Abstract
Objective
Adolescents who regularly use marijuana may be at heightened risk of developing subclinical and clinical psychotic symptoms. However, this association could be explained by reverse causation or other factors. To address these limitations, we examined whether adolescents who engage in regular marijuana use exhibit a systematic increase in subclinical psychotic symptoms that persists during periods of sustained abstinence.
Method
The sample comprised 1,009 boys who were recruited in 1st and 7th grades. Self-reported frequency of marijuana use, subclinical psychotic symptoms, and several time-varying confounds (e.g., other substance use, internalizing/externalizing problems) were collected annually from age 13 to 18. Fixed effects (within-individual change) models examined whether adolescents exhibited an increase in their subclinical psychotic symptoms as a function of their recent and/or cumulative history of regular marijuana use, and whether these effects were sustained following abstinence. Models controlled for all time-stable factors (default) and several time-varying covariates as potential confounds.
Results
For each year adolescents engaged in regular marijuana use, their expected level of subsequent subclinical psychotic symptoms rose by 21% (p <.05) and their expected odds of experiencing subsequent paranoia or hallucinations rose by 133% (p < .001) and 92% (p < .05), respectively. The effect of prior regular marijuana use on subsequent subclinical psychotic symptoms persisted even when adolescents stopped using marijuana for a year. Findings remained significant after controlling for all time-stable and several time-varying confounds, as well as possible reverse causation.
Conclusions
These results suggest that regular marijuana use may significantly increase the risk that an adolescent will experience persistent subclinical psychotic symptoms.
As a growing number of states are legalizing medicinal and recreational marijuana use, it is increasingly important to understand the consequences that regular use may have on physical and mental health. One area of particular concern is the effect that adolescent marijuana use may have on the development of psychotic symptoms (e.g., hallucinations, paranoia). Multiple longitudinal and cross-sectional studies have reported that marijuana use, particularly during adolescence, is related to acute psychotic episodes and future psychotic disorders (1–4). More recent research suggests that regular marijuana use might increase adolescents’ risk for developing a psychotic illness by causing them to experience persistent subclinical psychotic experiences, which are typically transitory and fairly common during adolescence (5). However, no published longitudinal studies have examined whether adolescents who regularly use marijuana (i.e., weekly or more often) over several years exhibit a systematic increase in their subclinical psychotic symptoms that persists during periods of sustained abstinence.
One critical issue is to determine whether a prior history of regular marijuana use, independent of current use, increases an adolescent’s risk for experiencing persistent psychotic symptoms. Experimental administration of the primary psychoactive chemical in marijuana, tetrahydrocannabinol (THC), can cause acute feelings of paranoia and other features of psychosis in healthy volunteers (6–9), but these symptoms largely subside when acute intoxication wanes (10). Many negative effects of adolescent marijuana use on cognitive functioning also tend to dissipate following a few months of abstinence (11). Nearly all prior longitudinal studies examining the association between marijuana use and future psychotic symptoms have not controlled for recent patterns of use (12–17), have not repeatedly assessed marijuana use across adolescence, or have combined prior and recent use (18). Therefore, it is impossible to delineate the enduring effect that regular use has on emergent psychotic symptoms, and whether this effect is sustained when individuals remain abstinent for several months.
A second issue is whether the association between adolescent marijuana use and later psychotic symptoms is causal or a function of confounding factors (19–23). Together, prior studies have collectively controlled for approximately 60 confounding factors (e.g., other substance use, mental health problems), with estimates suggesting that these factors account for nearly half of the association between marijuana use and psychosis (3). Although no single study can accurately, comprehensively, and directly quantify the myriad pre-existing individual differences that may explain the linkage between marijuana use and psychosis, it is possible to eliminate these factors as potential confounds by using within-individual change models. By examining the association between changes in marijuana use and psychotic symptoms within individuals over time, all pre-existing time-stable factors (whether measured or not) are eliminated as potential confounds (24). Moreover, only within-individual change models can address two key questions regarding the association between adolescent marijuana use and psychotic symptoms: 1) During periods of regular marijuana use, do adolescents experience an increase in their psychotic symptoms (concurrent effect)?; and 2) Do adolescents who engage in regular marijuana use across multiple years exhibit an incremental and sustained increase in their psychotic symptoms that remains even during periods of abstinence (cumulative/sustained effect)?
The only published longitudinal study examining the association between within-individual changes in marijuana use and subclinical psychotic symptoms reported that adolescents experienced a significant increase in their psychotic symptoms during years when they increased their marijuana use; however, whether this increase was short-lived or persisted across multiple years was not examined (25).
The direction of any causal association between marijuana use and psychotic symptoms is also debated. For example, the self-medication hypothesis suggests that adolescents may begin engaging in regular marijuana use as a way to cope with prodromal psychotic symptoms. Studies directly examining the self-medication hypothesis have produced mixed findings. Fergusson and colleagues (25) found that within-individual increases in subclinical psychotic symptoms were unrelated to concurrent changes in adolescent marijuana use after controlling for several time-varying confounds. In contrast, another longitudinal study found evidence of a bidirectional association between psychosis vulnerability and marijuana use in adolescence (26).
The present study was designed to address several key issues regarding the association between adolescent marijuana use and subclinical psychotic symptoms. Using within-individual change modeling, analyses examined whether adolescents experienced a systematic increase in their subclinical psychotic symptoms during periods when they used marijuana regularly (concurrent effect), and whether this increase persisted over time, even during periods of sustained abstinence (cumulative/sustained effect). These effects were further examined for different features of psychosis (e.g., paranoia, hallucinations, bizarre behavior). Analyses controlled for all time-stable factors and several potential time-varying confounds (e.g., other substance use, externalizing and internalizing problems). The possibility of reverse causation was also examined by examining marijuana use as an outcome of (rather than predictor of) subclinical psychotic symptoms.
