Abstract
High frequency repetitive Transcranial Magnetic Stimulation (rTMS) over the left dorsolateral prefrontal cortex (DLPFC) has been found to alleviate depressive symptoms. However, the mechanisms driving these effects are still poorly understood. In the current study, we tested the idea that this intervention protects against negative mood shifts following emotional provocation. We furthermore explored changes in EEG activity (frontal alpha asymmetry) and effects on attentional processing (emotional Stroop). To this end, 23 healthy individuals participated in two sessions separated by one week, whereby they once received 15 min of 10Hz rTMS stimulation (1500 pulses) at 110% of the individual motor threshold, and once sham stimulation. Then, negative mood was induced using sad movie clips. The results revealed a significantly stronger mood decline following rTMS compared to sham stimulation. No changes were observed in frontal alpha asymmetry and attentional processing. Our findings are at odds with the view that high frequency rTMS over the left DLPFC directly protects against the induction of negative mood, but rather suggest that it enhances the effects of emotional provocation. Possibly, in healthy young individuals, this stimulation protocol heightens susceptibility to mood induction procedures in general.
Keywords: repetitive Transcranial Magnetic Stimulation, DLPFC, negative mood induction, depression, alpha asymmetry
Introduction
Depression is expected to become the leading cause of disabilities worldwide with about 20% of the population suffering from a mood disorder at least once in a lifetime (Kessler et al., 2005; de Graaf et al., 2012). Even more alarmingly, depression is characterized by high relapse rates, which increase steeply with every subsequent depressive episode, even following psychotherapy (Steinert et al., 2014). These findings underline the importance of improving existing treatments and emphasize the necessity of effective prevention instruments to interrupt this vicious circle.
One technique that has gained attention as a potential treatment adjunct for depression and as a tool for relapse prevention is repetitive transcranial magnetic stimulation (rTMS). rTMS is a non-invasive brain stimulation technique, whereby trains of brief magnetic fields are generated in a coil to induce electric fields in the nerve tissue underneath the coil, leading to neuronal depolarization (Hallett, 2007). When applied at high frequencies of ≥10 Hz (HF), rTMS has been shown to increase neuronal excitability of the stimulated site, which outlasts the stimulation itself (Fitzgerald et al., 2006). Results from meta-analyses have demonstrated that HF rTMS to the left frontal cortex ameliorates depressive symptoms with comparable effect sizes as observed in psychotherapy and pharmacological treatments (for reviews see Fitzgerald et al., 2003; Schutter, 2009; Chen et al., 2013).
In healthy samples, early studies found an increase in negative mood after stimulation of the left prefrontal cortex (George et al., 1996; Pascual-Leone et al., 1996; Dearing Martin et al., 1997), while more recent studies did not detect any changes in mood as a result of the stimulation itself (Mosimann et al., 2000; Grisaru et al., 2001; Padberg et al., 2001; Baekenet al., 2006, 2008; Baeken et al., 2014; Moulier et al., 2016). Schaller et al. (2011) demonstrated that several sessions of HF rTMS attenuated only depression ratings of healthy individuals as assessed with the Becks Depression Inventory, while subjective mood ratings remained stable. A recent review concluded that a single session stimulation is not sufficient to alter mood states directly in healthy individuals (Remue et al., 2016).
Summarizing, despite the mood enhancing effects of HF rTMS in patient samples, the underlying working mechanisms are not yet fully understood. Studies investigating the effects of DLPFC stimulation on cognitive and emotional processing found that HF rTMS over the left DLPFC can improve task-switching abilities of depressed individuals (Vanderhasselt et al., 2009), and that the same protocol can alter attentional processing of emotional stimuli in healthy women (De Raedt et al., 2010). In the latter study, the authors used an exogenous cueing task that assessed facilitated attentional engagement with angry faces, and difficulties disengaging attention from them. Whereas HF stimulation of the right DLPFC resulted in impaired disengagement from negative faces, stimulation of the left DLPFC resulted in reduced engagement towards these stimuli. Moreover, on the neuronal level, these effects were accompanied by changes in PFC activity.
This suggests that rTMS modulates a neuronal network involved in emotion regulation, thereby attenuating the negative impact of adverse events. Indeed, the PFC has been linked to the regulation of negative emotions in numerous studies (Ochsner et al., 2002, 2004; Phan et al., 2005; Johnstone et al., 2007). Summarizing, different lines of research suggest that HF rTMS over the left DLPFC ameliorates cognitive functions that have been found to be impaired in depressed individuals and have been linked to the emergence and recurrence of depression (Hammar and Ardal, 2009; Snyder, 2013). In light of these findings, it is conceivable that HF rTMS over the left DLPFC has protective effects against a subsequent negative event. Indeed, recent studies have found that HF rTMS over the left DLPFC prior to a stress-inducing task attenuates subsequent decreases in heart rate variability (Remue et al., 2015) and stress hormonal responses (Baeken et al., 2014), as compared to stimulation over the right DLPFC and/or sham stimulation. Based on these considerations, the present study aims at investigating the effects of rTMS stimulation over the left DLPFC on affective responses to a negative mood induction and on attentional processing, which are both of direct relevance to emotion regulation in depression.
