Abstract
Interpersonal violence (IPV) is one of the most frequent causes for the development of posttraumatic stress disorder (PTSD) in women. Trauma-related triggers have been proposed to evoke automatic emotional responses in PTSD. The present functional magnetic resonance study investigated the neural basis of trauma-related picture processing in women with IPV-PTSD (n = 18) relative to healthy controls (n = 18) using a newly standardized trauma-related picture set and a non-emotional vigilance task. We aimed to identify brain activation and connectivity evoked by trauma-related pictures, and associations with PTSD symptom severity. We found hyperactivation during trauma-related vs neutral picture processing in both subcortical [basolateral amygdala (BLA), thalamus, brainstem] and cortical [anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), insula, occipital cortex] regions in IPV-PTSD. In patients, brain activation in amygdala, ACC, insula, occipital cortex and brainstem correlated positively with symptom severity. Furthermore, connectivity analyses revealed hyperconnectivity between BLA and dorsal ACC/mPFC. Results show symptom severity-dependent brain activation and hyperconnectivity in response to trauma-related pictures in brain regions related to fear and visual processing in women suffering from IPV-PTSD. These brain mechanisms appear to be associated with immediate responses to trauma-related triggers presented in a non-emotional context in this PTSD subgroup.
Keywords: PTSD, neurocircuitry, amygdala, functional connectivity, symptom severity
Introduction
The World Health Organization (2004) defines violence as “the intentional use of physical force or power, threatened or actual, against oneself, another person or against a group or community that either results in or has a high likelihood of resulting in injury, death, psychophysiological harm, maldevelopment or deprivation” (p. 1). Interpersonal violence (IPV) is described as violence between individuals performed by family members, intimate partners or the community. The US Department of Justice estimates that 85% of IPV victims are women (U.S. Department of Justice, 2012), who additionally have a significantly elevated risk to develop posttraumatic stress disorder (PTSD) after a traumatic event, for example IPV (Golding, 1999; Dutton et al., 2006). In addition to the substantial burden of IPV in women for mental health systems (e.g. Center of Disease Control and Prevention, 2003), women with IPV-PTSD suffer from intense symptoms, such as vivid re-experiencing of the traumatic event, avoidance of trauma-relevant cues and persistent hypervigilance in consequence of the traumatic event (American Psychiatric Association, 2000). The diagnosis of PTSD focuses on the prominent role of altered processing of trauma-related triggers (Criterion B; DSM-IV-R & DSM-5; American Psychiatric Association, 2000, 2013), such as being alone in the dark or seeing a person who looks like the offender.
According to the cognitive model of PTSD by Ehlers and Clark (2000), trauma-related triggers provoke sudden, intense and automatic emotional responses. These responses lead to cognitive evaluation of the traumatic memory and as a consequence to dysfunctional reactions such as safety and avoidance behavior. From a neuroscientific perspective, the question which neurobiological mechanisms lead to hypervigilant responding to trauma-related cues is central. This question has been investigated predominantly in combat veterans for traumata related to war, and partially also in survivors of traffic or mining accidents (Bremner et al., 1999; Hayes et al., 2011; Hou et al., 2007). Moreover, several important studies have investigated PTSD in female victims of IPV, mostly using unspecific threat stimuli (faces; Fonzo et al., 2010; eye contact; Steuwe et al., 2015; negative words; Thomaes et al., 2009), but also trauma-related and more complex stimuli (e.g. video clips; Moser et al., 2015).
Neuroscientific studies in PTSD patients investigating responses to trauma-related instead of unspecific threatening stimuli are critical in order to understand the specific neural responses to triggers directly involved in the traumatic event. Furthermore, neural correlates of trauma-related automatic processing triggered by trauma-related pictures that did not require the in-depth evaluation of their emotional content should be assessed. This is especially relevant for the cognitive model of PTSD by Ehlers and Clark (2000) according to which automatic processing of trauma-related triggers plays a decisive role in the symptomatology of the disorder.
Neuroimaging studies have yielded amygdala hyperactivation during automatic trauma-related stimulus processing in combat veterans suffering from PTSD (Liberzon et al., 1999; Pissiota et al., 2002; Vermetten et al., 2007) and in PTSD patients with other kinds of traumata (Protopopescu et al., 2005; Hou et al., 2007). These findings, together with the amygdala’s ability to influence neuroendocrine, autonomic, and motor responses, indicate a key role for the amygdala in threat processing (Kim et al., 2011). The importance of the amygdala is further underlined by findings demonstrating a positive correlation between amygdala activity and symptom severity in PTSD (Rauch et al., 1996; Shin et al., 2004; Protopopescu et al., 2005). It has been suggested that especially the basolateral nucleus of the amygdala (BLA) plays an important role in PTSD (Furini et al., 2014; Nicholson et al., 2015; Perusini et al., 2015; Veer et al., 2015), for example due to its involvement in fear regulation and extinction, both of which are thought to be altered in PTSD (Furini et al., 2014; Jovanovic and Ressler, 2010).
Further studies investigating automatic trauma-related stimulus processing in PTSD have found decreased activation in ventral/pregenual parts of anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) (Bremner et al., 1999; Shin et al., 2001; Sakamoto et al., 2005; Hou et al., 2007). Dorsal parts of these regions have been found to be hyperactivated at least during unspecific threat processing in PTSD (Bryant et al., 2005; Felmingham et al., 2009; Shin and Liberzon, 2010). Due to different roles of ventral/pregenual and dorsal parts of ACC and mPFC, it is necessary to distinguish these regions in order to gain a better understanding of fear processing in PTSD. In addition, hippocampus and insula showed increased activation in PTSD patients during processing of trauma-related triggers (Sakamoto et al., 2005; Hou et al., 2007; Vermetten et al., 2007; Thomaes et al., 2009).
Taken together, the neurocircuitry of PTSD is marked by hyperactivation (amygdala, insula, hippocampus) and hypoactivation (ventral mPFC, pregenual ACC) in different fear-related regions. Hyperactivations in (para-)limbic structures are directly associated with PTSD symptoms (e.g. intrusions), whereas hypoactivations of frontal regions are assumed to reflect emotion regulation deficits (Patel et al., 2012).
Apart from the frequently reported brain regions described above, additional regions involved in processing and integration of (sensory) information are important in PTSD. Occipital regions have been proposed to be relevant for abnormal visual processing of fear-related stimuli (Bremner et al., 2003; Hendler et al., 2003). In addition, thalamus and brainstem have been reported to be involved in several PTSD-related symptoms (Kemp et al., 2009; Patel et al., 2012; Weston, 2014; Duval et al., 2015; Mahabir et al., 2015).
To the best of our knowledge, to date, only one study has investigated neural responses during automatic processing of briefly presented trauma-related triggers in women who suffered from IPV-PTSD (Protopopescu et al., 2005). In this functional magnetic resonance imaging (fMRI) study by Protopopescu et al. (2005), trauma-relevant negative words were used and the authors found amygdala hyperactivation in the first half of their experiment. They suggested that in PTSD patients, the amygdala response is immediately present and stays constant or diminishes over time (Protopopescu et al., 2005).
Investigation of altered functional connectivity patterns in PTSD aids the understanding of disturbed brain networks in this disorder. Several previous studies have taken the interplay of the amygdala and other brain regions into account while investigating stimulus processing in PTSD (e.g. Osuch et al., 2008; Rabinak et al., 2011). Functional connectivity analyses have yielded increased covariation between amygdala and insula (Osuch et al., 2008; Rabinak et al., 2011) and both heightened (with dorsal mPFC) and reduced (with ventral mPFC) amygdala-mPFC functional connectivity (Bryant et al., 2008). Furthermore, Sripada et al. (2012) described reduced covariation for the amygdala–hippocampus connection, whereas Osuch et al. (2008) found heightened connectivity. With regard to the ACC, Sripada et al. (2012) found reduced covariation between the amygdala and the rostral ACC, whereas Osuch et al. (2008) reported increased covariation between these regions. A model postulated by Weston (2014) emphasized the role of amygdala–brainstem as well as amygdala-occipital regions covariation patterns with regard to hypervigilance as well as vivid re-experiencing symptoms in PTSD. Overall, to date, functional connectivity findings in PTSD are partially inconsistent and further work is clearly needed.
