Abstract
Next to social problems, individuals with autism spectrum disorder (ASD) often report severe sensory difficulties. Altered processing of touch is however a stronger mediator of social symptoms’ severity than altered processing of for instance vision or audition. Why is this the case? We reasoned that sensory difficulties may be linked to social problems in ASD through insufficient self-other distinction centred on touch. We investigated by means of EEG whether the brain of adults with ASD adequately signals when a tactile consequence of an observed action does not match own touch, as compared to the brain of matched controls. We employed the action-based somatosensory congruency paradigm. Participants observed a human or wooden hand touching a surface, combined with a tap-like tactile sensation that either matched or mismatched the tactile consequence of the observed movement. The ASD group showed a diminished congruency effect for human hands only in the P3-complex, suggesting difficulties with signalling observed action-based touch of others that does not match own touch experiences. Crucially, this effect reliably correlated with self-reported social and sensory everyday difficulties in ASD. The findings might denote a novel theoretical link between sensory and social impairments in the autism spectrum.
Keywords: Autism spectrum disorder, high functioning autism, touch processing, self-other distinction, P3, sensory evoked potential
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by, amongst others, persistent difficulties in social communication and social interaction (DSM-5; American Psychiatric Association, 2013). This is often reflected in a reduced knowledge of others’ state of mind (Baron-Cohen et al., 1985) and an egocentric bias within the normal reciprocity of social interactions (American Psychiatric Association, 2013). Apart from social impairments, people with ASD often show sensory abnormalities in their daily life. For instance, feelings of sensory overload and other hypersensitivities are repeatedly reported (for reviews see Donohue et al., 2012; Iarocci and McDonald, 2006; Marco et al., 2011) and sensory problems have recently been formally included in the diagnostic criteria of ASD in the DSM-5 (American Psychiatric Association, 2013). For this reason, an increasing number of researchers consider compromised sensory processing an essential aspect of ASD, and have tried to link it conceptually to social impairments (Pellicano and Burr, 2012; van Boxtel and Lu, 2013; Van De Cruys et al., 2014). More specifically, it was suggested that social difficulties in ASD might merely result from the sensory complexity inherent to social situations, leading to attempts to avoid these overstimulating situations (Simmons et al., 2009; Van De Cruys et al., 2014).
Importantly, altered processing of tactile information is more strongly related to social symptoms’ severity than altered processing of distal senses such as vision and audition (Hilton et al., 2010; Lundqvist, 2015). But why is this the case? Why does touch seem to be so strongly related to social symptoms of ASD? We think that this may have to do with the important role of tactile information when distinguishing between self and other (Deschrijver et al., 2015). Recently, it has been argued that self-other distinction plays a crucial role in social cognition because one crucial requirement of almost all social abilities is to distinguish between self and other (Brass et al., 2009; Paladino et al., 2010; Spengler et al., 2010; Spengler et al., 2009). Self-other distinction based on distal senses, however, proves quite difficult. What I see when I am moving my hand for instance, is relatively similar to what I see when someone else is moving his/her hand. This is however not the case for the sense of touch: when I feel touch when moving my hand (e.g. when grasping a glass), this tactile experience is completely different to what I feel when another person is performing the same movement, as only in the former case my skin is involved.
This is noteworthy, because research has shown that we do involve our own touch-processing systems to simulate tactile experiences of others (Keysers et al., 2004; Gazzola and Keysers, 2009; Keysers et al., 2010). When we observe an action, we simulate the tactile consequences of this action in our somatosensory cortices (next: action-based touch simulation; Gazzola and Keysers, 2009; Keysers et al., 2010). This suggests that while interacting with others, we are ‘feeling’ what others feel while they are acting (Keysers et al., 2004; Gazzola and Keysers, 2009; Keysers et al., 2010). However, as outlined earlier, the experience arising from these simulated touch representations of others does not quite resemble feeling own touch. Moreover, the simulated touch mostly does not readily correspond to one’s experience of own touch at any given time. One could consequently reason that detecting the mismatch between felt touch and simulated other-related touch could be useful to distinguish self from other when one is observing action-based touch: If the visual movement that I am seeing is produced by me, I should feel corresponding tactile feedback. If I detect own touch that is incongruent, instead, someone else must have produced the movement. Interestingly, previous research has shown already that comparing simulated touch to felt touch crucially contributes to the neural representation of the self as similar or distinct of its environment (Tsakiris and Fotopoulou, 2008; Paladino et al., 2010; Tsakiris, 2010; Ionta et al., 2011; Blanke, 2012; Deschrijver et al., 2015 ). The sense of touch might thus be of paramount importance for understanding that tactile consequences of an observed hand action are other-related, more so than distal senses such as vision and audition. Impaired ability to signal simulated touch of others that does not match own touch, in contrast, might result in severe impairments in both the representation of self and others (Lombardo and Baron-Cohen, 2010, 2011). In sum, we reasoned that insights into the neural interplay of observed touch and felt touch in individuals with ASD may prove crucial for our understanding of both social and sensory aspects of the disorder.
Recently we developed and validated a paradigm to investigate the interaction of felt touch and observed action-based touch (the action-based somatosensory congruency paradigm) and tested it in neurotypical adults using electroencephalography (EEG) measures (Deschrijver et al., 2015). In this paradigm, picture sequences of human and wooden hands touching a surface with the index or middle finger are presented, while simultaneously applying a ‘tap-like’ tactile stimulation to the corresponding or non-corresponding finger of the participant. Using EEG, we observed effects at early low-level stages of somatosensory processing (sensory evoked potentials; SEPs P50, N100 and N140, see also Deschrijver et al., 2015; Popovich and Staines, 2015) as well as at high-level stages of processing (P3-complex comprising an early centro-parietal P3-component and a later more posterior parietal P3 component, e.g. Verleger et al., 2005). Previous research in the field of social neuroscience has associated the P3-component with self versus other-related processes (for a review, see Knyazev, 2013). Studies have for instance reported amplified P3 components for hearing one’s own name or seeing one’s own face (as compared to hearing/seeing another (familiar) person’s name/face (Perrin et al., 2005; Holeckova et al., 2006; Tacikowski and Nowicka, 2010; Tacikowski et al., 2011; Cygan et al., 2014; Tacikowski et al., 2014 ), for touch experienced by another body that is congruent to own touch (Longo et al., 2012; Deschrijver et al., 2015), and for observed actions that are compatible to own (intended) actions (de la Asuncion et al., 2015; Ruissen and de Bruijn, 2015). We therefore reasoned in our earlier study that a P3-effect in the current paradigm might reflect self-other distinction centred on touch processes (Deschrijver et al., 2015). The findings revealed that participants process a tactile stimulus differently depending on whether the sensation matches or mismatches an observed human hand’s finger tap. As predicted, we observed a congruency effect for human hands only in the P3, suggesting that the neurotypical human brain signals simulated action-based touch that does not match felt touch at high-levels of processing.
