Abstract
Besides the prefrontal cortex, the insula and medial structures of the temporal lobe are thought to be involved in risky decision-making. However, their respective contributions to decision processes remain unclear due to the lack of studies involving patients with isolated insular damage. We assessed adult patients who underwent resection of the insula (n = 13) or of the anterior temporal lobe (including medial structures) (n = 13) as part of their epilepsy surgery, and a group of healthy volunteers (n = 20), on the Iowa Gambling Task (IGT) and on the Cups Task. Groups were matched on sociodemographic, estimated-IQ and surgery-related factors. On the IGT, patients with temporal lobe resection performed significantly worse than both the insular and healthy control groups, as they failed to learn which decks were advantageous on the long-term. On the Cups Task, the insular and temporal groups both showed impaired sensitivity to expected value in the loss domain, when compared with healthy controls. These findings provide clinical evidence that the insula and mesiotemporal structures are specifically involved in risky decision-making when facing a potential loss, and that temporal structures are also involved in learning the association between behavior and consequences in the long-term.
Keywords: amygdala, decision-making, epilepsy, gambling, insula, neuropsychology, temporal lobe
Introduction
Decisions are often the result of both rational and emotional drives (Tversky and Kahneman, 1981). According to the somatic marker hypothesis (Damasio, 1994), emotions influence the decision process through internal sensations, visceral and musculoskeletal physiologic changes called ‘body states’, which are associated with reinforcing stimuli. These states are thought to be represented in several brain regions, including the amygdala, the insula, somatosensory parietal regions, the hypothalamus and in the brain stem (Bechara and Damasio, 2005). These somatic markers are then signaled to the ventromedial prefrontal cortex (vmPFC) where they are integrated. The somatic marker hypothesis predicts that damage within the somatic marker circuitry results in impaired decision-making. Accordingly, several studies have reported that patients with vmPFC damage show deficits in gambling tasks aimed at simulating real-life risky decision-making (Bechara et al., 1999; Bar-On et al., 2003; Studer et al., 2013; Clark et al., 2014).
The insula is a multisensory brain area involved in visceral sensation processing and in sensing the physiological condition of the body (Craig, 2002). In line with the somatic marker hypothesis, several functional imaging studies have reported signal change in the insula during decision-making under risk (Preuschoff et al., 2008; Liu et al., 2011; Studer et al., 2012; Werner et al., 2013), supporting a contribution of this structure to decision processes. However, because of the very low prevalence of cerebral damage confined to the insula (Cereda et al., 2002), the specific role of the insula in decision-making remains poorly understood. A few studies conducted with small groups of patients suggest that insular lesions lead to impaired risk adjustment on gambling tasks (Clark et al., 2008, 2014; Weller et al., 2009). These studies, however, are limited by the extent of cerebral damage, which in some cases largely exceeded the insula—sometimes extending to adjacent regions such as the somatosensory cortex, frontal lobes or internal capsule and putamen, which may also have contributed to the observed deficits.
Medial structures of the temporal lobe, including the amygdala and hippocampus, are also typically activated in functional neuroimaging studies on risky decision-making (Coricelli et al., 2005; Fukui et al., 2005; De Martino et al., 2006; Cohen et al., 2008; Li et al., 2010). Lesion studies involving subjects with damage to the amygdala show impairments on gambling tasks aimed at measuring risky decision-making (Bechara et al., 1999, 2003; Brand et al., 2007; Weller et al. 2007). Epileptic patients with unilateral damage of anterior mesiotemporal structures have been found to show no preference for advantageous decks on the Iowa Gambling Task (IGT) (Bonatti et al., 2009; Labudda et al., 2009; Delazer et al., 2010). These results suggest that these structures are important for learning from feedback and in making decisions in uncertain, ambiguous situations. When examining decision-making, De Martino et al. (2010) found a specific reduction in aversion to loss in two patients with a very rare genetic disease with symmetrical and bilateral damage to the amygdala, suggesting a role of this structure in preventing actions with a potentially deleterious outcome.
It has been proposed that risky decision-making relies on different neural circuits depending on whether the potential outcome is a gain or a loss (Levin et al., 2012). In their review of the literature, Levin and colleagues proposed that both the insula and the amygdala—as a result of their greater involvement in negative emotional experience—are more prominently involved in decision-making under risk in a context of potential loss than possible gain. Although based mostly on functional imaging studies, this hypothesis has received little support from lesion studies and in some case results were contradictory (Weller et al., 2007, 2009). Furthermore, it is not known whether insular and amygdala lesions lead to similar or different impairments in decision-making.
Recent works by our group and others suggest that the insula is involved in the epileptogenic zone of a non-negligible proportion of drug-resistant epileptic patients (Isnard et al., 2004; Nguyen et al., 2009) and that in these cases insular resection may lead to seizure control without major neurological complications (Malak et al., 2009) or neuropsychological complications (Boucher et al, 2015b). The medial structures of the temporal lobe are often resected as part of temporal lobe epilepsy surgery (Wiebe et al., 2001). This study examines risky decision-making in patients with insular or median temporal lobe damage as a result of neurosurgery for drug-resistant epileptic seizures. We predict that, in comparison with healthy controls, patients with damage to either of these regions will show similar deficits in decision-making, and that they will be more specifically impaired when facing a potential loss.
Methods
Participants and procedure
Adult patients who underwent partial or complete insular resection for control of drug-resistant epilepsy in our epilepsy service, during the period extending from November 2004 to June 2014, were all invited to participate in a study on the neuropsychology of the insula, except for one patient who presented with behavioral problems prior to his surgery. All of the 19 patients accepted our invitation. Two were excluded after data collection because they had an additional resection involving a significant part of the PFC that may have affected their results, and four additional patients were excluded because they had temporal lobe resection in addition to insular resection. Figure 1 depicts resection overlap among insular patients. These patients were matched with a group of patients who had their epilepsy surgery in the anterior temporal lobe that spared the insula. Among temporal lobe patients, 10 had selective amygdalo-hippocampectomy, and the remaining three had a standard anterior temporal lobectomy combined with amygdalo hippocampal resection (see Boucher et al., 2015a for more details on surgical procedures). Representative cases are illustrated in Figure 2. Participants (IQ > 80) were recruited such that they were comparable to the insular group with regards to age, gender, education, hemisphere of resection and time since surgery. Table 1 describes the final sample of insular patients (n = 13) along with information on their surgeries; the same information for temporal patients (n = 13) is listed in Table 2. Finally, a group of 20 healthy volunteers matched to the experimental groups by age, sex and years of education was recruited. These control participants were recruited using ads published on the hospital’s intranet page with the following selection criteria: aged between 18 and 55 years and no history of neurological problems. Comparisons between groups on sociodemographic variables, estimated-IQ and epilepsy-related factors revealed no significant differences (Table 3).
