Abstract
Rumination, and particularly ruminative brooding, perpetuates dysphoric mood states and contributes to the emergence of depression. Studies of adults and older adolescents have characterized the association between rumination and intrinsic functional connectivity within default mode (DMN), salience (SN) and executive control (ECN) networks; we know little, however, about the brain network basis of rumination during early puberty, a sensitive period for network reorganization. 112 early puberty boys and girls completed resting-state scans, the Ruminative Response Scale, and the Youth Self-Report questionnaire. Using independent components analysis and dual regression, we quantified coherence for each individual in networks of interest (SN, ECN, DMN) and in non-relevant networks (motor, visual) in which we predicted no correlations with behavioral measures. Boys and girls did not differ in levels of rumination or internalizing symptoms, or in coherence for any network. The relation between SN network coherence and rumination; however, and specifically ruminative brooding, was moderated by sex: greater SN coherence was associated with higher levels of brooding in girls but not in boys. Further, in girls, brooding mediated the relation between SN coherence and internalizing symptoms. These results point to coherence within the SN as a potential neurodevelopmental marker of risk for depression in early pubertal girls.
Keywords: intrinsic functional connectivity, rumination, puberty, anterior cingulate, salience network
Introduction
Rumination is defined as ‘a mode of responding to distress that involves repetitively and passively focusing on symptoms of distress and on the possible causes and consequences of these symptoms’ (Nolen-Hoeksema, 1991, p. 569). Although ruminating, individuals appraise their problems as unsolvable (Lyubomirsky et al., 1999), generate less effective solutions to problems (Lyubomirsky and Nolen-Hoeksema, 1995; Lyubomirsky et al., 1999), and show low motivation to implement strategies that are generated (Wenzlaff et al., 1988; Lyubomirsky and Nolen-Hoeksema, 1993). Further, individuals who ruminate are less likely to reach out for social support (Nolen-Hoeksema and Davis, 1999) and report high levels of social friction (Nolen-Hoeksema and Davis, 1999). Importantly, longitudinal studies indicate that both youth and adults who ruminate are more likely to subsequently develop depression (Nolen-Hoeksema et al., 2008) and other forms of psychopathology (Joormann et al., 2006; McLaughlin and Nolen-Hoeksema, 2011).
Recently, investigators have characterized brain networks associated with high levels of rumination. These studies have capitalized on the recognition that functional MRI signals acquired when individuals are at rest exhibit reproducible oscillatory dynamics (Biswal et al., 1995). By associating time series of these oscillatory patterns, it is possible to identify a network of regions that activate in tandem and, therefore, likely have a history of functional co-activation (Buckner et al., 2013). Importantly, the networks identified in these intrinsic (or resting-state) functional connectivity analyses are reliable across time (Zuo et al., 2010; Thomason et al., 2011; Somandepalli et al., 2015) and reflect known structural connections (Greicius et al., 2009). Further, they correspond to patterns of task-based activation that have been reported across a variety of paradigms (Smith et al., 2009), including induced rumination (Berman et al., 2014). Researchers have conducted functional connectivity analyses on these intrinsic networks to probe the network basis of rumination in the default mode network (DMN), salience network (SN) and executive control network (ECN). These networks been implicated in psychopathology across all age groups (Menon, 2011; Hamilton et al., 2013), and may be involved in rumination. The DMN is involved in self-referential and memory consolidation processes, SN is implicated in orienting towards novel, potentially threatening, stimuli, and ECN is involved in inhibiting the persevative thinking that characterizes rumination and shifting individuals’ cognitive sets to another line of thought (Menon, 2011). The most robust evidence linking intrinsic networks with rumination has been documented for the DMN; adult studies converge to indicate that high ruminators have stronger connectivity between regions within the DMN than do their low-rumination counterparts (Berman et al., 2011, 2014; Hamilton et al., 2011; Piguet et al., 2014; Luo et al., 2015). High ruminating adults also evidence greater connectivity within the SN (Kuhn et al., 2014) and decreased connectivity within the ECN (Kuhn et al., 2012).
Importantly, rumination is a multidimensional construct consisting of two components, ruminative brooding and ruminative reflection (henceforth, ‘brooding’ and ‘reflection’), and it is possible that brooding and reflection are differentially related to brain networks. Brooding involves passively comparing one’s current situation to an unachieved standard, whereas reflection involves intentionally turning inward to alleviate dysphoric mood. Although neither type of rumination is considered to be adaptive, brooding has been associated with the emergence of higher levels of depressive symptoms than has reflection (Treynor et al., 2003). Indeed, all of the studies with adults described above that examined the network basis of rumination reported differential associations for brooding and reflection (Berman et al., 2011; Hamilton et al., 2011; Kuhn et al., 2014; Luo et al., 2015), highlighting the importance of examining these two components of rumination separately.
Only two studies to date have examined the brain network basis of rumination in youth, and these assessed a sample of older (13–17 years) depressed adolescents (Connolly et al., 2013; Ho et al., 2015); researchers have not yet characterized the brain basis of rumination in a sample prior to the typical onset of depression. This is particularly important given that the transition from childhood to adolescence appears to be a sensitive period both for the emergence of rumination and for the development of brain networks that support this cognitive process in adults. Indeed, levels of rumination increase during this period (Hampel and Petermann, 2005), and connections within DMN, SN, and ECN continue to mature (Satterthwaite et al., 2013b).
The emergence of sex differences in brain and behavior also occurs during the transition to adolescence. By definition, adolescence begins with the onset of puberty, during which concentrations of circulating gonadal hormones rise and bind to receptors that are present throughout the brain and influence subsequent brain development (Sisk and Zehr, 2005). This, in turn, contributes to sexual dimorphisms in the brain (Lenroot et al., 2007; Goddings et al., 2014) and may also shape behaviors—such as rumination—that differ by sex. Indeed, sex differences in rumination (Johnson and Whisman, 2013) do not emerge until later in puberty (Burwell and Shirk, 2007; Rood et al., 2009; Abela and Hankin, 2011; Hamilton et al., 2015). Given this timing, it is possible that sex differences in brain network functioning set the stage for later-emerging sex differences in rumination.
To examine these issues, we recruited a sample of early-pubertal youth in which boys and girls were matched on pubertal status rather than on age, given theories suggesting that it is pubertal maturation specifically—rather than other age-related factors—that contributes to the emergence of sex differences in internalizing symptomatology in mid-adolescence (Sisk and Zehr, 2005; Patton and Viner, 2007; Blakemore et al., 2010); this allowed us to ensure that sex differences are not confounded by girls’ earlier entry into puberty. We assessed the relations among connectivity in three intrinsic networks (DMN, SN and ECN), levels of ruminative brooding and reflection (the latter is reported in the supplement), and levels of internalizing symptoms that are posited to arise from ruminative thinking, as well as in two non-relevant networks (motor, visual). Based on previous findings, we predicted, first, that ruminative brooding would be associated with internalizing symptomatology. Second, we predicted that although boys and girls would not differ with respect to rumination or internalizing symptoms at this early pubertal stage, they would differ in the coherence (strength of within-network connectivity) of brain networks, setting the stage for sex differences in levels of rumination and internalizing symptoms that emerge later in puberty. Third, we predicted that ruminative brooding, but not reflection, would be associated with patterns of activation in the three intrinsic brain networks—most strongly in DMN, which has the strongest evidence for this relation, but not in the motor or visual networks. If so, we sought to examine whether ruminative brooding would mediate a relation between network coherence and internalizing symptoms. Finally, we explored sex differences in the network basis of rumination.