In the current work, we aimed to evaluate this process in a group of adults with high functioning autism (HFA), while comparing their EEG responses to those of a neurotypical control group (CON). We hypothesized that individuals with ASD might experience difficulties in signalling human action-based touch that does not match own felt touch, indicated by a reduced P3 effect, as a reflection of deficient self-other distinction based on touch processes. Such a finding would suggest that representations of the tactile consequences of an observed other’s actions may not be identified as other-related in the brain of individuals with ASD. Furthermore, we hypothesized that such a problem might be rooted in sensory difficulties related to the spectrum and, in turn, may be associated with social difficulties by affecting how individuals with ASD represent one’s self as well as others. We therefore additionally hypothesized that the P3 effect might correlate with self-report measures of social autistic difficulties and of sensory hypersensitivity/avoidance at an individual level in the HFA group. Such findings would help to understand the relation of sensory and social difficulties in the autism spectrum.
Methods and materials
Subjects
We recruited 23 adults with HFA by means of our own research database and a recruiting announcement distributed by the Flemish Autism Association. Each participant was then matched with a neurotypical control participant (CON) on demographic measures of age (±5 years), intelligence (±20 total IQ-score points), gender and handedness (as measured by the Edinburgh Handedness Inventory; Oldfield, 1971). Participants in the CON group were screened on several exclusion criteria prior to participation (neurological, psychiatric, sensory or motoric problems and the use of psychiatric medication). All participants with HFA had received a formal diagnosis of ASD (including autism, Asperger’s syndrome and PDD-NOS) from an independent clinician or multidisciplinary team and were free of any additional neurological disorder. They completed the Autism Diagnostic Observational Schedule (ADOS; Lord et al., 2000) Module 4 with a trained researcher. The local Ghent University ethics committee approved the study. All participants were financially compensated for their participation and gave written informed consent before participation.
Similar to previous studies on HFA (Magnée et al., 2008; Zwickel et al., 2011), we included in our data analyses HFA participants who scored above or one point below cut-off on one subscale of the ADOS and attained an ADOS score of minimum 6. It is not uncommon for individuals with a diagnosis of ASD that are high-functioning to score just below ADOS cut-off criteria (Magnée et al., 2008; Zwickel et al., 2011). As such, the data of 19 pairs of participants were included in our analyses (with 14 HFA participants meeting full ADOS criteria). Two participants from the CON group were additionally excluded from analyses, one because artefact rejections retained less than 10% of one individual’s data and one because of displaying a P3-complex that strongly differed from all other participants in terms of morphology and topography. The remaining participant groups (CON: n = 17; HFA: n = 19), were well matched for gender (CON: 11 males; HFA: 13 males), handedness (right-handed CON: 16 persons; right-handed HFA: 17 persons), age (CON: M = 31.35, SD = 6.63; HFA: M = 32.95 years, SD = 6.26), and full-scale IQ score (CON: M = 118.76, SD = 14.16; HFA: M = 110.95, SD = 14.64). Finally, all participants filled out self-report questionnaire forms: the Edinburgh Handedness Inventory (Oldfield, 1971), the Autism Spectrum Quotient (AQ; Baron-Cohen et al., 2001), the Social Responsiveness Scale—Adult version (SRS-A; Bölte et al., 2008), and the Sensory Profile-NL (SP; Dunn and Westman, 1997). While the AQ is a self-report measure of general autistic traits, the SRS-A specifically measures social difficulties characteristic of the autism spectrum. The SP, on the other hand, focuses on sensory processing peculiarities within daily life situations, and yields four quadrant scores (Sensation Hypersensitivity, Sensation Avoidance, Low Registration and Sensation Seeking). Due to missing data, the SRS-A questionnaire data of three individuals (one from the HFA group and two from the CON group), the AQ questionnaire data of two participants with HFA and the Sensory Profile-NL data of one individual with HFA could not be included. T-tests confirmed that no significant demographic differences existed between groups. Individuals in the HFA group scored well above ADOS and autism cut-offs of the AQ on average (Lord et al., 2000; Baron-Cohen et al., 2001). As one could expect, t-tests showed highly significant differences between the mean total dimensional scores on the SRS-A and on the AQ questionnaires. Participant characteristics and statistics are summarized in Table 1.
Table 1.
Participant details
| HFA | CON | P-value | |
|---|---|---|---|
| Male participants, n | 13.00 | 11.00 | 0.81 |
| Right-handed participants, n | 17.00 | 16.00 | 0.61 |
| Mean age, years (SD) | 32.95 (6.26) | 31.35 (6.63) | 0.46 |
| Mean full‐scale IQ (SD) | 110.95 (14.64) | 118.76 (14.16) | 0.11 |
| Mean ADOS communication (SD) | 2.58 (1.07) | N.A. | N.A. |
| Mean ADOS reciprocal social interaction (SD) | 6.16 (2.17) | N.A. | N.A. |
| Mean total score AQ (SD) | 32.11 (8.44) | 11.18 (3.92) | 0.00*** |
| Mean total score SRS-A (SD) | 159.33 (35.02) | 92.67 (14.40) | 0.00*** |
| SP-NL, Hypersensitivity quadrant mean score (SD) | 42.70 (11.89) | 33.41 (8.41) | 0.01** |
| SP-NL, Avoidance quadrant mean score (SD) | 44.10 (10.78) | 31.71 (6.32) | 0.00*** |
Standard deviances are noted between brackets where applicable. T‐tests or Chi-Square tests were used whenever appropriate.