In order to get a better understanding of the neurophysiological effects of rTMS on attentional processing and emotion regulation, the electroencephalogram (EEG) is a non-invasive way to study electrocortical field potentials recorded over the left and right frontal cortex. In particular, examining hemispheric differences in alpha power (8–12 Hz) is a commonly used correlate of underlying cortical activity (Coan and Allen, 2004). Stronger right-sided alpha power (conventionally interpreted as reflecting more left-sided brain activity; Henriques and Davidson, 1990; Gotlib et al., 1998) is thought to reflect stronger approach and weaker withdrawal motivation, as well as stronger positive affect (Nusslock et al., 2015). This marker has been linked to current and lifetime depression, as well as to the prospective risk to develop depression (for review, see Allen and Reznik, 2015). Moreover, stronger left lateralized alpha at rest and in response to emotional provocation has been found to predict stronger mood decline and hormonal stress responses (Papousek et al., 2014; Quaedflieg et al., 2015). Furthermore, increased left-lateralized alpha following neurofeedback attenuates subjective habituation to laboratory stress induction (Quaedflieg et al., 2016). Together, these findings show striking parallels with the above-mentioned effects of rTMS over the left DLPFC. Therefore, the current study explored whether the potential effects of rTMS on affective responding are accompanied by changes in frontal alpha asymmetry. In addition, we also assessed asymmetries in the beta band (i.e. 13–30 Hz) as these might be similarly related to the attentional avoidance of angry faces (Schutter et al., 2001, 2008).
This exploratory study investigated whether mood responses to a negative event can be modulated by HF rTMS over the left DLPFC, using a counterbalanced within-subject crossover design. In particular, healthy individuals received a single session of 10 Hz rTMS, before inducing a negative mood by means of a short movie sequence. Compared to a session of sham rTMS, we expected HF rTMS to attenuate mood decline in response to the movie. Furthermore, we explored whether these potential effects are accompanied by changes in frontal alpha asymmetry, that is, a reduction in relative left lateralized alpha. Finally, as stimulation of the DLPFC affects attentional processing of emotional faces, an emotional Stroop task (van Honk et al., 2002) was used to assess biased processing of angry and sad faces. In particular, we anticipated attenuated interference of emotional (i.e. sad and angry) faces compared to neutral faces after HF rTMS than after sham stimulation.
Materials and methods
Participants
In this exploratory study, 23 right-handed students participated (Age: M = 21.5, SD = 3.0) in return for 50€. Participants were screened for contra-indications to rTMS according to the recommendations by the TMS Consensus Group (Rossi et al., 2009, 2011). Prior to inclusion, we screened participants, using the following exclusion criteria: (1) metal in cranium, (2) use of psychotropic drugs (e.g. cannabis, XTC), (3) a history of severe neurological problems (e.g. epilepsy, head injury or head surgery and brain infarction), (4) history of psychiatric disorders, (5) medication use (i.e. benzodiazepines, antidepressants and neuroleptica), (6) severe physical illness (e.g. heart disease) and (7) pregnancy. Moreover, participants were excluded if they had heightened scores (i.e. above 13) on the Beck’s Depression Inventory (BDI-II; Beck et al., 1996), which was administered during the screening prior to the study. This study was conducted in accordance to the Declaration of Helsinki and approved by the local medical ethical committee (CMO Region Arnhem-Nijmegen, the Netherlands). Participants gave written informed consent.
Material and instruments
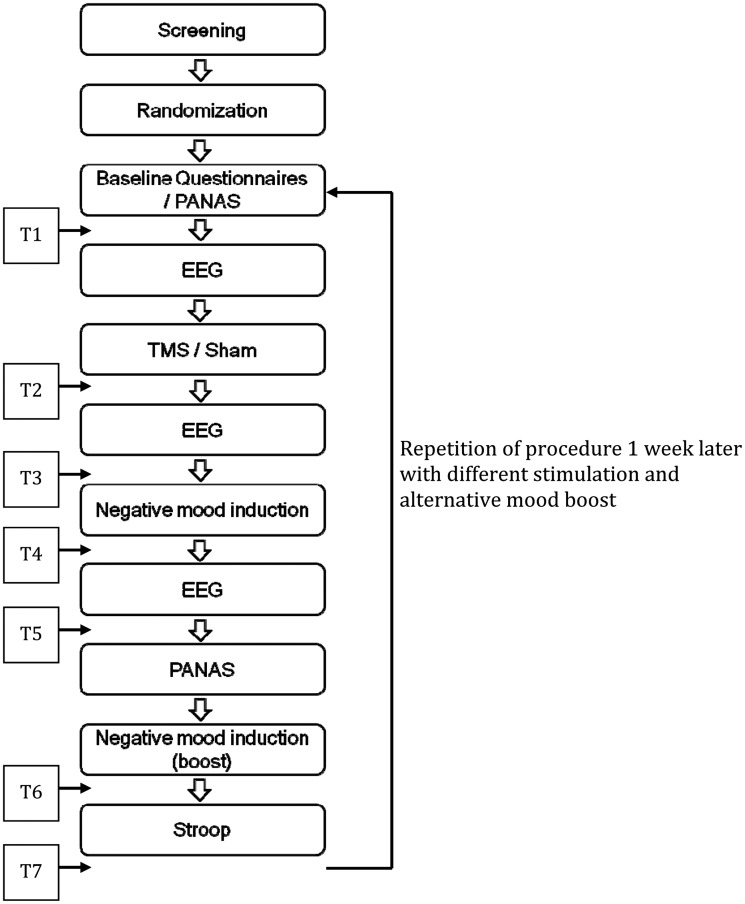
Questionnaires. In order to assess the time-course of affective responding, two 10-point Likert scales were administered at seven different time points throughout each session (see Figure 1 for an overview). In analogy to previous studies with a similar mood induction procedure (Vrijsen et al., 2013), we asked participants to indicate how happy and how sad they currently felt (anchors: 1 = not at all; 10 = extremely). The scales were combined to a sum score, such that higher values represent a more positive mood state.