Fig. 1.
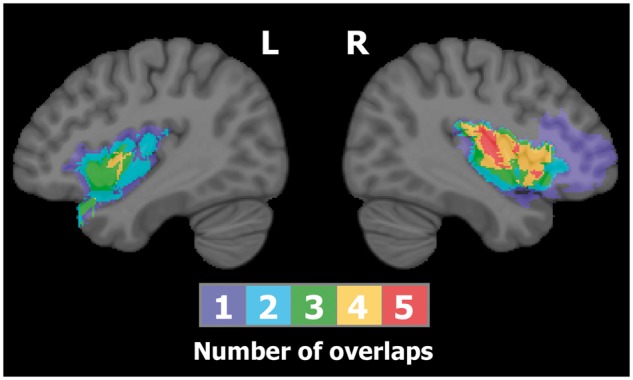
Overlap of resections conducted within the insular group. The color bar indicates the number of overlapping cases at each voxel. Maximum lesion overlap is found in the right insular cortex.
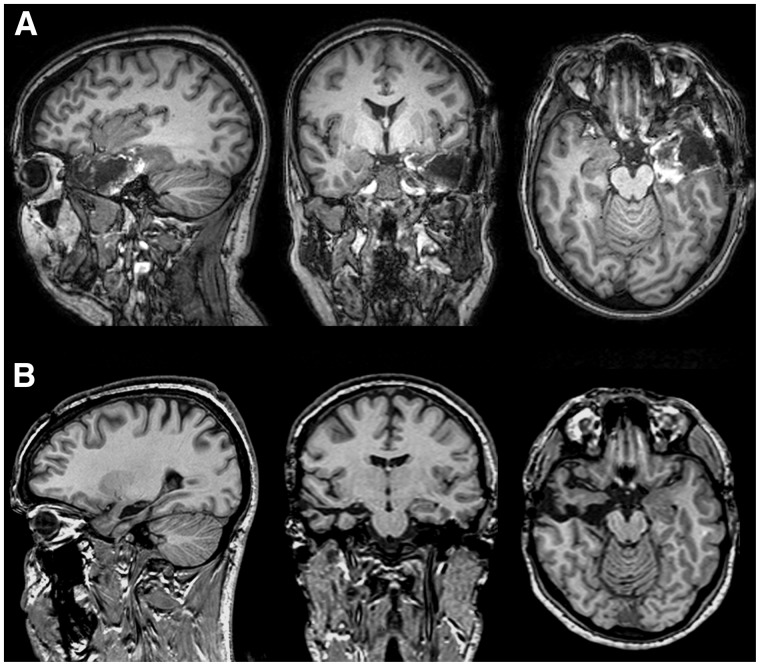
Fig. 2.
Post-operative T1-weighted sagittal, coronal and axial MRI scans from representative cases of the temporal group. In (A) anterior temporal lobectomy. In (B) selective amygdalohippocampectomy.
Table 1.
Characteristics of insular patients
| Pt. | Age at first seizures (yrs) | Age at surgery (yrs) | Time since surgery (yrs) | Pre-surgery MRI | Resection |
Engel et al.’s (1993) classification of outcome | ||
|---|---|---|---|---|---|---|---|---|
| Side | Insular area | Other areas | ||||||
| I1 | 31 | 47 | 1.1 | Normal | L | Posterior | Temporo-parietal opercula | Class I |
| I2 | 5 | 23 | 0.6 | R insular tuber | R | Complete | Fronto-parieto-temporal opercula | Class I |
| I3 | 5 | 38 | 0.5 | Normal | L | Anterior | Temporal operculum | Class I |
| I4 | 21 | 36 | 0.4 | Normal | R | Posterior | Parieto-temporal opercula | Class II |
| I5 | 30 | 35 | 2.7 | Normal | L | Anterior | Class II | |
| I6 | 26 | 36 | 1.6 | Normal | R | Anterior–superior | Frontal opercula | Class I |
| I7 | 9 | 27 | 1.6 | Normal | R | Anterior | Orbitofrontal operculum | Class I |
| I8 | 33 | 39 | 4.0 | Normal | L | Anterior | Temporal operculum | Class III |
| I9 | 0 | 32 | 0.5 | Possible subtle R operculo-insular CD | R | Posterior | Parietal operculum, inferior post-central gyrus | Class I |
| I10 | 31 | 34 | 8.8 | Normal | L | Posterior | Class I | |
| I11 | 4 | 38 | 6.7 | R insular CD | R | Complete | Fronto-parietal opercula | Class I |
| I12 | 13 | 49 | 0.5 | Normal | R | Superior | Frontal operculum | Class I |
| I13 | 4 | 34 | 0.5 | L insular CD | L | Superior | Frontal operculum | Class I |
CD, cortical dysplasia; HS, hippocampal sclerosis; L, left; R, right.
Table 2.
Characteristics of temporal patients
| Pt. | Age at first seizure (yrs) | Age at surgery (yrs) | Time since surgery (yrs) | Pre-surgery MRI | Resection |
Engel et al.’s (1993) classification of outcome | |
|---|---|---|---|---|---|---|---|
| Side | Type | ||||||
| T1 | 17 | 21 | 7.0 | R HS | R | SAH | Class I |
| T2 | 18 | 25 | 7.1 | L HA | R | SAH | Class II |
| T3 | 5 | 20 | 7.7 | L HS | L | SAH | Class I |
| T4 | 30 | 47 | 2.8 | L HS | L | SAH | Class I |
| T5 | 19 | 34 | 4.4 | Normal | L | SAH | Class I |
| T6 | 41 | 52 | 1.2 | L HS | L | ATL | Class I |
| T7 | 1 | 32 | 0.3 | R HA | R | ATL | Class I |
| T8 | 2 | 43 | 1.5 | R HA | R | SAH | Class I |
| T9 | 10 | 19 | 2.7 | R HS | R | SAH | Class I |
| T10 | 11 | 43 | 7.9 | R HS | R | SAH | Class I |
| T11 | 1 | 47 | 2.0 | L HA, T1C | L | ATL | Class I |
| T12 | 26 | 32 | 2.7 | R HS | R | SAH | Class I |
| T13 | 5 | 18 | 10.8 | L HS | L | SAH | Class I |
ATL, anterior temporal lobectomy; HA, hippocampal atrophy; HS, hippocampal sclerosis; L, left; R, right; SAH, selective amygdalohippocampectomy; T1C, type 1 Chiari malformation.