Surprisingly, studies investigating functional connectivity patterns in PTSD patients during processing of trauma-related stimuli are as yet lacking. Trauma-specific approaches are necessary to understand the natural processing and the underlying neural correlates of specific triggers in IPV-PTSD patients. This study investigated trauma-related trigger processing in an implicit emotional task in women suffering from IPV-PTSD using interpersonal threat stimuli. For this purpose, we developed a new standardized Trauma-Related Assault Picture Set (TRAPS-M) for patients suffering from PTSD after IPV. In accordance with previous work, we hypothesized to find (a) hyperactivation in amygdala, hippocampus, insula, dorsal ACC, dorsal mPFC, occipital regions, thalamus and brainstem, and hypoactivation of pregenual ACC and ventral mPFC in response to trauma-related vs neutral cues. Furthermore, we investigated functional connectivity during the processing of trauma-related pictures in PTSD. Based on recent proposals, (b) we expected decreased connectivity between amygdala and mPFC (Bryant et al., 2008), reflecting emotion regulation deficits in PTSD patients. Further, we expected increased connectivity of amygdala with insular cortex, occipital regions and brainstem as postulated by Osuch et al. (2008) and the theoretical model by Weston (2014). Since connectivity patterns between amygdala and other regions are heterogeneous, this study aims to clarify the interplay of amygdala and other regions in IPV-PTSD. Furthermore, (c) we additionally investigated the relationship between brain activation and symptom severity.
Materials and methods
Subjects
Eighteen female PTSD patients and 18 female healthy controls (HC) participated in this study (group characteristics are summarized in Table 1). As investigating neural correlates of trauma-related processing in IPV-PTSD patients was central to this study, the experience of a trauma related to IPV (e.g. rape, sexual, physical abuse) at least once during the lifespan was an inclusion criterion for the patient group. All PTSD patients fulfilled the diagnostic criteria for PTSD as primary diagnosis according to the DSM-IV-TR (American Psychiatric Association, 2000), as assessed by the German version of the Structured Clinical Interview for DSM-IV (SCID; Wittchen et al., 1997). Comorbid diagnoses in PTSD patients were social anxiety disorder (n = 4), specific phobia (n = 1), recurring (n = 1) depressive disorder (n = 1), chronic pain disorder (n = 2) and eating disorder (n = 2). Two PTSD patients had been under stable SSRI-medication (selective serotonin reuptake inhibitor) for at least 3 months. All participants had normal or corrected-to-normal vision and were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Written informed consent was obtained from each subject. Participants were recruited via public announcements in newspapers and were paid for participation. All experimental procedures were approved by and conducted in accordance with guidelines of the ethics committee of Muenster (Germany). The study conforms to the Declaration of Helsinki.
Table 1.
Demographic and clinical characterization
| IPV-PTSD M ± SD (Range) | HC M ± SD (Range) | t-value | P-value | |
|---|---|---|---|---|
| n | 18 | 18 | – | – |
| Age (years) | 26.61 ± 5.78 | 26.33 ± 8.83 | t(34) = −0.112 | P = 0.912 |
| (19–38) | (18–51) | |||
| Level of education (years in school) | 12.69 ± 1.20 | 12.83 ± 0.51 | t(32) = 0.471 | P = 0.641 |
| (10–14) | (12–14) | |||
| Questionnaire data | ||||
| PDSa | 23.50 ± 9.88 | 0.50 ± 0.71 | t(34) = −9.84 | P < 0.050 |
| (7–43) | (0–2) | |||
| PTCIb | 3.35 ± 1.31 | 1.12 ± 0.16 | t(34) = −7.15 | P < 0.050 |
| (1.39–5.33) | (1–1.55) | |||
| FDS-20c | 2.20 ± 1.20 | 0.24 ± 0.29 | t(34) = −4.13 | P < 0.050 |
| (0.25–7.05) | (0–1.05) | |||
| BDI-IId score | 19.72 ± 10.58 | 1.44 ± 1.42 | t(34) = −7.26 | P < 0.050 |
| (5–35) | (0–4) | |||
| Comorbidities (DSM-IV) | ||||
| Pain disorder (DSM 307.8) | 1 | |||
| Anorexia nervosa (DSM 307.1) | 1 | |||
| Social anxiety disorder (DSM 300.23) | 2 | |||
| Recurrent mild depression (DSM 296.31) | 1 | |||
IPV-PTSD, interpersonal violence posttraumatic stress disorder; HC, healthy controls.
German version by Anke Ehlers (unpublished) of the Posttraumatic Diagnostic Scale (Foa et al., 1997).
German version by Anke Ehlers (unpublished) of the Posttraumatic Cognition Inventory (Foa et al., 1999).
German version (Spitzer et al., 2005) of the Dissociative Experiences Scale (Bernstein and Putnam, 1986).
German version (Hautzinger et al., 2009) of the Beck Depression Inventory (Beck et al., 1996).
Stimuli and paradigm
In order to develop a trauma-related picture set for patients suffering from IPV-PTSD (Trauma-Related Assault Picture Set Muenster; TRAPS-M), 20 pictures depicting assault scenes were collected from the International Affective Picture Set (IAPS; Lang et al., 2008), 2 were collected from the Emotional Picture Set (EmoPics; Wessa et al., 2010) and 72 from the Internet (by searching for assault-related terms, e.g. “attack”, “weapon”, “violence”), and additional 10 pictures were custom-made, resulting in a pilot study set of 104 pictures. All 104 pictures referred to situations of (threatening) IPV, but depicted different situations (e.g. men hitting women; person directing a knife against another person; assault situation; beaten persons, etc.) in order to cover a sufficient, but necessary range of possible interpersonal-violence situations. The pictures were rated by a group of psychologists and clinical experts who were asked to indicate on 4-point Likert scales (1 = not suitable, 4 = fully suitable) to which degree each of the 104 pictures was suitable to elicit anxiety in PTSD patients after IPV (procedure adapted from Hauschildt et al., 2012). For the final TRAPS-M, 50 negative pictures were selected that were rated as most suitable (M ≥ 2.5) and checked for variability of picture content in order to ensure a heterogenous set. Subsequently, 50 neutral pictures from the IAPS and EmoPics were matched for color, luminance, central object/person, number of persons, facial expression, depicted location (inside/outside) and complexity (all P > 0.050). Stimuli characteristics and description of the TRAPS-M are summarized in Table 2.
Table 2.
Characteristics of the trauma-related affective picture set—muenster (TRAPS-M)
| TRAPS-Ma | Trauma-related pictures M (SD) | Neutral pictures M (SD) | P-value | |
|---|---|---|---|---|
| Content (χ2-test) | Central focusb | 1.32 (.47) | 1.34 (.48) | P = 1.00 |
| Number of peoplec | 1.56 (1.49) | 1.54 (1.72) | P = 0.367 | |
| Central faced | 2.32 (.84) | 2.3 (.79) | P = 0.509 | |
| Complexitye | 2.24 (.43) | 2.36 (.49) | P = 0.275 | |
| Locationf | 1.6 (.49) | 1.72 (.45) | P = 0.291 | |
| Picture properties (t-test; df = 98) | RGB | 94.75 (28.72) | 103.61 (19.42) | P = 0.074 |
| Red | 102.49 (31.50) | 110.98 (17.39) | P = 0.098 | |
| Green | 93.88 (29.05) | 103.12 (20.93) | P = 0.071 | |
| Blue | 87.87 (30.62) | 95.80 (24.37) | P = 0.155 | |
| Luminance | 95.78 (28.90) | 105.11 (18.90) | P = 0.059 | |
Trauma-Related Affective Picture Set – Muenster (developed in Muenster, Germany).
1—people, 2—object, 3—animal, 4—nature.
Number of people on the picture: 0, 1, 2, 3, 4, 5, 6–15 (counted as “6”), >15 (counted as “7”).
1—yes (emotional expression in the foreground), 2—no (no face in the foreground), 3—no face.
1—central object, simple background, 2—central object, complex background or many objects, simple background, 3—many objects, complex background.
1—inside, 2—outside.
