Abstract
Pleasurable stimuli, including reward, inhibit pain, but the level of the neuraxis at which they do so and the cerebral processes involved are unknown. Here, we characterized a brain circuitry mediating pain inhibition by reward. Twenty-four healthy participants underwent functional magnetic resonance imaging while playing a wheel of fortune game with simultaneous thermal pain stimuli and monetary wins or losses. As expected, winning decreased pain perception compared to losing. Inter-individual differences in pain modulation by monetary wins relative to losses correlated with activation in the medial orbitofrontal cortex (mOFC). When pain and reward occured simultaneously, mOFCs functional connectivity changed: the signal time course in the mOFC condition-dependent correlated negatively with the signal time courses in the rostral anterior insula, anterior-dorsal cingulate cortex and primary somatosensory cortex, which might signify moment-to-moment down-regulation of these regions by the mOFC. Monetary wins and losses did not change the magnitude of pain-related activation, including in regions that code perceived pain intensity when nociceptive input varies and/or receive direct nociceptive input. Pain inhibition by reward appears to involve brain regions not typically involved in nociceptive intensity coding but likely mediate changes in the significance and/or value of pain.
Keywords: cognitive-emotional pain modulation, functional magnetic resonance imaging, pain biomarker, psychological pain modulation
Introduction
Interactions between the two fundamental motivators pain and reward modulate our perceptions and behavior (Leknes and Tracey, 2008, for review; Talmi et al., 2009; Becker et al., 2013). The prospect of reward, say a sport’s trophy, renders pain, of for example an injury, less intense and less significant compared to situations in which the same nociceptive input lacks association with reward. The influence of reward on pain is conceptualized in Field’s motivation-decision model (Fields, 2007), postulating that reward decreases pain signals when the motivation to obtain reward is prioritized over pain avoidance. Pain-inhibiting effects of reward have been confirmed experimentally in rodents (Dum and Herz, 1984) and humans (Becker et al., 2013), with the latter study also showing pain-facilitatory effects of punishment.
Pleasure is an integral component of reward and passively experiencing pleasurable stimuli such as seeing positively valenced pictures or smelling pleasant odors decreases pain (e.g. Villemure et al., 2003; Rhudy et al., 2005) as would be expected for rewarding stimuli. Pleasure-induced pain inhibition correlates with increased activation in the orbitofrontal cortex (OFC; Roy et al., 2009; Younger et al., 2010). Outside pain, the OFC is involved in determining the subjective and context-dependent value of reward (Grabenhorst and Rolls, 2011, for review). Thus, the OFC is a prime candidate region for mediating pain-inhibitory effects of reward. What remains completely unknown is how activation of the OFC is translated into pain reduction. Such pain reduction could be exerted via inhibition of ascending nociceptive input, e.g. via descending pathways as suggested by the Motivation-Decision Model. Alternatively, perceptual pain reduction could be mediated supraspinally without altered spinal pain processing (cf. Wiech and Tracey, 2009).
Anatomically, the OFC has extensive reciprocal connections to several regions that are involved in the processing of painful stimuli, including the insular cortex, the anterior cingulate cortex (ACC) and somatosensory areas. Connections with the insular cortex are organized in an anterior-posterior fashion, possibly with stronger connections to the anterior compared to the posterior insula (Mufson and Mesulam, 1982; Cavada et al., 2000, for review). Anterior parts of the insula are important for emotional-cognitive pain modulation (e.g. Kong, 2006; Zeidan et al., 2011), but only the posterior insula receives direct spinothalamic nociceptive input (Dum et al., 2009). The OFC has also strong reciprocal connections with several sub-regions of the ACC (Carmichael and Price, 1995; Ongür and Price, 2000, for review; for subdivisions of the ACC see Etkin et al., 2011). Work in monkeys shows that both the posterior-dorsal and anterior-dorsal ACC (pdACC and adACC; posterior and anterior midcingulate cortex according to Vogt, 2016) receive spinothalamic input (Dum et al., 2009). However, while the pdACC tracks perceived pain intensity (Wager et al., 2013) and is perhaps implicated in processing the affective dimension of pain (Apkarian et al., 2005, for review), the anterior-dorsal portion of the ACC plays a role in additional processes relevant to this study, including pain modulation, reward processing and the detection of conflict (e.g. Braver et al., 2001, for review; Petrovic et al., 2008; Beckmann et al., 2009; Talmi et al., 2009). Somatosensory brain regions have been shown to be important for sensory-discriminative aspects of pain perception. Primary somatosensory cortex (SI) seems to be particularly important for stimulus localization (Vierck et al., 2013, for review) and thereby for thread localization. Thus, its anatomical connections place the OFC in a position to mediate pain-inhibitory effects via a circuitry including the insula, ACC and somatosensory regions.
The aim of this study was to characterize the brain circuitry mediating pain inhibition in the presence of a rewarding stimulus. Specifically, we wanted to test at which level of the neuraxis this modulation is exerted. Therefore, we first assessed, using a standard general linear model analysis as well as comparison to the ‘neurological pain signature’ (NPS), a network sensitively tracking perceived pain intensity related to changes in nociceptive input (Wager et al., 2013), whether pain inhibition by reward was associated with decreased activation in pain intensity coding regions and/or regions receiving direct nociceptive input, i.e., the posterior insula, adACC and pdACC, SI, SII and thalamus. Next, we tested how between-subject variance of this pain modulation is reflected in the brain and whether pain inhibition by monetary wins or pain facilitation by monetary losses is the driving factor. Using a psycho-physiological interaction (PPI) analysis, the functional connectivity of the region identified in the between-subject analysis was investigated on the individual level. Pain modulation was achieved by combining a wheel of fortune game with experimental pain stimuli in healthy volunteers undergoing functional magnetic resonance imaging (fMRI).
Materials and methods
Participants
Thirty-one healthy volunteers were recruited for the experiment. Exclusion criteria were any present or past pain condition, psychiatric disorders, excessive gambling, substance abuse behaviors, alcohol consumption of more than 100 ml alcohol per week, tobacco use, regular night shifts or sleep disorders. The study was approved by the McGill University Institutional Review Board and informed consent was obtained from all participants according to the revised Declaration of Helsinki (2008).
Six participants1 participated only in a pre-scan familiarization session and not in the fMRI session. Data from one participant were excluded after the fMRI session without any analysis because of intense pain at the head during data acquisition caused by an uncomfortable position. Thus, the final sample consisted of 24 volunteers (12 females; age M = 23.7 years, SD = 3.6 years).
General design
The study followed a within-subject design with the two factors ‘stimulation intensity’ and ‘outcome’ of the wheel of fortune game (see below). Before the fMRI session, participants underwent a pre-scan familiarization session. In the pre-scan session, participants were familiarized with the thermal stimuli, the rating scale and the wheel of fortune game to decrease unspecific effects of novelty and saliency. Further, thermal pain thresholds were assessed to determine the stimulation intensities for the wheel of fortune game. At the beginning of the fMRI session, participants were reminded of the wheel of fortune game and, if necessary, stimulus intensities were adjusted to achieve mildly and moderately painful sensations.