Materials and methods
Participants
Participants were native English speakers recruited from throughout the San Francisco Bay Area community through online posts to Craigslist and to parent listservs. Advertisements targeted an age group corresponding to the early stages of puberty in boys and girls. Participants’ pubertal status was assessed by parent report of pubertal status and by subsequent confirmation upon visit to the laboratory. Children were excluded if they reported neurological (including severe head injuries), cardiovascular, or any other major medical problems. The study complied with Institutional Review Board guidelines, and, in accordance with the Declaration of Helsinki, participants and their parents provided written informed assent and consent, respectively. Participants were compensated for their participation. 139 children completed a neuroimaging scan session. Scans from 27 participants were excluded from data analyses due to scanner problems (n= 12), administration of an incorrect protocol (n= 1), unscorable cardiac physiological data (n= 6), unusable structural data (n= 2), and excessive motion during the scan (n= 6); Thus, data from 112 children (53 boys and 59 girls) were included in the final analyses. The racial and ethnic demographics of this sample were representative of the Bay Area: 47% Caucasian, 6% African-American, 10% Hispanic, 17% Asian, 3% Native American and 16% multiracial.
Measures
Questionnaires. We administered a ten-item Rumination Response Scale—Adolescent Version (RRS-A) (Burwell and Shirk, 2007) and examined items from the five-item brooding and the five-item self-reflection subscales (Treynor et al., 2003). Brooding and self-reflection are conceptualized to be two distinct subtypes of rumination. Brooding involves passive comparisons to an unachieved standard and items on this scale include ‘Think about a recent thing that happened and wish it had gone better’ and ‘Think “Why do I always react this way?”’ Reflection involves a purposeful turning inward to problem-solve in order to alleviate dysphoric mood; items on this scale include ‘Write down what you are thinking about and analyze it’ and ‘Analyze recent events to try to understand why you are sad or upset’.
Participants used the Youth Self-Report questionnaire (Achenbach, 1991; Achenbach and Rescorla, 2001) to report about problem behaviors over the previous six months. We chose to use this questionnaire because it captures a broad spectrum of functioning, both within and outside of the clinical range (Achenbach and Rescorla, 2001). We analyzed the 16-item Anxious/Depressed Problems Subscale.
Pubertal status. Stage of pubertal maturation was determined using self-reported Tanner Staging (Marshall and Tanner, 1968), a reliable and valid metric (Slora et al., 2009). Girls rated both their pubic hair and breast development, and boys rated both their pubic hair and penile/testicular development, on five-point scales. A composite Tanner Stage score was computed for each participant by averaging the two scores.
Clinical interview. Trained interviewers assessed the diagnostic status of participants by administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version to both the child and parent (Kaufman et al., 1997).
Neuroimaging data acquisition. We present details of neuroimaging data acquisition in the Supplemental Information.
Data pre-processing
We implemented a preprocessing stream that could be applied at the individual, rather than the group, level of analysis; we selected this stream on the basis of data comparing approaches to minimize confounds without introducing noise to the data (Hallquist et al., 2013; Jo et al., 2013; Satterthwaite et al., 2013a; Power et al., 2014). This research guided decisions about the best approaches to use in order to address confounds generated by motion (Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012; Yan et al., 2013) and to ensure appropriate ordering of preprocessing steps, particularly bandpass filtering relative to nuisance regression (Hallquist et al., 2013; Jo et al., 2013). In cases in which there were controversies about how best to deal with confounds, we selected an approach that had been demonstrated to be effective in similar data sets of youth (Jo et al., 2013; Satterthwaite et al., 2013a). We present details regarding our approach to addressing confounds and pre-processing in the Supplementary Materials.
Operationalizing network coherence
We defined coherence as the strength of the association between timecourses of all voxels within an identified network. Following preprocessing, we obtained a metric of resting-state functional connectivity for each individual within each intrinsic network of interest using a combination of group probabilistic independent components analysis (ICA) and dual regression analyses. Group ICA is a multivariate signal processing method (Kiviniemi et al., 2003; Beckmann et al., 2005) that we selected over seed-based correlation because it accounts for multiple voxel-voxel relations in order to obtain an interacting network of voxels, it permits data-driven exploration of the spatial-temporal properties of functional neuroimaging data, and the group ICA followed by dual regression approach has been shown to have higher short- and long-term test-retest reliability than seed-based analysis (Zuo et al., 2010; Smith et al., 2014).
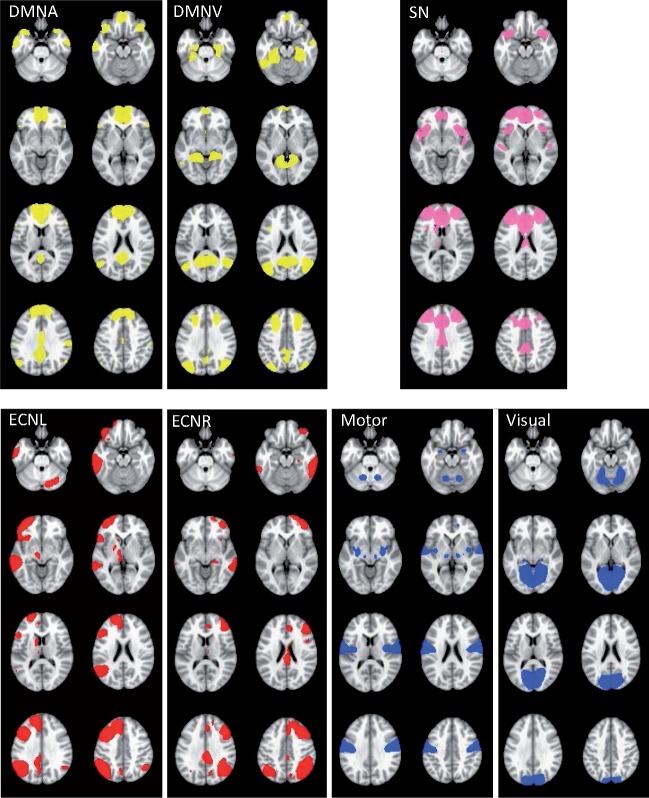
We conducted group ICA using FSL 5.0.6 MELODIC software version 3.14, specifying 25 components. Specifically, a set of ICA components derived from the whole sample was generated and used to elicit individual maps of a comparable network for statistical analysis. Group components that were identified from group ICA were visually inspected and networks comprising the networks of interest (DMN, SN and ECN) and non-relevant networks (motor, visual) were visually identified on the basis of their neuroanatomical components by trained raters (MG, SO). These networks are visualized in Figure 1 and include one network comprising the anterior subdivision of the DMN (DMNA), another network comprising a ventral subdivision (DMNV), a single SN, a right and left ECN (ECNR; ECNL) and visual and motor networks.