Method
Participants and Procedures
Participants were 1,009 boys (55.1% Black; 41.1% White; 3.8% other) who were recruited from a list of students enrolled in the 1st and 7th grades (referred to as the youngest and oldest cohorts) in Pittsburgh public schools in 1987–1988. Students enrolled in classes for severe intellectual and physical disabilities were not eligible for the study. A random sample of boys enrolled in each grade was selected for a multi-informant (parents, teachers, self-report) screening to assess conduct problems (e.g., fighting, stealing). Parental consent rates for the screening were 84.6% (N=849) and 83.9% (N=855) for the youngest and oldest cohorts, respectively. Boys who scored in the upper 30th percentile on the screener within each grade were selected for longitudinal follow-up (youngest N=256; oldest N=257), along with an approximately equal number of boys who were randomly selected from those scoring below the 70th percentile (youngest N=247; oldest N=249). At screening, boys in the youngest (Age: M=6.96, SD=.55) and oldest (Age: M=13.38, SD=.79) cohorts were predominately living with their biological mother (95% and 92%, respectively), and approximately half had a biological father living in the home (42% and 44%, respectively). In both the youngest and oldest cohorts, approximately one-fifth of mothers (20.6% & 22.8%, respectively) and fathers (16.9% & 23.3%, respectively) living in the home had not graduated high school. The proportion of families with no employed parental figure in the oldest and youngest cohorts was 14.5% and 12%, respectively. Boys in the follow-up sample were not significantly different from the screening sample in terms of race, family composition, and parental education and employment (27).
Following screening, the youngest cohort was interviewed every six months for four years, followed by nine annual assessments and follow-ups at ages 26 and 29. Following screening, the oldest cohort was assessed every six months for 30 months and then annually for ten years, and again at age 36. Here, data from the two cohorts were combined by aligning assessments by participant age at the time of the interview, resulting in overlapping annual assessments from ages 13–18. We focused on this age range because: 1) The last annual assessment for participants in the 1st grade cohort was conducted when they were approximately 18 years old; and 2) prior studies have suggested that regular marijuana use in adolescence is most strongly associated with psychotic symptoms and other forms of cognitive impairment (1, 3, 12, 28). Further details about sample and study methodology are available elsewhere (27).
Measures
Regular marijuana use
Marijuana use was assessed with the youth-reported Substance Use Questionnaire (SUQ)(29). At each annual assessment, youth reported the number of days they used marijuana in the past year. Adjacent 6-month assessments for the oldest cohort were summed to index past year use. A binary variable, created at each age to index at least weekly marijuana use (i.e., >=52 times), was used given evidence that weekly use before age 18 might be associated with longer term impairments in cognitive functioning (28). A cumulative history of marijuana use was indexed at each age by counting the number of prior years participants reported weekly use. Cumulative years of weekly use was truncated at 2 years because only 3.8% had 3+ years of prior weekly use by age 18.
Subclinical psychotic symptoms
Five items from the Youth Self Report (YSR) (30) were used to index subclinical psychotic symptoms in the past year at each age. Consistent with prior studies, a symptom was considered present if youth rated it as occurring “sometimes” or “often” (31). The items indexed feelings of paranoia (“I feel that others are out to get me”), hallucinations (“I see things that other people think aren’t there” “I hear sounds or voices that other people think aren’t there”) and bizarre thinking (“I have thoughts that other people would think are strange” “I do things that other people thing are strange”). A count of past year psychotic symptoms was calculated for each age, as well as three binary variables indexing whether participants experienced each symptom subtype (i.e., paranoia, hallucinations, bizarre thinking). Longitudinal evidence indicates that adolescents who endorse experiencing these subclinical symptoms are at increased risk for developing a psychotic disorder later in life (32).
Time-Varying Covariates
Prior subclinical psychotic symptoms (T-1)
Psychotic symptoms at the prior assessment wave (T-1) were included as covariates. For total psychotic symptoms, the number of symptoms at T-1 was truncated at 2 (i.e., upper 9–20% at each age). For analyses involving symptom subtypes, binary items indexing the presence/absence of the symptom at T-1 were used.
Other substance use
The SUQ was used to assess the number of days participants used alcohol, tobacco, and other illicit drugs (tranquilizers, barbiturates, or codeine without prescription; other prescription drugs without prescription; amphetamines; hallucinogens; cocaine; crack; heroin; PCP) in the past year. As with marijuana use, alcohol use was dichotomized to index at least weekly use (>=52 days). Tobacco use was dichotomized to index near daily or daily use (>=312 days). Other illicit drug use was dichotomized to index use or non-use due to the low prevalence at each age (i.e., 2–6%). Variables indexing a prior history of use were created for each age by summing the number of prior years of weekly alcohol use (truncated at 2; 3.8% used weekly 3+ years by age 18), daily tobacco use (truncated at 3; 5.8% used daily 4+ years by age 18), and other illicit drug use (truncated at 1; 5.0% used 2+ years by age 18).
Internalizing and externalizing problems
The extensively validated internalizing and externalizing problems scales from the YSR were used to control for fluctuations in other forms of psychopathology (33). Participants rated items using a 3-point scale (0=not true to 2=very true/often true), and items were summed to generate internalizing (34 items) and externalizing (34 items) problem scores. The item “I feel that others are out to get me” was excluded from the internalizing scale because it was used as an index of paranoia.
Data Analysis Plan
Fixed effects regressions in Stata 14.0 (34) were used to examine the within-individual association between changes in weekly marijuana use and psychotic symptoms from age 13–18. Because these models focus exclusively on modeling within-individual change, all time-stable factors that may vary between individuals are ruled out as potential confounds (24). Poisson (total subclinical symptoms) and logistic (binary symptom subtypes) fixed effects regression models were used. Incidence rate ratios (IRR) are reported for total symptoms and odds ratios (OR) are reported for symptom subtypes. A series of three models were run for each outcome (For formulas, see Supplemental Table 1S). The base model examined the effects of concurrent weekly marijuana use and prior years of weekly marijuana use on subclinical psychotic symptoms, after controlling for age-related changes in subclinical psychotic symptoms. Next, all time-varying covariates were added to the model to control for potential confounds (i.e., psychotic symptoms at T-1, concurrent internalizing and externalizing problems, concurrent and prior use of tobacco, alcohol, and illicit drugs). In the final model, the number of years of prior marijuana use was subdivided into two orthogonal variables to delineate the impact of prior use on subsequent psychotic symptoms during years when adolescents reported no marijuana use versus years when they reported some use. This model tested whether the cumulative effect of prior marijuana use dissipated or remained significant during subsequent periods of year-long abstinence.
