influenced this safety profile included dexamethasone at a dose of 0 to 4 mg per day (Fig. S2D in the Supplementary Appendix), divalproex at a dose of 750 mg twice a day for seizure control, and acetaminophen as needed.
CLINICAL RESPONSE
At the time of treatment, the patient presented with a highly aggressive recurrent glioblastoma with features indicating a poor prognosis, including multifocal leptomeningeal disease (Figs. S3 and S4 in the Supplementary Appendix), histologic features of dedifferentiated glioblastoma (Fig. S5 in the Supplementary Appendix), and a proliferative rate of more than 60%. Expression of IL13Rα2 was heterogeneous but similar in the primary tumor and the recurrent tumors (tumors 1, 2, and 3); the mean (±SD) H scores in the primary tumor and in the recurrent tumors were 100 and 88±20, respectively (Figs. S1 and S5 in the Supplementary Appendix). Chromosomal analysis of the recurrent tumors also showed a clonal relationship with the primary tumor, albeit with some minor variations (Fig. S7 in the Supplementary Appendix).
During the six weekly intracavitary infusions (cycles 1 through 6) (Fig. 1A), the treated tumor in the temporal–occipital region (tumor 1) remained stable for more than 45 days after surgery without evidence of disease progression (Fig. 1B). However, MRI revealed that nonresected tumors in the left temporal lobe (tumors 4 and 5) and new tumors (tumors 6 and 7) near the resected lesions 2 and 3 were progressing (Fig. 1B). In addition, new metastatic lesions in the spine causing leg numbness, including one large tumor that was 18 mm in diameter and several smaller tumors (≤4 mm), were detected (Fig. 2C, and Fig. S2A in the Supplementary Appendix). These results suggested that although intracavitary administration of IL13BBζ–CAR T cells may have prevented tumor recurrence locally (at the tumor 1 injection site), these infusions were not sufficient to effectively control tumor progression at distant sites.
Subsequently, we administered 10 intraventricular infusions of IL13BBζ–CAR T cells into the right lateral ventricle without any other therapeutic interventions (Fig. 2A). After the first three intraventricular infusions (on day 133), we observed a dramatic reduction in the size of all intracranial and spinal tumors, and after the fifth intraventricular infusion (on day 190), all tumors had decreased by 77 to 100% (Table S3 in the Supplementary Appendix).
Five additional intraventricular infusions were administered (cycles 12 through 16), and during this consolidation phase, all lesions continued to resolve. The tumors were not measurable by means of MRI and remained undetectable by means of PET (Fig. 2B through 2E, and Table S3 in the Supplementary Appendix). Most remarkably, after intraventicular delivery of IL13BBζ–CAR T cells, all metastatic tumors in the spine were completely eliminated (Fig. 2D and 2E). During intraventricular treatment (day 108 through day 284), systemic dexamethasone was gradually eliminated (Fig. S2D in the Supplementary Appendix), and the patient returned to normal life and work activities.
This dramatic clinical response was sustained for 7.5 months after the initiation of CAR T-cell therapy, and none of these initial tumors (tumors 1 through 7 and spinal tumors) recurred. These results show that treatment with IL13BBζ–CAR T cells mediated a complete response according to Response Assessment in Neuro-Oncology criteria (see the Supplemental Methods section in the Supplementary Appendix).16 Unfortunately, this patient’s disease eventually recurred after cycle 16 (228 days after the first CAR T-cell treatment) at four new locations that were distinct and non-adjacent to tumors 1 through 7 and the spinal tumors. The cause of this tumor recurrence is currently under investigation, with preliminary results suggesting decreased expression of IL13Rα2 (Fig. S8 in the Supplementary Appendix).