Table 3.
Description of the study sample
| Insular patients (n = 13) |
Temporal patients (n = 13) |
Healthy controls (n = 20) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± s.d. | Range | % | Mean ± s.d. | Range | % | Mean ± s.d. | Range | % | P-value |
| Age (yr) | 38.5 ± 7.7 | 23–49 | 38.0 ± 10.6 | 22–54 | 36.1 ± 10.2 | 24–52 | 0.749 | |||
| Gender (% women) | 69.2 | 53.8 | 50.0 | 0.538 | ||||||
| Education (yr) | 13.5 ± 2.0 | 11–18 | 13.4 ± 3.0 | 8–20 | 13.5 ± 1.8 | 11–18 | 0.983 | |||
| Estimated-IQ | 102.7 ± 10.9 | 88–120 | 96.96 ± 11.1 | 83–113 | 104.5 ± 7.9 | 88–120 | 0.102 | |||
| Age at first seizure | 17.4 ± 11.6 | 4–33 | 18.4 ± 14.1 | 1–43 | 0.845 | |||||
| Age at surgery (yr) | 36.7 ± 6.8 | 23–48 | 33.8 ± 12.2 | 18–52 | 0.453 | |||||
| Time since surgery (yr) | 2.3 ± 2.7 | 0.4–8.8 | 4.5 ± 3.3 | 0.3–10.8 | 0.073 | |||||
| Hemisphere (% right) | 53.8 | 53.8 | 1.000 | |||||||
Note. Statistical comparisons between groups were conducted using non-parametric Chi-square (gender and hemisphere) tests and analyses of variance.
Assessments were conducted by a licensed neuropsychologist (O.B.), after obtaining informed written consent from the study participant. Patients were assessed at least 4 months after surgery. Two patients (one in the insular group, one in the temporal group) were assessed in English because they were native English speakers; all the other assessments were conducted in French. The assessment also comprised other tasks of neuropsychological function, which have been reported in a separate article (Boucher et al., 2015c). A 50$ financial compensation was given to each participant at the end of the assessment. The study protocol was approved by the Ethics committee of the Centre Hospitalier de l’Université de Montréal.
Experimental tasks
Iowa Gambling Task
The IGT (Bechara et al., 1994; Bechara, 2007) is a computer task aimed at measuring decision-making deficits by simulating real-life decision-making. Four decks of cards (labeled A–D) are presented to the participant, who is asked to pick one card at a time by clicking on any deck (100 trials total). After a card is picked, a message is displayed on the screen to indicate the amount of money that was won. For some cards, however, the participant wins money but must also pay a penalty. The amount of money accumulated during the task is illustrated by a green bar placed on the top of the computer screen. Decks A and B are said to be disadvantageous, because they are associated with higher gains, but with even heavier penalties, and thus result in overall money loss in the long-term. In contrast, decks C and D are advantageous because, although they are associated with lower gains, they result in an overall gain on the long-term because of lighter penalties. After a learning phase, healthy participants typically come to select more cards from decks C and D, resulting in overall money gain, whereas patients with decision-making deficits fail to learn which decks are advantageous and thus show either no deck preference or select more cards from decks A and B (Bar-On et al., 2003; Clark et al., 2003). The raw score represents the number of cards selected from advantageous decks (C and D) minus the number selected from disadvantageous decks (A and B). Responses are grouped by blocks of 20 consecutive trials. The first 40 trials represent the learning phase and are thus analyzed separately from trials 41 to 100, which represent the ‘test phase’.
Cups Task
Decision-making for risky gains and losses was assessed using a homemade computerized version of the Cups Task (Levin et al., 2007). In this task, two arrays of cups are presented to the participant. Each cup contains an amount of money. During each trial, the participant is asked to choose between the two arrays of cups to gain money or to avoid losing money. After the response, a cup from the selected array is randomly lifted up by the computer, and the participant gains/loses the amount of money that was hidden in this cup. One array of cups is riskless: each cup contains the same small amount of money ($±1.00). The other array represents a risky choice with only one cup containing any amount of money (either $±2.00, $±3.00 or $±5.00) while the other cups have $0.00. Both arrays have the same number of cups, i.e. either 2, 3 or 5. Thus, when selecting the risky array, chances are either 50, 33 or 20% that the cup associated with an amount be lifted up.
Half trials were gain trials (i.e. with a positive amount of money), the other half were loss trials (i.e. with a negative amount of money). The entire task comprised 54 trials. In each condition, there were an equal number of risk-advantageous, risk-disadvantageous and equal expected value (EV) trials. Trials with equal EV for the risky and riskless options were 50% × ±$2.00, 33% × ±$3.00 and 20% × ±$5.00: on these trials, risky responses are neither advantageous nor disadvantageous on the long-term. Risk-advantageous trials were: 50% × +$5.00, 50% × +$3.00, 33% × $5.00 on gain trials and 33% × −$2.00, 20% × −$2.00, 20% × −$3.00. Risk-disadvantageous trials were: 33% × +$2.00, 20% × +$2.00, 20% × $3.00 on gain trials and 50% × −$5.00, 50% × −$3.00, 33% × −$5.00. The outcome (amount of money won/lost) was presented for 400 ms and was preceded by a 1 s blank period. The screen was then left blank for another 2 s before the next trial. Participants were only informed of the total money they accumulated over the trials at the end of the experiment. They were asked to do the best they could to gain as much money as possible and were encouraged to respond as they would do if they used their own money. The task was preceded by a two-item demonstration trial. Sensitivity to EV value (nb. advantageous risky decisions—nb. disadvantageous risky decisions) was computed separately for the Gain and Loss conditions.