Fig. 1.
Procedural overview. Note. T1-T7: 7 assessment points of Likert scales.
In addition, the German and the Dutch translation of the Positive Affect Negative Affect Schedule (Watson et al., 1988; Krohne et al., 1996) were used to assess changes in positive (PA) and negative affect (NA) in response to the mood induction procedure. These scales were administered twice during each session, first at baseline and second after the EEG measure that followed the first mood induction movie (Figure 1).
rTMS procedure. Repetitive transcranial magnetic stimulation was administered using a MagStim Rapid2 (Magstim, UK) with a figure-of-eight coil. During the active treatment, participants received 60 trains of 5-s 10 Hz stimulation with an inter-train interval of 25 s. The intensity was set to 110% of the resting motor threshold of the right abductor pollicis brevis. The site of stimulation was F3 according to the international 10–20 system (American Electroencephalographic Society, 1994). We chose for this procedure, as it is the most accurate way to identify the DLPFC without using a neuro-navigation system (Fitzgerald and Daskalakis, 2012). For the sham-stimulation, these parameters were held constant, while only the orientation of the coil was tilted for 45° away from the cortex.
Mood induction. The mood induction procedure was adapted from Fitzgerald et al. (2011), selecting three clips from the movie ‘Sophie’s Choice’ with different durations (i.e. 12, 6 and 5 min). The first clip was used to induce negative mood, while the two shorter clips were presented to boost the negative mood. Participants were instructed to place themselves into the position of the main actor in order to allow them to fully perceive the negative emotion.
Resting state EEG. At three time points throughout each session, resting state EEG was recorded. Each recording consisted of four blocks of 1 min, with alternating instructions to keep the eyes closed or open. EEG was measured using a standard 32-channel setup (BrainVision QuickAmp, Brain Products) according to the international 10–20 system, using 32 electrodes. The signal ground was placed on the nose, the online reference was located at the left mastoid (A1) while the right mastoid (A2) was serving as additional recording channel. Four electrodes were placed at the eyes to control for horizontal and vertical eye-movement. All electrode impedances were kept below 5 kΩ. Data was recorded at a frequency of 500 Hz applying a band pass filter of 0.3–70 Hz.
For data reduction, all electrodes were re-referenced to the overall mean. Due to presence of excessive muscle activity over T7 and T8 in a large proportion of subjects, these electrodes were excluded from the average reference. An offline filter with 1–30 Hz was applied, before dividing the signal into 75% overlapping epochs of each 2 s, and removing epochs carrying artifacts (i.e. amplitude ± 75 μV; maximal voltage step exceeding 50 μV/ms; maximal change in amplitude exceeding 100 μV/500 ms). A Fast-Fourier Transformation (Hanning window: 100% length) was conducted on the remaining epochs, and mean values of the FFT were calculated, separately for α (8–12 Hz) and β (13–30 Hz) frequency bands. A lateralization score for the different frequencies was calculated by subtracting the summed signal from the left frontal electrodes (i.e. F3, F7 and FC5) from the summed signal of the right frontal electrodes (i.e. F4, F8 and FC6) and dividing this by the sum of these six electrodes. More positive values represent relatively stronger right-lateralized power. As previous studies (Boytsova and Danko, 2010; Ben-Simon et al., 2013) suggest that the EEG signal assessed with eyes closed represents a different attentional state from eyes open (i.e. invert directed attention versus outwards direct attention), eyes-open/eyes closed condition was used as an additional within-subjects factor in all subsequent analyses.
Emotional Stroop Task. An emotional Stroop task (van Honk et al., 2002) was used to assess individual differences in attentional bias for emotional faces. Pictures of oval-cut faces from 10 different characters (5 female, 5 male), each displaying three different emotional expressions (i.e. anger, sadness and neutral), were taken from Ekman and Friesen (1976). Two versions of each face, one colored in blue and one in yellow, were presented on a black background. All combinations were presented twice, resulting in 120 trials. Every trial started with a white fixation-cross, replaced by a single face after a random interval of 1500–2500 ms. Participants had to indicate the color as fast as possible by pressing a corresponding button on the keyboard. Latencies above or below 3 SD of the individual mean were removed. Bias scores were calculated, separately for sad and angry faces, by subtracting the median reaction time on trials with neutral faces from the median reaction time on emotional faces. Thus, higher values indicate a stronger interference, which indicates an attentional bias towards the respective emotional expression.
