Abstract
Rewards reliably elicit ventral striatum activity. More recently studies have shown that vicarious rewards elicit similar activation. Ventral striatum responses to rewards for self peak during adolescence. However, it is currently not well understood how ventral striatum responses to vicarious rewards develop. In this study, we test this question using behavioral and fMRI data. A total of 233 participants aged 9–26 years old played a gambling game in the scanner in which they could win or lose money for themselves, their best friend and mother. Participants rated how close they felt to their friend and mother and how much they liked winning for them. These ratings were positively correlated. On the neural level males showed higher responses to winning for a friend, but there were no age differences. In contrast, there was a quadratic effect of age when winning for mother, showing heightened ventral striatum activity in mid-adolescence. Furthermore, there was an interaction between age and sex; for females responses to winning for friends become stronger with age relative to winning for mothers. In conclusion, this study provided evidence for elevated ventral striatum responses for mothers in mid-adolescence, and a shift in ventral striatum responses towards peers in girls.
Keywords: fMRI, adolescence, friend, mother, vicarious reward
Introduction
Prior neuroimaging studies have consistently demonstrated that experiencing rewards for self is associated with robust activity in the ventral striatum, specifically in the nucleus accumbens (Nacc) (Delgado, 2007) and that the strength of the neural response in ventral striatum is positively related to the subjective experience of the reward (Galvan and McGlennen, 2013). Adolescents show higher activity in the ventral striatum relative to children and adults when winning money for themselves (Braams et al., 2015; Silverman et al., 2015). However, adolescence is also a crucial time for social development. As adolescents assert greater independence from their parents, peers become an important social group. Adolescents form more complex friendships with their peers (Steinberg and Morris, 2001). Especially relationships with best friends are found to be more intimate (Degirmencioglu et al., 1998), positive and reciprocal (De Goede et al., 2009b). How adolescents experience rewards for close others is not yet understood.
There is evidence that sharing feelings with friends or winning money for friends is associated with activity in the ventral striatum, also known as vicarious rewards. For example, adults show stronger activity in the ventral striatum both when they win money for themselves as well as when they win money for their friend, relative to someone they dislike (Braams et al., 2014a; Fareri et al., 2012) and these responses are moderated by relationship closeness (Fareri et al., 2012). A recent meta-analysis confirmed that receiving rewards for self is related to activation in the ventral striatum, but vicarious rewards for others were more strongly related to activation in prefrontal areas such as the dorsomedial prefrontal cortex, ventromedial prefrontal cortex and medial prefrontal cortex (Morelli et al., 2015). Activity in the ventral striatum response is possibly dependent on experienced closeness with the beneficiary. An important question that remains unanswered is how responses to vicarious rewards in the ventral striatum for friends change over adolescent development.
Another important social context during adolescence is the parent–child relationship. As adolescence gain independence and autonomy from their parents, this is often thought to lead to a decline in parent–child closeness. Indeed, one study found that mother cohesion decreased between 9th and 12th grade and stabilized after 12th grade (Tsai et al., 2013). Even though adolescents become more independent, parents continue to provide an important social context and parent–child relationships become more egalitarian as adolescents get older (De Goede et al., 2009a, b). A previous study investigating neural responses to donations to family members found that donating money to family members was associated with increased activity in the ventral striatum (Telzer et al., 2010). A longitudinal follow up showed that higher ventral striatum responses to family money donations were related to decreases in risk taking behavior 1 year later (Telzer et al., 2013).
In the current study, we test and compare responses to vicarious rewards for friends and parents using behavioral as well as neural measures. In this study mothers were chosen as beneficiary since mothers are most often indicated as primary parent (Telzer et al., 2015). To investigate how behavioral and neural measures develop over age we included children, adolescents and adults between the ages of 9 and 26 years. Participants played a well-validated reward task in which they could win and lose money for themselves, their best friend and their mother. In a previous study using the same paradigm, we found that ventral striatum responses showed a different pattern of results for friends and disliked others (Braams et al., 2014a,2014b), which indicates that this task is suitable to detect differences in neural responses to vicarious rewards for different beneficiaries. After the task, participants rated how much they liked to win for each of the beneficiaries.
We hypothesized that self-report relationship closeness with friends increases with age (Steinberg and Morris, 2001) and that this would be mirrored by higher striatum responses to winning money for friends with age. For mother, we hypothesized that self-report relationship closeness decreases or remains stable over development and this would be mirrored by a decrease or no change in striatum responses over age for mothers.
There are indications that the experience of friendship and family cohesion differs for boys and girls. Girls are often thought to experience more intimate friendships. Girl dyads are characterized by self-disclosure, empathy and interdependence (Colarossi and Eccles, 2000; Helsen et al., 2000), whereas boy dyads have higher levels of negative interaction and issues of dominance occur more often (Jenkins et al., 2002; Updegraff et al., 2004). Furthermore, a prior study found that girls reported higher mother cohesion than boys (Tsai et al., 2013). Based on these prior studies, we predicted that, compared to males, females would report higher relationship quality for friends (Colarossi and Eccles, 2000; Helsen et al., 2000) and mothers (Tsai et al., 2013). We explored whether sex differences would also be present in vicarious rewards for friends and mothers.
Materials and methods
Participants
Participants were part of a large, longitudinal study called Braintime. Participants were scanned three times, with a 2-year interval. The current study reports only data from the second time point since participants played the gambling game for their mother only on this time point. Results from the self-condition reported in this study have previously been published separately as part of a longitudinal study (Braams et al., 2015; Peper et al., 2013; Peters et al., 2014).
Data were used from participants who generated usable fMRI data. In total, 254 participants performed the fMRI session. Ten participants were excluded for moving more than 1 voxel. An additional eight participants were excluded for not finishing the task, technical problems and/or artifacts during data collection. An additional three participants were excluded since they could not play for their mother. The total number of participants for whom fMRI data was available was therefore 233 (117 females). Participants were aged between 9.9 years old and 26.6 years old (Mage = 16.1, SDage = 3.7). Two subscales, picture completion and vocabulary, of the Wechsler Intelligence Scale for Adults (WAIS-III) or the Wechsler Intelligence Scale for Children (WISC-III) (Wechsler, 1997) were used to determine an approximation of IQ. The estimated IQ scores fell within the high to normal range (MIQ = 108.2, SDIQ = 10.46). Informed consent was obtained from adult participants and parental permission and minor assent was obtained for the children and adolescents. Participants were screened for MRI contra indications and were free of neurological and psychiatric disorders. All procedures were reviewed and approved by the university medical ethical committee. Participants received a compensation for their participation in a larger scale study (EUR 60 for adult, EUR 25 for participants aged 12–17 and EUR 20 for participants younger than 12).
