Abstract
Social anxiety disorder (SAD) involves abnormalities in social motivation, which may be independent of well-documented differences in fear and arousal systems. Yet, the neurobiology underlying motivational difficulties in SAD is not well understood. The aim of the current study was to spatiotemporally dissociate reward circuitry dysfunction from alterations in fear and arousal-related neural activity during anticipation and notification of social and non-social reward and punishment. During fMRI acquisition, non-depressed adults with social anxiety disorder (SAD; N = 21) and age-, sex- and IQ-matched control subjects (N = 22) completed eight runs of an incentive delay task, alternating between social and monetary outcomes and interleaved in alternating order between gain and loss outcomes. Adults with SAD demonstrated significantly reduced neural activity in ventral striatum during the anticipation of positive but not negative social outcomes. No differences between the SAD and control groups were observed during anticipation of monetary gain or loss outcomes or during anticipation of negative social images. However, consistent with previous work, the SAD group demonstrated amygdala hyper-activity upon notification of negative social outcomes. Degraded anticipatory processing in bilateral ventral striatum in SAD was constrained exclusively to anticipation of positive social information and dissociable from the effects of negative social outcomes previously observed in the amygdala. Alterations in anticipation-related neural signals may represent a promising target for treatment that is not addressed by available evidence-based interventions, which focus primarily on fear extinction and habituation processes.
Keywords: social anxiety disorder, fMRI, reward, monetary incentive delay, nucleus accumbens, threat
Introduction
Social anxiety disorder (SAD) is a common and debilitating psychiatric disorder predominantly characterized by persistent fear of one or more social or performance situations (American Psychiatric Association and American Psychiatric Association and DSM-5 Task Force, 2013). Previous behavioral and neuroimaging studies of SAD have highlighted a central role for negative affects and threat-related neural circuits in symptom expression, primarily including a limbic–medial prefrontal circuit that involves enhanced processing of threat stimuli (Phan et al., 2006; Goldin et al., 2009; Etkin, 2010; Schmidt et al., 2010; Hattingh et al., 2013). Additionally, recent work has also suggested that SAD may be uniquely characterized by diminished positive affect (Brown et al., 1998; Kashdan, 2007; Alden et al., 2008; Morrison and Heimberg, 2013; Weeks, 2015), which may mechanistically relate to its development and maintenance (Caouette and Guyer, 2014; Haller et al., 2015) and cannot be accounted for by depressive symptomatology (Kashdan, 2007; Eisner et al., 2009; Weeks, 2015). Despite the increasing interest in characterizing psychiatric disorders in terms of positively and negatively valenced motivational systems (Insel et al., 2010; Casey et al., 2014; Insel, 2014) and the potential to inform treatment development, few studies have examined the mechanisms of positive affect deficits in SAD within the functional neurobiology of reward.
Reward processing in typically developing humans and non-human primates is mediated by dense dopaminergic projections originating from the ventral tegmental area (VTA) that project to the striatum, the orbitofrontal cortex (OFC), the ventromedial prefrontal cortex (vmPFC) and the anterior cingulate cortex (Haber and Knutson, 2010). Collectively, these regions form a mesolimbic dopamine pathway that is sensitive to both the magnitude and the probability of reward (Schultz, 1998; Ikemoto and Panksepp, 1999; Schultz, 2000; Berridge et al., 2009; Saddoris et al., 2015). In particular, patterned firing of dopaminergic neurons in the nucleus accumbens (NAc) is thought to encode incentive motivation related to approach behaviors toward salient goals (Knutson et al., 2001; Knutson and Cooper, 2005; Kim et al., 2006; Bjork and Hommer, 2007; Forbes et al., 2009). Comparative research further suggests that dopamine-mediated responses in NAc are increased during anticipation of learned cue-outcome associations (Martin and Ono, 2000; Melendez et al., 2002), which are thought to be broadly reflective of the motivational relevance of upcoming events (Carter et al., 2009) and related to the modulation and planning of complex motivated behavior (Mogenson et al., 1980; Roesch et al., 2009). Social interaction mobilizes the same mesolimbic network that is active while processing non-social rewards such as food, money, sex and drugs of addiction (Koob and LeMoal, 1997; Izuma et al., 2008; Rilling et al., 2008; Spreckelmeyer et al., 2009; Rademacher et al., 2010; Trezza et al., 2011). Such reward network responses toward social information are also present during both anticipatory and outcome periods (Hayden et al., 2007; Winston et al., 2007; Rademacher et al., 2010), suggesting that behavior is strongly guided by both the motivation to attain social rewards and the enjoyment of such rewards once received (Ruff and Fehr, 2014).
While convergent evidence suggests that in typically developing individuals, social cohesion and affiliation are associated with increase in striatal responses to social cues, this perspective also suggests that reduced striatal activation during the assignment of values to social stimuli ought to be associated with degraded social affiliation and perceptions of weaker social bond formation, both of which are observed in SAD (Mathew et al., 2001; Fox and Kalin, 2014; Haller et al., 2015). Consistent with this conceptualization, relatively early PET imaging studies demonstrated altered striatal functions in SAD, which may be rooted in abnormal central dopamine function, linked to dopamine D2 receptor and dopamine transporter (DAT) availability in the striatum (Tiihonen et al., 1997; Schneier et al., 2000; Schneier et al., 2008) (also see Schneier et al., 2009). In behavioral terms, when compared with non-anxious counterparts, adults with SAD report that positive events are less likely to occur (Gilboa-Schechtman et al., 2000), report greater difficulty expressing positive emotions (Turk et al., 2005) and demonstrate a reduced tendency to sustain or savor positive affect once experienced (Eisner et al., 2009).
A small number of neuroimaging studies have probed the neurobiological substrates of dampened positive affect in SAD. For example, Sripada et al. (2013) reported that the experience of cooperative social reciprocity in an economic exchange (‘trust’) game was associated with increased striatal activity in control subjects but absent in SAD, potentially reflecting diminished valuation of positive partner feedback. Richey et al. (2014) observed diminished NAc activation in SAD compared to controls and adults with autism during anticipation of social but not monetary outcomes in the Monetary Incentive Delay (MID) task (Knutson et al., 2001, 2008). This work illustrated comparatively reduced striatal activity during anticipation of non-emotional (neutral) faces from the NimStim face set (Tottenham et al., 2009), which was interpreted as a social-specific effect because it was not observed during anticipation of non-social (i.e. monetary) gains. Consistent with these results, Maresh et al. (2014) reported no differences between adults with and without SAD during anticipation of monetary gains. Collectively, this pattern of results suggests that while perceptions of non-social reward may be preserved in SAD, abnormalities in incentive motivation may be constrained exclusively to social stimuli.
Alterations in value-related neural signals may mechanistically relate to disorder development and maintenance by compromising the ability to select between social options that should be pursued or avoided. However, a lack of appetitive motivation toward social reward does not rule out the possibility that diminished ‘seeking’ of social incentives in SAD may in fact be attributable to enhanced avoidance motivation. Previous animal and human work has demonstrated a role for ventral striatum in aspects of aversive motivation and avoidance (Reis et al., 2004; Oleson et al., 2012), indicating that cues preceding stressors can also increase dopamine (DA) release and furthermore that DA depletion can impair active avoidance in rats (Mccullough et al., 1993). Although this question has not been specifically examined in anxious samples, prior neuroimaging studies have used incentive tasks (including the MID) to determine whether cues predicting appetitive (gain) and aversive (loss) outcomes are encoded by separate systems. Knutson and Greer (2008) reported results from a meta analysis of 21 studies using the MID, which indicated that gain anticipation contrasts showed greater activation in NAc than loss anticipation contrasts of the same magnitude. These authors framed this result in terms of an anticipatory affect model of NAc function, in which positive (gain) and negative (loss) outcomes recruit spatiotemporally distinct functions within the mesolimbic circuit (NAc and insular cortices, respectively). Under this anticipatory affect account, valence-specific effects for cue-driven striatal responses in the MID appear to be indexing a particular component of accumbens DA function in the form of activational or instrumental seeking behaviors, but not the preparation of an avoidance behavior. Thus, the differentiation between enhanced avoidance and diminished approach motivation appears to be testable within incentivized reaction time tasks such as the MID.