Each of the 50 trauma-related and 50 neutral pictures was presented once during the event-related functional run (8 min 19 s). Pictures were presented for 800 ms. Order was counterbalanced and pseudo-randomized with optseq (http://www.surfer.nmr.mgh.harvard.edu/optseq/), which implements temporal jitter to increase signal discriminability (Dale et al., 1999). Between picture presentations, a white fixation cross occurred for an average interval of 3915 ms (jittered between 1280 and 15320 ms). A vigilance task was implemented to ensure that participants looked at the pictures, without any requirement to process the emotional content of pictures in depth (non-emotional low level context). To this end, participants were told to press a button with their right index finger whenever a blurred picture occurred (overall five trials). Blurred pictures [originally EmoPics, see Wessa et al., 2010, blurred with Adobe Photoshop CS6 (version 13.0.1, Adobe Systems Inc., San Jose, CA)] were randomly presented during the experiment. This task aimed to create a situation in which a naturalistic and immediate response to unpredictably appearing trauma-triggers in a non-emotional context could be investigated. Stimuli were presented using Presentation 17.2 (Neurobehavioral Systems, Inc, Albany, CA) and rear-projected via a Liquid Crystal on Silicon projector (DLA-RSxx, JVCKenwood USA Corporation, USA) onto a screen, which the participants saw through a mirror on the magnetic resonance imaging head coil. Before fMRI-scanning, participants were trained on a PC to perform the task in a 5-min training session outside the scanner. Within 1 week after the fMRI-scanning session, participants rated the pictures on 9-point-Likert scales with regard to valence (1= negative to 9 = positive), arousal (1 = calm to 9 = intense) and anxiety (1 = no anxiety to 9 = high anxiety). Right after this rating session, all PTSD patients were invited to talk about their experiences and emotional pressure during the days after scanning.
Rating data analysis
Rating data were analyzed with repeated-measures analyses of variance (ANOVAs). For each rating scale (valence, arousal, anxiety), we used a 2 × 2 design, with between-subject factor group (PTSD patients, HC) and within-subject factor emotion (trauma-related, neutral). For ANOVAs, a probability level of P < 0.050 was considered statistically significant.
fMRI image acquisition and analysis
Scanning was conducted on a 3-T scanner (Magnetom Prisma; Siemens, Erlangen, Germany). First, a T1-weighted anatomical scan was acquired. The functional run consisted of 255 volumes (36 axial slices per volume, thickness = 3 mm, gap = 0.3 mm, in-plane resolution = 2.26 × 2.26 mm, orientated at a tilted angle to the anterior–posterior commissural plane) using a T2*-weighted echo planar sequence. The first 10 volumes were discarded to ensure steady-state tissue magnetization. Image preprocessing and analyses were performed using BrainVoyager QX 2.8.4 (Brain Innovation, Maastricht, The Netherlands). All volumes were realigned to the first volume, corrected for slice time errors, and spatially (6-mm full-width half-maximum isotropic Gaussian kernel) and temporally (high-pass filter, 10 cycles per run; low-pass filter, 2.8 s) smoothed. Anatomic and functional images were co-registered and normalized to Talairach space (Talairach and Tournoux, 1988). Statistical analysis was performed using multiple linear regression of the signal time course at each voxel. The blood oxygen level-dependent (BOLD) signal change was modeled with a two gamma hemodynamic response function (HRF) for each event type. We conducted a small-volume correction analysis for apriori defined regions of interest (ROIs)—amygdala, ACC, mPFC, hippocampus, insula, occipital cortex, thalamus and brainstem—to replicate earlier findings in PTSD patients. All ROIs were created according to the Automated Anatomical Labeling atlas (AAL; Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003) and were transformed into the Talairach space (according to Lancaster et al., 2007) using ICBM2TAL in Matlab (MATLAB 2012b, The MathWorks, Inc., Natick, MA). A ROI for the brainstem was downloaded from the digitized version of the Talairach atlas (http://www.talairach.org/nii/gzip/). For ROI analyses, we used a random-effects model (z-transformation) with three predictors (blurred pictures, trauma-related pictures and neutral pictures) and a constant predictor for regression estimation. Based on this regression model for each patient, we calculated a 2 (trauma-related/neutral) × 2 (PTSD patients/HC) design for group comparison. First, voxel-level threshold was set at P < 0.005 (uncorrected). Thresholded maps were then submitted to an ROI-based correction criterion based on the estimate of the map’s spatial smoothness and on an iterative procedure (Monte Carlo simulation as implemented in BrainVoyager). After 1000 iterations, the minimum cluster size threshold that yielded a cluster-level false-positive rate of 1.67% (Bonferroni correction for three tests) was applied to the statistical maps. For cluster thresholding, we used three masks [(a) a ROI-mask comprising ACC, mPFC, insula, hippocampus, thalamus, brainstem, occipital region; (b) an amygdala-mask comprising amygdala; (c) a whole brain-mask]. Cluster size for amygdala was checked with an amygdala ROI (dilating factor of 1 mm) due to its small size and to avoid missing relevant activation. Amygdala subnuclei investigation was implemented by maximal voxel analysis using anatomical probability maps of the Anatomy toolbox (Amunts et al., 2005; Eickhoff et al., 2005). ACC and mPFC clusters were subdivided into ventral/pregenual and dorsal parts (Shin et al., 2007; Shin and Liberzon, 2010). Planned comparisons were realized using SPSS software (Version 23; SPSS, Inc., Chicago, IL).
For connectivity analysis, we used psychophysiological interaction (PPI; O’Reilly et al., 2012) as implemented in Neuroelf (neuroelf.net). To determine whether or not the BOLD changes in the BLA (using the significant differential amygdala effect as seed region) were related to BOLD changes in other regions (mPFC, ACC, insula, hippocampus, occipital regions, thalamus, brainstem), we defined a functional volume of interest based on the relevant contrast effect in the left BLA. First, we extracted the BOLD signal time course from this seed region (physiological predictor). Then, we contrasted the HRF representing trauma-related pictures and the HRF representing neutral pictures (psychological predictor). Lastly, we convolved the physiological regressor with the psychological regressor in order to obtain a psychophysiological predictor. This psychophysiological as well as the physiological predictor were included in the random-effects model specified above. Checking for significant cluster size was performed as described above. To resolve PPI group effects, planned comparisons were conducted for HC and PTSD separately using one-sample t-tests at α < 0.025. Furthermore, we calculated a ROI-based parametric modulation of anxiety ratings to confirm PPI ROI results. To this end, we performed a time course modulation of trauma-related picture presentation by individual anxiety rating data. Correlations between symptom severity in PTSD and brain activity during processing of trauma-related triggers were analyzed with an analysis of covariance (ANCOVA) within PTSD patients using a German version (Ehlers et al., unpublished, 1996) of the Posttraumatic Diagnostic Scale (Foa et al., 1997) as a covariate.
Results
Subjects
PTSD patients and HC did not differ regarding age (t(34) = −0.11, P = 0.912), education (number of years in school; t(34) = 0.45, P = 0.641) and handedness (t(34) = 0.79, P = 0.431). PTSD patients as compared to HC revealed significantly higher scores in PTSD symptom severity and PTSD-associated symptoms (Posttraumatic Diagnostic Scale (PDS): t(34) = −9.84; Posttraumatic Cognition Inventory (PTCI): t(34) = −7.15; Dissociative Experiences Scale (FDS-20): t(34) = −4.13, all P < 0.050). As expected, PTSD patients as compared with HC self-rated their depressive symptoms as more severe, as indicated by significantly higher scores on the Beck Depression Inventory (BDI-II; t(34) = −7.26, P < 0.050).