Experimental task
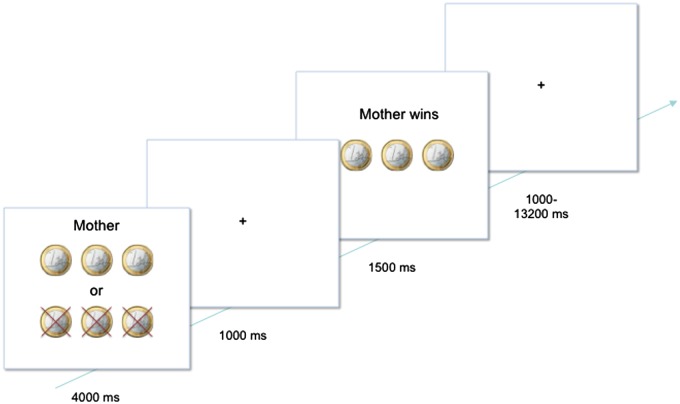
Participants played a heads or tails gambling game in which they could win or lose money (Braams et al., 2014a,b). On each trial, participants guessed whether the computer would pick heads or tails and they won when the computer selected the chosen side of the coin. Each trial started with a trial onset screen (4000 ms) during which the participant indicated their choice to play for heads or tails. On the trial onset screen, the participants also saw how much they could win or lose on that trial, explained in more detail below. The trial onset screen was followed by a fixation screen (1000 ms) and after that a feedback screen, which showed whether participants won or lost on that trial (1500 ms). Trials ended with a variable jitter (Mjitter = 2298 ms, range 1000–13 200 ms), see Figure 1. Trial sequence and timing was optimized using OptSeq (Dale, 1999); see also (http://surfer.nmr.mgh.harvard.edu/optseq). The probability for winning was 50%. Three different distributions of coins were included; trials on which two coins could be won and five lost, trials on which three coins could be won or three lost and finally trials on which five coins could be won or two could be lost. These different distributions of coins were included to keep participants engaged in the task, but were not analyzed separately (see also Braams et al., 2014b). Participants were informed about the different distributions of coins and were familiarized with them during the practice task. Participants were explained that the coins won during the experiment translated to real money at the end of the experiment and that one of the beneficiaries would be randomly selected to receive the money. Participants received 4, 5 or 6 euro’s at the end of the task and they were asked to provide the beneficiary with this money if they did not win money for themselves. Unbeknownst to the participants, the total earnings on the task did not relate to the amount won during the task but were chosen at random. Participants played 30 trials in the gambling game for themselves, 30 trials for their best friend and 30 trials for their mother person. Trials were presented in random order. Best friends were same-sex peers and participants were asked to provide the name of their best friend before the experimental session.
Fig. 1.
Example of a trial. On stimulus onset participants were presented with a screen indicating how much they could win or lose and for whom they were playing on that trial. During this time, they chose to play for heads or tails by pressing the corresponding button. After 4000 ms a fixation cross was presented for 1000 ms, after which the outcome screen was presented for 1500 ms. The outcome screen indicated how many coins the participant had won or lost and for whom. A trial ended with a fixation cross shown for a variable delay between 1000 and 13 200 ms.
Self-report measures
Subjective ratings. After the scanning session participants indicated how much they liked it when they won for themselves and when their friend and mother won. Ratings were made on a 10-point scale ranging from (0) ‘not at all’ to (10) ‘very much’. The mean score for this rating was 7.6 (SD = 1.9; range = 1–10) for self, 7.3 (SD = 1.7; range = 1–10) for friend and 7.4 (SD = 1.8; range = 0–10) for mother. In total 231 participants provided ratings for how much they liked it when they won for themselves, 228 participants provided ratings for when their friend won and 224 participants provided ratings for how much they liked it when their mother won. Ratings for some participants were not recorded due to equipment problems.
Inclusion of Other in Self Scale (IOS). The IOS is a single item measure to assess relationship closeness (Aron et al., 1992). Participants are presented with seven Venn diagrams with varying overlap. In each Venn diagram, one circle represents the participant, the other circle represents another person. Scores for the IOS range from one to seven, with one representing the lowest relationship closeness and seven the highest. The Inclusion of Other in Self scale has been used in other studies with an adolescent population starting at age 12 (see for instance Lourenco et al., 2015). In the current study, participants filled out the IOS twice, once in which the other circle represented their best friend and once when the other circle represented their mother. Mean scores for experienced relationship closeness was 5.34 (SD = 1.31) for friend and 5.52 (SD = 1.63) for mother.
Procedure
Participants received instructions regarding the testing session in a quiet laboratory room. Participants were familiarized with the MRI scanner with a mock scanner. Next the gambling game was explained and participants performed six practice trials. After the scanner session, WISC-III or WAIS-III were administered. Participants filled out the IOS and the ratings directly after the scan.