CAR T-CELL PERSISTENCE AND CENTRAL NERVOUS SYSTEM INFLAMMATORY RESPONSE
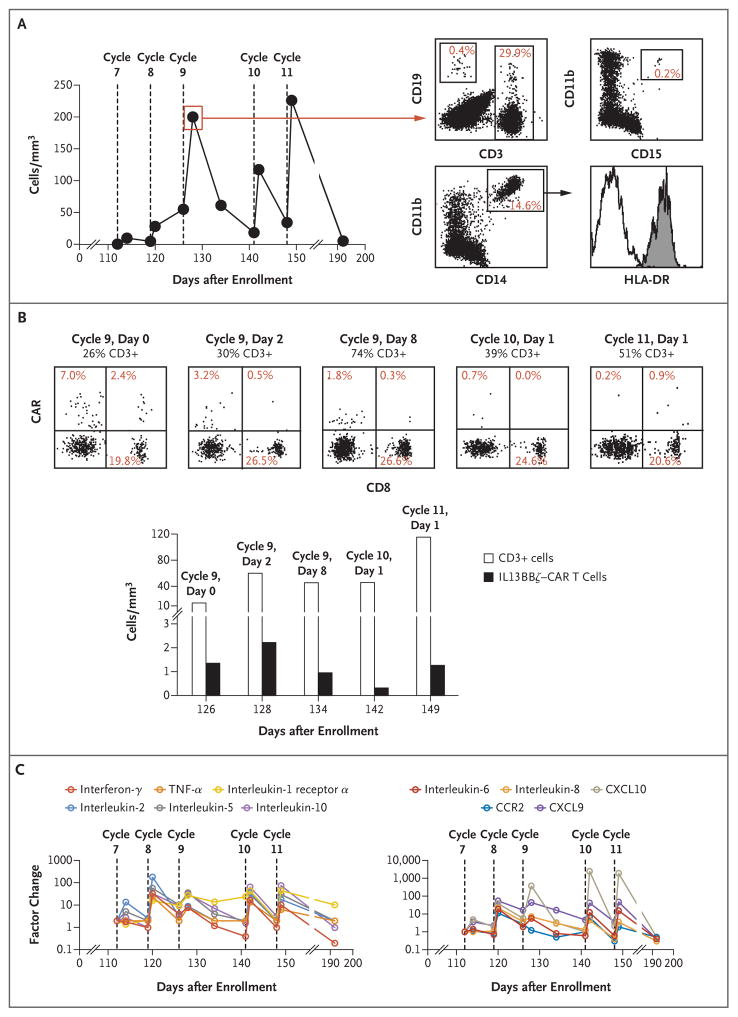
Immediately after each intraventricular infusion (on day 1 or 2), the total number of immune cells per cubic millimeter of cerebrospinal fluid increased by a mean (±SD) factor of 7.0±3.6, as compared with preinfusion levels (on day 0 of each cycle), and typically decreased over the 7-day treatment cycle (Fig. 3A). Evaluation on day 2 of cycle 9 showed that the immune-cell populations in the cerebrospinal fluid included both endogenous and CAR-expressing CD3+ T cells, CD14+CD11b+HLA-DR+ mature myeloid populations, CD19+ B cells, and few CD11b+CD15+ granulocytes (Fig. 3A). Consistent with flow-cytometric data, cytopathological analysis of the cerebrospinal fluid on day 1 of cycle 11 confirmed the presence of reactive lymphocytes, monocytes, and macrophages (data not shown).
Figure 3. Cerebrospinal Fluid (CSF) Analysis of Immune-Cell Populations, CAR T-Cell Persistence, and Inflammatory Cytokines during Intraventricular Therapy.
