Abstract
Dopamine plays an important role in goal-directed behavior, through its modulatory influence on striatal neurons. It is unclear whether tonic dopamine levels, which regulate the vigor of acting, interact with the phasic dopamine response to reward that drives instrumental behavior. In a randomized placebo-controlled study in healthy volunteers, we show that methylphenidate, a drug that increases tonic dopamine levels, systematically reduced striatal phasic BOLD responses to gain and loss in a gambling task as measured with fMRI. It also increased response vigor and reward expectancy-related BOLD signals in the ventral striatum. These findings suggest that striatal tonic dopamine levels constitute an average reward expectation signal that modulates the phasic dopaminergic response to reward. This offers opportunities for treatment of behavioral disorders associated with abnormal reward sensitivity.
Keywords: dopamine, Pharmacological fMRI, feedback, gambling
Introduction
The basal ganglia and prefrontal cortex play a critical role in voluntary behavior and reward-based learning. Both actions are modulated by dopamine that is released by midbrain neurons into the striatum. A range of studies show that tonic dopamine in the striatum facilitates action initiation and performance vigor. Drugs that increase tonic dopamine in the striatum, such as cocaine and methylphenidate (MPH), increase motor responsiveness and action readiness (Advokat, 2010; Spronk et al., 2013). Low levels of striatal dopamine on the other hand decrease response vigor and may cause loss of voluntary movement as in Parkinson’s disease (Antonelli and Strafella, 2014).
Electrophysiological studies of reward-based learning have shown that dopaminergic midbrain neurons also encode reward prediction error (RPE): when an outcome is better than expected their firing temporarily rises above baseline (burst firing), and when an outcome is worse than expected their firing falls below baseline (Schultz, 2007). Burst firing leads to a temporary increase in dopamine release (phasic dopamine) in dopaminergic projection regions such as the ventral striatum.
Niv (2007) suggested that the role of dopamine in voluntary behavior and reward-based learning are not independent. They hypothesized that tonic dopamine, besides controlling response vigor, also represents an average reward expectancy signal reflecting past reward history. Frequent release of phasic dopamine, following abundant rewards, leads to dopamine spill-over and thereby increases tonic dopamine and action readiness. In other words, a rich reward context promotes high action utility. Similarly, sporadic reward would decrease tonic dopamine, signaling a low action utility in a poor reward context. Several studies confirmed that response vigor fluctuates as a function of both reward history (Guitart-Masip et al., 2011) and drug induced changes in tonic dopamine (Beierholm et al., 2013).
Cools et al. (2011) subsequently proposed that tonic dopamine, as a signal of reward expectancy, would also interfere with reward learning mechanisms governed by phasic dopamine. The phasic dopamine response reflects the discrepancy between expected and received reward, and a change in tonic dopamine would alter the magnitude of this discrepancy. Low reward expectancy and low tonic dopamine will allow for a large phasic dopamine response to unexpected reward. In contrast, high reward expectancy and high levels of tonic dopamine will only allow for a small phasic response to unexpected reward. Understanding the relationship between tonic and phasic dopamine would have important implications for predicting dopaminergic drug effects in patients with abnormal reward sensitivity.
Our aim was to obtain direct evidence of this relationship between tonic and phasic dopamine response in a pharmacological functional MRI (fMRI) study. Twenty healthy participants entered a double blind study and performed a gambling task (Camara et al., 2010; Gehring and Willoughby, 2010) during fMRI scanning sessions after receiving a single dose of placebo and MPH 40 mg. Relative to previous studies (Knutson et al., 2004; Pessiglione et al., 2006; Dodds et al., 2008), we introduced two modifications to increase design sensitivity. First, previous studies defined the reward-related BOLD response as the difference between the least and the most appealing reward condition, obscuring the parametric relation of the BOLD signal to the RPE. Instead, we created a full-range parametric variation of the RPE from unexpected high punishment to unexpected large reward and measured the phasic dopamine response for each level. Second, previous studies used task paradigms in which participants learn to improve performance. Such tasks engage other systems beyond the striatum that modulate striatal activity and are themselves modulated by dopaminergic manipulations. In our gambling paradigm (Camara et al., 2010), participants chose between two levels of reward amplitude on each trial. Although this choice induced reward expectancy, at feedback participants were passively subjected to arbitrary gains or losses. Thus, before entering the scanner participants had learned that their choice is arbitrary and had no implications for the outcome. As a consequence, phasic DA release could be expected to reliably reflect the parametrically manipulated feedback that we provided.