Yet, despite clear theoretical predictions the landscape of reward circuitry deficits in SAD remains largely unexplored. Accordingly, the primary purpose of this study was to determine whether SAD is characterized by deficiencies in reward processing that are not addressed by currently available evidence-based treatments, which focus primarily on fear extinction and habituation processes as their central mechanisms of action (Feske and Chambless, 1995; Blanco et al., 2010; Hofmann et al., 2012). By manipulating the valence (positive, negative) and type of incentives (social, non-social) in a sample of adults with SAD and healthy control subjects during an incentivized reaction time-task (Knutson et al., 2000, 2008), we sought to identify the dissociable contributions of reward- and threat-related neural systems to social incentive processing in SAD. Given previous findings of reduced NAc activation in response to social, but not monetary rewards in SAD (Maresh et al., 2014; Richey et al., 2014) and cognitive and behavioral research indicating that this disorder is characterized by circumscribed deficits in positive affects (Brown et al., 1998; Kashdan, 2004), we predicted that SAD would be characterized by diminished activation in the ventral striatum during anticipation of positive social outcomes and not enhanced striatal activation during anticipation of negative social outcomes, in keeping with the anticipatory affect account of striatal functions (Knutson and Greer, 2008). We further predicted that these effects (degraded approach motivation and lack of enhanced avoidance motivation) would be constrained exclusively to social incentives and would not be observed during monetary incentive processing. Second, given the framework of the present study that reward network dysfunction may be dissociable from threat-related processing of social stimuli, we also predicted that hypo-activation of NAc during anticipatory processing of positive social incentives in NAc can be distinguished temporally from amygdala hyper-activation during negative social outcomes. We further hypothesized that individual estimates of NAc activity during positive social incentives would be related to symptom severity in the SAD group.
Method
Participants
The total sample comprised 43 adults (control N = 22, SAD N = 21) recruited from the community. All participants provided written informed consent, as reviewed and approved by the local Institutional Review Board (IRB). Participants were matched on age, sex and IQ. Information on group size, age and gender distributions are summarized in Table 1 and further described in the supplementary materials. Participants were right-handed and had normal or corrected to normal vision. All participants were assessed for preliminary exclusion criteria, including MRI contraindications, uncontrolled epilepsy (seizure within 6 months prior to consent), history of neurological injury (head trauma), current use of psychotropic medications (past 3 months) and color blindness as measured by the Ishihara Color Plate Test (Ishihara, 1990).
Table 2.
Clusters showing significant group differences in ANOVA models.
| Talairach–Tournoux atlas label | Brodmann areaa | Size (mm3) | t mean | t max | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Anticipation Phase | |||||||
|
Reward Type [Money, Faces] X Valence [Gain, Loss] X Possible Win [Win, Non-win] | |||||||
| Control > SAD; Clusters significant at P < 0.005; FDR corrected at P < 0.05 | |||||||
| Right posterior cingulate | 30 | 20 985 | 3.1495 | 4.6284 | 23.7 | −60.0 | 13.7 |
| Postcentral gyrus | 7 | 5405 | 3.0387 | 4.0141 | −25.8 | −52.2 | 66.4 |
| Right orbital gyrus | — | 4807 | 3.1262 | 4.4526 | 1.5 | 52.1 | −23.8 |
| Right superior temporal gyrus | 22 | 4180 | 3.1934 | 4.5182 | 54.0 | 0.5 | −0.6 |
| Right subcallosal gyrus | 47b | 2656 | 3.0752 | 4.1761 | 21.7 | 25.0 | −17.0 |
| Right medial frontal gyrus | 6 | 1935 | 3.0029 | 4.1564 | 11.6 | −15.5 | 77.0 |
| Right lentiform nucleus | — | 1878 | 3.0782 | 4.5594 | 7.6 | −6.3 | −10.5 |
| Left middle occipital gyrus | 18 | 1131 | 3.0755 | 3.9644 | −24.7 | −96.6 | 2.0 |
| Right insula | 38 | 976 | 2.9797 | 3.8351 | 39.1 | 4.1 | −18.2 |
| Left pyramid of vermis | — | 840 | 2.9759 | 3.9305 | −1.5 | −80.3 | −41.0 |
| Left precuneus | 7 | 698 | 3.2157 | 4.2233 | −14.6 | −64.3 | 38.5 |
| Left precentral gyrus | 6 | 620 | 2.9269 | 3.5014 | −62.1 | −13.8 | 41.2 |
| Left inferior temporal gyrus | 20 | 538 | 2.8732 | 3.3393 | −43.9 | −10.9 | −21.5 |
| Left superior temporal gyrus | 22 | 530 | 3.0179 | 3.6064 | −53.0 | 3.6 | −0.4 |
| Right medial frontal gyrus | 10 | 430 | 2.9675 | 3.5237 | 10.6 | 70.3 | 8.6 |
| Right postcentral gyrus | 5 | 425 | 3.0765 | 3.9269 | 31.8 | −47.2 | 69.9 |
| Left declive | 37 | 409 | 2.9744 | 4.0045 | −54.0 | −63.3 | −28.1 |
| Right lingual gyrus | 18 | 399 | 2.8101 | 3.1349 | 22.7 | −80.5 | −11.2 |
| Left superior temporal gyrus | 22 | 398 | 2.831 | 3.1299 | −54.0 | −10.3 | 9.8 |
| Left middle temporal gyrus | 39 | 365 | 2.9147 | 3.3478 | −55.1 | −68.0 | 8.9 |
| Left insula | 13 | 307 | 2.9292 | 3.3453 | −36.9 | −3.5 | −2.0 |
| Left subcallosal gyrus | 25b | 302 | 2.8414 | 3.1191 | −8.0 | 13.1 | −13.2 |
| Right inferior semi-lunar lobule | — | 273 | 2.8314 | 3.1293 | 20.7 | −84.8 | −56.7 |
| Right superior frontal gyrus | 6 | 256 | 2.9377 | 3.4283 | 9.6 | 7.1 | 56.4 |
| SAD > control | |||||||
|
No clusters significant at P < 0.005, FDR corrected at P < 0.05 | |||||||
|
Outcome Phase | |||||||
|
Reward Type [Money, Faces] X Valence [Gain, Loss] X Possible Win [Win, Non-win] | |||||||
| Control > SAD; Clusters significant at P < 0.005; FDR corrected at P < 0.05 | |||||||
| Right cingulate gyrus | 8c | 1040 | 3.2575 | 4.9365 | 18.2 | 17.9 | 43.1 |
| Right medial frontal gyrus | 9 | 745 | 3.0694 | 3.8223 | 24.2 | 43.4 | 19.7 |
| Right orbital gyrus | 11c | 630 | 3.2491 | 4.7119 | 16.2 | 38.5 | −23.5 |
| Right cerebellar tonsil | — | 628 | 3.5349 | 5.3472 | 21.2 | −46.6 | −44.4 |
| Left superior frontal gyrus | 10 | 438 | 3.0484 | 3.4366 | −17.2 | 44 | 30.6 |
| Right superior parietal lobule | 7 | 433 | 2.9559 | 3.4125 | 39.4 | −58.1 | 50.3 |
| Left superior frontal gyrus | 32 | 335 | 3.0703 | 3.5477 | −8.1 | 7.7 | 55.9 |
| Right postcentral gyrus | 3 | 261 | 3.0162 | 3.3558 | 64.6 | −20.3 | 35.9 |
| Right cerebellar tonsil | — | 257 | 3.1064 | 3.8123 | 45.5 | −49 | −60 |
| SAD > control | |||||||
| No clusters significant at P < 0.005, FDR corrected at P < 0.05 | |||||||
BA label is indicated in table if the focus point of cluster within <5 mm of BA label boundary.
Indicates clusters displayed in Figure 4a and b.
Indicates clusters displayed in Figure 5a.
Table 3.