Fig. 1.
ICA—identified networks of interest.
The set of spatial maps from the group-average analysis was used to generate individual-specific versions of the spatial maps and associated timeseries using dual regression (Beckmann et al., 2009; Filippini et al., 2009). First, for each individual, the group-average set of spatial maps is regressed (as spatial regressors in a multiple regression) into the individual's 4D space-time dataset. This results in a set of individual-specific timeseries, one per group-level spatial map. Next, those timeseries are regressed (as temporal regressors) into the same 4D dataset, resulting in a set of individual-specific spatial maps, one per group-level spatial map. Each spatial map contains regression weights that serve as a measure of each voxel’s functional connectivity with the identified network while controlling for the influence of other networks, some of which may reflect artifacts such as physiological noise. Z-scores that normalize by the residual within-subject noise were used. We then applied the group mask for a given component to the individual-level spatial map for the corresponding component, and values within this mask were averaged to produce a metric of network coherence for each individual. Our approach is consistent with published approaches (Van Duijvenvoorde et al., 2015).
Statistical analyses
A priori statistical analyses were conducted using SPSS software version 23. We examined individual coherence metrics for each a priori identified network as well as for behavioral variables to ensure normality of data distributions. We used t-tests to test hypotheses concerning sex differences. We used correlational analyses to examine the relation between rumination and anxious/depressed symptoms. We examined brain-behavior relations by (i) correlating each network coherence estimate with brooding and (ii) using ordinary least squares multiple regression to test whether sex moderated the relation between network coherence and brooding. Based on the findings described below, we tested a mediational model relating SN coherence, brooding, and anxious/depressed symptoms in females only (associations were not significant in males) using path-analytic approaches implemented in PROCESS for SPSS and utilizing 95% bias-corrected bootstrap confidence intervals for the indirect effect based on 10 000 bootstrap samples (MacKinnon, 2008; Hayes, 2013). Finally, we combined moderation and mediation results by estimating the conditional indirect effects of SN coherence on anxious/depressed symptoms through brooding as a function of sex, using a moderated mediation approach. This was implemented using SPSS PROCESS using a modification to handle a dichotomous moderator (Preacher et al., 2007) and using 10 000 bootstrap estimates for the construction of 95% bias-corrected bootstrap confidence intervals for the conditional indirect effects (Hayes, 2013).
Results
Demographic variables
As expected given the study design, boys and girls were in the early stages of puberty and did not differ on pubertal status; also as expected, boys were older than girls (see Table 1).
Table 1.
Demographic features and sex differences in behavioral outcomes, motion during scan, and network coherence.
| Boys | Girls | Cohen’s d | Test statistic | P | |
|---|---|---|---|---|---|
| n | 53 | 59 | |||
| Tanner stage | 1.92 (0.68) | 2.14 (0.78) | −0.301 | t (110) = −1.581 | 0.117 |
| Age | 11.90 (0.82) | 11.18 (1.05) | 0.764 | t(110) = 4.016 | 0.000*** |
| Reflection | 8.86 (2.56) | 9.42 (3.06) | −0.199 | t(103) = −1.009 | 0.315 |
| Brooding | 10.30 (3.71) | 9.58 (3.20) | 0.208 | t(103) = 1.065 | 0.289 |
| YSR Anxious/Depressed Total Score | 5.23 (4.26) | 5.58 (4.84) | −0.077 | t(109) = −0.397 | 0.692 |
| Motion (RMS Relative Motion; mm2) | 0.050 (0.025) | 0.051 (.025) | −0.040 | t(110) = −0.154 | 0.878 |
| ECNL coherence | 3.046 (0.500) | 3.212 (0.467) | −0.350 | t(110) = −1.816 | 0.072+ |
| ECNR coherence | 3.094 (0.519) | 3.245 (0.599) | −0.285 | t(110) = −1.419 | 0.159 |
| SN coherence | 3.581 (0.677) | 3.654 (0.630) | −0.107 | t(110) = −0.591 | 0.556 |
| DMNA coherence | 3.181 (0.495) | 3.043 (0.478) | 0.289 | t(110)= 1.502 | 0.136 |
| DMNV coherence | 3.665 (0.576) | 3.867 (0.637) | −0.344 | t(110) = −1.75 | 0.083+ |
| Motor coherence | 3.985 (0.922) | 4.100 (0.811) | −0.127 | t(110) = −0.702 | 0.484 |
| Visual coherence | 4.848 (1.133) | 4.466 (0.911) | 0.370 | t(110)= 1.972 | 0.051+ |
Values denote mean (±SD) or number of subjects; P-values refer to t-test.
P < 0.001, **P < 0.010, *P < 0.050, + P < 0.100.
Rumination and anxious/depressed symptoms
As shown in Table 1, girls and boys did not differ in levels of reflection, brooding, or anxious/depressed symptoms. Brooding was significantly associated with anxious/depressed symptoms in the whole sample [r(105) = 0.632, P = 0.000], and separately in both boys [r(50) = 0.729, P = 0.000] and girls [r(55) = 0.568, P = 0.000]. Fisher r- to z-transformations indicated no trend-level sex differences in the association between brooding and anxious/depressed symptoms (z = −1.40, P = 0.162). Ruminative reflection results are in Section 4 of the Supplementary Materrials.