Rating data
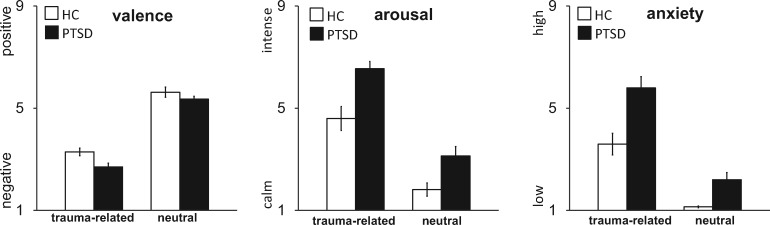
Analyses of post-scanning rating data showed that both PTSD patients and HC subjects rated trauma-related pictures as more unpleasant (as shown in significantly lower valence ratings for trauma-related vs neutral pictures; F[1,34] = 214.15, P < 0.005), more arousing (F[1,34] = 164.25, P < 0.005), and more anxiety-eliciting (F[1,34] = 128.43, P < 0.005) than neutral pictures. PTSD patients rated all pictures as more unpleasant (as shown in significantly lower valence ratings by PTSD patients vs HC; F[1,34] = 10.86, P < 0.005), more arousing (F[1,34] = 15.44, P < 0.005), and more anxiety-eliciting (F[1,34] = 18.35, P < 0.005) than controls. For anxiety ratings, we found a group × emotion interaction (F[1,34] = 5.73, P = 0.022). Figure 1 shows that the interaction effect is driven by the higher anxiety ratings of PTSD patients relative to HC for trauma-related vs neutral. Taken together, these results indicate that the TRAPS-M picture set is suitable to elicit anxiety in PTSD patients.
Fig. 1.
Ratings of trauma-related and neutral pictures on the dimensions valence, arousal and anxiety, shown separately for patients suffering from PTSD and HC. Graphs show means (± SD). 9-point Likert scales were as follows: valence—1 = negative, 5 = neutral, 9 = positive; arousal—1 = calm, 9 = intense; anxiety – 1 = low, 9 = high.
fMRI data
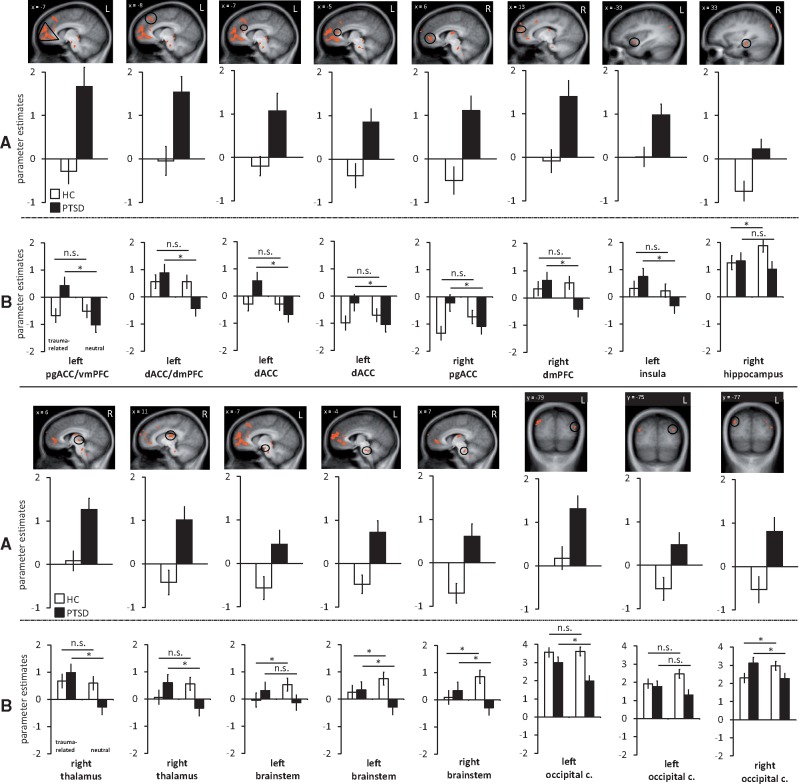
ROI analysis revealed an emotion × group interaction in left BLA, left dorsal ACC/mPFC, left ventral/pregenual ACC/mPFC, left dorsal ACC, right pregenual ACC, right dorsal mPFC, left insula, right hippocampus, bilateral brainstem, right thalamus and bilateral occipital cortex (left middle and superior cluster, right superior cluster) (Table 3, Figures 2 and 3). Planned comparisons for trauma related as compared with neutral pictures revealed significant activation differences in the IPV-PTSD but not the HC group in the following ROIs (Table 3): left BLA, left dorsal ACC/mPFC, left ventral/pregenual ACC/mPFC, left dorsal ACC, right pregenual ACC, right dorsal mPFC, left insula, right thalamus and left middle occipital cortex. Right hippocampus and left brainstem showed significant activation differences in HC but not in IPV-PTSD for the relevant contrast. In bilateral brainstem and right superior occipital cortex, activation differences were found in both groups (PTSD: trauma-related > neutral; HC: neutral > trauma-related). Planned comparison in left superior occipital cluster failed to reach significance in both groups.
Table 3.
ROI analysis: significant hyperactivations for trauma-related compared with neutral pictures for IPV-PTSD patients compared with healthy controls
| Region | Laterali- zation | Talairach coordinates of peak voxel |
Cluster size (mm3) | t-value average | t-value maximum | t-value IPV-PTSD df(17) | t-value HC df(17) | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| ACC/mPFC (ventral) | L | −11 | 48 | 6 | 9093 | 3.09* | 4.33 | 3.56** | 0.03 |
| ACC/mPFC (dorsal) | L | −9 | 24 | 42 | 416 | 2.96* | 3.41 | 3.37** | −0.58* |
| ACC (dorsal) | L | −6 | 28 | 27 | 80 | 2.89* | 3.16 | 3.47** | −0.03* |
| ACC (dorsal) | L | −4 | 32 | 16 | 192 | 2.91* | 3.18 | 2.48** | −1.10** |
| ACC (ventral) | R | 14 | 40 | −1 | 1137 | 3.07* | 3.91 | 2.69** | −2.01** |
| mPFC (dorsal) | R | 15 | 50 | 21 | 276 | 3.01* | 3.71 | 3.75** | −0.87* |
| Insula | L | −33 | 8 | −5 | 104 | 3.03* | 3.56 | 3.88** | 0.44 |
| Hippocampus | R | 32 | −24 | −19 | 144 | 3.27* | 4.08 | 1.31** | −3.32** |
| Thalamus | R | 6 | −20 | 2 | 176 | 3.05* | 3.71 | 4.80** | 0.37 |
| Thalamus | R | 10 | −16 | 19 | 469 | 3.19* | 4.17 | 3.25** | −2.02** |
| Occipital cortex | L | −32 | −74 | 29 | 64 | 2.83* | 2.99 | 1.71** | −2.06** |
| Occipital cortex | L | −40 | −78 | 15 | 134 | 2.99* | 3.30 | 3.36** | −.23* |
| Occipital cortex | R | 38 | −82 | 25 | 772 | 2.96* | 3.37 | 2.32** | −2.46** |
| Brainstem | L | −6 | −12 | −13 | 64 | 2.97* | 3.29 | 1.39** | −2.20** |
| Brainstem | L | −2 | −30 | −23 | 190 | 2.95* | 3.24 | 2.55** | −2.49** |
| Brainstem | R | 4 | −30 | −21 | 469 | 2.93* | 3.18 | 2.15** | −3.63** |
| Amygdala | L | −22 | −8 | −9 | 136 | 3.07* | 3.47 | 4.44** | 0.29 |
ROI, region of interest; IPV-PTSD, interpersonal violence posttraumatic stress disorder; HC, healthy controls; L, left; R, right; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; P ≤ 0.005 uncorrected, and P ≤ 0.050 corrected; *significant interaction; **significant planned comparisons.
Fig. 2.
(A) Estimated brain activation for differential effects (trauma-related vs neutral pictures) in patients suffering from PTSD and HC in a priori defined regions of interest (all P <0.050 corrected). (B) Estimated brain activation separately for trauma-related and neutral pictures in PTSD and HC. Note that occipital cortex figures are scaled from −2 to +4. Parameter estimates for HC are shown in white, for PTSD in black. PTSD patients showed increased activation in left pregenual ACC/ventral medial prefrontal cortex (mPFC), bilateral dorsal ACC, right dorsal mPFC, left insula, right thalamus, bilateral brainstem and occipital cortex. HC showed increased activation in right hippocampus, bilateral brainstem and occipital cortex. Pg, pregenual; c, cortex; n.s., not significant.
Fig. 3.