To examine the possibility of reverse causation, logistic fixed effects regressions examined whether changes in concurrent and prior subclinical psychotic symptoms predicted changes in weekly marijuana use. A variable indexing a cumulative history of subclinical psychotic symptoms was created by counting the number of prior years with 2 or more subclinical symptoms. The same covariates were included in these models, except that marijuana use at the preceding assessment (T-1) was included instead of prior psychotic symptoms, and only concurrent substance use variables were included.
Missing Data
Models were run using conditional maximum likelihood estimation, which uses all available information in a time series to generate model parameters rather than resorting to complete case analysis. The parameters are unbiased when data in a time series are missing at random (35), meaning the probability that the dependent variable Y is missing is not associated with the value of Y after controlling for all observed covariates (24).
Sample retention at the measurement occasions used in the present study ranged from 83%–99%. Of the original 1009 participants, 70.0% (N=702) had no missing data, 14.4% (N=145) were missing one year of data, 5.0% were missing two years (N=50), 4.8% were missing three years (N=48), 2.8% were missing four years (N=28), 1.6% were missing five years (N=16), and 2.0% were missing all six years (N=20). Compared to participants with complete data, individuals with missing data were more likely to be Black and to have higher levels of subclinical psychotic symptoms, internalizing problems, and externalizing problems. However, these variables were only weakly associated with missingness (rs from .07–.19), suggesting that any deviation from the missing at random assumption was likely minor.
Results
Descriptive statistics
Table 1 contains descriptive statistics for substance use and subclinical psychotic symptoms by age. Between ages 13–18, the prevalence of psychotic symptoms gradually declined. By the last assessment, 695 participants had reported at least one subclinical psychotic symptom, 391 had reported paranoia, 231 had reported hallucinations, and 574 had reporting bizarre thinking.
Table 1.
Descriptive Statistics for Substance Use and Subclinical Psychotic Symptoms
| Age 13 | Age 14 | Age 15 | Age 16 | Age 17 | Age 18 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| Substance use frequency | ||||||||||||
| Days using marijuana | 3.8 | (27.9) | 10.8 | (47.9) | 16.9 | (62.1) | 27.0 | (81.1) | 33.5 | (89.0) | 51.8 | (110.8) |
| Days using alcohol | 4.6 | (24.7) | 10.5 | (38.3) | 13.8 | (41.9) | 20.7 | (56.8) | 31.4 | (70.0) | 49.6 | (91.6) |
| Days using tobacco | 24.3 | (83.7) | 50.3 | (118.5) | 73.3 | (137.8) | 89.6 | (149.8) | 107.3 | (159.6) | 135.5 | (170.2) |
| Days using other illicit drugs | 0.2 | (2.5) | 0.3 | (3.2) | 0.5 | (8.6) | 0.7 | (9.4) | 1.3 | (14.4) | 0.7 | (5.7) |
| % | N | % | N | % | N | % | N | % | N | % | N | |
| Weekly marijuana use | 1.7% | 15 | 5.0% | 47 | 7.8% | 71 | 10.6% | 93 | 12.9% | 112 | 20.3% | 177 |
| Weekly alcohol use | 1.9% | 17 | 5.0% | 47 | 7.4% | 67 | 10.6% | 93 | 17.1% | 148 | 24.4% | 213 |
| Daily tobacco use | 5.0% | 44 | 11.3% | 106 | 16.5% | 150 | 21.5% | 189 | 25.9% | 224 | 33.9% | 296 |
| Other illicit drug use | 2.0% | 18 | 3.4% | 32 | 3.7% | 34 | 4.9% | 43 | 5.5% | 48 | 6.1% | 53 |
| Subclinical psychotic symptoms | ||||||||||||
| Paranoia | 18.5% | 164 | 17.3% | 162 | 14.3% | 130 | 12.5% | 110 | 12.8% | 111 | 13.4% | 117 |
| Hallucinations | 8.9% | 79 | 9.4% | 88 | 7.9% | 72 | 6.2% | 55 | 4.7% | 41 | 4.2% | 37 |
| Bizarre thinking | 34.8% | 309 | 30.5% | 286 | 24.3% | 221 | 19.5% | 172 | 16.3% | 141 | 17.0% | 148 |
| Any symptom | 45.5% | 404 | 41.0% | 384 | 34.0% | 309 | 28.4% | 250 | 25.8% | 223 | 27.0% | 236 |
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| Total symptoms | 0.73 | (0.96) | 0.66 | (0.98) | 0.53 | (0.89) | 0.44 | (0.84) | 0.39 | (0.79) | 0.41 | (0.79) |
Note. M=Mean; SD=standard deviation. Original sample included 1,009 boys. Sample size for each age: N=888 (age 13); N=937 (age 14); N=908 (age 15); N=881 (age 16); N=866 (Age 17); N= 873 (age 18).
As expected, substance use increased from age 13–18. By the last assessment, 270 participants had used marijuana weekly, 325 had used alcohol weekly, 377 had used tobacco daily, and 134 had used other illicit drugs at least once. The average age of onset was 16.1 (SD=1.6) for weekly marijuana use, 16.3 (SD=1.6) for weekly alcohol use, 15.6 (SD=1.7) for daily tobacco use, and 15.7 (SD=1.7) for any other illicit drug use.
Predicting changes in psychotic symptoms
The primary results for total subclinical psychotic symptoms and symptom subtypes are presented in Tables 2 and 3, respectively. Sample sizes for these analyses vary because participants with no variation in the dependent variable (i.e., no within-individual change) are excluded by default from Poisson and logistic fixed effects models (24). Findings related to significant model covariates are included in Supplemental Table 2S.
Table 2.