Sex differences in network coherence
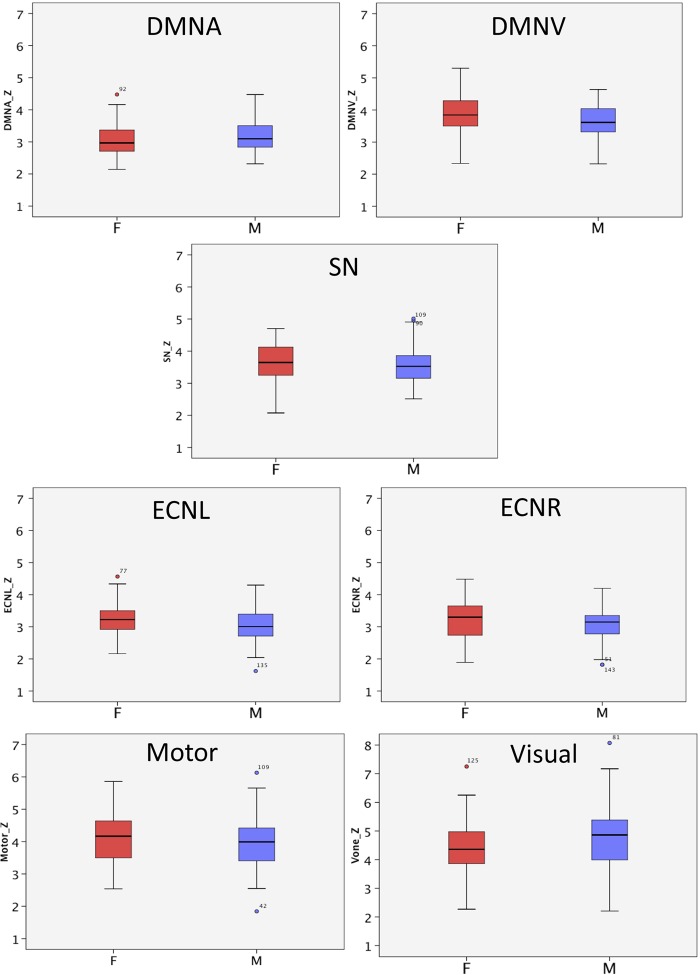
There were no sex differences in motion (see Table 1). Comparisons of network coherence in boys and girls (see Table 1 and Figure 2) yielded no sex differences in networks of interest, although there was a trend for greater ECNL and DMNV coherence in girls and greater visual network coherence in boys. There were no sex differences in motor or visual network coherence.
Fig. 2.
Sex differences in network coherence.
Brain-behavior relations
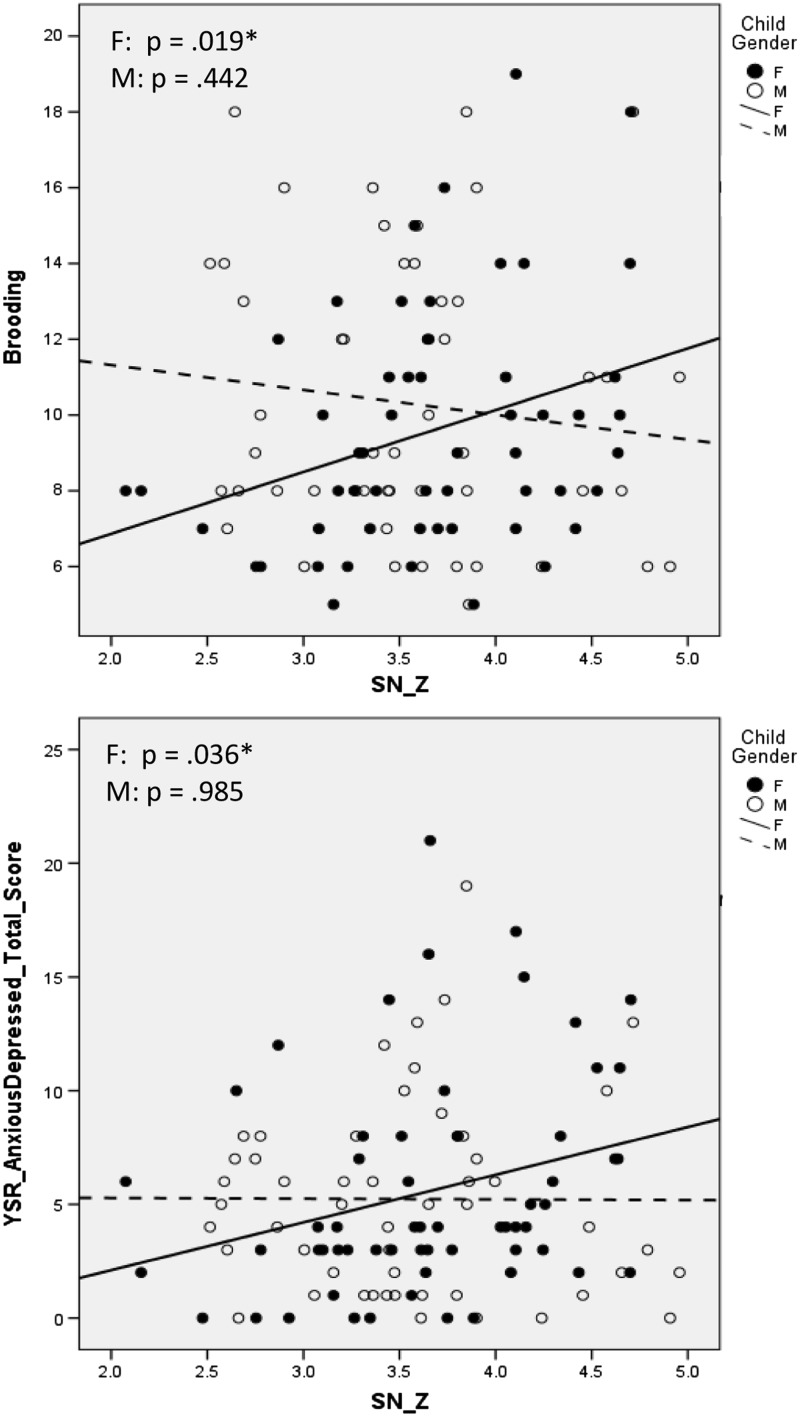
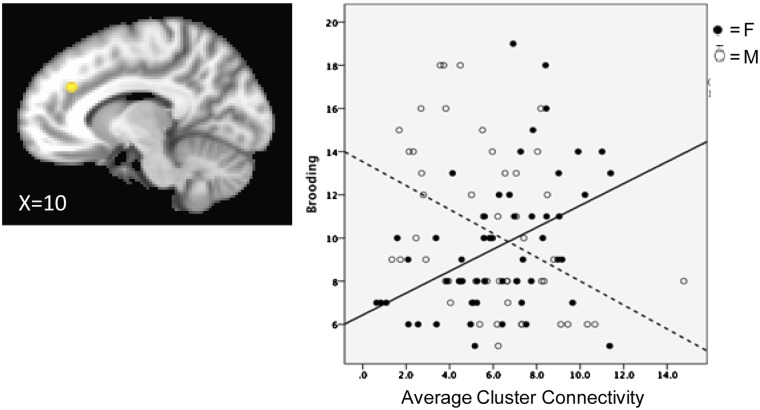
Network associations with motion are described in the Supplementarty Materials, as are network associations with ruminative reflection. Across the whole sample, brooding was not associated with coherence in networks of interest [|rs|(105) < 0.147, Ps > 0.134] or non-relevant networks [|rs|(105) < 0.047, Ps > 0.631]. As presented in Table 2, sex moderated the relation between brooding and SN coherence. The interaction of sex and SN continued to predict brooding even after controlling for age [B = 1.139 (0.526), P = 0.033, ΔR2 = 0.044], which, as previously noted, differed by sex. Follow-up, within-sex analyses indicated that there was no significant relation between brooding and SN coherence in boys [r(50) = −0.117, P = 0.419]; in girls, however, greater brooding was associated with increased coherence in SN [r(55) = 0.317, P = 0.019]. Sex differences in the relation between SN and ruminative brooding are presented in Figure 3.