Rating scale
Participants rated the perceived intensity of each thermal stimulus using a horizontally orientated VAS. The VAS ranged from 0 ‘no sensation’ to 200 ‘most intense pain tolerable’ with 100 being the pain threshold, to differentiate between non-painful and painful sensations (Villemure and Bushnell, 2009). In the pre-scan session, participants were trained to rate the perceived intensity of the thermal stimuli only, not of the combination of pain and monetary wins or losses as presented later in the wheel of fortune game, to allow for testing of effects of monetary wins and losses on the perception of thermal stimuli.
Thermal stimulation
Heat stimuli were applied to the thenar eminence of participants’ non-dominant hand using a 27-mm diameter contact thermode (Contact Heat Evoked Potentials, CHEPS; PATHWAY Pain & Sensory Evaluation System, Medoc Ltd. Advanced Medical System, Israel). To standardize thenar stimulation, participants placed their hand on a hemisphere made of Styrofoam®, in which the thermode was embedded. Baseline thermode temperature was set to 32 °C; rise rate to 20 °C/s, and return rate to 30 °C/s. The target temperature was held constant for three seconds. Participants’ pain thresholds were assessed using a modified staircase method. The stimulation intensities for the wheel of fortune game were: (i) pain threshold plus 1.5 °C to provoke mildly painful sensations; and (ii) pain threshold plus 2.5 °C to provoke moderately painful sensations. Resulting average temperatures for the mildly painful stimuli were 47.6°C (SD = 1.6°C; rated as on average 118.46, SD = 17.37, in the neutral condition on the visual analogue scale; VAS), and 48.6°C (SD = 1.6°C; rated as 134.98 on average, SD = 19.99) for the moderately painful stimuli.
Wheel of fortune game
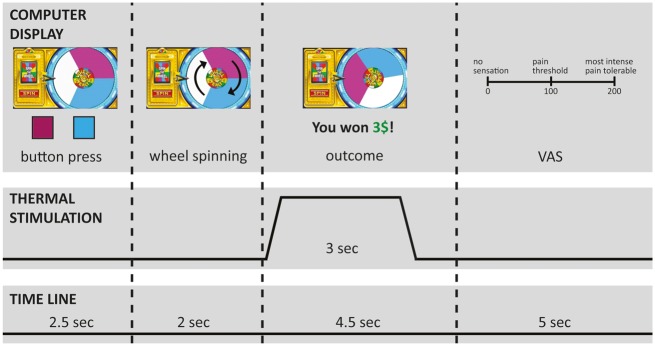
A wheel of fortune game, adapted from previous versions (Becker et al., 2013), served to manipulate participants’ perception of painful thermal stimuli by experiencing monetary wins and losses. Participants were presented on a computer screen with a wheel of fortune display (Figure 1) that was divided into three sections of equal size but different color. The game comprised two types of trials: choice trials, in which participants selected one of two colors of the wheel of fortune by pressing a corresponding button on a keyboard and neutral trials, in which participants had to press a button corresponding to the white section of the wheel that was not associated with any wins or losses. In both types of trials, the wheel started spinning after participants pressed a button. When the wheel came to a stop, the color under the cursor determined the outcome (Figure 1). If the wheel landed on the color the participant had selected, the participant won a certain amount of money (winning condition); if the wheel landed on the color the participant had not selected, the participant lost a certain amount of money (losing condition). In the neutral condition, the wheel always landed on white and an outcome of $0 was displayed. To make the wheel more realistic for the participants, the wheel also landed in some of the choice trials on white (dummy trials) with an outcome of $0 displayed. For trials with thermal stimulation (see below), the thermal stimulus was administered simultaneously with the monetary outcome.
Fig. 1.
Time line of one trial of the wheel of fortune game with simultaneous thermal stimulation. VAS: visual analogue scale. Depicted in the figure is an example of the wining condition. In the losing condition, participants saw the message in the outcome phase of the game that they lost a certain amount of money. In the neutral trials, no pointer was displayed on the wheel.
The losing and the neutral conditions served as control conditions for comparison with the winning condition. The losing condition was included to ensure that any pain-inhibitory effects are not simply caused by unspecific arousal because both winning and losing are associated with arousal (Sokol-Hessner et al., 2009, 2013). Losing trials also made the game more realistic, thereby increasing participants’ engagement.
During the functional scan of the MRI session, participants played in total 102 trials of the wheel of fortune game: 30 per condition (winning, losing, neutral) with 10 of each condition combined with the low stimulation intensity, 10 with the high stimulation intensity and 10 with no stimulation; 10 dummy trials (participants had a choice but the wheel landed on white) and 2 extra trials to adjust the net monetary outcome of the game. Except the two extra trials, which were always performed at the end of the game, trials were presented in pseudorandom order. The trials without stimulation were included to reduce skin sensitization. To further reduce skin sensitization, thermal stimulation never occurred in more than five consecutive trials. Immediately after each thermal stimulus, participants rated the perceived intensity of the thermal stimulus using the VAS (Figure 1).
To maximize the salience of the wins for the effect of interest (pain inhibition by reward), higher outcomes were used for trials with simultaneous thermal stimulation. In trials with thermal stimulation, participants received between $8.50 and $9.50 in the winning condition. A 4:3 relationship between wins and losses was used, based on work showing that losses loom larger than wins with respect to saliency (Kahneman and Tversky, 1979) as well as sympathetic arousal (Hochman and Yechiam, 2011). Thus, participants lost between $6.38 and $7.13 with thermal stimulation. In trials without stimulation, participants won or lost between $1 and $6. Different magnitudes of monetary outcome in trials with and without stimulation do not pose a problem because condition effects were analyzed across trials with stimulation or across trials without stimulation. Unbeknownst to the participants, the outcome of a trial was not related to their color selection because outcomes for each trial occurred in a predetermined, pseudorandom order. By this, other processes that might influence overlapping neural correlates such as learning and associated meaningful choice behavior were purposefully excluded, as confirmed by analysis of participants’ choice behavior.
The game was set-up in a way that participants made a monetary net win, which they were paid at the end of the experiment. Participants were informed before playing the game that they would receive this net win in real money at the end of the fMRI session. The net win was varied (extra trials of the game) between participants to keep a realistic impression of the game in case participants discussed the experiment with each other.
MRI data acquisition
Imaging data were acquired on a 3 T Siemens TRIO MRI scanner at the McConnell Brain Imaging Center, Montreal Neurological Institute (MNI) using a 32-channel head coil. A gradient-echo planar imaging (EPI) sequence covering the whole brain was used for the functional scan (TR = 2.37 s, TE = 30 ms, flip angle = 90 degree, 45 3 mm thick axial slices, descending acquisition, field of view (FoV) 192 mm × 192 mm, matrix 64 × 64, resulting in an in-plane resolution of 3 × 3 mm2, 562 image volumes). Slices were titled 30° clockwise from the AC-PC plane to reduce signal drop-out in orbitofrontal areas. The first two images were discarded to allow steady state magnetization. High-resolution, anatomical T1-weighted images (RF spoiled, pre-scan normalized MPRAGE sequence, TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°, FoV 176 × 256 × 256 mm, matrix 176 × 256 × 256, resulting in a voxel size of 1 mm3) were acquired for each subject for co-registration purposes. Field maps were obtained using a gradient echo sequence (TE = 30 ms, 0.25 ms dwell time, FoV and matrix identical to EPI).
