Abstract
Ideas spread across social networks, but not everyone is equally positioned to be a successful recommender. Do individuals with more opportunities to connect otherwise unconnected others—high information brokers—use their brains differently than low information brokers when making recommendations? We test the hypothesis that those with more opportunities for information brokerage may use brain systems implicated in considering the thoughts, perspectives, and mental states of others (i.e. ‘mentalizing’) more when spreading ideas. We used social network analysis to quantify individuals’ opportunities for information brokerage. This served as a predictor of activity within meta-analytically defined neural regions associated with mentalizing (dorsomedial prefrontal cortex, temporal parietal junction, medial prefrontal cortex, /posterior cingulate cortex, middle temporal gyrus) as participants received feedback about peer opinions of mobile game apps. Higher information brokers exhibited more activity in this mentalizing network when receiving divergent peer feedback and updating their recommendation. These data support the idea that those in different network positions may use their brains differently to perform social tasks. Different social network positions might provide more opportunities to engage specific psychological processes. Or those who tend to engage such processes more may place themselves in systematically different network positions. These data highlight the value of integrating levels of analysis, from brain networks to social networks.
Keywords: mentalizing, social influence, social networks, information brokerage, Facebook, betweenness centrality, recommendations
Online social networks, such as Facebook, play an increasingly dominant role in influencing the spread of ideas and behaviors (Simmons et al., 2011; Bakshy et al., 2012; Guille et al., 2013). Within social networks, some individuals are in a better position than others to encounter, adopt and share new ideas or information (Burt et al., 2013). Do such individuals with more opportunities to connect otherwise unconnected others—high information brokers—use their brains differently than low information brokers?
Although separate bodies of research have characterized neurocognitive processes involved in communication and social influence (Cascio et al., 2015) and social network effects on behavior (Smith and Christakis, 2008), these lines of work have not yet been integrated (c.f., Zerubavel et al., 2015). Here, we test a novel proposition concerning the relationship between personal social networks and core mental processes, or ‘network cognition’ (c.f. Brashears and Quintane, 2015). Specifically, we test the hypothesis that people with more opportunities for information brokerage may use brain systems implicated in considering the thoughts, perspectives, and mental states of others (i.e. ‘mentalizing’) more when spreading ideas. We focus on recommendation behavior as one key social process and combine tools from social network analysis (SNA) with functional neuroimaging to gain insight into how broader social environments interact with key neural systems during social transmission of information.
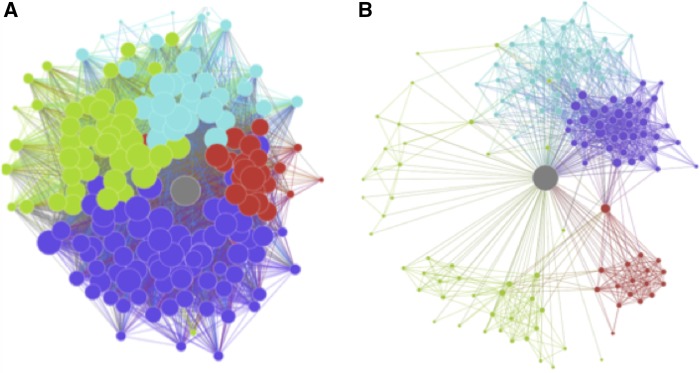
SNA of logged data (e.g. from Facebook) provides an objective and rich set of measures and techniques to quantify the size, structure and scope of an individual’s social environment (Borgatti et al., 2009; Burt et al., 2013). In doing so, these measures allow researchers to operationalize classic sociological concepts such as opportunities to connect otherwise unconnected others, often termed ‘information brokerage’ (Arnaboldi et al, 2013; Burt et al., 2013). This characteristic can be stated mathematically by ‘ego betweenness centrality’ (Freeman, 1979; Marsden, 2002; Everett and Borgatti, 2005), which is typically associated with greater opportunities to pass information and exert influence between others (Figure 1). Individuals whose social network structures present more brokerage opportunities should likewise be exposed to more perspectives of others, and these past experiences may guide how they engage with new social situations (Burt et al., 2013). In particular, higher information brokerage may afford more opportunities to practice mentalizing, as when translating ideas between different individuals or groups.
Fig. 1.
Example ego networks for participants high and low in brokerage opportunities. Gray node is the ego, i.e. the Facebook user. Node size represents the number of connections to other nodes in the network. Other node colors represent community membership according to a community detection algorithm. Networks contain similar numbers of friends but high and low opportunities for brokerage. Network A contains 184 nodes with ego betweenness score of 0.06. Network B contains 140 nodes with ego betweenness of 0.59.