Table 2.
Sex moderation of the relation between network coherence and behavioral outcomes
| Brooding |
|||
|---|---|---|---|
| B (SE) | P | ΔR2 | |
| ECNL coherence | 0.480 (0.707) | 0.498 | 0.005 |
| ECNR coherence | −0.775 (0.620) | 0.214 | 0.015 |
| SN coherence | 1.144 (0.522) | 0.031* | 0.045 |
| DMNA coherence | 0.663 (0.691) | 0.339 | 0.009 |
| DMNV coherence | 0.948 (0.572) | 0.100 | 0.026 |
| Motor coherence | 0.397 (0.396) | 0.319 | 0.009 |
| Visual coherence | 0.521 (0.336) | 0.124 | 0.023 |
Betas reported are interaction terms from a regression that includes main effect of network coherence, sex, and network coherence by sex interaction. All main effect terms are centered.
Fig. 3.
SN coherence is associated with ruminative brooding and depressive symptoms in females but not males.
We also examined the relation between network coherence and anxious/depressed symptomatology. Across both sexes, there were no associations between anxious/depressed symptoms and coherence in networks of interest [|rs|(111) < 0.151, Ps > 0.113] or non-relevant networks [|rs|(55) < 0.033, Ps > 0.733]. As presented in Table 2, sex did not moderate the relation between anxious/depressed symptoms and network coherence. Given the significant relation between brooding and anxious/depressed symptoms and our subsequent test of mediation below, we explored within-sex associations between SN coherence and anxious/depressed symptomatology. In boys, there was no association between anxious/depressed symptoms and SN coherence [r(50) = −0.005, P = 0.974]. However, in girls, increased anxious/depressed symptoms was associated with increased coherence in SN [r(59) = 0.273, P = 0.036]. The differential relation between SN and anxious/depressed symptoms for boys and girls is presented in Figure 3.
Mediation
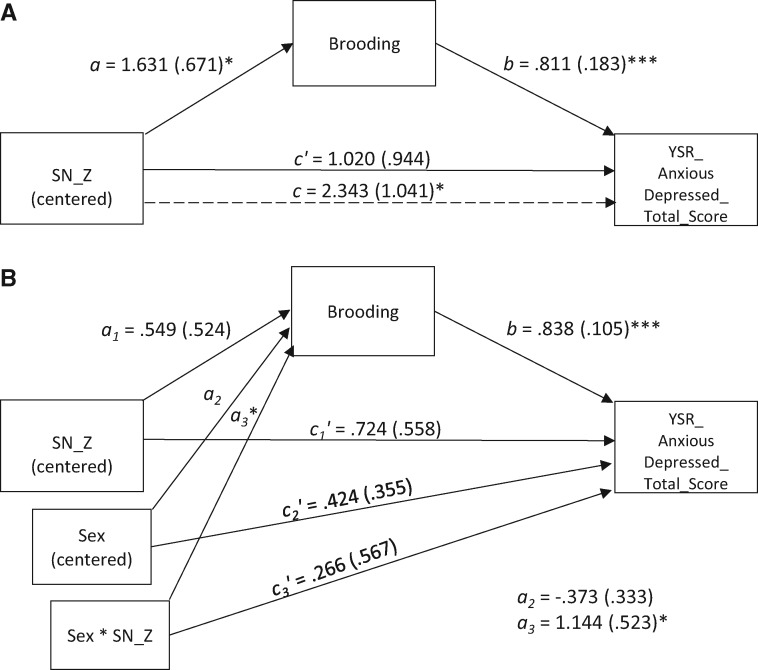
Given that brooding is posited to elicit depressive symptomatology, combined with the significant associations in girls between SN coherence and both brooding and depressive symptomatology, we examined whether brooding mediates the relation between SN coherence and depressive symptomatology in girls using the methods described earlier. As shown in the path estimates, standard errors, and bootstrapped 95% CIs for the indirect effect in Figure 4A, we found that brooding does indeed mediate this relation. To ensure that these results were not driven by the small subsample of individuals who met diagnostic criteria for depression in this sample, we re-ran the mediational model excluding individuals who met a liberal criteria for depression—either the parent or child reported that they met criteria or subthreshold (missing one symptom to meet criteria) for depression. Again, brooding did mediate the relation between SN coherence and depressive symptomatology (see Figure 4B for path estimates, standard errors and bootstrapped 95% CIs for the indirect effect). Given that sex moderated the relation between SN coherence and brooding, we formally integrated our moderation and mediation results by testing whether sex moderated the aforementioned mediational models (full sample and without those meeting criteria for depression). In both cases, sex moderated the effect of SN coherence on anxious/depressed symptoms via brooding (see Figure 4B for path estimates, standard errors, and bootstrapped 95% confidence intervals for the conditional indirect effect).
Fig. 4.
(A) In girls only, ruminative brooding mediates the relation between SN coherence and anxious/depressed symptoms. The relation is present even after excluding participants who meet criteria for major depressive disorder. Indirect effect is significant: ab = 1.323, SEab = 0.651, 95% bias-corrected CI = 0.351, 2.991. When the model is run excluding n = 9 participants who met criteria for MDD, the indirect effect remains significant: ab = 1.226, SEab = 0.652, 95% bias-corrected CI = 0.270, 2.881. (B) A moderated mediation model including both boys and girls reveals a significant conditional indirect effect of SN coherence on anxious/depressed symptoms through brooding. This relation is significant even after excluding participants who meet criteria for major depressive disorder. Conditional indirect effect of SN coherence on anxious/depressed symptoms through brooding is significant:ω = 1.918, SEω = 0.900, 95% bias-corrected CI = 0.302, 3.877. When excluding n = 9 participants who met criteria for MDD, the conditional indirect effect remains significant: ω = 1.849, SEω = 0.905, 95% bias-corrected CI = 0.233, 3.808. *P < 0.05, **P < 0.01, ***P < 0.001.
Exploratory analyses
We conducted follow-up voxelwise regression to probe which regions within SN showed significant interaction of sex and brooding. As shown in Figure 5, a significant cluster was found within the left dorsal anterior cingulate cortex (dACC). Values extracted from the cluster reveal that, in girls, stronger dACC connectivity relative to the rest of the SN is associated with increased brooding [β = 0.507, t(53) = 3.410, P = 0.001, pr2 = 0.180]; however, in males, stronger dACC connectivity relative to the rest of the SN is associated with decreased brooding [β = −0.553, t(48) = −3.056, P = 0.004, pr2 = 0.163].
Fig. 5.