Procedure
Participants were invited to two laboratory sessions separated by one week. The procedure of both sessions was nearly identical, except that they once underwent real rTMS and once sham TMS (order was counterbalanced across participants). After providing informed consent, they were given the set of questionnaires including the PANAS. This set of questionnaires also contained the BDI and the Spielberger State-Trait Anxiety Inventory (STAI-T; Spielberger et al., 1983), which are not reported here. Subsequently, the baseline EEG was administered followed by the (sham) rTMS stimulation. Next, the second EEG assessment took place after which the first mood induction movie was presented on the computer screen. Subsequently, the last EEG assessment took place, followed by the PANAS and one of the two mood boosters (counterbalanced across sessions), and finally the emotional Stroop task. During each session, mood scales were administered at baseline (T1), after the stimulation (T2), after the first EEG assessment (T3; about 10 min after stimulation), after the first mood induction movie (T4, about 30 min after stimulation), after the second EEG assessment (T5; about 40 min after stimulation), after the mood booster (T6; about 50 min after stimulation) and after the emotional Stroop task (T7; about 60 min after stimulation). At the end of the second session, participants were fully debriefed and paid. One session lasted about 3 h in total. See Figure 1 for an overview of the procedure.
Data analysis
To investigate changes in mood across the seven measurements (i.e. using the two Likert scales), we performed the following two analyses: First, immediate effects of rTMS on mood were tested by a 2 (stimulation: rTMS, Sham) × 2 (time: T1, T2) repeated-measures (RM) analysis of variance (ANOVA). Second, preventive effects of the stimulation on mood decline were tested in a 2 (stimulation: rTMS, Sham) × 4 (time: T3, T4, T5, T6) RM ANOVA. Additionally, stimulation dependent changes in positive and negative affect in response to the mood induction were tested in 2 (stimulation: rTMS, Sham) × 2 (time: T1, T5) RM ANOVAs, separately for PANAS-PA and PANAS-NA scores.
Bias scores of the Stroop task were subjected to a 2 (stimulation: rTMS, Sham) × 2 (valence: angry, sad) RM ANOVA. Finally, changes in alpha asymmetry were explored by means of a 2 (stimulation: rTMS, Sham) × 2 (condition: eyes-open, eyes-closed) × 3 (time: baseline, post-stimulation, post-mood induction) repeated measures ANOVA. In order to assess potential order effects, the analyses were repeated with order (rTMS first, sham first) as a between-subjects factor. Where assumptions of sphericity were violated, we reported Greenhouse–Geisser corrected statistics.
Results
Effects of stimulation on mood
A 2 (intervention: rTMS, Sham) × 2 (time: T1, T2) RM ANOVA on mood scales revealed no significant interaction, F(1,22) = 0.51, P = 0.484, indicating that the stimulation itself did not alter the mood state. There was a marginal significant overall drop in mood, F(1,22) = 3.95, P = 0.059, η2 = 0.15. See Table 1 for an overview of the mood ratings.
Table 1.
Mean (SD) mood ratings as assessed with the Likert scales
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|
| rTMS | 16.43 (1.75) | 15.43 (2.48) | 16.13 (2.18) | 13.57 (3.13) | 15.91 (1.91) | 10.68 (4.35) | 13.87 (3.14) |
| Sham | 16.65 (1.90) | 16.22 (2.63) | 16.35 (1.90) | 14.43 (2.61) | 15.78 (1.93) | 12.00 (3.78) | 15.09 (2.52) |
Note. T1–T7: seven assessment points of mood scales.
In order to test whether the stimulation modified mood changes in response to the mood induction, a 2 (intervention: TMS, Sham) × 4 (time: T3, T4, T5, T6) RM ANOVA was conducted. This analysis revealed a significant effect of time [F (1.42, 29.84) = 44.83, P < 0.001, η2 = 0.68], which was moderated by intervention [F (2.36, 49.47) = 3.32, P = 0.037, η2 = 0.14). Post-hoc tests indicated that the mood state was comparable between interventions before the mood induction, t(22) = 0.49, P = 0.630, and at the subsequent two assessment points, T4: t(22) = 1.53, P = 0.142, T5: t(22) = 0.38, P = 0.710. After the second mood boost at T6, mood was significantly lower after the rTMS stimulation as compared to the sham stimulation, t(22) = 2.5, P = 0.030, d = 0.51 (Table 1).
Next, changes in positive affect and negative affect as a result of the mood induction were investigated. A 2 (intervention: TMS, Sham) × 2 (time: T1, T5) RM ANOVA on PANAS-PA scores did only reveal a significant main effect of time, F(1,22) = 26.49, P < 0.001, η2 = 0.55, indicating an overall drop in positive affect (T1: M = 35.41, SD = 1.35; T5: M = 29.07, SD = 1.82). The remaining main and interaction effects were all not significant (P > 0.5). The same analysis with PANAS-NA scores also indicated a general increase in negative affect (T1: M = 11.74, SD = 0.56; T5: M = 13.98, SD = 0.9, F(1,22) = 10.6, P = 0.004, η2 = 0.33), with no difference between intervention or moderation by intervention (P > 0.245).
Effects on attentional processing
A 2 (Intervention: TMS, Sham) × 2 (Valence: sad, angry) RM ANOVA revealed neither a significant effect of intervention F(1, 21) = 0.15, P = 0.699, nor an effect of valence, F(1, 21) = 0.44, P = 0.513, or an interaction of both, F(1, 21) = 0.45, P = 0.507.