Based on the hypotheses described earlier and the task paradigm used, we made the following predictions regarding the striatal BOLD signal. First, rewards received in previous trials were expected to increase tonic striatal DA levels and therefore reward expectation signals in the current trial. This should be most evident in the time window following choice, and we tested this in statistical model 3 (cf. below). Second, the increase of tonic DA after MPH administration was also expected to heighten reward expectation, again leading to increased striatal BOLD signal following choice. The prime effect of MPH on reward expectation, independent of trial history related expectation was tested in statistical model 4 (cf. below). Choice and feedback related expectations were basically zero in this model because participants were unable to learn choice dependent outcome expectations and because BOLD signal estimates were averaged over trials which nullified the impact of random reward expectations from preceding trials. This approach therefore allowed independent assessments of trail induced (model 3) and drug induced changes (model 4) in tonic DA on reward expectations. We predicted that MPH would decrease the striatal phasic BOLD response to feedback, because of a higher tonic dopamine and thus higher reward expectancy level in the drug condition.
Materials and methods
Participants
Twenty-four healthy women and men (23–35 years) participated in this study. Inclusion criteria: right handedness, no history of mental illness or neurological disorders, no history of drug or alcohol abuse, and no use of medication. After inclusion, participants completed a thorough medical questionnaire and a full physical was performed. Exclusion criteria: abnormal blood pressure (more than 160/95 mm Hg), or pulse rate, MRI contra-indications, cardiovascular abnormalities as assessed by a 12-lead ECG, hematology and biochemistry values indicative of illness or pregnancy, positive results on the drug screen, excessive drinking (>20 alcohol consumptions a week), and contraindications to MPH.
FMRI results reported are based on data from twenty participants (10 men; mean age 23; SD =1.6). Data from three other participants were excluded due to sleep, panic, and nervousness during scanning. FMRI data of one other subject was excluded because of large ventricles. Her behavioral data however was included for data analysis (21 behavioral datasets).
This study was approved by the local medical ethical committee and carried out in accordance with the declaration of Helsinki (1991). Participants gave informed consent and were compensated for participation.
Procedures
Participants were tested after administration of placebo and MPH 40 mg according to a randomized double-blind within-subject design (order 1, placebo first; order 2, MPH first; half of the participants received order 1 and the other half order 2). When participants arrived at the laboratory (T0), they completed the Bond and Lader Questionnaire [(1); for description see section ‘Subjective Measures’ below], a blood sample was taken, and the encapsulated MPH or placebo was administered. At the end of a 1 h waiting period (T1), participants again completed the questionnaire and a second blood sample was taken. The scan session started on average 108 (SD = 14) and ended on average 197 (SD = 16) minutes after drug administration (calculated for participants who’s fMRI data were analyzed). After scanning (T2) participants again completed the questionnaire and a third blood sample was taken.
Plasma values
Plasma MPH and ritalinic acid were determined with liquid chromatography-mass spectrometry. A repeated-measures ANOVA (RM-ANOVA) with Treatment (two levels) and Time (three levels) as within-subject factors was carried out for plasma MPH and ritalinic acid (SPSS, version 21).
Subjective measures
The Bond and Lader Questionnaire (Bond and Lader, 1974) consists of 16 bipolar set of adjectives loading onto three subscales: alertness, contentedness and calmness. A RM-ANOVA with Treatment and Time as within-subject variables and Order (two levels: placebo or MPH first) as covariate was carried out. To correct for multiple testing, effects were considered significant when P < 0.01.
Gambling task
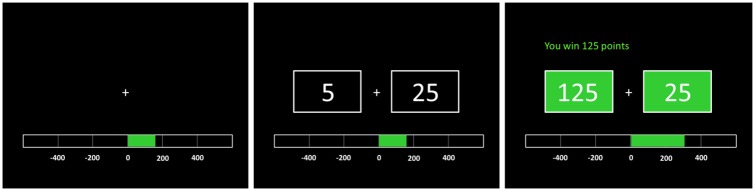
During fMRI scanning participants performed a gambling task (see Figure 1 for details). To allow investigation of the parametric variation of BOLD signal change with the size and valence of the RPE the following ordered sequence of event types was created: Gain 125, Gain 7/27 (Gain 7 and 27 combined), Gain 25, Gain 5, Loss 5, Loss 25, Loss 7/27, Loss 125. In addition, Choice 5, and Choice 25 events represented the response of participants relative to the cue stimulus. In total, 600 forced choice and 60 dummy trials (fixation cross for 4 s) were randomly presented across 5 runs (40 min in total).