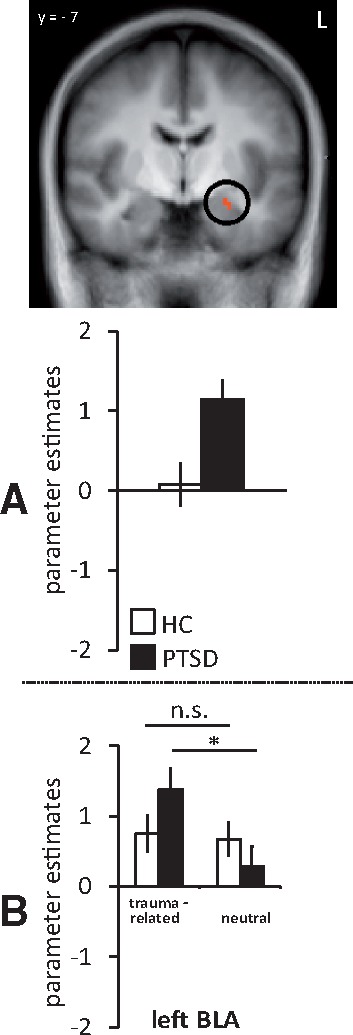
(A) Estimated brain activation for the differential effect (trauma-related vs neutral pictures) in patients suffering from PTSD and HC in a priori defined amygdala region of interest (P <0.050 corrected). (B) Estimated brain activation separately for traumarelated and neutral pictures in PTSD and HC. Parameter estimates for HC are shown in white, for PTSD in black. PTSD patients showed increased activation in left BLA. n.s., not significant.
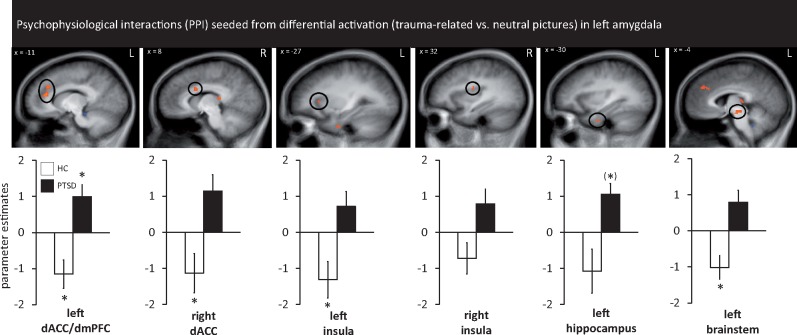
PPI analysis revealed higher connectivity in PTSD patients as compared with HC between the left amygdala seed region (BLA) and the following regions: left dorsal ACC/mPFC, right dorsal ACC, bilateral insula, left hippocampus and left brainstem (Table 4, Figure 4). We did not find any hypoconnectivities between left amygdala and other relevant brain regions for the trauma-related vs neutral picture contrast. The connectivity pattern between left amygdala and left dorsal ACC/mPFC was driven by coupling in the PTSD group and decoupling in the HC group. Connectivity patterns between left amygdala and the following regions were driven by coupling in the HC group: right dorsal ACC, left insula, left brainstem. Left amygdala and left hippocampus activation was marginally significant for a coupling in the PTSD group (P = 0.026). Left amygdala and right insula connectivity could not be explained by different coupling patterns within each group.
Table 4.
PPI analysis: significant differences in connectivity patterns for the contrast trauma-related > neutral scenes in IPV-PTSD patients vs HC
| Seed region | PPI region | Lateralization | Talairach coord. of peak voxel |
Cluster size (mm3) | t-value average | t-value maximum | t-value IPV-PTSD df(17) | t-value HC df(17) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | ||||||||
| Amygdala (left) BLA | ACC/mPFC (dorsal) | L | −10 | 33 | 18 | 1432 | 3.21* | 4.52 | 2.71** | −2.84** |
| ACC (dorsal) | R | 9 | 10 | 29 | 168 | 3.06* | 3.67 | 2.10** | −2.68** | |
| Insula | L | −23 | 23 | 11 | 184 | 3.24* | 4.03 | 1.86** | −2.87** | |
| Insula | R | 34 | −8 | 21 | 72 | 2.85* | 3.00 | 1.26** | −2.32** | |
| Hippocampus | L | −28 | −6 | −27 | 144 | 2.92* | 3.16 | 2.43(* | −2.35** | |
| Brainstem | L | −2 | −16 | −7 | 240 | 3.27* | 3.96 | 2.25** | −2.74** | |
PPI, psychophysiological interaction; IPV-PTSD, interpersonal violence posttraumatic stress disorder; HC, healthy controls; coord., coordinates; L, left; R, right; BLA, basolateral nucleus; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; P ≤ 0.005 uncorrected, and P ≤ 0.050 corrected; *significant interaction; **significant planned comparisons; (**) P = 0.026.
Fig. 4.
PPIs seeded from differential activation (trauma-related vs neutral pictures) in left amygdala (as shown in Figure 3). Regions showing higher PPI connectivity (all P <0.050 corrected) between patients suffering from PTSD and HC with the seed region in left amygdala: significant differences were found in left dorsal ACC/dorsal medial prefrontal cortex (mPFC) for both groups, right dorsal ACC for HC, left insula for HC, left hippocampus (marginally significant for PTSD) and left brainstem (HC). Asterisks mark significance against baseline.
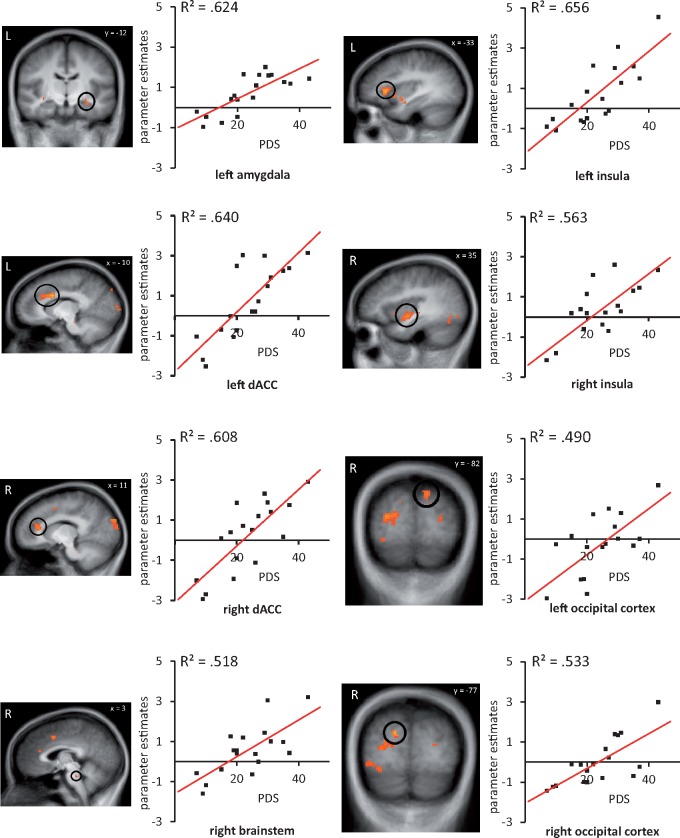
Correlation analysis revealed positive correlations between symptom severity in PTSD patients and different brain ROIs: left amygdala, bilateral dorsal ACC, bilateral insula, bilateral occipital cortex and right brainstem (Table 5, Figure 5). Further correlation analysis revealed no significant correlations between symptom severity and other regions in the whole brain analysis. These results indicate that greater symptoms severity in PTSD is associated with increased neural activity in a-priori defined regions.
Table 5.
