Abstract
Rules, whether in the form of norms, taboos or laws, regulate and coordinate human life. Some rules, however, are arbitrary and adhering to them can be personally costly. Rigidly sticking to such rules can be considered maladaptive. Here, we test whether, at the neurobiological level, (mal)adaptive rule adherence is reduced by oxytocin—a hypothalamic neuropeptide that biases the biobehavioural approach-avoidance system. Participants (N = 139) self-administered oxytocin or placebo intranasally, and reported their need for structure and approach-avoidance sensitivity. Next, participants made binary decisions and were given an arbitrary rule that demanded to forgo financial benefits. Under oxytocin, participants violated the rule more often, especially when they had high need for structure and high approach sensitivity. Possibly, oxytocin dampens the need for a highly structured environment and enables individuals to flexibly trade-off internal desires against external restrictions. Implications for the treatment of clinical disorders marked by maladaptive rule adherence are discussed.
Keywords: oxytocin, norms, rule adherence, need for structure, behavioural approach
Introduction
Human societies rely on rules that enable individuals to predict what others will do, and to avoid activities that are considered socially inadequate, poorly appreciated or even deviant. Rules come in many different forms. Examples include widely shared taboos like the prohibition of incest or homicide, norms like being fair and honest or society specific laws like driving on the right side of the street. Adhering to rules ensures individuals to be included rather than excluded from groups and societies (Tyler, 1997), fulfils an epistemic need for predictability and structure (Szechtman and Woody, 2004; Merwin et al., 2010) and enables individuals to coordinate with others and to constructively regulate social life.
Notwithstanding its functionality to both individuals and their groups, obediently and mindlessly following rules can also be maladaptive and dysfunctional. First, obediently following rules undermines the individual’s ability to adapt to changing circumstances, to adequately solve new problems and to seize on emerging opportunities (Wertheimer, 1945). Second, sometimes rules are arbitrary and following them benefits neither the individual nor society. Classic illustrations include Milgram’s obedience-to-authority experiments (Milgram, 1963), where many participants obeyed the experimenter’s rule to administer ostensibly painful electric shocks to another individual, and Asch’s work showing that individuals often follow majority opinions that are obviously wrong (Bond and Smith, 1996).
Interestingly, maladaptive rule adherence is also a trademark of diverse clinical disorders, including obsessive compulsive disorder, anorexia nervosa and autism spectrum disorder, in which individuals rigidly obey to often self-imposed and highly arbitrary rules (Lewis and Bodfish, 1998; Szechtman and Woody, 2004; Merwin et al., 2010). A promising hypothesis in the literature is that these different psychopathologies share a deficit in oxytocin at the neurophysiological level (Modahl et al., 1998; Kim et al., 2014; Hofmann et al., 2015). Here we examine the possibility that oxytocin conditions adherence to arbitrary rules that hurt self-interest without providing clear benefits. Evidence for such a possibility would further our understanding of the biological underpinnings of (mal)adaptive rule following, and point to possible treatments of the aforementioned clinical disorders and related psychopathologies.
Oxytocin is a nine-amino acid produced in the hypothalamus, that functions as both a hormone and neurotransmitter (Gimpl and Fahrenholz, 2001). Upon its release from the hypothalamus, oxytocin affects a wide array of areas of the central nervous system, including the brainstem, hippocampus, amygdala and the striatum (Bethlehem et al., 2013). In addition to its well-known role in reproduction and pair-bond formation (Donaldson and Young, 2008), more recent work suggests that oxytocin acts on (i) the cortico-amygdala circuitry to reduce withdrawal from (social) threat, permitting alternative responses to danger than flight and submission (Kemp and Guastella, 2011; Striepens et al., 2012; Harari-Dahan and Bernstein, 2014), and (ii) the ‘wanting’ mesocorticolimbic circuitry promoting approach behaviour especially when targets or events have positive valence (Lukas et al., 2011; Harari-Dahan and Bernstein, 2014; Meziane et al., 2015).
Because of these psychobiological pathways we expected oxytocin to reduce rule adherence. First, rules often demand the restriction of spontaneous approach behaviour. For example, waiting in front of a red traffic light or standing in a queue in the supermarket interferes with the internal goal to proceed towards one’s destination, or to not waste more time than strictly necessary. The oxytocin-biased biobehavioural approach might thus manifest itself in enhanced salience of appealing internal goals and reduced fear for possible sanctions that may follow from failure to adhere to a rule. The net result would be that individuals given oxytocin, rather than placebo, adhere less to arbitrary rules that are personally costly to follow. Second, there is some evidence that in healthy individuals, oxytocin increases divergent thinking and creative performance by violating the usual pattern and norm (De Dreu et al., 2014) and in patients diagnosed with autistic spectrum disorder, oxytocin has been shown to reduce the need for structure expressed by obsessive ordering and repetitive behaviour (Hollander et al., 2003; Anagnostou et al., 2014).
Our main prediction is therefore grounded in the idea that oxytocin biases biobehavioural approach, and reduces need for structure. While both approach and need for structure can be state-dependent, there is good evidence that individuals also differ in chronic approach (vs avoidance) sensitivity (Spielberg et al., 2011), as well as in their personal need for structure, order and preference for a predictable environment (Neuberg and Newsom, 1993). Whereas individuals with heightened approach sensitivity have a relatively flexible processing style for whom set-switching is relatively easy (Baas et al., 2008), those with high personal need for structure are relatively rigid in their thinking and relying on tried-and-true rules and schemata (Rietzschel et al., 2007). Accordingly, following the logic of a moderation-of-process design (Spencer et al., 2005), oxytocin should reduce rule following especially among individuals scoring high on approach sensitivity, and high on personal need for structure. Evidence for such treatment-by-trait interactions would resonate with the more general observation that oxytocin effects often depend on personality differences (Bartz et al., 2011a), and would also shed light on when and why oxytocin reduces rule following.