Changes in Current and Cumulative Weekly Marijuana Use Influencing Fluctuations in Subclinical Psychotic Symptoms from Ages 13 to 18
| Total subclinical psychotic symptoms | ||||
|---|---|---|---|---|
| Without covariates | With covariates | |||
| IRR | [95% C.I.] | IRR | [95% C.I.] | |
| Current weekly marijuana use | 1.37*** | [1.16, 1.62] | 1.12 | [0.93, 1.35] |
| Years of prior weekly use | ||||
| 0 years | --- | --- | ||
| 1 year | 1.20 | [0.97, 1.48] | 1.15 | [0.91, 1.46] |
| 2+ years | 1.45* | [1.09, 1.93] | 1.51* | [1.08, 2.11] |
| Test of linear trend | 1.20** | [1.05, 1.38] | 1.21* | [1.03, 1.42] |
Note. IRR=Incidence rate ratio. Sample size is 657 because participants who experienced no within-person change in subclinical symptoms are dropped from the analysis by default. Total number of observations = 3,545. Age and age2 are included as predictors in all models. Covariates were psychotic symptoms at T-1, concurrent internalizing and externalizing problems, concurrent and cumulative years of weekly alcohol use, daily tobacco use, and any other illicit drug use. Contrasts for prior weekly use represent the predicted change in adolescents’ subclinical psychotic symptoms following 1 year and 2+ years of weekly use relative to years preceding the initiation of weekly use, after controlling for other model covariates. For linear trend analysis, the number of years of prior use was treated as a continuous predictor.
p<.05;
p<.01;
p<.001
Table 3.
Changes in Current and Cumulative Weekly Marijuana Use Influencing Fluctuations in Psychotic Symptom Subtypes from Ages 13 to 18
| Without covariates | With covariates | |||
|---|---|---|---|---|
| OR | [95% C.I.] | OR | [95% C.I.] | |
| Paranoia (N = 343)a | ||||
| Current weekly marijuana use | 2.46*** | [1.69, 3.58] | 2.04** | [1.29, 3.23] |
| Years of prior weekly use | ||||
| 0 years | --- | --- | --- | --- |
| 1 year | 1.93** | [1.20, 3.08] | 2.75** | [1.53, 4.94] |
| 2+ years | 2.23* | [1.19, 4.20] | 4.96*** | [2.14, 11.50] |
| Test of linear trend | 1.57** | [1.16, 2.12] | 2.33*** | [1.55, 3.49] |
| Hallucinations (N = 218)a | ||||
| Current weekly marijuana use | 1.49 | [0.85, 2.62] | 0.94 | [0.49, 1.82] |
| Years of prior weekly use | ||||
| 0 years | --- | --- | --- | --- |
| 1 year | 1.88† | [0.92, 3.84] | 1.94 | [0.87, 4.35] |
| 2+ years | 2.60† | [0.99, 6.77] | 3.64* | [1.07, 12.38] |
| Test of linear trend | 1.67* | [1.06, 2.62] | 1.92* | [1.09, 3.38] |
| Bizarre thinking (N = 511)a | ||||
| Current weekly marijuana use | 1.34 | [0.93, 1.93] | 0.90 | [0.59, 1.39] |
| Years of prior weekly use | ||||
| 0 years | --- | --- | --- | --- |
| 1 year | 0.98 | [0.61, 1.59] | 0.88 | [0.50, 1.53] |
| 2+ years | 1.34 | [0.70, 2.60] | 1.41 | [0.63, 3.16] |
| Test of linear trend | 1.11 | [0.82, 1.52] | 1.10 | [0.75, 1.62] |
Note. OR=odds ratio. Total number of observations = 1,908 (paranoia), 1,175 (hallucinations), 2,796 (bizarre behavior). Age and age2 are included as predictors in all models. Covariates were psychotic symptoms at T-1, concurrent internalizing and externalizing problems, concurrent and cumulative years of weekly alcohol use, daily tobacco use, and other illicit drug use. Contrasts for prior weekly use represent the predicted change in adolescents’ subclinical psychotic symptoms following 1 year and 2+ years of weekly use relative to years preceding the initiation of weekly use, after controlling for other model covariates. For linear trend analysis, the number of years of prior use was treated as a continuous predictor.
Sample sizes vary because participants who experience no within-person change in the dependent variable are dropped from the analysis by default.
p<.09;
p<.05;
p<.01;
p<.001
Changes in concurrent weekly marijuana use and the number of prior years of weekly use were both significantly associated with increases in total subclinical psychotic symptoms before covariate adjustment (Table 2). However, only the cumulative effect of prior weekly marijuana use remained significant after controlling for time-varying confounds. In the model with covariates, linear trend analysis indicated that for each additional year adolescents engaged in weekly marijuana use, their expected number of subsequent psychotic symptoms rose by 21%.
Models examining specific symptom subtypes indicated concurrent weekly use and the number of prior years of weekly marijuana use were both significantly associated with paranoia, before and after controlling for time-varying covariates (Table 3). Linear trend analysis in the model with covariates indicated that for each additional year adolescents engaged in weekly marijuana use, their predicted odds of experiencing subsequent paranoia rose by 133%. Similarly, the number of prior years adolescents engaged in weekly marijuana use was significantly associated with subclinical hallucinations, although the effect of concurrent weekly was not significant (Table 3). Linear trend analysis in the model with covariates indicated that for each additional year adolescents engaged in weekly marijuana use, their expected odds of experiencing future hallucinations rose by 92%. Concurrent and prior marijuana use were not associated with changes in bizarre thinking (Table 3).
Cumulative Effect of Weekly Marijuana Use Following Abstinence
Results indicated that the linear effect of the number of prior years of weekly marijuana use on total subclinical psychotic symptoms, paranoia, and hallucinations persisted even when adolescents stopped using marijuana for a year (see Table 4 and Figure 1). Results from the model with covariates indicated that for each additional year adolescents engaged in weekly marijuana use, their expected total subclinical psychotic symptoms rose by 29% during subsequent periods of yearlong abstinence, and their expected odds of experiencing paranoia and hallucinations rose by 112% and 158%, respectively.
Table 4.