Clusters showing significant group differences in social-only trials.
| Control > SAD; Clusters significant at P < 0.005; FDR corrected at P < 0.05 | |||||||
|---|---|---|---|---|---|---|---|
| Talairach-Tournoux atlas label | Brodmann areaa | Size (mm3) | t mean | t max | MNI coordinates |
||
| X | Y | Z | |||||
| Outcome Phase | |||||||
| Reward Type [Money, Faces] X Valence [Gain, Loss] X Possible Win [Win, Non-win] | |||||||
| Right cingulate gyrus | 8c | 1040 | 3.2575 | 4.9365 | 18.2 | 17.9 | 43.1 |
| Right medial frontal gyrus | 9 | 745 | 3.0694 | 3.8223 | 24.2 | 43.4 | 19.7 |
| Right orbital gyrus | 11c | 630 | 3.2491 | 4.7119 | 16.2 | 38.5 | −23.5 |
| Right cerebellar tonsil | — | 628 | 3.5349 | 5.3472 | 21.2 | −46.6 | −44.4 |
| Left superior frontal gyrus | 10 | 438 | 3.0484 | 3.4366 | −17.2 | 44 | 30.6 |
| Right superior parietal lobule | 7 | 433 | 2.9559 | 3.4125 | 39.4 | −58.1 | 50.3 |
| Left superior frontal gyrus | 32 | 335 | 3.0703 | 3.5477 | −8.1 | 7.7 | 55.9 |
| Right postcentral gyrus | 3 | 261 | 3.0162 | 3.3558 | 64.6 | −20.3 | 35.9 |
| Right cerebellar tonsil | — | 257 | 3.1064 | 3.8123 | 45.5 | −49 | −60 |
| SAD > control | |||||||
| No clusters significant at P < 0.005, FDR corrected at P < 0.05 | |||||||
BA label is indicated in table if the focus point of cluster within <5 mm of BA label boundary.
Indicates clusters displayed in Figure 4a and b.
Indicates clusters displayed in Figure 5a.
Table 1.
Mean (SDs) age and symptom profiles.
| Control (n = 22) | SAD (n = 21) | t or χ2, (P) | |
|---|---|---|---|
| Age | 26.50 (7.98) | 25.67 (7.61) | 0.35 (0.73) |
| Sex (F/M) | 10/12 | 8/13 | χ2(1) = 0.03(0.86) |
| WASI (full-scale) | 118.2 (7.93) | 117.2 (10.9) | 0.33 (0.73) |
| WASI (performance) | 118 (8.89) | 118.1 (12.3) | 0.1 (0.99) |
| WASI (verbal) | 114.7 (10) | 113.1 (11) | 0.46 (0.64) |
| LSAS total score | 17.94 (11.62) | 68.05 (18.4) | 9.15 (<0.00001) |
| LSAS fear subscale | 12.44 (7.64) | 35.57 (9.61) | 8.21 (<0.00001) |
| LSAS avoidance subscale | 8.72 (8.05) | 32.0 (11.07) | 7.39 (<0.00001) |
| BDI total score | 5.65 (4.56) | 7.2 (6.25) | 0.91 (0.37) |
Both groups completed: (1) The Weschler Abbreviated Scale of Intelligence (WASI); (2) The Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987), a measure designed to assess social fear and avoidance; (3) the Beck Depression Inventory-II (BDI; Beck, 1961; Beck Steer and Garbin, 1998), administered to assess the overall severity of depressive symptoms and to verify that the SAD group did not have significant mood symptoms.
fMRI task
The fMRI task was modified from the Monetary Incentive Delay (MID) task as first described in Knutson et al. (2000). Task conditions and trial timings are summarized in Figure 1. Runs began with an instructional screen indicating the forthcoming run type. Events (money [or] faces, gain [or] loss) were segregated into separate runs (36 trials per run), in order to minimize the number of cues to be memorized, resulting in four distinct run types: (1) Money ‘gain’[+ $1], (2) Money ‘loss’[−$1], (3) Face ‘gain’[happy] and (4) Face ‘loss’[angry]. Eight total runs, two of each type as depicted in Figure 2b were presented in counterbalanced order across subjects. Additional task details are provided in the supplementary materials.
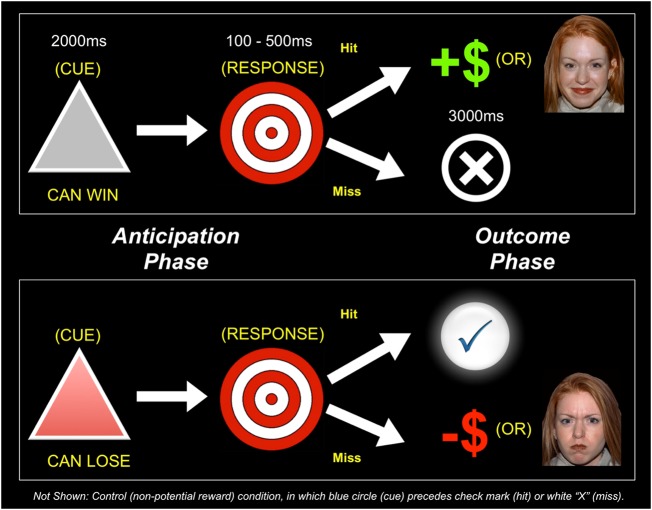
Fig. 1.
Schematic depiction of the modified MID task. Participants alternated between ′money′ and ′face′ runs, and ′win′ and ′loss′ runs, denoted by an instructional screen at the start of each run. Each trial consisted of a cue (i.e. a gray triangle indicates a ‘win’ trial and a red triangle indicates a ‘loss’ trial), an anticipatory delay, a target and outcome feedback. Not shown: in all runs, a blue circle cue appeared in 50% of trials, indicating a non-incentivized trial in which a ‘hit’ resulted in a check mark and a ‘miss’ in an ‘X’.
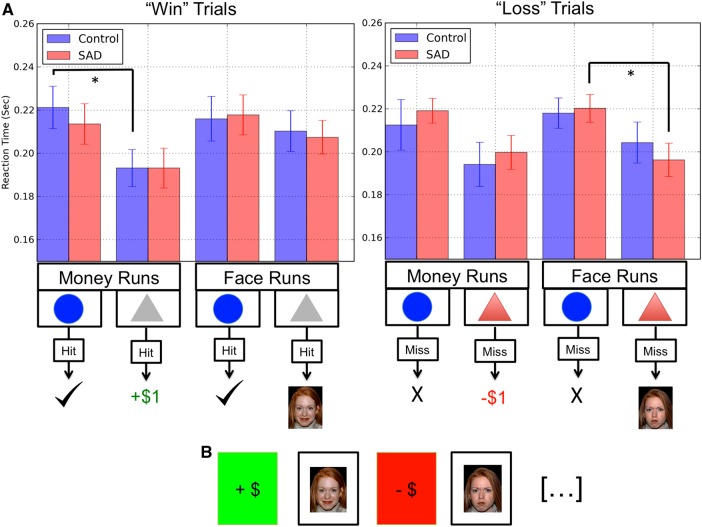
Fig. 2.
Average reaction times during face and money conditions for both potential reward ('Rew') and non-potential reward ('Non') trials. Error bars represent the standard errors of the mean.
Measures
Diagnostic assessment
All subjects completed the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV) (Brown et al., 1994), administered by research reliable doctoral-level assessors and reviewed by a Ph.D. level clinical psychologist (J.A.R.).
Cognitive assessment
Subjects completed the Wechsler Abbreviated Scale of Intelligence-II (WASI-II) (Wechsler, 2011), a clinician-administered measure of general cognitive function and IQ.
Self-reported symptoms
All subjects completed the Liebowitz Social Anxiety Scale – LSAS (Liebowitz, 1987), which comprises 24 items and uses a 0 to 3 rating for both fear (ranging from 'no fear' to 'severe fear') and avoidance (ranging from 'never' to 'usually'), for different social situations (Cronbach's α for the current sample = 0.93). Participants completed the Beck Depression Inventory – BDI-II (Beck et al., 1996), a 21-item self-report measure of depression symptomatology (Cronbach's α for the current sample = 0.77).