Fig. 1.
During fMRI scanning participants performed a gambling task with the intent to win as many points as possible. A fixation cross was presented for 0.5 s followed by the numbers ‘5’ and ‘25’ (choice cue). The left/right position of these cues was randomized. Participants chose a number by pressing the corresponding button. One second later, the boxes around the numbers turned both green (indicating gain) and both red (indicating loss) and stayed on the screen for 1.5 s. The intertrial interval was 3 s plus RT. When participants chose ‘5’, they could gain or lose 5 (80% of trials, expected small gain or loss), 7 (10%, unexpected small gain or loss) or 125 points (10%, unexpected large gain or loss); when they chose ‘25’, they could win or lose 25 (80%, expected small gain or loss), 27 (10%, unexpected small gain or loss), or 125 points (10%, unexpected large gain or loss). In the present example, the participant chose ‘5’, and gained 125 points. The color bar below indicated the total amount of points collected so far.
To increase motivation, two modifications to the Camara et al. (2010) task were made: (i) at the bottom of the screen a bar showed the total score (red for negative and green for positive), (ii) at the top of the screen for each individual trial the points lost or gained were presented.
Performance analysis
Response times
The average response time (RT) on the subsequent trial was calculated for each event type. Two participants responded extremely slowly (average RT > 2 SDs above mean). They were considered outliers and their data was excluded to avoid violation of parametric assumptions.
A RM-ANOVA, with Treatment and Event Type (eight levels) as within subject variables, and Order (two levels) as covariate, was carried out. A Greenhouse-Geisser correction was applied when appropriate.
Choice behavior
For each event trial, the percentage of subsequent trials on which ‘25’ was chosen was calculated. RM-ANOVAs with Treatment and Event Types as within subject variable and Order as covariate, was carried out.
Image acquisition
MRI images were acquired with a Siemens 3T head only scanner (Magnetom Allegra, Siemens Medical Systems, Erlangen, Germany). Whole brain EPI images: TR = 2.0 s; TE = 30 ms; FOV = 224 × 224 mm; flip angle = 90°; interleaved slice acquisition; 32 oblique slices; 64 × 64 matrix; voxel size = 3.5 × 3.5 × 3.5 mm. T1-weighted anatomical scan: TR = 2.25 s; TE = 2.6 ms; flip angle = 9°, 256 × 256 matrix, 192 sagittal slices; voxel size = 1 × 1 × 1 mm.
Analysis of the fMRI data
SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) was used for preprocessing and analysis. Preprocessing steps: (i) the first three volumes were deleted, (ii) slice-time correction, (iii) within run realignment, (iv) for each session, volumes from run 2 till 5 were coregistered to the volumes of run 1, (v) spatial normalization of functional images (parameters estimated without segmentation onto the MNI EPI template and images resliced to 2 × 2 × 2 mm) and (vi) 4 mm smoothing.
The following events were modeled: Choice 5 (‘at choice response onset’), Choice 25, Gain 125 (at feedback onset), Gain 7/27 (Gain 7 and 27 combined), Gain 25, Gain 5, Loss 5, Loss 25, Loss 7/27, Loss 125. Events were modeled with a standard canonical HRF and no time and dispersion derivatives were included. The time series were high pass filtered (128 s).
For each individual, four GLMs were built to study the effects of MPH on the BOLD response. In each GLM, 6 realignment parameters (RPs) were added as regressors of no interest. The RPEs used in model 1–3 were calculated with a Toolbox for Estimating Parameters of Reinforcement Learning Models created by Jee Hoon Yoo, University of Bristol, September 2008. The indirect actor model was used, which uses a standard basic reinforcement learning model. Updating procedure: mi = mj + εδ where δ = rj – mj. In the equation, i indexes the current trial and j the preceding trial. The variable mi is the action value of the chosen alternative in the current trial. ε denotes the learning rate, and δ represents the difference between received reward rj and action value mj. in the previous trial. We also investigated the effect of MPH on the negative likelihood (a measure of the total model fit), the learning rate and the exploration parameter.
In demonstrating RPE effects at the time of feedback, it has become common practice to split up the RPE into the reward expectation, which negatively correlates with RPE effects, and the actual reward received, which positively correlates with any RPE effect (Rushworth and Behrens, 2008; Rutledge et al., 2014). However, because in our random-feedback paradigm participant learn that the outcome is random, their stimulus-related reward expectancy at the moment of feedback is constant and RPE will co-vary with the amount of reward received. Hence, we did not split up the RPE.