MRI data acquisition
Scanning was performed on a 3 Tesla Philips scanner, with a standard whole-head coil. The functional scans were acquired using T2*-weighted echo-planar imaging (EPI; TR = 2.2 s, TE = 30 ms, sequential acquisition, 38 slices of 2.75 mm, field of view 220 mm, 80 × 80 matrix, in-plane resolution 2.75 mm). The first two volumes were discarded to allow for equilibration of T1 saturation effects. After the functional runs, a high-resolution 3D T1-weighted anatomical image was collected (TR = 9.751 ms, TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 mm × 0.875 mm × 1.2 mm, and FOV = 224 × 168 × 177). Visual stimuli were displayed on a screen in the magnet bore. A mirror attached to the head coil allowed participants to view the screen. Foam inserts inside the coil were used to limit head movement.
fMRI pre-processing
All data were analyzed with SPM8 (Wellcome Department of Cognitive Neurology, London). Images were corrected for slice timing acquisition and differences in rigid body motion. Structural and functional volumes were spatially normalized to T1 templates. Translational movement parameters never exceeded 1 voxel (<3 mm) in any direction for any participant or scan. The normalization algorithm used a 12-parameter affine transform together with a nonlinear transformation involving cosine basis functions and resampled the volumes to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). Functional volumes were spatially smoothed with a 6 mm FWHM isotropic Gaussian kernel. Statistical analyses were performed on individual subjects data using the general linear model in SPM8. The fMRI time series were modeled as a series of zero duration events convolved with the hemodynamic response function (HRF). On trial onset events were modeled separately for playing for self, friend and mother. On feedback onset winning and losing for self, friend and mother were modeled. This resulted in three conditions at trial onset (self, friend, mother) and six conditions at feedback onset (self win, self lose, friend win, friend lose, mother win, mother lose). Trials on which the participants failed to respond were modeled separately as covariate of no interest and were excluded from further analyses. The modeled events were used as regressors in a general linear model, along with a basic set of cosine functions that high-pass filtered the data (cut off 120), and a covariate for session effects. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pair-wise contrasts. The resulting contrast images, computed on a subject-by-subject basis, were submitted to random-effects group analyses.
Region of interest analysis
Parameter estimates were extracted from a priori defined anatomical Nacc clusters for left and right Nacc. Anatomical Nacc clusters were extracted from the Harvard-Oxford subcortical atlas and were thresholded at 40%. The region of interest was chosen since the Nacc is a primary reward area (Delgado, 2007) and previous studies using the same task showed peak activation when receiving rewards for self in the Nacc (Braams et al., 2014a,b, 2015). Region of interest analysis was performed using the MarsBar toolbox ((Brett et al., 2002) (http://marsbar.sourceforge.net/) for SPM8. To check whether whole brain results for the contrasts win > lose for each beneficiary showed significant activation in the a priori defined Nacc cluster we performed whole brain analyses. On the group level contrasts for win > lose for each beneficiary (self, friend, mother) were calculated. Whole brain results were regarded significant when they exceeded a threshold of P < 0.05, Family Wise Error corrected at the voxel level. As expected results showed robust activation in the bilateral ventral striatum for the contrasts win> lose for self, win > lose for friend and win > lose for mother (Figure 2 and Table 1). See whole brain tables for the contrasts comparing all three beneficiaries in Supplementary Table S1.
Fig. 2.
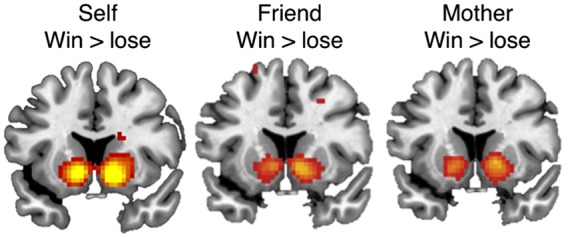
Whole brain contrasts for win> lose for Self, win > lose for Friend and win > lose for Mother. Contrasts were FWE corrected with a threshold of P < 0.05. All contrasts resulted in significant activation in the bilateral striatum, including the nucleus accumbens. Results are projected on a slice with MNI coordinate y = 12.
Table 1.
Whole brain table for neural activation for the contrast win > lose when playing for friend and win > lose when playing for mother
| MNI |
||||||
|---|---|---|---|---|---|---|
| Region | R/L | x | y | z | T(232) | Voxels |
| Win > Lose Self | ||||||
| Putamen | R | 15 | 14 | −5 | 13.07 | 680 |
| Superior Medial Gyrus | L | −3 | 56 | 4 | 7.39 | 380 |
| Superior Frontal Gyrus | L | −21 | 35 | 49 | 7.05 | 235 |
| Calcarine Gyrus | L | −6 | −58 | 10 | 6.24 | 125 |
| Angular Gyrus | L | −48 | −64 | 49 | 5.73 | 66 |
| Paracentral Lobule | L | −3 | −25 | 58 | 5.63 | 176 |
| Post-central Gyrus | L | −27 | −34 | 58 | 5.54 | 32 |
| Superior Frontal Gyrus | R | 21 | 32 | 52 | 5.46 | 29 |
| Anterior Cingulum | R | 15 | 32 | 25 | 5.35 | 12 |
| Middle Frontal Gyrus | L | −39 | 53 | 1 | 5.34 | 45 |
| Cuneus | R | 3 | −82 | 25 | 5.09 | 20 |
| Cerebellum | R | 33 | −85 | −23 | 5.03 | 17 |
| Win > Lose Friend | ||||||
| Caudate Nucleus | R | 9 | 14 | −2 | 9.77 | 213 |
| Caudate Nucleus | L | −9 | 14 | −2 | 8.56 | 122 |
| Superior Frontal Gyrus | R | 21 | 26 | 55 | 6.83 | 92 |
| Superior Medial Gyrus | R | 3 | 53 | 1 | 6.64 | 167 |
| Middle Frontal Gyrus | L | −24 | 20 | 58 | 6.38 | 98 |
| Angular Gyrus | R | 45 | −73 | 46 | 6.27 | 91 |
| Inferior Parietal Cortex | L | −48 | −61 | 52 | 6.15 | 67 |
| Middle Cingulate Gyrus | R | 0 | −37 | 43 | 5.08 | 18 |
| Precuneus | R | 6 | −52 | 19 | 5.04 | 18 |
| Win > Lose Mother | ||||||
| Caudate Nucleus | R | 15 | 14 | −5 | 9.30 | 244 |
| Caudate Nucleus | L | −15 | 11 | −5 | 8.15 | 135 |
| Middle Frontal Gyrus | L | −24 | 20 | 58 | 5.26 | 26 |
| Anterior Cingulate Cortex | R | 6 | 44 | 13 | 5.39 | 15 |
Reported clusters survive Family Wise Error correction, P < 0.05. Only clusters comprised of 10 voxels or more are reported.