Picture ratings
After scanning, participants completed a picture-rating task outside of the scanner. In this task, participants provided a rating (0–8 point scale) reflecting valence and arousal ratings for each face stimulus using a self-assessment mannequin (Bradley and Lang, 1994).
fMRI data analysis
fMRI acquisition and preprocessing details are outlined in the supplementary material. Onset times and durations of events were used to model a signal response containing a regressor for each response type, win and non-win (w, n) trials, in the context of social and monetary (Soc, Mon) outcomes, separately for reward and punishment (R, P): [1] wSocR, [2] nSocR, [3] wSocP, [4] nSocP, [5] wMonR, [6] nMonR, [7] wMonP and [8] nMonP. The eight event types reflect the 2 × 2 × 2 factorial nature of the design, in which win possibility was crossed with the stimulus type and valence of outcome. Events were time-locked to the onset of the cue (for the analysis of win vs non-win trials) and of the outcome (in contrast to [Soc, Mon] and [R, P] trials). Group-wise activation and deactivation images were obtained by a mixed effect higher level analysis using a whole-brain univariate GLM in which we regressed the preprocessed and spatially smoothed BOLD signal on the anticipatory and outcome phases of the task.
Consistent with previous studies using the MID in clinical samples (Guyer et al., 2006; Beck et al., 2009; Delmonte et al., 2012), the primary group-level (random effects) method of fMRI data analysis was a mixed model ANOVA (cf. Henson and Penny, 2005; equations (21)–(25)), with one between-subjects factor: group (SAD, Control) and three within-subjects factors: 2 (reward potential [win, non-win]), × 2 (stimulus type [Social, Monetary]), × 2 (outcome type [Reward, Punishment]), applied separately for the anticipatory and outcome phases of the task. In addition, we sought to characterize neural responses to social outcomes and to replicate previous findings in SAD, indicating amygdala activation when viewing angry faces (Phan et al., 2006; Evans et al., 2008). Because amygdala activity is most likely to be observed in SAD for social conditions only and thought to be less related to non-social (monetary) manipulations (cf. Maresh et al., 2014), we examined the outcome phase for social-only trials. In this planned contrast, we opted to code non-social conditions in this model, to avoid their inclusion in implicit baseline parameters, which would likely add noise to subsequent parameter estimates. The specific interaction of interest within this mixed model ANOVA was a 2 (group) × 2 (reward and punishment [R, P]) × 2 (hit, miss) for social (Soc) outcomes.
For all whole-brain results, statistical parameter maps were thresholded at P < 0.005 and false discovery rate (FDR) corrected at P < 0.05. Clusters that passed corrections for height and multiple comparisons were further scrutinized by extracting subject- and condition-specific signal intensity coefficients to evaluate simple effects. Relations between neural responses to rewards and social anxiety symptoms from the LSAS were assessed in SAD by using group-level activation maps to extract mean subject-specific parameter estimates that were then analyzed using Scientific and Numeric Python (SciPy and NumPy, respectively) (Oliphant, 2007; van der Walt et al., 2011). For these exploratory correlational analyses, we did not correct for multiple comparisons.
Results
MID reaction times
In-scanner reaction times (RTs) to task targets are depicted in Figure 2. Full details regarding analysis and interpretation of RT data are provided in the supplementary materials.
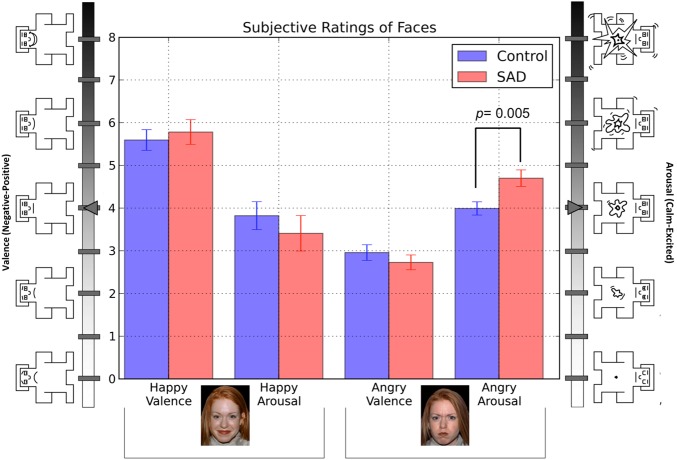
Picture ratings
Valence and arousal ratings of faces are depicted in Figure 3. Full analysis and interpretation of face rating data are provided in the supplementary materials.
Fig. 3.
Average valence and arousal ratings of faces. Valence = 0 (extremely unpleasant) to + 8 (extremely pleasant); arousal = 0 (not at all aroused) to + 8 (extremely aroused).
Cognitive functioning and self-reported symptoms
Results for self-reported symptoms and IQ measurements are reported in Table 1.
fMRI statistical comparisons
Anticipatory phase
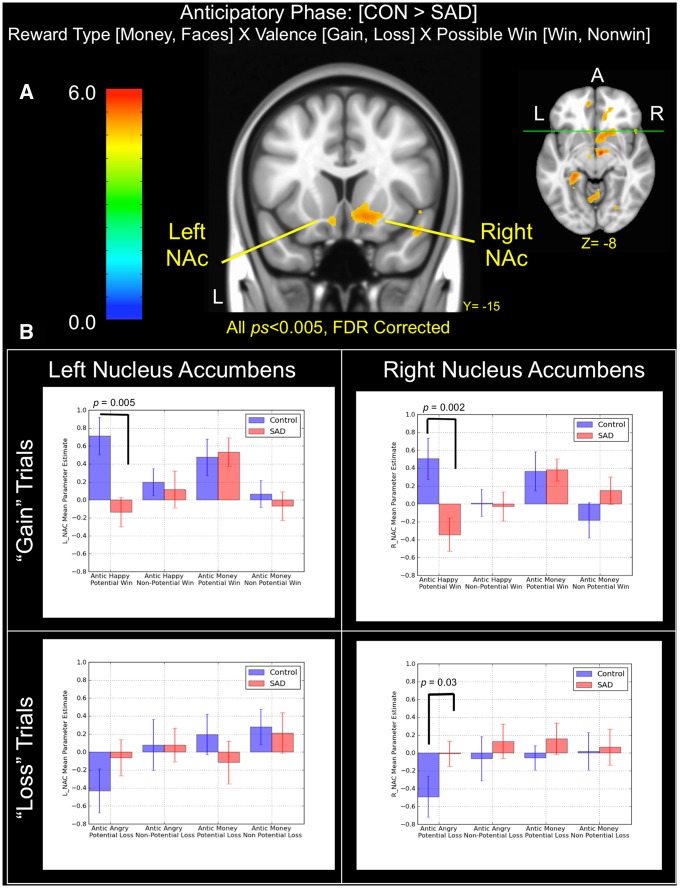
A whole-brain mixed-model ANOVA compared voxel-wise activity of controls vs SAD for each stimulus category during the anticipatory period of the MID (i.e. subsequent to the cue period and prior to target presentation). Main effect images and corresponding activation tables are provided in the supplementary material. In the mixed-model ANOVA, a significant interaction was observed in bilateral NAc for the contrast controls > SAD during anticipation (P < 0.005 FDR corrected at P < 0.05; left k = 302, peak voxel MNI[x, y, z]: −8, 13, −13, t = 3.11; right k = 2,696, peak voxel MNI: 22, 25, −17, t = 4.17), as well as in left and right temporal cortex, thalamus and insula (Figure 4a). No significant clusters were observed at a similar threshold for the contrast SAD > controls. Following upon significant clusters in a priori regions of interest (ROIs) in left and right NAc, we followed the procedure outlined in Knutson et al. (2008) by placing 8 mm spheres (e.g. 4 mm radius) with centroids at the following coordinates (MNI: ±10, 10, −2). We further constrained each ROI to include only voxels that demonstrated significant activation (P < 0.005; FDR at P < 0.05) in the group-level ANOVA model. Case- and event-wise parameter estimates were extracted for each ROI and aggregated into separate models for comparison of simple effects within gain and loss trials (Figure 4b). In line with initial predictions, planned posthoc tests of simple effects revealed that controls demonstrated significantly greater activation in bilateral NAc during anticipation of positive social images than SAD subjects (left NAC: t[39] = 3.11 and P = 0.005; right NAc: t[39] = 3.22 and P = 0.002), an effect that appeared to be exclusive to the anticipation of positive social images and was not observed between groups during anticipation of monetary gains. However, we did find evidence for comparatively reduced NAc activation in controls relative to SAD during anticipation of angry faces for right (t[39] = −2.17 and P = 0.03) but not left NAc (t[39] = −1.46 and P = 0.15), although the SAD group was not significantly different from zero in either case.
