Abstract
Streptococcus pneumoniae is a human pathogen responsible for the majority of childhood pneumonia and media otitis cases worldwide. The diversity of its capsular polysaccharides (CPS) results in more than 91 serotypes of which at least 23 are virulent. Various CPS conjugated to immunogenic carrier proteins are currently licensed and provide protection against the infection caused by the respective serotypes but not against new and emerging virulent serotypes. In this study, we considered the conserved protein antigen PsaA, the pneumococcal surface adhesin A, in order to overcome the limitations of CPS antigens. The PsaA was translationally fused to a polyhydroxybutyrate (PHB) synthase which mediated production of PsaA displayed on PHB inclusions in recombinant Escherichia coli. This suggested that the PsaA fusion to the PHB synthase did not interfere with PHB synthase activity and its ability to mediate formation of nano-sized inclusions composed of a PHB core surrounded by the PHB synthase fused to PsaA. Isolated PHB beads showed a negative surface charge. Transmission electron microscopy analysis suggested that the PsaA fusion to the PHB synthase reduced the size of PHB beads from about 500 nm to 100 nm. The integrity and antigenicity of the fusion protein attached to isolated PHB beads was confirmed by SDS-PAGE, tryptic peptide fingerprinting analysis using MALDI-TOF-MS/MS and immunoblotting using a monoclonal anti-PsaA antibody. Mice immunized with PsaA displaying PHB beads produced high and specific IgG levels dominated by IgG1 isotype. While IgG1 titer were similar between soluble and insoluble PsaA, the IgG2 titers were strongly increased upon vaccination with insoluble PsaA i.e. PsaA displayed on PHB beads. Particulate PsaA-PHB beads elicited IgG antibodies recognizing PsaA in whole cell lysates of seven different serotypes of S. pneumoniae. This study suggested that PHB beads are suitable carriers for PsaA in order to induce a significant and specific Th-2-type immune response.
Keywords: Immunology, Vaccines, Biochemistry, Pharmaceutical science
1. Introduction
Streptococcus pneumoniae is considered as most important pathogen causing severe pneumonia, meningitis and middle ear inflammation [1]. The cell surface of S. pneumoniae contains various proteins and capsular polysacharides (CPS) which play an important role in pathogenicity such as attachment and immune system evasion. The diversity of CPS contributes to more than 91 serotypes, while cell surface proteins were found to be more conserved and less variable [2]. Hence, S. pneumoniae cell surface proteins are increasingly considered as vaccine candidate antigens.
All licensed vaccines are conjugated CPS based and induce a specific serotype dependent protective immune response profile i.e. a strong and specific Th2-type response [3]. However, the emergence of new invasive serotypes not covered by existing vaccines demands attention.
Subunit vaccines based on conserved proteins/epitopes could induce serotype independent and more broadly protective immunity [3]. Due to their relevance for pathogenicity, cell surface proteins such as pneumococcal surface protein A (PspA), pneumococcal surface antigen A (PsaA) and pneumolysin (Ply) are currently being considered for vaccine development [4, 5, 6]. As subunit vaccines are often less immunogenic, adjuvant and/or immunogenic delivery systems are needed. Recently, polyhydroxybutyrate (PHB) beads (< 1 μm) displaying specific antigens had been demonstrated as effective antigen delivery system in the context of the intracellular pathogens [7, 8]. PHB is a polyester naturally produced by various bacteria [9]. Introducing PHB biosynthesis genes into heterologous expression hosts, allows the intracellular formation of discrete and spherical PHB inclusions [10]. This also resulted in PHB inclusions densely coated with proteins of interest [11, 12, 13, 14, 15, 16]. Translational fusion of proteins of interest to PHB synthase, PhaC, retained its PHB bead forming activity displaying the protein of interest at the PHB bead surface [13, 17]. PHB beads were bioengineered to display antigens from intracellular pathogens like Mycobacterium tuberculosis and Hepatitis C virus. These particulate vaccine candidates elicited both Th1 and Th2 antigen specific immune responses resulting in protective immunity [8, 18, 19]. In this study it was conceived to engineer PHB beads displaying PsaA as possible antigen delivery system to develop a particulate vaccine against the extracellular pathogen S. pneumoniae.
2. Materials and methods
2.1. Bacterial strains, oligonucleotides, plasmids and cultivation condition
Bacterial strains, plasmids and primers used in this study are listed in Table 1. E. coli XL 1 blue was grown at 37 °C in Luria Bertani (LB) in presence of ampicillin (100 μg/mL). PHB beads and recombinant soluble protein was produced in recombinant ClearColi [20]. ClearColi was grown in LB Miller media supplemented with glucose 1% (w/v), ampicillin (100 μg/mL). Chloramphenicol (50 μg/mL) was only added to media used for PHB bead production.
Table 1.
Description of bacterial strains, plasmids and oligonucleotides used in this study.
| Strains, plasmid and primers | Characteristics | Review |
|---|---|---|
| Strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| ClearColi | F– ompT hsdSB (rB − mB −) gal dcm lon λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) msbA148 ΔgutQ ΔkdsD ΔlpxL ΔlpxM ΔpagP ΔlpxP ΔeptA (LPS negative) | [18] |
| Plasmids | ||
| pET-14b | ApR and T7 promoter | Novagen |
| pET-14b-phaC | pET-14b version, holding phaC gene fragment | [21] |
| pUC57-psaA | pUC57 version, ColE1 origin, ApR holding NdeI/psaA gene/SpeI. | GenScript |
| pET-14b-psaA-phaC | pET-14b-phaC version, holding psaA gene fused to 3′ end of phaC gene | This study |
| pMCS69 | CmR; T7 promoter, pBBR1MCS derivative containing phaA and phaB genes from Ralstonia eutropha co-downstream to lac promoter | [22] |
| pET14b_NanA_PhaC (reversed) | ApR and T7 promoter, containing nanA gene cloned to 3′ end of phaC gene | [23] |
| pET14b- his6-psaA | ApR and T7 promoter, containing the his6-psaA gene inserted into the NdeI//BamHI sites of pET14b. | This study |
| Primers | ||
| psaA fwr (NdeI, underline restriction site) | 5′AAACATATGCACCACCACCACCACCACTCGGGCAAAAAGGATACGACCTCGG3′ | This study |
| psaA rev (BamHI, underline restriction site) | 5′ AAAGGATCCTCATTTTGCCAGACCTTCAGCGATTTTGTCCAG3′ | This study |
2.2. Construction of plasmids mediating production of PHB beads displaying PsaA
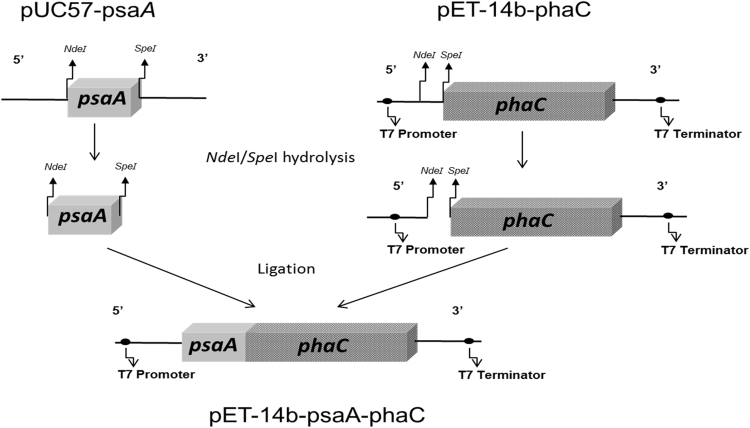
The gene encoding PsaA (amino acids 22–309) was synthetized by Genscript Corporation (USA) employing codon optimization for E. coli [24]. This hybrid gene encoding a translational fusion of PsaA with PhaC was constructed and cloned into plasmid pET-14b as outlined in Fig. 1.
