Abstract
Humans often give in to temptations that are in conflict with valuable long-term goals like health or saving for the future. Such willpower failures represent a prevalent problem in everyday life and in many psychiatric disorders. Strategies that increase resistance to temptations could therefore improve overall societal well-being. One important strategy is to voluntarily precommit, i.e. to restrict one’s future action space by removing the tempting short-term option from the choice set, thereby leaving only the long-term option for implementation. The neural mechanisms necessary to implement precommitment have remained unknown. Here, we test whether anodal transcranial direct current stimulation (tDCS) over the frontopolar cortex (FPC) can improve precommitment. Participants performed a self-control task in which they could precommit to obtain a delayed larger reward by removing an immediately available smaller reward from the future choice options. We found that anodal stimulation over FPC selectively increased the propensity to precommit. In contrast, tDCS had no effects on non-binding decisions, impulse control or reward preference. Our data establish a causal role for the FPC in the implementation of precommitment, revealing a novel route to improving resistance against temptations.
Keywords: impulse control, prefrontal cortex, self-control, tDCS, temporal discounting
Introduction
Homer’s Odysseus ordered his crew to tie him to the mast in order to resist the Sirens’ deadly temptation. Such voluntary restrictions of one’s future choice options in order to avoid anticipated willpower failures are referred to as “precommitment.” The need for strategies to resist temptations stems from the limited ability to resist appealing immediate rewards (Muraven and Baumeister, 2000). Caving into temptation hampers the achievement of higher-order goals like health or saving money for the future, causing great costs to individual and societal well-being. Moreover, self-control problems are among the defining symptoms of several psychiatric disorders, including addiction and obesity (Kirby et al., 1999; Bickel and Marsch, 2001; Baumeister and Vonasch, 2015; Stutzer and Meier, 2015). Thus, finding means to facilitate the implementation of higher-order goals has both clinical and societal relevance.
While most research on self-control has focused on the ability to resist temptation impulses through willpower, there is evidence that precommitment represents an effective alternative strategy that enables humans to make binding choices and thereby avoid impulse control failures in many situations of everyday life (Rachlin and Green, 1972; Kalenscher and Pennartz, 2008; Fujita, 2011 ). For example, many humans buy unhealthy food only in smaller quantities to restrict their access to such “vices” (Wertenbroch, 1998), voluntarily impose strict deadlines in order to avoid procrastination (Ariely and Wertenbroch, 2002) or invest money in inaccessible retirement funds. Enhancing precommitment therefore has the potential to increase individual and societal well-being in many respects, especially for impulsive agents (Kurth-Nelson and Redish, 2010, 2012). In line with this assumption, gambling machines requiring the gambler to preset a maximum loss limit reduced the expenditure of pathological gamblers (Ladouceur et al., 2012).
Despite its societal and clinical relevance, the neurocognitive mechanisms underlying precommitment are largely unknown. One functional magnetic resonance imaging (fMRI) study (Crockett et al., 2013) has identified regions that may play a role in making binding choices. In contrast to conditions in which no precommitment was possible, precommitment choices activated the frontopolar cortex (FPC). The authors concluded that the FPC might be involved in monitoring the expected value of precommitment. In this view, FPC biases decisions in favor of precommitment when the expected value of precommitment exceeds the costs of restricting one’s choice options. If this assumption is correct, it should be possible to improve precommitment by stimulating the FPC with anodal transcranial direct current stimulation (tDCS), because anodal tDCS is thought to increase the neural excitability of the stimulated brain region (Nitsche and Paulus, 2000). Increasing the excitability of the FPC should enhance its sensitivity to the expected value of precommitment, which in turn should facilitate the decision to precommit to the delayed reward. In addition, we tested for potential effects of cathodal tDCS over FPC, which decreases neural excitability and may thus reduce precommitment (but note that cathodal tDCS tends to show weaker effects on cognitive functions than anodal tDCS; see Jacobson et al., 2012). We expected anodal tDCS over FPC to increase the number of precommitment choices in a self-control task (Crockett et al., 2013). Such a finding would indicate a causal link between FPC activation and precommitment and provide insights into potential ways of improving precommitment in disorders related to self-control problems.
Importantly, we assessed the specificity of FPC stimulation on the willingness to engage in precommitment by testing for tDCS effects on other cognitive processes that may play a role in precommitment. In particular, the propensity to make precommitment choices for delayed rewards might also be influenced by the propensity to make nonbinding choices of delayed rewards, by the ability to resist temptation impulses or by the preference for larger, relative to smaller, rewards. Therefore, we examined whether tDCS over FPC affects nonbinding choices of delayed rewards, impulse control and preference for larger rewards. Because our neuroimaging results ascribe the FPC a specific role in making precommitment choices (Crockett et al., 2013), we hypothesized that anodal tDCS over FPC would selectively improve precommitment without affecting choices in the control conditions.
Materials and methods
Participants
Seventy-eight healthy heterosexual males (mean age = 23.1 year, age range 18–38 years) volunteered in the study after having given informed consent. Two further subjects were excluded for not following task instructions, i.e. for not permanently keeping their response fingers on the response keys during the delay phases. Three further subjects were removed from the analysis because they showed a preference for the smaller reward in the no-delay condition (the inclusion of all of these subjects, however, did not qualitatively change the results). The study was approved by the local ethics committee of the Canton Zurich.