Fig. 4.
(A) Brain areas showing significant group (SAD, control) × reward type (money, faces) × valence (gain, loss) × possible win (win, non-win) interactions during the anticipatory phase of the task. (B) Bar graphs depict mean parameter estimates (±1 standard error) by group and trial types in left and right NAc clusters. Note that all results displayed were thresholded at P < 0.005 and FDR corrected at P < 0.05.
We also observed significant clusters in the bilateral insula during anticipatory processing. We examined casewise parameter estimates in each cluster by placing an 8-mm sphere (e.g. 4 mm radius) at the peak voxel and extracting subject-wise activation estimates for each ROI and event in the same manner used for NAc clusters. The results indicated that both left and right insula demonstrated significantly increased activity relative to the implicit baseline for potential gain in social and monetary incentives for both controls (left insula: t[39] = 2.75 and P = 0.01; right insula: t[39] = 2.19 and P = 0.04, respectively) and SAD (t[39] =2.39 and P = 0.03; t[39] = 2.23 and P = 0.03, respectively). However, we did not observe increased responses in the left insula during monetary gain in either group (all Ps > 0.16), nor during any loss trial type (all Ps > 0.08), suggesting that only the right anterior insula was recruited during anticipation of potential gain for both social and non-social incentives. Unlike statistical maps in left/right NAc, casewise parameter estimates extracted from either insula cluster did not support a robust interaction of diagnostic status with responses to social or monetary incentives.
Outcome phase
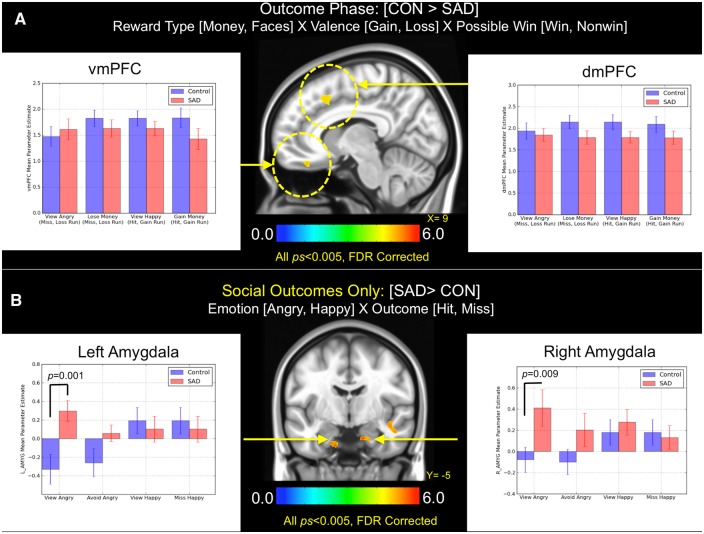
The application of the same mixed-model ANOVA to the outcome phase indicated greater activation in controls within two major clusters that extended into four reward-related regions: dorsomedial prefrontal cortex (dmPFC), vmPFC and bilateral OFC during feedback (P < 0.005, whole-brain FDR corrected at P < 0.05; Figure 5). Given previous findings that the mesial PFC preferentially tracks rewarding outcomes, we directly analyzed casewise estimates at peak activation coordinates within the dmPFC and vmPFC (note that we excluded ‘miss’ outcomes from gain trials [e.g. white 'X' displayed] and ‘hit’ outcomes from loss trials [white check mark displayed] from this analysis in order to evaluate absolute effects related to reward or loss [cf. Patel et al., 2013; Delmonte et al., 2012]). The results from follow-up tests of simple effects in the dmPFC and vmPFC indicated that controls and SAD displayed similar patterns of activation across all event types (all Ps > 0.24), suggesting no diagnostic differences in either region during outcome notification.
Fig. 5.
(A) Brain areas showing significant group (SAD, control) × reward type (money, faces) × valence (gain, loss) × possible win (win, non-win) interactions during the outcome phase of the task. The bar graph depicts mean parameter estimates by group and trial types in vmPFC and dmPFC clusters. (B) Brain areas showing significant group (SAD, control) × emotion (angry, happy) × outcome (hit, miss) interactions during social-only trials in the outcome phase. Bar graphs indicate mean parameter estimates (±1 standard error). Note that all results displayed were thresholded at P < 0.005 and FDR corrected at P < 0.05.
Direct comparison of positive vs negative social outcomes
To examine the outcome phase for social-only trials, we constructed another mixed model ANOVA by again modeling both social and non-social outcomes, but in this case examining only the 2 (group) × 2 (reward and punishment [R, P]) × 2 (hit, miss) interaction. The results of this analysis revealed significant activation in bilateral amygdala, which prompted follow-up pairwise t tests to determine the direction and magnitude of simple effects that produced this result (Figure 5b). Clusters identified in this analysis included voxels that fell within and also outside of predefined amygdala1 ROIs from the Harvard-Oxford subcortical atlas (Frazier et al., 2005; Makris et al., 2006). Planned posthoc tests revealed that SAD subjects demonstrated greater bilateral activation in amygdala exclusively when viewing angry faces (left amygdala: t[39] = 3.49 and P = 0.001; right amygdala: t[39] = 3.31 and P = 0.009), whereas no other between-group differences for the remaining three event types were observed in either ROI (all Ps > 0.28).
Inclusion of Beck Depression Inventory (BDI) scores as a covariate
Given previous reports indicating an association between depression and abnormal striatal function (Pizzagalli et al., 2009), all analyses, for both anticipatory and outcome phases of the MID, were repeated with casewise scores on BDI covaried (see the supplementary materials for full details). In short, the principal results reported above remained unchanged, with the exception of clusters in the bilateral insula, which were regressed out entirely, indicating that the variance in these voxels could be substantially accounted for by the variance contained in the BDI.
Finite impulse response model: direct dissociation of reward- vs threat-related ROIs
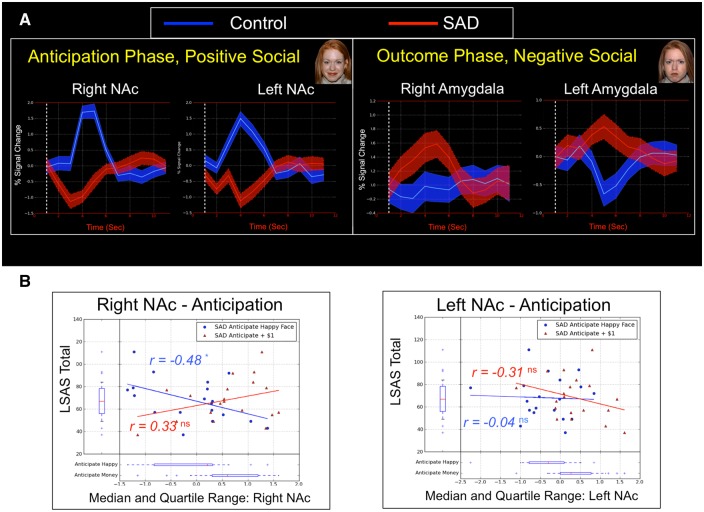
To enhance the comparability of results across ROIs and to rule out the possibility that regional differences in the shape or temporal dynamics of evoked BOLD responses were driving between-group differences observed in the univariate GLM, we conducted an unbiased finite impulse response (FIR) model comparison of average signal intensity in the neural responses observed in ventral striatum and amygdala during anticipatory and outcome periods (respectively; Figures 4 and 5; full details provided in the supplementary materials). Overall results of this analysis indicated that relative to controls, SAD subjects showed a significantly diminished response in the ventral striatum during anticipation of social reward images, which was dissociable from significant hyper-activation in amygdala observed subsequently in the outcome phase while viewing an angry face (Figure 6a).
Fig. 6.