Statistical analysis
The first three trials with thermal stimulation (conditions winning with mild pain, neutral with mild pain and winning with moderate pain in the order of their appearance) were excluded from analysis because ratings were on average higher (> the 95th percentile) than the rest of the trials. In addition, trials in which participants failed to choose a color were excluded (59 out of 2448 trials).
Behavioral data
Before testing the effects of monetary wins on the perception of nociceptive stimuli, it was ensured that the wheel of fortune game did indeed not allow meaningful choice behavior. Frequencies of choice repetitions were analyzed using a repeated measures analysis of variance (ANOVA) design with the two within-subjects factors ‘stimulation intensity’ (with the levels no stimulation, mildly and moderately painful) and ‘outcome’ (with the levels winning, losing, neutral) by mixed model procedures.
To test the effects of monetary wins and losses on the perception of nociceptive stimuli, VAS ratings of perceived intensity of the thermal stimuli were analyzed, after confirming normality (Shapiro-Wilk test), with a repeated measurement ANOVA design using mixed model procedures with the factors ‘stimulation intensity’ and ‘outcome’. Mixed model procedures were followed by post hoc pairwise comparisons, when appropriate.
In a previously published study, we observed that pain modulation by monetary wins increases with increased perceived intensity of nociceptive stimuli: stimuli perceived as more painful were more inhibited by monetary wins (Becker et al., 2013). We analyzed whether this finding was replicated in the present study by using Pearson’s correlation between the average of the perceived intensity of the mildly painful stimuli in the neutral condition and the difference between the winning and neutral condition after testing for multivariate outliers with Mahalanobis distance.
The significance level was set to 5%. All statistical analyses were performed using PASW Statistics 21 (SPSS Inc. Chicago).
fMRI data
All image processing and statistical analysis was performed using the software package FSL (FMRIB's Software Library; http://www.fmrib.ox.ac.uk/fsl; Smith et al., 2004).
Single subject analysis. The following preprocessing steps were applied to each functional dataset: manual denoising using multivariate exploratory linear optimized decomposition into independent components (MELODIC) to remove motion artifacts and physiological noise indicated by spatial maps (e.g. ring around head, component primarily in ventricles), time courses (large spikes) and power spectra (predominantly high frequency content) of the components, spatial smoothing (Gaussian kernel, full width at half-maximum: 5 mm), motion correction and temporal highpass filtering (Gaussian-weighted least-squares straight line fitting with sigma = 100s). Susceptibility-related distortions were corrected using FSL field map correction routines.
A general linear model (GLM) was applied to each functional dataset, modeling the outcome interval (Figure 1) of each of the nine conditions of the wheel of fortune game (outcome conditions ‘winning’, ‘losing’ and ‘neutral’, each combined with stimulation intensities ‘no stimulation’, ‘mild pain’ and ‘moderate pain’, termed in the following: win_no, win_mild, win_moderate, lose_no, lose_mild, lose_moderate, neutral_no, neutral_mild, neutral_moderate). Intervals in which the wheel was spinning, dummy trials, the extra trials at the end of the game and motion outliers were modeled in regressors of no interest. The model regressors were convolved with a gamma hemodynamic response function and the first temporal derivatives were included. Voxel-wise parameter estimates were derived using the appropriate contrasts. Individuals’ functional images were registered to their own anatomical scan and subsequently to the linear ICBM 152 template in MNI standard space using linear transformations (FLIRT; Jenkinson et al., 2002).
Group level analysis. Second level analyses were performed using a mixed-effects model, implemented in FLAME (Beckmann et al., 2003). In a first model (Model 1), the parameter estimates and the corresponding estimates of the variance from the individuals’ functional scans were merged on a group level. In a second model (Model 2), one regressor modeling the group mean (intercept) and a demeaned regressor containing each subject’s behavioral index of pain modulation was included to test specifically for neural correlates of inter-individual differences in perceptual modulation. To maximize the effects of the modulation and to control for unspecific effects of arousal, the behavioral index of pain modulation was calculated as the individual difference between VAS ratings in the winning and losing conditions during mild pain. For Model 2, a mask of typical pain (Treede et al., 1999) and reward (Liu et al., 2011) processing regions was used, comprising primary (SI) and secondary (SII) somatosensory cortex, insula, ACC, thalamus, OFC, medial PFC, putamen, caudate nucleus, ventral striatum and amygdala (volume of mask: 466 040 mm3). Statistical inference was based on a voxel-based threshold of z = 2.3, cluster corrected at P < 0.05 (whole brain for Model 1 and across the mask for Model 2).
Neurological pain signature. We tested whether the different stimulation intensities and monetary wins and losses had a modulatory effect on the so-called ‘Neurological Pain Signature’ (NPS; Wager et al., 2013). The NPS is a distributed pattern of fMRI activations, defined previously in an independent dataset by multivariate pattern analysis, which sensitively and specifically tracks changes in perceived pain intensity in response to changes of nociceptive input, including when controlling for stimulus intensity (Wager et al., 2013). Our rationale for testing any modulatory influences on the NPS was its high sensitivity and the fact that it does not respond to non-painful emotional events that otherwise activate similar brain areas. Modulation of the NPS by monetary wins would be consistent with the hypothesis that reward decreases activation in pain intensity coding regions. Lack of effects on the NPS, in conjunction with no change in the magnitude of pain-related activation in areas receiving ascending nociceptive input, would point to reward-induced pain modulation by another cerebral system, possibly related to valuation of simultaneously present pain and reward.
For calculating the strength of the NPS response in the different conditions of the wheel of fortune game, a voxelwise pattern of regression weights defined previously in an independent dataset (Wager et al., 2013) was used. For each subject and each condition of interest (win_mild, win_moderate, neutral_mild, neutral_moderate, lose_mild, lose_moderate), these pre-defined regression weights were multiplied with the parameter estimates obtained from the single subject analysis (the condition-specific brain activation maps) for each voxel and summed up across all voxels (i.e. the scalar product was calculated). This calculation resulted in one value for each subject and condition, describing the accordance with the NPS. These values were analyzed for differences between conditions with a repeated measurement ANOVA design using mixed model procedures with the factors ‘stimulation intensity’ and ‘outcome’.
Connectivity analyses. To investigate the influence of brain regions identified in Model 2 on other brain areas, a psychophysiological interaction analysis (PPI; Friston et al., 1997) was performed. PPI analyses provide a model of how a psychological context (i.e., obtaining monetary wins) changes the influence one brain area has on another area and is regarded as a measure of effective connectivity (Stephan and Friston, 2010). The area of significant activity related to the regressor of behavioral pain modulation in Model 2 served as the seed for the PPI analysis. Two PPI regressors were computed, each as the scalar product of the time course averaged across the voxels in the seed and a vector coding the wining condition with mild pain (win_mild) as the regressor of interest or coding the losing condition with mild pain (lose_mild) as a regressor of no interest. Both PPI regressors were included in the same model to model the full space of the pain modulation conditions (McLaren et al., 2012). In addition, regressors coding all nine conditions, the time course averaged across the seed voxels and the same regressors of no interest as above were included to ensure that the variance explained by the PPI regressors is not confounded by main effects of the conditions or other factors (O’Reilly et al., 2012). Statistical inference was based on a voxel-based threshold of z = 1.6 and cluster threshold of P < 0.05 within the regions of interest (RoIs) anterior insula (volume of mask: 3840 mm3), ACC (50120 mm3) and contralateral SI (postcentral gyrus; 29864 mm3) (Worsley et al., 1996). RoIs were anatomically defined using the Harvard-Oxford Cortical Structural Atlas implemented in FSL (signal intensity minimum at 30%). The insula RoI was manually restricted on the ICBM 152 template to the anterior part of the insula (the three short insular gyri) based on emotional-cognitive pain modulation being represented in anterior but not posterior parts.