Stimuli
Subjects performed a decision task requiring them to make choices between smaller sooner (SS) and larger later (LL) rewards. As the task included relatively short delays in the range of 4–10 s, we used erotic stimuli as primary reinforcers consumable at the time of delivery (Prevost et al., 2010; Crockett et al., 2013). In order to approximately match the subjective value of SS and LL rewards across subjects, all subjects had to rate the attractiveness of 300 pictures of women in lingerie on a Likert-scale of 0–10 some days (mean = 3.2 days) before the main experimental session. Based on these ratings, we constructed individualized stimulus sets by first removing all pictures rated as not attractive (ratings of 0 or 1) and then splitting the remaining pictures at each individual’s median. Pictures rated above the median were designated as LL rewards (mean attractiveness rating = 7.1) and those below were SS rewards (mean attractiveness rating = 4.2). Thus, an individualized set of SS and LL pictures was used for each subject in the main experimental session. Moreover, the same picture was never shown more than once in order to avoid saturation effects.
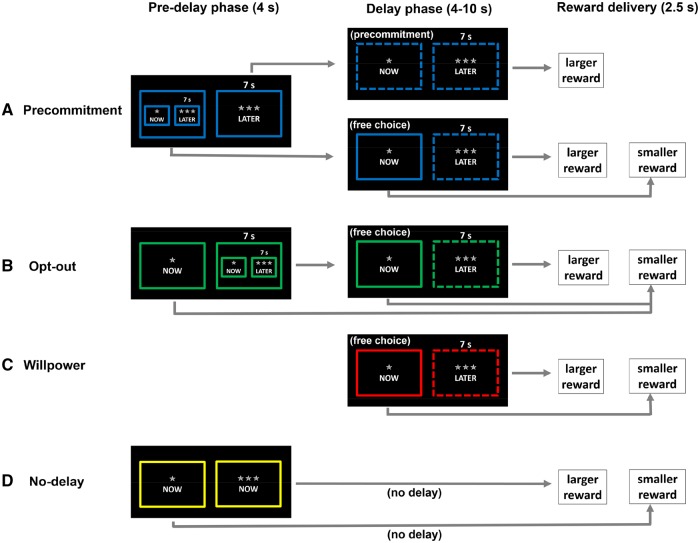
The task included the following four conditions: precommitment condition, opt-out condition, willpower condition and no-delay condition (Figure 1). The precommitment condition started with a pre-delay phase (4 s): subjects decided whether to have a free choice between the SS and the LL option during a subsequent delay phase or to precommit to the LL option, removing the SS option from the delay phase. The LL reward was presented automatically after the indicated temporal delay, except if the SS option was chosen before (in which case the SS reward was presented immediately). Subjects indicated their choice by pressing the left control key (for the option shown on the left side of the screen) or the right control key (for the option on the right side of the screen) on a QWERTZ keyboard. The assignment of options to screen sides was counterbalanced across subjects. If subjects decided to have the free choice (i.e. not to precommit), then a delay phase started after the pre-delay phase. In contrast, if subjects decided to commit to the LL option in the pre-delay phase, the SS reward was not available for selection during the delay phase, such that subjects could not reverse their decision for the LL reward during the delay. Depending on subjects’ choices, either the SS or the LL reward was presented for 2.5 s, followed by a variable inter-trial interval (ITI) in which a fixation cross was presented on the center of the screen. If subjects chose the LL option, the length of the ITI was 1.5 s, whereas in case of SS choices the remaining duration of the terminated delay phase was added to these 1.5 s. This was done to ensure that subjects could not finish the task more quickly by choosing the SS reward, and we informed subjects about this procedure before the start of the experiment.
Fig. 1.
Schematic illustration of task conditions and trial procedure. In all task conditions, subjects chose between a SS and a LL option. When making their choices, subjects did not know which pictures exactly would be presented and could only decide between a more attractive (LL) and a less attractive (SS) option. The duration of the delay subjects had to wait in order to obtain the LL reward was indicated above the LL option (4, 7 or 10 s). Solid lines indicated that the option was currently available for selection, whereas dashed lines indicated that the option was currently not available for selection. (A) The precommitment condition included a pre-delay phase in which subjects decided whether or not to make a precommitment choice for the LL reward. If subjects decided to precommit to the LL reward, then the SS option was not available during the delay phase, such that subjects could not reverse their initial choice and the LL reward was delivered automatically after the delay. If subjects preferred not to precommit but to have the free choice between SS and LL options, then the SS reward was available for selection during the delay phase, presumably requiring subjects to suppress the impulse to select the SS reward while waiting for the LL reward. If subjects chose the SS option during the delay phase, the reward was delivered immediately, otherwise, the LL reward was delivered automatically after the indicated delay. (B) In the pre-delay phase of the opt-out condition, subjects decided between obtaining the SS reward immediately or waiting for the LL reward. During the delay phase, the SS option remained available for selection, such that (contrary to the precommitment condition) choices were not binding and subjects could reverse their initial preference for the LL option. (C) The willpower condition consisted of the delay phase of the precommitment task. Thus, there was no pre-delay phase, the SS reward was available for selection, while in case of inaction the LL reward was presented automatically after the indicated delay. (D) The no-delay condition included only a pre-delay but no delay phase. The chosen smaller or larger reward option was presented immediately after the decision phase.