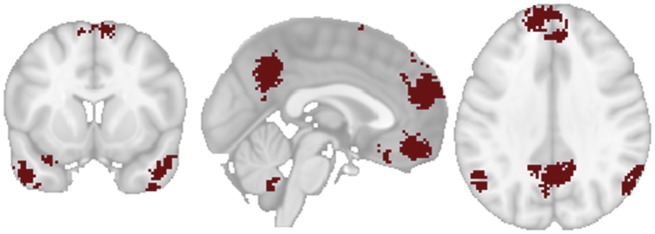
Past neuroimaging research demonstrates that successful transmission of ideas covaries with activity within brain regions that are also associated with mentalizing (Dietvorst et al., 2009; Falk et al, 2012, 2013). In other work, mentalizing skills (via a behavioral task) have been tied to larger support network size (Stiller and Dunbar, 2007). Mentalizing commonly recruits the dorsomedial prefrontal cortex (DMPFC), the temporal parietal junction (TPJ) and precuneus/posterior cingulate cortex (PC/PCC) (Saxe and Kanwisher, 2003; Saxe and Powell, 2006; Decety and Lamm, 2007). In addition, studies have implicated middle and ventral areas of the medial prefrontal cortex (MPFC) and bilateral superior temporal sulci (STS) and temporal pole in mentalizing (Frith and Frith, 2003; Schurz et al, 2014; Denny et al, 2012). Indeed, an automated meta-analysis of 124 studies that include the term ‘mentalizing’ produces a reverse inference (RI) map including clusters in the DMPFC, bilateral TPJ, PC/PCC, middle temporal gyrus (MTG) and MPFC (Figure 2). However, the relationship between social network characteristics and engagement of brain regions implicated in mentalizing during recommendations has not been studied.
Fig. 2.
Regions of interest in the mentalizing network. A Neurosynth (Yarkoni et al., 2011) RI map of the term ‘mentalizing’, based on 124 studies FDR 0.01 corrected was used to create this ROI mask.
In addition, previous work on the relationship between social network characteristics and cognition has focused primarily on network size or ‘ego degree’ (Dunbar, 2012; c.f., Molesworth et al., 2015). For example, neurocognitive research has considered certain aspects of brain structure and function, such as correlations between network size and the size of specific brain regions (Bickart et al., 2011; Dunbar, 2012). In the current investigation, we go beyond network size to consider the network ‘structure’ in the form of information brokerage. Information brokerage may be particularly important for facilitating the spread of information, and may also be systematically related to cognitive tendencies (Burt et al., 2013), such as the tendency to consider the mental states of others. We examined whether neural activity within the mentalizing system differs as a function of an individual’s potential information brokerage capacity within their social network, operationalized by ego-betweenness centrality. In doing so, we tested a novel social network cognition account of influence: that individuals with greater ego-betweenness centrality would show more mentalizing activity when considering and using social feedback to make recommendations to others.
The combination of neural and SNA metrics thus offers the opportunity to analyze links between mechanisms involved in sharing and the positions occupied by individuals in social networks. We focused on logged social network data obtained from the Facebook accounts of a sample of adolescents, a group in which social ties and interactions are particularly salient. Facebook networks have been validated as reasonable proxies for offline interaction networks in related groups (La Gala et al., 2012; Kane et al., 2014) and may be especially important within adolescent groups. In parallel, neural measures can offer a non-invasive way to simultaneously interrogate multiple neurocognitive processes that may be key to sharing information. We examined neural activity during a mobile game recommendation task that simulates the experience of learning about and recommending online applications (i.e. a task that is similar to what adolescents might do in day to day life, while still maintaining a high degree of experimental control (Cascio et al., 2015).
Materials and methods
Participants
Sixty-five adolescent males were recruited from the Michigan Driver History Record via the University of Michigan Transportation Research Institute as part of a larger series of studies examining male adolescent driving behavior. All participants were between the ages of 16–17 (M = 16.9, SD = 0.31) and male, right-handed, did not suffer from claustrophobia, were not currently taking any psychological medications, had normal (or corrected to normal) vision, did not have metal in their body that was contraindicated for fMRI, and did not typically experience motion sickness. Legal guardians provided written informed consent following telephone discussion with a trained research assistant, and teens provided written assent. 50 of these 65 participants both completed the online social network survey, including providing access to their Facebook network data and had usable neuroimaging data from the fMRI App Recommendation Task (described below; Figure 3).
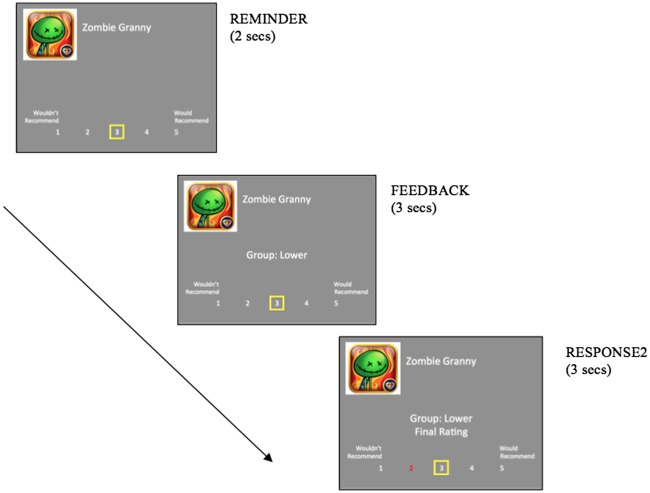
Fig. 3.
App Recommendation Task (Cascio, et al. 2015). Prior to the fMRI scan participants saw descriptions of each app and made an initial rating (RESPONSE1). Participants then completed the social feedback portion of the App Recommendation Task during the fMRI session. Ratings were given on a five-point Likert scale, where 1 = wouldn’t recommend and 5 = would recommend. Ratings were based on exposure to peer group feedback (higher, lower, same or not rated) in conjunction with a reminder of the participant’s initial rating.
