Abstract
Cortical networks underpinning attentional control and mentalizing converge at the right temporoparietal junction (rTPJ). It is debated whether the rTPJ is fractionated in neighboring, but separate functional modules underpinning attentional control and mentalizing, or whether one overarching cognitive mechanism explains the rTPJ’s role in both domains. Addressing this question, we combined attentional control and mentalizing in a factorial design within one task. We added a social context condition, in which another individual’s mental states became apparently task-relevant, to a spatial cueing paradigm. This allowed for assessing cue validity- and context-dependent functional activity and effective connectivity of the rTPJ within corresponding cortical networks. We found two discriminable rTPJ subregions, an anterior and a posterior one. Yet, we did not observe a sharp functional dissociation between these two, as both regions responded to attention cueing and social context manipulation. The results suggest that the rTPJ is part of both the ventral attention and the ToM network and that its function is defined by context-dependent coupling with the respective network. We argue that the rTPJ as a functional unit underpins an overarching cognitive mechanism in attentional control and mentalizing and discuss how the present results help to further specify this mechanism.
Keywords: anterior cingulate cortex, attention, dynamic causal modeling, right temporoparietal junction, Theory of Mind
Phylogenetic and ontogenetic development of the human brain forms cortical networks to serve cognitive mechanisms that are essential for survival (Bullmore and Sporns, 2012). One of these is the ventral attention network, necessary for directing the attentional focus towards unexpected salient and behaviorally relevant objects in the environment (Corbetta et al., 2008). Another one is the Theory of Mind (ToM) network, which underpins mentalizing, the ability to attribute mental states to other individuals in order to explain, predict, and manipulate their behavior (Van Overwalle, 2009).
Surprisingly, although these cognitive mechanisms apparently differ substantially, evidence from almost two decades of research in both domains converged on a key role of the right temporoparietal junction (rTPJ) in both networks and associated cognitive processes. It was proposed that the rTPJ, together with the middle and inferior frontal gyrus, the frontal operculum, and the anterior insula, constitute the ventral attention network. Activity of this network disrupts the current attentional focus in situations when an unattended but relevant object is detected. This allows for disengaging from the current attentional set and reorienting attention towards the new object (Corbetta et al., 2008).
It was also suggested that the rTPJ, together with the left TPJ, the bilateral posterior superior temporal sulcus, the bilateral dorsal and ventral part of the medial prefrontal cortex and adjacent anterior cingulate cortex (ACC), the bilateral dorsolateral prefrontal cortex, and the precuneus, form the ToM network. Within the ToM network, the rTPJ processes models of others’ and one’s own mental states and their relation to the environment (what do I/does someone else see, know, believe in a certain situation; Decety and Sommerville, 2003; Mar, 2011).
Based on the premise that there is a one-to-one mapping of cognitive mechanisms and brain structures (Henson, 2005), heated debates on the rTPJ’s cognitive function arose. One position, termed ‘fractionation view’, claimed that neighboring, but structurally distinct functional modules within the rTPJ underpin attentional control and mentalizing (e.g. Nelson et al., 2012; Scholz et al., 2009). Specifically, connectivity-based parcellation studies identified an anterior rTPJ subregion associated with attentional control, and a posterior rTPJ subregion related to mentalizing (Mars et al., 2012; Bzdok et al., 2013). In contrast, recent integrative accounts, adopting an ‘overarching view’, try to characterize one common cognitive mechanism to explain the rTPJ’s function in both domains (Cabeza et al., 2012a; Carter and Huettel, 2013; Geng and Vossel, 2013; Kubit and Jack, 2013; Krall et al., 2016).
Several attempts were made to empirically clarify the rTPJ’s cognitive role in attentional control and mentalizing. However, so far, neither within-subject conjunct analyses of functional activity in a spatial cueing and a ToM task (Mitchell, 2008; Scholz et al., 2009; Lee and McCarthy, 2016), nor meta-analyses of neuroimaging studies on attention and ToM (e.g. Decety and Lamm, 2007; Cabeza et al., 2012a; Kubit and Jack, 2013; Krall et al., 2014) have solved this issue. One reason for this is an inferential barrier from which both approaches suffer. Comparing rTPJ activity obtained from two distinct tasks (and in meta-analyses also from different participants) is unsuited to identify a rTPJ cluster that simultaneously engages in both attentional control and mentalizing. This requires a factorial combination of both cognitive processes within the same task and participant. Such a design would allow for the conclusion whether the rTPJ underpins one but not the other cognitive mechanism, or whether both domains rely on a common cognitive mechanism.
Here, we introduced a context factor to an fMRI version of the Posner spatial cueing paradigm. The participants reacted to targets that were validly or invalidly cued. Using a cover story, we manipulated the participants’ belief about the origin of the cue. In a non-social context, they were convinced that the cues were computer-based (i.e. a computer-based prediction of the following target’s appearance). In a social context, they believed that cues were sent by a confederate outside the scanner, thus inducing a context in which another’s mental states became apparently task-relevant (i.e. the confederate’s prediction of target appearance). Previous research demonstrated that in such a task manipulation, participants adopt an intentional stance, thereby considering another individual’s mental states (Gallagher and Frith, 2003; Teufel et al., 2010; Wykowska et al., 2014). The appreciation of another’s mental state in an interactive situation is an essential form of mentalizing. Other processes that are circumscribed by the umbrella term mentalizing, are reasoning about another’s false or true beliefs (e.g. Schuwerk et al., 2014), or visual perspective taking (e.g. Schurz et al., 2015).
According to the fractionation view, distinct rTPJ subregions should be engaged in either attentional control or mentalizing, without any interaction with the respective other domain. In contrast, if the rTPJ underpins one overarching cognitive mechanism relevant for both domains, we expect to find non-separable functional activity of the rTPJ associated with attentional control and mentalizing.
It was previously suggested that the rTPJ’s function could only be understood by investigating its task-dependent interactions with other brain regions within respective brain networks (Seghier, 2013). Thus, in addition to the analysis of functional activity, we investigated in what way invalid cueing and social context modulated effective connectivity between an anterior and a posterior rTPJ subregion, related to attentional control and/or mentalizing, and the ACC, an important node within the ToM network that engages in processing mental states in interactive social contexts (Gallagher et al., 2002, Haroush and Williams, 2015). This made it possible to investigate how cognitive mechanisms are instantiated by the interplay of brain regions in and between task-dependent networks.
Materials and Methods
Participants
Twenty-four adults took part in this study (Mage = 22.8 years, s.d. = 3.0 years; 12 female). One additional participant had to be excluded due to excessive scan-to-scan head movement. All participants were unambiguously right-handed (indexed by a handedness inventory; Oldfield, 1971), had no history of neurological or psychiatric condition, had normal or corrected-to-normal vision, gave informed written consent and received monetary compensation. The study was approved by the University Medical Center Regensburg Ethics Committee.