The opt-out condition tested whether stimulation effects on LL choice depended on the binding nature of the precommitment option. The opt-out condition was closely matched to the precommitment condition in terms of choice structure and choice complexity, with the exception that participants could not precommit themselves to the LL reward. Thus, just as precommitment trials, opt-out trials, too, started with a pre-delay phase. In this pre-delay phase, subjects chose whether to see the SS reward immediately after the pre-delay phase or to wait for the LL reward. If subjects decided to wait, then the pre-delay phase was followed by the same delay phase as in the precommitment condition after a non-commitment choice, in which the SS reward remained available for selection. Thus, in contrast to the precommitment condition, subjects’ choice of the LL reward in the pre-delay phase of the opt-out condition was not binding as subjects could reverse their decisions during the delay phase and select the SS reward.
The willpower condition assessed stimulation effects specifically when subjects were exerting self-control. Trials in this condition included no pre-delay phase but started with a delay phase, in which only the SS reward was available for immediate selection, whereas the LL reward was presented automatically after the indicated temporal delay. This delay phase was terminated immediately if subjects chose the SS option (in this case, the remaining part of the terminated delay phase was again added to the ITI). Thus, the willpower condition required subjects to suppress the temptation to choose the SS reward in order to obtain the LL reward, allowing us to control for potential stimulation effects on impulse control.
Finally, the no-delay condition assessed whether stimulation affected preference for larger rewards irrespective of temporal delay. In trials of this condition, both the smaller and the larger rewards were available for selection during the pre-delay phase. The chosen reward was presented immediately (i.e. without temporal delay) after the pre-delay phase. In the sham group, this condition allowed us also to validate the effectiveness of our reward manipulation by testing whether subjects indeed show a preference for the larger over the smaller rewards when delays are absent. In addition, it allowed us to control for potential effects of tDCS over FPC on reward preference.
Before the start of the stimulation, subjects obtained detailed task instructions and performed six trials of each task condition as practice. During stimulation, we presented a total of four blocks per condition, with each block containing six trials (with balanced numbers of 4, 7 and 10 s delays). Thus, 24 trials were presented per condition, resulting in a total of 96 trials. The experimental blocks were presented in randomized order. The total duration of the task was 23 min. In order to increase the distinctiveness of the conditions, the frames of the available and unavailable options were shown in different colors (the assignment of conditions to colors was counterbalanced across subjects). In addition, before the start of a block, a cue presented for 2 s alerted subjects to the upcoming condition (e.g. “blue task”).
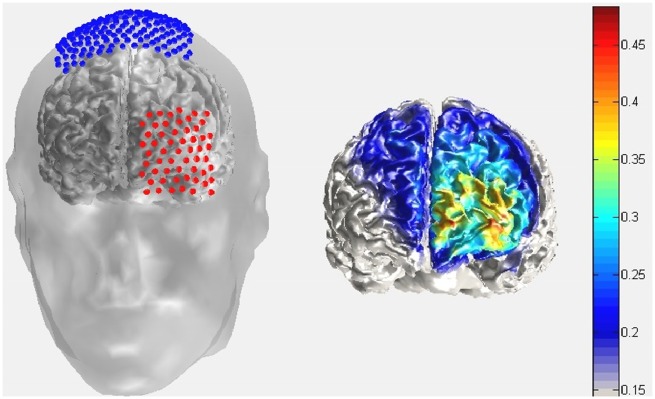
tDCS protocol. We applied anodal, cathodal or sham tDCS using a multi-channel stimulator over the left FPC region showing the strongest BOLD response during binding relative to non-binding choices (peak activation: x = −34, y = 58, z = −8) in the study by Crockett et al. (2013). Figure 2 illustrates electrode positioning and the modeled current density for anodal stimulation (Jung et al., 2013). In 31 participants, we used T1-scans of the individual subject to determine the center point of the frontal electrode over the left FPC with Brainsight 2.0 frameless stereotaxy. As we found the center of the frontal electrode to be reliably placed at electrode position LL1 on a Waveguard Duke 128 channels cap in these subjects, we fixed the electrode at this electrode position for the remaining subjects. The vertex electrode was placed over electrode position Z7 on the 128 channels cap. As frontal and vertex electrodes, we applied standard 5 × 5 and 10 × 10 cm electrodes, respectively, fixed by rubber straps. We used larger vertex than frontal electrodes to minimize the stimulation effect at the control vertex site relative to the FPC site.
Fig. 2.
Illustration of electrode positioning and modeled current density for the anodal stimulation over the left FPC.
During task performance, we stimulated subjects with 1 mA current strength in the anodal and cathodal groups, while in the sham group, the current was turned off after 30 s. Such sham tDCS protocols have been shown to reliably blind participants (Woods et al., 2016). To account for possible delays in the onset of tDCS effects, subjects had to wait 4 min following stimulation onset before they started the self-control task.