Material and methods
Ethics and subjects
Predictions were tested in a randomized double-blind placebo controlled between-subjects experiment. The experiment was approved by the University of Amsterdam ethics committee (file WOP-2015-4100) and complied with Helsinki protocols and the APA-ethics guidelines. Subjects filled out a medical screening, provided written informed consent prior to participation and received remuneration along with a written debriefing upon completion of the study.
Statistical power and sample size
There is some concern about the robustness and replicability of oxytocin effects on human behaviour (Nave et al., 2015; Walum et al., 2016). One reason is that, as in many other (neuropharmacology) studies, many experiments on oxytocin have rather low sample sizes and comparatively low statistical power. For example, the median sample size across 40 human intranasal oxytocin studies investigating the influence of oxytocin on a variety of psychological processes like trust, emotion recognition and face recognition was 49 participants (with the largest sample size being n = 112; Van IJzendoorn and Bakermans-Kranenburg, 2012; Shahrestani et al., 2013; Van IJzendoorn, 2013; Nave et al., 2015; Walum et al., 2016). In addition to a parsimonious experimental setup with only one outcome variable (rule following, see below), we recruited a larger sample size compared to previous studies (Walum et al., 2016). Specifically, we aimed to test our predictions with a statistical power of β = 0.80 (and α = 0.05). In estimating the required sample size, we used a recent study from our own laboratory in which we used similar procedures, considered a similar type of effect (‘divergent thinking’ the generation of ideas that deviate from an implicit prime) and studied subjects from a similar population (albeit a few years earlier, the composition of the research population tends to remain relatively stable). This study was Experiment 4 in De Dreu et al. (2014) and had an ηp2 = 0.061 with N = 62. G-Power 3.1 (Faul et al., 2007) indicated a required sample size of N = 123. Because De Dreu et al., (2014) relied on male participants only, and oxytocin effects in females may be weaker due to their higher concentrations of endogenous oxytocin, we recruited a sample of 139 undergraduate students (mean age = 21.5 ± 3.4, 99 female) for this study. Participants were randomly assigned to treatment conditions. There was no systematic difference in the sex proportions across treatments (χ2-test (1) = 0.52, P = 0.47).
The study was advertised as a study on ‘medication and decision making’ via the Research Institute’s on-line recruiting system. Participants filled out an on-line medical screening. Exclusion criteria were significant medical or psychiatric illness, medication, smoking more than five cigarettes per day and drug or alcohol abuse. Eligible subjects were scheduled for a session and instructed to refrain from smoking or drinking (except for water) for 2 h before the experiment.
Medication
Participants self-administered a single intranasal dose of 24 IU of oxytocin (Syntocinon spray; Novartis; three puffs per nostril, each with 4 IU of oxytocin) or placebo 30 min before the start of the experimental tasks (for similar procedures and rationale, see e.g. Baumgartner et al., 2008; De Dreu et al., 2010; Stallen et al., 2012). To avoid any subjective effects (for example, olfactory effects) other than those caused by oxytocin, the placebo contained all of the active ingredients except for the neuropeptide (De Dreu et al., 2010). Thus, the only difference between the placebo and treatment was the absence versus presence of the active neuropeptide.
Experimental procedure
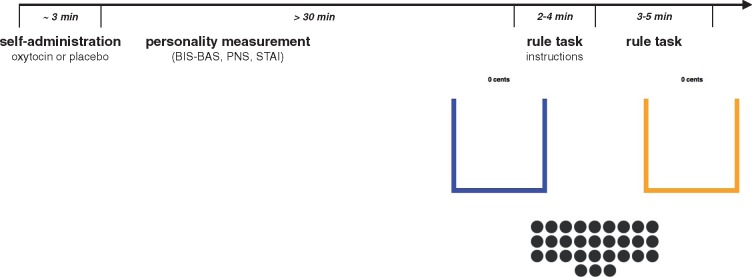
Figure 1 summarizes the timeline and experimental tasks. Upon arriving at the laboratory, subjects were seated in individual cubicles in front of a computer. After signing the informed consent forms subjects were instructed to self-administer the medication under experimenter supervision. Neither the participant nor the experimenter knew about the medication type (placebo or oxytocin). Although there are several possible pathways through which intranasal oxytocin affects brain and behaviour (Born et al., 2002; Gossen et al., 2012; Striepens et al., 2013; Leng and Ludwig, 2016), research typically finds effects 30 min post-administration. Accordingly, we followed the procedure used before in our own lab and that of others (Baumgartner et al., 2008; De Dreu et al., 2010). After administration subjects proceeded for 30 min with a series of personality and state questionnaires. More specifically, and because oxytocin has been implicated in fear modulation (Kirsch et al., 2005; Labuschagne et al., 2010), subjects reported their current anxiety level (STAI; Spielberger and Sydeman, 1994). The state STAI is a 20 item questionnaire asking respondents to rate statements like ‘I am tense’ or ‘I feel calm’ on a 4-point Likert scale (1 = not at all, to 4 = very much so). The scale had a good internal consistency in our sample (Cronbach α = 0.89). They then responded to Dutch translations of questionnaires measuring behavioural inhibition and behavioural approach tendencies (BIS-BAS; Carver and White, 1994), and need for structure (PNS: Kivimäki et al., 1996). If time remained, they were provided with the 60-item NEO-questionnaire which measures five broad dimensions of personality (Hofstee et al., 1995; Denissen et al., 2008). Because items were administered randomly and none of the subjects completed the entire measure before the 30-min loading time was finished, data were incomplete and further ignored.