Follow-up analyses revealed a cluster (x = 10, y = −36, z = 30) within the left dACC of the SN shows a significant sex moderation of the relation between network coherence and brooding. A voxelwise regression with centered sex, brooding, and interaction terms was run; clusters for the interaction term were identified using a P < 0.001 voxelwise and P < 0.01 cluster threshold. Coordinates are in RAI
Discussion
This is the first study to examine the network basis of ruminative brooding during early puberty, a period prior to the typical period of onset of depression when levels of brooding increase and sex differences in brain and behavior emerge. We matched boys and girls on the basis of pubertal status rather than age in order to ensure that sex differences were not confounded by pubertal status. This study yielded three important findings. First, similar to results reported in studies of older youth and adults, brooding was related to internalizing symptoms. Second, as we hypothesized, boys and girls did not differ with respect to levels of brooding or internalizing symptoms; however, we also did not find the predicted sex differences in the coherence of brain networks that we posited would set the stage for the well-documented differences in brooding and internalizing symptoms that emerge later in puberty. Third, we found a sex-specific association between network coherence in SN and ruminative brooding in females. This finding was driven by dACC connectivity with the rest of the SN, suggesting that dACC is driving brooding-related network activity. Importantly, we did not find associations in two non-relevant networks, providing another metric to validate statistical thresholding. Most notably, mediation was moderated by sex, such that, in girls, greater SN coherence contributes to increased rumination, which in turn is associated with internalizing symptoms, even in girls who do not yet show signs of clinically significant depression.
Stronger SN coherence in young females who brood may presage subsequent depression
Despite evidence implicating all three networks in rumination in adults (Berman et al., 2011, 2014; Hamilton et al., 2011; Kuhn et al., 2012, 2014; Piguet et al., 2014; Luo et al., 2015), we found that coherence of SN, but not of DMN or ECN, was associated with brooding. The SN, which includes the anterior insula, dACC, and frontopolar regions, receives afferent inputs from subcortical and limbic structures involved in emotion processing and in motivation (Menon, 2011), as well as autonomic inputs (Craig, 2002). Not surprisingly, the SN has been implicated in orienting to and monitoring internal and external stimuli that threaten homeostasis, in assigning saliency to ambiguous material, and transmitting this information to other networks to guide goals and response generation (Craig, 2002; Critchley, 2005). Individuals with greater SN coherence attend more to threatening stimuli (Carlson et al., 2013) and report higher levels of anxiety (Seeley et al., 2007); further, SN node neurofeedback training can reduce emotional responses to negative self-relevant stimuli (Hamilton et al., 2016). SN is more coherent in depressed adults and adolescents than in controls (Hamilton et al., 2012; Kerestes et al., 2014). Further, meta-analytic evidence suggests that, in depression, the SN exhibits potentiated responses to negative information as a result of high baseline pulvinar activity; then, low striatal dopamine levels prevent this viscerally charged information from being propagated up to the dorsolateral prefrontal cortex (Hamilton et al., 2012). This may bias depressed individuals to attend to threatening, painful, and negative self-relevant information and prevent it from being reappraised. Similarly, the triple network model of psychopathology proposes that aberrant SN detection and mapping of external and internal stimuli give rise to abnormal engagement of the ECN and DMN, which compromises goal-relevant behaviors and self-referential and memory consolidation processes, respectively (Menon, 2011).
Our finding that coherence in SN, but not in ECN or DMN, was associated with ruminative brooding in early-pubertal girls suggests that the development of ruminative brooding begins with aberrant SN coherence, potentially setting the stage for processing to go awry later in the DMN and ECN. In the context of Response Styles Theory, which posits that individuals—especially females—who brood are at higher risk for the onset of depression, it makes sense that early pubertal girls who brood frequently show patterns of network activity consistent with the initial stages of the pathological process that leads to depression. Research on healthy development also indicates that, with age, not only does the SN become more coherent (Sole-Padulles et al., 2016), but it also becomes increasingly integrated (i.e. functionally coupled) with DMN and ECN (Marek et al., 2015). Importantly, the connections between SN and the other networks are among the last to stabilize (Marek et al., 2015); this is important because it suggests that aberrant development of SN before it integrates with other networks could presage maladaptive network imbalances. Indeed, high-ruminating adults exhibit stronger SN-ECN connectivity (Kuhn et al., 2012) than do their low-ruminating peers. Thus, it is possible that in girls who exhibit higher levels of brooding, when the SN, which is already strongly coherent, integrates with the DMN and ECN, the DMN and ECN may not be equipped for the strength and potentiation of the SN activation, which may explain the over-coherence within DMN and under-coherence within ECN that has been found to be associated with ruminative brooding in adults (Berman et al., 2011; Hamilton et al., 2011, 2014; Kuhn et al., 2012; Piguet et al., 2014; Luo et al., 2015).
dACC integrates emotional and cognitive inputs to guide behavior and is strongly connected to SN in high-brooding girls
Importantly, our exploratory analyses indicated that girls with higher levels of brooding show a stronger connection between the dACC and the rest of SN. This region of the dACC, the anterior section of the mid-cingulate cortex (Shackman et al., 2011), plays a central role in ruminative brooding. It links the SN, DMN and ECN (Sheline et al., 2009), and meta-analytic evidence indicates it is unique among all other brain regions in it that processes pain, threat, and uncertainty, generates negative emotions, but also exerts cognitive control (Shackman et al., 2011). Thus, the dACC is the seat of ‘hot’ higher-level cognition because it guides behavior towards instrumental goals in the context of negative emotions or pain, uncertainty, or threat (Critchley, 2005; Shackman et al., 2011; Shenhav et al., 2013). It is not surprising, therefore, that the dACC is related to brooding, a process in which individuals respond to distress or emotional pain by repeatedly seeking to understand its possible causes and consequences.
Indeed, researchers have suggested that hyperconnectivity between the dACC and all other networks is a distinguishing feature of depression (Anand et al., 2009; Sheline et al., 2009; Kenny et al., 2010; Ye et al., 2012; Zhu et al., 2012; Admon et al., 2015). Interestingly, this hyperconnectivity is also associated with increased rumination (Spati et al., 2015). Further, graph theoretic metrics indicate that the dACC is more strongly integrated with and more centrally positioned among other nodes of depressed individuals’ neural networks (Onoda and Yamaguchi, 2015), suggesting that, in depression, networks are functionally organized to readily send salient information to other networks for further consolidation and goal modulation. This formulation is consistent with behavioral evidence that depressed individuals exhibit a negative attention bias (Gotlib et al., 2004; Maalouf et al., 2012) that is conceptualized to underlie memory biases and impair emotion regulation (Gotlib and Joormann, 2010). In the high-brooding girls, who are at the greatest risk for depression, strong dACC-SN connectivity may facilitate SN inputs to disproportionately influence memory-consolidation/self-directed DMN processes and goal-directed ECN activities.