Data analysis. We used mixed general linear models (MGLMs) as implemented in IBM SPSS 22 to analyze subjects’ choices in the four experimental conditions. We excluded all trials in which subjects failed to make a choice within the duration of the 4 s pre-delay phase of the precommitment (mean dropout rate = 1.5%), opt-out (1.6%) and no-delay (1.3%) conditions. The advantage of MGLMs over analyses of variance is that they allow to flexibly control for potentially confounding effects of covariates (see below) when modeling the effects of the experimental manipulations. Specifically, we analyzed the effects of anodal and cathodal tDCS on choices in the pre-delay phases of the precommitment, opt-out and no-delay conditions, and in the delay phase of the precommitment, opt-out and willpower condition. We predicted subjects’ propensity to make a binding choice for the larger reward in the precommitment condition and to choose the larger reward (percentage of choices of larger reward relative to all choices) in the other conditions using stimulation predictors for active, i.e. anodal and cathodal, tDCS. Importantly, our results are robust against analyzing choices instead of mean choice probabilities. The two stimulation predictors were dummy variables that were set to 1 if a subject received anodal or cathodal tDCS, respectively, otherwise their value was set to 0. Thus, they allowed us to test for effects of anodal and cathodal tDCS relative to baseline performance in the sham condition. In conditions where the larger reward was delivered after a temporal delay (i.e. precommitment, opt-out and willpower), we included also predictors for delay length (4, 7 or 10 s) and the interactions between delay length and anodal or cathodal tDCS. Because the willingness to precommit depends on individual differences in delay discounting and impulsiveness (Noor, 2007; Kurth-Nelson and Redish, 2012; Crockett et al., 2013), and because delay discounting varies across the lifespan (Steinberg et al., 2009), we included covariates for delay discounting, age and impulsiveness as predictors of no interest. Delay discounting and impulsiveness were measured using the delay discounting questionnaire of Kirby et al. (1999; German version by Forstmeier and Maercker (2011)) and the Barratt Impulsiveness Scale (BIS-15; Spinella, 2007; German version by Meule et al., 2011), respectively. All predictors for tDCS, delay length and the covariates represented fixed effects. In addition, a subject regressor represented a random effect. The observed result pattern was robust to excluding these covariates from the model. Parameter estimates were obtained using maximum-likelihood estimation and tested for significance using F-statistics.
Results
Baseline measures and task validation
We randomly assigned 78 subjects to one of three between-subjects experimental groups that received anodal (27 subjects), cathodal (26 subjects) or sham (25 subjects) tDCS over the FPC while performing the self-control task. The three experimental groups were well balanced with respect to age as well as baseline measures of impulsiveness (Spinella, 2007) and delay discounting (Kirby et al., 1999), all F < 2, all P > 0.14. In addition, to control for potential tDCS effects on subjects’ emotional state, we measured their mood, alertness and calmness before the start and before the end of stimulation using the multidimensional mood state questionnaire (Steyer et al., 1994). Again, there were no significant differences between tDCS groups, all F < 1, all P > 0.43. These data suggest that potential tDCS effects are not confounded with baseline group differences in delay discounting, impulsivity or mood.
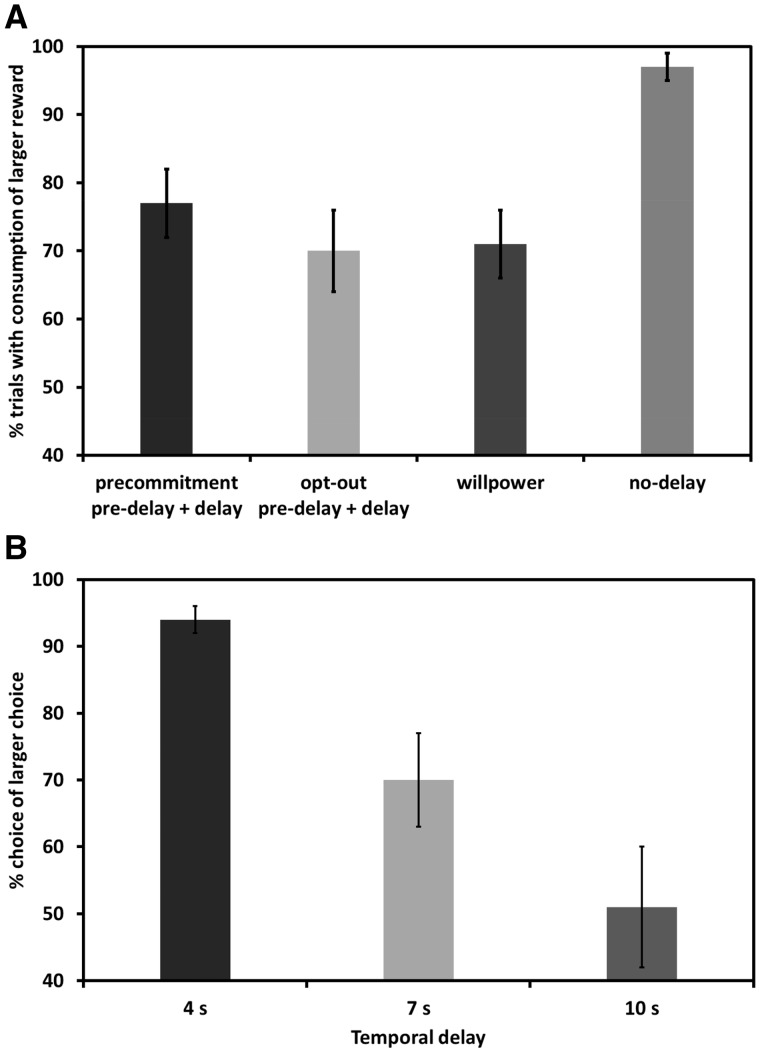
First, we evaluated the effectiveness of our task manipulations by examining choice behavior of the sham group. In the no-delay condition, subjects predominantly chose the high-rated pictures (mean = 97%, standard error of the mean (SEM) = 2%), suggesting a strong preference for the large rewards over the small rewards when both were available immediately. Note that this also holds when the three subjects with a preference for the low-rated images (see Materials and methods) were included into the analysis (mean = 95%, SEM = 3%). Compared to the no-delay condition, preference for the larger reward option significantly dropped in the precommitment (mean = 76%, SEM = 5%; collapsing over pre-delay and delay phase); opt-out (mean = 68%, SEM = 5%; collapsing over pre-delay and delay phase); and willpower (mean = 69%, SEM = 6%) conditions, all t(24) > 4.56, all P < 0.001 (Figure 3A). Thus, whenever the LL reward was available only after a temporal delay, subjects were more likely to choose SS compared to when the LL reward was presented without a delay.