Further, correlations of pain modulation by monetary wins or losses (indexed by the difference in VAS ratings between winning or losing and neutral with mild pain) with the connectivity strengths between the mOFC and the PPI clusters were calculated.
Results
Winning vs Losing money with simultaneous pain
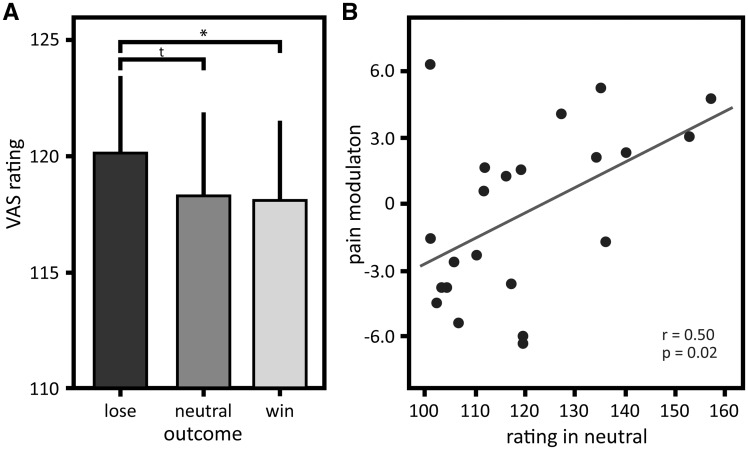
The outcome of the wheel of fortune game modulated pain perception dependent on stimulus intensity (interaction ‘stimulation intensity’ × ‘outcome’: F23 = 4.50, P = 0.022), but did not show an overall effect on perceived pain (main effect ‘outcome’: F23 = 0.28, P = 0.75), while the different stimulus intensities were perceived as different (main effect ‘stimulation intensity’: F23 = 58.10, P < 0.001). Post hoc comparisons revealed that monetary wins and losses modulated the perceived intensity of the mildly painful stimuli (pain intensity was rated as less intense in the winning condition compared with the losing condition, P = 0.03; Figure 2A). Post hoc comparisons did not show a difference in the VAS ratings between the winning and the neutral condition with mild pain (P = 0.89), while there was a trend between the losing and neutral condition with mild pain (P = 0.05). Moderately painful stimuli were not modulated by monetary wins and losses (post hoc comparisons winning vs losing P = 0.30, winning vs neutral P = 0.99, losing vs neutral P = 0.45). Therefore, only conditions with mildly painful stimulation are considered in the following analyses of pain modulation by monetary wins and losses.
Fig. 2.
Perceived pain intensity and pain modulation by monetary wins as a function of perceived intensity of nociceptive stimuli. (A) Means and standard errors of intensity ratings for the thermal stimuli in the winning, losing and neutral condition in the mildly painful stimulation category; (B) Correlation of pain modulation displayed as the difference between ratings in the winning and neutral condition in the mildly painful stimulation category (y-axis) as a function of perceived intensity of the stimuli in the neutral condition (x-axis). Successful modulation (i.e. inhibited perception by monetary wins) is displayed as positive values. Post hoc comparisons * P < 0.05, tP < 0.10.
Further, pain inhibition by monetary wins was greater the higher subjects rated the nociceptive stimuli in the mildly painful category in the neutral condition (r = 0.50, P = 0.02; Figure 2B; one multivariate outlier excluded, Mahalanobis distance P = 0.04).
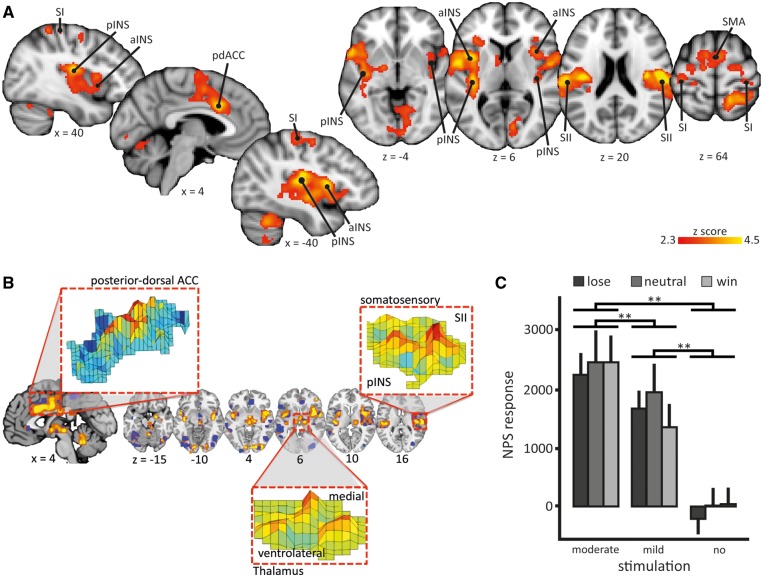
Before addressing whether pain-inhibitory effects of winning are mediated by (i) inhibition of pain intensity coding regions and/or direct inhibition of ascending input, or (ii) a cerebral circuitry outside these typical pain processing areas, we interrogated the main effects of Model 1 to assess activations associated with each condition (win_no, lose_no, win_mild and lose_mild). Each condition was associated with robust brain activation compared with rest (Supplementary Figure S1 and Supplementary Table S1). Also, as expected, moderate pain was associated with significantly higher activation in typical pain processing regions compared to mild pain, including posterior and anterior insula, pdACC, SII and supplementary motor cortex (Figure 3A;Table 1), indicating that increases in perceived intensity were associated with increased brain activation.
Fig. 3.
Neurological pain signature response and brain correlates of moderate compared to mild pain. (A) An univariate analysis showed that moderate pain was associated with higher brain activations compared to mild pain (contrast: [win_mod + neut_mod + lose_mod] > [win_mild + neut_mild + lose_mild]): anterior insula (aINS), posterior insula (pINS), primary somatosensory cortex (SI), secondary somatosensory cortex (SII), posterior-dorsal anterior cingulate cortex (pdACC) and supplementary motor area (SMA). Images are displayed in neurological convention, i.e. right side of the brain is on the right. Coordinates are given in MNI space. Statistical inference was based on a voxel-based threshold of z = 2.3, cluster corrected at p <.05 on a whole brain level. For details see Table 1; (B) A priori defined pattern of the Neurological Pain Signature (NPS). The inset show examples of the pattern distribution of voxel weights within certain brain areas; (C) NPS responses in the winning, losing, and neutral condition with mildly painful, moderately painful, or no stimulation of the wheel of fortune game; mean scalar values expressing the NPS across subjects; error bars: standard error of the mean. Post hoc comparisons ** P < 0.01. Scaling of the NPS values depends on many factors such as voxel size, contrast weight, field strength, etc. Because only a within-study comparison was of interest here, we did not attempt to equate scaling of the NPS values with previous studies.