Fig. 1.
Schematic presentation of the construction of plasmid pET-14b-psaA-phaC encoding the PsaA-PhaC fusion protein for formation of PHB beads in recombinant ClearColi.
2.3. Construction of the plasmid encoding N-terminally His-tagged PsaA for production of soluble PsaA
The gene encoding PsaA was amplified from pUC57-psaA by PCR using the corresponding primers psaA-fwr and psaA-rev. This introduced 6 histidine residues into the N terminus of PsaA in order to enable purification by immobilized Ni2+ affinity chromatography. The gene encoding His6-PsaA was inserted into pET-14b_NanA_PhaC using hydrolysis with NdeI and BamHI to replace nanA.
2.4. Production, isolation and purification of PHB beads
ClearColi harboring pMCS69 was transformed with pET-14b-psaA-phaC (encoding PsaA-PhaC fusion protein for production of PsaA displaying PHB beads) and pET14b-PhaC (PhaC wildtype control for production of PHB beads). Cells were cultivated and subjected to mechanical cell disruption. Beads were isolated and sterilized as previously described [25, 26].
2.5. Production, isolation and purification of recombinant soluble protein
ClearColi was transformed with pET-14b-his6-psaA (encoding His6-PsaA). Cells were cultivated and lysed for purification of His6-PsaA using the Ni-NTA Fast Start Kit (Qiagen, Germany).
2.6. Confirmation of the PhaC in vivo activity using transmission electron microscopy (TEM)
Cells harboring plasmid pET-14b-psaA-phaC and pET-14b-phaC, respectively, were analysed by TEM as described previously [20] to demonstrate the presence of PHB inclusions inside cells which is indicative of in vivo functionality of PhaC and its fusion protein variants.
2.7. Protein analysis
PsaA-PhaC and PhaC beads as well as soluble His6-PsaA were analysed by SDS-PAGE as previously described [27]. Immunoblot analysis was conducted as perviously described [28]. A monoclonal anti-PsaA antibody (Steroid & Immunobiochemistry Laboratory, Canterbury Health Laboratories, Christchurch, New Zealand) was used to identify the PsaA. All images were obteined using the GEL-DOC 2000 (Bio-Rad Laboratories, USA) and analysed using Image Lab Software (Version 3.0 build 11, Bio-Rad Laboratories, USA). Proteins were further identified by MALDI-TOF/MS. To confirm the PHB bead surface display and identity of PsaA, ELISA using goat anti-mouse IgG peroxidase conjugate (Sigma-Aldrich, St. Louis, MO) as secondary antibody as well as CLSM (confocal laser scanning microscopy) using a fluorescently (Alexa fluor 488) labelled goat anti-mouse antibody (Sigma-Aldrich, St. Louis, MO) as secondary antobody were employed as previously described [29].
2.8. Mesurement of the PHA bead size distribution and zeta potential
Size distribution of the particles and the zeta potential were mesured using the Mastersizer 3000 particle sizer (Malven instrument, United Kingdon) and the Zetasizer Nano ZS (Malven instrument, United Kingdon), respectively. Samples were prepared as 0.1% (w/v) of the wet PHB beads in saline solution. The pH values were adjusted with HCl.
2.9. Analysis of immunological properties of PHB beads
2.9.1. Immunization schedule
Four groups were prepared containing each 6 animals between 5–6 weeks old (male, Balb/c mice). Mice were acquired from CENPALAB (Centro Nacional para la Producción de Animales de Laboratorio, La Habana, Cuba). Three doses of 4 μg of antigen plus 100 μg of alum salt (ALHYDROGEL, Brenntang Biosector, Denmark) were subcutaneously administrated to each animal. Animals of the placebo group received only alum salt. Immunizations occurred at 0, 14 and 21 d. Blood samples were collected from the retro-orbital plexus at days 0, 14, 21 and 28 d after vaccination. Group 1 (G1) received 4 μg of PsaA displayed on PHBs, Group 2 (G2) received 4 μg of His6-PsaA antigen as pure soluble protein, Group 3 (G3) received 4 μg of PhaC wild type PHB beads. Group 4 received only alum salts.
2.9.2. Assessment of anti-PsaA antibody titers in mice
Anti-PsaA and anti-PhaC IgG levels were measured using an indirect ELISA. Maxisorp 96-well plates (NUNC) were coated using 0.5 μg/ml of His6-PsaA and incubation overnight at 4 °C. The plates were then washed with PBS containing 0.05% (v/v) Tween 20 (PBS-T) and blocked for 30 min at 37 °C with PBS containing 1% (w/v) BSA. After three washes with PBS-T, plates were incubated with dilutions of serum samples from individual mice in PBS-T containing 1% (w/v) BSA and 12.6 mM of EDTA (PBS-TB), for 60 min at room temperature (RT). The initial dilution factor (DF) of all samples was 1600. After three washes with PBS-T, plates were incubated for 60 min with the secondary antibody, the goat anti-mouse IgG peroxidase conjugate (Sigma-Aldrich, St. Louis, MO) diluted 1:10,000 in PBS-TB. Plates were developed using the peroxidase substrate as described elsewhere [30]. A serum was considered as positive if the initial absorbance at a wavelength of 570 nm was equal or greater twofold of the pre-immune serum value. Results were displayed as the reciprocal antibody titres, representing the DF required to obtain half of the maximum level of absorbance.