Fig. 3.
Validation of experimental manipulations. (A) At baseline (sham tDCS), subjects showed a strong preference for the larger reward in the no-delay condition, while choices of the larger reward significantly dropped when it was available only after a temporal delay. However, the possibility to precommit resulted in a higher number of choices of the LL reward relative to the opt-out and willpower conditions. (B) In the willpower condition, the preference for the LL reward decreased as a function of temporal delay. Error bars indicate standard error of the mean.
Next, we tested the efficacy of precommitment as a self-control strategy by comparing the consumption of larger later rewards in the precommitment condition with the opt-out and willpower conditions. Note that for this analysis (as for the preceding one), we collapsed data across the pre-delay and delay phases of the precommitment and opt-out conditions to obtain an indicator of the overall consumption of larger reward images. As hypothesized, the possibility to make binding choices in the precommitment condition resulted in a significantly higher number of LL choices relative to both the willpower and opt-out conditions, all t(24) > 2.47, all P < 0.05, confirming previous findings that precommitment is an effective self-control strategy (Noor, 2007; Kurth-Nelson and Redish, 2012; Crockett et al., 2013). As the preference for the larger reward dropped in conditions where the rewarding stimulus was presented after a temporal delay, we further tested how the length of the delay affected reward preference. We found that delay length (4, 7 or 10 s) predicted preference for the LL reward in the willpower task, F(1,75) = 4.93, P < 0.001, indicating that the subjective value of the LL reward was discounted as a function of increasing temporal delay (Figure 3B). This confirms the effectiveness of the willpower manipulation and suggests that whenever the SS reward remained obtainable, longer temporal delays required subjects to suppress the temptation to select the SS reward instead of waiting for the preferred LL reward. Taken together, the result pattern in the baseline group was qualitatively similar to the behavioral results of Crockett et al. (2013), even though subjects tended to be more patient across all task conditions in the current sample (which may be explained by cultural differences; see Wang et al., 2016).
Anodal tDCS over FPC promotes precommitment
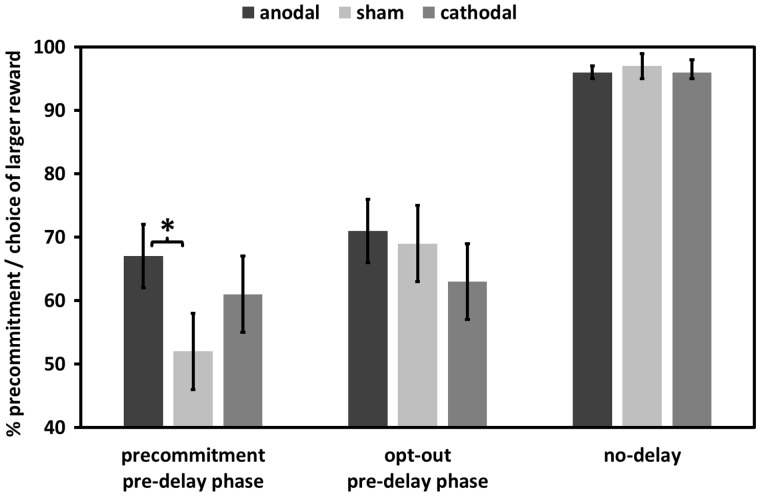
The main goal of the current study was to test whether anodal stimulation of the FPC enhances the willingness of our subjects to engage in precommitment. If our hypothesis is correct, anodal stimulation should lead to more precommitment choices in the pre-delay phase of the precommitment condition. We tested this with a MGLM that predicted the number of precommitment choices using dummy variables for the effect of anodal tDCS and cathodal tDCS relative to sham tDCS. In addition, we included predictors for delay length and the interactions between delay length and anodal or cathodal tDCS. Importantly, we found that subjects receiving anodal stimulation made significantly more precommitment decisions (mean = 67%, SEM = 5%) compared with subjects receiving sham stimulation (mean = 52%, SEM = 6%), F(1,75) = 4.83, P = 0.036, while the data revealed no significant effect of tDCS in subjects receiving cathodal stimulation (61%), F(1,78) = 1.5, P = 0.27 (Figure 4). The mean number of precommitment choices was non-significantly higher in the cathodal tDCS than in the baseline sham tDCS group. There were no interactions between delay length and anodal or cathodal stimulation, all F < 1.34, all P > 0.26. This finding supports our hypothesis that anodal FPC stimulation increases the propensity to precommit.