Limitations and future directions
This study examined the network basis of rumination in the early stages of puberty in order to gain a better understanding of antecedents of depression. As youth transition through puberty, gonadal hormones increase in concentration and bind to receptors in the brain to influence subsequent neural development. Longitudinal studies that follow youth who are recruited on the basis of their pubertal status must test whether SN becomes increasingly coherent over the course of pubertal development and potentiates responding in ECN and DMN. Such studies can also investigate whether the dACC becomes more strongly connected not only to the rest of SN, but also to the ECN and DMN—particularly in individuals who go on to develop depression. Our use of an ICA-based approach enabled us to examine within-network connectivity (i.e. coherence) and its association with ruminative brooding and internalizing symptoms, but limited our ability to examine between-network associations. In light of proposals that the SN is involved in dynamically coordinating activation of the DMN and ECN to guide goal-directed cognition (Uddin, 2015), future studies should focus on examining between-network associations in order to test specifically whether stronger connectivity between SN and DMN, as well as between SN and ECN, is associated higher levels of ruminative brooding and internalizing symptoms. In our data-driven ICA maps, dACC segregated with the SN, but future cross-sectional and longitudinal studies using seed-based or graph-theoretical approaches to test a priori hypotheses about the degree to which the dACC is coupled with multiple networks can provide a fuller picture of the network basis of rumination in youth.
One remaining question concerns the network basis of ruminative brooding in boys. Indeed, compared with girls, boys report equivalent levels of brooding and exhibit comparable levels of activation in the networks of interest. Thus, while boys do indeed brood, the network basis of this brooding is not clear. One possibility is that, at least during early puberty, boys exhibit a more diffuse pattern of network function than do girls, relying on a combination of SN, DMN, and/or ECN. Interestingly, our finding that, in boys, higher levels of dACC-to-SN coherence is associated with lower levels of brooding suggests that the dACC may serve a more cognitive-control related function. Indeed, in this sample, boys were older than girls; in light of evidence that the capacity of the dACC to support cognitive control continues to improve as a function of age during adolescence (Ordaz et al., 2013), it may be that the dACC is better able to exert cognitive control over the emotional inputs it receives in boys than in girls. Finally, the RRS-A asked about the frequency of one’s ruminative cognitions in the circumstance when negative emotions arise. It is possible that boys’ negative emotions do not last as long or occur as often as is the case in girls; as a result, boys may spend less time engaged in ruminative cognitions overall, despite ruminating at similar frequencies to girls in negative emotional situations. In this case, girls’ more frequent and consistent activation of rumination-supporting networks would lead to a strengthened association between network coherence and rumination. Future research probing the network basis of ruminative brooding in boys over time should test these possibilities more explicitly and systematically.
Although we have suggested that puberty influences brain networks through the actions of gonadal hormones acting on receptors in the brain, it is clear that environmental influences, such as social interactions with peers or with parents, also influence brain development (Whittle et al., 2014, 2016). Using growth curve modeling with longitudinal data, investigators should examine whether positive peer relations and/or authoritative parenting buffer maladaptive trajectories of SN development. Finally, growth curves can highlight the periods within pubertal development where rates of change are the fastest, suggesting optimal periods for preventative intervention.
Supplementary Material
Acknowledgement
We thank Tiffany Ho, Aarthi Padmanbhan, and Collin Price for helpful discussions about the intrinsic functional connectivity analyses. We also thank Cat Camacho, Monica Ellwood, Meghan Goyer, Emily Livermore, Elaine Patten, Holly Phan, and Sophie Schouboe for their help in running participants through the study protocol. Last, we are most appreciative of the participants and their families for the time and their commitment to our research.
Funding
This work was supported by the National Institutes of Health (R01-MH101495 to IHG, K01-MH106805 to S.J.O., T32-MH019938 to Alan Schatzberg (funding S.J.O.), F32-MH102013 to J.L., U54-EB020403 to GP); the Brain & Behavior Research Foundation [Young Investigator Awards to S.J.O. (23582) and J.L. (2233)]; the Alzheimer’s Association, Michael J Fox. Foundation for Parkinson’s Research, and W. Garfield Weston Foundation (Foundation Biomarkers Across Neurodegenerative Diseases to G.P.), the National Science Foundation (Graduate Research Fellowship Program to N.C.), and a Klingenstein Third Generation Foundation (Fellowship Award to S.J.O.).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Abela J.R., Hankin B.L. (2011). Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: a multiwave longitudinal study. Journal of Abnormal Psychology, 120(2), 259–71. [DOI] [PubMed] [Google Scholar]
- Achenbach T.M. (1991) Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles, Burlington, VT: University of Vermont, Department of Psychology. [Google Scholar]
- Achenbach T.M., Rescorla L.A. (2001) Manual for the ASEBA School-Age Forms and Profiles, Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families.
- Admon R., Nickerson L.D., Dillon D.G., et al. (2015). Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychological Medicine, 45(1), 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. (2009). Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research, 171(3), 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Presented at the Annual Meeting of the Philosophical Transactions of the Royal Society London B: Biological Science, 360(1457), 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay N., Filippini N., Smith S.M. (2009). Group comparison of resting-state fMRI data using multi-subject ICA and dual regression. Presented at the Annual Meeting of the Organization of Human Brain Mapping. [Google Scholar]
- Berman M.G., Misic B., Buschkuehl M., et al. (2014). oes resting-state connectivity reflect depressive rumination? A tale of two analyses. Neuroimage, 103, p 267–79. [DOI] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance Medicine, 34(4), 537–41. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Yeo B.T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature of Neuroscience, 16(7), 832–7. [DOI] [PubMed] [Google Scholar]
- Burwell R.A., Shirk S.R. (2007). Subtypes of rumination in adolescence: associations between brooding, reflection, depressive symptoms, and coping. Jorunal of Clinical Child and Adolescent Psychology, 36(1), 56–65. [DOI] [PubMed] [Google Scholar]
- Carlson J.M., Cha J., Mujica-Parodi L.R. (2013). Functional and structural amygdala - anterior cingulate connectivity correlates with attentional bias to masked fearful faces. Cortex, 49(9), 2595–600. [DOI] [PubMed] [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., et al. (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry, 74(12), 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology, 493(1), 154–66. [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciecnes of the United States of America, 106(17), 7209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. (2014). The influence of puberty on subcortical brain development. Neuroimage, 88, 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. (2010). Cognition and depression: current status and future directions. Annual Revies of Clinical Psychology, 6, 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Krasnoperova E., Yue D.N., Joormann J. (2004). Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology, 113(1), 121–35. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex, 19(1), 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage, 82, 208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.L., Stange J.P., Abramson L.Y., Alloy L.B. (2015). Stress and the development of cognitive vulnerabilities to depression explain sex differences in depressive symptoms during adolescence. Clinical Psychological Science, 3(5), 702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen M.C., Gotlib I.H. (2013). Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of Disease, 52, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry, 169(7), 693–703. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Glover G.H., Bagarinao E., et al. (2016). Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Research, 249, 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel P., Petermann F. (2005). Age and gender effects on coping in children and adolescents. Journal of Youth and Adolescence, 34(2), 73–83. [Google Scholar]