(A) Finite impulse response models for anticipation and outcome phases demonstrate dissociable response patterns in NAc and amygdala, depending on emotional valence of social incentives. (B) Scatterplots depict correlations between social anxiety symptom severity as measured by the LSAS total scale score and casewise parameter estimates during anticipation of social and monetary rewards in left and right NAc clusters that differentiated controls from SAD during the anticipatory phase.
fMRI-symptom associations
The results of follow-up correlational analyses indicated that neither left nor right NAc2 demonstrated any relationship with the LSAS total scale score during monetary anticipation (within potential win trials only). However, we observed a significant correlation between right (r = −0.48 and P = 0.04) but not left (r = −0.04 and P = 0.96) NAc activation and LSAS total scale score, indicating that more severe SAD symptoms were associated with lower levels of right NAc activation exclusively during anticipation of positive faces (Figure 6b). No significant correlations were observed between LSAS scores and NAc parameter estimates during anticipation of potential loss trials for either social or monetary incentives (all Ps > 0.21).
Discussion
This study was designed to dissociate neural responses in social and non-social rewards and punishments in a sample of non-depressed adults with SAD compared with healthy control subjects. Results collectively indicated diminished anticipatory BOLD responses in ventral striatum that were social-specific as well as spatially and temporally independent of enhanced BOLD responses observed in threat and arousal systems. These findings provide evidence for a reward-centric mechanism of functional pathophysiology in SAD that is not currently incorporated into major treatment approaches, which instead focus primarily on fear extinction and habituation processes.
Because individuals with social anxiety have been found to report reduced social approach motivation and diminished positive affect, we predicted that diminished striatal function would be observed in the SAD group exclusively during anticipation of positive social incentives. Consistent with prediction, a significant decrement in NAc activity was observed during anticipation of positive social incentives, an effect that was distinguishable from responses to monetary incentives and from later stages of reward processing when notification of outcomes occurred. When considered together, the effect of positive and negative social images produced a dissociation in the spatial and temporal domains, such that the direction of striatal and amygdala responses in the SAD group depended on the emotional valence (positive/negative) and temporal period (anticipation/notification) under consideration. Interestingly, this specific combination of effects was observed against the backdrop of no between-groups differences in the vmPFC and dmPFC during outcome processing, suggesting that the subjective value of both monetary and social incentives are not neurally differentiable once achieved. Nor did we observe differences between groups in insula activation during incentive processing. Considering research linking insula activity to visceral and autonomic arousal (Eickhoff et al., 2006), this suggests that the intensity of internal states of arousal did not differ between groups during anticipation of social incentives regardless of valence. This finding is in contrast with a number of prior studies that have found enhanced insula activity in SAD samples while viewing social stimuli. However these studies have exclusively focused on direct viewing of social images (Straube et al., 2004; Shah et al., 2009) and social feedback (Peterburs et al., 2016) and not anticipation of such stimuli as was the case here, suggesting that the characteristics of anticipatory processing in SAD may differ substantially from outcome processing along dimensions of both motivation and arousal.
Moreover, the double dissociation of striatal and amygdala responses may offer novel insights into how the particular dimensions of affect and arousal may be separable and also mechanistically altered in SAD. Knutson et al. (2014) recently proposed a testable quantitative model for inferring affect from certain combinations of results in the MID. At the core of this model is theoretical space defined by orthogonal axes of ‘positive arousal’ and ‘negative arousal.’ Specific combinations of brain activity in NAc and insula appear to track closely with self-reported positive affect and overall arousal (respectively) during anticipatory processing in the MID. The scheme for affective inference contained in this circumplex model of positive and negative arousal, when applied to the results observed here, yields the inference that differences in positive affect (as indexed by NAc activation) but not general arousal (indexed by insular cortex) may uniquely characterize SAD during incentive processing, which in turn lends support to the hypothesis that social-motivational deficits in SAD may be due to trait-like differences in approach-related motivation, rather than general levels of anticipatory arousal.
We further sought to determine whether diminished pursuit of social incentives in SAD might be due to (1) diminished approach motivation or (2) enhanced avoidance-related motivation, as indexed by between-groups differences in striatal activation during anticipation of social reward and punishments, respectively. Relative to baseline, we found evidence for significant reductions in striatal activation bilaterally in controls during anticipation of negative social images (Figure 4b). However, SAD subjects did not display a similar pattern of diminished striatal activity relative to baseline and indeed parameter estimates were not different from zero for either trial type in the SAD group. In contrast to initial prediction, a direct group-wise comparison of responses in striatum during anticipation of negative social outcomes revealed that the SAD group demonstrated comparatively enhanced activity in right (P = 0.03) but not left (P = 0.15) striatum. Along these same lines, reaction time data in our study indicated significantly faster responses to social punishment trials in the SAD group. We thus found only partial support for the hypothesized lack of enhanced avoidance motivation. Instead, this pattern of results appears to support a model in which the striatum is disinhibited by cues preceding avoidable social punishment in SAD. Although unexpected, this finding is not without precedent. For example, animal research has indicated that DA neurons appear to respond more selectively to rewards and normally show weak responses or even inhibition to aversive stimuli (Mirenowicz and Schultz, 1996; Ungless et al., 2004). However, elevated DA levels have been observed in NAc in response to conditioned stimuli when associations are well learned (Samejima et al., 2005; Eldar et al., 2016). In relation to the current study, it is therefore possible that cue-outcome associations were learned more rapidly by the SAD group, thus bolstering striatal responses over time. Supporting this account, Levita et al. (2012) examined striatal responses during a well-learned conditioned avoidance task and found evidence for greater activation of NAc during active avoidance and furthermore found that individual differences in NAc activity positively correlated with individual differences in self-reported anxiety. Although the MID is not a conditioned learning task and thus does not directly measure learning rate, we did select for individuals high and low in anxiety. Consequently, if SAD subjects learned the cue-outcome associations more rapidly than controls in social (but not non-social) punishment trials, this may explain the comparatively greater striatal responses exclusively during social avoidance. Thus, a potentially profitable line of future work might examine whether such striatal responses in SAD are directly tracking with an enhanced rate of avoidance learning during aversive social conditioning.
In total, results from this study also have a number of mechanistic implications for intervention. Prior treatment development efforts have largely fallen in line with major theoretical approaches that feature negative valence systems at their core (Schneider et al., 1999). Yet, current evidence-based treatments for SAD such as Cognitive Behavioral Therapy (CBT) and selective-serotonin reuptake inhibitors (SSRIs), which focus on fear extinction and serotonergic systems, respectively, produce only modest effects, with between 40 and 60% of SAD patients remaining symptomatic after treatment (Heimberg et al., 1998; Stein et al., 1999; Davidson et al., 2004; Liebowitz et al., 2005). Results from our study point toward a separate pathophysiologic mechanism that is not currently incorporated into CBT and related families of interventions. In light of these results, it is possible that targeting hedonic function in SAD may result in provable gains in treatment and reduce rates of dropout. Consistent with this possibility, treatment of hedonic deficits, separate from negative affect has proven successful in other psychiatric disorders characterized by reward circuitry dysfunction (Dimidjian et al., 2006; Johnson et al., 2011; Kuyken et al., 2015).
Results and conclusions presented herein should be evaluated in light of study limitations. First, although the MID is a reliable and well-validated index of reward-circuitry function, we are observing posthoc the generative model of payoffs, where prior beliefs are being described by the task. This allowed us to determine correlational relationships to symptom severity, but does not allow a definitive answer to the question of whether social-motivational deficits are developmentally linked to striatal abnormalities (see supplementary materials for additional discussion). Second, we note that in comparison to some clinical neuroimaging reports, our sample size was not large. However, we also note that studies employing the MID often report 20 participants or fewer and a recent review of this task by its developer suggests that this phenomenon may be due to the relatively large effect sizes typically observed in the MID (Knutson and Heinz, 2015). Third, we note that although we are evaluating potential underlying affective dimensions of psychopathology, the initial characterization of patients was performed along DSM-IV lines and not a dimensional approach based on underlying constructs. Finally, the salience of static face images from the NimStim set (Tottenham et al., 2009) is not entirely understood as it relates to a truly social form of incentive. Future studies should therefore consider using either dynamic social images or perhaps genuine social feedback in real-time, in order to gain a more comprehensive understanding of incentive processing in naturalistic settings. In conclusion, the current results highlight a novel pattern of reduced positive affect that is not well represented in currently available evidence-based treatments and, if addressed in the context of intervention, may improve treatment response and reduce high rates of relapse.