Results
Self-report measures
Relations between subjective ratings and age
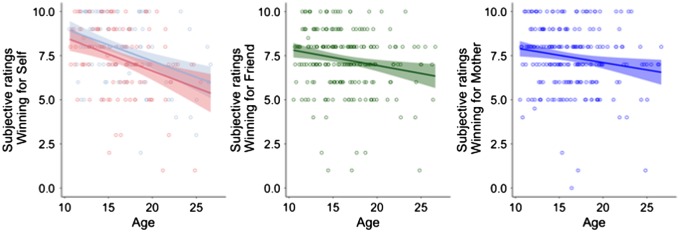
First, we tested general developmental patterns of subjective ratings for winning for self, friend and mother. We used regression models to first test linear and quadratic effects of age and then test whether adding a main or interaction effect of sex improved model fit. If including effects for sex did not improve model fit, they were removed for the final model. For all three beneficiaries, the subjective rating of how much the participant liked to win for this person decreased linearly with age. Only for the rating for self the main effect of sex was significant, males rated winning for self as more pleasurable than females [self: age βlinear = −10.5, t(227) = −5.83, P = <0.001, sex β = 0.54, t(227) = 2.29, P = 0.022; friend age βlinear = −5.12, t(225) = −2.92, P = 0.004; mother age βlinear = −4.69, t(221) = −2.55, P = 0.012; Figure 3]. This indicates that even though participants of all ages rated the rewards as pleasurable (average scores > 7 on a scale of 1–10), participants rated winning in the task as less pleasurable with increasing age.
Fig. 3.
Subjective ratings for winning for Self, Friend and Mother, plotted as a function of age. The ratings for Self showed a main effect of sex. The ratings for Self are plotted in blue for males and red for females.
Second, to investigate the value of winning for a friend and mother, relative to winning for self, we calculated difference scores between pleasure scores when winning for friend vs winning for self, and winning for mother vs winning for self. In these analyses, we used winning for self as a baseline to account for any general age effects of reward experience on the task. For this purpose, we performed regression analyses with the difference score [winning for friend − winning for self] and [winning for mother − winning for self] as dependent variable. We first tested for linear and quadratic effects of age and then included main and interaction effects of sex. Sex was removed from the final model if inclusion did not result in improved model fit.
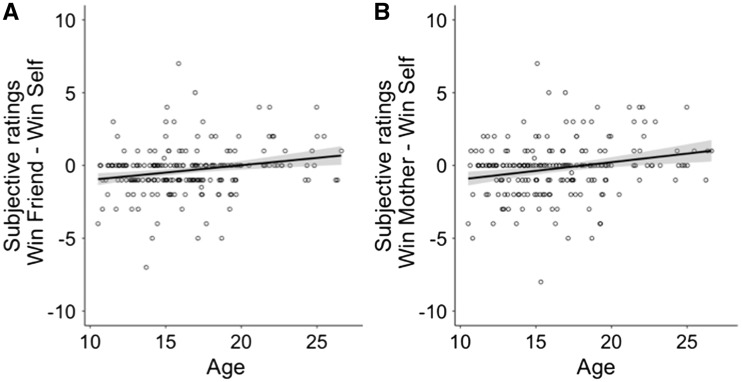
Results showed a positive linear regression coefficient for age and the difference score [winning for a friend – winning for self] [age βlinear = 5.34, t(225)=3.42, P < 0.001; Figure 4A]. These results indicate that winning for a friend is rated as relatively more pleasurable with increasing age. The main effect of sex was significant [sex β = .528, t(225) = −2.58, P = 0.011]. Females rated their winning for a friend relative to winning for self higher than males. There was also a positive linear effect of age for the difference score [winning for mother-winning for self] [age βlinear = 5.63, t(222) = 3.04, P = 0.003; Figure 4B]. Quadratic effects were not significant for any of the models. The effect of sex was not significant. Again, this result shows that the winning for mother is rated as relatively more pleasurable with increasing age. No age or sex effects were observed for the differences between [winning for friend – winning for mother] [age βlinear = −0.54, t(219) = −0.374, P = 0.709].
Fig. 4.
Regression analysis with age as predictor and the difference score for the subjective ratings [winning for friend − winning for self] (panel A) and [winning for mother – winning for self] (panel B) as dependent variable. Results showed a positive relation between age and the difference scores for both friend and mother.
Relations between the inclusion of other in self scale and age
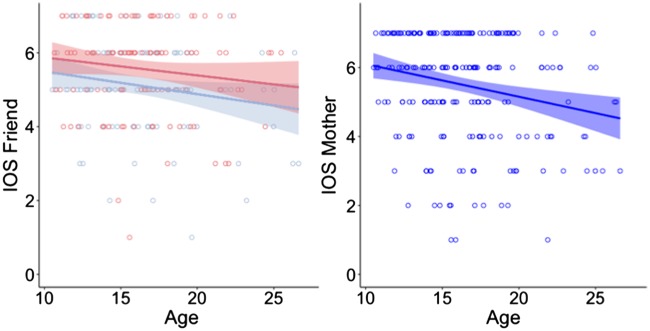
First, we tested for general age effects on relationship closeness. Again we first tested linear and quadratic developmental patterns, and then tested whether inclusion of a main and interaction effect of sex improved model fit. For both friend and mother, the best fitting model to describe the relation between relationship closeness and age was a linear model, indication that relationship closeness decreased with age. For friend we also found a significant main effect of sex, females rated the closeness to a friend higher than males [friend age βlinear = −3.14, t(225) = −2.44, P = 0.015, sex β = −0.46, t(225) = −2.71, P = 0.007; mother age βlinear = −5.34, t(227) = −3.41, P < 0.001; Figure 5].
Fig. 5.
Ratings on the Inclusion of Other in Self scale for Friend and Mother, plotted as a function of age. Ratings for Friend are plotted in blue for males and red for females.