Acquisition of online social network
Participants completed a survey in which they were asked whether they had a Facebook account. If so, they were asked to login to their account and add an application that requests access to information regarding their Facebook activity, friends and links between their friends using the Facebook OpenGraph API. These data were anonymized and used to compute betweenness centrality as a measure of information brokerage within the participants’ networks. Participants had an average of 510 friends in their full Facebook networks (range = 84–1548; SD = 317.3). Ego betweenness was calculated for the full networks as cB(v) = and ranged between 0.035 and 0.75 (normalized using the total number of possible brokerage opportunities, i.e. the number of possible edges, to range between 0 and 1 using the formula 2/((n−1)(n−2)), where n is the number of nodes in the graph; see (Freeman, 1979; Brandes, 2001) as implemented in (Hagberg et al., 2008), where higher values indicate more opportunities to broker information between more different groups. Social network size was also recorded as the number of confirmed friendships in the participant’s Facebook network.
Task and procedure
The App Recommendation Task was designed to examine social influence on recommendation behavior in the fMRI environment (O’Donnell, et al., 2015). The task captures neural processes associated with sharing recommendations, ostensibly for a mobile game website and manipulates social feedback regarding the recommendations of peers. The task stimuli consist of icons representing real puzzle based game apps and their associated descriptions acquired from the iTunes App Store, but unknown to most people (i.e. not culturally popular games that many people have already encountered). Actual apps from the App Store were used to maintain a sense of realism and relevance to the adolescent participants. As part of the task, participants were exposed to information that is available at the App Store, namely, game titles, logos, and brief descriptions of the games. Games from one category (puzzle-based games) were used to reduce strong preferences for one particular game genre over another (e.g. shooter game vs sports games) and all game descriptions were limited to a consistent two sentence structure (e.g. Zombie Grandmother: ‘Fight your way through the army of the Undead blasting them with fireballs, cutting ropes and breaking chains. Defeat your main target, the Zombie Grandmother!’).
Participants completed two rounds of the App Recommendation Task. Prior to the fMRI scan, participants learned about 80 previously unknown puzzle games from the iTunes App store and responded on a five-point scale to the question ‘I would recommend this to a friend’. During the fMRI session they were reminded of their initial rating, then they received experimentally manipulated feedback indicating how a group of their peers who had already participated in the study had ostensibly rated the same app (feedback was of one of four conditions: (i) NOT RATED—no group feedback, (ii) SAME—group agreed, (iii) HIGHER—group average was higher than participant’s initial rating or (iv) LOWER—group average was lower than participant’s initial rating). The social feedback ratings were pseudo randomly generated to maintain 20 trials for each feedback type. Finally, participants were given an opportunity to update their initial recommendations if they wished, and to lock in a final response in the scanner. In other words, during the fMRI portion of the task, each game rating block consisted of three parts. First participants saw a reminder of the game using the title and logo along with a reminder of how they initially rated the game (2 s). Next participants were exposed to manipulated peer group recommendations relative to their own (higher, lower or same) or no peer group feedback (not rated) (3 s). Finally, participants were asked to lock in a final recommendation for each game for the website (3 s; Figure 3). A fixation cross was displayed between each 8-s trial (mean 2.5 s, range 0.12–5.4, SD 1.47).
Social influence effects were examined using R Core Team (2014). For each participant we computed: the proportion of trials when they made a change to their initial rating for an app after receiving social feedback that was different (either higher or lower) from their initial rating and the proportion of trials where they made a change despite receiving supportive social feedback (i.e. that peers rated the app the same).
MRI data acquisition and preprocessing
Imaging data were acquired using a 3 Tesla GE Signa MRI scanner. Functional images were recorded using a reverse spiral sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, 43 axial slices, FOV = 220 mm, slice thickness = 3mm; voxel size = 3.44 × 3.44 × 3.0 mm). We also acquired in-plane T1-weighted images (43 slices; slice thickness = 3 mm; voxel size = 0.86 × 0.86 × 3.0 mm) and high-resolution T1-weighted images (SPGR; 124 slices; slice thickness = 1.02 × 1.02 × 1.2 mm) for use in coregistration and normalization.
Functional data were pre-processed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). To allow for the stabilization of the BOLD signal, the first four volumes (8 s) of each run were discarded prior to analysis. Functional images were despiked using the 3dDespike program from the AFNI toolbox. Next, data were corrected for differences in the time of slice acquisition using sinc interpolation; the first slice served as the reference slice. Data were then spatially realigned to the first functional image. We then co-registered the functional and structural images using a two-stage procedure. First, in-plane T1 images were registered to the mean functional image. Next, high-resolution T1 images were registered to the in-plane image. Following coregistration the high-resolution T1 images were segmented into white and gray matter allowing the skull to be removed. Structural and functional images were then normalized to the skull-stripped MNI template provided by (FMRIB Software Library v5.0) FSL (‘MNI152_T1_1mm_brain.nii’). In the final pre-processing step the functional images were smoothed using a Gaussian kernel (8 mm FWHM).
Regions of interest
We used Neurosynth (Yarkoni et al., 2011) to carry out an automated meta-analysis of the functional neuroimaging literature on ‘mentalizing’. We queried the Neurosynth database (updated as of August 2016) for published studies containing the term ‘mentalizing’ which resulted in 124 studies from which associated MNI coordinates were extracted. These underwent a Neurosynth meta-analysis and a FDR 0.01 corrected RI map was created and saved as an ROI mask (see Figure 2). This map included subregions in the DMPFC, bilateral TPJ, PC/PCC, MTG and MPFC.
fMRI data analysis
Data were modeled at the single subject level using the general linear model as implemented in SPM8. The four peer group feedback conditions (not rated, same, higher and lower) were combined with outcomes pertaining to whether participants updated their initial recommendation or not following feedback about group recommendations (change and no change) as regressors in the model. The 6-s period including peer feedback (FEEDBACK) and the final rating (RESPONSE2) were modeled together in a single regressor.