Changes on EEG frequencies bands
A 2 (Intervention: TMS, Sham) × 3 (time: baseline, post TMS, post mood induction) × 2 (EEG condition: eyes open, eyes closed) RM ANOVA on alpha asymmetry scores revealed no three way interaction effect, F(1.59, 34.92) = 0.45, P = 0.598, or intervention dependent changes over time, F(1.53, 33.63) = 0.06, P = 0.894. Moreover, there was no overall difference between the two interventions, F(1, 22) = 2.70, P = 0.114. Only the EEG conditions displayed a marginally significant difference, F(1, 22) = 4.12, P = 0.055, η2 = 0.16, with stronger left lateralized alpha asymmetry with open eyes. All other main and interaction effects were not significant either (P > 0.350).
A 2 (Intervention: TMS, Sham) × 3 (Time: baseline, post-TMS, post-mood induction) × 2 (EEG condition: eyes open, eyes closed) RM ANOVA to explore changes in beta asymmetry scores revealed no intervention dependent changes over time, F(2, 21) = 0.61, P = 0.553, nor a three way interaction effect, F(2, 21) = 0.74, P = 0.491. All other main and interaction effects were not significant either (P > 0.391).
Order effects
The distribution of male and female participants was equal across order of sessions χ2(1, N = 23) = 0.38, P = 0.537. Mood scores did not differ between interventions at baseline, t(22) = 0.59, P = 0.565, however, during the first session participants had higher baseline positive affect and higher negative affect, suggesting generally increased arousal (PA: session 1: M = 36.43, SD = 6.00, session 2: M = 34.39, SD = 7.60, t(22) = 2.27, P = 0.033; NA: session 1: M = 12.35, SD = 2.98, session 2: M = 11.13, SD = 2.74, t(22) = 3.03, P = 0.006).
Out of the 23 participants, 15 reported that this study was about the impact of TMS on mood, however, none of these participants made a guess about the direction of this relation. The blinding was successful for the first session, with 11 out of the 23 participants correctly guessing their condition, χ2(1, N = 23) = 0.05, P = 0.827. During the second session, 16 participants correctly guessed their condition, which is above chance level, χ2(1, N = 23) = 4.68, P = 0.031. This effect might be explained by priming, as they were able to use the first session as reference. However, to assure that order of sessions does not account for the findings, all main analyses were repeated with order of sessions as between-subjects factor.
Only in the analyses assessing changes in negative affect, order revealed an interaction with the intervention, F(1, 21) = 8.14, P = 0.010. This interaction was driven by higher levels of negative affect during the first session for both orders [TMS first: session 1: M = 13.46, SD = 1.2, session 2: M = 12.46, SD = 1.16, F(1, 10) = 3.86, P = 0.078, η2 = 0.28; Sham first: session 1: M = 13.42, SD = 0.92, session 2: M = 12.13, SD = 0.7, F(1, 11) = 4.47, P = 0.058, η2 = 0.29]. In all remaining analyses, the results remained the same and order did not interact with the intervention.
Discussion
Aim of this exploratory study was to investigate whether a single session of HF rTMS over the left DLPFC alters the impact of a negative mood induction procedure in healthy individuals. Contrary to our hypothesis, the results point to a stronger mood decline after the negative mood induction as a result of rTMS.
This mood decline only occurred in response to the mood induction procedure, but not directly after the stimulation itself, pointing to an interaction of rTMS and mood induction. These findings are thereby in line with recent TMS studies showing that (a single session of) HF rTMS to the left prefrontal cortex does not change mood states in healthy individuals (Mosimann et al., 2000; Grisaru et al., 2001; Padberg et al., 2001; Baeken et al., 2006, 2008, 2014; Moulier et al., 2016; for review see, Remue et al., 2016). However, after presenting the mood induction, mood decline was amplified as a function of rTMS. In order to confirm that this change is not the result of deteriorating mood independent of the mood induction, as indicated by the study by George et al. (1996), subsequent studies may want to control for the natural course of mood state.
It is important to note that participants received the instructions to place themselves in the perspective of the main character of the movie to fully perceive the negative emotion. Hence, this enhanced mood decline after rTMS compared to sham rTMS may be interpreted as improved emotion regulation. That is, participants may have been better able to follow the instructions after receiving the real stimulation. The role of dorsal regions of the PFC in emotional regulation has been demonstrated in previous studies (for review, see Mitchell, 2011), showing the involvement of the left PFC during mood regulation in general, and during the up regulation of mood in particular (Ochsner et al., 2004). Hence, HF rTMS over the DLPFC may have modulated the susceptibility to emotional processing, while the instructions to up-regulate the negative emotion determined the directionality of mood change. Support for this explanation comes from a study by Feeser et al. (2014) who found a strengthening in reappraisal of negative emotions after transcranial direct current stimulation (tDCS) over the right DLPFC. Furthermore, Padberg et al. (2001) also found an increase in laughing frequency in response to a humorous movie clip, after receiving HF rTMS to the left as compared to the right prefrontal cortex. These changes in facial expression point to an increase in positive mood and thus to a possible up-regulation of positive emotions. However, no changes in subjective mood ratings have been found in this study.