Procedure
For both groups, the EEG-experiment was the first in a larger battery of tasks (not presented here), which took place in two experimental sessions (with approximately 3 weeks time in between). Both sessions took place in a dimly lit and sound-attenuated room. In the first session, the EEG-data were gathered, with this study being the first of two EEG-studies. In the second session, each participant completed two behavioural tasks (not reported here) and demographic data were gathered. If no recent standardized cognitive assessment was performed (within 5 years prior to participation), we derived the participants’ status as ‘high functioning’ from an IQ-score estimation using the KAUFMAN 2 short form Wechsler Adult Intelligence Scale III (full scale IQ ≥ 85; see Minshew et al., 2005 for the use in ASD). After this, participants belonging to the HFA group received the ADOS-Module 4 (Lord et al., 2000), administered by a formally trained researcher.
Stimuli and task
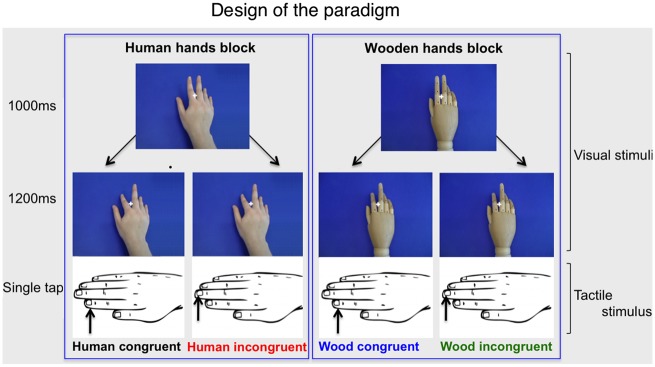
All materials and the design were identical to those described in our earlier study with the action-based congruency paradigm (Deschrijver et al., 2015; Figure 1). All visual stimuli were 640 × 380 pixels large and were centrally presented at approximately 60 cm distance from the participant on a 17 inch monitor. We placed the participant’s right hand in a natural palm-down position on the table surface. A custom made tactile stimulator (Dancer Design; www.dancerdesign.co.uk) with two independently controlled piezo-electric electrodes delivered supra-threshold ‘single tap’ stimuli to about 1 cm2 of the tip of the participant’s index and middle fingers of this hand, consistent with the location of the observed action-based touch. The fingers were loosely fixed to the electrodes with tape and then covered with a dark cloth, preventing the participant from seeing his hand. Within reach of the left hand, a keyboard was situated. Before the start of the experiment was announced, we applied 30 tactile sensations to the index finger and an equal amount to the middle finger of the participant, which were randomly intermixed and identical to the ones used in the experiment. We used an interstimulus interval of 700 ms, while continuously showing a white fixation cross in the middle of a black screen. Data acquisition and stimulus delivery were accomplished via the programme Presentation (Neurobs), ran on a HP Compaq desktop with Windows XP driver.
Fig. 1.
Design of the paradigm.
In the experiment, each trial started with showing a hand in a neutral posture from a first-person perspective, corresponding to the participant’s own right hand (1000 ms). We then presented an index or middle finger in a tapping position (1200 ms) and ended with a black screen (700 ms). A ‘single tap’ tactile stimulation to the index or middle finger of the participant was synchronized with the onset of the tapping position frame. To achieve this, an audio file containing a single sawtooth waveform drove the piezzo-element of the tactile stimulator. In congruent trials, the observed hand executed a finger movement that would naturally lead to the tactile sensation at the corresponding finger of the participant, when executed by one’s self. In incongruent trials, the tactile sensation was incompatible with the observed hand movement. For human hand stimuli, we used the right hand of a Caucasian female, whereas a right mannequin hand was used for wooden hand stimuli. A central fixation mark (‘+’ sign) was continuously presented during trials. In 10% of the trials of each condition, the fixation mark changed colours to red, designating the trial to be a target. Participants were instructed to count the number of red fixation crosses they had seen. During the breaks, the participant was required to give in this count with his left hand via the number keys on the keyboard. After this, accuracy feedback was offered.
The design consisted of two within-subjects factors and one between-subjects factor: Animacy (human vs wood), Congruency [congruent (C) vs incongruent (I)] and Group (CON vs HFA), respectively. The within-subjects factors led to four experimental conditions: human congruent, human incongruent, wood congruent and wood incongruent. We presented human and wooden hand trials in separate blocks, of which the order was counterbalanced across participants. Within each animacy block, we randomly presented 200 congruent and 200 incongruent trials, leading to 800 trials in total. Index finger and middle finger movements were equiprobable per condition. After every 40 trials, self-paced breaks occurred. The experiment proper lasted about 50 min.
EEG recording and analyses
EEG was recorded with a Biosemi Activetwo amplifier at a sampling rate of 1024 Hz from 64 active electrodes, placed according to the international 10/20 setting. For offline re-referencing, two electrodes were placed on the mastoids. To measure horizontal eye movements, bipolar electrodes with left and right cantal montage were placed, whereas electrodes above and below the left eye served to measure eye blinks. We held electrode offsets between −25 and 25 µV at each electrode.
The EEG data were analysed with BrainVision Analyzer 2 (BVA; Brain Products). The average of the left and right mastoid was used as a reference for the raw data. A high pass filter of 0.1 Hz, a low pass filter of 30 Hz and a notch filter of 50 Hz were performed. Epochs were defined from −100 ms to 400 ms around the onset of the tactile stimulation. The ERPs were automatically corrected for eye movement artefacts. Additionally, we applied an automatic artefact rejection including a minimum/maximum amplitude check (−100 µV and 100 µV, respectively), a gradient check (maximum allowed voltage step: 50 µV/ms within 200 ms before and after the locked event), a low activity check (0.5 µV within an interval length of 100 ms) and a baseline correction. Because we were interested in congruency-related processes, the data were collapsed over left and right finger movements observations. Target trials were not considered for analyses.