Participants rarely changed their recommendation when the group rating was consistent with the participant’s initial recommendation and when no rating was available; thus, these conditions did not have sufficient instances across participants to be modeled on their own and were combined with trials where no response was recorded under an ‘OTHER’/nuisance regressor condition. The initial reminder period was modeled as a condition of no interest. The six rigid-body translation and rotation parameters derived from spatial realignment were also included as nuisance regressors. Data were high-pass filtered with a cutoff of 128s.
Two different focal contrasts at the participant level were compared with baseline activity: (i) trials in which participants were presented with feedback that the peer recommendations differed from their own ([group rated higher + group rated lower ] > rest), and separately (ii) trials in which participants were presented with feedback that the group’s recommendation differed from their own and they updated their final recommendations ([group rated higher and participant changed their final rating + group rated lower and participant changed their final rating] > rest).
Next, we created random effects models at the group level that averaged across each participant level contrast of interest. Parameter estimates of activity from our hypothesized, meta-analytically derived functional mentalizing network were extracted in units of percent signal change, using Marsbar (Brett et al., 2002). We then ran multiple regression analyses in R Core Team (2014), in which neural activity within the mentalizing network was predicted from participants’ ego betweenness centrality, as well as social network size. This allowed us to examine the interaction between social feedback and information brokerage position on neural activity during decision making and to determine whether activity in regions associated with mentalizing during social decisions was associated with either social network size or structure. In addition, we ran parallel regression analyses predicting brain activity during trials in which participants received feedback that the peer group rating differed from their own and they changed their initial rating, controlling for neural activity during trials in which they received feedback indicating the group agreed with their initial rating and they did not change this rating. We also ran a similar analysis controlling for neural activity during trials in which participants received feedback that the group rating differed from their own and did not change their rating. These analyses allow us to determine whether there is an effect of social network structure on brain activity within our regions of interest that is related to using divergent peer feedback to update recommendations, that goes above and beyond (i) making a rating that agrees with the group opinion, and (ii) receiving feedback that diverges from one’s own opinion.
Finally, to check for significant activation outside of our a priori hypothesized regions of interest (i.e. to determine the specificity of our ROI results), we ran a group level model with each participant’s information brokerage (i.e. betweenness centrality) score used as a regressor for whole brain effect for trials in which participants were presented with feedback that the group’s recommendation differed from their own and they updated their final recommendations. We ran a parallel analysis for network size. Both whole brain analyses were thresholded at P < 0.001 with a cluster size of 76 corresponding to P < 0.05 corrected based on 3dClustSim (Version AFNI_16.2.02).
Results
Behavioral data
As expected, participants updated their recommendations significantly more often when receiving feedback that peer recommendations differed from their initial ratings than when peer feedback reinforced their initial ratings [paired t(49) = 12.22, P < 0.001, r2 = 0.75 CI (0.32, 0.45)] (O’Donnell, et al., 2015). We observed no difference, however, in tendencies to change ratings after receiving differing feedback according to social network size, controlling for behavior after receiving reinforcing feedback [β = −0.02, t(47) = −0.12, P = 0.909, r2 = 0.00, CI (−0.0002, 0.0001)], or tendencies to change ratings after any form of feedback [β = −0.04, t(48) = −0.25, P = 0.808, r2 = 0.00, CI (−0.0001, 0.0001]). We observed marginal differences in tendencies to change ratings after receiving differing feedback according to brokerage position (ego betweenness), controlling for behavior after receiving reinforcing feedback [β = −0.24, t(47) = −1.73, P = 0.090, r2 = 0.06, CI (−0.66, 0.05)], and in the tendency to change ratings after any form of feedback [β = −0.23, t(48) = −1.60, P = 0.117, r2 = 0.05, CI (−0.48, 0.06)]. In other words, those high and low in network ties and information brokerage were equally likely to update their recommendations in response to peer feedback, with those lower in brokerage updating marginally more often overall.
fMRI data
We tested the hypotheses that (i) participants with larger Facebook networks (i.e. higher in network ties), and separately (ii) those higher in information brokerage would show increased activity in the network comprised of DMPFC, bilateral TPJ, MPFC, PCC and MTG when encountering opinions that differed from their own. We first examined social network size in relation to activity within brain regions derived from a Neurosynth automated meta-analysis of the term ‘mentalizing’ (Figure 2), including DMPFC, bilateral TPJ, MPFC, PCC, and MTG. Social network size was not significantly related to activity in this network of interest in response to peer feedback that differed from their initial recommendations [β = 0.006, t(48) = 0.04, P = 0.967, r2 = 0.00, CI (−0.0001, 0.0001)]. We next examined information brokerage in relation to this same neural activity. Participants higher in ego betweenness centrality showed greater activity in the network of interest when receiving peer feedback that differed from their initial recommendations [β = 0.312, t(48) = 2.274, P = 0.028, r2 = 0.097, CI (0.031, 0.503)].