Relations between subjective ratings and inclusion of other in self scale
The next question was how winning pleasure ratings on the gambling task related to relationship closeness as measured with the IOS. To test this relationship, we fitted separate regression models for each of the ratings. In these models the IOS rating was the dependent variable. We included a linear effect of age and the winning pleasure ratings as independent variables. Based on our hypothesis, we only tested for linear effects and then tested whether including a main and interaction effect of sex improved model fit. Results showed a positive relationship between the IOS and the rating participants provided for winning for their mother [βlinear = 0.130, t(219) = 2.26, P = 0.025]. In other words, those participants who indicated that they liked it better when their mother won also indicated that they experienced more relationship closeness with their mother. Results did not show a similar relationship for friend [βlinear = 0.048, t(222) = 0.96, P = 0.337]. There were no interaction effects between the subjective ratings and Sex (main effects of Sex on the Inclusion of Other in Self scale have been reported in a previous paragraph).
fMRI
Developmental effects
The first question we aimed to answer concerned general developmental effects of neural responses to rewards for winning for friend and mother. Neural responses to rewards for self are described in two separate publications (Braams et al., 2014a, 2015). These publications show that neural responses to rewards for self peak in adolescence. To test age related effects, we extracted parameter estimates for the contrasts win > lose for friend and win > lose for mother from a priori anatomically defined left and right Nacc clusters. We used regression models to first test linear and quadratic effects of age and then test whether adding a main or interaction effect of sex improved model fit. If including effects for sex did not improve model fit, they were removed for the final model. Two separate models were fit, one for contrast values win > lose for friend and one for contrast values win > lose for mother.
For friend, there were no linear or quadratic effects of age in the Nacc. The best fitting model was a model with only a main effect of sex [left Nacc: βsex = 0.741, t(231) = 2.44, P = 0.016; right Nacc: βsex = 0.992, t(231) = 3.11, P = 0.002]. Male participants showed higher ventral striatum responses to winning for friends than female participants, see Table 2. We then tested how neural responses to vicarious rewards for friends (win > lose for friend) develop respective to neural responses to winning for self (win > lose for self). We calculated a differences score between win > lose friend and win > lose self, higher scores indicate higher ventral striatum responses to winning for a friend. A model with a linear regressor for age and a main and interaction term for sex showed the best fit. The interaction effect of age and sex was significant in the left Nacc and trending in the right Nacc [left Nacc: interaction Age × Sex β= −0.226, t(229) = −2.169, P = 0.031; right Nacc: interaction Age × Sex β= −0.208, t(229) = −1.69, P = 0.091). To follow up on this interaction, we tested developmental effects of age for both sexes separately. For girls, there was a significant increase in Nacc responses to win > lose for friend compared to win > lose for self [left Nacc: age βlinear = 0.210, t(114) = 2.86, P = 0.005; right Nacc: age βlinear = 0.190, t(114) = 2.03, P = 0.045]. During childhood and early adolescence, the left Nacc shows more activation for win > lose for self, whereas in late adolescence and adulthood the Nacc shows higher responses to win > lose for friend. For boys, there was no effect of age (P’s > 0.82; Figure 6).
Table 2.
Regression coefficients (B), standard error, t values and P values for the models for friend win > lose and mother win > lose for the left and right Nacc
| B | Std Error | t value | P value | |
|---|---|---|---|---|
| Friend win > lose | ||||
| Left Nacc | ||||
| Intercept | 0.55 | 0.21 | 2.56 | 0.011* |
| Age Linear | 4.63 | 3.49 | 1.33 | 0.186 |
| Age Quadratic | −3.69 | 3.70 | −1.00 | 0.320 |
| Sex | 0.81 | 0.31 | 2.66 | 0.008** |
| Age Linear*Sex | −4.00 | 4.70 | −0.85 | 0.395 |
| Age Quadratic*Sex | 4.25 | 4.79 | 0.89 | 0.376 |
| Right Nacc | ||||
| Intercept | 0.72 | 0.22 | 3.24 | 0.001** |
| Age Linear | 3.85 | 3.64 | 1.06 | 0.291 |
| Age Quadratic | −6.75 | 3.86 | −1.75 | 0.081 |
| Sex | 1.08 | 0.32 | 3.37 | 0.001** |
| Age Linear*Sex | −2.36 | 4.91 | −0.48 | 0.631 |
| Age Quadratic*Sex | 7.54 | 5.00 | 1.51 | 0.133 |
| Mother win > lose | ||||
| Left Nacc | ||||
| Intercept | 1.10 | 0.18 | 6.08 | <0.001*** |
| Age Linear | −3.94 | 2.95 | −1.34 | 0.182 |
| Age Quadratic | −5.58 | 3.12 | −1.79 | 0.075† |
| Sex | −0.19 | 0.26 | −0.72 | 0.470 |
| Age Linear*Sex | 7.50 | 3.97 | 1.89 | 0.060 |
| Age Quadratic*Sex | 2.49 | 4.04 | 0.62 | 0.538 |
| Right Nacc | ||||
| Intercept | 1.27 | 0.20 | 6.27 | <0.001*** |
| Age Linear | −3.86 | 3.29 | −1.18 | 0.241 |
| Age Quadratic | −7.60 | 3.48 | −2.18 | 0.030* |
| Sex | −0.32 | 0.29 | −1.12 | 0.265 |
| Age Linear*Sex | 6.62 | 4.43 | 1.50 | 0.136 |
| Age Quadratic*Sex | 2.29 | 4.51 | 0.51 | 0.612 |
Indicators of significance: †P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 6.
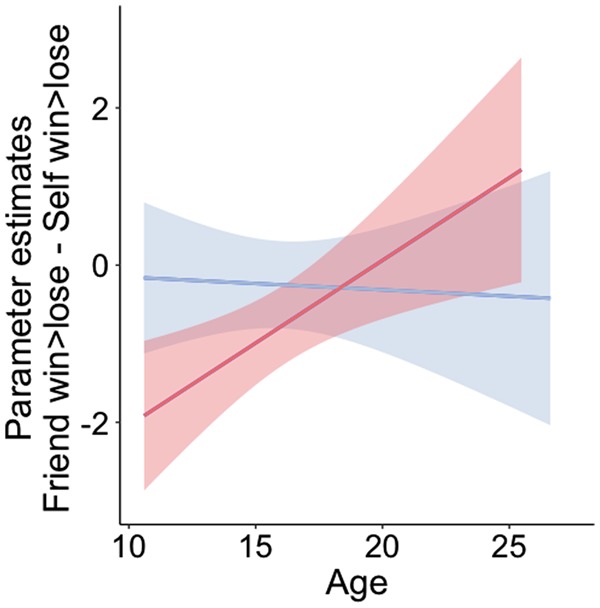
Interaction effect of age and sex for the difference in parameter estimates for win > lose for friend and win > lose for self in the left nucleus accumbens (nacc).