All statistical analyses were conducted with SPSS Statistics 22 on exported mean area amplitudes. We used time frames and electrode positions of interest identical to those reported in our earlier study (Deschrijver et al., 2015): 45–55 ms at electrode sites AF3, AFz, F3 and F1 for the P50 component; 85–100 ms at identical electrode sites for the N100 component; 105–120 ms at electrode sites FC3, FC5, C3 and C5 for the N140 component; 230–270 ms and 310–360 ms at electrodes Cz, CPz and Pz for the early and late P3 component, respectively. For each ERP-component of interest, we pooled the data over the relevant electrode positions and performed a 2 × 2 × 2 mixed-model ANOVA (with the within-subjects factors Animacy and Congruency and a between-subjects factor Group). Where needed, Greenhouse-Geisser-corrected analyses were reported. All EEG-data of the CON group (but none of the HFA group) were included as a part of our first manuscript on the action-based sensory congruency paradigm (Deschrijver et al., 2015).
Results
Behavioural results
For the CON group, a correct counting response was given on 91.11% of break questions on average (SD = 8.15%), whereas this was the case for 89.47% of the break questions in the HFA group (SD = 14.03%). A Chi-Square test on these data yielded no group difference (Χ2 = 0.48, P = 0.49). This suggests a similar task-involvement for both groups.
EEG results
SEPs
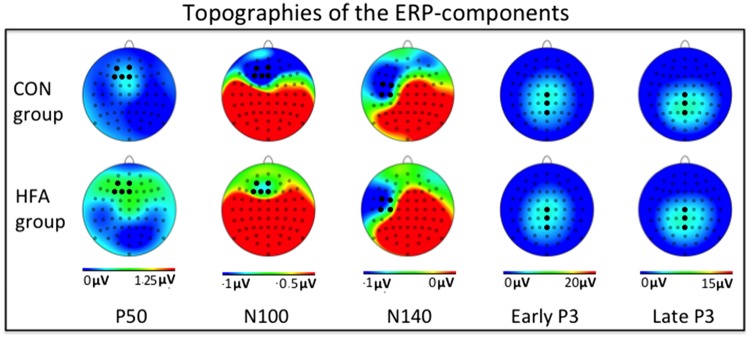
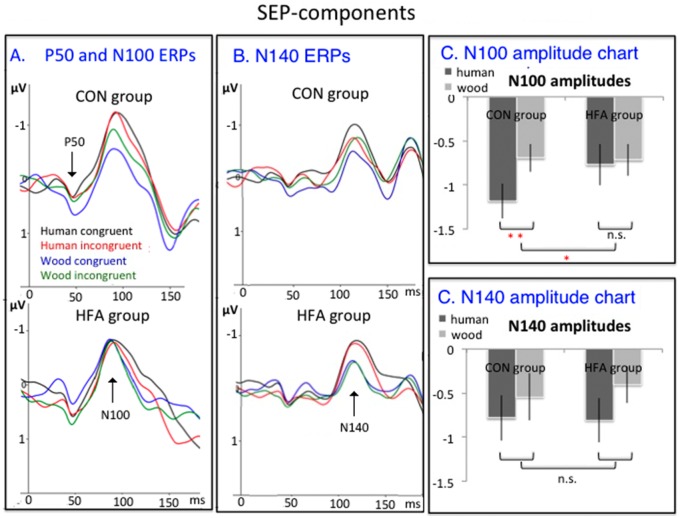
P50. Individuals in the CON group showed numerically larger P50 amplitudes in wooden congruent trials as compared to wooden incongruent trials, with no congruency difference in human hand trials. For individuals with HFA, the congruency effect for wooden hand trials numerically reversed (Figures 2 and 3A). However, the ANOVA showed only a marginally significant three-way interaction of Animacy, Congruency and Group [F(1,34) = 3.66, P = 0.06]. Because we did not have a priori hypotheses about a P50 group difference, we did not investigate this marginally significant effect any further.
Fig. 2.
Topography maps of the ERP effects. Electrodes of interest are highlighted in black.
Fig. 3.
P50, N100 and N140 components. ERP-waves, pooled per group and per condition over relevant electrodes. (A) P50 and N100 ERPs (upper: CON; lower: HFA). (B) N140 ERPs (upper: CON; lower: HFA). (C) N100 amplitude charts (error bars denote standard errors; ** p < 0.01; ** p < 0.05; n.s. non significant). (D) N140 amplitude charts (error bars denote standard errors; n.s. non significant).
N100. The ANOVA on N100 amplitudes yielded a significant main effect of Animacy [F(1,34) = 8.50, P < 0.01; Figures 2 and 3AC] and an Animacy × Group interaction effect [F(1,34) = 5.40, P < 0.05). Control participants showed larger N100 amplitudes for human hand trials as compared to wooden hand trials [t(16) = −3.81, P < 0.01, animacy effect M = 0.26 µV], whereas this animacy difference was not present in the HFA group [t(18) = −0.41, P = 0.69; animacy effect: M = 0.04 µV]. No other effects approached significance (all P’s > 0.10).
N140. The ANOVA identified a reliable main effect of Animacy in the N140 [F(1,34) = 10.12, P < 0.005; Figures 2 and 3BD]. The interaction effect of Animacy and Group was far from significant [F(1,34) = 0.26, P = 0.61]. This suggests that both groups showed a larger N140 for human than for wooden hand stimuli (pooled animacy effect for CON: M = 0.82 µV; for HFA: M = 0.50 µV).