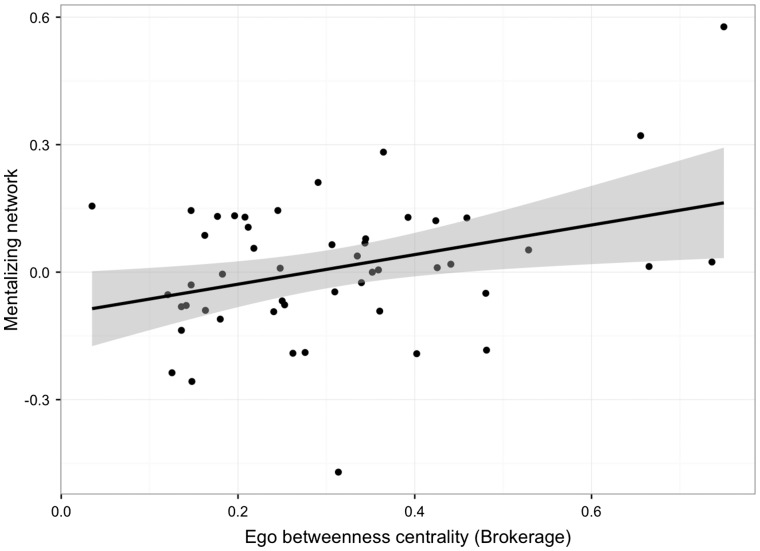
Next, we examined whether this increased brain activity was associated with conforming to the peer feedback. Social network size was not significantly related to activity in the hypothesized mentalizing network of interest when participants received and conformed to divergent peer feedback [β = 0.105, t(48) = 0.728, P = 0.47, r2 = 0.011, CI (−0.00009, 0.0002)]. In contrast, those with higher betweenness centrality in their Facebook networks showed greater activity within the hypothesized mentalizing network during trials when they received and conformed to divergent peer feedback [β = 0.343, t(48) = 2.526, P = 0.015, r2 = 0.117, CI (0.07, 0.63); Figure 4]. This result also held controlling for individual differences in activity within the mentalizing ROIs when participants received feedback indicating either (i) peer agreement and did not change their behavior [β = 0.283, t(47) = 2.123, P = 0.039, r2 = 0.199, CI (0.02, 0.56)] or (ii) that the group opinion differed from their initial rating and did not change their behavior [β = 0.346, t(47) = 2.504, P = 0.016, r2 = 0.118, CI (0.07, 0.63)]. Thus, participants who were higher in information brokerage were especially likely to show increased activity in meta-analytically defined sub-regions of DMPFC, bilateral TPJ, MPFC, PCC and MTG when they changed their minds to incorporate peer feedback, whereas network size was not associated with activity in these regions. Additionally, these effects held when controlling for individual differences in neural activity within the network of interest associated with maintaining the same opinion as the peer group, and separately receiving divergent peer feedback but not updating one’s final recommendation to conform to peers.
Fig. 4.
Association between information brokerage and neural activity in hypothesized mentalizing network of interest when receiving peer feedback and finalizing recommendations incorporating social feedback.
We also ran whole brain regressions of participants’ network size and information brokerage (i.e. ego betweenness centrality) against neural activity during trials where peer feedback differed from initial rating and led to a change of rating to explore other neural activity outside of meta-analysis mentalizing ROI. This analysis confirmed the relationship between activity in the hypothesized network of interest and information brokerage (see Figure 5 and Table 1). However, consistent with the ROI results there were no significant activations associated with network size. Together, these results demonstrate that the underlying neural mechanisms that lead to social influence on recommendation behavior covary with opportunities for information brokerage in personal social networks.
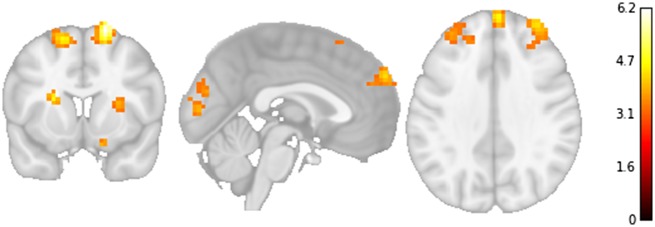
Fig. 5.
Whole brain analysis showing association between information brokerage and neural activity during social feedback leading to recommendation change. Note: x = 0, y = 10, z = 34; threshold = P < 0.001, k ≥ 76, where k is the number of voxels per cluster based on a 3dClustSim simulation corresponding to P < 0.05, corrected.
Table 1.
Positive associations between information brokerage and neural activity during social feedback leading to behavior change
| Region | hemisphere | x, y, z | size | t-stat |
|---|---|---|---|---|
| DMPFC | R | 5 60 34 | 76 | 4.59 |
| SFG (BA 6) | R | 18 8 70 | 276 | 6.24 |
| DMPFC | R | 11 29 61 | 3.27 | |
| SFG | L | −16 8 64 | 4.61 | |
| DLPFC (BA 10) | R | 39 43 25 | 467 | 5.36 |
| Insula | R | 29 -9 16 | 5.18 | |
| Putamen | L | −23 8 16 | 267 | 4.72 |
| Anterior insula | L | −30 32 10 | 3.27 | |
| DLPFC (BA9) | L | −33 46 37 | 3.27 | |
| Insula | R | 32 15 −14 | 87 | 4.65 |
| Temporal pole | R | 42 22 −20 | 4.49 | |
| Calcarine/Cuneus | R | 1 −88 7 | 100 | 3.92 |
Note: Threshold, P < 0.001, k = 76, where k is the number of voxels per cluster based on a 3dClustSim simulation corresponding to P < 0.05, corrected. DMPFC, dorsomedial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus. Note: no negative associations were observed between information brokerage and neural activity during social feedback leading to behavior change. Likewise, no whole-brain associations were observed with social network size.