The use of the catheter device enabled evaluation of the CSF during the intraventricular treatment course. Samples of CSF were obtained just before and 1 to 2 days after each intraventricular infusion during cycles 7 through 11, and additional samples were collected 8 days after the cycle 9 infusion and 44 days after the cycle 11 infusion. Panel A shows the cell counts in the CSF over the intraventricular treatment course. These counts were elevated after each infusion (P = 0.009 calculated with the use of a ratio paired t-test comparing the infusion values before [day 0] and after [day 1 or day 2] each cycle). Flow-cytometric analysis of cell populations on day 2 of cycle 9 show evidence of CD19+ B cells, both CAR+ (i.e., CD19t+) and nonengineered CD3+ T cells, CD11b+CD15+ granulocytes, and CD14+CD11b+HLA-DR+ mature myeloid populations. Panel B shows the results of serial evaluation of the CD3+ T-cell population in the CSF for the presence of IL13BBζ–CAR T cells (i.e., CD19t+). CD3-gated cells from the CSF samples obtained at the indicated day of cycles 9, 10, and 11 were costained with CD19 and CD8 (top histograms). Percentages of CD3 cells that were CAR+ (CD19t+) were as follows: day 0 of cycle 9, 9.4%; day 2 of cycle 9, 3.7%; day 8 of cycle 9, 2.1%; day 1 of cycle 10, 0.7%; and day 1 of cycle 11, 1.1%. Percentages of immunoreactive cells were then used to calculate the numbers of total CD3+ T cells and CD19+ CD3+ cells (IL13BBζ–CAR T cells) per cubic millimeter of CSF at each time point. Because of low cell recovery in the CSF, the persistence of CAR T cells could not be evaluated for cycles 7 and 8 and day 0 of cycles 10 and 11. Panel C shows the factor change in cytokine levels with intraventricular treatment during cycles 7 through 11. Only cytokines that were assessed with the use of a multiplex assay and that had a change by a factor of 10 or more as compared with pretreatment levels at one or more time points are shown. Baseline values (on day 0 of cycle 7) used to calculate factor changes were as follows: interferon-γ, 8.2 pg per milliliter; tumor necrosis factor α (TNF-α), 1.6 pg per milliliter; interleukin-1 receptor α, 50.1 pg per milliliter; interleukin-2, 0.6 pg per milliliter; interleukin-5, 0.5 pg per milliliter; interleukin-10, 4.4 pg per milliliter; interleukin-6, 56.5 pg per milliliter; interleukin-8, 226.2 pg per milliliter; C-X-C motif chemokine ligand 10 (CXCL10), 161.4 pg per milliliter; CCR2, 1660.6 pg per milliliter; and C-X-C motif chemokine ligand 9 (CXCL9), 82.9 pg per milliliter (Table S4A in the Supplementary Appendix). For all cytokines except interleukin-1 receptor α and CCR2, P<0.05, calculated with the use of a ratio paired t-test comparing the infusion values before (day 0) and after (day 1 or day 2) all cycles in which 10 × 106 CAR+ T cells were infused (cycles 8 through 11).
After the administration of intraventricular therapy (cycles 7 through 11), CAR+ T cells were detected at all time points evaluated (Fig. 3B). CAR+ T cells persisted in the cerebrospinal fluid for at least 7 days, as indicated by their presence 7 days after cycle 8, before the cycle 9 infusion (CAR+ T cells composed 9.4% of CD3 cells on day 0 of cycle 9). We observed only small increases in levels of CAR+ T cells in the cerebrospinal fluid, with an increase by a factor of 1.6 after cycle 9 (day 2 of cycle 9 vs. day 0 of cycle 9), followed by a decrease by a factor of 2.3 over 8 days (as assessed on day 8 of cycle 9). In later cycles (e.g., on day 1 of cycle 10 and on day 1 of cycle 11), when the tumor burden was significantly lower, the numbers of CAR+ T cells in the cerebrospinal fluid after infusion decreased.
Each intraventricular infusion of IL13BBζ–CAR T cells in cycles 7 through 11 was associated with significantly elevated levels of cytokines in the cerebrospinal fluid. The measured levels and calculated factor change for 30 cytokines are provided in Tables S4A and S4B in the Supplementary Appendix. Levels of 11 inflammatory cytokines increased by a factor of more than 10 from preinjection baseline levels (on day 0 of cycle 7) immediately after at least one infusion. These cytokines included interferon-γ, tumor necrosis factor α, interleukins 2, 10, 5, 6, and 8; chemokines C-X-C motif chemokine ligand 9 (CXCL9) (or monokine induced by interferon-γ[MIG]), C-X-C motif chemokine ligand 10 (CXCL10) (or IP-10), and CCR2 (monocyte chemoattractant protein 1 [MCP-1]); and soluble receptor interleukin-1 receptor α (Fig. 3C). Increases in levels of inflammatory cytokines appeared to correspond with the incidence of grade 1 and 2 symptoms such as fever, fatigue, and myalgia (Table S2 in the Supplementary Appendix), and these levels returned to near baseline levels between weekly treatment cycles.