Table 1.
Brain activation in response to moderately vs mildly painful stimulation
| MNI peak coordinates in mm |
|||||
|---|---|---|---|---|---|
| Brain region | Cluster size (mm3) | z score peak | x | y | z |
| Cluster spanning: | 42938 | 4.71 | −36 | 6 | 10 |
| central opercular cortex, | 4.71 | −36 | 6 | 10 | |
| insula, | 4.59 | −40 | −20 | 12 | |
| SII | 4.57 | −54 | 0 | 8 | |
| Cluster spanning: | 41520 | 4.46 | −4 | 14 | 32 |
| pdACC, | 4.46 | −4 | 14 | 32 | |
| parietal cortex, | 3.95 | 16 | −48 | 64 | |
| SMA | 3.55 | −8 | −2 | 60 | |
| cerebellum | 36816 | 4.01 | −32 | −50 | −50 |
| Cluster spanning: | 30520 | 5.34 | 54 | −14 | 18 |
| SII, | 5.34 | 54 | −14 | 18 | |
| central opercularcortex, | 4.90 | 38 | −16 | 18 | |
| insula | 3.94 | 34 | 14 | 6 | |
Brain areas that were more activated by moderately compared to mildly painful stimulation (significant on a whole brain-level, voxel-based threshold z = 2.3 and cluster-based threshold P < 0.05). Local maxima within the clusters are given for individual anatomical areas. SII, secondary somatosensory cortex; pdACC, posterior-dorsal anterior cingulate cortex; SMA, supplementary motor area.
In contrast, activation did not differ between win_mild and lose_mild, controlled for win_no and lose_no (contrast [win_mild – win_no] – [lose_mild – lose_no]; to ensure that significant brain activation in this contrast was not driven by deactivations, only z-values greater than zero were entered into the contrast calculation). To reduce the probability of a Type II error, we also interrogated a lenient voxel-based threshold of z > 1.6 uncorrected for cluster extent, which neither revealed any activation in this contrast. We conclude that brain activation was not decreased in win_mild compared to lose_mild, indicating that behavioral pain inhibition by monetary wins relative to losses did not change the magnitude of pain-related brain activation, despite lower ratings of perceived pain.
To further test the hypothesis that pain modulation by monetary wins and losses modulates activation in pain intensity coding regions, we tested whether the NPS, a brain system that sensitively scales with perceived pain related to changes in nociceptive input (Figure 3B), varied across conditions (cf. Woo et al., 2015). No differences in the NPS were found for the different outcomes of the wheel of fortune game (main effect ‘outcome’ F184 = 1.37, P = 0.26) nor an interaction between stimulus intensities and outcomes (F184 = 0.71, P = 0.57; Figure 3C). However, in line with the univariate results of Model 1, the NPS response increased significantly for moderately compared to mildly painful stimuli across the outcomes of the wheel of fortune game (main effect ‘stimulus intensity’ F184 = 92.22, P < 0.001; all post hoc comparisons P < 0.001; Figure 3C).
Mediation of the pain-inhibitory effects of winning
Inter-individual differences in the magnitude of behavioral pain modulation, indexed by the difference in VAS ratings between winning and losing with mild pain, correlated with the activation in the medial OFC (mOFC) in win_mild (between-subject analysis) (peak 10, 42, -16; z = 3.34; medial orbital gyrus, Rolls et al., 2015) (Model 2; Figure 4). No other brain region showed this behavior. This correlation was only present in the winning condition with mild stimulation; no correlation between activation and behavioral pain modulation was found for any of the other conditions. This suggests that the mOFC specifically mediated pain inhibition by monetary wins relative to monetary losses.
Fig. 4.
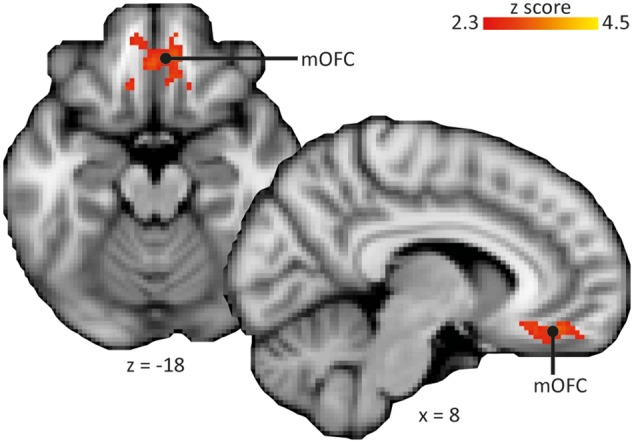
Brain correlates of pain inhibition by reward. Behavioral pain modulation correlated with brain activation in the medial orbitofrontal cortex (mOFC) in the condition with monetary wins and mild pain. Images are displayed in neurological convention, i.e. right side of the brain is on the right. Statistical inference was based on a voxel-based threshold of z = 2.3, cluster corrected at P <.05 across the mask comprising typical pain and reward processing regions (see section ‘Statistical analysis’). Coordinates are given in MNI space.
In order to characterize the brain circuitry mediating pain-inhibitory effects of monetary wins, a PPI analysis was performed with the mOFC as seed (within-subject analysis). The mOFC condition-dependent increased negative connectivity with all three RoIs (Figure 5; Table 2): a rostrally located cluster in the anterior insula, the hand area of SI contralateral to stimulation site and the adACC. Outside the RoIs, no region showed increased connectivity with the mOFC, significant at a whole brain level. The increased negative connectivity of the mOFC was specific to the condition in which participants won money and received mild pain at the same time, as ensured by the PPI design by including a second PPI regressor for losing money with simultaneous mild pain. No condition-dependent changes in connectivity with any of the three RoIs (anterior insula, adACC, SI) were obtained for this second PPI regressor when participants lost money and received mild pain simultaneously.
Fig. 5.
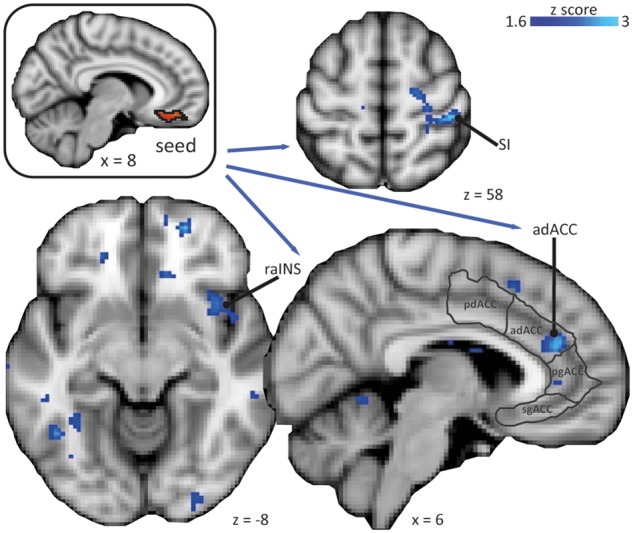
PPI analysis of connectivity of the medial orbitofrontal cortex (mOFC). The mOFC as the seed shows increased negative connectivity with the right primary somatosensory cortex (SI) in the hand area contralateral to the stimulation site, the right rostral anterior insula (raINS), and the anterior-dorsal anterior cingulate cortex (adACC). Subdivision of the ACC according to Etkin et al. (2011); pd, posterior-anterior; pg perigenual; sg, subgenual. Images are displayed in neurological convention, i.e. right side of the brain is on the right. Statistical inference was based on a voxel-based threshold of z = 1.6 and cluster threshold of P < 0.05 within the regions of interest anterior insula, ACC, and SI (postcentral gyrus). Coordinates are given in MNI space.