2.9.3. Analysis of the isotype IgG profile in sera
The isotype IgG profile against PsaA was analysed by using an indirect ELISA as described above. Pools of sera from all animals at time 28 d from G1 and G2 were prepared and diluted 1:1600. To detect the various IgG isotypes, plates were incubated with the respective anti-IgG1, IgG2a, IgG2b and IgG3 antibodies (derived from goat) (Sigma-Aldrich, St. Louis, MO) using a DF of 1:2500. Plates were developed using the anti-goat IgG peroxidase conjugate and the respective substrate as described above. The results from duplicate experiments are represented as mean values plus standard deviation (Mean ± STD).
2.9.4. Serotype coverage of induced anti-PsaA antibodies
The specificity of sera from animals immunized with PsaA-PhaC PHB beads for PsaA in whole cell lysates of pathogenic S. pneumoniae serotypes 1, 3, 5, 6b, 7F, 14 and 23F was assessed by immunoblotting. Cell suspension were prepared in PBS (pH 7.0) and adjusted to an OD620 0.8–1. Then 300 μl of these suspensions were mixed with denaturing buffer (DF 2). Samples were incubated at 95 °C for 20 min. Twenty μl of soluble sample were separated by SDS-PAGE. For immunoblotting pooled sera from all animals of G1 (DF: 2000) and G2 (DF: 500) were used to assess whether induced antibodies would detect PsaA in various S. pneumoniae serotypes.
2.10. Statistical analyses
Graph Pad Prism 4.03 (San Diego, USA) software was used for statistical analysis of data. Results were reported as results of 6 animals and significant differences were calculated by the Kruskal–Wallis non-parametric test. When significant differences were found, the Dunn’s post-test was used, considering significant differences when P was less than 0.05.
3. Results
3.1. Construction of plasmids mediating production of the PsaA-PhaC fusion protein and His6-PsaA
The strategy for construction of plasmids encoding PsaA from S. pneumoniae translationally fused to PhaC from Ralstonia eutropha for production of particulate PsaA is presented in Fig. 1.
In this study, the amino acid sequence of PsaA, the manganese ABC transporter, a manganese-binding adhesion lipoprotein, was selected from S. pneumoniae CGSP14 (GenBank number: ACB90875.1). This sequence has 100% identity with orthologous proteins found in strains R6 and TIGR4, and 98–99% identity with the other orthologues of various S. pneumoniae strains deposited in NCBI database. The gene encoding PsaA without its predicted signal sequence (1–21 amino acid residues) was synthetized as codon–optimized for E. coli (GenScript, USA). This synthetic gene was used to construct pET-14b-psaA-phaC. The recombinant soluble version of PsaA was produced as N-terminally His6-tagged protein and purified Ni2+ affinity chromatography.
3.2. Production and characterization of PsaA displaying PHA beads
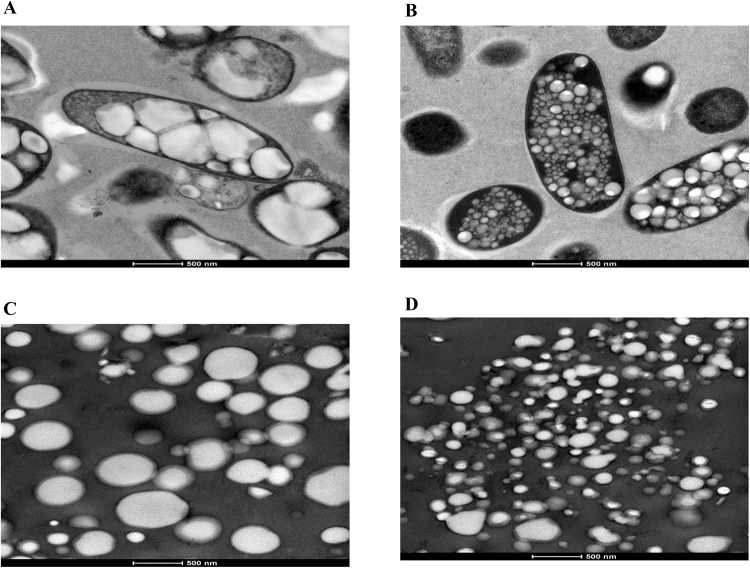
To produce PHB beads, ClearColi harbouring pMCS69 (Table 1) were transformed with either pET-14b-psaA-phaC or pET-14b-phaC (non-antigen displaying negative control). To confirm the formation of PHB inclusions indicative of PhaC functionality, cells and purified beads were observed by TEM (Fig. 2). Results showed that both plasmids mediated formation of distinct inclusions. However, PHB inclusions which formation was mediated by the PsaA-PhaC were smaller and more abundant when compared to inclusions formed by only PhaC (Fig. 2).
Fig. 2.
TEM analysis of recombinant Clearcoli cells (pMCS69) harboring various plasmids and respective isolated PHB beads. A, cells harboring pET-14b-phaC (PhaC wildtype PHB beads); B, cells harboring pET-14b-psaA-phaC (PsaA-PhaC PHB beads); C, PHB beads isolated from cells harboring pET-14b-phaC; D, PHB beads isolated from cells harboring pET-14b-psaA-phaC.
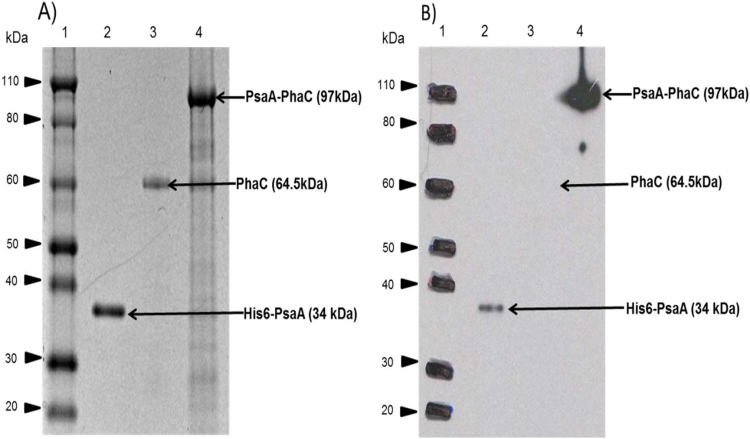
After isolation and purification, the protein profile of PHB beads and His6-PsaA was analysed by SDS-PAGE and immunoblotting (Fig. 3).