For mother, the best fitting model was model with a linear and quadratic regressor for age. Results showed a significant quadratic relationship with age for the contrast win > lose for mother for the right Nacc and this relationship was significant at trend level for the left Nacc [left Nacc: age βquadratic = −3.83, t(230) = −1.95, P = 0.052; right Nacc: age βquadratic = −6.13, t(230) = −2.79, P = 0.006; Figure 7, Table 3). We then compared win > lose for mother and win > lose for self. There were no significant linear or quadratic age effects and no main or interaction effect with sex.
Fig. 7.
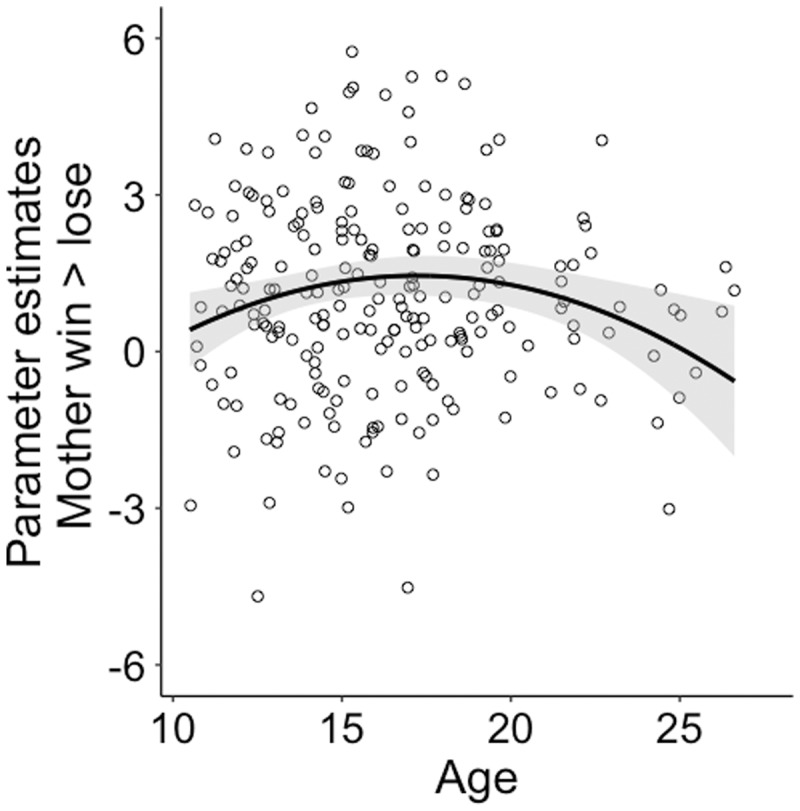
Quadratic regression with age and the parameter estimate for the contrast mother win > lose for the right nucleus accumbens (nacc).
Table 3.
Regression coefficients (B), standard error, t values and P values for the models for the difference score mother win > lose – friend win > lose
| B | Std Error | t value | P value | |
|---|---|---|---|---|
| Left Nacc | ||||
| Intercept | 0.55 | 0.26 | 2.12 | 0.035* |
| Age Linear | −8.57 | 4.24 | −2.02 | 0.044* |
| Age Quadratic | −1.89 | 4.49 | −0.42 | 0.674 |
| Sex | −1.00 | 0.37 | −2.69 | 0.008** |
| Age Linear*Sex | 11.50 | 5.71 | 2.02 | 0.045* |
| Age Quadratic*Sex | −1.76 | 5.81 | −0.30 | 0.763 |
| Right Nacc | ||||
| Intercept | 0.54 | 0.29 | 1.90 | 0.059 |
| Age Linear | −7.72 | 4.66 | −1.66 | 0.099 |
| Age Quadratic | −0.85 | 4.93 | −0.17 | 0.864 |
| Sex | −1.40 | 0.41 | −3.42 | 0.001** |
| Age Linear*Sex | 8.98 | 6.27 | 1.43 | 0.153 |
| Age Quadratic*Sex | −5.25 | 6.39 | −0.82 | 0.412 |
Indicators of significance: †P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001.
Differences in neural responses to rewards for mother and friend
The next question was whether the relative differences for neural responses to winning for mother and friend were different for any of the ages. To answer this question, we calculated a difference score between contrast values for win > lose for mother and win > lose for friend. Positive values indicate higher neural responses for mother and negative values indicate higher neural responses for friend. Again we tested a model that included linear and quadratic effects of age, and main and interaction effects of sex.
We found a significant linear main effect of age [left Nacc: age βlinear = −10.43, t(227) = −2.48, P = 0.014; right Nacc: age βlinear = −9.01, t(227) = −1.95, P = 0.052], a main effect of sex [left Nacc: sexβ = −0.982, t(227) = −2.66, P = 0.008; right Nacc: sexβ = −1.423, t(227) = −3.52, P = 0.0005] and an interaction effect between the linear regressor of age and sex [left Nacc: interaction age × sex β = 14.80, t(227) = 2.61, P = 0.009; right Nacc: interaction age × sexβ = 11.18, t(227) = 1.79, P = 0.074]. This interaction effect indicates that neural responses to rewards for friends and mother follow a different trajectory over age for males and females. To follow up on this interaction, we tested the age effect separately for females and males. For females, neural responses to winning shifted from a stronger response for mothers in childhood and early adolescence to a stronger response for friends in late adolescence and early adulthood [left Nacc: age βlinear = −0.18, t(114) = −2.42, P = 0.017; right Nacc: age βlinear = −0.15, t(114) = −1.93, P = 0.057] whereas for males neural responses to winning for mother and friend were similar across ages [left Nacc: βlinear = 0.070, t(115) = 1.07, P = 0.289; right Nacc: βlinear = 0.028, t(115) = 0.37, P = 0.709; Figure 8).
Fig. 8.