Table 2.
Brain regions showing increased negative connectivity with the medial orbitofrontal cortex (mOFC) when winning money and receiving pain simultaneously
| MNI peak coordinates in mm |
|||||
|---|---|---|---|---|---|
| Brain region | Cluster size (mm3) | z score peak | x | y | z |
| adACC | 1824 | 2.78 | 8 | 38 | 20 |
| SI (hand area) | 1400 | 2.88 | 42 | −30 | 58 |
| rostral anterior insula | 584 | 2.30 | 38 | 14 | −10 |
Brain areas that showed increased negative connectivity with the mOFC in the PPI analysis when receiving monetary reward and pain at the same time (significant on a voxel-based threshold of z = 1.6 and cluster threshold of P < 0.05 within the regions of interest (RoIs) anterior insula, ACC, and postcentral gyrus (Worsley et al. 1996)). Local maxima within the clusters are given for individual anatomical areas. SI, primary somatosensory cortex; adACC, anterior-dorsal anterior cingulate cortex.
Because negative connectivity between the mOFC and the PPI clusters in the adACC, the SI and the rostral anterior insula increased specifically in the winning condition with mild pain, we tested whether the connectivity strength between the mOFC and these PPI clusters was uniquely associated with pain inhibition by monetary wins. Pain inhibition by monetary wins was indexed by the difference in VAS ratings between winning with mild stimulation and neutral with mild stimulation. Connectivity strength between mOFC and adACC in the winning condition with mild pain correlated significantly with the degree of pain inhibition by monetary wins (r = 0.48, P = 0.02). In contrast, connectivity strength between mOFC and SI or rostral anterior insula did not correlate with pain inhibition by monetary wins. Further, no correlation was found for the connectivity strength between mOFC and adACC, SI or rostral anterior insula and pain facilitation by monetary losses (indexed by the difference in VAS ratings between losing and neutral with mild stimulation).
Discussion
The present results extend the existing literature by identifying a brain circuitry that inhibits pain when reward is simultaneously present. The pain inhibitory effects of reward were mediated by the mOFC: activation in this region correlated positively across subjects with the individuals’ perceived changes in pain intensity when obtaining monetary wins relative to losses. Interestingly, outcomes of the wheel of fortune task did not change the magnitude of activation in a pain intensity coding brain network or in typical pain processing areas, including those that receive direct nociceptive input. Rather than changing the magnitude of activation, monetary wins influenced the functional coupling between the OFC and typical pain processing regions: on an individual level, the signal time course in the mOFC condition-dependent correlated negatively with the signal time courses in the rostral anterior insula, adACC and primary somatosensory cortex when pain and reward occurred simultaneously. We interpret this as moment-to-moment down-regulation of these regions by the mOFC, which might be the mechanism by which the OFC achieved pain inhibition.
To our knowledge, the present results describe for the first time a cerebral network that might mediate pain inhibition by reward and provide insight into at which level of the neuraxis this modulation is achieved (cf. Leknes and Tracey 2008). The central role of the mOFC identified in this study is in line with previous results indicating that the OFC is important for pain inhibition by pleasure (Roy et al., 2009; Younger et al., 2010) as well as the reward literature that identifies the mOFC as monitoring the valence of reward values (Berridge and Kringelbach, 2015, for review). Pleasure is one component of reward for which hedonic experience or pleasure ("liking") and motivation to obtain reward (‘wanting’) are differentiated (Berridge et al., 2009). The design of the present study included a stimulus related to behavior and inducing approach behavior (White, 1989) to incorporate motivational aspects of reward in addition to the ‘liking’ of the reward for a more naturalistic reward stimulus. However, the current design does not allow disentangling specific contributions of motivation and pleasure. It would be interesting to investigate in future studies potential differential contributions of the mOFC in motivational and affective aspects of pain-reward interaction.
Extending studies on pain inhibition by pleasurable stimuli by means of a connectivity analysis, the present study describes a mechanism by which the OFC might induce pain inhibition: the results suggest that the OFC down-regulated, or at least was negatively correlated with, activation in the rostral anterior insula, adACC and SI. We suggest that the modulation of activity in these three regions by the OFC reflects different aspects of the interaction between reward and pain.
Both the rostral anterior insula and the adACC are implicated in cognitive-emotional pain modulation (e.g. Rainville, 1997; Petrovic et al., 2002; Jasmin et al., 2004; Zeidan et al., 2011). In addition, the anterior insula has been conceptualized as a key area processing pain as a homeostatic behavioral drive, linking it to the emotional and motivational aspects of pain (Craig, 2003). This role of the anterior insula is in line with the present results: winning as a motivational event associated with positive emotions induced pain inhibition through involvement of the anterior insula. Interestingly, we found no involvement of insular sub-regions in pain modulation by monetary wins and losses that are part of the NPS, i.e. a network of brain regions tracking perceived pain intensity related to changes in nociceptive input or the part of the insula receiving direct nociceptive input, i.e. the dorsolateral posterior insula (Dum et al., 2009). Previously, it was demonstrated that pain-related activity in the rostral anterior insula, in close proximity to the cluster found in the present study, modulates the representation of reward in the mOFC: increased OFC activation related to increased rostral anterior insula activation associated with participants valuing pain avoidance higher than a potential reward (Talmi et al., 2009). Thus, together the present study and the study by Talmi et al. describe a reciprocal relationship between the OFC and the rostral anterior insula with the directionality possibly depending on whether reward or pain avoidance is prioritized.