Discussion
We show that social network structure, but not size, is associated with brain activity in meta-analytically defined subregions of DMPFC, bilateral TPJ, MPFC, PCC, and MTG during social judgments. In particular, information brokers—individuals with more opportunities to connect otherwise unconnected others in their social networks—show increased activity in these regions when learning that others’ opinions differed from their own. Moreover, higher brokers, relative to lower brokers, also show increased activity within these regions when updating their recommendations to realign with peer recommendations.
DMPFC, bilateral TPJ, MPFC, PCC and MTG were identified using Neurosynth’s RI map of ‘mentalizing’ (Yarkoni et al., 2011). In parallel, previous studies have linked brain activity within each of these regions to mentalizing and social judgments (Frith and Frith, 2001; Saxe and Kanwisher, 2003; Amodio and Frith, 2006; Saxe and Powell, 2006). Understanding others’ mental states may be particularly important for successfully communicating information to others, and specifically making successful recommendations. In line with this view, previous neuroimaging research has linked brain activity in TPJ to successful recommendation behavior and social sharing (Dietvorst et al., 2009; Falk et al., 2013; O’Donnell, et al., 2015). Individuals who engaged the bilateral TPJ more while considering novel ideas in a recommendation task were better at convincing others to adopt their opinion (Falk et al., 2013) and this was reflected in the way they presented the ideas to others (Falk et al., 2012; O’Donnell et al., 2015). Likewise, salespeople who communicated more effectively with their clients also showed increased activity within TPJ and MPFC (Dietvorst et al., 2009). More broadly, our findings reinforce an expanded view of brain regions within the hypothesized mentalizing system not only to include simulating the mental states of others in direct interaction (Saxe and Kanwisher, 2003; Decety and Lamm, 2007), but also to include the use of these regions to incorporate peer feedback in recommendations made to others (Falk et al., 2013; O’Donnell, et al., 2015). This may include simulating both the mental states of peers (whose feedback is incorporated) as well as imagined recipients of the recommendations.
A separate line of research has documented that information brokers have particular advantages in situations that require access to novel information (for a review, see Burt et al., 2013). Might these advantages relate to the fact that higher information brokerage is associated with greater activity in regions of interest implicated in mentalizing during social decision making? One possibility is that opportunities to broker information between diverse groups require thinking about what is important to both parties and how to best present (frame) information for them, affording those within such social network positions more practice in this domain. It is also possible that people who tend to take the perspectives of others are more likely to form relationships with diverse groups, predisposing them to being information brokers (Kalish and Robins, 2006; Burt et al., 2013). In both cases, the socio-cognitive process of making and updating recommendations differs based on the degree to which an individual interfaces with external perspectives in daily life. This highlights the importance of considering broader social environments, and social network position in particular, in understanding the brain bases of social transmission.
More broadly, our data add to previous theories suggesting that an individual’s social network structure and position can be conceptualized as an individual difference variable that influences and is influenced by psychological tendencies (Burt, 2012; Burt et al., 2013). This would imply that the spread of ideas through networks depends not only on brokerage positioning, but also on the social cognitive framework tied to being a broker. Yet, past research has not considered how those with higher brokerage in their personal networks may use their brains differently in the context of social decision making. We found that individuals whose social networks provide them with more opportunities to share novel information or make connections between other pairs of individuals in their networks (i.e. high information brokerage) showed greater levels of activity in regions associated with mentalizing while considering social feedback concerning mobile game app recommendations. These findings resonate with past research linking ‘self-monitoring’—the tendency to adapt one’s self-presentation to the current context—to higher network brokerage (Oh and Kilduff, 2008). Our data are also consistent with recent findings demonstrating that more popular individuals show heightened activity in DMPFC, PC, and TPJ during social judgments (Zerubavel et al., 2015). More generally, these results bolster the emerging idea that cognitive factors underlie social network structure (Brashears and Quintane, 2015), and highlight the value of studying the neurocognitive factors in parallel with personal network structure. Given that both high and low information brokers tend to update their recommendations in response to peer feedback to similar degrees, social network differences may manifest in ways that are not apparent with self-report or behavioral measures alone. In other words, the effects of brokerage on cognitive processes were more clearly observed in the neural processes underlying decision making than in the decision outcome itself.
In line with the importance of considering social network structure (in addition to size), we do not find a significant relationship between network size and activity within our hypothesized mentalizing network when receiving divergent peer feedback and incorporating it into recommendations. Recent neurocognitive studies have reported links between social network size and amygdala size (Bickart et al., 2011), grey matter volume of social cognitive regions (Kanai et al., 2012), and functional resting-state connectivity between the amygdala and social perception regions (Bickart et al., 2012). In contrast to such work, we observe a clear connection between social network structure (brokerage role) and activity within hypothesized mentalizing regions during an active recommendation task. One possibility is that the task presented to our participants (i.e. incorporating divergent peer opinions into a final recommendation) may capture a different type of processing than the processes reflected during resting state, and hence covary with different network metrics. This follows as making recommendations is one important way that ideas spread and information is brokered. In addition, prior studies have also relied upon self-report data of social resources. In contrast, we have made use of objectively measured social network data and considered a different social resource, i.e. information brokerage. Such networks and log data of online interactions can serve as rich proxies for social resources and thus present new opportunities to address questions about the brain bases of social interaction. In combination with past work, the significant role of social network structure—and not size—during the active task of using social information to make recommendations highlights the value of using a broad range of social network metrics for studying network cognition.