Task and design
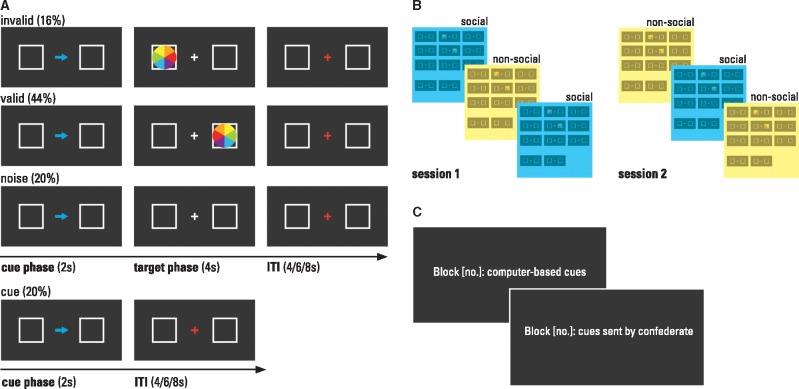
The set-up of the current Posner spatial cueing paradigm (Posner, 1980) closely followed descriptions of previous fMRI versions of this task (Corbetta et al. 2000; Mitchell 2008; Scholz et al. 2009; for details on conditions and timings see Figure 1A and Supplementary Materials). To introduce a social vs non-social context, we manipulated the participants’ belief about the origin of the cue. As a result of the cover story and the training phase (described in detail in Supplementary Materials), the participants were convinced that the arrow was either sent by a confederate outside the scanner to cue target appearance or that it was a computer-based cue of the following target. The trials were blocked according to this context factor (see Figure 1B). The order of social versus non-social context blocks was counterbalanced between participants. To keep the context type salient in each trial throughout the whole experimental block, the arrow color (yellow/blue, counterbalanced across participants) indicated the supposed source of the cue. Thus, the only difference between social and non-social context conditions was the subject’s belief about the origin of the cues. Physical properties of stimuli and trial timings were identical.
Fig. 1.
Task and experimental design. (A) Examples of stimuli and trial types. (B) Design: the context factor was blocked (counterbalanced across subjects) and blocks were separated in two sessions. (C) Blocks were preceded by an instruction screen (presented for 30s) which indicated block number and context type. The participants completed a total of six blocks (50 trials per block, alternating contexts, order counterbalanced across subjects), divided by a short break into two scan sessions. The total task duration was 59 min.
Procedure
We used a cover story to convince the participants that they would receive either computer-based cues or cues from a human confederate outside the scanner. Prior to the scan session, we employed the following briefing protocol: The participants were told that this study was being conducted in cooperation with a German automobile manufacturer to test how humans react to novel alert systems during driving. Particularly, two systems under development were being tested: First, new sensor technology that informs the driver about potential dangers, such as a damaged road surface. Second, via a display in the car, a technology, which enables other road users to inform the driver about potential threats, e.g., an accident at the next intersection. Furthermore, they were told that to investigate how the human brain reacts to those different sources of information, we would test how fast and accurately participants reacted to computer- or human-based cues in an attention paradigm. Thus, in half of the blocks, the cue indicating target appearance (the arrow in the Posner spatial cueing paradigm), would be computer-based (non-social context). In the other half of the blocks, a confederate outside the scanner would send the cue (social context). When the cue is computer-based, the likelihood of target appearance results from information sampled by sensor technology. In the other condition, the confederate interprets information about the likelihood of target appearance and determines the side on which the arrow is most likely to appear by sending the arrow cue to the participant inside the scanner. They were also told before each block, it would be indicated what source of information comes next. Additionally, arrow color (blue or yellow; counterbalanced across participants) would indicate the nature of the cue. Results from a debriefing protocol completed after scanning showed that all participants were convinced by the cover story. Details on this procedure and on the debriefing protocol are provided in Supplementary Materials.
Behavioral data analysis
We analyzed reaction times (from target onset until button press) performing a 2 × 2 repeated measures analysis of variance (ANOVA). Within subject factors were context (social vs non-social) and validity (invalid vs valid). The significance level was set at P ≤ 0.05.
Imaging and image analyses
The participants were scanned employing a 3-Tesla head scanner (Siemens Allegra, Erlangen, Germany). The functional blood oxygenation level dependent images were obtained using a T2*-weighted EPI sequence (TR = 2 s, TE = 0.05 s, 90° flip angle, FoV = 192 mm, plane matrix 64 × 64, voxel size 3 × 3 × 3 mm, 32 axial slices per volume). We acquired 1800 volumes (900 in each session). For structural imaging we used a T1*-weighted MPRAGE sequence (TR = 2.25 s, TE = 0.026 s, TI = 0.9 s, FoV = 256 mm, voxel size 1 × 1 × 1 mm, 160 axial slices). The first four functional volumes at the beginning of each run were discarded to allow for T1 equilibration.
The images were preprocessed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) running in MATLAB 8 (The MathWorks Inc., Natick, MA). Statistical analyses were performed in SPM12. For details on preprocessing and first level analysis see Supplementary Methods. In brief, on the individual level, we generated statistical parametric maps (SPMs) by t-statistics derived from contrasts utilizing the HRF. The current design aimed to isolate brain regions associated with attentional orienting towards invalidly and validly cued targets in a social and non-social context, respectively. To this end, we obtained the closest possible contrast by calculating participant-specific contrast images for social invalid vs social noise, social valid vs social noise, non-social invalid vs non-social noise and non-social valid vs non-social noise.
These four contrasts were entered into a second-level 2 × 2 within-subjects ANOVA (flexible factorial design including an additional subject factor; see Glascher and Gitelman, 2008) with the factors context (social vs non-social) and validity (invalid vs valid). The resulting SPM maps were thresholded at P < 0.001, uncorrected. The reported significant voxels from t-statistics survived a threshold of P < 0.05, FWE (family-wise error)-corrected for multiple comparisons on cluster level.
Dynamic causal modeling
Effective connectivity was analyzed using DCM12 implemented in SPM12. Our analyses adhered to previously described theoretical and methodological recommendations (Friston et al., 2003; Stephan et al., 2010). First, volumes of interests were defined following a combination of structural and functional criteria. Participant-specific time series were extracted from regions of interest in the rTPJ/right middle temporal gyrus (rMTG), rTPJ/right superior temporal gyrus (rSTG) and the ACC. After model space specification and model estimation, Bayesian model selection (BMS) and Bayesian model averaging (BMA) were employed to optimally characterize resulting average model parameters. Supplementary Methods give further information on this procedure.