Fig. 4.
Effects of anodal and cathodal tDCS relative to sham stimulation on choices in the pre-delay phases of the precommitment, opt-out, and no-delay conditions. The y-axis indicates the percentage of precommitment choices in the precommitment condition and the percentage of choices of the larger reward in the opt-out and no-delay conditions. Relative to sham tDCS, anodal FPC stimulation increased the number of precommitment decisions but showed no effects on the control conditions. There were no significant effects of cathodal tDCS on task performance. An asterisk (*) indicates a significant effect at a threshold of P < 0.05. Error bars indicate SEM.
To investigate the specificity of the anodal stimulation effect on decisions to precommit, we analyzed the effects of anodal stimulation also during the pre-delay phase of the opt-out and no-delay conditions (Figure 4). In the opt-out condition, where the SS option remained available during the later delay phase, we found a main effect of delay length, F(2,155) = 45.80, P < 0.001, but no further effect passed the statistical threshold, all F < 1, all P > 0.54. There was also no evidence for significant tDCS effects on choices in the no-delay condition in which the chosen image was presented without delay, all F < 1, all P > 0.57. To test specificity more stringently, we examined whether the effects of anodal tDCS were significantly stronger in the precommitment condition than in the opt-out and no-delay conditions. For that purpose, we computed a further MGLM including predictors for anodal tDCS, task condition (a dummy-coded predictor that was 1 for the precommitment condition and 0 for the opt-out and no-delay conditions), and the interactions between these factors. This MGLM revealed a significant anodal tDCS × task condition interaction, F(1,104) = 4.68, P < 0.05, confirming that anodal stimulation effects were significantly stronger in the precommitment condition than in the opt-out and no-delay conditions. These data suggest that FPC tDCS has little effect on choices of the larger reward when no precommitment was possible and support our hypothesis that the FPC plays a crucial role specifically in making precommitment choices.
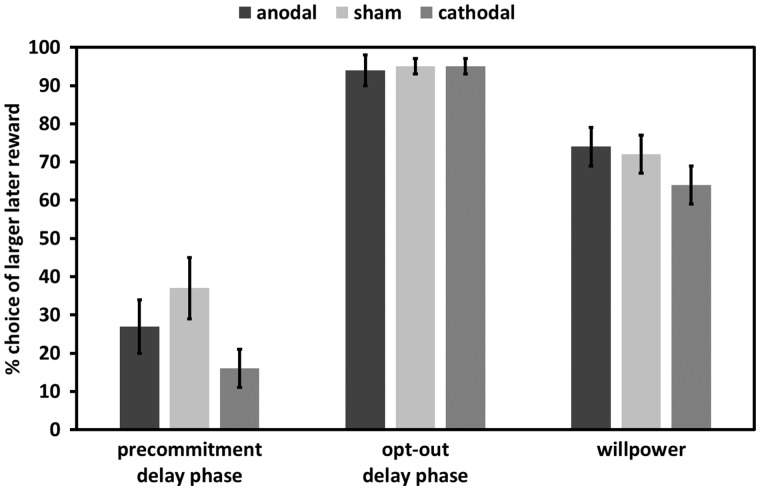
Given the specific tDCS effects on precommitment decisions in the pre-delay phase, we also tested whether tDCS affected choices in the delay phases of the precommitment, opt-out and willpower conditions (Figure 5). In order to obtain the LL reward in the delay phases of these conditions, participants presumably had to suppress the temptation to select the immediately available SS reward while waiting for the delivery of the LL reward. For each of these conditions, we computed a MGLM testing whether tDCS predicted subjects’ ability to wait for the LL reward during the delay phase of the willpower condition (again including predictors for delay length and its interactions with tDCS). None of these task conditions showed significant effects of anodal or cathodal tDCS nor interactions between tDCS and delay length, all F < 2.66, all P > 0.1. In order to provide evidence for the specificity of the anodal tDCS effects on making binding choices, we tested whether anodal tDCS effects were significantly stronger in the pre-delay phase of the precommitment condition than in the delay phases of the precommitment, opt-out and willpower conditions. We computed an MGLM with predictors for anodal tDCS, delay length, and task condition (which was 1 for the pre-delay phase of the precommitment condition and 0 for the delay phases of the precommitment, opt-out and willpower conditions) and the interactions between these factors. Again, we found a significant anodal tDCS × task condition interaction, F(1,572) = 6.15, P < 0.05. Thus, we can also rule out the possibility that tDCS over FPC improved precommitment by affecting impulse control in general.
Fig. 5.
tDCS effects on choices of the larger-later reward in the delay phases of the precommitment, opt-out, and willpower conditions. Note that in the delay phase no decision to precommit to the larger later reward was possible. There were no significant tDCS effects on task performance. Error bars indicate SEM.