Model 1. RPE. Parameters: feedback (all feedback events; modeled to feedback onset), RPE as parametric modulator, which scales the amplitude of the hemodynamic response during feedback, 6RPs.
Model 2. Absolute RPE. The absolute value of the PE is a measure for the salience of feedback. Parameters: feedback, absolute value of the RPE as parametric modulator and 6RPs
Model 3. RPE History. The receipt of a large reward raises the expectancy to receive reward on the next trial. In other words, the BOLD response in the ventral striatum (reward expectancy) associated with choice, depends on the RPE received on the previous trial. Parameters: Choice (button press), RPE of the preceding trial as parametric modulator and 6RPs
Model 4. This model was used to plot the percent signal changes for all modeled events for clusters were effects of MPH were found. This model allowed assessing the influence of MPH on reward expectation, independent of any reward history related expectations. That is, gains and losses assigned after a choice were completely arbitrary and as a consequence subjects were not able to learn any systematic choice-outcome contingencies. In addition, any random expectations to may have occurred based on rewards received in preceding trials were also neutralized by averaging BOLD signal estimates over trials in each event type. Therefore, MPH changes in tonic DA was the sole determinator of reward expectation in the feedback estimates. These estimates were used to check for MPH effects on the average BOLD response (contrast 1), and to examine the task-related BOLD response to feedback in general (contrast 2). Parameters: all event types separately, Fixation, Choice 5, Choice 25 and 6RPs
For each model contrast images from the individual analysis were used as input for dependent t-tests, in which Order was added as a covariate. Firstly, task-related effects were examined within the placebo-condition using a whole brain analysis. A second set of whole brain analyses investigated the effects of MPH for the different models. For both analysis we used PFWE-corrected at cluster level < 0.05 with an extend threshold of > 5 voxels. MNI coordinates were labeled according to the AAL regions (Tzourio-Mazoyer et al., 2002). We used the CA-CP line as a pragmatic guideline to define the ventral striatum. Striatal clusters with a negative z-value are considered ventral striatum (including ventral putamen and caudate; in line with the ventral ROI used in e.g. Hiebert et al., 2014). The SPM toolbox RFX-plot (Gläscher, 2009) was used to extract percent signal changes.
A slope analysis was carried out to examine if MPH changes the relationship between the parametric manipulations of RPEs and the event-related BOLD response. For this analysis, the analysis event types were recoded as follows: Gain 125 to 4, Gain 7/27 to 3, Gain 25 to 2, Gain 5 to 1, Loss 5 to −1, Loss 25 to −2, Loss 7/27 to −3 and Loss 125 to -4.
Results
Effectiveness of psychopharmacological manipulation
Control measures showed that MPH manipulations were successful. Statistical analysis of the plasma MPH concentrations (n = 19) showed a main effect of Treatment [F(1,18) = 33.8; P < 0.001] and Time [F(2,36) = 15.0; P < 0.001], and an interaction between Treatment and Time [F(2,36) = 15.0; P < 0.001]. For plasma Ritalinic Acid concentrations (n = 19), a main effect of Treatment [F(1,18) = 104.0; P < 0.001] and Time [F(2,36) = 33.7; P < 0.001], and an interaction between Treatment and Time [F(2,36) = 33.6; P < 0.001] was found. Post hoc t-tests showed that 40 mg Ritalin successfully increased plasma MPH and Ritalinic Acid.
Analysis of the Bond and Lader Alert subscale showed a main effect of Treatment [F(1,18) = 10.2; P < 0.01]. Participants reported to be more alert in the MPH than in the placebo session. No other effects were found.