Results
Behavior
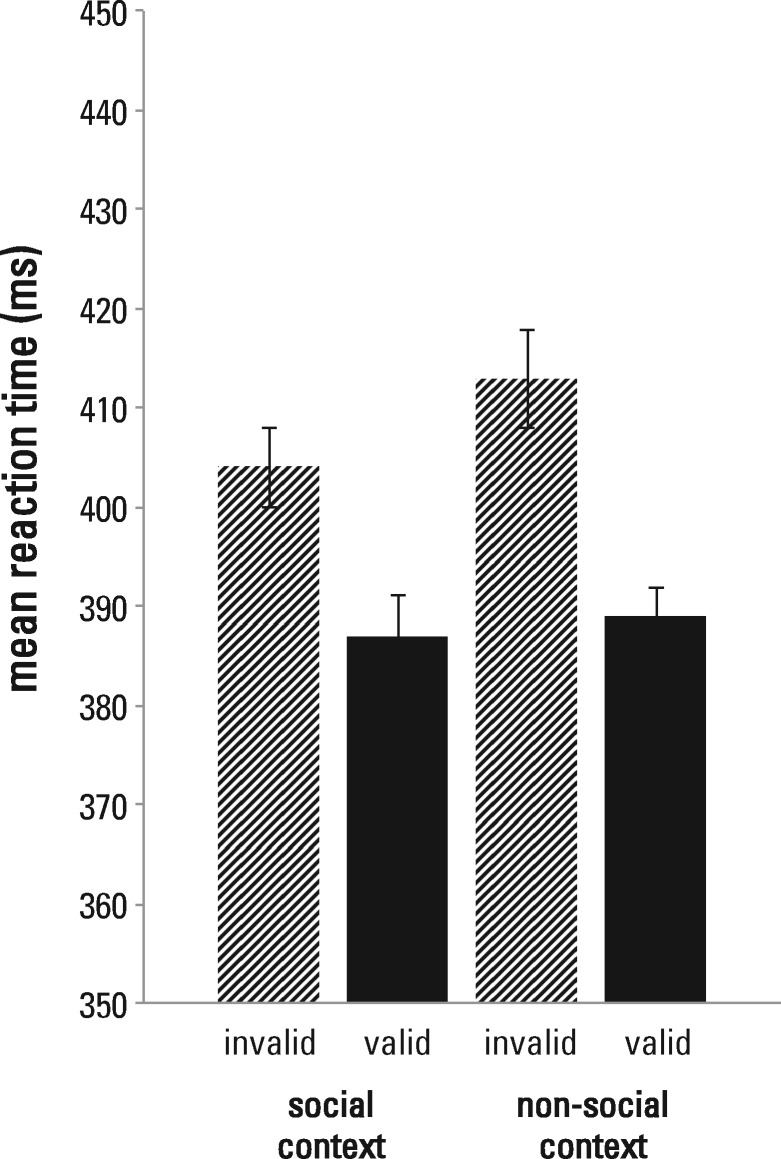
On average, participants missed 1.4% (SE = 0.3%) of the responses, indicating high overall accuracy for target detection. Mean reaction times and standard errors for each condition are provided in Figure 2. The 2 (context: social vs non-social) × 2 (validity: invalid vs valid) repeated measures ANOVA revealed a significant main effect of validity, F(1,23) = 28.35, P < 0.001, ηp2 = 0.55, showing that the participants oriented their spatial attention according to the cue. Their reaction times were faster for validly (M = 388 ms, SE = 18 ms) than for invalidly (M = 408, SE = 18 ms) cued targets. Neither a significant main effect of context, F(1,23) = 2.81, P = 0.107, ηp2 = 0.11, nor a significant interaction between context and validity, F(1,23) = 1.32, P = 0.262, ηp2 = 0.05, were observed. This suggests that believing the arrow cues were computer-based or sent by a confederate, did not affect response latencies for target detection.
Fig. 2.
Behavioral results. Averaged mean reaction times (±SEM, corrected for between-subject variability) for invalid and valid trials in the social and non-social context. We found a main effect of validity. Neither a significant main effect of context, nor a significant validity × context interaction were observed.
Imaging
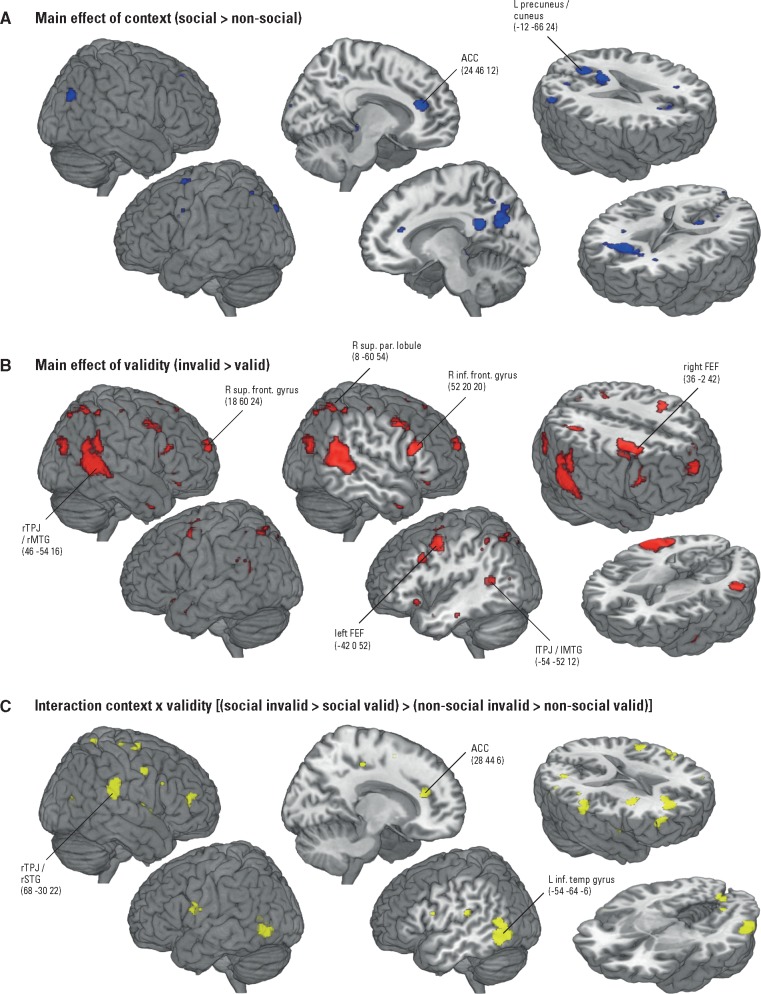
Main effect of context. The main effect of context (social > non-social; Table 1A, Figure 3A) revealed significantly increased functional activity in the bilateral medial prefrontal cortex, namely in the right superior frontal lobule [Brodmann area (BA) 10], and the left and right ACC (BA 32). This activity closely relates to a region associated with the processing of mental states in interactive contexts, identified by a recent meta-analytic analysis of ToM network activity (Schurz et al., 2014). Further, the cuneus/precuneus (BA 7/18) was significantly activated. In the reverse contrast (non-social > social) we observed a significant cluster in the left middle temporal pole (BA 38; Table 1B). Notably, this region was previously reported in fMRI studies on ToM, but its activity was not associated with core mentalizing processes (Mar, 2011). For example, Frith and Frith (2003) concluded that left temporal pole activity reflects the retrieval of semantic scripts that support mentalizing. Therefore, we concluded that the observed activity in the current contrast might reflect semantic processing of abstract, conceptual properties of our stimuli (Peelen and Caramazza, 2012). However, as this inference is reverse and we lack hypotheses for this contrast and region, we do not discuss this finding in more detail.
Table 1.