Fig. 1.
Timeline of the experiment. After self-administration of either placebo or oxytocin participants waited for at least 30 min before receiving instructions for the rule-task. In the rule task, each ball has to be dragged into either the blue or the yellow bucket. The rule is to put each ball into the blue bucket, which only yields €0.05 per ball, whereas putting a ball into the yellow bucket yields €0.10.
BIS-BAS is a 24 item questionnaire with items like ‘I worry about making mistakes’ (behavioural inhibition) or ‘I often act on the spur of the moment’ (behavioural approach) (1 = very false for me, to 5 = very true for me). The BAS-scale can further be divided into three subscales. Since the focus of this study was approach sensitivity as a general construct, and to prevent multiple testing of the same hypothesis, the BAS score was not further subdivided into subscale-scores. Both BIS and BAS had good internal consistencies in our sample (BIS: Cronbach α = 0.83, BAS: Cronbach α = 0.80). PNS is a 12 item questionnaire in which participants rate items like ‘I enjoy being spontaneous’ (low structural need) or ‘I find that a consistent routine enables me to enjoy life more’ (high structural need) on a 6-point scale (1 = strongly disagree, to 6 = strongly agree; Cronbach α = 0.81).
The computer was configured to switch to the experimental task only 30 min after administration. In this decision-making task, participants saw 30 balls on the computer screen. They had to drag each ball individually in either the blue or yellow bucket with their mouse. In the instructions, it was explained to them that for each ball they put in the blue bucket they would receive 0.05€ and for each ball they put in the yellow bucket they would receive 0.10€. Then they read that ‘the rule is to put the balls in the blue bucket’. Above each bucket there was a counter showing the amount of money already accumulated (Kimbrough and Vostroknutov, 2015). Thus, subjects would maximize their payoff by putting all balls in the yellow bucket. Yet completely adhering to the rule would earn them only half of the money they could earn would they consistently violate this rule. No reason was given for following the rule and participants would not face any negative consequences for violating the rule.
Data analytic strategy
Compared to most studies on intranasal oxytocin (Walum et al., 2016), we only test the effect of intranasal oxytocin on one outcome variable, thereby avoiding the risk of false positive effects due to treatment comparisons across multiple outcome variables in one sample. Predictions were tested in a single regression model, with rule following (number of balls in the blue bucket) as criterion and as predictors main effects for treatment (0 = placebo; 1 = oxytocin), BIS-BAS and PNS, as well as the two-way interactions between treatment on the one hand and BIS-BAS and PNS on the other. Significant two-way interactions were probed using simple slope analyses for treatment at ±1 s.d. of the critical trait (Aiken and West, 1999). To facilitate interpretation of interactions, and to reduce possible multicollinearity, all continuous variables were mean-centred. Because of the censoring of the dependent variable, we performed censored regression but note that using a linear OLS regression model did not alter the statistical conclusions reported here. Significance was decided with P ≤ 0.05 (two-tailed) and only in case of predicted effects, we cautiously interpreted effects at P ≤ 0.10 (two-tailed). In light of the on-going debate about the robustness of intranasal oxytocin effects on human behaviour, we further corrected all hypothesized regression main effects and interactions for multiple comparisons using false discovery rate (FDR; Benjamini and Hochberg, 1995) to reduce the probability of type I errors. We also checked whether obtained results were robust to control variables like age, sex, type of contraception and hormonal cycle. Types of contraception were obtained through self-report in the medical screening. Female participants further indicated their first and last day of their last menstruation from which luteal and follicular phase were inferred.
Results
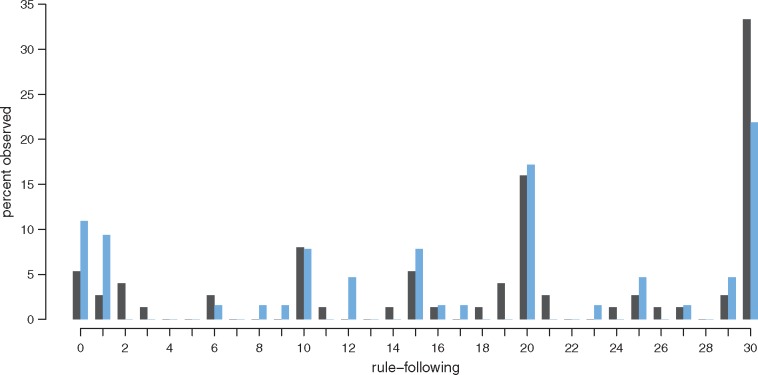
As the rule following task is relatively new and few studies used it, in a first analysis, we probed the general tendency for oxytocin to influence rule following over and beyond individual differences in approach sensitivity and need for structure. Figure 2 shows that, first of all, rule following in our sample is varied, with only few participants strictly following the (arbitrary) rule and only few subjects disobeying completely. Most participants tried to find some middle-ground between strict compliance and complete disobeying, and thus sometimes did, and sometimes did not follow the rule. Furthermore, as can also be seen in Figure 2, oxytocin treatment appeared to influence rule following. While 33% of the sample decided to adhere fully to the rule in the placebo condition by placing all balls in the bucket that would only yield half of the payment, only 22% did so in the oxytocin condition. On the other extreme, 11% of the participants in the oxytocin condition violated the rule maximally by putting all balls in the bucket they were not supposed to. Only 5% of the participants did so in the placebo condition (overall univariate effect of oxytocin on rule adherence: Mann–Whitney U = 2797, P = 0.08).