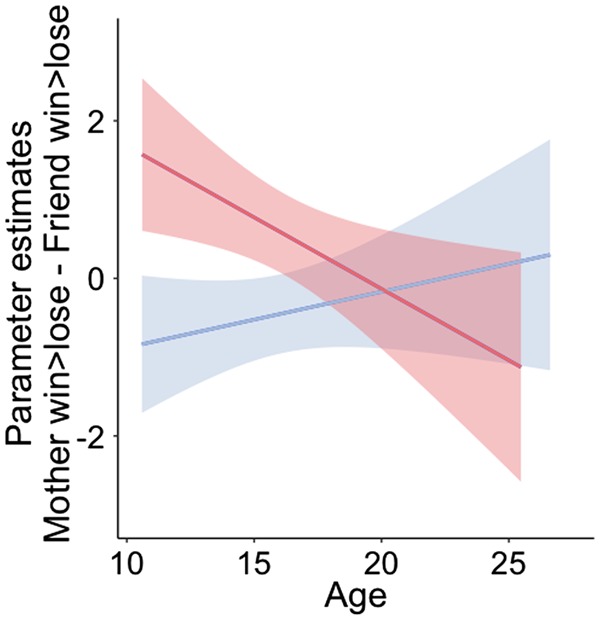
Interaction effect between age and sex in the left nucleus accumbens. The dependent variable was a difference score between parameter estimates for the contrast [win > lose for mother] – [win > lose for friend]. Positive values indicate higher neural responses for mother and negative values indicate higher neural responses for friend. For females, neural responses to winning shifted from a stronger response for mother in childhood/early adolescence to a stronger response for friends in young adulthood. In contrast, for males neural responses to winning for mother and friend do not significantly differ with age.
Relations with self-report measures
Finally, we tested for relations between neural activation for the contrasts win > lose for friend and win > lose mother with self-reported winning pleasure and IOS scales. We fitted separate regression models for the IOS rating for mother and friend. In these models, the IOS rating was the dependent variable and age, sex and an interaction term for age and sex as well as a regressor for the parameter estimates win > lose were included as independent variables. There were no relationships between neural activation and self-report measures.
Discussion
In this study, we tested how responses to vicarious rewards for friends and mothers change over adolescent development. To do so, we used a combination of behavioral and neural measures. Results showed that both subjective behavioral ratings for winning (i.e. winning pleasure) for friends and mothers, and relationship closeness with mothers and friends decreased with age. When controlling for age, relationship closeness and winning pleasure for mothers were positively related. On the neural level, whole brain contrasts for winning > losing money for peers and mothers resulted in robust activation in the ventral striatum, consistent with prior research (Braams et al., 2014; Fareri et al., 2012; Telzer et al., 2010). Age and sex related changes in neural responses for mothers and friends were also present and these results are discussed in more detail below.
Winning vs losing for friend and mother
Interestingly, there were no age-related differences in ventral striatum responses in adolescence when playing for friends, even though peers are a very salient social context during this life period (Degirmencioglu et al., 1998; Steinberg and Morris, 2001). When playing for mother we found a quadratic effect of age, with the highest contrast values in mid-adolescence. These findings mirror previous results showing heightened bilateral ventral striatum activity in mid adolescence relative to younger and older participants when playing for self (Braams et al., 2015). The similarity between these patterns indicates that the heightened reward sensitivity in mid adolescence is not limited to hedonic (i.e. self related) rewards. These findings fit well with recent insights showing that heightened rewards activity in mid adolescence is not only associated with negative outcomes, such as increased risk taking (Galvan et al., 2007) or alcohol use (Braams et al., 2016), but can also be related to greater motivation to being prosocial towards family (Telzer, 2016). The neural responses to rewards for mothers may coincide with stronger prosocial values towards family members (Telzer, 2016).
Further support for the notion that the ventral striatum response is linked to prosocial values comes from a study in which adolescents could win money for their family members. Adolescents who report feeling happier on days that they help their family, also show higher responses in the ventral striatum when winning money for their family (Telzer et al., 2010, 2011).
Sex differences when winning vs losing for a friend
Even though, there were no general age differences in neural activity in the ventral striatum when winning vs losing for friends, interesting sex differences were found in behavioral ratings and neural responses. First, girls reported higher levels of closeness to their friends than boys, consistent with prior research showing that girls often experience a more intimate relationship with their best friend, whereas boys often interact with larger groups of peers (Galambos, 2004; Maccoby, 1990).
At the neural level, however, boys showed in general higher neural responses in the ventral striatum when winning for friends than girls. Prior studies have suggested that there may be differences between prosocial motivation (feeling closeness to friends) and prosocial actions. A behavioral study found that boys were more willing to share resources with unknown others when this resulted in mutual benefit, whereas girls preferred equity of outcomes (Meuwese et al., 2015). Possibly, girls experience more prosocial intentions and motivations, whereas boys experience more reward when actually sharing with friends. This gender discrepancy between prosocial motivation and prosocial outcomes, and the relation with neural activity, should be examined in more detail in future studies.
Two additional developmental sex differences emerged. First, when comparing winning for friends with winning for self, there was an age-related increase in winning for friends relative to self, specifically for girls. Second, when comparing winning for friends with winning for mothers, again there was an age-related increase in winning for friends relative to mother, specifically for girls. Together these findings suggest that there may be a developmental change in prosocial motivations related to friends in adolescent girls, such that vicarious rewards for friends start to play a larger role in late adolescence and adulthood. Future studies should test the relation between vicarious rewards, behavior, and self-reported friendship relations in more detail.
Limitations
The current study also had limitations that should be addressed in future research. First, the current paradigm was not set up to distinguish between developmental trajectories of neural responses to wins and losses separately. Future studies could include a baseline condition, such as a zero win or loss condition, to investigate whether striatum responses to wins peak in adolescence or adolescents are more sensitive to losses. For the current data set, we can only interpret the relative differences between winning and losing, therefore it remains to be determined if the results are reflecting an increase for rewards or a decrease for losing over time (see also Galvan and McGlennen, 2013).
Second, the adolescents in this sample overall reported high closeness to their mothers. This may explain the absence of a relationship between the neural measures and behavioral ratings, i.e. possibly the measures were not sensitive enough to detect individual differences. It will be important in future studies to have a larger battery of behavioral measures to link neural activity for vicarious rewards to behavioral measures.
A third limitation of the current study is the cross-sectional design. Future studies should test if these results are predictive of developmental outcomes later in time.