Whole brain imaging results for the 2 (context: social vs non-social) × 2 (validity: invalid vs valid) flexible factorial design
| MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Contrast/Brain region | BA | x | y | z | Cluster sizea | T-valueb |
| (A) Main effect of context (social > non-social) | ||||||
| R superior frontal lobule (ACC) | 10 | 24 | 46 | 12 | 333 | 5.79 |
| R ACC | 32 | 6 | 32 | 16 | 5.76 | |
| L ACC | −12 | 32 | 16 | 3.39 | ||
| L cuneus/precuneus | 18 | −12 | −66 | 24 | 183 | 4.80 |
| L precuneus | 7 | −10 | −68 | 38 | 3.46 | |
| (B) Main effect of context (non-social > social) | ||||||
| L middle temporal pole | −42 | 0 | −30 | 210 | 5.76 | |
| L middle temporal pole | 38 | −52 | 6 | −26 | 5.14 | |
| L middle temporal pole | −34 | −6 | −24 | 3.59 | ||
| (C) Main effect of validity (invalid > valid) | ||||||
| rTPJ/rMTG | 39 | 46 | −54 | 16 | 1944 | 7.18 |
| rTPJ/rMTG | 21 | 58 | −44 | 4 | 6.54 | |
| R middle occipital gyrus | 19 | 44 | −78 | 24 | 6.06 | |
| R superior frontal gyrus | 10 | 18 | 60 | 24 | 174 | 5.35 |
| R frontal superior medial gyrus | 9 | 8 | 56 | 36 | 3.76 | |
| L precentral gyrus (left FEF) | 6 | −42 | 0 | 52 | 383 | 5.08 |
| L precentral gyrus | 6 | −38 | 0 | 34 | 4.59 | |
| −28 | 4 | 42 | 3.79 | |||
| R precentral gyrus (right FEF) | 36 | −2 | 42 | 554 | 4.69 | |
| R precentral gyrus | 6 | 42 | 2 | 48 | 4.23 | |
| R middle frontal | 26 | 2 | 36 | 4.00 | ||
| R superior parietal lobule | 7 | 8 | −60 | 54 | 821 | 4.61 |
| R superior parietal lobule | 7 | 32 | −58 | 58 | 4.38 | |
| R angular gyrus | 39 | 32 | −54 | 44 | 4.33 | |
| lTPJ/lMTG | 39 | −54 | −52 | 12 | 190 | 4.49 |
| lMTG | 39 | −60 | −54 | 22 | 3.38 | |
| R inferior frontal gyrus | 44 | 52 | 20 | 20 | 234 | 4.43 |
| R inferior frontal gyrus | 44 | 44 | 18 | 28 | 3.65 | |
| (D) Interaction context × validity [(social-invalid > social-valid) > (non-social invalid > non-social valid)] | ||||||
| rTPJ/rSTG | 40 | 68 | −30 | 22 | 195 | 4.86 |
| rSTG | 22 | 56 | −32 | 18 | 4.18 | |
| R middle frontal lobule (ACC) | 10 | 28 | 44 | 6 | 198 | 4.75 |
| R superior frontal lobule | 10 | 22 | 46 | 14 | 4.49 | |
| R ACC | 9 | 14 | 34 | 18 | 3.99 | |
| L inferior temporal gyrus | 37 | −54 | −64 | −6 | 267 | 4.72 |
| lMTG | 39 | −50 | −60 | 8 | 3.90 | |
| lMTG | 19 | −52 | −70 | 2 | 3.33 | |
Peak activations (PFWE-corr < 0.05, cluster level) for the (A, B) main effect of context (social > non-social and non-social > social), (C) the main effect of validity (invalid > valid) and the (D) interaction validity × context [(social-invalid > social-valid) > (non-social-invalid > non-social-valid)]. Reverse contrasts are only reported if significantly increased functional activity was observed.
Notes: BAs are approximate. L, left; R, right; ACC, anterior cingulate cortex; r/lTPJ, right/left temporoparietal junction; FEF, frontal eye field; r/lMTG, right/left middle temporal gyrus; rSTG, right superior temporal gyrus.
Number of activated voxels per cluster.
Peak T-value in activated cluster.
Fig. 3.
Findings of the 2 (context: social vs non-social) × 2 (validity: invalid vs valid) flexible factorial design, shown at voxel-wise P < 0.001 and PFWE-corr < 0.05 on cluster level, overlayed onto a MRI brain template and displayed in neurological convention. (A) Main effect of context: Significant activations (in blue) for the social vs non-social context condition. In the social context condition the participants believed the cues were sent by the confederate outside the scanner, in the non-social context condition the participants believed the cues were computer-based. (B) Main effect of validity: Significantly increased functional activity (in red) in response to invalidly cued targets. C. Interaction context × validity: Significant activations (in yellow) related to detecting invalidly cued targets, modulated by social vs non-social context.
Main effect of validity. For the main effect of validity (invalid > valid; Table 1C, Figure 3B) we found activations in the posterior part of the rMTG and in the adjacent occipital gyrus (BA 19/21/39). This area closely corresponds to the previously rTPJ-labeled region associated with attentional control in response to the appearance of an invalidly cued target (for meta-analyses see, Decety and Lamm, 2007; Kubit and Jack, 2013). We will refer to it as rTPJ/rMTG. We further observed activations in the right superior frontal gyrus (BA 10) and adjacent right frontal superior medial gyrus (BA 9), left and right precentral gyrus (BA 6; previously referred to as left and right frontal eye field (FEF); e.g. Shulman et al. 2003; DiQuattro et al. 2013), right superior parietal lobule (BA 7) extending into the right angular gyrus (BA 39), lTPJ/lMTG (BA 39), and right inferior frontal gyrus (BA 44). The reverse contrast (valid > invalid) yielded no significantly increased functional activity.
Context × validity interaction. We found a significant activation in the rSTG (BA 22/40) for the interaction between context and validity [(social-invalid > social-valid) > (non-social invalid > non-social valid), Table 1D, Figure 3C]. This cluster is more anterior to the rTPJ/rMTG region observed in the main effect of validity. Because it also falls within the region named rTPJ in previous literature (Kubit and Jack 2013), we labeled it rTPJ/rSTG accordingly. The interaction also revealed significant activations in the right ACC (BA 9/10) and in the left inferior and middle temporal gyrus (MTG) (BA 19/37/39). This shows that activity in these regions in response to invalidly and validly cued targets was influenced by the participant’s belief about cue source. The reverse interaction showed no significant activations.
Dynamic causal modeling
The whole brain findings (i) replicated the rTPJ/rMTG’s involvement in attentional control during the detection of invalidly cued targets, (ii) revealed significantly increased ACC activity in a social vs non-social context and (iii) suggested that social context modulates activity in the rTPJ/rSTG and ACC during invalidly cued target detection. Crucially, the functional imaging results revealed two separate clusters within the rTPJ: the more posterior rTPJ/rMTG in the main effect of validity, and the more anterior rTPJ/rSTG in the context × validity interaction.
Consequently, we performed two DCM analyses modeling connectivity of each rTPJ subregion with the ACC under the modulatory influence of invalid cues and social context. We were interested if and how each of the rTPJ subregions interact during the detection of an invalidly vs validly cued target in a social vs non-social context. In DCM analysis 1, we modeled the connectivity between the more posterior rTPJ/rMTG and the ACC. In the complementary DCM analysis 2, connectivity between the more anterior rTPJ/rSTG and the ACC was investigated.
For DCMs, three parameters are specified: (i) Intrinsic parameters that describe the latent connectivity among neural populations in the experiment, (ii) driving inputs, characterizing the response of a region to stimulus presentation and (iii) modulatory parameters, representing context-dependent changes of intrinsic connectivity (Friston et al., 2003; Stephan et al., 2010). After model space definition and model estimation, we combined family level random-effects BMS and BMA within the resulting winning family to optimally characterize model parameters Penny et al., 2010). See Supplementary Materials for details on time series extraction, model space definition, BMS and BMA.