As the insula, the ACC can be subdivided in different functional parts along the anterior-posterior axis (Beckmann et al., 2009; Etkin et al., 2011). While the posterior-dorsal part of the ACC is part of the NPS (Wager et al., 2013) and the main part of the ACC activated in pain fMRI studies (Vogt, 2005; Dum et al., 2009), the anterior-dorsal part of the ACC, i.e. the part involved in pain inhibition by monetary wins relative to losses in this study, has been implicated in pain inhibition e.g. by placebo (Petrovic et al., 2002). Interestingly, this part of the ACC is also commonly activated by reward processing and the detection of conflict (e.g. Braver et al., 2001, for review; Petrovic et al., 2008; Beckmann et al., 2009; Talmi et al., 2009). Although participants could not actively avoid pain in the present study, experiencing pain and reward simultaneously induces a conflict between negative and positive motivational systems, conceptualized in the Motivation-Decision Model (Fields, 2007). Our data suggest a mechanism of how this conflict is dealt with by the brain: the connectivity strength between the mOFC and the adACC correlated with the behavioral pain inhibition by monetary wins. By decreasing the perceived pain, the conflict between negative and positive motivational systems is reduced. Thus, increased OFC activation when pain and reward co-occur would not only lead to pain inhibition but also to reduction of the perceived conflict via down-regulation of activation in the adACC. A reduction of perceived conflict is further supported by the behavioral result that participants who perceived the nociceptive stimulation as more painful showed more pain inhibition by monetary wins, replicating an earlier study (Becker et al., 2013). The interpretation that the adACC reflects the conflict induced by simultaneously present pain and reward is supported by a recent meta-analysis suggesting that this part of the ACC monitors survival-relevant goal conflicts including nociceptive processes (Lieberman and Eisenberger, 2015). More intense pain should induce a higher conflict than less intense pain, at least until a point when the pain becomes too strong a motivator. This might explain why we found no pain modulation by monetary wins and losses with more intense pain, i.e. the moderately painful stimulation category, a result that also replicates previous findings (Becker et al., 2013). A similar observation of reduced pain modulation when the pain gets too intense has been made with pain-inhibitory effects of distraction (McCaul and Haugtvedt, 1982). A mix of within-subject and between-subject effects might contribute to the potential paradox that pain modulatory effects for mildly painful stimuli were greater the higher subjects rated the pain and that moderately painful stimuli were not modulated. The first is a between-subject phenomenon whereas the second is a within-subject effect.
The third region modulated by the mOFC was the hand region of the SI contralateral to the side of thermal stimulation, i.e. the SI region that received input from the heat stimuli. SI is involved in the coding of the sensory-discriminative dimension of pain, including stimulus localization (Vierck et al., 2013, for review). Thus, SI activation by pain is likely related to the alerting and warning function of pain, for which precise localization is important (Apkarian et al., 2005; Vierck et al., 2013). Inhibition of SI by the OFC as found here might diminish the alerting function of nociceptive stimulation, thereby biasing an organism towards enduring pain to obtain reward, in line with predictions of the Motivation-Decision model.
The Motivation-Decision model suggests that pain inhibition by reward is achieved via a circuitry descending from brainstem structures to second order nociceptive neurons in the spinal dorsal horn (Fields, 2007). Such pain inhibition should result in a decrease of pain signals ascending via the spinothalamic tract, the main tract to relay nociceptive information from the spinal cord to the brain (Dum et al., 2009). Here, we did not find evidence for decreased activation in any structure being targeted by the spinothalamic tract, including the thalamus, the posterior insula/SII, the ACC, or SI. In addition, the NPS, which sensitively and specifically tracks perceived pain intensity in response to changes in nociceptive input (Wager et al., 2013), was not modulated by the outcomes of the wheel of fortune game. Further, the regions that were found to be modulated by the OFC, i.e. rostral anterior insula, adACC and SI, are not part of the NPS nor do they receive spinothalamic input, except for SI (which arguably is not part of the NPS for methodological reasons because pain-related activation in SI largely depends on stimulation site and protocol, Vierck et al., 2013). We interpret these findings to mean that pain inhibition by reward did not decrease nociceptive processing and is therefore not achieved via descending pain inhibitory systems. In contrast, we found clear evidence for supraspinal mechanisms without altered spinal pain processing. Importantly, the fact that pain can be modulated via different mechanisms sounds a note of caution when considering brain activation patterns as pain biomarkers (Davis et al., 2012).
Reward-induced pain inhibition as described here fits the concept of higher-order modulation mediated by purely supraspinal mechanisms. Of course, modulation of participants’ ratings by such higher-order modulation might be criticized as simply representing response bias. However, higher-order pain modulation does not contradict perceptual modulation and might represent a process at a cognitive level (cf. Buechel et al., 2014). In line with this reasoning, it has been argued that there is no clear distinction between sensory and cognitive processing such as decision-making within the pain system and that these components cannot be separated in a meaningful way (Buechel et al., 2014). Further, a dissociation between perceptual and cognitive components of the pain experience has been described as a factor contributing to the development and maintenance of chronic pain, resulting in ‘exaggerated’ pain perception in chronic states (Lethem et al., 1983). We interpret the relationship in the present study between the behavioral pain modulation and the cerebral mechanism of reward-induced pain inhibition on an individual level as contradicting the conjecture of a simple response bias.
Reward is an important mechanism shaping behavior and as such reward has been applied successfully in the treatment of chronic pain patients, e.g. in the context of operant pain therapy (e.g. Nicholas et al., 1991; Thieme et al., 2003). In operant pain therapy, reward is used to increase desirable health behaviors (e.g. increase of activity) and decrease maladaptive pain behaviors (e.g. activity avoidance). However, the immediate pain-relieving aspects of reward are typically not taken into account. Perhaps the acute pain relieving effects of reward could be exploited to generate a self-sustaining circuit of reward-induced pain relief, which in turn acts a reinforcer for the behavior preceding the reward. However, cumulating animal and human evidence shows alterations in the mesolimbic reward circuitry in chronic pain (Becker et al., 2012; Borsook et al., 2016; Mitsi and Zachariou, 2016; Taylor et al., 2016), hinting at impaired reward processing in chronic pain. This suggests that efficacy of operant pain therapy might be enhanced by directly targeting altered pain-reward interactions, for example by increasing saliency and processing of reward through mindfulness-based approaches (e.g. Garland et al., 2015).
It should be noted that although statistically significant, behavioral pain modulation was small in the present study. Further, the behavioral results on a group level appear to be driven by pain-facilitating effects of losing compared to the neutral condition. In contrast to the behavioral results, brain results indicate that the cerebral circuitry identified here is indeed likely driven by pain inhibition by reward. The discrepancy between the behavioral and imaging results could be due to difficulties of appropriate neutral conditions, i.e. conditions that only differs in the aspect of interest but not in any other aspect. For example, studies on emotional pain modulation often use pictures, but neutral pictures (e.g. mushrooms, furniture) are typically associated with lower arousal compared to positively or negatively valenced pictures (Rhudy et al., 2005).
In summary, our results indicate that the mOFC mediates pain inhibition by reward by influencing brain regions that are likely concerned with the relative value and importance of pain, rather than nociceptive processing. To further expand these findings and to allow implementation in pain therapy, future studies could investigate whether the brain mechanism of pain-reward interaction are altered in chronic pain patients.
Supplementary Material
Acknowledgements
This work was supported by an IASP Collaborative Research Grant to SB and PS, a Postdoctoral Fellowship for Leading Early Career Researchers funded by the Baden-Württemberg Foundation, a research fellowship by the German Research Foundation to SB, a Merit Scholarship Program for Foreign Students (Ministère de l’Éducation et de l’Enseignement supérieur, MELS, Quebec), a Quebec Bio-Imaging Network (QBIN) Scholarship for foreign students and a The Louise and Alan Edwards Foundation’s Edwards PhD. Studentships in Pain Research to WG, and a Canadian Institutes of Health Research (CIHR) Operating Grant to PS. The authors declare no competing financial interests. We thank Yan Jun Chen and Alysha-Karima Ahmed for their support with subject recruitment and data entry.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Footnotes
For three participants, no stimulation intensities that were rated as mildly and moderately painful could be established within the safety range; one participant did not make any choices during the familiarization with the wheel of fortune game; for one participant it was not possible to schedule the fMRI session; one participant did not show up for the fMRI session.