Despite this value, the current data should be considered in light of strengths and limitations to the social and psychological inferences that can be drawn from these correlational data. This study balances external and internal validity; the task used mirrors situations in which individuals make online recommendations (e.g. product ratings) by incorporating product information in conjunction with existing social feedback (e.g. ‘like’, ±1 or thumbs up/down ratings of reviews). For example, when making product recommendations people are often exposed to the recommendations of others in the process, thus undergoing some degree of social influence on our own recommendations. Thus, we used a task designed to replicate making online recommendations (ratings for mobile game apps) and receiving social feedback about these decisions (O’Donnell et al., 2015). After making their initial recommendations, we systematically manipulated the social feedback provided to participants to allow for the comparison of supportive (i.e. group rating was SAME) vs differing (i.e. group rating was HIGHER or LOWER) feedback. That said, one major limitation is that our approach relies on RI to draw conclusions about potential cognitive processes that may differ by network position. The brain regions we focus on for their role in mentalizing (DMPFC, bilateral TPJ, PC/PCC, MPFC and MTG) are also implicated in other cognitive functions beyond mentalizing; hence, our interpretations of associations with this brain activity should be considered one of a wide range of possible psychological interpretations. However, the use of a large-scale automated meta-analysis to derive clusters preferentially associated with mentalizing allows us to take one step toward testing the theoretical claim that information brokerage c=10?>co-varies with activity within this brain system.
Our data should also be interpreted within the constraints of the sample studied—adolescent males. Gender may be associated with general network characteristics and network cognition tendencies. Most notably, women tend to have larger networks, greater proportion of family ties, and greater capacity to recall social network features (see Brashears, 2008 and Brashears et al., 2016, for reviews on gender differences in networks). As such, gender differences should be considered in future work on the correlates of ego betweenness and in investigations that combine brain and social network analyses more broadly. There is also evidence that personal network structure shifts over the course of the human lifespan. For example, Wrzus et al. (2013) review how personal friendship networks increase in size through young adulthood, before dropping steeply thereafter. In parallel, a growing body of literature has documented dynamic changes in the structure and function of teenage brains as well as in how the brain responds to social and emotional tasks across development (Crone and Dahl, 2012). As such, studies that combine SNA and neuroimaging during social and cognitive tasks across development may be particularly fruitful. Likewise, focus on multiple operationalizations of social networks (e.g. communication networks vs. friendship networks) may also be particularly informative in relation to the brain’s response to social tasks.
More broadly, our findings lay the foundation for further study of how brain network and social network dynamics may interact in the context of social interactions and decision making. Our data demonstrate how an individual’s position in a network may not only affect the interactional dynamics with existing network ties (Burt et al., 2013); rather, social network position appears to be associated with different neurocognitive processing at a more basic level. In turn, this may affect how new social interactions unfold and how social network structures evolve.
Funding
The research was supported by (i) the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development contract no. HHSN275201000007C (PI:Bingham); (ii) A University of Michigan Injury Center Pilot Grant (PI:Falk); and (iii) An NIH Director’s New Innovator Award no. 1DP2DA03515601 (PI Falk).
Acknowledgments
The authors gratefully acknowledge the Communication Neuroscience lab for research assistance and the staff of the University of Michigan fMRI Center as well as C. Raymond Bingham, Jean T. Shope, Marie Claude Ouimet, Anuj K. Pradhan, Bruce G. Simons-Morton, Kristin Shumaker, Jennifer LaRose, Farideh Almani and Johanna Dolle for collaboration on a larger study from which these data were drawn and assistance with data collection.
Conflict of interest. None declared.
References
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77. 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arnaboldi V., Guazzini A., Passarella A. (2013). Egocentric online social networks: analysis of key features and prediction of tie strength in Facebook. Computer Communications, 36(10–11), 1130–44. [Google Scholar]
- Bakshy E., Rosenn I., Marlow C., Adamic L. (2012). The role of social networks in information diffusion. arXiv, 1201.4145 [physics] Available: http://arxiv.org/abs/1201.4145.
- Bickart K.C., Wright C.I., Dautoff R.J., Dickerson B.C., Barrett L.F. (2011). Amygdala volume and social network size in humans. Nature Neuroscience, 14(2), 163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti S.P., Mehra A., Brass D.J., Labianca G. (2009). Network analysis in the social sciences. Science, 323(5916), 892–5. 10.1126/science.1165821. [DOI] [PubMed] [Google Scholar]
- Brandes U. (2001). A faster algorithm for betweenness centrality. Journal of Mathematical Sociology, 25(2), 163–77. [Google Scholar]
- Brashears M. (2008). Gender and homophily: differences in male and female association in Blau space. Social Science Research, 37(2), 400–15. [DOI] [PubMed] [Google Scholar]
- Brashears M.E., Quintane E. (2015). The microstructures of network recall: How social networks are encoded and represented in human memory. Social Networks, 41, 113–26. [Google Scholar]
- Brashears M., Hoagland E., Quintane E. (2016). Sex and network recall accuracy. Social Networks, 44(1), 74–84. [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. (2002). Region of interest analysis using an spm toolbox. Presented at the The 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan.