Fig. 3.
SDS-PAGE and immunoblot analysis of proteins attached to PHB beads and purified His6-PsaA. A, Comassie blue stained SDS-PAGE gel; B, immunoblot of (A) developed using monoclonal anti-PsaA antibodies. Lane 1, Molecular weight (MW) standard (Novex® Sharp Pre-Stained Protein Standard, Invitrogen); lane 2, purified His6-PsaA (MW: 34 kDa) derived from cells harboring pET14b-his6-psaA; lane 3, PHB beads isolated from cells harboring pET-14b-phaC encoding PhaC (MW: 64.5 kDa); lane 4, PHB beads isolated from cells harboring pET-14b-psaA-phaC encoding PsaA-PhaC fusion protein (MW: 97 kDa). The arrows indicate the protein band of interest with the corresponding theoretical MW.
In Fig. 3A dominating protein bands are observed which correspond to the theoretical molecular weights of the various proteins of interest such as His6-PsaA (34 kDa) (soluble antigen control, PhaC (MW: 66 kDa) (mediating non-antigen displaying PHB bead formation) and PsaA-PhaC fusion protein (MW: 97 kDa) (mediating PsaA displaying PHB bead formation). The identity of proteins was further confirmed by immunoblotting using a specific anti-PsaA antibody (Fig. 3B) and by tryptic peptide fingerprinting analysis using Maldi-TOF/MS (Table 2).
Table 2.
Tryptic peptide fingerprinting analysis (MALDI-TOF/MS).
| Protein | Peptides fragment identified by MALDI-TOF/MS. |
|---|---|
| PsaA-PhaC fusion protein | N28-K58,G114-K132,V228-K243,V228-K243,T244-K267,V311-R326,I353-R364,D368-K380,A381-R391,A381-R391,R391-R398,F392-R398,T399-R404,F405-R415,F436-R459,F436-R459,L460-R470,I485-R497,N498-K524,Y540-R553,H554-R568,N569-R589,N569-R589,D596-R625,I603-R625,E661-R676,G677-K703,L746-R768,E769-K788,F791-K808,A854-R863,A866-R873,A866-R873. |
The SDS-PAGE analysis also showed a strong enrichment of the target proteins while the immunoblot confirmed identity and stability of the target protein with no obvious proteolytic truncation (Fig. 3). Densitometry suggested a purity of ≥95%.
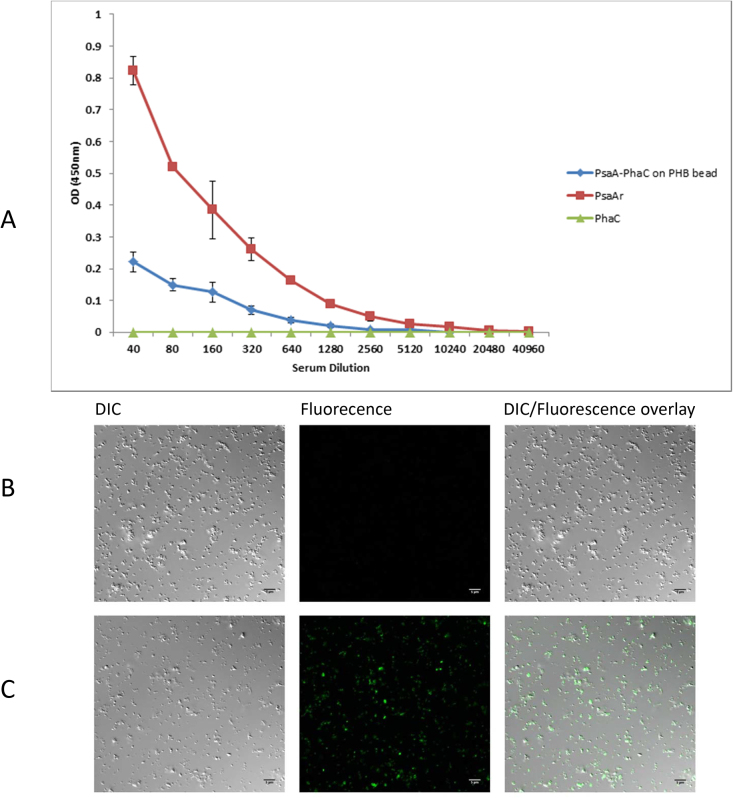
In order to determine whether PsaA is displayed on the surface of the PHB beads, an ELISA and CLSM was employed which showed binding of the anti-PsaA antibody to the surface of PHB beads which formation was mediated by the PsaA-PhaC fusion protein (Fig. 4)
Fig. 4.
Immunological assessment of PsaA display on the PHB bead surface. The specific monoclonal anti-PsaA antibody (see Materials and Methods) was used to detect PsaA at the surface of PHB beads by ELISA (A) and CLSM (B and C). A, Plates were coated with PsaA-PhaC beads, PhaC beads and soluble His6-PsaA. Anti-IgG HRP-conjugated secondary antibody was used to detect the bound primary anti-PsaA antibody. Data showed that the anti-PsaA antibody bound to PsaA on PHB beads as well as soluble PsaA while PhaC bead (negative control) did not show any binding. Each data point represented the mean of two replicates plus standard deviation. CLSM was further used to demonstrated PsaA surface display by detecting bound anti-PsaA antibodies with a secondary fluorescently labelled (Alexa fluor 488) antibody. PhaC beads (B) and PsaA-PhaC beads (C) were, after incubation with primary and seconday antibody, observed by CLSM. Fluorescent label of beads in the middle column (B, C) is indicative of PsaA display. DIC, differential interference contrast.
To determine the amount of PsaA- PhaC fusion protein attached to PHB beads, SDS-PAGE analysis including defined amount of BSA for generation of a densitometry based standard curve was applied. Densitometry using Image Analysis Software served to deduce the amount of protein per amount of PHB bead. The amount of PsaA-PhaC or PhaC per PHB bead is shown in Table 3. These data were used to calculate the amount of PHB beads to be injected per dose.
Table 3.