BMS and BMA
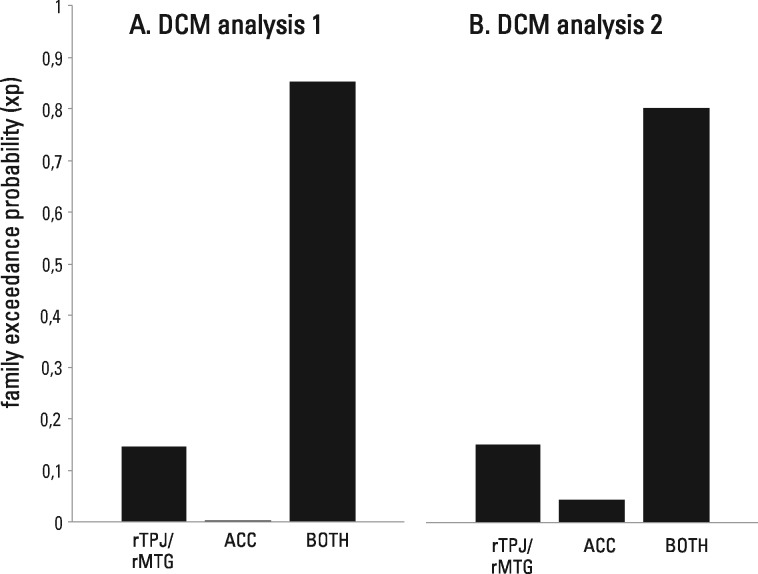
DCM analysis 1: rTPJ/rMTG and ACC. Three model families, defined by the region(s) that received driving input from experimental stimulation (rTPJ/rMTG, ACC, BOTH), were compared. The model family BOTH (with driving input to rTPJ/rMTG and ACC) had the highest exceedance probability (rTPJ/rMTG: xp = 0.1459, ACC: xp = 0.0015, BOTH: xp = 0.8526; Figure 4A). In other words, models in which experimental stimulation entered both the rTPJ/rMTG and the ACC had the best fit to the observed time series. The direct comparison of exceedance probabilities between models yielded positive to very strong evidence for preferring model family BOTH (Penny et al., 2004).
Fig. 4.
BMS results (family exceedance probability) of model families defined by region(s) that received driving input of experimental stimulation. The family of models receiving driving input to the rTPJ/rMTG and the ACC (BOTH) had the highest model evidence in DCM analysis 1 and 2.
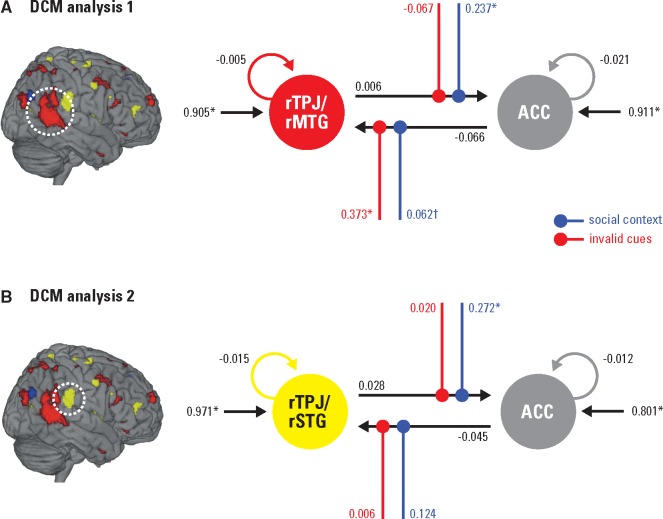
To specify the parameter values of the winning models, we applied BMA within the winning family BOTH. Finally, the resulting connection strength of individual parameters was tested for statistical significance against zero by one-sample t-tests. Figure 5A displays the structure of the average model of all participants and averaged connection strengths. Table 2 provides statistical details of averaged model parameters. This procedure revealed a low intrinsic connectivity between the rTPJ/rMTG and the ACC: Both the intrinsic parameter for the connection rTPJ/rMTG→ACC and for the connection ACC→rTPJ/rMTG did not significantly differ from zero. This suggests no or weak latent connectivity between the attention network and the ToM network in the current task. Driving input was significant and positive for the rTPJ/rMTG and the ACC. Thus, not only the rTPJ/rMTG, a region processing sensory input (Beauchamp et al., 2004) responded to stimulus presentation, but also the ACC, a region relatively higher in the cortical hierarchy (Friston et al., 2010). In the averaged model we observed a social context-dependent positive modulatory change in the upward connection rTPJ/rMTG→ACC (upward within the cortical hierarchy). When the participants believed that the cues were sent by the confederate outside the scanner, the upward connection strength from the rTPJ/rMTG to the ACC increased. The downward connection ACC→rTPJ/rMTG (downward within the cortical hierarchy) was significantly modulated only on an uncorrected threshold. Thus, the downward connection from the ACC to the rTPJ/rMTG tended to be positively strengthened in the social context. In contrast, invalid cues exhibited a significant modulatory effect on the downward connection ACC→rTPJ/rMTG. The connection from the ACC to the rTPJ/rMTG became more positive through invalid cueing. The upward connection rTPJ/rMTG→ACC was not significantly modulated by invalid cues.
Fig. 5.
BMA results of both DCM analyses. Parameter estimates characterizing the respective winning model family structure. Asterisks indicate connection strength differing significantly from zero. The cross marks one parameter that was significant on an uncorrected threshold. Modulatory parameters are depicted in color.
Table 2.
BMA results both DCM analyses: effective connectivity parameters of the respective winning model family, averaged over all participants
| Parameter estimates (Hz) |
|||||||
|---|---|---|---|---|---|---|---|
| Connection | M | SE | t(23) | P-valuea | |||
| DCM analysis 1: rTPJ/rMTG and ACC | |||||||
| Intrinsic connectivity | rTPJ/rMTG | → | rTPJ/rMTG | −0.005 | 0.016 | −0.30 | 1.000 |
| ACC | → | ACC | −0.021 | 0.020 | −1.06 | 0.600 | |
| rTPJ/rMTG | → | ACC | 0.006 | 0.020 | 0.33 | 1.000 | |
| ACC | → | rTPJ/rMTG | −0.066 | 0.041 | −1.60 | 0.244 | |
| Modulatory effect of social context | rTPJ/rMTG | → | ACC | 0.237 | 0.081 | 2.94 | 0.014 |
| ACC | → | rTPJ/rMTG | 0.062 | 0.028 | 2.22 | 0.072b | |
| Modulatory effect of invalid cueing | rTPJ/rMTG | → | ACC | −0.067 | 0.044 | −1.51 | 0.290 |
| ACC | → | rTPJ/rMTG | 0.373 | 0.071 | 5.24 | < 0.001 | |
| Driving input on rTPJ/rMTG | 0.905 | 0.315 | 2.87 | 0.018 | |||
| Driving input on ACC | 0.911 | 0.191 | 4.80 | < 0.001 | |||
| DCM analysis 2: rTPJ/rSTG and ACC | |||||||
| Intrinsic connectivity | rTPJ/rSTG | → | rTPJ/rSTG | −0.015 | 0.013 | −1.08 | 0.582 |
| ACC | → | ACC | −0.012 | 0.017 | −0.70 | 0.980 | |
| rTPJ/rSTG | → | ACC | 0.028 | 0.017 | 1.67 | 0.219 | |
| ACC | → | rTPJ/rSTG | −0.045 | 0.028 | −1.60 | 0.244 | |
| Modulatory effect of social context | rTPJ/rSTG | → | ACC | 0.272 | 0.066 | 4.13 | <0.001 |
| ACC | → | rTPJ/rSTG | 0.124 | 0.084 | 1.48 | 0.308 | |
| Modulatory effect of invalid cueing | rTPJ/rSTG | → | ACC | 0.020 | 0.021 | 0.92 | 0.734 |
| ACC | → | rTPJ/rSTG | 0.006 | 0.046 | 0.14 | 1.000 | |
| Driving input on rTPJ/rSTG | 0.971 | 0.250 | 3.90 | 0.002 | |||
| Driving input on ACC | 0.801 | 0.184 | 4.36 | < 0.001 | |||
Notes. BMA, Bayesian model averaging; DCM, Dynamic causal modeling; Hz, Hertz; SE, standard error of the mean; rTPJ/rMTG, right temporoparietal junction/right middle temporal gyrus; rTPJ/rSTG, right temporoparietal junction/right superior temporal gyrus; ACC, anterior cingulate cortex.