Discussion
The current study assessed whether anodal stimulation over FPC can improve self-control by enhancing subjects’ willingness to precommit. In line with our hypothesis, we found that anodal tDCS over left FPC increased the number of precommitment choices for the LL reward in the precommitment condition. This result confirms the neuroimaging-inspired hypothesis of a causal role of the FPC in precommitment (Crockett et al., 2013) and suggests that anodal tDCS over FPC may be a promising tool to improve self-control. Importantly, the data revealed no effects of tDCS over FPC on non-binding choices of LL rewards (opt-out condition), on impulse control (willpower condition), or on subjects’ preference for the larger reward when delivered immediately (no-delay condition). The specificity of our FPC tDCS findings on precommitment suggests that the increased number of binding choices is unlikely to be caused by improved impulse control or changes in the value representation of the LL and SS options. Thus, the current data provide causal evidence for a functional role of the FPC in precommitment and demonstrate that anodal tDCS can effectively foster the ability to resist temptations by voluntarily restricting one’s choice options.
The FPC tDCS effects on precommitment may be explained within the framework of a recent neural model of precommitment (Crockett et al., 2013), which assumes that FPC biases the decision to precommit as a function of the expected value of precommitment. According to this neural model, anodal tDCS may have improved precommitment by increasing the FPC’s sensitivity to the expected value of precommitment. This interpretation is in line with previous studies relating the FPC to metacognitive functions like counterfactual thinking (Daw et al., 2006; Rushworth et al., 2011). As an individual’s sensitivity to the expected value of precommitment depends on the anticipation of counterfactual events in the future (e.g. “If I went to the bar, I’d drink. Therefore, I’d better not go.”), such metacognitive functions may play an important role in precommitment (Kurth-Nelson and Redish, 2010, 2012). Thus, our results are consistent with current theories on FPC functioning and suggest that anodal tDCS over FPC may have increased sensitivity to the expected value of precommitment by increasing metacognitive functioning.
Note that one critical issue with regard to interpreting tDCS findings arises from its relatively low spatial resolution (Bestmann et al., 2015; Woods et al., 2016). Thus, the observed effects could in principle result from stimulation effects on brain regions adjacent to FPC, such as ventromedial prefrontal cortex (vmPFC) and lateral prefrontal cortex. However, it is important to note that the current data provide no evidence for stimulation effects on subjective value representations (no-delay condition) or impulse control (willpower condition), which are related to ventromedial prefrontal cortex (vmPFC) and lateral prefrontal cortex activity, respectively (Figner et al., 2010; Rushworth et al., 2011;Bartra et al., 2013). Moreover, given that our previous fMRI study found that precommitment choices selectively activated FPC (Crockett et al., 2013), the specificity of our tDCS effects on precommitment suggests that tDCS exerted its effects by modulating this neural activity in the FPC rather than in other brain regions that were not activated by the current task.
In addition, a recent theoretical model of tDCS effects has debated the directionality of tDCS (Bestmann et al., 2015). In line with this, a meta-analysis showed that only 16% of the published studies investigating tDCS effects on cognitive functions reported both an excitatory effect of anodal tDCS and an inhibitory effect of cathodal tDCS (Jacobson et al., 2012). The majority of studies find only facilitatory anodal effects, with cathodal tDCS showing little effects on performance. Jacobson et al. (2012) proposed that in such cases the non-stimulated hemisphere might compensate the effects of unilateral tDCS. Consistent with this proposal, FPC activity during precommitment is bilateral (Crockett et al., 2013), whereas in the current study, we stimulated only the left FPC. Alternatively, cathodal tDCS might even improve cognitive functioning by decreasing neural competition (Antal et al., 2004). This might explain why we found a higher number of precommitment decisions in the cathodal tDCS than in the sham tDCS group, even though this difference was clearly non-significant. While our data do not support the assumption that cathodal tDCS impairs FPC excitability, the result pattern is consistent with the conclusions of meta-analyses reporting more reliable effects for anodal than cathodal tDCS (Jacobson et al., 2012). Moreover, although the directionality of tDCS is still a matter of debate, it is important to note that the finding of enhanced precommitment during anodal tDCS over FPC is consistent with the neuroimaging findings of increased FPC activation for precommitment decisions (Crockett et al., 2013).
It is also worth mentioning that recent meta-analyses have suggested that tDCS may have small or inconsistent effects on physiological measures and cognition (Horvath et al., 2015a, 2015b). However, these meta-analyses are themselves debated since they are based on low numbers of studies with heterogeneous electrode montages (Nitsche et al., 2015; Santarnecchi et al., 2015). Given the ongoing debate on how tDCS may best be used for modulating cognition and behavior, the results of the current study may be taken as first, tentative evidence that precommitment can be improved by FPC stimulation. The finding that precommitment can be facilitated with brain stimulation may be of relevance for the treatment of psychiatric disorders involving self-control problems (Kirby et al., 1999; Bickel and Marsch, 2001; Baumeister and Vonasch, 2015;). For example, patients with gambling addiction were found to benefit from precommitment (Ladouceur et al., 2012). Thus, enhancing precommitment may be a promising tool for reducing willpower failures in these clinical populations. The current data deepen our understanding of the neural mechanisms underlying precommitment and suggest that the use of precommitment strategies may be enhanced by FPC stimulation.
Acknowledgement
We also acknowledge the Neuroscience Center Zurich.
Funding
This work was supported by grants PP00P1_150739, CRSII3_141965, and 00014_165884 from the Swiss National Science Foundation.