PHB bead yield and composition.
| Sample (Plasmids present in production strain) | Culture volume (L) | Wet biomass (g) | Wet PHA bead mass (g) | Antigen % in fusion protein | Antigen/wet Bead (μg/mg) |
|---|---|---|---|---|---|
| pET-14b-psaA-phaC | 4 | 32.8 | 1.63 | 34 | 0.39 |
| pET-14b-phaC | 4 | 29.4 | 5.51 | 0 | 1 |
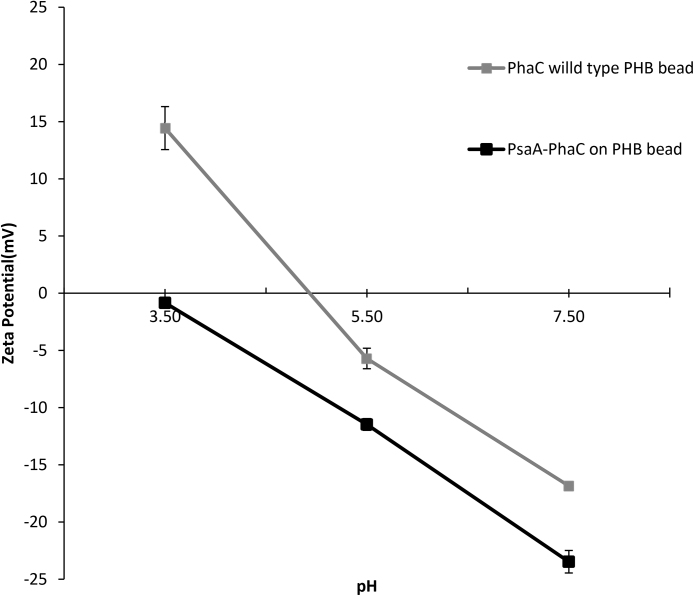
The Zeta potential of PHB beads was measured as a function of the pH (pH 3–7.5) suggesting a negative surface charge for both PHB beads (Fig. 5). However, display of PsaA further increased the negative surface charge.
Fig. 5.
Correlation between zeta potential and pH of various PHB beads. Error bar represent the mean plus standard deviation between three measurements.
3.3. Humoral immune response
During the immunization schedule all animals remained healthy and alive; they were gaining weight and showed no abnormal behaviour (data not shown). In the groups (G1 and G3) immunized with PsaA-PhaC and PhaC PHB beads small granulomas were detected (about 2 mm) at injection sites but no suppuration was observed. After euthanasia, the liver, lung, spleen and kidneys of the animals from groups G1, G2 and G3 showed no differences when compared with organs of animals which received the Placebo (G4).
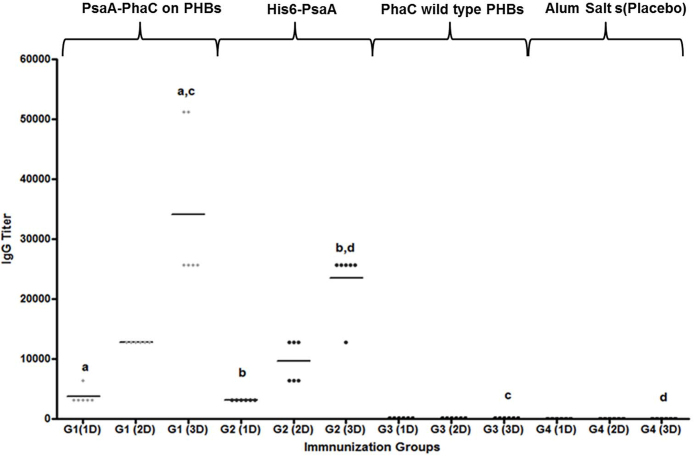
IgG titers specific towards PsaA after each immunization dose are shown in Fig. 6. The absorbance value obtained in G1 and G2 showed induction of specific anti-PsaA antibodies when compared with control groups G3 and G4. Besides, the tendency of an increase in IgG titer after each dose was evident and significant for G1 and G2 (first dose vs third dose with (p < 0.001)). As expected the highest titers for these groups were achieved after the third dose presenting significant differences in comparison with G3 and G4 ((p < 0.01 and (p < 0.05)), respectively. No statistical significant differences were found between G1 and G2.
Fig. 6.
Anti-PsaA IgG antibody response. Anti-PsaA IgG antibody titers were obtained by ELISA. G1 (grey diamond) group immunized with 4 μg of PsaA fused to PhaC and displayed on PHA beads, G2 (black circle) group immunized with 4 μg of His6-PsaA, G3 (black triangle) group immunized with 4 μg of PhaC wild type bead (no antigen PHB bead negative control) and G4 (black square) (placebo) group immunized with only 100 μg Alum salt. All of the immunogens were extracted from E. coli (Clearcoli, LPS free E. coli strain). Data are shown as reciprocal antibody titres, representing the dilution required to obtain half of the maximal OD value at 450/570 nm. a,bStatistical significant difference (p < 0.001). cSignificantly greater than the third dose of placebo and PhaC immunized control group (p < 0.01), dSignificantly greater than the third dose of placebo and PhaC immunized control group (p < 0.05). No statistical differences were found after third dose between G1 and G2 (p = 0.240).
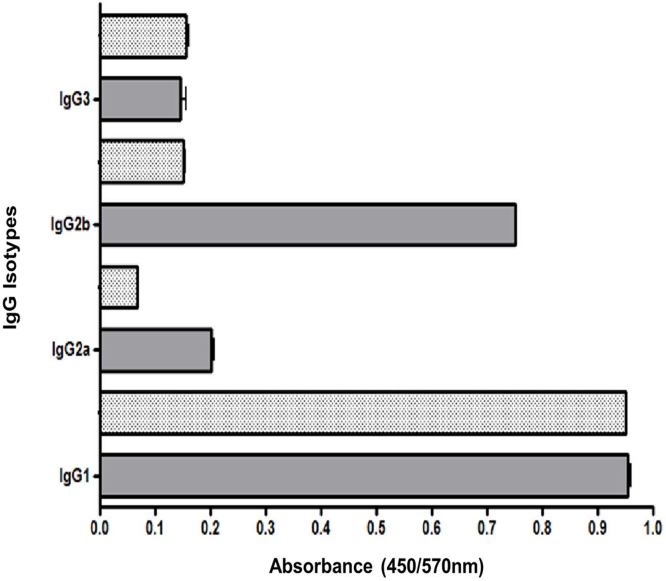
The humoral response was further studied, evaluating the IgG subclass profile in sera of animals 7 d after the last immunization. This analysis included subclasses IgG1, IgG2a, IgG2b and IgG3. IgG1 was the prevalent isotype elicited in G1 and G2. Interesting in the group immunized with PsaA-PhaC fusion protein on PHB beads IgG2b was the second prevalent subtype showing higher titers than the other isotypes (Fig. 7).