Another explanation is related to the concept of homeostatic metaplasticity (Ziemann and Siebner, 2008). It is possible that rTMS resulted in perturbations of the neural system of healthy individuals, which tried to go back into its baseline homeostatic default-state after the stimulation, and thereby amplified the mood decline. According to this line of thought, low frequency (e.g. 1 Hz) stimulation protocol, which initially reduces excitability prior to a negative mood induction, might attenuate the impact of the negative mood induction. However, one may also argue that the perturbations due to rTMS may interfere with mood regulation in general, resulting in an amplified mood decline.
It is important to mention that the differential effects on mood decline occurred only after the negative emotion-boost towards the end of the session, whereas they were not yet evident directly after the first mood induction clips. This pattern was also reflected in changes on the PANAS scale, which showed a significant mood deterioration that remained unaffected by the rTMS intervention. Crucially, the time point of administering the PANAS might not have been suited to identify such changes, since the post assessment took place before the booster of negative mood. At that moment, recovery from the negative mood might have minimized the mood differences between the stimulation conditions.
In contrast to our expectations, EEG analyses did not reveal any reliable changes in frontal alpha (or beta) asymmetry in response to the emotional provocation. This suggests that the effects of left PFC stimulation on mood were not mediated by a change in frontal alpha asymmetry. However, this does not rule out the possibility of rTMS to affect frontal asymmetry at other time points during the session. Indeed, our subjective mood data suggests that the critical rTMS effects may have taken place after the second mood induction (i.e. the mood booster) rather than directly after the first mood induction. Moreover, it is possible that there were more short-lived rTMS effects on frontal asymmetry that went undetected. For instance, Papousek et al. (2014) found that emotional films acutely induced more right-sided alpha activity that corresponded with deteriorated mood, but these effects had vanished after the movie. This pattern aligns with the capability model of frontal alpha asymmetry (Coan et al., 2006), according to which this EEG marker is most sensitive to affective responding and to individual differences in emotional vulnerability when measured during an emotional challenge (for review, see Allen and Reznik, 2015).
It is also conceivable that the effects of the stimulation are detectable at other cortical regions. The right parietal cortex is one potential region of interest for the effects of neuro-stimulation in depression. Based on the functional connectivity between left prefrontal and right parietal cortex (for review, see Schutter and van Honk, 2005), Schutter et al. demonstrated beneficial effects of 2 Hz rTMS over this region on depression ratings and cognitive markers of depression (Schutter et al., 2009, 2010). Taken together, the potential effects of rTMS over the DLPFC on frontal asymmetry remains to be investigated. A particularly promising avenue would be to focus on effects that take place during the mood induction or in response to the negative mood booster, next to exploring rTMS effects beyond the frontal cortex.
There are two reasons why we expected attenuations of the interference of negative pictures during the Stroop task, as a function of rTMS. First, we thought that a dampening of the mood decline would lead to weaker interference effects due to the mood congruent processing style (Gilboa-Schechtman et al., 2000). In other words, the less sad participants are, the weaker the impact of negative faces during the task, and hence, the weaker the interference effect. Second, stimulation of the DLPFC is expected to affect cognitive control processes (Vanderhasselt et al., 2006, 2009) that modulate performance during the Stroop task. The high frequency stimulation may have resulted in a reduction of the interference due to an increase in cognitive control. The combination of both aspects might explain why no effects were found on the Stroop task, as they might have canceled out each other. Thus, the current stimulation protocol might have improved cognitive control and reduced positive mood at the same time, both leading to opposite Stroop effects.
The results of this exploratory study should be carefully interpreted, taking several limitations into account. First of all, the crossover design allowed participants to experience both stimulation conditions and thereby to detect slight differences in scalp sensations. However, despite this factor limiting the blinding, our analyses did not indicate any order effects. Still, improved control procedures or study designs might be preferable. Second, MRI based localization might be considered as alternative to determine the target location (Sparing et al., 2008; Ahdab et al., 2010). Third, this study was conducted with healthy individuals, which have been shown to differ in functional connectivity from depressed patients (for review, see Pizzagalli, 2010). Hence, the neural effects of stimulation may differ between these populations. Fourth, replication with larger samples is essential before drawing solid conclusions. We recommend that these replication studies include a positive mood induction procedure and a contrasting rTMS stimulation protocol (i.e. low frequency stimulation) for which opposite effects would be expected, to disentangle the working mechanism of the current effects. Furthermore, the up- and down-regulation of emotional information by means of neurostimulation is a promising method with potential therapeutic value, worthy of investigation. Finally, more narrowly tracking the time course of changes in alpha asymmetry and other neural correlates may help to better understand the working mechanisms relevant for the current study. For instance, future studies should consider assessing frontal asymmetry during an emotional task, rather than during rest.
In conclusion, this study indicates that rTMS can modulate the impact of a negative mood induction. Contrary to our expectations, HF rTMS over the left DLPFC seems to increase the susceptibility to mood responses in general, while the context determines the directionality of this change. Hence, due to the negative mood induction procedure in the current study, HF rTMS might have been related to a stronger mood decline. These findings provide a new perspective on the effects of rTMS that require subsequent research on possibilities to prevent depressive episodes. As pointed out, potential approaches could focus on low frequency stimulation to prevent a mood decline or on using a positive mood induction after a high frequency rTMS treatment, in order to target the absence of positive affect in depression.
Acknowledgements
We would like to thank Jan Leijtens for his support with setting up and conducting this study.
