Abstract
Cognitive models posit that social anxiety disorder (SAD) is associated with and maintained by attentional bias (AB) for social threat. However, over the last years, it has been suggested that AB in SAD may result from a decreased activation of the left prefrontal cortex, and particularly of its dorsolateral part (dlPFC). Accordingly, a transient increase of neural activity within the left dlPFC via non-invasive brain stimulation decreases AB in non-anxious control participants. Yet, none of these studies focused on SAD. This is especially unfortunate as SAD constitutes the main target for which a genuine reduction of AB may be most appropriate. In this experiment, we sought to investigate the causal influence of left dlPFC neuromodulation on AB among 19 female individuals with a DSM-5 diagnosis of SAD. We adopted a double-blind within-subject protocol in which we delivered a single-session of anodal versus sham transcranial Direct Current Stimulation (tDCS) over the left dlPFC during the completion of a probe discrimination task assessing AB. Consistent with our hypothesis, participants demonstrated a significant decrease in AB during the anodal tDCS over the left DLPFC relative to the sham stimulation. These findings value tDCS as an innovative procedure to gain new insight into the underlying mechanisms of SAD.
Keywords: neuromodulation, transcranial direct current stimulation, attention bias modification, social anxiety disorder, prefrontal cortex, attentional bias
Introduction
Social anxiety disorder (SAD) is a common disorder with a lifetime prevalence of more than 12% (Kessler et al., 2005). SAD is characterized by intense fear and avoidance of social situations, causing considerable distress and impaired daily functioning. It has an early age onset and tends to follow a chronic and debilitating course if untreated (Hayward et al., 2008).
As highlighted by Hirsch and Clark (2004), a curious feature of this disorder is that it persists even if most individuals with SAD perform naturalistic exposure to at least some feared social situations on a regular basis in their daily life. One possibility is that people with chronic SAD process information in ways that maintain their anxiety. Accordingly, laboratory studies involving probe detection and probe discrimination tasks indicate that people with SAD respond faster to probes replacing social threat stimuli, such as faces expressing anger or disgust, than to probes replacing neutral cues, thereby exhibiting an attentional bias (AB) for social threat that is absent in non-anxious control participants (for a meta-analysis, see Bantin et al., 2016). As suggested by prominent cognitive models of SAD (Rapee and Heimberg, 1997; Heimberg et al., 2010; for a review, see Wong and Rapee, 2016), AB may causally contribute to increased anxiety proneness, and thereby figures prominently in the maintenance, and perhaps the etiology, of SAD. Accordingly, reducing AB via attention bias modification (ABM) procedures—a novel computer-based treatment approach designed to reduce AB for threat by repeatedly directing participants’ attention towards neutral stimuli—may yield clinical benefits vis-à-vis SAD symptoms (for a meta-analysis, see Heeren et al., 2015a). Likewise, transiently fostering AB promotes anxiety proneness among non-anxious controls ( MacLeod et al., 2002; Heeren et al., 2012a).
The aforementioned findings thus suggest that AB for threat can be modified so that, when successfully modified, it can reduce SAD symptoms. However, despite the promising initial results, modifying AB had only a very small effect—albeit significant—on reducing AB and SAD symptoms (for a meta-analyses, Mogoaşe et al., 2014; Heeren et al., 2015a; Linetzky et al., 2015). Yet, as highlighted by Grafton and MacLeod (2016), most of the studies that failed to replicate the initial promising effects of ABM on symptoms also failed to induce the targeted change in AB. Consequently, one can argue that the current ABM procedure might be suboptimal to successfully alter AB for threat as intend. Therefore, research should focus on the development of novel procedures able to robustly achieve the intended change, i.e. a reduction of AB for threat via an increased selective attention to non-threat (Clarke et al., 2014a). This way, we argued that the best way to achieve this goal is to target the basic mechanisms, particularly at the neural circuitry level, that are assumed to be involved in the modification of AB (Heeren et al., 2013; De Raedt et al., 2015).