Supplementary measures
IQ was estimated using the average scaled scores obtained on the matrix reasoning and similarities subtests from the Wechsler Adult Intelligence Scales—Third Edition (Wechsler, 1997). Furthermore, given the presumed role of mesiotemporal structures in learning processes and to test the hypothesis that differences between the insular and temporal groups on gambling performance were attributable to differences in learning abilities, we reviewed post-operative neuropsychological assessment results from the clinical file of each patient a posteriori to obtain information on memory function. Performance on the Rey Auditory Verbal Learning Test (RAVLT) was available for all insular patients and for all but two temporal patients and was thus used in our study. The RAVLT (Rey, 1970; Crawford et al., 1989) is a 15-item word learning task which includes five consecutive learning trials, one interference list recall, one immediate recall trial after the interference list and delayed recall and recognition trials after a 20-min delay. Since the gambling tasks used in our study do not rely on long-term memory, only the learning (total number of words recall over the five learning trials) and immediate recall (number of words recalled from the initial 15-word list) trials were considered. Mean duration between RAVLT administration as part of the standard post-operative neuropsychological assessment and the other tasks administered for the aims of this study did not differ between the insular group (mean = 1.0 years, s.d. = 1.6, range = 0.0–5.3) and the temporal group (mean = 1.7 years, s.d. = 2.1, range = 0.0–5.0) [F(1,22) = 1.07, P = 0.313].
Statistical analyses
Because outcomes variables were not normally distributed, non-parametric statistical tests were used to compare performance between groups. Kruskal-Wallis tests were performed to compare groups on IGT performance [raw scores during the learning phase (trials 1–40) and raw scores during the test phase (trials 41–100)] and on the Cups Task (sensitivity to EV in the Gain and in the Loss conditions, separately). For each significant difference, Mann–Whitney non-parametric tests were performed post hoc to compare performance between each pair of groups. Mann–Whitney non-parametric tests were also used to compare the insular and temporal groups on RAVLT performance. Differences were considered significant at P < 0.05. Furthermore, we examined whether each group of participants improved from the learning phase to the test phase of the IGT by comparing mean raw scores during both phases [e.g. (learning phase raw score/2) vs (test phase raw score/3)] using Wilcoxon non-parametric tests. Finally, we used Chi-square tests to compare the proportions of participants in each group failing to show any preference for advantageous over disadvantageous decks on the IGT (i.e. sum of raw score for trials 41–100 ≤ 0) and obtaining a raw score of 3 or less in sensitivity to EV for the gain and loss conditions on the Cups Task. Statistical analyses were performed using SPSS 22.0 software (SPSS, Chicago, IL).
Results
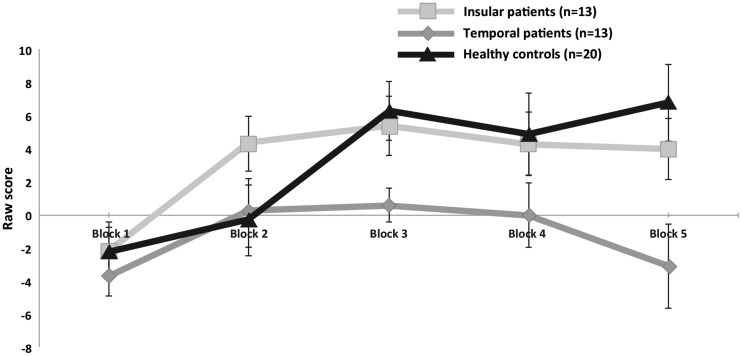
Gambling tasks results from each group are reported in Table 4. Comparisons between the three groups revealed statistically significant differences for two outcomes: test phase on IGT and sensitivity to EV in the loss condition on the Cups Task. For the IGT—Test phase performance, post hoc comparisons revealed that temporal patients performed significantly worse than both the healthy controls (U = 57.0, P = 0.006) and the insular patients (U = 28.0, P = 0.003). There was no significant difference between the healthy controls and the insular patients (U = 98.5, P = 0.250). IGT performance over time for each group is illustrated in Figure 3. The ability to learn to select cards from the advantageous decks over time on the IGT was assessed separately for each group using intragroup Wilcoxon non-parametric tests on mean raw scores during the learning and test phases. Analyses showed that both the healthy controls (Z = −3.21, P = 0.001) and the insular patients (Z = −2.24, P = 0.025) improved significantly between the learning and test phases but the temporal patients failed to learn to select cards from advantageous decks (Z = −0.46, P = 0.646).
Table 4.
Performance of each study group on gambling tasks
| Insular patients |
Temporal patients |
Healthy controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | P-value | Posthoc comparisons |
| IGT | |||||||||||
| Learning phase (trials 1–40) | 13 | 2.2 | 11.3 | 13 | −3.5 | 8.2 | 20 | −2.5 | 9.4 | 0.500 | |
| Test phase (trials 41–100) | 13 | 13.7 | 16.2 | 13 | −2.5 | 15.9 | 20 | 18.0 | 21.9 | 0.005 | C > T; I > T |
| Cups Task | |||||||||||
| Sensitivity to EV − Gain | 12 | 4.6 | 2.6 | 13 | 4.7 | 2.9 | 20 | 4.6 | 3.2 | 0.996 | |
| Sensitivity to EV − Loss | 12 | 4.3 | 2.1 | 13 | 3.6 | 3.5 | 20 | 6.4 | 2.2 | 0.016 | C > I; C > T |
Note. One participant from the insular group could not complete the Cups Task due to a lack of time during the assessment session. P-values obtained from non-parametric Kruskal-Wallis tests for differences between the three groups. Posthoc comparisons indicate the direction of significant (P < 0.05) differences revealed by non-parametric Mann–Whitney tests performed between each pair of groups. C, healthy controls; I, insular patients; T, temporal patients.
Fig. 3.
Mean raw score (advantageous—disadvantageous decks) on the IGT as a function of block (1–5), for each group. Error bars represent standard errors of the mean. (light gray = insula; dark gray = temporal lobe; black = healthy controls).