P3
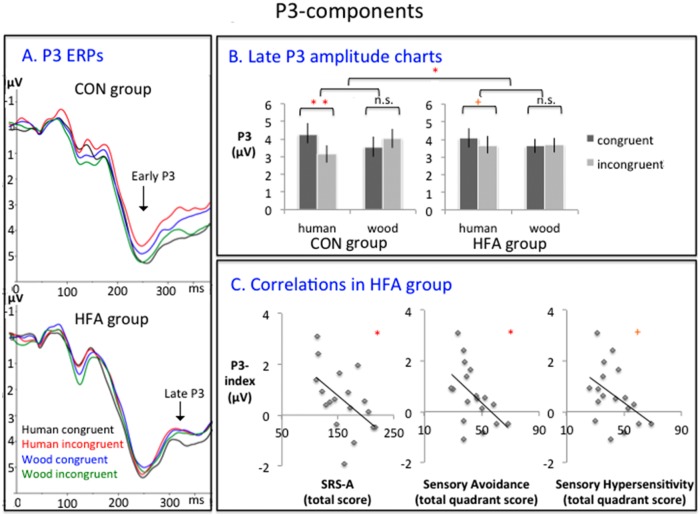
Early P3. In the early P3, the ANOVA yielded a strong interaction effect of Animacy and Congruency [F(1,34) = 9.40, P < 0.005; Figures 2 and 4A]. A marginally significant interaction effect of Animacy, Congruency and Group was also present [F(1,34) = 3.19, P = 0.083]. Given our a priori hypotheses regarding the P3-effects, we tentatively investigated this effect further. The CON group showed a highly significant difference between congruent and incongruent human hand trials [t(16) = 4.48, P < 0.001; mean difference M = 0.71 µV] whereas this difference was not significant for the HFA group [t(18) = 0.85, P = 0.40; mean difference M = 0.16 µV]. Neither of the groups showed a significant congruency difference in the wooden hand trials [for CON: t(16) = −1.04, P = 0.31, mean difference M = 0.34 µV; for HFA: t(18) = −0.69, P = 0.50, mean difference M = 0.12 µV).
Fig. 4.
P3 components. (A) P3 ERPs, pooled per group and per condition over electrodes Cz, CPz, Pz (upper: CON; lower: HFA). (B) late P3 amplitude charts. (left: CON; right: HFA; error bars denote standard errors; ** p < 0.01; * p < 0.05; +p < 0.10). (C) Correlations of the P3-index in the HFA group with respectively the SRS-A (left), the Sensory Avoidance subscale of the Sensory Profile-NL (middle) and the Sensory Hypersensitivity subscale of the Sensory Profile-NL (right) (regression lines are noted; * p < 0.05; +p < 0.10).
Late P3. In the late P3-component, the ANOVA revealed an almost significant main effect of Congruency [F(1,34) = 4.06, P = 0.05; Figures 2 and 4AB] and a strong continuation of the earlier described Congruency × Animacy interaction effect [F(1,34) = 16.73, P < 0.001; Figure 3A and B]. Importantly, we also detected a three-way interaction of Animacy, Congruency and Group [F(1,34) = 4.45, P < 0.05]. Again, paired comparisons showed that the CON group distinguished clearly between congruent and incongruent human hand trials [t(16) = 4.01, P = 0.001; mean congruency difference M = 1.13 µV], while this effect was smaller and only marginally significant in the HFA group [t(18) = 1.8, P = 0.09; mean congruency difference: M = 0.44µ]. Neither of the groups showed a congruency effect in wooden hand trials: t(16) = −1.73, P = 0.10 for CON and t(18) = −0.38, P = 0.71 for HFA.
Correlation results
Given our strong hypothesis that the P3 effect in the HFA group would show relationships with self-reported measures of social autistic difficulties and sensory avoidance/hypersensitivity, we computed for each individual in the HFA group an index of this effect [P3-index = (human congruent – human incongruent) − (wood congruent – wood incongruent)]. The P3-index and in the HFA group showed a negative relationship with the Sensation Avoidance quadrant of the SP-NL (r = −0.51, P < 0.05), a marginally significant negative relationship with the Sensation Sensitivity quadrant (r = −0.44, P = 0.07). While sensory processing theories of ASD have mainly focused on explaining sensory hypersensitivity and avoidance behaviours (Van De Cruys et al., 2014), it might not surprise that the other two quadrants of the SP-NL yielded relatively high though insignificant correlations (for Low Registration: r = −0.32, P = 0.20; for Sensation Seeking: r = 0.42, P = 0.08). As such, we focused on the relationships with the Sensations Sensitivity and Sensation Avoidance quadrants (Figure 4C). In addition, we observed a significant relationship between the P3-index and the SRS-A (r = −0.53, P < 0.05; Figure 4C). The SRS-A scale and the Sensory Sensitivity/Avoidance quadrants of the SP-NL correlated highly positively (r = 0.74, P = 0.001 and r = 0.68, P < 0.005, respectively). These results suggest that a reduced P3 in HFA is related to sensory and social difficulties in the daily life of individuals with HFA. While such correlations should also be expected in the normal population overall, the limited range of autism scores limits the possibility that such relationships would be detectable in the current CON group. Additional correlational analyses in the CON group were therefore not conducted.
Discussion
In the current study, we explored how adults with HFA use the sense of touch to process simulated action-based touch of others. More specifically, by means of the action-based somatosensory congruency paradigm (Deschrijver et al., 2015) and EEG, we examined neuronal comparison processes of action-based touch observation and own touch. The HFA group showed altered neural processing of the stimuli at early stages of own somatosensory processing (N100 SEP effects) and at late stages of more high-level processing (reflected in the P3-complex). Crucially, as predicted, a congruency effect for human hands within the amplitudes of the late P3 component was present for the control group, but diminished for the HFA group. In addition, this effect showed negative correlations with both social and sensory difficulties experienced by the HFA group in everyday life.