Performance
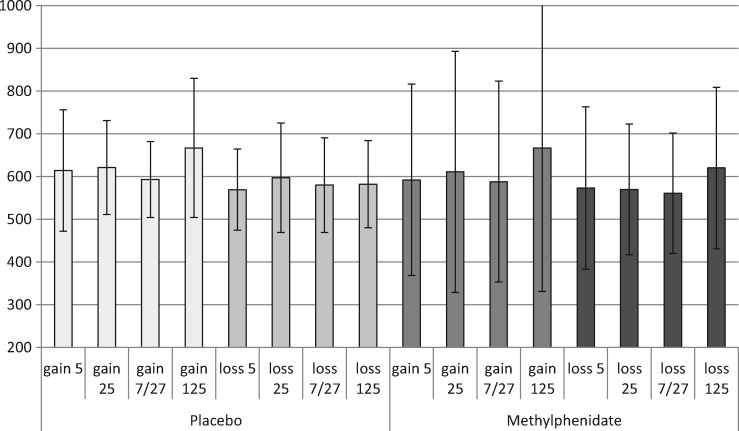
Response speed analysis confirms that MPH increases response vigor. Repeated measures ANOVA on the reaction times (n = 19) showed a main effect of Treatment [F(1,17) = 4.9; P = 0.04]. MPH reduced the average RT across FB types from on average 603 (SE = 24) ms in the placebo session to 598 (SE = 46) ms in the MPH session. Although the reaction time values did not show evidence of a significant interaction between feedback type and drug condition [F(1,17) = 0.213, P = 0.650], the evidence for increased vigor was more clear when looking at the bulk of the more neutral feedback trials (gain 5/7/25/27 or loss 5/7/25/27). In these gain trails RT dropped from 615 in placebo to 595 ms under MPH, and in these loss trials it dropped from 583 to 570 ms, respectively (see Figure 2). In line with Camara et al. (2010), overall participants chose the 25 more often (in 58%, SD = 14) than the 5 (42%, SD = 14) [T(18) = 2.4; P = 0.03]. However, there was no significant difference in the RT for choosing the 25 points option compared with the five points option [F(1,17) = 0.6, P = 0.461]. No effects of MPH on choice behavior were found.
Fig. 2.
The average reaction time (in ms) on the next trial for the different gain and loss events, during placebo and MPH (with SDs).
Task-related brain activation during placebo
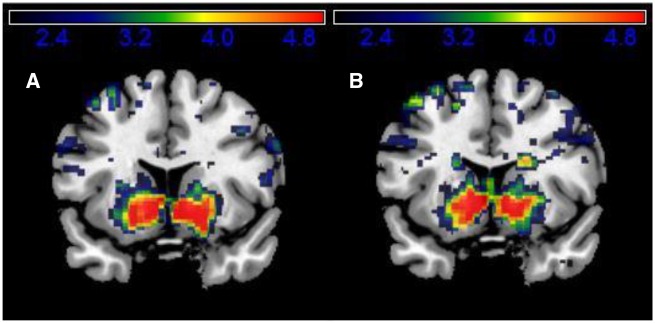
During placebo, the relative RPE sizes correlated positively with the BOLD signal in the ventral striatum during feedback delivery (model 1; Tpeak voxel = 7.68, PFWE-corrected = 0.000) and also during choice of the next trial (model 3; Tpeak voxel = 7.01, PFWE-corrected = 0.000) (see Table 1 and Figure 3 for striatal activations and Supplementary Table S1 for a complete overview of task-related brain activity). The latter finding confirms the hypothesis that reward history adjusts current reward expectation. In contrast, the salience of the RPE (i.e. regardless of its valence; model 2) did not modulate striatal activity (Table 1).
Table 1.
Task-related BOLD responses in the striatum in the placebo session, based on whole brain analysis (for a complete list of the results of this whole brain analysis see Supplementary Table S1)
| Regions | BA | PFWE-corrected at cluster level | Peak Coordinates x, y, z | Peak T-value | Cluster size |
|---|---|---|---|---|---|
| A. Feedback-related BOLD responses that correlate positively with the size of the RPE | |||||
| Ventral striatum | 0.000 | −19, 4, −13 | 7.68 | 1380 | |
| 11, 10, −5 | 7.23 | ||||
| −9, 8, −7 | 6.89 | ||||
| B. Feedback-related BOLD responses that correlate positively with the size of the salience of feedback (absolute value of the RPE) | |||||
| No significant striatal cluster was found | |||||
| C. Choice-related BOLD response that correlates positively with RPE history. | |||||
| Ventral striatum | 0.000 | 11, 6, −5 | 7.01 | 1838 | |
| −7, 10, −5 | 6.77 | ||||
| 1, −4, 9 | 5.85 | ||||
| Regions | BA | PFWE-corrected at cluster level | Peak Coordinates x, y, z | Peak T-value | Cluster size |
| D. Regions in which MPH reduced the BOLD response during feedback (across feedback types) | |||||
| Ventral striatum | 0.000 | −19, 6, −17 | 4.70 | 131 | |
| −23, 12, 3 | 4.70 | ||||
| −23, 16, −9 | 4.35 | ||||
| Ventral striatum | 0.012 | 25, 12, −7 | 4.61 | 70 | |
| 21, 20, −11 | 3.43 | ||||
| 21, 10, −15 | 3.43 | ||||
Effects of MPH on the BOLD response during feedback (across feedback types). Local maxima more than 8.0 mm apart are shown.