Funding
This project was financially supported by the Behavioral Science Institute, Nijmegen and by the Radboud UMC, Nijmegen, the Netherlands.
References
- Ahdab R., Ayache S.S., Brugières P., Goujon C., Lefaucheur J.P. (2010). Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Clinical Neurophysiology, 40(1), 27–36. [DOI] [PubMed] [Google Scholar]
- Allen J.J.B., Reznik S.J. (2015). Frontal EEG asymmetry as a promising marker of depression vulnerability: summary and methodological considerations. Current Opinion in Psychology, 4, 93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Electroencephalographic Society. (1994). Guideline thirteen: Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology, 11(1), 111–3. [PubMed] [Google Scholar]
- Baeken C., Leyman L., De Raedt R., Vanderhasselt M.A., D’haenen H. (2006). Lack of impact of repetitive high frequency transcranial magnetic stimulation on mood in healthy female subjects. Journal of Affective Disorders, 90(1), 63–6. [DOI] [PubMed] [Google Scholar]
- Baeken C., Leyman L., De Raedt R., Vanderhasselt M.A., D’haenen H. (2008). Left and right high frequency repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not affect mood in female volunteers. Clinical Neurophysiology, 119(3), 568–75. [DOI] [PubMed] [Google Scholar]
- Baeken C., Vanderhasselt M.A., Remue J., et al. (2014). One left dorsolateral prefrontal cortical HF-rTMS session attenuates HPA-system sensitivity to critical feedback in healthy females. Neuropsychologia, 57, 112–21. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R., Brown G. (1996). Beck Depression Inventory-II. San Antonio. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ben-Simon E., Podlipsky I., Okon-Singer H., et al. (2013). The dark side of the alpha rhythm: fMRI evidence for induced alpha modulation during complete darkness. The. European Journal of Neuroscience, 37(5), 795–803. [DOI] [PubMed] [Google Scholar]
- Boytsova Y.A., Danko S.G. (2010). EEG differences between resting states with eyes open and closed in darkness. Human Physiology, 36(3), 367–9. [Google Scholar]
- Chen J., Zhou C., Wu B., et al. (2013). Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Research, 210, 1260–4. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1–2), 7–49. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Allen J.J.B., McKnight P.E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf R., ten Have M., van Gool C., van Dorsselaer S. (2012). Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Social Psychiatry and Psychiatric Epidemiology, 47(2), 203–13. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Leyman L., Baeken C., et al. (2010). Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biological Psychology, 85(3), 487–95. [DOI] [PubMed] [Google Scholar]
- Dearing Martin J., George M.S., Greenberg B.D., et al. (1997). Mood effects of prefrontal repetitive high-frequency TMS in healthy volunteers. CNS Spectrums, 2(01), 53–68. [Google Scholar]
- Ekman P., Friesen W.V. (1976). Measuring facial movement. Environmental Psychology and Nonverbal Behavior, 1(1), 56–75. [Google Scholar]
- Feeser M., Prehn K., Kazzer P., Mungee A., Bajbouj M. (2014). Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimulation, 7, 105–12. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D.A., Arnold J.F., Becker E.S., et al. (2011). How mood challenges emotional memory formation: an fMRI investigation. NeuroImage, 56(3), 1783–90. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Brown T.L., Marston N.A.U., Daskalakis Z.J., De Castella A., Kulkarni J. (2003). Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Archives of General Psychiatry, 60(10), 1002–8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Daskalakis Z.J. (2012). A practical guide to the use of repetitive transcranial magnetic stimulation in the treatment of depression. Brain Stimulation, 5(3), 287–96. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Fountain S., Daskalakis Z.J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–96. [DOI] [PubMed] [Google Scholar]
- George M.S., Wassermann E.M., Williams W.A., et al. (1996). Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. The Journal of Neuropsychiatry and Clinical Neurosciences, 8(2), 172–80. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E., Revelle W., Gotlib I.H. (2000). Stroop interference following mood induction: emotionality, mood congruence, and concern relevance. Cognitive Therapy and Research, 24, 491–502. [Google Scholar]
- Gotlib I.H., Ranganath C., Rosenfeld J.P. (1998). Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion, 12(3), 449–78. [Google Scholar]
- Grisaru N., Bruno R., Pridmore S. (2001). Effect on the emotions of healthy individuals of slow repetitive transcranial magnetic stimulation applied to the prefrontal cortex. The Journal of ECT, 17(3), 184–9. [DOI] [PubMed] [Google Scholar]
- Hallett M. (2007). Transcranial magnetic stimulation: a primer. Neuron, 55(2), 187–99. [DOI] [PubMed] [Google Scholar]
- Hammar A., Ardal G. (2009). Cognitive functioning in major depression—a summary. Frontiers in Human Neuroscience, 3, 26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques J.B., Davidson R.J. (1990). Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology, 99(1), 22–31. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience, 27(33), 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Krohne H., Egloff B., Kohlmann C., Tausch A. (1996). Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule” (PANAS). Diagnostica, 42(2), 139–56. [Google Scholar]
- Mitchell D.G.V. (2011). The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behavioural Brain Research, 217(1), 215–31. [DOI] [PubMed] [Google Scholar]
- Mosimann U.P., Rihs T. a., Engeler J., Fisch H.U., Schlaepfer T.E. (2000). Mood effects of repetitive transcranial magnetic stimulation of left prefrontal cortex in healthy volunteers. Psychiatry Research, 94(3), 251–6. [DOI] [PubMed] [Google Scholar]