Fig. 2.
Rule following. Share of participants in the oxytocin (blue) and placebo (black) condition as a function of how many of the 30 balls have been placed according to the rule.
To examine the proposed trait-interaction responsible for oxytocin-reduced rule-following, we computed a censored regression model with rule following as the criterion and treatment (dummy coded 0 = placebo; 1 = oxytocin), BIS, BAS, PNS and the interactions among treatment and personality traits as predictors, controlling for sex, age and state anxiety (STAI). Obtained P-values for the main treatment effect and the three interaction terms were FDR-corrected to reduce the probability of type I errors.
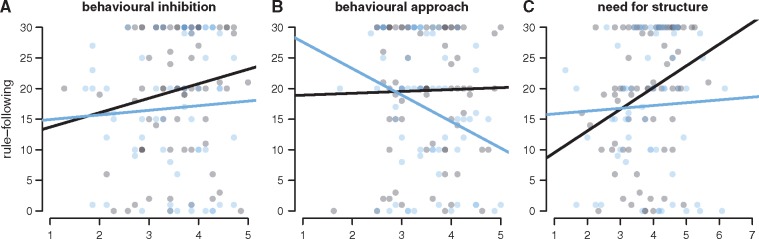
Results are summarized in Table 1. There was no significant interaction of rule following and behavioural inhibition, regardless of treatment (Figure 3A and Table 1; b(BIS) = 0.41, t = 0.13, P = 0.90, two-sided; b(treatment × BIS) = 1.10, t = 0.24, P = 0.81). Second, and as predicted, subjects with high behavioural approach sensitivity violated the rule more frequently when given oxytocin (Figure 3B and Table 1; b(treatment × BAS) = −12.76, t = −2.34, P = 0.04). Post-hoc probes of this interaction (Holmbeck, 2002) revealed that under oxytocin the higher the behavioural approach sensitivity, the more often the rule was violated (b = −8.12, t = −1.97, P = 0.04); under placebo, there was no significant change in rule violation across behavioural approach sensitivity (b = 4.64, t = 1.28, p = 0.20). This fits the hypothesis that oxytocin biases biobehavioural approach, and suggests that oxytocin further activates and accelerates inherent tendencies towards behavioural approach.
Table 1.
Censored (below 0 and above 30) regression results predicting the propensity of rule adherence (i.e. the number of balls put into the blue bucket) as a function of treatment and personality traits (mean-centred)
| B | SE | t | P ≤ | |
|---|---|---|---|---|
| Intercept | 22.93 | 2.15 | 10.68 | 0.01 |
| Treatment1,a | −3.97 | 2.71 | −1.47 | 0.19 |
| Sex2 | −2.78 | 3.23 | −0.86 | 0.39 |
| Age | −0.41 | 0.44 | −0.94 | 0.35 |
| State Anxiety (STAI) | −3.01 | 2.68 | −1.12 | 0.26 |
| Behavioural Inhibition (BIS) | 0.41 | 3.13 | 0.13 | 0.90 |
| Behavioural Approach (BAS) | 4.64 | 3.61 | 1.28 | 0.20 |
| Need for Structure (PNS) | 6.13 | 2.77 | 2.21 | 0.03 |
| Treatment × BISa | 1.10 | 4.53 | 0.24 | 0.81 |
| Treatment × BASa | −12.76 | 5.45 | −2.34 | 0.04 |
| Treatment × PNSa | −9.15 | 3.66 | −2.50 | 0.04 |
Note: 0 = placebo, 1 = oxytocin; 0 = female, 1 = male.
FDR corrected P-value.
Fig. 3.
Moderators of rule following. Rule following depending on (A) behavioural inhibition (BIS), (B) behavioural approach (BAS) and (C) need for structure (PNS) in the oxytocin treatment (blue dots) and placebo treatment (black dots). Lines indicate the best linear fit.
From the fitted regression line shown in Figure 3B, it may appear as if participants with very low approach sensitivity are more rule-following under oxytocin than placebo. However, we note that such an inference is problematic, as most participants reported behavioural approach scores between 3 and 5. Finally, whereas individuals under placebo adhered more to the rule the higher their need for structure (Table 1; b(PNS) = 6.13, t = 2.21, P = 0.03), oxytocin dampened rule adherence in particular in participants high on need for structure (Figure 3C and Table 1; b(treatment × PNS) = −9.15, t = −2.50, P = 0.04). In participants with a high need for structure, oxytocin thus led to more rule breaking. This was confirmed by post-hoc probes of this interaction (Holmbeck, 2002). While need for structure significantly predicted higher rule-following under placebo (b = 6.13, t = 2.21, P = 0.03), this was not the case under oxytocin (b = −3.01, t = −1.21, P = 0.23).
Robustness checks
Since personality traits have been measured directly after intranasal oxytocin administration, we cannot rule out the possibility that these measures were already influenced by early psychophysiological effects of the oxytocin administration. We therefore tested for possible effects of treatment on behavioural approach, behavioural inhibition, need for structure and state anxiety. Table 2 shows the means and standard deviations. Participants in the oxytocin group did not significantly differ in either of the four traits/state from participants in the placebo group (t-tests, BAS: t(137) = 0.10, P = 0.92; BIS: t(137) = 0.47, P = 0.64; PNS: t(137) = −0.47, P = 0.64; STAI: t(137) = −0.88, P = 0.38). Looking at the correlational structure among the personality measures, it appeared that participants with high behavioural inhibition or low behavioural approach were also more likely to report a high need for structure (Table 2).
Table 2.