Effect of Prior Weekly Marijuana Use on Psychotic Symptoms During Subsequent Years of Abstinence Versus Continued Use
| Past year marijuana use | ||||
|---|---|---|---|---|
| No use | Some use | |||
| IRR or ORa | [95% C.I.] | IRR or ORa | [95% C.I.] | |
| Total symptoms (N = 657)b | ||||
| Years of prior weekly use | 1.29* | [1.00, 1.66] | 1.19* | [1.00, 1.41] |
| Paranoia (N = 343)b | ||||
| Years of prior weekly use | 2.12* | [1.16, 3.89] | 2.41*** | [1.55, 3.74] |
| Hallucinations (N = 218)b | ||||
| Years of prior weekly use | 2.58* | [1.07, 6.20] | 1.73 | [0.93, 3.22] |
| Bizarre thinking (N = 511)b | ||||
| Years of prior weekly use | 1.16 | [0.64, 2.09] | 1.08 | [0.71, 1.64] |
Note. Total number of observations = 1,908 (paranoia), 1,175 (hallucinations), 2,796 (bizarre behavior).
Incidence rate ratio (IRR) is reported for total symptoms (fixed effects Poisson regression) and odds ratios (OR) are reported for symptom subtypes (fixed effects binary logistic regressions). Effects represent the linear association between the number of years of prior weekly marijuana use and changes in psychotic symptoms for subsequent years when adolescents reported no marijuana use versus years when they used at least once. Effects are after controlling for age, age2, psychotic symptoms at T-1, concurrent internalizing and externalizing problems, and concurrent and cumulative years of weekly alcohol use, daily tobacco use, and other illicit drug use.
Sample sizes vary because participants who experience no within-person change in the dependent variable are dropped from the analysis by default.
p<.05;
p<.001
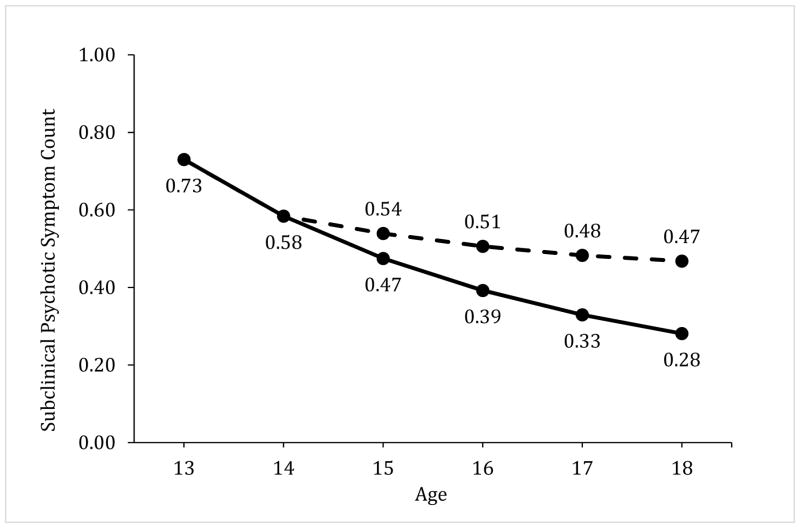
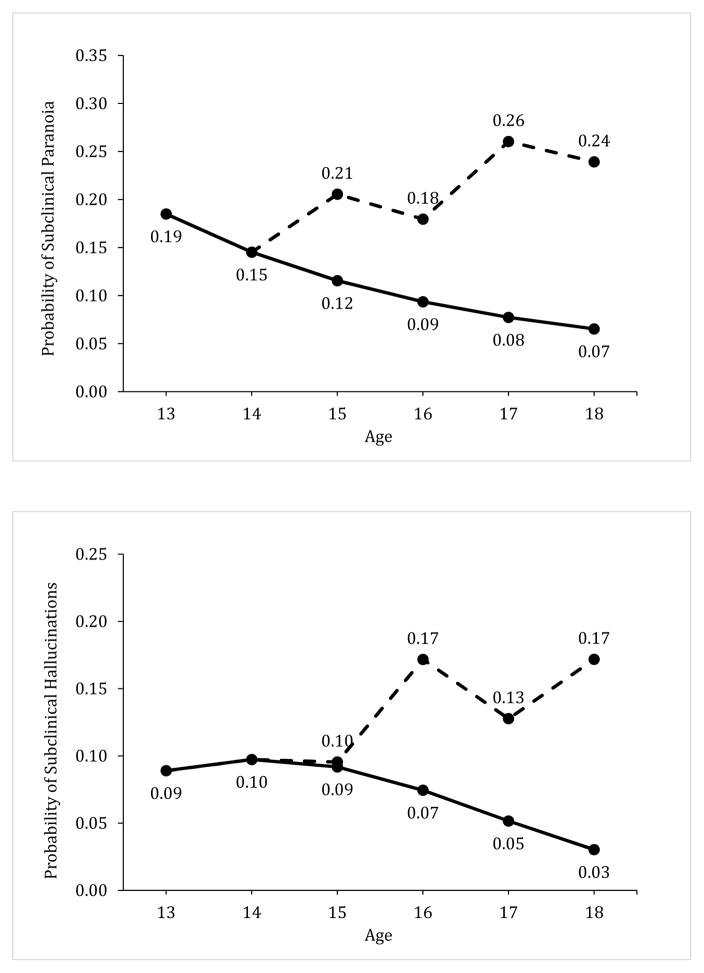
Figure 1.
Illustration of the model-predicted association between regular adolescent marijuana use and changes in subclinical psychotic symptoms. Graphs show the predicted count of subclinical psychotic symptoms (top) and the predicted probability of subclinical paranoia (middle) and hallucinations (bottom) across adolescence. The solid line represents the predicted levels of these outcomes if an adolescent never engaged in weekly marijuana use and the dashed line indicates the predicted outcomes for the same adolescent if he used marijuana weekly at ages 15 and 17, but abstained from marijuana use during the past year at ages 16 and 18. The sample mean for total subclinical psychotic symptoms (.73) and the sample prevalence of paranoia (18.5%) and hallucinations (8.9%) at age 13 were used as baselines for illustration purposes. Predicted values assume all other model covariates remained invariant over time. The age-related patterns for the outcomes show some differences in relation to the timing of weekly marijuana use and abstinence; however, each graph illustrates that adolescents are more likely to exhibit a chronic pattern of elevated subclinical psychotic symptoms when they engage in weekly marijuana use, and this higher level of symptoms persists despite year-long periods of abstinence.