Biological attention differences in early somatosensory processing: SEP-results
The SEP data of the current study suggested that adults with HFA show an altered interplay of observed action-based touch and felt touch already at an early low-level stage of processing, as compared to neurotypical controls (Allison et al., 1992; Hilton et al., 2010; Lundqvist, 2015). Earlier studies related facilitation of the SEPs around 100 ms to attentional demands in somatosensory processes in the primary somatosensory cortex (Allison et al., 1992; Eimer and Driver, 2000; Eimer and Forster, 2003; Schubert et al., 2006; Schubert et al., 2008; Deschrijver et al., 2015; Popovich and Staines, 2015), whereas N140 SEP amplitudes are usually modulated by processes in the secondary somatosensory cortex that are considered as independent of attention processes (Allison et al., 1992; Popovich and Staines, 2015). The lacking animacy effect in the N100 may thus suggest a failure to direct attention to human hands (Popovich and Staines, 2015), while the attention-independent somatosensory animacy processing in the N140 was well preserved. This interpretation fits well within current views on ASD that highlight difficulties to direct attention to biological stimuli (Annaz et al., 2012; Chevallier et al., 2012; Jones and Klin, 2013). Our HFA group seemed able to compensate for this within somewhat later somatosensory processes (N140).
Compromised higher-order self-related processes: P3 results
In social neuroscience research, modulation of the P3 component has been related to self versus other-related processes (for a review, see Knyazev, 2013). Studies have for instance reported amplified P3 components for hearing one’s own name or seeing one’s own face as compared to hearing/seeing another person’s name/face (Perrin et al., 2005; Holeckova et al., 2006; Tacikowski and Nowicka, 2010; Tacikowski et al., 2011; Cygan et al., 2014; Tacikowski et al., 2014), for touch experienced by another body that is congruent to own touch (Longo et al., 2012; Deschrijver et al., 2015), and for observed actions that are compatible with own (intended) actions (de la Asuncion et al., 2015; Ruissen and de Bruijn, 2015). In the current study, we found that adults with HFA show deficits in signalling simulated action-based touch that does not match felt own touch, as reflected in a reduced P3. If a modulation of the P3-component within this task indeed reflects self-other distinction processes, the current P3 findings may suggest that ASD is associated with difficulties in distinguishing self from other based on touch processes. Indeed, when observed action-based touch that does not match own touch is not adequately signalled in the brain, it might become difficult for the brain to determine when observed (tactile consequences of) actions are not one’s own. In the social world, individuals with HFA might thus not only experience a distorted sense of self, but also a inaccurate sense of others (Lombardo et al., 2010; Paladino et al., 2010; Lombardo and Baron-Cohen, 2011). While the ability to distinguish between self and others is extremely crucial for social understanding (Brass et al., 2009; Spengler et al., 2009; Paladino et al., 2010; Spengler et al., 2010), it might not surprise that the late P3-effect reliably correlated with social impairments in the HFA group (see also further).
Our findings link two lines of research that have recently attested self-other distinction deficits in ASD. First, studies have demonstrated that effects of the rubber hand illusion (Tsakiris and Haggard, 2005) vary subtly along the non-clinical and clinical autism spectrum (Cascio et al., 2012; Paton et al., 2012; Palmer et al., 2013). These findings hint towards compromised self-other distinction mechanisms centred on passive touch processes. In addition, the current results relate to studies that suggest difficulties of individuals with HFA in distinguishing the self from others on a motor level (Brass et al., 2009; Spengler et al., 2010). In sum, the neuronal representation of the self as similar or distinct of others seems to rely heavily on higher-order comparison processes of own and simulated sensorimotor information, which may be compromised in ASD (Brass et al., 2009; Spengler et al., 2009; Cascio et al., 2012; Paton et al., 2012; Palmer et al., 2013; Deschrijver et al., 2015; Palmer et al., 2015; yet see also Gowen et al., 2008; Grecucci et al., 2013; Press et al., 2010; Sowden et al., 2016).
Exploring the relationship between the P3 effect and sensory difficulties in ASD
More and more researchers suggest that the cognitive system of individuals with ASD is hypersensitive for incoming sensory information (Pellicano and Burr, 2012; Palmer et al., 2013; van Boxtel and Lu, 2013; Van De Cruys et al., 2014; Palmer et al., 2015), while social difficulties of individuals might arise as a consequence of this (Van De Cruys et al., 2014). Confirming our last more exploratory hypothesis, we showed that sensory and social difficulties of individuals with HFA both showed an inverse relationship with the strength of the late P3-effect. This suggests that the individuals in the HFA group who reported the most severe hypersensitivity/avoidance and social difficulties, showed the weakest interaction effect in the late P3 (suggesting more severely compromised self-other distinction abilities). While experimental research is needed to explore the causal directionality within the observed relationships, we can for now only speculate about their functional meaning. However, it is interesting to note that P3 components have been associated to sensory unexpectedness (Escera et al., 2003; Friedman et al., 2001). Therefore, we think that the uncertainty accompanying simulated touch information might interfere with the ability to adequately distinguish simulated from own somatosensory information, which may consequently relate to social difficulties. Interestingly, some authors have argued that self-other distinction abilities (based on motor representations) may underlie crucial social-cognitive mentalizing abilities in typically developing individuals as well as in individuals with ASD (Spengler et al., 2009, 2010). In the same respect, one might tentatively reason that deficient self-other distinction centred on action-based touch in individuals with ASD may be related to mentalizing abilities, which are known to be affected in at least some individuals with ASD (Baron-Cohen et al., 1985; Deschrijver et al., 2016). As such, compromised self-other distinction abilities centred on touch might denote a crucial theoretical link between sensory and social impairments in the autism spectrum.
Conclusions
In the current study, we investigated whether adults with HFA show difficulties in matching experienced and simulated action-based touch. We showed altered processing in the HFA group at an early stage of somatosensory processing (N100) and to a late stage of higher-order self-other distinction (early and late P3). At low-level somatosensory stages, individuals with HFA did not direct somatosensory attention to biological stimuli (as reflected in altered N100 SEP effects), while they might have compensated for this in attention-independent somatosensory processes (intact N140 effects; Popovich and Staines, 2015). At high-levels stages of self-related processing, individuals with HFA were less able to signal observed action-based touch that does not match one’s own sensation of touch (reflected in an altered P3 interaction effect). This effect reliably correlated to sensory and social difficulties of individuals with HFA. In sum, the results contain the first demonstration of an atypical interplay of action-based touch simulation and actual experience of touch in adults with HFA, while showing a functional relationship with both social and sensory ideosyncracies related to ASD.