Correlation analysis of symptom severity (PDSb) and brain activation of trauma-related compared with neutral pictures in IPV-PTSD patients
| Region | Lateralization | Talairach coordinates of peak voxel |
Cluster size (mm3) | r average | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ACCa (dorsal) | L | −9 | 9 | 28 | 1660 | 0.80* |
| ACCa (dorsal) | R | 17 | 33 | 24 | 1173 | 0.78* |
| ACC (dorsal) | R | 5 | 7 | 42 | 412 | 0.71* |
| Insulaa | L | −35 | 21 | 6 | 1345 | 0.81* |
| Insula | L | −35 | 11 | −4 | 1139 | 0.77* |
| Insulaa | R | 35 | −7 | −4 | 634 | 0.75* |
| Insula | R | 45 | 13 | 4 | 104 | 0.64* |
| Insula | R | 27 | −21 | 18 | 152 | 0.72* |
| Insula | R | 31 | 9 | 4 | 146 | 0.71* |
| Occipital cortexa | L | −12 | −91 | 26 | 1256 | 0.70* |
| Occipital cortex | L | −45 | −65 | 0 | 338 | 0.66* |
| Occipital cortex | L | −27 | −82 | 12 | 176 | 0.66* |
| Occipital cortexa | R | 15 | −77 | 25 | 118 | 0.73* |
| Occipital cortex | R | 9 | −90 | 28 | 3595 | 0.71* |
| Occipital cortex | R | 47 | −71 | −6 | 589 | 0.64* |
| Occipital cortex | R | 31 | −79 | −10 | 232 | 0.70* |
| Brainstema | R | 7 | −29 | −20 | 152 | 0.72* |
| Amygdalaa | L | −25 | −11 | −11 | 183 | 0.79* |
IPV-PTSD, interpersonal violence posttraumatic stress disorder; L, left; R, right; ACC, anterior cingulate cortex; P ≤ 0.005 uncorrected, and P ≤ 0.050 corrected.
Correlation pictures in Figure 4; *significant correlation.
German version by Anke Ehlers (unpublished) of the Posttraumatic Diagnostic Scale (Foa et al., 1997).
Fig. 5.
Correlations between brain activation in patients suffering from PTSD for trauma-related vs neutral pictures and symptom severity (assessed with the Posttraumatic Disorder Scale, PDS). Scatterplots represent correlations for the respective clusters. Table 5 lists a summary of all correlation clusters.
fMRI data—additional analysis
ROI-based parametric analysis of anxiety ratings
ROI-based parametric analysis of anxiety ratings to resolve PPI results confirmed nearly all ROIs as revealed in PPI analysis, namely the following regions: left dorsal ACC/mPFC, right dorsal ACC, left insula and left brainstem. Parametric analysis of anxiety ratings confirmed missing connectivity between left amygdala and right insula as well as left amygdala and left hippocampus as reported in the PPI analysis. Results of the ROI-based parametric analysis of anxiety ratings can be found in S-Table 6.
Whole brain analysis of the emotion × group interaction
Whole brain analysis revealed increased activity in the following regions for trauma-related vsneutral processing: bilateral superior temporal gyrus (STG), left fusiform face area (FFA), left middle temporal gyrus, bilateral middle frontal gyrus, bilateral superior frontal gyrus, left inferior frontal gyrus, bilateral medial frontal gyrus, right postcentral gyrus, right parahippocampal gyrus, bilateral subcallosal gyrus, bilateral precuneus, bilateral posterior cingulate cortex(PCC)/precuneus, left PCC, left caudatus and left putamen. All regions of the ROI analysis were confirmed by the whole brain analysis. Only one of these regions was significantly correlated with symptom severity (inferior frontal gyrus). Whole brain results for trauma-related vs neutral picture processing can be found in the supplement (S-Figure 6, S-Table 7).
Whole brain analysis of PPI analysis
Whole brain results of PPI analysis with the left amygdala seed region revealed the following regions: increased connectivity pattern with right STG, bilateral BA28, right parahippocampal gyrus, right middle temporal gyrus, bilateral middle frontal gyrus, right medial frontal gyrus, right inferior temporal gyrus, left inferior parietal gyrus and decreased connectivity pattern with right BA7, right middle temporal gyrus and right postcentral gyrus. Whole brain results of PPI analysis are found in the supplement (S-Figure 7, S-Table 8).
Whole brain analysis of main effects of emotion and group
Results of effects of emotion (trauma-related vs neutral) and group (PTSD vs HC) are found in supplementary material (S-Table 9, S-Table 10).
Discussion
The aim of this study was to investigate brain activation and functional connectivity patterns during processing of visual trauma-related triggers in female patients suffering from IPV-PTSD using a novel set of standardized trauma-related pictures presented during a non-emotional vigilance task. Results revealed increased activation to trauma-related vs neutral pictures in the BLA, dorsal mPFC, dorsal and pregenual ACC, insula, occipital regions, brainstem and thalamus in IPV-PTSD as compared with controls. Correlation analysis yielded a positive correlation between symptom severity in patients with IPV-PTSD and brain activation in amygdala, ACC, insula, occipital cortex and brainstem. Functional connectivity analysis showed higher connectivity between left BLA and other fear-related regions.
Overall, the current findings of amygdala hyperactivation support a key role of the amygdala in PTSD (Liberzon et al., 1999; Rauch et al., 2000; Jovanovic and Ressler, 2010; Patel et al., 2012). Moreover, our findings corroborate the results of Protopopescu et al. (2005), which revealed amygdala activation in response to trauma-related words in female IPV-PTSD patients. Particularly, the BLA appears to be involved in PTSD or PTSD-related symptoms during visual implicit trauma-related trigger processing in this study. The BLA is known as the main entrance center for information about the external environment from sensory regions (e.g. thalamus) and therefore plays an important role in detecting relevant stimuli. Due to its strong connections to the amygdala’s centromedial nucleus, which is crucial for behavioral output processes, the BLA is able to potentiate fear responses via these projections (Janak and Tye, 2015). In this study, we provide evidence for the BLA’s essential functioning in detecting trauma-related stimuli in an automatic context.
In addition to amygdala hyperactivation, increased activation was also found in the insula, in accordance with previous studies showing insula hyperactivation in PTSD patients in response to trauma-related (Vermetten et al., 2007) and trauma-unrelated stimuli (e.g. Whalley et al., 2009). Given the insula’s important role in processing the meaning and prediction of (aversive) bodily states (Paulus and Stein, 2006; Craig, 2009; Patel et al., 2012), this hyperactivation may reflect perception of aversive bodily changes during trauma-related trigger processing. This is in line with models of PTSD proposing that insula activation is associated with the experience and interpretation of interoceptive states as dangerous (Patel et al., 2012).
As expected, dorsal ACC/mPFC regions were hyperactivated in response to trauma-related pictures in IPV-PTSD patients in this study. This result is in line with previous PTSD studies reporting hyperactivity in dorsal parts of ACC/mPFC regions during general (Bryant et al., 2005; Felmingham et al., 2009; Shin and Liberzon, 2010) and trauma-related threat processing (Moser et al., 2015). It appears that dorsal parts of the ACC/mPFC are rather involved in emotion generation or processing, whereas ventral/pregenual parts are associated with emotion regulation processes (Devinsky et al., 1995; Viviani, 2014; Duval et al., 2015; Etkin et al., 2015). We found a significant correlation between brain activation in dorsal ACC and symptom severity in IPV-PTSD, suggesting that activation in this area is not simply driven by general differences between patients and controls but by the intensity of PTSD symptomatology. Furthermore, we found higher connectivity between BLA and dorsal ACC/mPFC. This is in line with prior findings, highlighting the role of these regions’ interplay during emotion processing by the excitatory influences of the amygdala on mPFC (Gilboa et al., 2004; Bryant et al., 2008). Although the direction of influence could not be detected with PPI, this coupling suggested an interplay between amygdala, involved in automatic stimulus detection, and medial prefrontal regions, involved in subsequent responses to trauma-relevant stimuli. Interestingly, we found activation in ventral/pregenual parts of ACC and mPFC, but did not observe altered connectivity between amygdala and ventral/pregenual ACC/mPFC regions or a correlation between ventral/pregenual ACC/mPFC activation and symptomatology in IPV-PTSD patients. To date, only one previous study had found increased ventral frontal activation using trauma-related smell as a cue in PTSD patients. The authors interpreted this activation as mediation of emotional processes related to behavior and emotion regulation (Vermetten et al., 2007). Previous studies, which reported ventral/pregenual mPFC/ACC deactivation, differ with regard to stimulus presentation time during the experiments, which ranged between 1.5 (Shin et al., 2001) and 30 s (Bremner et al., 1999). In this study, stimulus presentation time was much shorter (800 ms), suggesting early initial emotion regulation processes as shown by hyperactivation in ventral frontal regions in IPV-PTSD. In this study, participants were confronted with visual trauma-related triggers in a non-emotional vigilance task that initiated automatic threat processing. Thus, we assume that PTSD patients are trying to regulate emotional responses, but possibly with the need of higher effort and maybe in a dysfunctional way. This hypothesis is strengthened by the lack of connectivity between BLA and ventral/pregenual ACC/mPFC regions, suggesting a missing link between fear regulation processes in frontal regions and down-regulation of the amygdala during automatic trauma-related trigger processing (Etkin et al., 2015). Interestingly, in contrast to dorsal frontal activation, ventral frontal activation was independent of symptom severity in IPV-PTSD. This result indicates independence of symptom severity and early emotion regulation processes in IPV-PTSD.