Potential reverse causation
Models examining whether changes in current and prior psychotic symptoms predicted changes in weekly marijuana use are presented in Supplemental Table 3S. Controlling for time-varying covariates, changes in prior and current psychotic symptoms did not predict increases in weekly marijuana use. Instead, there was some indication that prior psychotic symptoms were associated with a reduced likelihood of engaging in weekly marijuana use.
Discussion
This study found evidence suggesting that regular marijuana use may increase an adolescent’s risk of experiencing persistent subclinical psychotic symptoms. This association remained significant after controlling for all stable between-individual factors and within-individual changes in current marijuana use, current and prior use of tobacco, alcohol and other illicit drugs, and internalizing and externalizing problems. Importantly, the effect of prior weekly marijuana use on subclinical psychotic symptoms did not dissipate when adolescents remained abstinent for a year. Moreover, no support was found for the self-medication hypothesis; adolescents were not more likely to engage in regular marijuana use following an increase in their psychotic symptoms. The cumulative effect of regular marijuana was most pronounced for subclinical symptoms of paranoia and hallucinations.
Transient Versus Sustained Effect of Regular Marijuana Use
Consistent with prior studies, the prevalence of subclinical psychotic symptoms tended to decrease from early to late adolescence (36–39). Despite this normative developmental decline, our findings indicate that regular marijuana use increases the likelihood that teens will experience persistent subclinical psychotic symptoms across adolescence, particularly paranoia. These findings build upon experimental work showing that acute administration of THC can cause paranoia among healthy adult volunteers in laboratory settings (10). Although feelings of paranoia typically subside within 24 hours in experimental studies, our findings suggest that as adolescents regularly use marijuana over multiple years, their odds of experiencing chronic paranoia increases. More concerning, evidence suggests that this effect persists even when adolescents abstain from using marijuana for one year. A cumulative history of regular marijuana use also increased adolescents’ risk of experiencing subclinical hallucinations, which also persisted during periods of year-long abstinence. This effect is particularly concerning as evidence suggests that adolescents who report chronic subclinical hallucinations are at heightened risk for developing psychotic disorders by young adulthood (31).
Concurrent and cumulative marijuana use were not associated with an increase in strange thoughts or behaviors. The prevalence of these symptoms was fairly high in the current sample, with approximately one-third of participants reporting some odd thinking or behavior during the early teenage years. This finding might reflect the fact that participants were asked to indicate whether “others” viewed their thoughts or behaviors as strange. During adolescence, many youth may feel misunderstood by parents and other authority figures as they begin establishing their autonomy. As a result, endorsement of these items may be more indicative of a normative individuation process than emergent psychopathology.
Accounting for Potential Confounds
An area of contentious debate is whether the well-established link between adolescent marijuana use and features of psychosis is due to pre-existing individual differences (19–23). By focusing on the association between changes in marijuana use and changes in subclinical psychotic symptoms within-individuals, the current study eliminated all pre-existing time-stable individual differences as potential confounds. In these models, for example, a genetic predisposition toward schizophrenia cannot directly explain why an adolescent’s psychotic symptoms fluctuate from year to year (within-individual change), although it may explain why some people are more likely than others to develop psychotic symptoms or are more susceptible to environmental risk factors (between-individual differences). Furthermore, by including a series of time-varying confounding variables in the analysis, the findings indicated that this association was not accounted for by recent and cumulative changes in other substance use. In fact, adolescents who used other substances did not experience increased subclinical psychotic symptoms over time. The unique linkage between regular marijuana use and increased subclinical psychotic symptoms was further exemplified by the fact that the significant associations remained after controlling for other forms of psychopathology.
Accounting for Potential Reverse Causation
Consistent with prior work, the present study also found no evidence supporting a possible reverse pathway model (14, 25). After controlling for several time-varying covariates, results indicated that adolescents were not more likely to engage in weekly marijuana use during years when their subclinical psychotic symptoms increased. Furthermore, no consistent evidence supported a systematic association between a cumulative history of psychotic symptoms and an increased risk for engaging in weekly marijuana use.
Limitations and Future Directions
The findings presented here must be considered in the context of several limitations. First, findings were based on a longitudinal sample of urban boys followed from age 13–18 in one geographical area. Future research should investigate whether these findings hold for girls and adults living in geographically diverse locales, and whether the effect of regular adolescent marijuana use on subclinical psychotic symptoms persists into adulthood.
It is also important to note that this study investigated subclinical symptoms using a self-reported rating scale. Although evidence suggests that adolescents who display subclinical symptoms are at a heightened risk for developing psychotic disorders later in life (31, 32, 36), these symptoms are transient for many youth and may reflect accurate appraisals of reality rather than cognitive distortions. Consistent with this notion, data collected on participants at follow-up assessments in adulthood indicated that only 2.1% of participants in the current study had developed a psychotic disorder by their late 20s/early 30s (Supplemental Table 5). Although adolescents who engaged in heavy marijuana use across multiple years and those who experienced chronic paranoia and hallucinations were at significantly greater risk for developing a psychotic disorder, this adverse outcome only occurred for a small proportion of these participants (Supplemental Table 5). It will be important for future studies with much larger samples to examine why only a subset of adolescents who engage in regular marijuana use develop chronic subclinical psychotic symptoms, why an even smaller portion develop psychotic disorders, and whether these linkages remained significant after controlling for potential confounding factors.
Another limitation is that only self-reported frequency of marijuana use was assessed in the present study. Other factors, such as THC potency and mode of administration, may affect the association between marijuana use and psychotic symptoms. Given that the data presented here were collected in the mid-1990s and early-2000s, and that the THC concentration in marijuana has increased in recent years (40), our analysis might underestimate the risks associated with regular use. Future research should investigate how potent marijuana concentrates that can be vaporized, inhaled, or eaten may affect concurrent and future psychotic symptoms. Future experimental research should also investigate whether reducing the proportion of THC (and increasing the proportion of cannabidiol) in medical and recreational marijuana may reduce the risk of experiencing subclinical psychotic symptoms.