Group comparisons for the proportion of patients who did not show any preference for advantageous over disadvantageous decks on the IGT revealed a significant difference [χ2(2) = 11.14, P = 0.004]. According to post hoc comparisons, temporal patients (69.2%) were significantly more likely to show such deficit in comparison to both insular patients [15.4%; χ2(1) = 7.72, P = 0.005] and the healthy controls [20.0%; χ2(1) = 8.00, P = 0.005], whereas insular and control groups did not differ significantly [χ2(1) = 0.74, P = 0.737].
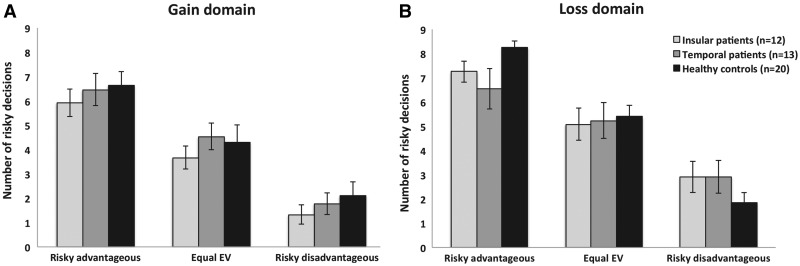
On the Cups Task, post hoc comparison for sensitivity to EV in the loss condition revealed that healthy controls adjusted their risky decisions significantly better than both insular (U = 51.5, P = 0.006) and the temporal (U = 74.0, P = 0.040) patients. There was no difference between insular and temporal patients on this outcome (U = 68.5, P = 0.611). The mean number of risky decisions according to EV level for each group is presented in Figure 4, separately for the gain (A) and the loss (B) conditions. As Figure 4 suggests, sensitivity to EV appears more pronounced in the loss condition compared with the gain domain in healthy controls, whereas this pattern is less obvious in both patient groups. Supplemental within-group Wilcoxon tests comparing sensitivity to EV in the gain vs the loss domains for each group separately confirmed this observation, as the controls had significantly higher sensitivity to EV scores in the loss condition (Z = −2.87, P = 0.004), whereas this difference was not observed in the insular (Z = −0.36, P = 0.719) and temporal (Z = −1.17, P = 0.240) groups.
Fig. 4.
Mean number of risky decisions on the Cups Task according to EV, for each group, in the Gain (A) and Loss (B) conditions. Error bars represent standard errors of the mean. light gray = insula; dark gray = temporal lobe; black - healthy controls).
Group comparisons for the proportion of participants with a sensitivity to EV score of 3 or less on the Cups Task revealed a significant difference in the loss condition [χ2(2) = 6.31, P = 0.043] but not in the gain condition [χ2(2) = 0.81, P = 0.666]. Post hoc comparisons with sensitivity to EV in the loss condition ≤ 3 showed that such performance occurred more frequently in the temporal (46.2%) and in the insular (41.7%) groups in comparison to healthy controls [10.0%; temporal vs controls: χ2(1) = 5.61, P = 0.018; insular vs controls: χ2(1) = 4.40, P = 0.036], whereas both groups of patients did not differ [χ2(1) = 0.82, P = 0.821].
Supplemental comparisons between the insular and temporal groups on learning performance on the RAVLT showed that insular patients tended to perform better than temporal patients for both the learning (insular: mean = 51.9, s.d. = 11.9; temporal: mean = 43.7, s.d. = 13.4; U = 44.5, P = 0.119) and immediate recall (insular: mean = 11.8, s.d. = 3.4; temporal: mean = 8.8, s.d. = 4.4; U = 41.0, P = 0.082) conditions, although these differences did not reach statistical significance.
Because of the more extended resection in the frontal cortex in one patient from the insular group (#I6), statistical analyses were rerun without this patient to exclude the possibility that frontal lobe damage accounts for our results with insular patients. All results remained virtually unchanged (not shown).
Discussion
This study examined risky decision-making in a sample of patients in whom the insula or anterior temporal lobe was removed as part of their neurosurgery for drug-resistant epileptic seizures. Patients with anterior temporal lobe resection showed altered performance in comparison to both the healthy controls and the insular patients when assessed on the IGT, in which participants must learn to select choices that are advantageous in the long run. Indeed, these patients failed to learn which decks were advantageous over time. In contrast, on the Cups Task in which the outcome probabilities are explicitly shown to the patients and which assesses risky decision-making separately for gains and losses, both groups of patients showed poorer performance than healthy controls in the loss condition. The insular and temporal patients adjusted their performance as a function of EV levels to a lesser extent than control participants when facing a potential loss. These results were not explained by group differences in estimated overall cognitive function.
Our results with insular patients are in line with the view that the insula is involved in decision-making by signaling the probability of a future punishment (Simmons et al., 2004; Liu et al., 2007, 2011; Preuschoff et al., 2008; Mohr et al., 2010). To our knowledge, this study is only the fourth to assess decision-making in a group of patients with damage to the insular cortex—the three previous ones were conducted by the same group of researchers. In the first study, Clark et al. (2008) compared 13 patients with focal insular lesion to 20 patients with lesion to the vmPFC and to matched controls on the Cambridge Gamble Task, and they found that the insular group was selectively poorer than the others to adjust their bets by the odds of winning/losing. Then, Weller et al. (2009) used the Cups Task to assess risk adjustment under gain and loss conditions separately, in a group of 10 patients with unilateral insular damage due to a middle cerebral artery stroke. They found that patients were severely impaired in adjustment to risk in both the gain and loss conditions when compared with healthy volunteers. More recently, Clark et al. (2014) assessed the gambler’s fallacy and near misses distortion effects on decision-making in patients with focal lesions to the vmPFC, insula or amygdala and in healthy controls. Although other groups all displayed these cognitive distortions, insular patients did not show such effects. In contrast with Weller’s results, in our study, patients for whom the insula was removed as part of their epilepsy surgery were only mildly impaired, and only in the loss condition. It is possible that in Weller’s insular group, damage to other brain structures including the vmPFC, accounts for the more severe and global effects on this task. Alternatively, it is possible that insular resection in epileptic patients lead to more subtle risk adjustment deficits than stroke due to compensation processes in long-term epileptic patients. Nevertheless, this study provides additional evidence that the Cups Task is sensitive to insular cortex damage. We are also the first to report performance in a group of patients with insular damage on the IGT. The lack of sensitivity of the IGT to insular damage in our study may be attributable to the lack of distinction between the gain and the loss domains in this task.