- Moulier V., Gaudeau-Bosma C., Isaac C., et al. (2016). Effect of repetitive transcranial magnetic stimulation on mood in healthy subjects. Socioaffective Neuroscience & Psychology, 6, 29672.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Walden K., Harmon-Jones E. (2015). Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: an RDoC Perspective. International Journal of Psychophysiology, 98, 249–261. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S. A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Padberg F., Juckel G., Prässl A., et al. (2001). Prefrontal cortex modulation of mood and emotionally induced facial expressions: a transcranial magnetic stimulation study. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(2), 206–12. [DOI] [PubMed] [Google Scholar]
- Papousek I., Weiss E.M., Schulter G., Fink A., Reiser E.M., Lackner H.K. (2014). Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: cardiac correlates and prediction of emotional impact. Biological Psychology, 103, 184–94. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Catalá M.D., Pascual A.P.L. (1996). Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology, 46(2), 499–502. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. a. (2010). Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology, 36(1), 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Meyer T., Smulders F.T.Y., Smeets T. (2015). The functional role of individual-alpha based frontal asymmetry in stress responding. Biological Psychology, 104, 75–81. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W.E.M., Smulders F.T.Y., Meyer T., Peeters F., Merckelbach H., Smeets T. (2016). The validity of individual frontal alpha asymmetry EEG neurofeedback. Social Cognitive and Affective Neuroscience, 11(1), 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remue J., Baeken C., De Raedt R. (2016). Does a single neurostimulation session really affect mood in healthy individuals? A systematic review. Neuropsychologia, 85, 184–98. [DOI] [PubMed] [Google Scholar]
- Remue J., Vanderhasselt M.A., Baeken C., Rossi V., Tullo J., Raedt R. (2015). The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology, 30, 759–66. [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. (2011). Screening questionnaire before TMS: an update. Clinical Neurophysiology, 122(8), 1686. [DOI] [PubMed] [Google Scholar]
- Schaller G., Lenz B., Friedrich K., et al. (2011). Repetitive transcranial magnetic stimulation influences mood in healthy male volunteers. Journal of Psychiatric Research, 45(9), 1178–83. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G. (2009). Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological Medicine, 39(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G., de Weijer A.D., Meuwese J.D.I., Morgan B., van Honk J. (2008). Interrelations between motivational stance, cortical excitability, and the frontal electroencephalogram asymmetry of emotion: a transcranial magnetic stimulation study. Human Brain Mapping, 29(5), 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J.L.G., Laman D.M., van Honk J., Vergouwen A.C., Koerselman G.F. (2009). Partial clinical response to 2 weeks of 2 Hz repetitive transcranial magnetic stimulation to the right parietal cortex in depression. The International Journal of Neuropsychopharmacology, 12(5), 643–50. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G., Putman P., Hermans E., van Honk J. (2001). Parietal electroencephalogram beta asymmetry and selective attention to angry facial expressions in healthy human subjects. Neuroscience Letters, 314(1–2), 13–6. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G., van Honk J. (2005). A framework for targeting alternative brain regions with repetitive transcranial magnetic stimulation in the treatment of depression. Journal of Psychiatry & Neuroscience, 30(2), 91–7. [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J.L.G., van Honk J., Laman M., Vergouwen A.C., Koerselman F. (2010). Increased sensitivity for angry faces in depressive disorder following 2 weeks of 2-Hz repetitive transcranial magnetic stimulation to the right parietal cortex. The International Journal of Neuropsychopharmacology, 13(9), 1155–61. [DOI] [PubMed] [Google Scholar]
- Snyder H.R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin, 139(1), 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R., Buelte D., Meister I.G., Paus T., Fink G.R. (2008). Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Human Brain Mapping, 29(1), 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, AC: Consulting Psychologist Press. [Google Scholar]
- Steinert C., Hofmann M., Kruse J., Leichsenring F. (2014). The prospective long-term course of adult depression in general practice and the community. A systematic literature review. Journal of Affective Disorders, 152–154, 65–75. [DOI] [PubMed] [Google Scholar]
- Van Honk J., Schutter D.J.L., D’Alfonso A.A., Kessels R.P., de Haan E.H. (2002). 1 hz rTMS over the right prefrontal cortex reduces vigilant attention to unmasked but not to masked fearful faces. Biological Psychiatry, 52(4), 312–7. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., De Raedt R., Baeken C., Leyman L., D’haenen H. (2006). The influence of rTMS over the left dorsolateral prefrontal cortex on Stroop task performance. Experimental Brain Research, 169(2), 279–82. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., De Raedt R., Baeken C., Leyman L., D’Haenen H. (2009). A single session of rTMS over the left dorsolateral prefrontal cortex influences attentional control in depressed patients. The World Journal of Biological Psychiatry, 10(1), 34–42. [DOI] [PubMed] [Google Scholar]
- Vrijsen J.N., Van Oostrom I., Speckens A., Becker E.S., Rinck M. (2013). Approach and avoidance of emotional faces in happy and sad mood. Cognitive Therapy and Research, 37, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Siebner H.R. (2008). Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimulation, 1(1), 60–6. [DOI] [PubMed] [Google Scholar]