Conclusion
This study demonstrates that adolescents are more likely to experience subclinical psychotic symptoms (particularly paranoia) during and after years of regular marijuana use. Perhaps the most concerning finding is that the effect of prior weekly marijuana use persists even after adolescents have stopped using for one year. For every additional year adolescents engage in regular marijuana use, their risk of exhibiting subclinical paranoia and hallucinations in future years increases in a linear manner, and the effect of cumulative use remains significant even during periods of abstinence lasting a year. Given the recent proliferation of marijuana legalization across the country, it will be important to enact preventative policies and programs to keep adolescents from engaging in regular marijuana use, as chronic use seems to increase their risk of developing persistent subclinical psychotic symptoms.
Supplementary Material
Table 5.
Number of Years of Weekly Marijuana Use and Subclinical Psychotic Symptoms from Age 13 to 18 Predicting the Emergence of a Psychotic Disorder by Young Adulthood (N=908)
| OR | [95% C.I.] | Lifetime Psychotic Disorder | ||||
|---|---|---|---|---|---|---|
| Yes (N=21) | No (N=887) | |||||
| N | % | N | % | |||
| Years of weekly marijuana use | ||||||
| 0 years | --- | --- | 14 | 2.1% | 649 | 97.9% |
| 1–2 years | 0.85 | [0.26, 2.78] | 3 | 1.6% | 183 | 98.4% |
| 3+ years | 3.63* | [1.22, 10.83] | 4 | 6.8% | 55 | 93.2% |
| Test of linear trend | 1.61 | [0.89, 2.93] | ||||
| Years with paranoia | ||||||
| 0 years | --- | --- | 6 | 1.1% | 546 | 98.9% |
| 1–2 years | 3.63* | [1.35, 9.75] | 10 | 4.0% | 243 | 96.1% |
| 3+ years | 4.69** | [1.48, 14.91] | 5 | 4.9% | 98 | 95.2% |
| Test of linear trend | 2.21*** | [1.30, 3.76] | ||||
| Years with hallucinations | ||||||
| 0 years | --- | --- | 12 | 1.7% | 677 | 98.3% |
| 1–2 years | 1.67 | [0.60, 4.61] | 5 | 2.7% | 178 | 97.3% |
| 3+ years | 7.50*** | [2.42, 23.31] | 4 | 11.1% | 32 | 88.9% |
| Test of linear trend | 2.43*** | [1.34, 4.39] | ||||
| Years with bizarre thinking | ||||||
| 0 years | --- | --- | 5 | 1.3% | 380 | 98.7% |
| 1–2 years | 2.21 | [0.78, 6.27] | 10 | 3.0% | 328 | 97.0% |
| 3+ years | 2.51 | [0.79, 7.91] | 6 | 3.2% | 179 | 96.8% |
| Test of linear trend | 1.57 | [0.91, 2.71] | ||||
| Years with any symptom | ||||||
| 0 years | --- | --- | 2 | 0.7% | 270 | 99.3% |
| 1–2 years | 3.20 | [0.79, 13.00] | 9 | 2.7% | 321 | 97.3% |
| 3–4 years | 2.74 | [0.61, 12.33] | 5 | 2.3% | 217 | 97.8% |
| 4+ years | 7.49** | [1.64, 34.08] | 5 | 6.0% | 79 | 94.1% |
| Test of linear trend | 1.69* | [1.09, 2.62] | ||||
Note. OR=odds ratio. Significance tests were calculated using Firth’s penalized likelihood estimation method for examining rare events. The contrast group was participants who had 0 years on each predictor. Lifetime prevalence of a psychotic disorder (i.e., schizophrenia, schizophreniform disorder, schizoaffective disorder, mood disorder with psychotic features, delusional disorder) was assessed using the Diagnostic Interview Schedule, which was administered on three occasions in the youngest cohort (mean ages = 20, 26, and 29) and twice in the oldest cohort (mean ages =26 and 36). Participants in both cohorts were also asked whether they were currently seeking treatment for schizophrenia at the last assessment. Participants were classified as having a lifetime psychotic disorder if they met diagnostic criteria on the DIS interview or reported receiving treatment for schizophrenia. Participants were included in the analysis if they completed at least one assessment from age 13 to 18 and at least one diagnostic interview in adulthood. Re-analyses limited to the 571 participants who completed all assessments produced comparable results and are available upon request.
p<.05;
p<.01;
p<.005
Acknowledgments
Manuscript preparation was supported by a grant award to Dr. Dustin Pardini from the National Institute on Drug Abuse [DA034608]. Data collection was supported by grants awarded to Dr. Rolf Loeber from the National Institute on Drug Abuse [DA411018], National Institute on Mental Health [MH 48890, MH 50778], Pew Charitable Trusts, Office of Juvenile Justice and Delinquency Prevention [96-MU-FX-0012], and the Pennsylvania Department of Health [SAP 4100043365].
Footnotes
Disclosures. All authors report no competing interests.