Descriptive statistics and zero-order correlations for personality traits, broken down by treatment
| Placebo | Oxytocin | |||||
|---|---|---|---|---|---|---|
| M (s.d.) | M (s.d.) | 1. | 2. | 3. | 4. | |
| STAI | 2.08 (0.49) | 2.16 (0.55) | – | 0.23 | −0.14 | 0.15 |
| BIS | 3.54 (0.72) | 3.47 (0.81) | – | −0.30 | 0.55 | |
| BAS | 3.81 (0.53) | 3.80 (0.54) | – | −0.41 | ||
| PNS | 3.79 (0.82) | 3.86 (1.03) | – |
Note: STAI = State Anxiety, BIS = Behavioural Avoidance, BAS = Behavioural Approach, PNS = Need for Structure.
Administration of oxytocin can differentially affect the behaviour of males and females (Rilling et al., 2014; Yao et al., 2014) and might interact with menstrual cycle and usage of contraceptives. Accordingly, we performed additional analyses to test the robustness of the reported results using stepwise inclusion of further control variables. Again, obtained P-values were FDR-corrected to reduce the type I error probability. First, we tested whether the effect of oxytocin on rule-following is sex specific, by allowing for an interaction of sex and drug-treatment on rule-following in the regression model (column 2, Table 3). There was no significant sex (b = −6.04, P = 0.11) or sex × drug interaction (b = 2.67, P = 0.65) in our sample. Also when entering the interaction terms between personality traits and treatment, there was no sex effect of oxytocin on rule following (column 3, Table 3), showing that the observed effect of oxytocin on rule-following was not significantly moderated by sex in our sample. Note however that most of our sample was comprised of female participants.
Table 3.
Censored (below 0 and above 30) regression results predicting the propensity of rule adherence (i.e. the number of balls put into the blue bucket) with stepwise included control variables
| (1) | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|
| B (P) | B (P) | B (P) | B (P) | B (P) | |
| Intercept | 22.59 (0.00) | 24.48 (0.00) | 23.84 (0.00) | 18.31 (0.00) | 18.00 (0.00) |
| Treatment1,a | −4.72 (0.08) | −5.79 (0.07) | −5.33 (0.14) | 0.57 (0.92) | 1.05 (0.84) |
| Sex2 | −6.04 (0.11) | −5.27 (0.26) | – | – | |
| Treatment × Sex | 2.67 (0.65) | 4.36 (0.51) | – | – | |
| BIS | −1.04 (0.76) | −0.54 (0.87) | −1.10 (0.75) | ||
| BAS | 4.61 (0.20) | 4.20 (0.26) | 4.04 (0.29) | ||
| PNS | 6.35 (0.02) | 6.16 (0.02) | 6.37 (0.02) | ||
| Treatment × BISa | 2.49 (0.60) | 3.15 (0.70) | 3.46 (0.67) | ||
| Treatment × BASa | −14.07 (0.02) | −12.19 (0.06) | −11.99 (0.07) | ||
| Treatment × PNSa | −9.69 (0.02) | −9.43 (0.04) | −9.00 (0.07) | ||
| STAI | −3.29 (0.23) | −3.93 (0.16) | |||
| Age | −0.46 (0.30) | −0.46 (0.32) | |||
| Contraception (Pill) | 5.49 (0.26) | 3.72 (0.58) | |||
| Contraception (Hormonal Spiral) | 5.43 (0.32) | 4.08 (0.52) | |||
| Treatment × Pill | −5.01 (0.47) | 2.64 (0.81) | |||
| Treatment × Hormonal Spiral | −8.03 (0.29) | −1.69 (0.87) | |||
| Follicular Phase | 0.64 (0.92) | ||||
| Luteal Phase | 5.63 (0.39) | ||||
| Treatment × Follicular Phase | −7.62 (0.44) | ||||
| Treatment × Luteal Phase | −12.90 (0.21) |
Note.10 = placebo, 1 = oxytocin; 20 = female, 1 = male. Contraception: all female participants indicated either to use the pill or a hormonal spiral—male participants are therefore coded as baseline.
FDR corrected P-value.
Since hormonal spiral and the use of birth control pill, as well as the menstrual cycle might affect the hormonal balance and moderate the effect of oxytocin on behaviour in female participants, we also included the type of contraception female participants used in our sample (column 4, Table 3) and the menstrual cycle (column 4, Table 3) together with all possible first-order interactions of drug treatment and these controls. The reported interactions between personality traits and oxytocin on rule-following are robust to these controls (columns 4 and 5, Table 3).
Discussion and conclusions
When given oxytocin, rather than matching placebo, individuals were less likely to follow an arbitrary rule that demanded them to forgo financial benefits. Consistent with the proposition that oxytocin reduces the demand for a structured environment and increases approach sensitivity (Kemp and Guastella, 2011; Harari-Dahan and Bernstein, 2014), we found that oxytocin reduced rule following especially among individuals with high need for structure, and high approach sensitivity.
Findings fit earlier work that oxytocin up-regulates creative thinking and reduces convergent processing (De Dreu et al., 2014). Yet they may appear at odds with work showing that oxytocin promotes social conformity, which can be seen as a tendency to follow rather than ignore social rules (Stallen et al., 2012; also see Ma et al., 2014; Edelson et al., 2015). The key difference between the current study and those on creativity, and the work on social conformity, is that the rule in this study was arbitrary and adhering to it had no benefits for either oneself or another group. In contrast, following the majority view within one’s group has a range of consequences to both oneself (e.g. being included in the group, benefitting from the ‘wisdom of the crowd’) and the group one affiliates with (e.g. fostering coordination and joint action). Possibly then, oxytocin enables individuals to focus on the benefits and costs of a particular rule, allowing them to flexibly adapt by either following the rule or complying with a norm, or not.