References
- Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9, 463–84. [DOI] [PubMed] [Google Scholar]
- Becker S., Gandhi W., Elfassy N.M., Schweinhardt P. (2013). The role of dopamine in the perceptual modulation of nociceptive stimuli by monetary wins or losses. European Journal of Neuroscience, 38, 3080–8. [DOI] [PubMed] [Google Scholar]
- Becker S., Gandhi W., Schweinhardt P. (2012). Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience Letters, 520, 182–7. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. (2003). General multilevel linear modeling for group analysis in FMRI. Neuroimage, 20, 1052–63. [DOI] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F.S. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience, 29, 1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. (2015). Pleasure systems in the brain. Neuron, 86, 646–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. (2009). Dissecting components of reward: "liking", "wanting", and learning. Current Opinion in Pharmacology, 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Linnman C., Faria V., Strassman A.M., Becerra L., Elman I. (2016). Reward deficiency and anti-reward in pain chronification. Neuroscience & Biobehavioral Reviews, 68, 282–97. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex, 11, 825–36. [DOI] [PubMed] [Google Scholar]
- Buechel C., Geuter S., Sprenger C., Eippert F. (2014). Placebo analgesia: a predictive coding perspective. Neuron, 81, 1223–39. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology, 363, 615–41. [DOI] [PubMed] [Google Scholar]
- Cavada C., Compañy T., Tejedor J., Cruz-Rizzolo R.J., Reinoso-Suárez F., Others. (2000). The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex, 10, 220–42. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2003). A new view of pain as a homeostatic emotion. Trends in Neurosciences, 26, 303–7. [DOI] [PubMed] [Google Scholar]
- Dum J., Herz A. (1984). Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacology Biochemistry & Behavior, 21, 259–66. [DOI] [PubMed] [Google Scholar]
- Dum R.P., Levinthal D.J., Strick P.L. (2009). The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. Journal of Neuroscience, 29, 14223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H.L. (2007). Understanding how opioids contribute to reward and analgesia. Regional Anesthesia and Pain Medicine, 32, 242–6. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Garland E.L., Froeliger B., Howard M.O. (2015). Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. Journal of Behavioral Medicine, 38, 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences, 15, 56–67. [DOI] [PubMed] [Google Scholar]
- Hochman G., Yechiam E. (2011). Loss aversion in the eye and in the heart: The autonomic nervous system’s responses to losses. Journal of Behavioral Decision Making, 24, 140–56. [Google Scholar]
- Jasmin L., Granato A., Ohara P.T. (2004). Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. Journal of Comparative Neurology, 468, 425–40. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Tversky A. (1979). Prospect theory: an analysis of decision under risk. Economic Journal, 47, 263–92. [Google Scholar]
- Kong J. (2006). Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. Journal of Neuroscience Research, 26, 381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S., Tracey I. (2008). A common neurobiology for pain and pleasure. Nature Reviews Neuroscience, 9, 314–20. [DOI] [PubMed] [Google Scholar]
- Lethem J., Slade P.D., Troup J.D., Bentley G. (1983). Outline of a fear-avoidance model of exaggerated pain perception-I. Behaviour Research and Therapy, 21, 401–8. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I. (2015). The dorsal anterior cingulate cortex is selective for pain: results from large-scale reverse inference submission. Proceedings of the National Academy of Sciences, 112, 15250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul K.D., Haugtvedt C. (1982). Attention, distraction, and cold-pressor pain. Journal of Personality and Social Psychology, 43, 154–62. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61, 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsi V., Zachariou V. (2016). Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience, 338, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E.J., Mesulam M.M. (1982). Insula of the old world monkey. II: afferent cortical input and comments on the claustrum. Journal of Comparative Neurology, 212, 23–37. [DOI] [PubMed] [Google Scholar]
- Nicholas M.K., Wilson P.H., Goyen J. (1991). Operant-behavioural and cognitive-behavioural treatment for chronic low back pain. Behaviour Research and Therapy, 29, 225–38. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E.J., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7, 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D., Price J.L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10, 206–19. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. (2002). Placebo and opioid analgesia– imaging a shared neuronal network. Science, 295, 1737–40. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Pleger B., Seymour B., et al. (2008). Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. Journal of Neuroscience, 28, 10509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P. (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 277, 968–71. [DOI] [PubMed] [Google Scholar]
- Rhudy J.L., Williams A.E., McCabe K.M., Nguyen M.A., Rambo P. (2005). Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology, 42, 579–87. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Joliot M., Tzourio-Mazoyer N. (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage, 122, 1–5. [DOI] [PubMed] [Google Scholar]
- Roy M., Piche M., Chen J.I., Peretz I., Rainville P. (2009). Cerebral and spinal modulation of pain by emotions. Proceedings of the National Academy of Sciences of the United States of America, 106, 20900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Woolrich M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–19. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P., Camerer C.F., Phelps E.A. (2013). Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Social Cognitive and Affective Neuroscience, 8, 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P., Hsu M., Curley N.G., Delgado M.R., Camerer C.F., Phelps E.A. (2009). Thinking like a trader selectively reduces individuals’ loss aversion. Proceedings of the National Academy of Sciences of the United States of America, 106, 5035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J. (2010). Analyzing effective connectivity with fMRI. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 446–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D., Dayan P., Kiebel S.J., Frith C.D., Dolan R.J. (2009). How humans integrate the prospects of pain and reward during choice. Journal of Neuroscience, 29, 14617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.M.W., Becker S., Schweinhardt P., Cahill C. (2016) Mesolimbic Dopamine Signaling in Acute and Chronic Pain. Pain Publish Ah:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme K., Gromnica-Ihle E., Flor H. (2003). Operant behavioral treatment of fibromyalgia: a controlled study. Arthritis & Rheumatology, 49, 314–20. [DOI] [PubMed] [Google Scholar]
- Treede R.D., Kenshalo D.R., Gracely R.H., Jones A.K. (1999). The cortical representation of pain. Pain, 79, 105–11. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Whitsel B.L., Favorov O.V., Brown A.W., Tommerdahl M. (2013). Role of primary somatosensory cortex in the coding of pain. Pain, 154, 334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C., Bushnell M.C. (2009). Mood influences supraspinal pain processing separately from attention. Journal of Neuroscience, 29, 705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemure C., Slotnick B.M., Bushnell M.C. (2003). Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain, 106, 101–8. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience, 6, 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.-W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. New England Journal of Medicine, 368, 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.M. (1989). Reward or reinforcement: what’s the difference?. Neuroscience & Biobehavioral Reviews, 13, 181–6. [DOI] [PubMed] [Google Scholar]
- Wiech K., Tracey I. (2009). The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage, 47, 987–94. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Roy M., Buhle J.T., Wager T.D. (2015). Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biology, 13, e1002036.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4, 58–73. [DOI] [PubMed] [Google Scholar]
- Younger J., Aron A., Parke S., Chatterjee N., Mackey S. (2010). Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One, 5, e13309.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Martucci K.T., Kraft R.A., Gordon N.S., McHaffie J.G., Coghill R.C. (2011). Brain Mechanisms Supporting the Modulation of Pain by Mindfulness Meditation. Journal of Neuroscience, 31, 5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.