Fig. 3.
A shows the ventral striatal cluster for which the BOLD response is positively correlated with the RPE in the placebo condition (model 1), and B shows the ventral striatal cluster for which the BOLD response correlates positively with the RPE history during placebo (model 3). Both clusters are shown at coronal slices at x = 15.
Effects of MPH on task-related brain activation
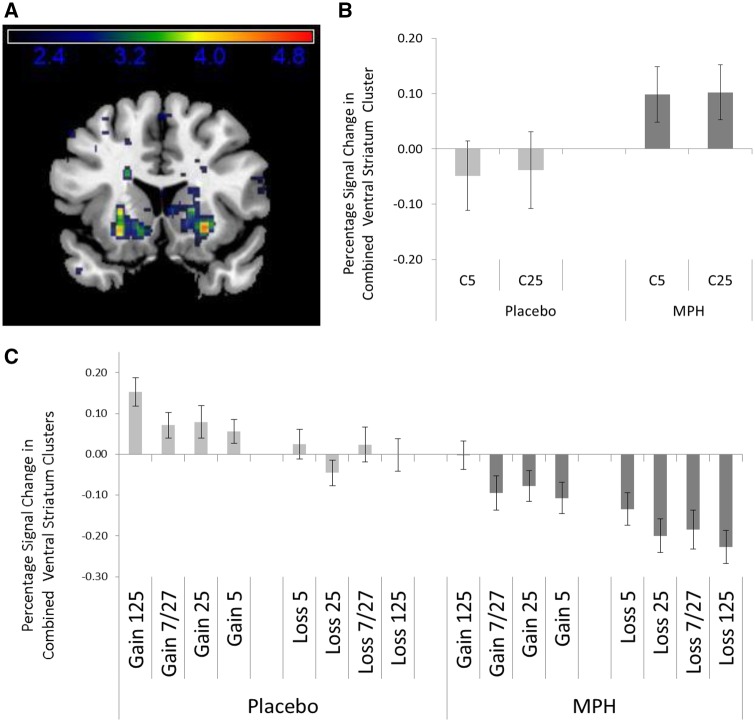
This relationship between the relative size of RPEs and the relative size of the event-related BOLD response was not altered by MPH as there was no significant MPH-related difference in the amount of modulation of the BOLD response estimated in either of the models (models 1–3). MPH did however change the absolute size of the phasic striatal BOLD response related to feedback (model 4). A whole brain analysis of the pooled response to all feedback types under MPH compared with placebo (model 4) revealed that MPH reduced the BOLD response bilateral in the ventral striatum (left ventral striatum: Tpeak voxel = 4.70, PFWE-corrected = 0.000; right ventral striatum: Tpeak voxel = 7.61, PFWE-corrected = 0.000; see Table 1 and Figures 4A and C).
Fig. 4.
The average percentage signal change (n = 20) with SEM across the two ventral striatal clusters (A) (coronal slices at x = 15) for Choice (B) and for the different types of feedback (C) during the placebo and MPH session (model 4).
Together with the finding that MPH does not change the nature of the relationship between BOLD response and RPE, this result suggests that the tonic increase in dopamine induced by MPH leads to a downward shift of the entire RPE-response curve by on average 0.17% signal change.
To confirm that MPH did not change the relationship between our parametric manipulations of RPEs and the event-related BOLD response, a follow-up slope analysis was carried out on the extracted ROI-based data. This linear regression analysis showed that the linear trend in the BOLD amplitudes over RPE categories, as opposed to amplitudes, was not significantly changed by drug condition (t = 0.96; P = 0.34).
Finally, we verified the influence of tonic dopamine on reward expectancy by comparing the percentage signal change between the two drug conditions at the time of the choice (estimated in model 4). As the choice for a reward level brings about a certain reward expectation, reflected in the striatal BOLD-response towards the choice cue, tonic dopamine increase through MPH should increase this average reward expectation signal. The ROI-based data extracted from the ventral striatum confirmed this (t = −1.93; P = 0.04; Figure 4B).
No effects of MPH were found on the negative likelihood (F(1,18) = 0.2; P > 0.1), the learning rate [F(1,18) = 1.47; P > 0.1), and the exploration parameter [F(1,18) = 0.0; P > 0.1]; output parameters of the reinforcement learning model.