Recently, Shalvi and De Dreu, (2014) found that oxytocin did not increase financially beneficial lying in a die rolling task. Lying can be interpreted as violating a rule and, as such, their finding may seem at odds with the current observation that oxytocin reduced rule adherence (given certain traits). However, lying arguably entails a higher psychological cost to the self-image (Shalvi et al., 2011; Gneezy et al., 2013; Abeler et al., 2014) and carries a moral dimension that is much less salient for arbitrary rules. As noted in Shalvi and De Dreu (2014; also see Shalvi et al., 2011), it requires additional justifications—such as that lying benefits one’s group rather than just oneself—to violate a morally laden rule such as ‘thou shall not lie’. Absent the need for such additional justification, we see that, especially for individuals with high approach sensitivity and need for structure, oxytocin appears to increase behavioural flexibility (also see De Dreu et al., 2014), suggesting that oxytocin reduces adherence to arbitrary rules that do not much more than hurting self-interest, and boosts adherence to rules that facilitate the regulation of social life.
A key finding was that effects of oxytocin emerged especially in subjects with high need for structure and high approach sensitivity. This not only fits the general observation that effects of oxytocin are strongly contingent on a broad range of states and traits (Bartz et al., 2011a), but also resonates with work suggesting that several clinical disorders, such as autism spectrum disorder, obsessive compulsive disorder and anorexia nervosa, seem to share important deficits in the oxytocinergic system. Low levels of oxytocin in blood plasma and the cerebrospinal fluid have been associated with autism spectrum disorder (Modahl et al., 1998), obsessive compulsive disorder (Leckman et al., 1994) and anorexia nervosa (Demitrack et al., 1990). Without exception these disorders are marked by rigid adherence to self-imposed rules, that seem arbitrary from an outside perspective, like arranging objects according to certain rules (Lam and Aman, 2006), or following harsh dietary restrictions (Garner and Bemis, 1982). In autism spectrum disorder and anorexia nervosa, treatment with oxytocin has some positive effects: it up-regulates social approach in autistic patients (Andari et al., 2010; Anagnostou et al., 2014), and reduces biased attention to palliative food items in patients with anorexia nervosa (Kim et al., 2014). Accordingly, present results suggest that treating these patients with oxytocin may reduce at least one critical, mostly maladaptive and often overlooked tendency—the rigid following of rules that have no clear function.
It is important to note that the effect of oxytocin, particular in participants with high trait approach sensitivity, might also bear negative consequences. For example, Bartz et al. (2011b) and Ebert et al. (2013) found that the administration of intranasal oxytocin to participants with borderline personality disorder—characterized by emotional instability and impulsiveness—led to less trusting and cooperative behaviour (also see Simeon et al., 2011). Together with the present results, these findings may point to an inverted U-shape relationship between oxytocin and rigid rule following on the one hand, and extreme emotional stability and impulsiveness on the other. With individuals rigidly following (arbitrary) rules, exogenous oxytocin may enable them to respond more flexibly; yet in individuals with a propensity for impulsivity, exogenous oxytocin may undermine adaptiveness and lead to flexibility that is erratic and maladaptive. It is noteworthy that a similar inverted U-shape has been noted between cognitive control and dopamine (Cools and D'Esposito, 2011; Piray et al., 2015), a neurochemical with strong connections to the oxytocinergic circuitry (Carter, 2014; De Dreu et al., 2014; also see Van Wimersma Greidanus et al., 1990). Future work is therefore needed to investigate the possible positive and negative consequences of intranasal oxytocin administration on rule-adherence in particular in clinical populations that are characterized by impulsiveness on the one side and rigid behaviour on the other side.
This study is the first to explore the connection of oxytocin and maladaptive rule adherence using a novel rule-following task. By recruiting a larger sample size than previous human oxytocin studies (Walum et al., 2016) in combination with a simple and straightforward task, testing our hypotheses on only one dependent variable and correcting results for multiple comparisons, we hope to at least partly address current concerns in the literature about a potentially high rate of false positive findings of intranasal oxytocin on human social behaviour (McCullough et al., 2013; Nave et al., 2015; Walum et al., 2016). However, as with any novel paradigm, our results should be seen as an early exploratory finding that warrants further replication, especially since our main finding rests on a treatment-by-trait interaction, that requires more statistical power to reliably detect than a simple main effect (Open Science Collaboration, 2015).
Although rules—including taboos, norms and laws—often are functional and needed for the regulation of human life, mindlessly adhering to any rule may be maladaptive and hurtful to oneself. Here we provided first evidence that in healthy individuals (maladaptive) rule adherence is attenuated by oxytocin, especially when individuals have high need for structure, and strong approach sensitivity.
Funding
This project was supported by grant NWO-08-032-002 from the Netherlands Organization for Scientific Research to CKWDD.
Conflict of interest. None declared.
Acknowledgements
We thank Tim de Wilde, Katie Daughters and Michael Giffin for data collection.