Patients with anterior temporal lobe resection also showed significant impairments on both gambling tasks used in this study. On the Cups Task, their performance was comparable to that of insular patients, i.e. they adjusted their risk-taking behavior according to EV to a lesser degree than healthy controls when facing a potential loss. These findings contrast with those of Weller et al. (2007), whose patients with amygdala damage were specifically impaired in risk adjustment in the gain but not the loss domain. Nevertheless, our results provide partial support for a greater involvement of the mesial temporal lobe structures (which include the amygdala) in processing negative emotions. Indeed, several studies have shown that the amygdala is involved in processing the negative valence of stimuli and has been implicated in choice behavior that is guided by a prospective negative outcome (Breiter et al., 1996; Morris et al., 1996, 1999; Phillips et al., 1997; Schneider et al., 1997; Whalen et al., 1998; Rotshtein et al., 2001; Kahn et al., 2002). This structure is also known to play a critical role in detecting threats and aversive events (Sehlmeyer et al., 2009; Schlund et al., 2010; LeDoux, 2012). On the IGT, unlike patients with insular resection, patients with anterior temporal lobe surgery were significantly impaired in their ability to learn to select cards from advantageous decks. This is congruent with previous studies conducted in patients with mesiotemporal damage (Bechara et al., 1999, 2003; Bonatti et al., 2009; Labudda et al., 2009; Delazer et al., 2010). Given the well-established roles of the hippocampus and amygdala in declarative and emotional learning (Phelps and LeDoux, 2005; Phelps, 2006), the different patterns of results obtained by insular and temporal lobe patients on the IGT may reflect a greater involvement of mesiotemporal regions in implicit learning, rather than different effects on risky decision-making. A sole memory effect would be sufficient to explain why patients from the temporal group were unable to learn the association between the decks and their consequences in the long-term, preventing them from differentiating between advantageous and disadvantageous options. Our findings with the RAVLT—a typical task of learning function—partly support this hypothesis. Indeed, temporal patients tended to perform poorer on this task in comparison to insular patients, although the difference did not reach the statistical significance threshold. We cannot exclude the possibility that mesiotemporal structures contribute to IGT performance beyond a role in implicit learning.
Results of this study are consistent with the hypothesis that different neural processes are involved in risky decision-making for gains and losses, and that the insula and median temporal lobe structures might be more especially involved in the latter (Mohr et al., 2010; Levin et al., 2012). Enhanced involvement of these structures in decision-making processes when facing a potential loss is supported by various neuroimaging studies (Kuhnen and Knutson, 2005; Yacubian et al., 2006; Knutson et al., 2007, 2008). Insular activations have also been associated with the magnitude of the anticipated loss during gambling tasks (Canessa et al., 2013) and have been recorded while experiencing negative feedback (Kuhnen and Knutson, 2005). This is also consistent with several studies suggesting that the insula and the amygdala are more specifically related to negative but not positive emotional experience (Anders et al., 2004; Brázdil et al., 2009; Nielen et al., 2009). Particularly, the amygdala has been related to fear processing (Bechara et al., 1995; LaBar et al., 1995, 1998), while the insula cortex is thought to be involved in disgust (Calder et al., 2000; Adolphs et al., 2003; Stark et al., 2007). Also, it is well-documented that negative emotions are particularly strongly associated with visceral changes (Brosschot and Thayer, 2003; Critchley et al., 2004). Given the role played by the insula in visceral sensations processing and interoception (Craig, 2002), a possible explanation for its increased contribution to risky decisions when facing a loss is related to the greater physiological changes associated with loss vs gain experience.
Results obtained on the Cups Task suggest that healthy control participants are more EV-sensitive when facing a potential loss than when facing a possible gain. Interestingly, this effect was not observed in the patient groups. This increased sensitivity to EV in the loss domain may be at least partly attributable to loss aversion. Loss aversion is a bias by which people tend to prefer avoiding losses to acquiring objectively commensurate gains, which results in a greater impact of losses on preferences (Tversky and Kahneman, 1991). The absence of a significant difference in EV sensitivity between the gain and loss conditions in the insular and temporal groups may reflect a role of the insula and of mesiotemporal structures in this cognitive bias, as suggested by recent neuroimaging studies. Indeed, in an fMRI study using a loss aversion paradigm, Canessa et al. (2013) found loss-specific activations in the amygdala and posterior insula tracking the magnitude of potential losses, which were also correlated with individual differences on a behavioral measure of loss aversion. Moreover, gray matter volume in the amygdala was also positively associated with behavioral loss aversion. Another study using voxel-based morphometry in healthy adults found that interindividual differences in loss aversion were associated with gray matter volume in the posterior insula in addition to the left medial frontal gyrus, although the relation was in the inverse direction (e.g. lower gray matter volume associated with higher loss aversion; Markett et al., 2016). Specific assessment of behavioral loss aversion in patients with insular and/or amygdala lesions would be required to better understand the contribution of these regions to loss aversion and to determine whether altered loss aversion is responsible for the present findings with the Cups Task.
Among the limitations of our study is the extent of resection among our epileptic patients. In the insular group, most resections were insular-opercular rather than purely insular. In the temporal group, resections always included amygdala and hippocampus resection, and in some cases they also included the lateral portion of the anterior temporal lobe, thus the specific contribution of each of these structures could only be inferred on the basis of the existing literature. Damage to other structures may have contributed to altered performance in some patients. Furthermore, not all insular resections included the same insular subregions (e.g. anterior vs posterior) and our sample size did not allow for more precise comparisons by subregion removed. In addition, we cannot exclude the possibility that anticonvulsant medication in epileptic participants adversely affects decision-making performance. Also, the gambling tasks used solicit several processes beyond decision-making. For instance, IGT performance may be influenced by implicit learning processes, which were not directly assessed and could only be estimated using a standard clinical verbal learning test. Future studies should try to prevent or control for these confounding factors. Finally, since patients were not assessed prior to their surgery with the experimental tasks under study, we cannot determine whether the observed deficits were directly caused by surgery or if they are the long-term effects of epilepsy on brain function already present before surgery was performed. Nevertheless, our study is unique in that it includes a relatively large number of patients with damage confined to the operculo-insular region, and our findings provide clinical evidence for the hypothesis that risky decision-making depends on distinct neural circuits depending on whether the individual is facing a potential gain or a potential loss (Levin et al., 2012).