In hippocampus, we detected a significant difference between IPV-PTSD and HC, with hyperactivation for trauma-related as compared with neutral pictures. Further analysis showed that this effect was mainly driven by reduced activation to trauma-related as compared with neutral stimuli in HC. In HC, hyperactivation during processing of neutral pictures might be associated with the hippocampus’ role in memory processes. Especially posterior parts of the hippocampus are involved in (autobiographical) memory functions (Fanselow and Dong, 2010; Bonnici et al., 2013). Thus, we speculate that hippocampal activation in HC might be explained by higher autobiographical relevance for neutral relative to IPV-PTSD trauma-related pictures in the current study. Concerning PTSD, a meta-analysis of Hayes et al. (2012) showed heterogenous findings with regard to hippocampal activations. The authors point out that it is necessary to create optimal tasks for eliciting hippocampal activation in these patients, such as learning or memory designs. Along these lines, the lack of significant hippocampal hyperactivation in PTSD in the current study could be explained in terms of a task that did not specifically elicit trauma-related memory processes. Interestingly, we could show a marginally significant hyperconnectivity between amygdala and hippocampus in PTSD patients relative to HC. Although this result has to be interpreted with caution, hyperconnectivity between amygdala and hippocampus might be related to the retrieval of trauma-related memories. We suggest that a coupling between amygdala and hippocampus might depict the pathway of trauma-related memory retrieval, which could potentially contribute to the intrusive nature of trauma recollections in PTSD (criterion B of PTSD, as described by the American Psychiatric Association, 2000; Patel et al., 2012).
Furthermore, as expected, we found hyperactivation in the visual cortex in IPV-PTSD as compared with HC. This result is in line with previous studies, suggesting an attention bias toward and heightened visual processing of trauma-related material in PTSD (e.g. Hendler et al., 2003; Weston, 2014). Furthermore, occipital regions have been shown to be associated with involuntary processing in PTSD (Bourne et al., 2013; Whalley et al., 2013), proposing involvement in re-experiencing processes of PTSD symptomatology (criterion B of PTSD, as described by the American Psychiatric Association, 2000). In addition, we found a modulation of occipital brain activation by symptom severity. This finding suggests an interaction of PTSD symptomatology and magnitude of visual processing of trauma-related triggers at early stages of stimulus processing (White et al., 2015).
Moreover, we observed increased responses in the thalamus for IPV-PTSD as compared with HC. Activation of the anterior part of the thalamus during implicit trauma-related trigger processing might be seen as a correlate of the PTSD patients’ “lower” threshold for the detection of threat in the environment even if no explicit emotional evaluation is required. This is in line with neurocircuitry models of PTSD which propose the thalamus to be an important subcortical key structure during sensory processing in PTSD (Patel et al., 2012). It should be noted that there was no significant correlation with thalamus activation in IPV-PTSD. It appears that relevant threat information exceeds a lower alertness threshold in PTSD without further modulation by symptom severity.
Remarkably, we detected an increased brainstem response in PTSD for trauma-related as compared with neutral pictures, while this pattern was reversed in HC. Hyperactivation in bilateral brainstem in PTSD might be associated with hyperarousal, a key symptom in PTSD (criterion D of PTSD, as described by the American Psychiatric Association, 2000; Weston, 2014; i.e. having the feeling to be always on the run). However, closer inspection of the data revealed a complex pattern of results with lower activation to trauma-related pictures in HC. Nevertheless, correlation analysis suggested a strong modulation of brainstem activation by symptom severity in IPV-PTSD patients.
As limitations of this study we have to consider the absence of a control group that was exposed to trauma without subsequent development of PTSD. However, strong correlations between symptom severity within PTSD patients and brain activation clearly indicate that our results are related to the pathology experienced by women suffering from IPV-PTSD. Thus, our findings are associated with symptoms in PTSD and could not simply be explained by trauma exposure. Furthermore, our sample included only female IPV-PTSD patients due to higher prevalence rates in women. Accordingly, we cannot address any gender differences that might exist in IPV-PTSD. With regard to sample characteristics, future studies should assess diagnoses of personality disorders, since especially PTSD and borderline personality disorder are frequently comorbid (Pagura et al., 2010). When focusing on processing of visual trauma-related triggers by using the TRAPS-M, future studies should additionally incorporate generally, non-trauma-related negative pictures into their design in order to further determine trauma-specificity of brain activity.
Taken together, this study advances our knowledge about neural circuits underlying automatic processing of trauma-related triggers in female IPV-PTSD patients. This study is the first to focus on women after IPV-PTSD by using a novel, anxiety-eliciting standardized trauma-related picture set for PTSD patients after IPV. We could show altered brain activation during automatic trauma-related picture processing in BLA, dorsal mPFC, dorsal and pregenual ACC, insula, occipital regions, brainstem and thalamus, with significant correlations between symptom severity and activation in all regions except pregenual ACC, mPFC and thalamus. In addition, increased functional connectivity between BLA and dorsal mPFC/ACC suggested an altered interplay between subcortical and cortical regions in the immediate response to trauma-related triggers in PTSD.
Overall, the present findings show involvement of subcortical and cortical regions in the processing of trauma-related visual stimuli in a non-emotional vigilance task in IPV-PTSD. The results suggest altered mechanisms of threat detection, visual processing and subsequent emotion generation and processing. Subcortical threat detection and cortical emotion-processing mechanisms are interrelated. Ventral frontal activation and lack of connectivity to amygdala supports the notion of initial early, but inaccurate, emotion regulation processes.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This study was supported by grants awarded to Prof. Dr. Thomas Straube by the German Research Society (Deutsche Forschungsgemeinschaft, DFG; SFB/TRR 58: C06, C07).
Conflict of interest. None declared.
Supplementary Material
References
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders (4th ed., Text Revision). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC: Author. [Google Scholar]
- Amunts K., Kedo O., Kindler M., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology, 210, 343–52. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Beck Depression Inventory – Second Edition. Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernstein E.M., Putnam F.W. (1986). Development, reliability, and validity of a dissociative scale. Journal of Nervous and Mental Disease, 174, 727–35. [DOI] [PubMed] [Google Scholar]
- Bonnici H.M., Chadwick M.J., Maguire E.A. (2013). Representations of recent and remote autobiographical memories in hippocampal subfields. Hippocampus, 23, 849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne C., Mackay C.E., Holmes E.A. (2013). The neural basis of flashback formation: the impact of viewing trauma. Psychological Medicine, 43, 1521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. (1999). Neural correlates of exposure to traumatic pictures and sound in vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry, 45, 806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., et al. (2003). Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry, 53, 879–89. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., Felmingham K.L., Kemp A.H., et al. (2005). Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biological Psychiatry, 58, 111–8. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., et al. (2008). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping, 29, 517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2003). Costs of Intimate Partner Violence against Women in the United States. Atlanta (GA: ): Centers for Disease Control and Prevention. [Google Scholar]
- Craig A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Greve D.N., Burock M.A. (1999). Optimal stimulus sequences for the event-related fMRI. Presented at the 5th international conference on functional mapping of the human brain, Duesseldorf, Germany.