References
- Abeler J., Becker A., Falk A. (2014). Representative evidence on lying costs. Journal of Public Economics, 113, 96–104. [Google Scholar]
- Aiken L.S., West S.G. (1999). Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage. [Google Scholar]
- Anagnostou E., Soorya L., Brian J., et al. (2014). Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Research, 1580, 188–98. [DOI] [PubMed] [Google Scholar]
- Andari E., Duhamel J.R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America, 107(9), 4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas M., De Dreu C.K.W., Nijstad B.A. (2008). A meta-analysis of 25 years of mood-creativity research: hedonic tone, activation, or regulatory focus? Psychological Bulletin, 134(6), 779–806. [DOI] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011a). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15(7), 301–9. [DOI] [PubMed] [Google Scholar]
- Bartz J., Simeon D., Hamilton H., et al. (2011b). Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience, 6(5), 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron, 58(4), 470–1. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, 57(1), 289–300. [Google Scholar]
- Bethlehem R.A.I., van Honk J., Auyeung B., Baron Cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38(7), 962–74. [DOI] [PubMed] [Google Scholar]
- Bond R., Smith P.B. (1996). Culture and conformity: a meta-analysis of studies using Asch's (1952b, 1956) line judgment task. Psychological Bulletin, 119(1), 111–37. [Google Scholar]
- Born J., Lange T., Kern W., McGregor G.P., Bickel U., Fehm H.L. (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience, 5(6), 514–6. [DOI] [PubMed] [Google Scholar]
- Carter C.S. (2014). Oxytocin pathways and the evolution of human behavior. Annual Review of Psychology, 65, 17–39. [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–33. [Google Scholar]
- Cools R., D'Esposito M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69(12), e113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C.K.W., Baas M., Roskes M., et al. (2014). Oxytonergic circuitry sustains and enables creative cognition in humans. Social Cognitive and Affective Neuroscience, 9(8), 7–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C.K.W., Greer L.L., Handgraaf M.J.J., et al. (2010). The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science, 328(5984), 1408–11. [DOI] [PubMed] [Google Scholar]
- Denissen J.J.A., Geenen R., van Aken M.A.G., Gosling S.D., Potter J. (2008). Development and Validation of a Dutch Translation of the Big Five Inventory (BFI). Journal of Personality Assessment, 90(2), 152–7. [DOI] [PubMed] [Google Scholar]
- Demitrack M.A., Lesem M.D., Listwak S.J., Brandt H.A., Jimerson D.C., Gold P.W. (1990). CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. American Journal of Psychiatry, 147(7), 882–6. [DOI] [PubMed] [Google Scholar]
- Donaldson Z.R., Young L.J. (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–4. [DOI] [PubMed] [Google Scholar]
- Ebert A., Kolb M., Heller J., Edel M.A., Roser P., Brüne M. (2013). Modulation of interpersonal trust in borderline personality disorder by intranasal oxytocin and childhood trauma. Social Neuroscience, 8(4), 305–13. [DOI] [PubMed] [Google Scholar]
- Edelson M.G., Shemesh M., Weizman A., Yariv S., Sharot T., Dudai Y. (2015). Opposing effects of oxytocin on overt compliance and lasting changes to memory. Neuropsychopharmacology, 40(4), 966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Garner D.M., Bemis K.M. (1982). A cognitive-behavioral approach to anorexia nervosa. Cognitive Therapy and Research, 6(2), 123–50. [Google Scholar]
- Gimpl G., Fahrenholz F. (2001). The oxytocin receptor system: structure, function, and regulation. Physiological Reviews, 81(2), 629–83. [DOI] [PubMed] [Google Scholar]
- Gneezy U., Rockenbach B., Serra-Garcia M. (2013). Measuring lying aversion. Journal of Economic Behavior & Organization, 93, 293–300. [Google Scholar]
- Gossen A., Hahn A., Westphal L., et al. (2012). Oxytocin plasma concentrations after single intranasal oxytocin administration—a study in healthy men. Neuropeptides, 46(5), 211–5. [DOI] [PubMed] [Google Scholar]
- Harari-Dahan O., Bernstein A. (2014). A general approach-avoidance hypothesis of Oxytocin: accounting for social and non-social effects of oxytocin. Neuroscience and Biobehavioral Reviews, 47, 506–19. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Fang A., Brager D.N. (2015). Effect of intranasal oxytocin administration on psychiatric symptoms: a meta-analysis of placebo-controlled studies. Psychiatry Research, 228(3), 708–14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hollander E., Novotny S., Hanratty M., et al. (2003). Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology, 28(1), 193–8. [DOI] [PubMed] [Google Scholar]
- Hofstee W.K.B., De Raad B., Goldberg L.R. (1992). Integration of the Big Five and circumplex approaches to trait structure. Journal of Personalify and Social Psychology, 63, 146–63. [DOI] [PubMed] [Google Scholar]
- Holmbeck G.N. (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27(1), 87–96. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Guastella A.J. (2011). The role of oxytocin in human affect a novel hypothesis. Current Directions in Psychological Science, 20(4), 222–31. [Google Scholar]
- Kim Y.R., Kim C.H., Cardi V., Eom J.S., Seong Y., Treasure J. (2014). Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology, 44, 133–42. [DOI] [PubMed] [Google Scholar]
- Kimbrough E.O., Vostroknutov A. (2016). Norms Make Preferences Social. The Journal of the European Economic Association, 14(3), 608–638. [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäki M., Elovainio M., Nord J. (1996). Effects of components of personal need for structure on occupational strain. The Journal of Social Psychology, 136(6), 769–77. [DOI] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology, 35(12), 2403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.S.L., Aman M.G. (2006). The repetitive behavior scale-revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(5), 855–66. [DOI] [PubMed] [Google Scholar]
- Leckman J., Goodman W., North W., et al. (1994). The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology, 19(8), 723–49. [DOI] [PubMed] [Google Scholar]