Funding
This study was funded by Fondation du CHUM (D.K.N.) and by post-doctoral grants from the Canadian Institutes of Health Research (MFE-115520 - O.B.).
Conflict of interest. None declared.
References
- Adolphs R., Tranel D., Damasio A.R. (2003). Dissociable neural systems for recognizing emotions. Brain and Cognition, 52, 61–9. [DOI] [PubMed] [Google Scholar]
- Anders S., Lotze M., Erb M., Grodd W. (2004). Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping, 23, 200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On R., Tranel D., Denburg N.L., Bechara A. (2003). Exploring the neurological substrate of emotional and social intelligence. Brain, 126, 1790–800. [DOI] [PubMed] [Google Scholar]
- Bechara A. (2007). Iowa Gambling Task Professional Manual. Lutz, FL: Psychologial Assessment Resources. [Google Scholar]
- Bechara A., Damasio A.R. (2005). The somatic marker hypothesis: a neural theory of economic decision. Games and Economic Behavior, 52, 336–72. [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. (2003). Role of the amygdala in decision- making. Annals of the New York Academy of Sciences, 985, 356–69. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R., Lee G.P. (1999). Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. The Journal of Neuroscience, 19(13), 5473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., Adolphs R., Rockland C., Damasio A.R. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–18. [DOI] [PubMed] [Google Scholar]
- Bonatti E., Kuchukhidze G., Zamarian L., et al. (2009). Decision making in ambiguous and risky situations after unilateral temporal lobe epilepsy surgery. Epilepsy & Behavior, 14(4), 665–73. [DOI] [PubMed] [Google Scholar]
- Boucher O., Dagenais E., Bouthillier A., Nguyen D.K., Rouleau I. (2015a). Different effects of anterior temporal lobectomy and selective amygdalohippocampectomy on verbal memory performance of patients with epilepsy. Epilepsy and Behavior, 52, 230–5. [DOI] [PubMed] [Google Scholar]
- Boucher O., Rouleau I., Escudier F., et al. (2015b). Pre- and post-operative neuropsychological function in epileptic patients undergoing partial or complete insulectomy. Epilepsy and Behavior, 43, 53–60. [DOI] [PubMed] [Google Scholar]
- Boucher O., Rouleau I., Lassonde M., Lepore F., Bouthillier A., Nguyen D.K. (2015c). Social information processing following resection of the insular cortex. Neuropsychologia, 71, 1–10. [DOI] [PubMed] [Google Scholar]
- Brand M., Grabenhorst F., Starcke K., Vandekerckhove M.M., Markowitsch H.J. (2007). Role of the amygdala in decisions under ambiguity and decisions under risk: evidence from patients with Urbach-Wiethe disease. Neuropsychologia, 45(6), 1305–17. [DOI] [PubMed] [Google Scholar]
- Brázdil M., Roman R., Urbánek T., et al. (2009). Neural correlates of affective picture processing: a depth ERP study. Neuroimage, 47, 376–83. [DOI] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., et al. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17, 875–87. [DOI] [PubMed] [Google Scholar]
- Brosschot J.F., Thayer J.F. (2003). Heart rate response is longer after negative emotions than after positive emotions. International Journal of Psychophysiology, 50, 181–7. [DOI] [PubMed] [Google Scholar]
- Calder A.J., Keane J., Manes F., Antoun N., Young A.W. (2000). Impaired recognition and experience of disgust following brain injury. Nature Neuroscience, 3, 1077–8. [DOI] [PubMed] [Google Scholar]
- Canessa N., Crespi C., Motterlini M., et al. (2013). The functional and structural neural basis of individual differences in loss aversion. Journal of Neuroscience, 33(36), 14307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda C., Ghika J., Maeder P., Bogousslavsky J. (2002). Strokes restricted to the insular cortex. Neurology, 59, 1950–5. [DOI] [PubMed] [Google Scholar]
- Clark L., Bechara A., Damasio H., Aitken M.R.F., Sahakian B.J., Robbins T.W. (2008). Differential effects of insula and ventromedial prefrontal cortex lesions on risky decision making. Brain, 131, 1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L., Manes F., Antoun N., Sahakian B.J., Robbins T.W. (2003). The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia, 41, 1474–83. [DOI] [PubMed] [Google Scholar]
- Clark L., Studer B., Bruss J., Tranel D., Bechara A. (2014). Damage to insula abolishes cognitive distortions during simulated gambling. Proceedings of the National Academy of Sciences, 111(16), 6098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X., Elger C.E., Weber B. (2008). Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. NeuroImage, 39, 1396–407. [DOI] [PubMed] [Google Scholar]
- Coricelli G., Critchley H.D., Joffily M., O’Doherty J.P., Sirigu A., Dolan R.J. (2005). Regret and its avoidance: a neuroimaging study of choice behavior. Nature Neuroscience, 8(9), 1255–62. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–66. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Stewart L.E., Moore J.W. (1989). Demonstration of savings on the AVLT and development of a parallel form. Journal of Clinical and Experimental Neuropsychology, 11, 975–81. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Öhman A., Dolan R.J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–95. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. (1994). Descartes’ Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam. [Google Scholar]
- De Martino B., Camerer C.F., Adolphs R. (2010). Amygdala damage eliminates monetary loss aversion. Proceedings of the National Academy of Sciences of the United States of America, 107, 3788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B., Kumaran D., Seymour B., Dolan R.J. (2006). Frames, biases, and rational decision-making in the human brain. Science, 313, 684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delazer M., Zamarian L., Bonatti E., et al. (2010). Decision making under ambiguity and under risk in mesial temporal lobe epilepsy. Neuropsychologia, 48, 194–200. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr, Van Ness P.C., Rasmussen T.B., Ojermann L.M. (1993). Outcome with respect to epileptic seizures In Engel J., Jr, editor. Surgical Treatment of the Epilepsies, 609–21, New York: Raven Press. [Google Scholar]
- Fukui H., Murai T., Fukuyama H., Hayashi T., Hanakawa T. (2005). Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage, 24, 253–9. [DOI] [PubMed] [Google Scholar]
- Isnard J., Guénot M., Sindou M., Mauguière F. (2004). Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia, 45, 1079–90. [DOI] [PubMed] [Google Scholar]
- Kahn I., Yeshurun Y., Rotshtein P., Fried I., Ben-Bashat D., Hendler T. (2002). The role of the amygdala in signaling prospective outcome of choice. Neuron, 33, 983–94. [DOI] [PubMed] [Google Scholar]