According to neurocognitive models of AB (Vuilleumier, 2005; Bishop, 2008, 2009), the deployment of attention in the presence of threat stimuli is regulated by two primary neural systems: (1) a bottom-up amygdala-based system that produces a signal reflecting the perceived salience of stimuli and directs attention toward salient stimuli (Adolphs et al., 1995; Davis and Whalen, 2001), and (2) a top-down system relying on the prefrontal (PFC) and anterior cingulate (ACC) cortices that both produces a signal when conflicting demands are made on attention (Bishop et al., 2004). According to this perspective, AB might result from a failure to recruit regulatory PFC regions that are mandatory to down-regulate amygdala activation in the presence of threat (Bishop, 2009). Accordingly, anxious individuals exhibit reduced left PFC activations, particularly of its dorsolateral (dlPFC; Bishop, 2009) and ventrolateral (vlPFC; Monk et al., 2006, 2008) sections, when performing tasks involving such a top-down control in the presence of threat. Moreover, reducing AB via ABM is associated with increased activation of the left dlPFC among healthy volunteers (Browning et al., 2010). Likewise, ABM boosted vlPFC activations (Taylor et al., 2014) and attenuated bilateral amygdala activations (Manson et al., 2013; Britton et al., 2014; Taylor et al., 2014) among individuals with SAD.
Beyond these previous findings, the more convincing line of evidence regarding the implications of PFC in AB arises from studies that used neuromodulation techniques to directly manipulate the activation of this brain region. These studies revealed that a single-session of high-frequency repetitive Transcranial Magnetic Stimulation (HF-rTMS) over the left dlPFC decreases AB (De Raedt et al., 2010), whereas HF-rTMS over the right dlPFC increases it (Leyman et al., 2009; De Raedt et al., 2010; Vanderhasselt et al., 2011). Yet, these studies were conducted among non-anxious healthy female participants. Moreover, as individuals with SAD differ from non-anxious controls in AB for social threat (Bantin et al., 2016), the modification of AB may operate differently among the former relative to the latter. Consequently, it remains decisive to test the effect of neurostimulation among individuals with SAD. Relevant to this issue, the level of situational anxiety at baseline did moderate the impact of HF-rTMS over the dlPFC on AB (Vanderhasselt et al., 2011).
Moreover, given that the effects of HF-rTMS only emerge after the stimulation, these aforementioned studies relied on an offline protocol—AB was assessed before and after brain stimulation—and not directly during the modulation of the dlPFC. Yet, a recent meta-analysis indicated that online stimulation protocols yield larger effect sizes vis-à-vis cognitive tasks than offline protocols do among individuals with psychiatric disorder (Dedoncker et al., 2016). As compared to HF-rTMS, transcranial Direct Current Stimulation (tDCS)—another non-invasive method of brain stimulation—renders possible the modulation of the cortical activities during the completion of a task. tDCS consists of the application of a weak (0.5–2 mA), direct current through electrodes positioned over one’s scalp which are able to reach the neuronal tissue and induce a polarization-shifts on the resting membrane potential (Nitsche et al., 2008). Anodal stimulation facilitates cortical activity, whereas cathodal tDCS has opposite effects. In contrast to TMS, tDCS has the advantage to be easier to use in double-blind sham-controlled studies. So far, only two tDCS studies focused on AB for threat. They combined tDCS with the ABM procedure among healthy undergraduate volunteers (Clarke et al., 2014b; Heeren et al., 2015b). Results indicated that increased activation within the left dlPFC using anodal tDCS combined with ABM is associated with larger reduction in AB, as compared to sham stimulation combined with ABM. Particularly, Clarke et al. (2014b) found that anodal tDCS combined with ABM reduced AB for threat via the promotion of attentional selectivity for non-threat cues. However, because none of these studies included a condition investigating the impact of tDCS without ABM, one cannot exclude that the effects of tDCS combined with ABM merely results from the anodal tDCS per se and not from the combination of tDCS and ABM.
Yet, notwithstanding these previous brain stimulation studies indicating that left dlPFC may figure prominently in the maintenance of AB, none of these studies was conducted among individuals with SAD. This is especially unfortunate as this disorder constitutes the main target in the previous ABM studies and the disorder for which the reduction of AB may be most appropriate, either as stand-alone treatment (Amir et al., 2011) or as integrated into a standard cognitive-behavioral treatment package (Rapee et al., 2013). Consequently, in the present study, we sought to manipulate the cortical excitability of the left dlPFC via tDCS during the completion of a probe discrimination task assessing AB for social threat among individuals with SAD. Following the above HF-rTMS (De Raedt et al., 2010) and tDCS studies (Clarke et al., 2014b; Heeren et al., 2015b), we decided to stimulate the left dlPFC, and not the vlPFC. This study therefore represents both an extension of the previous studies conducted among non-anxious female participants and also a critical step in translational research toward establishing the ability of anodal tDCS to reduce AB among individuals with SAD. In the present study, we adopted a double-blind within-subject protocol in which we delivered single-session of anodal vs sham tDCS over the left dlPFC during the completion of a probe discrimination task. Of primary interest was the reduction of AB for social threat during anodal tDCS relative to sham tDCS. Moreover, we reasoned that if anodal tDCS promotes attentional selectivity for non-threat among healthy volunteers when applied during ABM (Clarke et al., 2014b), then anodal tDCS should foster attentional selectivity for non-threat cues among individuals with SAD during the completion of a probe discrimination task, so that it culminates in the reduction of AB for threat.