Fig. 7.
Isotype IgG profile evaluated by direct ELISA using ELISA plates coated with 0.5 μg of soluble His6-PsaA. The results are expressed as the average plus STD of absorbance readings at 450/570 nm using pooled of sera (6 animals) from G1 and G2. The sera were collected 1 week after third dose. The STD bars are only indicated in plus direction. G1 (grey bars) group immunized with 4 μg of PsaA displayed on PHB beads, G2 (black point bars) group immunized with 4 μg of soluble His6-PsaA. IgG1 is the prevalent isotype in both groups.
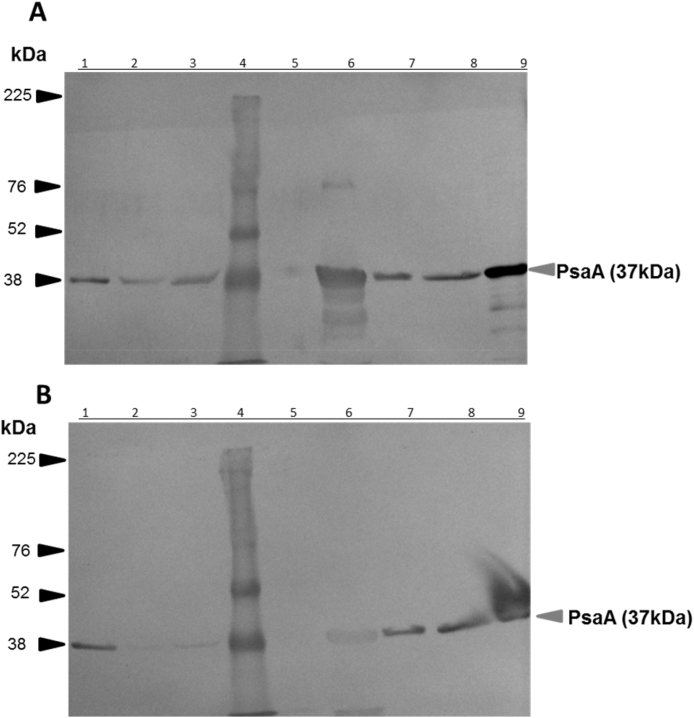
To assess whether induced anti-PsaA antibodies would recognize PsaA in whole cell lysates from different serotypes of S. pneumoniae, an immunoblot was performed using pooled sera from G1 and G2 obtained 7 d after the last immunization (Fig. 8). Antibodies in sera of both groups G1 and G2 were predominantly detecting a protein with an apparent molecular weight of 37 kDa which corresponds with the theoretical molecular weight of PsaA and aligned with the purified PsaA. Insterestingly, to get a visible band the sera from G2 had to be 4-fold more concentrated than from G1 suggesting enhanced production of the antibodies elicited after immunization with PsaA displayed on PHB beads versus its soluble counterpart.
Fig. 8.
Recognition of PsaA in various serotypes of S. pneumoniae by sera from mice immunized with PsaA displayed on PHB beads or soluble PsaA. A, immunoblots using sera (diluted 1:2000) from mice immunized with PHB beads displaying PsaA (G1). The corresponding SDS-PAGE of whole cell lysates of various S. pneumoniae serotypes is provided in Supplemental material (Fig. S1). B, immunoblot using sera (diluted 1:500) from mice immunized with soluble PsaA (G2). Lanes, 1, 2, 3, 5, 6, 7 and 8 correspond to cell lysates from S. pneumoniae serotypes: 1, 3, 5, 6b, 7F, 14, 23F, respectively; lane 4, MW standard and lane 9 soluble purified PsaA. The grey arrows indicate the protein corresponding to the molecular weight expected for PsaA.
4. Discussion
Current vaccines against S. pneumoniae infections are limited to protect against certain serotypes excluding important invasive pathogenic serotypes. Hence a serotype independent broadly protective vaccine, such as a vaccine based on conserved cell surface proteins could provide an alternative approach toward vaccine development.
PsaA has been considered as one of the most important vaccine candidate antigens as it plays a critical role during pathogenesis and is conserved among virulent strains. It induces both B-cell and T-cell responses. In this study we evaluated the concept of improving delivery and immunogenicity of PsaA by its display on PHB inclusions. Antigen displaying PHB beads (<1 μm) had been produced by engineered E. coli and induced both Th1 and Th2 immune responses as well as mediated protective immunity against tuberculosis and hepatitis C [8, 18, 31]. Antigen PHB beads are presumable taken up by phagocytosis of antigen presenting cells (APCs) which creates an antigen depot and allows cross presentation on MHC class I and class II, which would further enhance PsaA immunogenicity [8, 29, 31].
In addition, it was shown that the co-administration of Th1-promoting adjuvants to protein-based S. pneumoniae vaccine candidates induced protective immunity [32]. This suggested that the Th1 immune response might contribute to protective immunity against the extracellular pathogen S. pneumoniae. This did further justify considering particulate vaccine such as PHB beads, known to induce Th1 responses, for formulation of a S. pneumoniae vaccine.
To produce PsaA displaying PHB beads, a hybrid gene was constructed (Fig. 1) which encoded a single chain fusion protein of PsaA and the PHB forming enzyme, PhaC. The fusion protein mediated production of PHB inclusions in the ClearColi, an endotoxin-free mutant of E. coli [33] (Fig. 2). Interestingly, the fusion of PsaA to PhaC resulted in formation of smaller PHB beads (<200 nm) than observed with only PhaC suggesting an impact of PsaA on self-assembly of PHB beads inside the cell (Fig. 2). Protein and immunoblot analysis confirmed that PHB beads displayed the full-length PsaA at high-copy number with only minor indication of degradation (Figs. 3 and 4, Table 2). PHB beads as antigen carrier isolated from recombinant E. coli were recently demonstrated to induce only antigen specific responses, while potentially co-purifying E. coli proteins did not induce a detectable responses [19]. As the surface charge of particulate vaccine might impact cellular uptake and antigen processing, we assessed the Zeta potential of isolated PHB beads which resulted in anegative charge at physiological pH with an increased negative Zeta potential for the PsaA-PHB beads (Fig. 5). Particulate vaccine formulations which are designed for improved uptake of antigens by APCs are known to activate the NLRP3 inflammasome,which enhances efficacy of the vaccine [34]. Activation of the NLRP3 inflammasome was found to dependent on the surface charge of the vaccine particle.