Bonferroni-corrected for number of comparisons within each parameter class.
significant on uncorrected threshold.
DCM analysis 2: rTPJ/rSTG and ACC. As in DCM analysis 1, the model family BOTH had the highest exceedance probability (rTPJ/rSTG: xp = 0.1502, ACC: xp = 0.0457, BOTH: xp = 0.8041; Figure 4B), indicating positive to very strong evidence for model family BOTH in which the rTPJ/rSTG and the ACC received driving input from experimental stimulation.
BMA of models within the winning model family BOTH and the subsequent t-testing of averaged connection strength for statistical significance against zero was employed to characterize the average model of connectivity between the more anterior rTPJ/rSTG and the ACC. Figure 5B displays the model structure and averaged connection strengths, Table 2 reports statistical details. Analogous to the first DCM analysis with the more posterior rTPJ/rMTG, DCM analysis 2 revealed no or weak latent connectivity also between the more anterior rTPJ/rSTG and the ACC (connection strengths rTPJ/rSTG→ACC and ACC→rTPJ/rSTG did not significantly differ from zero). Again, driving input was significant and positive for both modeled regions, suggesting that the rTPJ/rSTG and the ACC responded to experimental stimulation. Crucially, social context again had a significant positive modulatory effect on the upward connection rTPJ/rSTG→ACC. The downward connection ACC→rTPJ/rSTG was not significantly modulated by social context. Contrary to DCM analysis 1, invalid cues had no significant modulatory influence, neither on the downward connection ACC→rTPJ/rSTG, nor on the upward connection rTPJ/rSTG→ACC. Thus, invalid cueing did not affect connectivity between the more anterior rTPJ/rSTG and the ACC.
Discussion
Recent work showed that cognitive mechanisms are instantiated by the dynamic interplay of brain regions forming task-dependent networks (see Rabinovich et al., 2015; Yeo et al. 2015). Here, we aimed to refine the rTPJ’s cognitive function in attentional control and mentalizing on such a network level.
In this study, we observed activity of the ventral attention network related to processing invalidly cued targets, independent of context (main effect of validity). Further, we found ToM network activity associated with information processing in the social context, independent of cue validity (main effect of context). This provides evidence for two distinct networks underpinning task-dependent cognitive processing. Crucially, we found that believing the arrow cues were computer-based or sent by a real person affected the rTPJ/rSTG’s response to cue validity (context × validity interaction). This demonstrates interplay of both networks at the rTPJ. Moreover, effective connectivity analyses revealed a social context-dependent modulation of both, the connection from the more anterior rTPJ/rSTG to the ACC and from the more posterior rTPJ/rMTG to the ACC. We begin by discussing distinct roles of the respective brain networks. In the remainder of the discussion, we address observed functional activity and effective connectivity of the rTPJ in the light of recent hypotheses on its fractionated or overarching cognitive role.
Context-dependent network activity
The present study replicated the attentional cueing effect on the behavioral and neural level. The participants reacted slower to invalidly than to validly cued targets. When targets appeared on the invalidly as compared with the validly cued side, we observed increased functional activity of the rTPJ/rMTG together with other regions from the ventral (and dorsal) attention network (Corbetta et al., 2008). This is in line with previous findings and the view that the rTPJ, as part of the ventral attention network, plays a role in directing attention towards unattended, but relevant stimuli (Corbetta et al., 2000; Shulman et al., 2003).
Additionally, we found increased functional activity of the ACC and the cuneus/precuneus, crucial nodes of the ToM network, when the participants believed they were receiving cues from the confederate outside the scanner in contrast to computer-based cues. The only difference between contexts was the participant’s belief that another’s mental state (the confederate’s prediction of target appearance) was task-relevant in the social context. Also, the debriefing confirmed that the participants were sufficiently convinced that both cue sources were equally error-prone and that they did not use different strategies in the social and non-social context. This rules out the possibility that observed functional activity can be attributed to the participant’s belief that cueing accuracy differed between contexts or to the application of different strategies for target detection in the social and non-social context.
This neuroimaging finding adds to previous evidence showing that introducing a context including another individual’s mental states influences performance in a spatial cueing task (Teufel et al., 2010; Wykowska et al., 2014). Moreover, the response of the ToM network, in particular of the ACC, is consistent with imaging studies employing similar procedures to make participants believe that they are interacting with a real person outside the scanner (Schurz et al., 2014). For example, in a study by Gallagher et al. (2002), participants played ‘rock, paper, scissors’ against a random sequence. When they thought they were playing with a person outside the scanner, increased functional activity of an ACC cluster, highly similar to the currently observed cluster, was found.
Building on past findings and the results of this study, we conclude that within the ToM network the ACC becomes active in contexts in which other’s mental states are (seemingly) relevant to create an interpersonal awareness (Decety and Sommerville, 2003). In other words, it establishes a mindset that allows for flexibly representing and juggling another’s mental state in relation to one’s own mental state and corresponding environmental states. This cognitive mechanism was previously described as ‘adopting an intentional stance’ (Dennett, 1987; Gallagher and Frith, 2003), which only occurs in the presence of another individual who is considered as having mental states such as beliefs, intentions or desires that determine his or her behavior.
The rTPJ as functional unit
In this study, we addressed a recent controversy on the rTPJ’s fractionated or overarching cognitive role in attentional control and mentalizing (e.g. Nelson et al., 2012; Cabeza et al., 2012a). Functional activity and effective connectivity findings revealed two discriminable rTPJ subregions, an anterior and a posterior region. Yet, we did not observe a sharp functional dissociation between these two (cf. Cabeza et al., 2012b), as both regions responded to attention cueing and social context manipulation. First, the interaction between context and validity at the rTPJ/rSTG suggests that this anterior rTPJ region participates in attentional control and mentalizing. This supports recent evidence by Krall et al. (2016), who found that continuous theta burst stimulation of the anterior rTPJ interfered with both attentional control and mentalizing.