These immunologic changes were restricted to the cerebrospinal fluid, since no notable increases in levels of cytokines (Tables S5 and S6 in the Supplementary Appendix) and no CAR+ T cells (data not shown) were detectable in the peripheral blood. Unfortunately, we were unable to recover fluid during the intracavitary infusions for comparison, since the catheter tip, which was placed at the edge of the resection cavity, prevented aspiration of tumor cyst fluid.
DISCUSSION
We report that autologous CAR T cells targeting IL13Rα2 mediated a transient complete response in a patient with recurrent multifocal glioblastoma, with dramatic improvements in quality of life, including the discontinuation of systemic glucocorticoids and a return to normal life activities. This clinical response was achieved despite the nonuniform tumor expression of IL13Rα2 and without previous chemotherapy designed for depletion of lymphocytes. Such chemotherapy has been used to augment systemic adoptive T-cell responses,17,18 but it was not incorporated in this study because of increased risks of bleeding and infection associated with surgical resection and uncertainty as to whether systemic depletion of lymphocytes would enhance responses to local delivery in the brain.
The use of CAR T cells for the treatment of solid tumors involves a unique set of challenges, including antigen validation, tumor trafficking and infiltration, tumor heterogeneity, and an immunosuppressive microenvironment.7,8 This study provides proof-of-principle data that confirm IL13Rα2 as a useful immunotherapeutic target in glioblastoma and establish that CAR T cells can mediate profound antitumor activity against a difficult-to-treat solid tumor.
Insights from this study are derived from the comparison in one patient of two intracranial CAR T-cell delivery routes — infusion into the resected tumor cavity and infusion into the ventricular system. In this patient, both routes (intracavitary and intraventricular) had similar low toxicity profiles, but they differed in their apparent ability to abrogate tumor growth at distant sites. While intracavitary therapy appeared to control local tumor recurrence, progression of glioblastoma at distant sites, including the appearance of new lesions, was observed. By contrast, after intraventricular administration of IL13BBζ–CAR T cells, regression of all central nervous system tumors, including spinal tumors, was achieved. It is possible that the leptomeningeal presentation of this patient’s recurrent glioblastoma, which is often seen in late-stage refractory disease, may have rendered the tumors more responsive to intraventricular therapy. Nevertheless, these data provide support for further exploration of the usefulness of intraventricular administration of CAR T cells for the treatment of malignant brain tumors.
This case also highlights the potential role of the endogenous immune system in CAR T-cell–mediated antitumor responses. Immediate increases in endogenous immune cells and inflammatory cytokines after each intraventricular infusion suggest recruitment and stimulation of the host immune system. This finding may explain how a complete response was achieved even though the tumor did not uniformly express the target. Correlative studies to evaluate the potential contributions of endogenous immune responses after administration of IL13BBζ–CAR T cells are under way.
This study showed antitumor activity against multifocal glioblastoma, with manageable therapy-related toxic effects, through targeting of the glioma-associated antigen IL13Rα2. The absence of systemic toxic effects is particularly noteworthy given the severe cytokine release syndrome and neurotoxicity that are often associated with anti-tumor responses against high disease burden in patients receiving CD19-targeted CAR T-cell therapy.19,20 After each intraventricular infusion of IL13BBζ–CAR T cells, we observed rapid and pleiotropic changes in levels of inflammatory cytokines in the cerebrospinal fluid, with significant increases in the interferon-γ–inducible chemokines CXCL9 and CXCL10, which have antitumor potential.21,22 These changes in cytokine levels did not affect neurologic function or the general well-being of the patient and thus provide an initial framework for defining the range of achievable therapy-related changes in cytokine levels in the cerebrospinal fluid without the development of dose-limiting toxic effects.