To compare immunological properties of soluble and particulate PsaA, the soluble PsaA was produced in ClearColi and purified by affinity chromatography to ≥95% purity (Fig. 3). Vaccination experiments showed that IgG levels elicited by PsaA on PHB beads were significantly greater than those induced by PHB beads and within the placebo group (p < 0.01 and p < 0.05, respectively). Antibodies against bacterial antigens like cell surface proteins (e.g. PsaA or PspA) were shown to interfere with binding and internalization by the mucosal-nasopharyngeal cells, impairing entrance of the pathogen into blood circulation [35, 36]. In particular, the results obtained by De et al. [37] after immunization with PsaA, reinforces the above stated. In vitro studies further demonstrated that anti-PsaA antibodies inhibited adherence of various S. pneumoniae serotypes to nasopharyngeal human carcinoma cells [38]. PsaA trapped in alginate microspheres was demonstrated to mediate reduction of S. pneumoniae colonization resulting in protection against pneumonia and septicaemia [39].
Production of human IgG1 was related to clearance of extracellular pathogens by activating phagocytosis of pathogens by macrophages (and other phagocytic cells) through Fc receptor antibody interaction. Here immunization with PsaA displaying PHB beads induced predominantly IgG1 (Figs. 6 and 7). This result agrees with the study presented by Palmiappan et al., [40] where oral immunization with a S. pneumoniae strain EF3030 promoted significant PsaA-specific titers of subclasses IgG1 and IgG2a [40]. However, PsaA displayed on PHB beads induced IgG2b as the second predominant IgG subclass (Fig. 7). These subclasses (IgG2a/b) of antibodies are generally identified as part of a Th1 immune response usually associated with an immune response to intracellular pathogens. However, studies on a murine pneumococcal carrier model illustrated the induction of IgG2b and IgG3 to be important against S. pneumoniae strain P1121 infection [41].
Due to their more conserved nature protein-based vaccines are potentially more broadly protective against various sertotypes of S. pneumoniae as they induce a serotype independent immune response. To assess whether PsaA on PHB beads induced production of cross-reactive antibodies, we tested reactivity of whole cell proteins of serotypes 1, 3, 5, 6b, 7F, 14, 23F with antibodies in pooled sera of vaccinated mice (Fig. 8). These data clearly suggested that serotype independent anti-PsaA antibodies were induced by PsaA displaying PHB beads and by soluble PsaA. However, anti-PsaA antibody titers in response to PsaA displaying PHB were greater than antibody titers induced by soluble PsaA. Our results agree with previous studies where PsaA was suggested to be conserved among serotypes suitable for use as antigen for broadly protective vaccines [42, 43, 44]. The PsaA displayed in the surface of PHB beads was selected from the strain CGSP14 which PsaA is identical to PsaA of strains R6 and TIGR4 (Serotype 14) and shows 98–99% amino acid sequence identity with all other deposited PsaA protein sequences in the current NCBI database [24, 45]. Overall, this study demonstrated that bioengineering can be used to produce immunogenic PsaA displaying PHB beads, which mediate a serotype independent PsaA specific antibody response. Hence the PHB bead based particulate vaccine approach holds the promise to be applicable not only for protection against intracellular pathogens but also extracellular pathogens such as S. pneumoniae.
Declarations
Author contribution statement
Majela González-Miro: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Laura Rodríguez-Noda, Mildrey Fariñas-Medina: Performed the experiments.
Dagmar García-Rivera: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vicente Vérez-Bencomo: Contributed reagents, materials, analysis tools or data.
Bernd H.A. Rehm: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the MacDiarmid Institute of Advanced Materials and Nanotechnology as well as Massey University (Institute of Fundamental Sciences).
Competing interest statement
The authors declare the following conflict of interests: Bernd H.A. Rehm is founding inventor, shareholder and CTO of PolyBatics Ltd which commercialises the PHA bead technology.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are also thankful for the provision the anti-PsaA antibody by John Lewis and Anja Werno (Canterbury Health Laboratories, NZ), the technical assistance by Maria Onelia Gonzalez Socarras, Alex Quintero Perez, Dr. Reinaldo Oliva Hernandez, Dr Juan Francisco Infante and Msc. Tamara Hernandez Salazar. We would also like to acknowledge the Manawatu Microscopy and Imaging Centre for preparation of electron microscopy sections, CLSM work, technical advice and use of their facility.
References
- 1.O'Brien K.L. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.van der Poll T., Opal S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374(9700):1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee L.H., Gu X.X., Nahm M.H. Towards New Broader Spectrum Pneumococcal Vaccines: The Future of Pneumococcal Disease Prevention. Vaccines (Basel) 2014;2(1):112–128. doi: 10.3390/vaccines2010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrieux M. Current status and perspectives on protein-based pneumococcal vaccines. Crit. Rev. Microbiol. 2015;41(2):190–200. doi: 10.3109/1040841X.2013.813902. [DOI] [PubMed] [Google Scholar]
- 5.Gor D.O. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 2005;73(3):1304–1312. doi: 10.1128/IAI.73.3.1304-1312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabors G.S. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18(17):1743–1754. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 7.Parlane N.A. Production of a particulate hepatitis C vaccine candidate by an engineered Lactococcus lactis strain. Appl. Environ. Microbiol. 2011;77(24):8516–8522. doi: 10.1128/AEM.06420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parlane N.A. Novel particulate vaccines utilizing polyester nanoparticles (bio-beads) for protection against Mycobacterium bovis infection - a review. Vet. Immunol. Immunopathol. 2014;158(1-2):8–13. doi: 10.1016/j.vetimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Rehm B.H. Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Curr. Issues Mol. Biol. 2007;9(1):41–62. [PubMed] [Google Scholar]
- 10.Lee S.Y. Comparison of recombinant Escherichia coli strains for synthesis and accumulation of poly-(3-hydroxybutyric acid) and morphological changes. Biotechnol. Bioeng. 1994;44(11):1337–1347. doi: 10.1002/bit.260441110. [DOI] [PubMed] [Google Scholar]