Conclusion
In conclusion, this study showed that neural responses to vicarious rewards for close others results in ventral striatum activation (Morelli et al., 2015). Furthermore, we show that the developmental trajectory of neural responses to vicarious rewards is dependent on the beneficiary. In girls, neural responses to vicarious rewards for friends compared to rewards for self increase over development, whereas neural responses to rewards for mothers peak in mid to late adolescence (16–17 years). The current results support the account that friends and mothers are important social factors across adolescence (Güroğlu et al., 2007).
Funding
This work was supported by a European Research Council (ERC) starting grant (ERC-2010-StG-263234) awarded to E.A.C.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
References
- Aron A., Aron E.N., Smollan D. (1992). Inclusion of other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology, 63(4), 596–612. [Google Scholar]
- Braams B.R., Güroğlu B., de Water E., et al. (2014a). Reward-related neural responses are dependent on the beneficiary. Social Cognitive and Affective Neuroscience, 9(7), 1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Peper J.S., van der Heide D., Peters S., Crone E.A. (2016). Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Developmental Cognitive Neuroscience, 17, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroğlu B., Crone E.A. (2014b). Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage, 100, 281–9. [DOI] [PubMed] [Google Scholar]
- Braams B.R., van Duijvenvoorde A.C., Peper J.S., Crone E.A. (2015). Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. Journal of Neuroscience, 35(18), 7226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. (2002). Region of interest analysis using an SPM toolbox. Neuroimage, 16(2), 497. [Google Scholar]
- Cocosco R.A., Kollokian V., Kwan R.K.S., Evans A.C. (1997). Brain web: online interface to a 3D MRI simulated brain database. Neuroimage, 5, S452. [Google Scholar]
- Colarossi L.G., Eccles J.S. (2000). A prospective study of adolescents' peer support: gender differences and the influence of parental relationships. Journal of Youth and Adolescence, 29(6), 661–78. [Google Scholar]
- Dale A.M. (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8(2–3), 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goede I.H., Branje S.J., Delsing M.J., Meeus W.H. (2009a). Linkages over time between adolescents' relationships with parents and friends. Journal of Youth and Adolescence, 38(10), 1304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goede I.H., Branje S.J., Meeus W.H. (2009b). Developmental changes in adolescents' perceptions of relationships with their parents. Journal of Youth Adolescence, 38(1), 75–88. [DOI] [PubMed] [Google Scholar]
- Degirmencioglu S.M., Urberg K.A., Tolson J.M., Richard P. (1998). Adolescent friendship networks: continuity and change over the school year. Merrill-Palmer Quarterly-Journal of Developmental Psychology, 44(3), 313–37. [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Niznikiewicz M.A., Lee V.K., Delgado M.R. (2012). Social network modulation of reward-related signals. Journal of Neuroscience, 32(26), 9045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos N.L. (2004). Gender and gender role development in adolescence. Handbook of Adolescent Psychology, 2, 233–62. [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. (2007). Risk-taking and the adolescent brain: who is at risk? Developmental Science, 10(2), F8–F14. [DOI] [PubMed] [Google Scholar]
- Galvan A., McGlennen K.M. (2013). Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience, 25(2), 284–96. [DOI] [PubMed] [Google Scholar]
- Güroğlu B., van Lieshout C.F.M., Haselager G.J.T., Scholte R.H.J. (2007). Similarity and complementarity of behavioral profiles of friendship types and types of friends: friendships and psychosocial adjustment. Journal of Research on Adolescence, 17(2), 357–85. [Google Scholar]
- Helsen M., Vollebergh W., Meeus W. (2000). Social support from parents and friends and emotional problems in adolescence. Journal of Youth and Adolescence, 29(3), 319–35. [Google Scholar]
- Jenkins S.R., Goodness K., Buhrmester D. (2002). Gender differences in early adolescents' relationship qualities, self-efficacy, and depression symptoms. Journal of Early Adolescence, 22(3), 277–309. [Google Scholar]
- Lourenco F.S., Decker J.H., Pedersen G.A., Dellarco D.V., Casey B.J., Hartley C.A. (2015). Consider the source: adolescents and adults similarly follow older adult advice more than peer advice. PLoS One, 10(6), e0128047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby E.E. (1990). Gender and relationships: a developmental account. American Psychologist, 45(4), 513.. [DOI] [PubMed] [Google Scholar]
- Meuwese R., Crone E.A., de Rooij M., Guroglu B. (2015). Development of equity preferences in boys and girls across adolescence. Child Development, 86(1), 145–58. [DOI] [PubMed] [Google Scholar]
- Morelli S.A., Sacchet M.D., Zaki J. (2015). Common and distinct neural correlates of personal and vicarious reward: a quantitative meta-analysis. Neuroimage, 112, 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Mandl R.C., Braams B.R., et al. (2013). Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cerebral Cortex, 23(7), 1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Koolschijn P.C., Crone E.A., Van Duijvenvoorde A.C., Raijmakers M.E. (2014). Strategies influence neural activity for feedback learning across child and adolescent development. Neuropsychologia, 62, 365–74. [DOI] [PubMed] [Google Scholar]
- Silverman M.H., Jedd K., Luciana M. (2015). Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage, 122, 427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. (2001). Adolescent development. Annual Review of Psychology, 52, 83–110. [DOI] [PubMed] [Google Scholar]
- Telzer E.H. (2016). Dopaminergic reward sensitivity can promote adolescent health: a new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galvan A. (2013). Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience, 3, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N.T., Qu Y. (2015). Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10(10), 1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2010). Gaining while giving: an fMRI study of the rewards of family assistance among White and Latino youth. Social Neuroscience, 5(5–6), 508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. Neuroimage, 58(1), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.M., Telzer E.H., Fuligni A.J. (2013). Continuity and discontinuity in perceptions of family relationships from adolescence to young adulthood. Child Development, 84(2), 471–84. [DOI] [PubMed] [Google Scholar]
- Updegraff K.A., Helms H.M., McHale S.M., Crouter A.C., Thayer S.M., Sales L.H. (2004). Who's the boss? Patterns of perceived control in adolescents' friendships. Journal of Youth and Adolescence, 33(5), 403–20. [Google Scholar]
- Wechsler D. (1997). Wechsler adult intelligence scale — third edition. Administration and scoring manual. The Psychological Corporation, San Antonio. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.