References
- Antal A., Nitsche M.A., Kruse W., Kincses T.Z., Hoffmann K.P., Paulus W. (2004). Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. Journal of Cognitive Neuroscience, 16(4), 521–7. [DOI] [PubMed] [Google Scholar]
- Ariely D., Wertenbroch K. (2002). Procrastination, deadlines, and performance: self-control by precommitment. Psychological Science, 13(3), 219–24. [DOI] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.F., Vonasch A.J. (2015). Uses of self-regulation to facilitate and restrain addictive behavior. Addictive Behavior, 44, 3–8. [DOI] [PubMed] [Google Scholar]
- Bestmann S., de Berker A.O., Bonaiuto J. (2015). Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cognitive Science, 19(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Bickel W.K., Marsch L.A. (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96(1),73–86. [DOI] [PubMed] [Google Scholar]
- Crockett M.J., Braams B.R., Clark L., Tobler P.N., Robbins T.W., Kalenscher T. (2013). Restricting temptations: neural mechanisms of precommitment. Neuron, 79(2), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N.D., O'Doherty J.P., Dayan P., Seymour B., Dolan R.J. (2006). Cortical substrates for exploratory decisions in humans. Nature, 441(7095), 876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B., Knoch D., Johnson E.J., et al. (2010). Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neuroscience, 13(5),538–9. [DOI] [PubMed] [Google Scholar]
- Forstmeier S., Maercker A. (2011). Self-control in older adulthood: a German vversion of the delay discounting test by Kirby. Psychotherapie Psychosomatik Medizinische Psychologie, 61(6),269. 274. [DOI] [PubMed] [Google Scholar]
- Fujita K. (2011). On conceptualizing self-control as more than the effortful inhibition of impulses. Personality and Social Psychology Review, 15(4), 352–66. [DOI] [PubMed] [Google Scholar]
- Horvath J.C., Forte J.D., Carter O. (2015a). Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia, 66, 213–36. [DOI] [PubMed] [Google Scholar]
- Horvath J.C., Forte J.D., Carter O. (2015b). Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimuli, 8(3),535–50. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Koslowsky M., Lavidor M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Jung Y.J., Kim J.H., Im C.H. (2013). COMETS: A MATLAB toolbox for simulating local electric fields generated by transcranial direct current stimulation (tDCS). Biomedical Engineering Letters, 3(1), 39–46. [Google Scholar]
- Kalenscher T., Pennartz C.M. (2008). Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Program in Neurobiology, 84(3), 284–315. [DOI] [PubMed] [Google Scholar]
- Kirby K.N., Petry N.M., Bickel W.K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology. General, 128(1), 78–87. [DOI] [PubMed] [Google Scholar]
- Kurth-Nelson Z., Redish A.D. (2010). A reinforcement learning model of precommitment in decision making. Frontiers in Behavioral Neuroscience, 4, 184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Nelson Z., Redish A.D. (2012). Don'T let me do that!—models of precommitment. Frontiers Neuroscience, 6, 138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur R., Blaszczynski A., Lalande D.R. (2012). Pre-commitment in gambling: a review of the empirical evidence. International Gambling Studies, 12(2), 215–30. [Google Scholar]
- Meule A., Vogele C., Kubler A. (2011). Psychometric evaluation of the German Barratt Impulsiveness scale - short version (BIS-15). Diagnostica 57(3), 126–33. [Google Scholar]
- Muraven M., Baumeister R.F. (2000). Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychological Bulletin, 126(2), 247–59. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Bikson M., Bestmann S. (2015). On the Use of Meta-analysis in Neuromodulatory Non-invasive Brain Stimulation. Brain Stimuli, 8(3), 666–7. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527 Pt 3, 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor J. (2007). Commitment and self-control. Journal of Economic Theory, 135(1), 1–34. [Google Scholar]
- Prevost C., Pessiglione M., Metereau E., Clery-Melin M.L., Dreher J.C. (2010). Separate valuation subsystems for delay and effort decision costs. Journal of Neuroscience, 30(42),14080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H., Green L. (1972). Commitment, choice and self-control. Journal of Experimental Analysis Behavior, 17(1),15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F., Noonan M.P., Boorman E.D., Walton M.E., Behrens T.E. (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–69. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E., Brem A.K., Levenbaum E., Thompson T., Kadosh R.C., Pascual-Leone A. (2015). Enhancing cognition using transcranial electrical stimulation. Current Opinion in Behavioral Sciences, 4, 171–8. [Google Scholar]
- Spinella M. (2007). Normative data and a short form of the Barratt Impulsiveness Scale. International Journal of Neuroscience 117(3), 359–68. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Graham S., O'Brien L., Woolard J., Cauffman E., Banich M. (2009). Age differences in future orientation and delay discounting. Child Development, 80(1), 28–44. [DOI] [PubMed] [Google Scholar]
- Steyer R., Schwenkmezger P., Notz P., Eid M. (1994). Theoretical analysis of a multidimensional mood questionnaire (MDBF). Diagnostica 40(4), 320–8. [Google Scholar]
- Stutzer A., Meier A.N. (2015). Limited self-control, obesity, and the loss of happiness. Health Economics, 25(11), 1409–24. [DOI] [PubMed] [Google Scholar]
- Wang M., Rieger M.O., Hens T. (2016). How time preferences differ: evidence from 53 countries. Journal of Economic Psychology, 52, 115–35. [Google Scholar]
- Wertenbroch K. (1998). Consumption self-control by rationing purchase quantities of virtue and vice. Marketing Science, 17(4), 317–37. [Google Scholar]
- Woods A.J., Antal A., Bikson M., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]