- 11.Hooks D. Polyhydroyxalkanoate synthase fusions as a strategy for oriented enzyme immobilisation. Molecules. 2014;19(6):8629. doi: 10.3390/molecules19068629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parlane N.A. Vaccines displaying mycobacterial proteins on biopolyester beads stimulate cellular immunity and induce protection against tuberculosis. Clin. Vaccine Immunol. 2012;19(1):37–44. doi: 10.1128/CVI.05505-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper J.L., Rehm B.H. Engineering bacteria to manufacture functionalized polyester beads. Bioengineered. 2012;3(4):203–208. doi: 10.4161/bioe.19567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grage K., Peters V., Rehm B.H.A. Recombinant protein production by in vivo polymer inclusion display. Appl. Environ. Microbiol. 2011;77(18):6706–6709. doi: 10.1128/AEM.05953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlane N.A. Self-assembled protein-coated polyhydroxyalkanoate beads: properties and biomedical applications. ACS Bio. Mater. Sci. Eng. 2016 doi: 10.1021/acsbiomaterials.6b00355. [DOI] [PubMed] [Google Scholar]
- 16.Rehm F.B., Chen S., Rehm B.H. Enzyme Engineering for In Situ Immobilization. Molecules. 2016;21(10) doi: 10.3390/molecules21101370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehm B.H. Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010;8(8):578–592. doi: 10.1038/nrmicro2354. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Donato G. Protective T Cell and Antibody Immune Responses against Hepatitis C Virus Achieved Using a Biopolyester-Bead-Based Vaccine Delivery System. Clin. Vaccine Immunol. 2016;23(4):370–378. doi: 10.1128/CVI.00687-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio Reyes P. Immunogencity of antigens from Mycobacterium tuberculosis self-assembled as particulate vaccines. Int. J. Med. Microbiol. 2016;306(8):624–632. doi: 10.1016/j.ijmm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Grage K., Rehm B.H. In vivo production of scFv-displaying biopolymer beads using a self-assembly-promoting fusion partner. Bioconjug. Chem. 2008;19(1):254–262. doi: 10.1021/bc7003473. [DOI] [PubMed] [Google Scholar]
- 21.Peters V., Rehm B.H. In vivo monitoring of PHA granule formation using GFP-labeled PHA synthases. FEMS Microbiol. Lett. 2005;248(1):93–100. doi: 10.1016/j.femsle.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Amara A.A., Rehm B.H. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues. Biochem. J. 2003;374(Pt 2):413–421. doi: 10.1042/BJ20030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooks D.O., Blatchford P.A., Rehm B.H. Bioengineering of bacterial polymer inclusions catalyzing the synthesis of N-acetylneuraminic acid. Appl. Environ. Microbiol. 2013;79(9):3116–3121. doi: 10.1128/AEM.03947-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larentis A.L. Cloning and optimization of induction conditions for mature PsaA (pneumococcal surface adhesin A) expression in Escherichia coli and recombinant protein stability during long-term storage. Protein Expr. Purif. 2011;78(1):38–47. doi: 10.1016/j.pep.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Thompson T. Google Patents; 2011. Compositions for separation methods. [Google Scholar]
- 26.Thompson T. Google Patents; 2013. Compositions for separation methods. [Google Scholar]
- 27.Hooks D.O., Blatchford P.A., Rehm B.H. Bioengineering of bacterial polymer inclusions catalyzing the synthesis of N-acetylneuraminic acid. Appl. Environ. Microbiol. 2013;79(9):3116–3121. doi: 10.1128/AEM.03947-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooks D.O., Rehm B.H. Insights into the surface topology of polyhydroxyalkanoate synthase: self-assembly of functionalized inclusions. Appl. Microbiol. Biotechnol. 2015;99(19):8045–8053. doi: 10.1007/s00253-015-6719-6. [DOI] [PubMed] [Google Scholar]
- 29.Parlane N.A. Bacterial polyester inclusions engineered to display vaccine candidate antigens for use as a novel class of safe and efficient vaccine delivery agents. Appl. Environ. Microbiol. 2009;75(24):7739–7744. doi: 10.1128/AEM.01965-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa-Viñals C. Validación y aplicación de un ELISA para la cuantificación de anticuerpos IgG contra polisacárido capsular Vi de Salmonella Typhi. Vaccimonitor. 2015;24(1):21–32. [Google Scholar]
- 31.Grage K. Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules. 2009;10(4):660–669. doi: 10.1021/bm801394s. [DOI] [PubMed] [Google Scholar]
- 32.Olafsdottir T.A. Novel protein-based pneumococcal vaccines administered with the Th1-promoting adjuvant IC31 induce protective immunity against pneumococcal disease in neonatal mice. Infect. Immun. 2012;80(1):461–468. doi: 10.1128/IAI.05801-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamat U. Endotoxin-free protein production—ClearColiTM technology. Nature Methods. 2013;10:9. [Google Scholar]
- 34.Neumann S. Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol. Cell Biol. 2014;92(6):535–542. doi: 10.1038/icb.2014.21. [DOI] [PubMed] [Google Scholar]
- 35.Johnson S.E. Inhibition of pneumococcal carriage in mice by subcutaneous immunization with peptides from the common surface protein pneumococcal surface adhesin a. J. Infect. Dis. 2002;185(4):489–496. doi: 10.1086/338928. [DOI] [PubMed] [Google Scholar]
- 36.Rajam G., Romero-Steiner S., Johnson S.E., Thompson T.A., Reed Y.M., Sampson J.S., Carlone G.M., Ades E.W. Immunogenicity and functional activity of multi-antigenic peptides (MAPs) of pneumococcal surface adhesin A (PsaA) in a rabbit model. In 105th General Meeting of ASM; Atlanta, GA; 2005. [Google Scholar]
- 37.De B.K. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine. 2000;18(17):1811–1821. doi: 10.1016/s0264-410x(99)00481-8. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Steiner S. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 2003;10(2):246–251. doi: 10.1128/CDLI.10.2.246-251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo J.Y. Cross-protective immunity of mice induced by oral immunization with pneumococcal surface adhesin a encapsulated in microspheres. Infect. Immun. 2002;70(3):1143–1149. doi: 10.1128/IAI.70.3.1143-1149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palaniappan R. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect. Immun. 2005;73(2):1006–1013. doi: 10.1128/IAI.73.2.1006-1013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rossum A.M., Lysenko E.S., Weiser J.N. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 2005;73(11):7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogunniyi A.D. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2000;68(5):3028–3033. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell H. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J. Clin. Microbiol. 1990;28(10):2191–2195. doi: 10.1128/jcm.28.10.2191-2195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampson J.S. Cloning and nucleotide sequence analysis of psaA: the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp adhesins. Infect. Immun. 1994;62(1):319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoskins J. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 2001;183(19):5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]