Supplementary Material
Acknowledgements
The authors wish to gratefully acknowledge the assistance of MRI techs and undergraduate research assistants who aided in the collection and organization of both neuroimaging and behavioral data for this study.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Footnotes
Note that the spatial extent of clusters observed bilaterally in the amygdala was further scrutinized in order to determine the amount of spatial overlap with pre-defined segmentations of left and right amygdala derived from standardized atlases of subcortical anatomy. We used the Harvard-Oxford subcortical atlas to calculate the number of voxels within significant clusters reported in Figure 6b that overlapped with left (k = 188) and right (k = 390) amygdala as defined by separate left and right amygdala segmentations within this atlas. ROI analyses reported here reflect BOLD signal activity extracted from the entire cluster, however supplemental analyses also scrutinized the extent to which event-wise patterns were similar when conducted only on voxels (1) within and (2) outside of Harvard-Oxford atlas labels. The results of this additional analysis indicated no differences in the patterns of observed effects for (1) or (2), suggesting that observed effects were consistent across the spatial extent of the cluster as defined by the Harvard-Oxford atlas (see the supplementary results for additional details).
All fMRI-symptom correlations reported here reflect calculations that take into account casewise scores on the BDI-II as a covariate, to guard against the possibility that depressive symptoms could be inflating relationships between NAc activation and LSAS scores. The authors wish to thank an anonymous reviewer for this suggestion.
References
- Alden L.E., Taylor C.T., Mellings T.M.J.B., Laposa J.M. (2008). Social anxiety and the interpretation of positive social events. Journal of Anxiety Disorders, 22(4), 577–90. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association, and DSM-5 Task Force. (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, Washington, D.C: American Psychiatric Association. [Google Scholar]
- Beck A., Schlagenhauf F., Wustenberg T., et al. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–42. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G. (1998). Psychometric properties of the Beck Depression Inventory. Twenty-five years of evaluation. Clinical Psychology review, 8, 77–100. doi: 10.1016/0272-7358(88)90050-5. [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Hommer D.W. (2007). Anticipating instrumentally obtained and passively-received rewards: A factorial fMRI investigation. Behavioural Brain Research, 177(1), 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Heimberg R.G., Schneier F.R., et al. (2010). A placebo-controlled trial of phenelzine, cognitive behavioral group therapy, and their combination for social anxiety disorder. Archives of General Psychiatry, 67(3), 286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Brown T.A., Chorpita B.F., Barlow D.H. (1998). Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology, 107(2), 179–92. [DOI] [PubMed] [Google Scholar]
- Brown T.A.D., Nardo P., Barlow D.H. (1994). Anxiety Disorders Interview Schedule for DSM-IV. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Caouette J.D., Guyer A.E. (2014). Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience, 8, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Maclnnes J.J., Huettel S.A., Adcock R.A. (2009). Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience, 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Oliveri M.E., Insel T. (2014). A neurodevelopmental perspective on the Research Domain Criteria (RDoC) framework. Biological Psychiatry, 76(5), 350–53. [DOI] [PubMed] [Google Scholar]
- Davidson J.R.T., Foa E.B., Huppert J.D., et al. (2004). Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of General Psychiatry, 61(10), 1005–13. [DOI] [PubMed] [Google Scholar]
- Delmonte S., Balsters J.H., McGrath J., et al. (2012). Social and monetary reward processing in autism spectrum disorders. Molecular Autism, 3(1), 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S., Hollon S.D., Dobson K.S., et al. (2006). Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology, 74(4), 658–70. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Lotze M., Wietek B., Amunts K., Enck P., Zilles K. (2006). Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. NeuroImage, 31(3), 1004–14. [DOI] [PubMed] [Google Scholar]
- Eisner L.R., Johnson S.L., Carver C.S. (2009). Positive affect regulation in anxiety disorders. Journal of Anxiety Disorders, 23(5), 645–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E., Hauser T.U., Dayan P., Dolan R.J. (2016). Striatal structure and function predict individual biases in learning to avoid pain. Proceedings of the National Academy of Science U. S. A., 113(17), 4812–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. (2010). Functional Neuroanatomy of Anxiety: A Neural Circuit Perspective. In: Stein M.B., Steckler T., editors. Behavioral Neurobiology of Anxiety and Its Treatment, 2: 251–77, Springer-Verlag Berlin Heidelberg. [DOI] [PubMed]
- Evans K.C., Wright C.I., Wedig M.M., Gold A.L., Pollack M.H., Rauch S.L. (2008). A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression Anxiety, 25(6), 496–505. [DOI] [PubMed] [Google Scholar]
- Feske U., Chambless D.L. (1995). Cognitive-behavioral versus exposure only treatment for social phobia – a metaanalysis. Behavior Therapy, 26(4), 695–720. [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Kalin N.H. (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171(11), 1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Chiu S.F., Breeze J.L., et al. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162(7), 1256–65. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Franklin M.E., Foa E.B. (2000). Anticipated reactions to social events: differences among individuals with generalized social phobia, obsessive compulsive disorder, and nonanxious controls. Cognitive Therapy and Research, 24(6), 731–46. [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. (2009). Neural bases of social anxiety disorder emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66(2), 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., et al. (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience, 26(24), 6399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P.W., Kadosh K.C., Scerif G., Lau J.Y.F. (2015). Social anxiety disorder in adolescence: how developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Developmental Cognitive Neuroscience, 13, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingh C.J., Ipser J., Tromp S.A., et al. (2013). Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Frontiers in Human Neuroscience, 6 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden B.Y., Parikh P.C., Deaner R.O., Platt M.L. (2007). Economic principles motivating social attention in humans. Proceedings of the Royal Society B-Biological Sciences, 274(1619), 1751–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg R.G., Liebowitz M.R., Hope D.A., et al. (1998). Cognitive behavioral group therapy vs phenelzine therapy for social phobia – 12-week outcome. Archives of General Psychiatry, 55(12), 1133–41. [DOI] [PubMed] [Google Scholar]
- Henson R., Penny W. (2005). ANOVAs in SPM. Technical report. Wellcome Department of Imaging Neuroscience, London.