Acknowledgements
The authors would like to express their gratitude to the Flemish Autism Association for its cooperation.
Funding
The research was supported by Research Foundation - Flanders (FWO; Grant FWO11/ASP/255 to E.D.).
Conflict of interest. Dr Eliane Deschrijver, Prof Dr Jan R. Wiersema and Prof Dr Marcel Brass reported no biomedical financial interests or potential conflicts of interest.
References
- Allison T., McCarthy G., Wood C.C. (1992). The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalography and Clinical Neurophysiology, 84, 301–14. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th edn Arlington, VA/Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Annaz D., Campbell R., Coleman M., Milne E., Swettenham J. (2012). Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders, 42(3), 401–8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. (1985). Does the autistic child have a “theory of mind”? Cognition, 21, 37–46. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The Autism-Spectrum Quotient (AQ): evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Blanke O. (2012). Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews. Neuroscience, 13(8), 556–71. [DOI] [PubMed] [Google Scholar]
- Bölte S., Poustka F., Constantino J.N. (2008). Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Research, 1, 354–63. [DOI] [PubMed] [Google Scholar]
- Brass M., Ruby P., Spengler S. (2009). Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 2359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.J., Foss-Feig J.H., Burnette C.P., Heacock J.L., Cosby a. (2012). The rubber hand illusion in children with autism spectrum disorders: delayed influence of combined tactile and visual input on proprioception. Autism, 16, 406–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cygan H.B., Tacikowski P., Ostaszewski P., Chojnicka I., Nowicka A. (2014). Neural correlates of own name and own face detection in autism spectrum disorder. PLoS One, 9(1), e86020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Asuncion J., Bervoets C., Morrens M., Sabbe B., De Bruijn E.R.A. (2015). EEG correlates of impaired self-other integration during joint-task performance in schizophrenia. Social Cognitive and Affective Neuroscience, 10(10), 1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschrijver E., Bardi L., Wiersema J.R., Brass M. (2016). Behavioral measures of implicit theory of mind in adults with high functioning autism. Cognitive Neuroscience, 7(1–4), 192–202 [DOI] [PubMed] [Google Scholar]
- Deschrijver E., Wiersema J.R., Brass M. (2015). The interaction between felt touch and tactile consequences of observed actions: an action-based somatosensory congruency paradigm. Social Cognitive and Affective Neuroscience, 11, 1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue S.E., Darling E.F., Mitroff S.R. (2012). Links between multisensory processing and autism. Experimental Brain Research, 222, 377–87. [DOI] [PubMed] [Google Scholar]
- Dunn W., Westman K. (1997). The sensory profile: the performance of a national sample of children without disabilities. American Journal of Occupational Therapy, 51(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Eimer M., Driver J. (2000). An event-related brain potential study of cross-modal links in spatial attention between vision and touch. Psychophysiology, 37, 697–705. [PubMed] [Google Scholar]
- Eimer M., Forster B. (2003). Modulations of early somatosensory ERP components by transient and sustained spatial attention. Experimental Brain Research, 151, 24–31. [DOI] [PubMed] [Google Scholar]
- Escera C., Yago E., Corral M.J., Corbera S., Nuñez M.I. (2003). Attention capture by auditory significant stimuli: semantic analysis follows attention switching. European Journal of Neuroscience, 18, 2408–12. [DOI] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y.M., Gaeta H. (2001). The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews, 25, 355–73. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Keysers C. (2009). The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cerebral Cortex, 19, 1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A., Brambilla P., Siugzdaite R., Londero D., Fabbro F., Rumiati F. I. (2013). Emotional resonance deficits in autistic children. Journal of Autism and Developmental Disorders, 43(3), 616–28. [DOI] [PubMed] [Google Scholar]
- Gowen E., Stanley J., Miall R. C. (2008). Movement interference in autism-spectrum disorder. Neuropsychologia, 46, 1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C.L., Harper J.D., Kueker R.H., et al. (2010). Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 40, 937–45. [DOI] [PubMed] [Google Scholar]
- Holeckova I., Fischer C., Giard M.H., Delpuech C., Morlet D. (2006). Brain responses to a subject’s own name uttered by a familiar voice. Brain Research, 1082, 142–52. [DOI] [PubMed] [Google Scholar]
- Iarocci G., McDonald J. (2006). Sensory integration and the perceptual experience of persons with autism. Journal of Autism and Developmental Disorders, 36(1), 77–90. [DOI] [PubMed] [Google Scholar]
- Ionta S., Heydrich L., Lenggenhager B., et al. (2011). Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron, 70(2), 363–74. [DOI] [PubMed] [Google Scholar]
- Jones W., Klin A. (2013). Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature, 504(7480), 427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. (2010). Somatosensation in social perception. Nature Reviews. Neuroscience, 11, 417–28. [DOI] [PubMed] [Google Scholar]
- Keysers C., Wicker B., Gazzola V., Anton J.L., Fogassi L., Gallese V. (2004). A touching sight: SII/PV activation during the observation and experience of touch. Neuron, 42, 335–46. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. (2013). EEG correlates of self-referential processing. Frontiers in Human Neuroscience, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Baron-Cohen S. (2010). Unraveling the paradox of the autistic self. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 393–403. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Baron-Cohen S. (2011). The role of the self in mindblindness in autism. Consciousness and Cognition, 20(1), 130–40. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., et al. (2010). Atypical neural self-representation in autism. Brain, 133, 611–24. [DOI] [PubMed] [Google Scholar]
- Longo M., Musil J., Haggard P. (2012). Visuo-tactile integration in personal space. Journal of Cognitive Neuroscience, 24(3), 543–52. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., et al. (2000). The Autism Diagnostic Schedule—Generic: a standard measures of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–23. [PubMed] [Google Scholar]