Although this patient had a remarkable clinical response, CAR T-cell accumulation and expansion in the cerebrospinal fluid in later cycles and over the 7-day infusion cycle were limited. This might be due to less antigen drive as a consequence of decreased tumor burden or to immune rejection of the therapeutic product — although clinical symptoms indicative of a rejection response23 were not observed. The use of our platform to manufacture central memory T cells12,13 and the 4-1BB–containing CAR design were based on data showing improved antitumor potency and persistence in preclinical models (Brown CE, et al.: unpublished data). However, how these platform modifications translate to persistence of IL13BBζ–CAR T cells and expansion in the cerebrospinal fluid, trafficking to tumor sites, or both in this patient remains uncertain.
The patient described here was a participant in this ongoing dose-escalation safety study to evaluate the role of intracranial CAR T-cell therapy targeting IL13Rα2 in patients with malignant gliomas. Among the seven patients who have received this therapy, this patient’s clinical experience of receiving both intracavitary and intraventricular administration of IL13BBζ–CAR T cells remains unique and has prompted the expansion of our phase 1 study to evaluate intraventricular administration in a larger cohort of patients. Overall, our clinical experience along with recent studies of human epidermal growth factor receptor 2–CAR T cells and epidermal growth factor receptor deletion mutant variant III (EGFRvIII)–CAR T cells against glioblastoma24,25 provide initial evidence of the safety and antitumor activity of CAR T-cell immunotherapy in patients with malignant brain tumors.
Supplementary Material
Acknowledgments
Supported by grants from Gateway for Cancer Research (G-14-600), the Food and Drug Administration (R01FD005129), the California Institute for Regenerative Medicine (CIRM; TR3-05641), the CIRM Alpha Stem Cell Clinics Network (AC1-07659), and the National Cancer Institute (NCI) and National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) (P30CA33572, R01CA155769, R21NS081594, and R21CA189223).
We thank Wen-Chung Chang, Nima Jamjampour, Alina Oancea, Alexandra Pike, Lauren Quezada, Laurelin Wolfenden, Sarah Wright, and Jingying Xu for T-cell manufacturing and product release; Alfonso Brito, Vivian Chiu, Cindy (Xin) Yang, and the staff at the City of Hope Clinical Immunobiology Correlative Studies Laboratory, Pathology Core and Cytogenetics Core Laboratory for their technical assistance; and Sandra Thomas for her critical review and editing of an earlier version of the manuscript.
Funded by Gateway for Cancer Research and others; ClinicalTrials.gov number, NCT02208362.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or of the California Institute for Regenerative Medicine or any other agency of the State of California.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Brown CE, Starr R, Aguilar B, et al. Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res. 2012;18:2199–209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–6. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 3.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–90. [PubMed] [Google Scholar]
- 4.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos CA, Savoldo B, Dotti G. CD19-CAR trials. Cancer J. 2014;20:112–8. doi: 10.1097/PPO.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–5. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priceman SJ, Forman SJ, Brown CE. Smart CARs engineered for cancer immunotherapy. Curr Opin Oncol. 2015;27:466–74. doi: 10.1097/CCO.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21:4062–72. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–8. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonnalagadda M, Mardiros A, Urak R, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid Fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23:757–68. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–98. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother. 2012;35:689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–43. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 15.Debinski W, Thompson JP. Retargeting interleukin 13 for radioimmunodetection and radioimmunotherapy of human high-grade gliomas. Clin Cancer Res. 1999;5(Suppl):3143s–3147s. [PubMed] [Google Scholar]
- 16.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 17.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerny C, Bronger H, Davoodi M, et al. The role of CXCR3/ligand axis in cancer. Int Trends Immun. 2015;3:46–52. ( http://researchpub.org/journal/iti/abstract/vol3-no2.html#paper3) [Google Scholar]
- 22.Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer (review) Oncol Lett. 2011;2:583–9. doi: 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed N, Brawley V, Hegde M, et al. Autologous HER2 CMV bispecific CAR T cells are safe and demonstrate clinical benefit for glioblastoma in a phase I trial. J Immunother Cancer. 2015;3(Suppl 2):O11. ( http://jitc.biomedcentral.com/articles/10.1186/2051-1426-3-S2-O11) [Google Scholar]
- 25.O’Rourke DM, Nasrallah M, Morrissette JJ, et al. Pilot study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. J Clin Oncol. 2016;34:2067. ( http://meetinglibrary.asco.org/content/171405-176) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.