- Hayes A.F. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis, New York, The Guilford Press. [Google Scholar]
- Ho T.C., Connolly C.G., Henje Blom E., et al. (2015). Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry, 78(9), 635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H.J., Gotts S.J., Reynolds R.C., et al. (2013). Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. Journal of Applied Mathematics, 2013, Article ID 935154, http://dx.doi.org/10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.P., Whisman M.A. (2013). Gender differences in rumination: a meta-analysis. Personality and Individual Differrence, 55(4), 367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Dkane M., Gotlib I.H. (2006). Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavioral Thereapy, 37(3), 269–80. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child Adolescent Psychiatry, 36(7), 980–8. [DOI] [PubMed] [Google Scholar]
- Kenny E.R., O’Brien J.T., Cousins D.A., et al. (2010). Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. The American Journal of Geriatric Psychiatry, 18(7), 643–51. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. (2014). Functional brain imaging studies of youth depression: a systematic review. NeuroImage: Clinical, 4, 209–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V., Kantola J.H., Jauhiainen J., Hyvarinen A., Tervonen O. (2003). Independent component analysis of nondeterministic fMRI signal sources. Neuroimage, 19(2 Pt 1), 253–60. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Vanderhasselt M.A., De Raedt R., Gallinat J. (2012). Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorder, 141(2–3), 352–60. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Vanderhasselt M.A., De Raedt R., Gallinat J. (2014). The neural basis of unwanted thoughts during resting state. Social Cognitive and Affective Neuroscience, 9(9), 1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage, 36(4), 1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Kong F., Qi S., You X., Huang X. (2015). Resting-state functional connectivity of the default mode network associated with happiness. Social Cognitive and Affective Neuroscience, 11(3), 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S., Nolen-Hoeksema S. (1993). Self-perpetuating properties of dysphoric rumination. Journal of Personal and Social Psychology, 65(2), 339–49. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S., Nolen-Hoeksema S. (1995). Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personal and Social Psychology, 69(1), 176–90. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S., Tucker K.L., Caldwell N.D., Berg K. (1999). Why ruminators are poor problem solvers: clues from the phenomenology of dysphoric rumination. Journal of Personal and Social Psychology, 77(5), 1041–60. [DOI] [PubMed] [Google Scholar]
- Maalouf F.T., Clark L., Tavitian L., Sahakian B.J., Brent D., Phillips M.L. (2012). Bias to negative emotions: a depression state-dependent marker in adolescent major depressive disorder. Psychiatry Research, 198(1), 28–33. [DOI] [PubMed] [Google Scholar]
- MacKinnon D.P. (2008) An Introduction to Statistical Mediation Analysis, Mahway, NJ, Routledge Academic. [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. (2015). The contribution of network organization and integration to the development of cognitive control. PLoS Biology, 13(12), e1002328.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. (1968). Growth and physiological development during adolescence. Annual Review of Medicine, 19, p 283–300. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Nolen-Hoeksema S. (2011). Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy, 49(3), 186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Science, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–82. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Davis C.G. (1999). “Thanks for sharing that”: ruminators and their social support networks. Journal of Personal and Social Psychology, 77(4), 801–14. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–24. [DOI] [PubMed] [Google Scholar]
- Onoda K., Yamaguchi S. (2015). Dissociative contributions of the anterior cingulate cortex to apathy and depression: Topological evidence from resting-state functional MRI. Neuropsychologia, 77, 10–8. [DOI] [PubMed] [Google Scholar]
- Ordaz S., Foran W., Velanova K., Luna B. (2013). Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience, 33(46), 18109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton G.C., Viner R. (2007). Pubertal transitions in health. Lancet, 369(9567), 1130–9. [DOI] [PubMed] [Google Scholar]
- Piguet C., Desseilles M., Sterpenich V., Cojan Y., Bertschy G., Vuilleumier P. (2014). Neural substrates of rumination tendency in non-depressed individuals. Biological Psychology, 103, 195–202. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Rucker D.D., Hayes A.F. (2007). Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behavioural Research, 42(1), 185–227. [DOI] [PubMed] [Google Scholar]
- Rood L., Roelofs J., Bogels S.M., Nolen-Hoeksema S., Schouten E. (2009). The influence of emotion-focused rumination and distraction on depressive symptoms in non-clinical youth: a meta-analytic review. Clinical and Psychological Review, 29(7), 607–16. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., et al. (2013a). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage, 64, 240–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., et al. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage, 60(1), 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Ruparel K., et al. (2013b). Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage, 83, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C.L., Zehr J.L. (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26(3–4), 163–74. [DOI] [PubMed] [Google Scholar]
- Slora E.J., Bocian A.B., Herman-Giddens M.E., et al. (2009). Assessing inter-rater reliability (IRR) of Tanner staging and orchidometer use with boys: a study from PROS. Journal of Pediatric Endocrinology and Metabolism, 22(4), 291–9. [DOI] [PubMed] [Google Scholar]
- Smith D.V., Utevsky A.V., Bland A.R., et al. (2014). Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage, 95, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the Naional Academy of Sciences of the United States of America, 106(31), 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C., Castro-Fornieles J., de la Serna E., et al. (2016). Intrinsic connectivity networks from childhood to late adolescence: Effects of age and sex. Developmental Cognitive Neuroscience, 17, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somandepalli K., Kelly C., Reiss P.T., et al. (2015). Short-term test-retest reliability of resting state fMRI metrics in children with and without attention-deficit/hyperactivity disorder. Developmental Cognitive Neuroscience, 15, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spati J., Hanggi J., Doerig N., et al. (2015). Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacology, 40(7), 1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dennis E.L., Joshi A.A., et al. (2011). ' Resting-state fMRI can reliably map neural networks in children. Neuroimage 55(1), 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. (2003). Rumination reconsidered: A Psychometric analysis. Cognitive Therapy and Research, 27(3), 247–59. [Google Scholar]
- Uddin L.Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Review of Neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C., Achterberg M., Braams B.R., Peters S., Crone E.A. (2016). Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage, 124(Pt A), 409–20. [DOI] [PubMed] [Google Scholar]
- Wenzlaff R.M., Wegner D.M., Roper D.W. (1988). Depression and mental control: the resurgence of unwanted negative thoughts. Journal of Personality and Social Psychology, 55(6), 882–92. [DOI] [PubMed] [Google Scholar]
- Whittle S., Simmons J.G., Dennison M., et al. (2014). Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Dennison M., et al. (2016). Observed measures of negative parenting predict brain development during adolescence. PLoS One, 11(1), e0147774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T., Peng J., Nie B., et al. (2012). Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. European Journal of Radiology, 81(12), 4035–40. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., et al. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry, 71(7), 611–7. [DOI] [PubMed] [Google Scholar]
- Zuo X.N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. (2010). Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage, 49(3), 2163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.