- Hofmann S.G., Asnaani A., Vonk I.J.J., Sawyer A.T., Fang A. (2012). The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy and Research, 36(5), 427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S., Panksepp J. (1999). The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews, 31(1), 6–41. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., et al. (2010). Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–51. [DOI] [PubMed] [Google Scholar]
- Insel T.R. (2014). The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. American Journal of Psychiatry, 171(4), 395–97. [DOI] [PubMed] [Google Scholar]
- Ishihara S. (1990) Ishihara's Tests for Color-Blindness (38 Plate Ed.), Tokyo/Kyoto, Kanehara, Shuppan Co. Ltd. [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–94. [DOI] [PubMed] [Google Scholar]
- Johnson D.P., Penn D.L., Fredrickson B.L., et al. (2011). A pilot study of loving-kindness meditation for the negative symptoms of schizophrenia. Schizophrenia Research, 129(2–3), 137–40. [DOI] [PubMed] [Google Scholar]
- Kashdan T.B. (2004). The neglected relationship between social interaction anxiety and hedonic deficits: differentiation from depressive symptoms. Journal of Anxiety Disorders, 18(5), 719–30. [DOI] [PubMed] [Google Scholar]
- Kashdan T.B. (2007). Social anxiety spectrum and diminished positive experiences: theoretical synthesis and meta-analysis. Clinical Psychology Review, 27(3), 348–65. [DOI] [PubMed] [Google Scholar]
- Kim H., Shimojo S., O'Doherty J.P. (2006). Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. Plos Biology, 4(8), 1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.H. (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Cooper J.C. (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–17. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport, 12(17), 3683–87. [DOI] [PubMed] [Google Scholar]
- Knutson B., Greer S.M. (2008). Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society, B, 363(1511), 3771–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Heinz A. (2015). Probing psychiatric symptoms with the monetary incentive delay task. Biological Psychiatry, 77(5), 418–20. [DOI] [PubMed] [Google Scholar]
- Knutson B., Katovich K., Suri G. (2014). Inferring affect from fMRI data. Trends in Cognitive Science, 18(8), 422–28. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–7. [DOI] [PubMed] [Google Scholar]
- Koob G.F., LeMoal M. (1997). Drug abuse: hedonic homeostatic dysregulation. Science, 278(5335), 52–8. [DOI] [PubMed] [Google Scholar]
- Kuyken W., Hayes R., Barrett B., et al. (2015). Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet, 386(9988), 63–73. [DOI] [PubMed] [Google Scholar]
- Levita L., Hoskin R., Champi S. (2012). Avoidance of harm and anxiety: a role for the nucleus accumbens. NeuroImage, 62(1), 189–98. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. (1987). Social Phobi. Modern Problems of Pharmacopsychiatry, 22, 141–73. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R., Gelenberg A.J., Munjack D. (2005). Venlafaxine extended release vs placebo, and paroxetine in social anxiety disorder. Archives of General Psychiatry, 62(2), 190–98. [DOI] [PubMed] [Google Scholar]
- Makris N., Goldstein J.M., Kennedy D., et al. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research, 83(2–3), 155–71. [DOI] [PubMed] [Google Scholar]
- Maresh E.L., Allen J.P., Coan J.A. (2014). Increased default mode network activity in socially anxious individuals during reward processing. Biology of Mood and Anxiety Disorders, 4, 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.D., Ono T. (2000). Effects of reward anticipation, reward presentation, and spatial parameters on the firing of single neurons recorded in the subiculum and nucleus accumbens of freely moving rats. Behavioural Brain Research, 116(1), 23–38. [DOI] [PubMed] [Google Scholar]
- Mathew S.J., Coplan J.D., Gorman J.M. (2001). Neurobiological mechanisms of social anxiety disorder. American Journal of Psychiatry, 158(10), 1558–67. [DOI] [PubMed] [Google Scholar]
- Mccullough L.D., Sokolowski J.D., Salamone J.D. (1993). A neurochemical and behavioral investigation of the involvement of nucleus-accumbens dopamine in instrumental avoidance. Neuroscience, 52(4), 919–25. [DOI] [PubMed] [Google Scholar]
- Melendez R.I., Rodd-Henricks Z.A., Engleman E.A., Li T.K., McBride W.J., Murphy J.M. (2002). Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcoholism-Clinical and Experimental Research, 26(3), 318–25. [PubMed] [Google Scholar]
- Mirenowicz J., Schultz W. (1996). Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature, 379(6564), 449–51. [DOI] [PubMed] [Google Scholar]
- Mogenson G.J., Jones D.L., Yim C.Y. (1980). From motivation to action – functional interface between the limbic system and the motor system. Progress in Neurobiology, 14(2–3), 69–97. [DOI] [PubMed] [Google Scholar]
- Morrison A.S., Heimberg R.G. (2013). Attentional control mediates the effect of social anxiety on positive affect. Journal of Anxiety Disorders, 27(1), 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson E.B., Gentry R.N., Chioma V.C., Cheer J.F. (2012). Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. Journal of Neuroscience, 32(42), 14804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T.E. (2007). Python for scientific computing. Computing in Science & Engineering, 9(3), 10–20. [Google Scholar]
- Patel K.T., Stevens M.C., Meda S.A., et al. (2013). Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biological Psychiatry, 74(7), 529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J., Sandrock C., Miltner W.H., Straube T. (2016). Look who's judging-feedback source modulates brain activation to performance feedback in social anxiety. NeuroImage, 133, 430–37. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry, 59(5), 424–29. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L., Krach S., Kohls G., Irmak A., Grunder G., Spreckelmeyer K.N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage, 49(4), 3276–85. [DOI] [PubMed] [Google Scholar]
- Reis F.L.V., Masson S., de Oliveira A.R., Brandao M.L. (2004). Dopaminergic mechanisms in the conditioned and unconditioned fear as assessed by the two-way avoidance and light switch-off tests. Pharmacology Biochemistry and Behavior, 79(2), 359–65. [DOI] [PubMed] [Google Scholar]
- Richey J.A., Rittenberg A., Hughes L., et al. (2014). Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Social Cognitive & Affective Neuroscience, 9(3), 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., King-Casas B., Sanfey A.G. (2008). The neurobiology of social decision-making. Current Opinion in Neurobiology, 18(2), 159–65. [DOI] [PubMed] [Google Scholar]
- Roesch M.R., Singh T., Brown P.L., Mullins S.E., Schoenbaum G. (2009). Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. Journal of Neuroscience, 29(42), 13365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff C.C., Fehr E. (2014). The neurobiology of rewards and values in social decision making. Nature Reviews in Neuroscience, 15(8), 549–62. [DOI] [PubMed] [Google Scholar]
- Saddoris M.P., Sugam J.A., Stuber G.D., Witten I.B., Deisseroth K., Carelli R.M. (2015). Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biological Psychiatry, 77(10), 903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K., Ueda Y., Doya K., Kimura M. (2005). Representation of action-specific reward values in the striatum. Science, 310(5752), 1337–40. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Mohr A., Miltner W.H.R., Straube T. (2010). Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biological Psychology, 84(2), 304–12. [DOI] [PubMed] [Google Scholar]
- Schneider F., Weiss U., Kessler C., et al. (1999). Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biological Psychiatry, 45(7), 863–71. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Abi-Dargham A., Martinez D., et al. (2009). Dopamine transporters, D-2 receptors, and dopamine release in generalized social anxiety disorder. Depression Anxiety, 26(5), 411–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier F.R., Liebowitz M.R., Abi-Dargham A., Zea-Ponce Y., Lin S.H., Laruelle M. (2000). Low dopamine D-2 receptor binding potential in social phobia. American Journal of Psychiatry, 157(3), 457–59. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Martinez D., Abi-Dargham A., et al. (2008). Striatal dopamine D-2 receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depression Anxiety, 25(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Schultz W. (1998). Predictive reward signal of dopamine neurons (vol. 80, pg 1, 1998). Journal of Neurophysiology, 80(6), U32.. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1(3), 199–207. [DOI] [PubMed] [Google Scholar]
- Shah S.G., Klumpp H., Angstadt M., Nathan P.J., Phan K.L. (2009). Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. Journal of Psychiatry & Neuroscience, 34(4), 296–302. [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., et al. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C., Angstadt M., Liberzon I., McCabe K., Phan K.L. (2013). Aberrant reward center response to partner reputation during a social exchange game in generalized social phobia. Depression Anxiety, 30(4), 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Fyer A.J., Davidson J.R.T., Pollack M.H., Wiita B. (1999). Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. American Journal of Psychiatry, 156(5), 756–60. [DOI] [PubMed] [Google Scholar]
- Straube T., Kolassa I.T., Glauer M., Mentzel H.J., Miltner W.H. (2004). Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry, 56(12), 921–30. [DOI] [PubMed] [Google Scholar]
- Tiihonen J., Kuikka J., Bergstrom K., Lepola U., Koponen H., Leinonen E. (1997). Dopamine reuptake site densities in patients with social phobia. American Journal of Psychiatry, 154(2), 239–42. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Damsteegt R., Achterberg E.J.M., Vanderschuren L.J.M.J. (2011). Nucleus accumbens mu-opioid receptors mediate social reward. Journal of Neuroscience, 31(17), 6362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk C.L., Heimberg R.G., Luterek J.A., Mennin D.S., Fresco D.M. (2005). Emotion dysregulation in generalized anxiety disorder: a comparison with social anxiety disorder. Cognitive Therapy & Research, 29(1), 89–106. [Google Scholar]
- Ungless M.A., Magill P.J., Bolam J.P. (2004). Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science, 303(5666), 2040–42. [DOI] [PubMed] [Google Scholar]
- van der Walt S., Colbert S.C., Varoquaux G. (2011). The NumPy array: a structure for efficient numerical computation. Computing in Science & Engineering, 13(2), 22–30. [Google Scholar]
- Wechsler D. (2011). Wechsler Abbreviated Scale of Intelligence (WASI) – II. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Weeks J.W. (2015). Replication and extension of a hierarchical model of social anxiety and depression: fear of positive evaluation as a key unique factor in social anxiety. Cognitive Behaviour Therapy, 44(2), 103–16. [DOI] [PubMed] [Google Scholar]
- Winston J.S., O'Doherty J., Kilner J.M., Perrett D.I., Dolan R.J. (2007). Brain systems for assessing facial attractiveness. Neuropsychologia, 45(1), 195–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.