- Leng G., Ludwig M. (2016). Intranasal oxytocin: myths and delusions. Biological Psychiatry, 79(3), 243–50. [DOI] [PubMed] [Google Scholar]
- Lewis M.H., Bodfish J.W. (1998). Repetitive behavior disorders in autism. Mental Retardation and Developmental Disabilities Research Reviews, 4(2), 80–9. [Google Scholar]
- Lukas M., Toth I., Reber S.O., Slattery D.A., Veenema A.H., Neumann I.D. (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology, 36(11), 2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Luo L., Geng Y., Zhao W., Zhang Q., Kendrick K.M. (2014). Oxytocin increases liking for a country's people and national flag but not for other cultural symbols or consumer products. Frontiers in Behavioral Neuroscience, 8, 193–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough M.E., Churchland P.S., Mendez A.J. (2013). Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neuroscience & Biobehavioral Reviews, 37(8), 1485–92. [DOI] [PubMed] [Google Scholar]
- Merwin R.M., Timko C.A., Moskovich A.A., Ingle K.K., Bulik C.M., Zucker N.L. (2010). Psychological inflexibility and symptom expression in anorexia nervosa. Eating Disorders, 19(1), 62–82. [DOI] [PubMed] [Google Scholar]
- Meziane H., Schaller F., Bauer S., et al. (2015). An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biological Psychiatry, 78(2), 85–94. [DOI] [PubMed] [Google Scholar]
- Milgram S. (1963). Behavioral Study of obedience. The Journal of Abnormal and Social Psychology, 76(4), 371–8. [DOI] [PubMed] [Google Scholar]
- Modahl C., Green L.A., Fein D., et al. (1998). Plasma oxytocin levels in autistic children. Biological Psychiatry, 43(4), 270–7. [DOI] [PubMed] [Google Scholar]
- Nave G., Camerer C.F., McCullough M. (2015). Does oxytocin increase trust in humans? A critical review of research. Perspectives on Psychological Science, 10(6), 772–89. [DOI] [PubMed] [Google Scholar]
- Neuberg S.L., Newsom J.T. (1993). Personal need for structure: individual differences in the desire for simpler structure. Journal of Personality and Social Psychology, 65(1), 113–31. [Google Scholar]
- Open Science Collaboration (2015). Estimating the reproducibility of psychological science. Science, 349(6251), aac4716. [DOI] [PubMed] [Google Scholar]
- Piray P., Ouden den H.E.M., van der Schaaf M.E., Toni I., Cools R. (2015). Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cerebral Cortex (New York, NY: 1991), bhv243.. 10.1093/cercor/bhv243. [DOI] [PubMed] [Google Scholar]
- Rietzschel E.F., De Dreu C.K.W., Nijstad B.A. (2007). Personal need for structure and creative performance: the moderating influence of fear of invalidity. Personality and Social Psychology Bulletin, 33(6), 855–66. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., et al. (2014). Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology, 39, 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S., Kemp A.H., Guastella A.J. (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology, 38(10), 1929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalvi S., De Dreu C.K.W. (2014). Oxytocin promotes group-serving dishonesty. Proceedings of the National Academy of Sciences of the United States of America, 111(15), 5503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalvi S., Dana J., Handgraaf M.J.J., De Dreu C.K.W. (2011). Justified ethicality: observing desired counterfactuals modifies ethical perceptions and behavior. Organizational Behavior and Human Decision Processes, 115(2), 181–90. [Google Scholar]
- Simeon D., Bartz J., Hamilton H., et al. (2011). Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology, 36(9), 1418–21. [DOI] [PubMed] [Google Scholar]
- Spencer S.J., Zanna M.P., Fong G.T. (2005). Establishing a causal chain: why experiments are often more effective than mediational analyses in examining psychological processes. Journal of Personality and Social Psychology, 89(6), 845–51. [DOI] [PubMed] [Google Scholar]
- Spielberg J.M., Miller G.A., Engels A.S., et al. (2011). Trait approach and avoidance motivation: lateralized neural activity associated with executive function. NeuroImage, 54(1), 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Sydeman S.J. (1994). State-trait anxiety inventory and state-trait anger expression inventory In: Maruish M.E., editor. The Use of Psychological Testing for Treatment Planning and Outcome Assessment (pp. 292–321). Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Stallen M., De Dreu C.K.W., Shalvi S., Smidts A., Sanfey A.G.A. (2012). The herding hormone: oxytocin stimulates in-group conformity. Psychological Science, 23(11), 1288–92. [DOI] [PubMed] [Google Scholar]
- Striepens N., Kendrick K.M., Hanking V., et al. (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N., Scheele D., Kendrick K.M., et al. (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings of the National Academy of Sciences of the United States of America, 109(44), 18144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H., Woody E. (2004). Obsessive-compulsive disorder as a disturbance of security motivation. Psychological Review, 111(1), 111–27. [DOI] [PubMed] [Google Scholar]
- Tyler T.R. (1997). The psychology of legitimacy: a relational perspective on voluntary deference to authorities. Personality and Social Psychology Review, 1(4), 323–45. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn M.H. (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational Psychiatry, 3, e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn M.H., Bakermans-Kranenburg M.J. (2012). A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology, 37(3), 438–43. [DOI] [PubMed] [Google Scholar]
- Van Wimersma Greidanus T.B., Kroodsma J.M., Pot M.L.H., Stevens M., Maigret C. (1990). Neurohypophyseal hormones and excessive grooming behaviour. European Journal of Pharmacology, 187(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Walum H., Waldman I.D., Young L.J. (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological Psychiatry, 79(3), 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer M. (1945). Productive Thinking. New York: Harper. [Google Scholar]
- Yao S., Zhao W., Cheng R., Geng Y., Luo L., Kendrick K.M. (2014). Oxytocin makes females, but not males, less forgiving following betrayal of trust. The International Journal of Neuropsychopharmacology, 17(11), 1785–92. [DOI] [PubMed] [Google Scholar]