- Burt R.S. (2012). Network-related personality and the agency question: Multirole evidence from a virtual world. American Journal of Sociology, 118(3), 543–91. [Google Scholar]
- Burt R.S., Kilduff M., Tasselli S. (2013). Social network analysis: foundations and frontiers on advantage. Annual Review of Psychology, 64(1), 527–47. [DOI] [PubMed] [Google Scholar]
- Cascio C.N., O’Donnell M.B., Bayer J., Tinney F., Falk E.B. (2015). Neural correlates of susceptibility to group opinions in online word-of-mouth recommendations. Journal of Marketing Research, 52(4), 559-75. [Google Scholar]
- Cascio C.N., Scholz C., Falk E.B. (2015). Social influence and the brain: persuasion, susceptibility to influence and retransmission. Current Opinion in Behavioral Sciences, 3, 51–7. [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–50. [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2007). The Role of the Right Temporoparietal Junction in Social Interaction: How Low-Level Computational Processes Contribute to Meta-Cognition. The Neuroscientist, 13(6), 580–93. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietvorst R.C., Verbeke W.J.M., Bagozzi R.P., Yoon C., Smits M., van der Lugt A. (2009). A Sales Force–Specific Theory-of-Mind Scale: Tests of Its Validity by Classical Methods and Functional Magnetic Resonance Imaging. Journal of Marketing Research, 46(5), 653–68. [Google Scholar]
- Dunbar R.I.M. (2012). The social brain meets neuroimaging. Trends in Cognitive Sciences, 16(2), 101–2. [DOI] [PubMed] [Google Scholar]
- Everett M., Borgatti S.P. (2005). Ego network betweenness. Social Networks, 27(1), 31–8. [Google Scholar]
- Falk E.B., Morelli S.A., Welborn B.L., Dambacher K., Lieberman M.D. (2013). Creating buzz: the neural correlates of effective message propagation. Psychological Science, 24(7), 1234–42. [DOI] [PubMed] [Google Scholar]
- Falk E.B., O’Donnell M.B., Lieberman M.D. (2012). Getting the word out: neural correlates of enthusiastic message propagation. Frontiers in Human Neuroscience, 6, 313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L.C. (1979). Centrality in social networks - conceptual clarification. Social Networks, 1, 215–39. [Google Scholar]
- Frith U., Frith C.D. (2001). The Biological Basis of Social Interaction. Current Directions in Psychological Science 10(5), 151–5. [Google Scholar]
- Frith, U., Frith, C. D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences, 358(1431), 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guille A., Hacid H., Favre C., Zighed D.A. (2013). Information diffusion in online social networks. A Survey. SIGMOD Rec 42(2), 17–28. [Google Scholar]
- Hagberg A.A., Schult D.A., Swart P.J. (2008). Exploring network structure, dynamics, and function using NetworkX. In: Proceedings of the 7th Python in Science Conference (SciPy2008) Pasadena, CA USA, 11–15.
- Kane G.C., Alavi M., Labianca G., Borgatti S.P. (2014). What’s different about social media networks? A framework and research agenda. MIS Quarterly 38(1), 275–304. [Google Scholar]
- Kalish Y., Robins G. (2006). Psychological predispositions and network structure: the relationship between individual predispositions, structural holes and network closure. Social Networks, 28(1), 56–84. [Google Scholar]
- Kanai, R., Bahrami, B., Roylance, R., Rees, G. (2012). Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences, 279(1732), 1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gala M., Arnaboldi V., Conti M., Passarella A. (2012). Ego-net digger: a new way to study ego networks in online social networks In: Proceedings of the First ACM International Workshop on Hot Topics on Interdisciplinary Social Networks Research. New York, NY, USA: ACM, 9–16. [Google Scholar]
- Marsden P.V. (2002). Egocentric and sociocentric measures of network centrality. Social Networks, 24(4), 407–22. [Google Scholar]
- Molesworth T., Sheu L.K., Cohen S., Gianaros P.J., Verstynen T.D. (2015). Social network diversity and white matter microstructural integrity in humans. Social Cognitive and Affective Neuroscience, 10(9), 1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M.B., Falk E.B., Lieberman M.D. (2015). Social in, social out: how the brain responds to social language with more social language. Communication Monographs, 82(1), 31–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Kilduff M. (2008). The ripple effect of personality on social structure: self-monitoring origins of network brokerage. Journal of Applied Psychology, 93(5), 1155.. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2014). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Available: http://www.R-project.org/ [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people The role of the temporo-parietal junction in "theory of mind." Neuroimage, 19, 1835–42. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. (2006). It’s the thought that counts specific brain regions for one component of theory of mind. Psychological Science, 17(8), 692–9. [DOI] [PubMed] [Google Scholar]
- Stiller, J., Dunbar, R. I. M. (2007). Perspective-taking and memory capacity predict social network size. Social Networks, 29(1), 93–104. [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Simmons M.P., Adamic L.A., Adar E. (2011). Memes online: extracted, subtracted, injected, and recollected In: Adamic L.A., Baeza-Yates R.A., Counts S., editors. Proceedings of The Fifth International Conference on Weblogs and Social Media. Menlo Park, CA: The AAAI Press, 353-360icwsm. The AAAI Press; Available: http://dblp.uni-trier.de/db/conf/icwsm/icwsm2011.html#SimmonsAA11. [Google Scholar]
- Smith K.P., Christakis N.A. (2008). Social networks and health. Annual Review of Sociology, 34, 405–29. [Google Scholar]
- Wrzus C., Hänel M., Wagner J., Neyer F.J. (2013). Social Network Changes and Life Events Across the Life Span: A Meta-Analysis. Psychological Bulletin, 139(1), 53–80. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerubavel N., Bearman P.S., Weber J., Ochsner K.N. (2015). Neural mechanisms tracking popularity in real-world social networks. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]