- Lundqvist L.O. (2015). Hyper-responsiveness to touch mediates social dysfunction in adults with autism spectrum disorders. Research in Autism Spectrum Disorders, 9, 13–20. [Google Scholar]
- Magnée M.J.C.M., De Gelder B., Van Engeland H., Kemner C. (2008). Audiovisual speech integration in pervasive developmental disorder: evidence from event-related potentials. Journal of Child Psychology and Psychiatry and Allied Disciplines, 49, 995–1000. [DOI] [PubMed] [Google Scholar]
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S. (2011). Sensory processing in autism: a review of neuropsychologic findings. Pediatric Research, 69(5), 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew N.J., Turner C. A., Goldstein G. (2005). The application of short forms of the Wechsler Intelligence scales in adults and children with high functioning autism. Journal of Autism and Developmental Disorders, 35(1), 45–52. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Paladino M.P., Mazzurega M., Pavani F., Schubert T.W. (2010). Synchronous multisensory stimulation blurs self-other boundaries. Psychological Science: A Journal of the American Psychological Society/APS, 21(9), 1202–7. [DOI] [PubMed] [Google Scholar]
- Palmer C.J., Paton B., Hohwy J., Enticott P.G. (2013). Movement under uncertainty: the effects of the rubber-hand illusion vary along the nonclinical autism spectrum. Neuropsychologia, 51(10), 1942–51. [DOI] [PubMed] [Google Scholar]
- Palmer C.J., Paton B., Kirkovski M., Enticott P.G., Hohwy J., Palmer C.J. (2015). Context sensitivity in action decreases along the autism spectrum: a predictive processing perspective. Proceedings. Biological Sciences/The Royal Society, 82(1802), pii: 20141557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton B., Hohwy J., Enticott P.G. (2012). The rubber hand illusion reveals proprioceptive and sensorimotor differences in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1870–83. [DOI] [PubMed] [Google Scholar]
- Pellicano E., Burr D. (2012). When the world becomes "too real": a Bayesian explanation of autistic perception. Trends in Cognitive Sciences, 16(10), 504–10. [DOI] [PubMed] [Google Scholar]
- Perrin F., Maquet P., Peigneux P., et al. (2005). Neural mechanisms involved in the detection of our first name: a combined ERPs and PET study. Neuropsychologia, 43, 12–9. [DOI] [PubMed] [Google Scholar]
- Popovich C., Staines W.R. (2015). The attentional-relevance and temporal dynamics of visual-tactile crossmodal interactions differentially influence early stages of somatosensory processing. Brain and Behavior, 4, 247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C., Richardson D., Bird G. (2010). Intact imitation of emotional facial actions in autism spectrum disorder. Neuropsychologia, 48(11), 3291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruissen M.I., de Bruijn E.R.A. (2015). Is it me or is it you? Behavioral and electrophysiological effects of oxytocin administration on self-other integration during joint task performance. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 70, 146–54. [DOI] [PubMed] [Google Scholar]
- Schubert R., Blankenburg F., Lemm S., Villringer A., Curio G. (2006). Now you feel it—Now you don’t: ERP correlates of somatosensory awareness. Psychophysiology, 43, 31–40. [DOI] [PubMed] [Google Scholar]
- Schubert R., Ritter P., Wüstenberg T., et al. (2008). Spatial attention related SEP amplitude modulations covary with BOLD signal in S1—a simultaneous EEG—fMRI study. Cerebral Cortex, 18, 2686–700. [DOI] [PubMed] [Google Scholar]
- Simmons D.R., Robertson A.E., McKay L.S., Toal E., McAleer P., Pollick F.E. (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–39. [DOI] [PubMed] [Google Scholar]
- Sowden S., Koehne S., Catmur C., Dziobek I., Bird G. (2016). Intact automatic imitation and typical spatial compatibility in autism spectrum disorder: challenging the broken mirror theory. Autism Research, 9(2), 292–300. [DOI] [PubMed] [Google Scholar]
- Spengler S., Bird G., Brass M. (2010). Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biological Psychiatry, 68, 1148–55. [DOI] [PubMed] [Google Scholar]
- Spengler S., Von Cramon D.Y., Brass M. (2009). Control of shared representations relies on key processes involved in mental state attribution. Human Brain Mapping, 30, 3704–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacikowski P., Cygan H.B., Nowicka A. (2014). Neural correlates of own and close-other’s name recognition: ERP evidence. Frontiers in Human Neuroscience, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacikowski P., Jednoróg K., Marchewka A., Nowicka A. (2011). How multiple repetitions influence the processing of self-, famous and unknown names and faces: an ERP study. International Journal of Psychophysiology, 79(2), 219–30. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Nowicka A. (2010). Allocation of attention to self-name and self-face: an ERP study. Biological Psychology, 84(2), 318–24. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. (2010). My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia, 48, 703–12. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Fotopoulou A. (2008). Is my body the sum of online and offline body-representations? Consciousness and Cognition, 17(4), 1317–20. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Haggard P. (2005). The rubber hand illusion revisited: visuotactile integration and self-attribution. Journal of Experimental Psychology. Human Perception and Performance, 31(1), 80–91. [DOI] [PubMed] [Google Scholar]
- van Boxtel J. J A., Lu H. (2013). A predictive coding perspective on autism spectrum disorders. Frontiers in Psychology, 4, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Cruys S., Evers K., Van Der Hallen R., Van Eylen L., Boets B., Wagemans J. (2014). Precise minds in uncertain worlds: predictive coding in autism. Psychological Review, 4, 1–36. [DOI] [PubMed] [Google Scholar]
- Verleger R., Jaśkowski P., Wascher E. (2005). Evidence for an integrative role of P3b in linking reaction to perception. Journal of Psychophysiology, 19(3), 165–81. [Google Scholar]
- Zwickel J., White S.J., Coniston D., Senju A., Frith U. (2011). Exploring the building blocks of social cognition: spontaneous agency perception and visual perspective taking in autism. Social Cognitive and Affective Neuroscience, 6, 564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]