- Knutson B., Rick S., Wimmer G.E., Prelec D., Loewenstein G. (2007). Neural predictors of purchases. Neuron, 53, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Wimmer G.E., Kuhnen C.M., Winkielman P. (2008). Nucleus accumbens acti- vation mediates the influence of reward cues on financial risk taking. Neuroreport, 19, 509–13. [DOI] [PubMed] [Google Scholar]
- Kuhnen C.M., Knutson B. (2005). The neural basis of financial risk taking. Neuron, 47, 763–70. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. (1998). Human amygdala activa- tion during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron, 20, 937–45. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., LeDoux J.E., Spencer D.D., Phelps E.A. (1995). Impaired fear conditioning follow- ing unilateral temporal lobectomy in humans. Journal of Neuroscience, 15, 6846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labudda K., Frigge K., Horstmann S., et al. (2009). Decision making in patients with temporal lobe epilepsy. Neuropsychologia, 47, 50–8. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2012). Rethinking the emotional brain. Neuron, 73, 653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I.P., Weller J.A., Pederson A.A., Harshman L. (2007). Age-related differences in adaptive decision making: sensitivity to expected value in risky choice. Judgment and Decision Making, 2, 225–3. [Google Scholar]
- Levin I.P., Xue G., Weller J.A., Reimann M., Lauriola M., Bechara A. (2012). A neuropsychological approach to understanding risk-taking for potential gains and losses. Frontiers in Neuroscience, 6:15, doi: 10.3389/fnins.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lu Z.L., D’Arbembeau A., Ng M., Bechara A. (2010). The Iowa Gambling Task in fMRI images. Human Brain Mapping, 31, 410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Powell D.K., Wang H., Gold B.T., Corbly C.R., Joseph J.E. (2007). Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. Journal of Neuroscience, 27(17),4587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malak R., Bouthillier A., Carmant L., et al. (2009). Microsurgery of epileptic foci in the insular region. Journal of Neurosurgery, 110, 1153–63. [DOI] [PubMed] [Google Scholar]
- Markett S., Heeren G., Montag C., Weber B., Reuter M. (2016). Loss aversion is associated with bilateral insula volume. A voxel based morphometry study. Neuroscience Letters, 619, 172–6. [DOI] [PubMed] [Google Scholar]
- Mohr P.N.C., Biele G., Heekeren H.R. (2010). Neural processing of risk. Journal of Neuroscience, 30, 6613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S., Frith C.D., Perrett D.I., et al. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383, 812–5. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Ohman A., Dolan R.J. (1999). A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Science of the United States of America, 96, 1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.K., Nguyen D.B., Malak R., et al. (2009). Revisiting the role of the insula in refractory partial epilepsy. Epilepsia, 50, 510–20. [DOI] [PubMed] [Google Scholar]
- Nielen M.M.A., Heslenfeld D.J., Heinen K., et al. (2009). Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain and Cognition, 71, 387–96. [DOI] [PubMed] [Google Scholar]
- Phelps M.E. (2006). Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Phelps M.E., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48, 175–87. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Senior C., et al. (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature, 389, 495–8. [DOI] [PubMed] [Google Scholar]
- Preuschoff K., Quartz S.R., Bossaerts P. (2008). Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience, 28, 2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. (1970). L’examen clinique en psychologie, 3rd edn Paris: Presses Universitaires de France. [Google Scholar]
- Rotshtein P., Malach R., Hadar U., Graif M., Hendler T. (2001). Feeling or features: different sensitivity to emotion in high-order visual cortex and amygdala. Neuron, 32, 747–57. [DOI] [PubMed] [Google Scholar]
- Schlund M.W., Siegle G.J., Ladouceur C.D., et al. (2010). Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. Neuroimage, 52, 710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F., Grodd W., Weiss U., et al. (1997). Functional MRI reveals left amygdala activation during emotion. Psychiatry Research, 76, 75–82. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C., Schöning S., Zwitserlood P., et al. (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One, 4, e5865.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Matthews S.C., Stein M.B., Paulus M.P. (2004). Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport, 15, 2261–5. [DOI] [PubMed] [Google Scholar]
- Stark R., Zimmermann M., Kagerer S., et al. (2007). Hemodynamic brain correlates of disgust and fear ratings. Neuroimage, 37, 663–73. [DOI] [PubMed] [Google Scholar]
- Studer B., Apergis-Schoute A.M., Robbins T.W., Clark L. (2012). What are the odds? The neural correlates of active choice during gambling. Frontiers in Neuroscience, 6:46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer B., Manes F., Humphreys G., Robbins T.W., Clark L. (2013). Risk-sensitive decision-making in patients with posterior parietal and ventromedial prefrontal cortex injury. Cerebral Cortex, doi:10.1093/cercor/bht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. (1981). The framing of decisions and the psychology of choice. Science, 211, 453–8. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. (1991). Loss aversion in riskless choice: a reference-dependent model. The Quarterly Journal of Economics, 106, 1039–61. [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale, 3rd edn San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weller J.A., Levin I.P., Shiv B., Bechara A. (2007). Neural correlates of adaptive decision making for risky gainsand losses. Psychological Science, 18, 958–64. [DOI] [PubMed] [Google Scholar]
- Weller J.A., Levin I.P., Shiv B., Bechara A. (2009). The effects of insula damage on decision-making for risky gains and losses. Social Neuroscience, 4, 347–58. [DOI] [PubMed] [Google Scholar]
- Werner N.S., Schweitzer N., Meindl T., Duschek S., Kambeitz J., Schandry R. (2013). Interoceptive awareness moderates neural activity during decision-making. Biological Psychology, 94, 498–506. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S., Blume W.T., Girvin J.P., Eliasziw M., et al. (2001). A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine, 345, 311–8. [DOI] [PubMed] [Google Scholar]
- Yacubian J., Gläscher J., Schroeder K., Sommer T., Braus D.F., Büchel C. (2006). Dissociable systems for gain- and loss-related value predic- tions and errors of prediction in the human brain. Journal of Neuroscience, 26, 9530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]