Discussion
The results presented here are in line with the predictions of Niv (2007). Not only was high tonic dopamine associated with increased response readiness, but it also enhanced the BOLD signal in the ventral striatum associated with reward expectation, i.e. at the time when participants chose for a particular reward level. This was evident when comparing drug conditions, but also on a trial by trial basis, as the amplitude of this BOLD response was systematically higher (or lower) depending on the size of the RPE in the previous trial. This confirms that the tonic dopamine level constitutes an average reward expectation signal.
Our results also provide direct support for the hypothesis of Cools et al. (2011) that the striatal tonic dopamine level affects the impact of the phasic RPE-related dopamine response. We observed that the tonic increase in dopamine induced by MPH, in contrast to the upward sizing of the BOLD signal at the time of choice, leads to a downward shift of the phasic BOLD response in the ventral striatum at the time of feedback. At the same time, the RPE-related modulation of the response is left intact. As there was no interaction of any kind between the drug conditions and the RPE size in the BOLD signal, we conclude that the tonic and phasic BOLD effects of our dopamine manipulation are additive, as if the tonic dopamine effect represents a constant value subtracted from the phasic BOLD response. This strongly suggests that these two phenomena arise from independent mechanisms.
It is not yet known what these mechanisms are. A tempting explanation is that these BOLD modulations result from a drug induced metabolic change. It has been demonstrated with glucose PET in humans that MPH lowers metabolic activity in the striatum (Wang et al., 1994; Volkow et al., 2008, 2013). A global, MPH-induced reduction in BOLD activation, independent of reward-related BOLD activity following phasic dopamine delivered in the striatum (Jonasson et al., 2014), would very well account for the results reported here. However, because the MPH-induced dopamine enhancement is tonic, the metabolic change it induces is tonic. Hence, it served as the baseline BOLD signal against which relative changes in phasic dopamine are contrasted during both placebo and MPH. The metabolic effect of MPH therefore remains invisible to BOLD-fMRI. Consequently, the global downward shift of the phasic BOLD response amplitude that we observed, can only emerge through a direct influence of the enhanced tonic dopamine level on the manifestation of the phasic dopamine response in the BOLD signal.
Three established mechanisms could explain the reduction of phasic dopamine due to an increase in tonic dopamine. First, a higher tonic dopamine level increases stimulation of presynaptic dopamine autoreceptors, which diminishes phasic dopamine release (Beaulieu and Gainetdinov, 2011). Second, increased tonic dopamine induces postsynaptic dopamine receptor desensitization, resulting in a reduced postsynaptic response to phasic dopamine (Jonasson et al., 2014). A third possibility relates to the suggested functional specificity of the striatal compartments. The striosome subsystem is the primary target of reward-related signals and provides inhibitory regulatory feedback to midbrain dopamine neurons (Canales, 2005). We hypothesize that the MPH-induced increase in average reward expectancy increases activity in striosome output neurons—detected as increased BOLD signal following the choice cue—which then down-regulate midbrain dopamine neurons, resulting in a reduced phasic dopamine response in the striatum.
An important implication of our results is that MPH could be used to down regulate the phasic reward response. In cocaine addicts, e.g. drug-related cues are associated with a phasic reward response in the ventral striatum (Stuber et al., 2005; Philips et al., 2013). Our results suggest that MPH could be used to suppress this response and reduce relapse rate. Furthermore, previous studies have shown abnormal reward sensitivity in ADHD (Furukawa et al., 2014) which can be normalized by MPH (Mizuno et al., 2013). Our findings suggest that MPH normalizes reward sensitivity by reducing the phasic dopamine response to immediate reward predicting cues. As a last example, individual differences in striatal reward responsiveness to food and sexual images have been shown to predict subsequent weight gain and sexual activity (Demos et al., 2012). Normalization of this heightened sensitivity to food or sex related cues might increase the success rate of behavioral control treatment schemes for indulgence in overeating and sexual activity.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Funding
E.A.T. Evers was funded by Marie Curie Grant (FP7, PIOF-GA-2009-251684).