- Devinsky O., Morrell M.J., Vogt B.A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118, 279–306. [DOI] [PubMed] [Google Scholar]
- Dutton M.A., Green B.L., Kaltman S.I., Roesch D.M., Zeffiro T.A., Krause E.D. (2006). Intimate partner violence, PTSD, and adverse health outcomes. Journal of Interpersonal Violence, 21, 955–68. [DOI] [PubMed] [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management, 11, 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Clark D.M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38, 319–45. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–35. [DOI] [PubMed] [Google Scholar]
- Etkin A., Buchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron, 65, 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K.L., Williams L.M., Kemp A.H., Rennie C., Gordon E., Bryant R.A. (2009). Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 173, 59–62. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Cashman L., Jaycox L., Perry K. (1997). The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychological Assessment, 9, 445–51. [Google Scholar]
- Foa E.B., Ehlers A., Clark D.M., Orsillo S.M. (1999). The Posttraumatic Cognitions Inventory (PTCI): Development and validation. Psychological Assessment, 11, 303–14. doi: 10.1037/1040-3590.11.3.303. [Google Scholar]
- Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. (2010). Exaggerated and disconnected insular-amygdalar BOLD response to threat-related emotional faces in women with intimate-partner violence PTSD. Biological Psychiatry, 68, 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini C., Myskiw J., Izquierdo I. (2014). The learning of fear extinction. Neuroscience and Biobehavioral Reviews, 47, 670–83. [DOI] [PubMed] [Google Scholar]
- Gilboa A., Shalev A.Y., Laor L., et al. (2004). Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry, 55, 263–72. [DOI] [PubMed] [Google Scholar]
- Golding J.M. (1999). Intimate partner violence as a risk factor for mental disorders: a meta-analysis. Journal of Family Violence, 14, 99–132. [Google Scholar]
- Hauschildt M., Peters M.J., Jelinek L., Moritz S. (2012). Veridical and false memory for scenic material in posttraumatic stress disorder. Consciousness and Cognition, 21, 80–9. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Keller F., Kühner C. (2009). BDI II. Beck Depressions-Inventar. Manual. Frankfurt a. M.: Pearson Assessment & Information GmbH. [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., LaBar K.S., McCarthy G., et al. (2011). Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research, 45, 660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T., Rotshtein P., Yeshurun Y., et al. (2003). Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. NeuroImage, 19, 587–600. [DOI] [PubMed] [Google Scholar]
- Hou C., Liu J., Wang K., et al. (2007). Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Research, 1144, 165–74. [DOI] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K.J. (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry, 167, 648–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A.H., Felmingham K.L., Falconer E., Liddell B.J., Bryant R.A., Williams L.M. (2009). Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: an fMRI study. Psychiatry Research: Neuroimaging, 174, 158–61. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., et al. (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research, 223, 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28, 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008). International Affective Picture System (IAPS): Affective Ratings of Pictures and Intruction Manual. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Liberzon I., Taylor S.F., Amdur R., et al. (1999). Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry, 45, 817–26. [DOI] [PubMed] [Google Scholar]
- Mahabir M., Tucholka A., Shin L.M., Etienne P., Brunet A. (2015). Emotional face processing in post-traumatic stress disorder after reconsolidation impairment using propranolol: a pilot fMRI study. Journal of Anxiety Disorders, 36, 127–33. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Moser D.A., Aue T., Suardi F., et al. (2015). Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Social Cognitive and Affective Neuroscience, 10, 645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., Frewen P.A., et al. (2015). The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology, 40, 2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E.J., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7, 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Osuch E.A., Willis M.W., Bluhm R.L., Group C.N.S., Ursano R.J., Drevets W.C. (2008). Neurophysiological responses to traumatic reminders in the acute aftermath of serious motor vehicle collisions using [15O]-H2O PET. Biological Psychiatry, 64, 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagura J., Stein M.B., Bolton J.M., Cox B.J., Grant B., Sareen J. (2010). Comorbidity of borderline personality disorder and posttraumatic stress disorder in the U.S. population. Journal of Psychiatry Research, 44, 1190–8. doi: 10.1016/j.jpsychires. 2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36, 2130–42. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. (2006). An insular view of anxiety. Biological Psychiatry, 60, 383–7. [DOI] [PubMed] [Google Scholar]
- Perusini J.N., Meyer E.M., Long V.A., et al. (2015). Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacology, 41, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A., Frans Ö., Fernandez M., von Knorring L., Fischer H., Fredrikson M. (2002). Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. European Archives of Psychiatry and Clinical Neuroscience, 252, 68–75. [DOI] [PubMed] [Google Scholar]
- Protopopescu X., Pan H., Tuescher O., et al. (2005). Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry, 57, 464–73. [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., et al. (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry, 2, 62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., van der Kolk B.A., Fisler R.E., et al. (1996). A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry, 53, 380–7. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry, 47, 769–76. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Fukuda R., Okuaki T., et al. (2005). Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. NeuroImage, 26, 813–21. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Bush G., Whalen P.J., et al. (2007). Dorsal anterior cingulate function in posttraumatic stress disorder. Journal of Traumatic Stress, 20, 701–12. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35, 169–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Orr S.P., Carson M.A., et al. (2004). Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female vietnam veterans with PTSD. Archives of General Psychiatry, 61, 168–76. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Whalen P.J., Pitman R.K., et al. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50, 932–42. [DOI] [PubMed] [Google Scholar]
- Spitzer C., Stieglitz R.-D., Freyberger H.-J. (2005). Fragebogen zu Dissoziativen Symptomen (FDS). Ein Selbstbe urteilungsverfahren zur syndromalen Diagnostik dissoziativer Phänomene. Deutsche Adaptation der Dissociative Experiences Scale (DES) von E. Bernstein-Carlson und F. W. Putnam. Testmanual zur Kurz- und Langform (FDS-20 aund FDS). Bern: Verlag Hans Huber. [Google Scholar]
- Sripada R.K., King A.P., Garfinkel S.N., et al. (2012). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: JPN, 37, 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C., Daniels J.K., Frewen P.A., Densmore M., Theberge J., Lanius R.A. (2015). Effect of direct eye contact in women with PTSD related to interpersonal trauma: psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Research: Neuroimaging, 232, 162–7. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme. [Google Scholar]
- Thomaes K., Dorrepaal E., Draijer N.P., et al. (2009). Increased activation of the left hippocampus region in complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Research, 171, 44–53. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Justice, Office of the Justice Programs, Bureau of Justice Statistics. (2012). Special report: Intimate partner violence, 1993 - 2010 (NCJ 239203).
- Veer I.M., Oei N.Y.L., van Buchem M.A., Spinhoven P., Elzinga B.M., Rombouts S.A.R.B. (2015). Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Research: Neuroimaging, 233, 436–42. [DOI] [PubMed] [Google Scholar]
- Vermetten E., Schmahl C., Southwick S.M., Bremner J.D. (2007). A positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacology Bulletin, 40, 8–30. [PMC free article] [PubMed] [Google Scholar]
- Viviani R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M., Kanske P., Neumeister P., Bode K., Heissler J., Schönfelder S. (2010). EmoPics: Subjektive und psychophysiologische Evaluationen neuen Bildmaterials für die klinisch-bio-psychophysiologische Forschung. Zeitschrift Für Klinische Psychologie Und Psychotherapie, Supplement 1/11, 77. [Google Scholar]
- Weston C.S.E. (2014). Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Frontiers in Psychiatry, 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley M.G., Kroes M.C.W., Huntley Z., Rugg M.D., Davis S.W., Brewin C.R. (2013). An fMRI investigation of posttraumatic flashbacks. Brain and Cognition, 81, 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley M.G., Rugg M.D., Smith A.P.R., Dolan R.J., Brewin C.R. (2009). Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain and Cognition, 69, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Pearce J., Morrison S., Dunstan F., Bisson J.I., Fone D.L. (2015). Risk of post-traumatic stress disorder following traumatic events in a community sample. Epidemiology and Psychiatric Sciences, 24, 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2004). Handbook for the Documentation of Interpersonal Violence Prevention Programmes. Geneva: World Health Organization. [Google Scholar]
- Wittchen H.U., Zaudig M., Fydrich T. (1997). Strukturiertes Klinisches Interview Für DSM-IV: SKID; Eine Deutschsprachige, Erweiterte Bearbeitung Der Amerikanischen Originalversion Des SCID. Göttingen [u.a.]: Hogrefe. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.