Interestingly, we observed an interaction between context and validity also in the left temporoparietal cortex. Just like the rTPJ, this region is consistently reported in studies on attention and ToM. In particular, one recent meta-analysis found an overlap of attention- and mentalizing-related activity also within this region, analogous to rTPJ findings (Lee and McCarthy, 2016). Together, this suggests that networks for attention and mentalizing also interact at the left temporoparietal cortex. Functional lateralization between the left and right temporoparietal cortex has been debated in attention and ToM research (Geng and Vossel, 2013; Schurz et al., 2013). Recently, Biervoye et al. (2016) suggested that the left temporoparietal cortex is particularly involved in the spontaneous appreciation of other’s mental states, without overt instruction to do so. As our participants were not explicitly instructed to consider the confederate’s mental state, the observed activity of the left temporoparietal cortex could be associated with this form of spontaneous mentalizing.
Second, effective connectivity analyses revealed that social context modulated both, the upward connection from the anterior rTPJ/rSTG to ACC, and from the posterior rTPJ/rMTG to ACC. Believing that the cues resembled another person’s prediction of target appearance increased the upward connection strength from both rTPJ subregions to the ACC. Additionally, modulatory influence of invalid cueing was only observed on downward connection strength from the ACC to the posterior rTPJ/rMTG. Notably, we did not observe a modulation of connection strength to or from the anterior rTPJ/rSTG by invalid cueing, although functional activity analysis revealed an interaction of validity and context at this region. A post hoc DCM analysis, including the modulation of input to the rTPJ/rSTG by invalid cueing, still revealed no significant influence of invalid cueing (details are reported in the Supplemental Materials). It could be, that a third brain region, not included in our models, is also interacting with the rTPJ/rSTG, and that this interaction is modulated by invalid cueing. This is a likely explanation of the observed interaction in the local response of the rTPJ/rSTG, as e.g. DiQuattro et al. (2013) reported that rTPJ activity is influenced by activity of the FEF during attentional reorienting. However, we refrained from following up on this finding out of the following reasons: First, including additional brain regions or modulatory influences would have resulted in an unreasonably large model space (in the example given above, 24.576 models per subject). Second, those model spaces would not have been well-motivated by a priori hypotheses, a crucial feature of powerful DCM analyses. Third, this unexplained variance does not affect the key findings and interpretations in this study and tracing back the lacking modulatory influence of invalid cueing in DCM analysis 2 falls beyond the scope the current study.
The pattern of functional activity and effective connectivity findings shows that in the current task attentional control and mentalizing relied on the same subregions within the rTPJ. This is incompatible with the fractionation view (Scholz et al., 2009; c.f. Krall et al., 2014) and supports previous experimental and meta-analytic findings reporting on overlapping rTPJ activity for attentional control and mentalizing which cannot be sharply dissociated (Decety and Lamm, 2007; Mitchell, 2008; Carter and Huettel, 2013; Kubit and Jack, 2013). Particularly, our findings are largely in line with the overarching account by Cabeza et al. (2012a), which can also explain why rTPJ activity for attention and mentalizing shifted between the anterior and posterior region depending on the applied contrast. They argue that activity within the region varies dependent on connectivity with the respective task-specific network. The rTPJ underpins an overarching cognitive mechanism, but different rTPJ subregions instantiate this processes within the respective domain and network. Unlike in previous studies using two separate tasks, social cognitive processing and attentional control were required simultaneously in our paradigm, possibly leading to the observed alternation between anterior and posterior activity.
Moreover, our results challenge conclusions derived from recent connectivity-based parcellation findings that two structurally distinct functional modules within the rTPJ underpin attentional control and mentalizing (Mars et al., 2012; Bzdok et al., 2013). Note that our functional findings are not incompatible with the view that the rTPJ is structurally separable. However, as already discussed by Bzdok et al., structural vicinity suggests related functions due to minimal transmission costs (Klyachko and Stevens, 2003; Sporns et al., 2004). Therefore, we propose that (i) the rTPJ can and should be treated as a functional unit which underpins one overarching cognitive mechanism necessary for attentional control and mentalizing (Cabeza et al., 2012a; Carter and Huettel, 2013; Geng and Vossel, 2013; Lee and McCarthy, 2016). (ii) The respective function of this cognitive mechanism is defined by context-dependent coupling with the respective network (Hein and Knight, 2008; Geng and Vossel, 2013; Seghier, 2013).
Towards a characterization of an overarching cognitive mechanism underpinned by the rTPJ
Our observed effective connectivity patterns are in line with two recently proposed accounts on the rTPJ’s overarching cognitive function. The ‘contextual updating hypothesis’ proposes that rTPJ activity during attentional control and mentalizing reflects one cognitive mechanism, namely the updating of expectations and internal models of task-relevant contexts in response to newly available information (Geng and Vossel, 2013). The same updating process handles internal models of environmental states and mental states, dependent on the co-activation of the rTPJ with context-specific networks (cf. Meinhardt et al., 2011). The social context-dependent modulation of the upward connection from both rTPJ subregions to the ACC might reflect such an updating process.
We speculate that the specific cognitive mechanism in our task is that—based on newly available sensory input—signals from the rTPJ to the ACC update the participant’s model of the confederate’s mental state. The arrow in the cueing phase (apparently) indicates the confederate’s prediction about target appearance (i.e. he believes it will appear on the left or on the right). In the target phase, the location of the target’s appearance provides new information to update the confederate’s prediction, namely whether it was invalid or valid. Crucially, this updating seems to occur only in the presence of another individual and not when the predictions were (presumably) computer-based. Adopting an intentional stance towards another agent might be a prerequisite for this contextual updating.
The observation that the rTPJ/rMTG responded earlier to social context than the ACC is also consistent with another integrative account of rTPJ function. In their nexus model, Carter and Huettel (2013) proposed that several functions, such as memory, attention and language, merge at the rTPJ and establish a social context that biases information processing. Intriguingly, the modulation of the downward connection from the ACC to the rTPJ/rMTG by invalid cueing may reflect such a biased information processing. The ACC, part of the ToM network and usually unrelated to attentional control, exerts top-down influence on the ventral attention network when mental states seem to play a role. This biased rTPJ/rMTG activity in its response to an invalidly cued target. Yet, the precise characterization of such an overarching mechanism falls beyond the scope of the current study and future research should empirically test integrative accounts proposed by Geng and Vossel (2013) or Carter and Huettel (2013).
In sum, the present work suggests that the rTPJ constitutes a relay between the ventral attention and the ToM network and that its function is defined by context-dependent coupling with the respective network. Our findings support the idea that the rTPJ as a functional unit underpins an overarching cognitive mechanism in attentional control and mentalizing.