References
- 1.Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35(8):1779–87. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: Systematic review. J Psychopharmacol (Oxf) 2005;19(2):187–94. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson ST, Radhakrishnan R, D’Souza DC. Impact of cannabis use on the development of psychotic disorders. Current Addiction Reports. 2014;1(2):115–28. doi: 10.1007/s40429-014-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. The Lancet. 2007;370(9584):319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 5.Kuepper R, van Os J, Lieb R, Wittchen H-U, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011:342. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers D, Ghodse A. Cannabis and psychotic illness. The British Journal of Psychiatry. 1992;161(5):648–53. doi: 10.1192/bjp.161.5.648. [DOI] [PubMed] [Google Scholar]
- 7.Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: Results of a clinical survey. Psychol Med. 1986;16(03):515–20. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot–A review of the association between cannabis and psychosis. Frontiers in Psychiatry. 2014:5. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thonicroft G. Cannabis and psychosis. Br J Psychiatry. 1990;157:25–33. doi: 10.1192/bjp.157.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Freeman D, Dunn G, Murray RM, Evans N, Lister R, Antley A, et al. How cannabis causes paranoia: using the intravenous administration of Δ9-tetrahydrocannabinol (THC) to identify key cognitive mechanisms leading to paranoia. Schizophrenia Bull. 2014:1–9. doi: 10.1093/schbul/sbu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardini D, White HR, Xiong S, Bechtold J, Chung T, Loeber R, et al. Unfazed or dazed and confused: does early adolescent marijuana use cause sustained impairments in attention and academic functioning? J Abnorm Child Psychol. 2015:1–15. doi: 10.1007/s10802-015-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: Longitudinal prospective study. Br Med J. 2002;325(7374):1212–3. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969:Historical cohort study. Bmj. 2002;325(7374):1–5. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen H-U, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj. 2004;330(7481):1–5. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42(06):1321–8. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 16.Andréasson S, Allebeck P, Rydberg U. Schizophrenia in users and nonusers of cannabis. Acta Psychiatr Scand. 1989;79(5):505–10. doi: 10.1111/j.1600-0447.1989.tb10296.x. [DOI] [PubMed] [Google Scholar]
- 17.Andréasson S, Engström A, Allebeck P, Rydberg U. Cannabis and schizophrenia A longitudinal study of swedish conscripts. The Lancet. 1987;330(8574):1483–6. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 18.Van Os J, Bak M, Hanssen M, Bijl R, De Graaf R, Verdoux H. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol. 2002;156(4):319–27. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- 19.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips P, Johnson S. How does drug and alcohol misuse develop among people with psychotic illness? A literature review. Soc Psychiatry Psychiatr Epidemiol. 2001;36(6):269–76. doi: 10.1007/s001270170044. [DOI] [PubMed] [Google Scholar]
- 21.Phillips LJ, Curry C, Yung AR, Yuen HP, Adlard S, McGorry PD. Cannabis use is not associated with the development of psychosis in an ‘ultra’high risk group. Aust N Z J Psychiatry. 2002;36(6):800–6. doi: 10.1046/j.1440-1614.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsuang MT, Simpson JC, Kronfol Z. Subtypes of drug abuse with psychosis: demographic characteristics, clinical features, and family history. Arch Gen Psychiatry. 1982;39(2):141–7. doi: 10.1001/archpsyc.1982.04290020013003. [DOI] [PubMed] [Google Scholar]
- 23.Sevy S, Robinson DG, Napolitano B, Patel RC, Gunduz-Bruce H, Miller R, et al. Are cannabis use disorders associated with an earlier age at onset of psychosis? A study in first episode schizophrenia. Schizophr Res. 2010;120(1):101–7. doi: 10.1016/j.schres.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison PD. In: Fixed effects regression models. Liao TF, editor. Thousand Oaks, CA: Sage Publications; 2009. [Google Scholar]
- 25.Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction. 2005;100(3):354–66. doi: 10.1111/j.1360-0443.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- 26.Griffith Lendering MF, Wigman JT, Prince van Leeuwen A, Huijbregts SC, Huizink AC, Ormel J, et al. Cannabis use and vulnerability for psychosis in early adolescence—a TRAILS study. Addiction. 2013;108:733–40. doi: 10.1111/add.12050. [DOI] [PubMed] [Google Scholar]
- 27.Loeber R, Farrington DP, Stouthamer-Loeber M, White HR. Violence and serious theft: Development and prediction from childhood to adulthood. New York, NY: Routledge; 2008. [Google Scholar]
- 28.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109(40):E2657–E64. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial behavior and mental health problems: Explanatory factors in childhood and adolescence. New York, NY: Routledge; 1998. [Google Scholar]
- 30.Achenbach TM. Integrative guide for the 1991 CBCL/4–18, YSR, and TRF profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 31.Welham J, Scott J, Williams G, Najman J, Bor W, O’Callaghan M, et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: A 21-year birth cohort study. Psychol Med. 2009;39(04):625–34. doi: 10.1017/S0033291708003760. [DOI] [PubMed] [Google Scholar]
- 32.Kaymaz N, Drukker M, Lieb R, Wittchen H-U, Werbeloff N, Weiser M, et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42(11):2239–53. doi: 10.1017/S0033291711002911. [DOI] [PubMed] [Google Scholar]
- 33.Ivanova MY, Achenbach TM, Rescorla LA, Dumenci L, Almqvist F, Bilenberg N, et al. The generalizability of the Youth Self-Report syndrome structure in 23 societies. J Consult Clin Psychol. 2007;75(5):729. doi: 10.1037/0022-006X.75.5.729. [DOI] [PubMed] [Google Scholar]
- 34.StataCorp. Stata statistical software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 35.Gardiner JC, Luo Z, Roman LA. Fixed effects, random effects and GEE: What are the differences? Stat Med. 2009;28(2):221–39. doi: 10.1002/sim.3478. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez M, Wichers M, Lieb R, Wittchen H-U, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophrenia Bull. 2011;37(1):84–93. doi: 10.1093/schbul/sbp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelleher I, Keeley H, Corcoran P, Lynch F, Fitzpatrick C, Devlin N, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: Evidence from four population-based studies. The British Journal of Psychiatry. 2012;201(1):26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- 38.De Loore E, Gunther N, Drukker M, Feron F, Sabbe B, Deboutte D, et al. Persistence and outcome of auditory hallucinations in adolescence: a longitudinal general population study of 1800 individuals. Schizophr Res. 2011;127(1):252–6. doi: 10.1016/j.schres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Mackie C, Castellanos-Ryan N, Conrod P. Developmental trajectories of psychotic-like experiences across adolescence: impact of victimization and substance use. Psychol Med. 2011;41(01):47–58. doi: 10.1017/S0033291710000449. [DOI] [PubMed] [Google Scholar]
- 40.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency Trends of Δ9 THC and Other Cannabinoids in Confiscated Cannabis Preparations from 1993 to 2008*. J Forensic Sci. 2010;55(5):1209–17. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.