Supplementary Material
References
- Advokat C. (2010). What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD). Neuroscience and Biobehavioral Reviews, 34(8), 1256–66. [DOI] [PubMed] [Google Scholar]
- Antonelli F., Strafella A.P. (2014). Behavioral disorders in Parkinson’s disease: the role of dopamine. Parkinsonism and Related Disorders, 20(Suppl 1), S10–2. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.M., Gainetdinov R.R. (2011). The physi ology, signaling, and pharmacology of dopamine receptors. Pharmacological Review, 63(1), 182–217. [DOI] [PubMed] [Google Scholar]
- Beierholm U., Guitart-Masip M., Economides M., et al. (2013). Dopamine modulates reward-related vigor. Neurop sychopharmacology, 38(8), 1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A., Lader M. (1974). The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology, 47, 211–8. [Google Scholar]
- Camara E., Krämer U.M., Cunillera T., et al. (2010). The effects of COMT (Val108/158Met) and DRD4 (SNP -521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb Cortex, 20, 1985–96. [DOI] [PubMed] [Google Scholar]
- Canales J.J. (2005). Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiology of Learning Memory, 83(2), 93–103. [DOI] [PubMed] [Google Scholar]
- Cools R., Nakamura K., Daw N.D. (2011). Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology, 36, 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos K.E., Heatherton T.F., Kelley W.M. (2012). Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. Journal of Neuroscience, 32(16), 5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds C.M., Müller U., Clark L., van Loon A., Cools R., Robbins T.W. (2008). Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. Journal of Neuroscience, 28(23), 5976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa E., Bado P., Tripp G., et al. (2014). Abnormal striatal BOLD responses to reward anticipation and reward delivery in ADHD. PLoS One, 9(2), e89129.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science, 295, 2279–82. 12 [DOI] [PubMed] [Google Scholar]
- Gläscher J. (2009). Visualization of group inference data in functional neuroimaging. Neuroinformatics, 7(1), 73–82. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Beierholm U.R., Dolan R., Duzel E., Dayan P. (2011). Vigor in the face of fluctuating rates of reward: an experimental examination. Journal of Cognitive Neuroscience, 23(12), 3933–8. [DOI] [PubMed] [Google Scholar]
- Hiebert N.M., Vo A., Hampshire A., Owen A.M., Seergobin K.N., MacDonald P.A. (2014). Striatum in stimulus-response learning via feedback and in decision making. Neuroimage, 101, 448–57. [DOI] [PubMed] [Google Scholar]
- Jonasson L.S., Axelsson J., Riklund K., et al. (2014). Dopamine release in nucleus accumbens during rewarded task switching measured by [11C]raclopride. Neuroimage, 99, 357–64. [DOI] [PubMed] [Google Scholar]
- Knutson B., Bjork J.M., Fong G.W., Hommer D., Mattay V.S., Weinberger D.R. (2004). Amphetamine modulates human incentive processing. Neuron, 43(2), 261–9. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Yoneda T., Komi M., et al. (2013). Osmotic release oral system-methylphenidate improves neural activity during low reward processing in children and adolescents with attention-deficit/hyperactivity disorder. Neuroimage: Clinical, 16(2), 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y. (2007). Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Annals of the New York Academy of Sciences, 1104, 357–76. [DOI] [PubMed] [Google Scholar]
- Pessiglione M., Seymour B., Flandin G., Dolan R.J., Frith C.D. (2006). Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature, 442(7106), 1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.E., Stuber G.D., Heien M.L., Wightman R.M., Carelli R.M. (2013). Subsecond dopamine release promotes cocaine seeking. Nature, 422(6932), 614–8. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Behrens T.E. (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11(4), 389–97. [DOI] [PubMed] [Google Scholar]
- Rutledge R.B., Skandali N., Dayan P., Dolan R.J. (2014). A computational and neural model of momentary subjective well-being. Proceedings of the National Academy of Sciences, 111(33), 12252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2007). Behavioral dopamine signals. Trends in Neuroscience, 30, 203–10. [DOI] [PubMed] [Google Scholar]
- Spronk D.B., van Wel J.H., Ramaekers J.G., Verkes R.J. (2013). Characterizing the cognitive effects of cocaine: a comprehensive review. Neuroscience and Biobehavioral Reviews, 37(8), 1838–59. [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Roitman M.F., Phillips P.E., Carelli R.M., Wightman R.M. (2005). Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology, 30(5), 853–63. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.J., et al. (2008). Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One, 3(4), e2017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Tomasi D., Wang G.J., et al. (2013). Predominance of D2 receptors in mediating dopamine's effects in brain metabolism: effects of alcoholism. Journal of Neuroscience, 33, 4527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.J., Volkow N.D., Fowler J.S., et al. (1994). MPH decreases regional cerebral blood flow in normal human subjects. Life Sciences, 54(9), PL143–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.