Supplementary Material
Acknowledgements
We are grateful to Valentin Böhm for his help with recruiting participants, data acquisition and analysis. We thank Helmut Nebl for his support in data analysis. Further, we thank Martin Bozek for his help in developing the context manipulation, as well as Beate Sodian, Josef Perner and Charlotte Grosse Wiesmann for helpful comments on the interpretation of the results.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Beauchamp M.S., Argall B.D., Bodurka J., Duyn J.H., Martin A. (2004). Interhemispheric integration of visual processing during task-driven lateralization. Nature Neuroscience, 7, 1190–2.15475952 [Google Scholar]
- Biervoye A., Dricot L., Ivanoiu A., Samson D. (2016). Impaired spontaneous belief inference following acquired damage to the left posterior temporoparietal junction. Social Cognitive and Affective Neuroscience, 11(10), 1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2012). The economy of brain network organization. Nature Reviews: Neuroscience, 13(5), 336–49. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Langner R., Schilbach L., et al. (2013). Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage, 81, 381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Moscovitch M. (2012a). Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends in Cognitive Sciences, 16(8), 338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Moscovitch M. (2012b). Response to Nelson et al.: ventral parietal subdivisions are not incompatible with an overarching function. Trends in Cognitive Sciences, 16(8), 400–1. [Google Scholar]
- Carter R.M., Huettel S.A. (2013). A nexus model of the temporal–parietal junction. Trends in Cognitive Sciences, 17, 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Ollinger J.M., McAvoy M.P., Shulman G.L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3, 292–7. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58, 306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist, 13, 580–93. [DOI] [PubMed] [Google Scholar]
- Decety J., Sommerville J.A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7, 527–33. [DOI] [PubMed] [Google Scholar]
- Dennett D.C. (1987). The Intentional Stance. Cambridge (MA: ): MIT Press. [Google Scholar]
- DiQuattro N.E., Sawaki R., Geng J.J. (2013). Effective connectivity during feature-based attentional capture: evidence against the attentional reorienting hypothesis of TPJ. Cerebral Cortex, 24, 3131–41. [DOI] [PubMed] [Google Scholar]
- Friston K., Daunizeau J., Kilner J., Kiebel S. (2010). Action and behavior: a free-energy formulation. Biological Cybernetics, 102, 227–60. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. (2003). Dynamic causal modelling. Neuroimage, 19, 1273–302. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 358(1431), 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. (2003). Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences, 7, 77–83. [DOI] [PubMed] [Google Scholar]
- Gallagher H.L., Jack A.I., Roepstorff A., Frith C.D. (2002). Imaging the intentional stance in a competitive game. Neuroimage 16, 814–21. [DOI] [PubMed] [Google Scholar]
- Geng J.J., Vossel S. (2013). Re-evaluating the role of tpj in attentional control: contextual updating?. Neuroscience and Biobehavioral Reviews, 37, 2608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J., Gitelman D. (2008). Contrast weights in flexible factorial design with multiple groups of subjects. Unpublished tutorial. Available: http://www.sbirc.ed.ac.uk/cyril/download/Contrast_Weighting_Glascher_Gitelman_2008.pdf [ May 21, 2015]
- Haroush K., Williams Z.M. (2015). Neuronal prediction of opponent's behavior during cooperative social interchange in primates. Cell, 160, 1233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Knight R.T. (2008). Superior Temporal Sulcus‚ It's My Area: Or Is It?. Journal of Cognitive Neuroscience, 20(12), 2125–36. [DOI] [PubMed] [Google Scholar]
- Henson R.N. (2005). What can functional neuroimaging tell the experimental psychologist?. Quarterly Journal of Experimental Psychology, 58, 193–233. [DOI] [PubMed] [Google Scholar]
- Klyachko V.A., Stevens C.F. (2003). Connectivity optimization and the positioning of cortical areas. Proceedings of the National Academy of Sciences of the United States of America, 100(13), 7937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall S.C., Rottschy C., Oberwelland E., et al. (2014). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure and Function, 220, 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall S.C., Volz L.J., Oberwelland E., Grefkes C., Fink G.R., Konrad K. (2016). The right temporoparietal junction in attention and social interaction: A transcranial magnetic stimulation study. Human Brain Mapping, 37(2), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubit B., Jack A.I. (2013). Rethinking the role of the rTPJ in attention and social cognition in light of the opposing domains hypothesis: findings from an ALE-based meta-analysis and resting-state functional connectivity. Frontiers in Human Neuroscience, 7, 323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., McCarthy G. (2016). Functional heterogeneity and convergence in the right temporoparietal junction. Cerebral Cortex, 26(3), 1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- Mars R.B., Sallet J., Schuffelgen U., Jbabdi S., Toni I., Rushworth M.F.S. (2012). Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex, 22, 1894–903. [DOI] [PubMed] [Google Scholar]
- Meinhardt J., Sodian B., Thoermer C., Döhnel K., Sommer M. (2011). True- and false-belief reasoning in children and adults: An event-related potential study of theory of mind. Developmental Cognitive Neuroscience, 1, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P. (2008). Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex, 18, 262–71. [DOI] [PubMed] [Google Scholar]
- Nelson S.M., McDermott K.B., Petersen S.E. (2012). In favor of a ‘fractionation’ view of ventral parietal cortex: comment on Cabeza et al. Trends in Cognitive Sciences, 16, 399–400. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Caramazza A. (2012). Conceptual object representations in human anterior temporal cortex. Journal of Neuroscience, 32, 15728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W.D., Stephan K.E., Daunizeau J., et al. (2010). Comparing families of dynamic causal models. PLoS Computational Biology, 6, e1000709.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W.D., Stephan K.E., Mechelli A., Friston K.J. (2004). Comparing dynamic causal models. Neuroimage, 22, 1157–72. [DOI] [PubMed] [Google Scholar]
- Posner M. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25. [DOI] [PubMed] [Google Scholar]
- Rabinovich M.I., Simmons A.N., Varona P. (2015). Dynamical bridge between brain and mind. Trends in Cognitive Sciences, 19, 453–61. [DOI] [PubMed] [Google Scholar]
- Scholz J., Triantafyllou C., Whitfield-Gabrieli S., Brown E.N., Saxe R. (2009). Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One, 4, e4869.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Schurz M., Aichhorn M., Anna M., Perner J. (2013). Common brain areas engaged in false belief reasoning and visual perspective taking: A meta-analysis of functional brain imaging studies. Frontiers in Neuroscience, 7, 712.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M. Kronbichler M. Weissengruber S. Surtees A.Samson, D., and Perner, J. (2015). Clarifying the role of theory of mind areas during visual perspective taking: Issues of spontaneity and domain-specificity. NeuroImage, 117, 389–96.. [DOI] [PubMed] [Google Scholar]
- Schuwerk T., Döhnel K., Sodian B., Keck I.R., Rupprecht R., Sommer M. (2014). Functional activity and effective connectivity of the posterior medial prefrontal cortex during processing of incongruent mental states. Human Brain Mapping, 35(7), 2950–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist, 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., McAvoy M.P., Cowan M.C., et al. (2003). Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology, 90, 3384–97. [DOI] [PubMed] [Google Scholar]
- Sporns O., Chialvo D.R., Kaiser M., Hilgetag C.C. (2004). Organization, development and function of complex brain networks. Trends in Cognitive Sciences, 8, 418–25. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Moran J.M., den Ouden H.E.M., Daunizeau J., Friston K. (2010). Ten simple rules for dynamic causal modeling. Neuroimage, 49, 3099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C., Alexis D.M., Clayton N.S., Davis G. (2010). Mental-state attribution drives rapid, reflexive gaze following. Attention, Perception, & Psychophysics, 72(3), 695–705. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykowska A., Wiese E., Prosser A., Muller H.J. (2014). Beliefs about the minds of others influence how we process sensory information. PLoS One, 9, e94339.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Eickhoff S.B., et al. (2015). Functional specialization and flexibility in human association cortex. Cerebral Cortex, 25(10), 3654–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.