Materials and methods
Participants
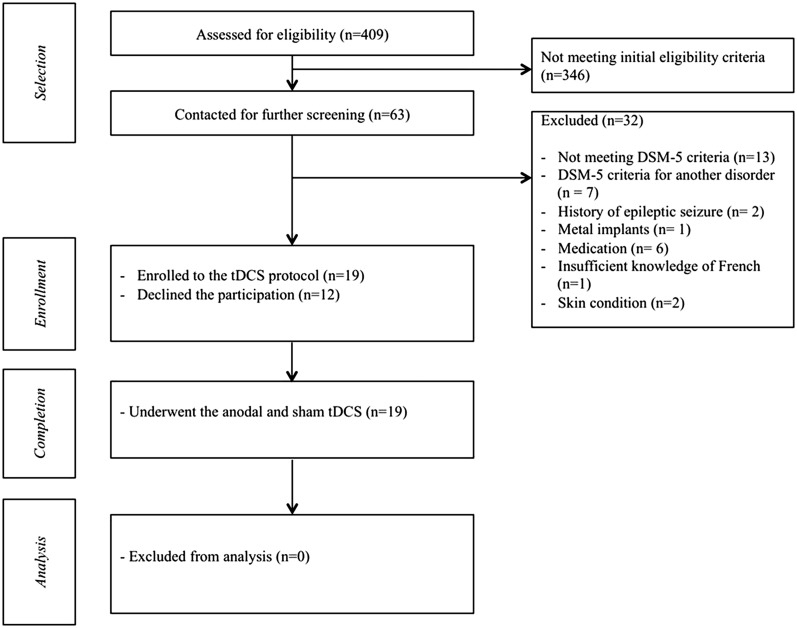
We recruited 19 right-handed female participants with a primary DSM-5 diagnosis of SAD (American Psychiatric Association, 2013) from the community of Walloon Brabant in Belgium. To be consistent with previous studies (De Raedt et al., 2010; Vanderhasselt et al., 2011; Heeren et al., 2015b), only female participants were included. Moreover, women exhibit higher prevalence rates of SAD than men (McLean et al., 2011; Xu et al., 2012). A total of 409 volunteers responded to our invitation to take part in a brain stimulation study among socially anxious women. They were first screened by email for initial eligibility criteria. As shown in Figure 1, 63 of these participants met the initial criteria assessed via online screening questionnaires. To be eligible, participants should (a) score above 56 (i.e. the cut-off score for probable diagnosis of SAD in the French version of the scale; Bouvard and Cottraux, 2010) on the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987), (b) be right-handed and (c) have normal or corrected-to-normal vision. During this preliminary contact, there were also given an introduction to the tDCS technique. All these participants were then contacted for a personal visit in the laboratory for further screening. Exclusion criteria were: (a) the absence of DSM-5 criteria for SAD, (b) the presence of additional psychiatric disorders, (c) current or past heart, respiratory, dermatological or neurological problems, (d) current pharmacological or psychological treatments, (e) the presence of metallic foreign particles around the head or a cardiac pacemaker, (f) pregnancy at the time of the testing and (g) insufficient knowledge of French language. These criteria were checked through a medical interview and using the Mini International Neuropsychiatric Interview (MINI; Lecrubier et al., 1998). A PhD level clinical psychologist completed all the interviews. Of these 63 participants, 32 were discarded as they reported at least one of the exclusion criteria and 12 declined participation. The remaining 19 participants were enrolled in the study. Table 1 shows their demographic and clinical characteristics.
Fig. 1.
Flowchart depicting passage of participants through the study.
Table 1.
Demographic and clinical measures for individuals with social anxiety disorder
| Mean (SD) | Cronbach’s alpha | |
|---|---|---|
| Demographic measures | ||
| Age | 24.16 (4.87) | |
| Educational level (in years) | 13.26 (2.42) | |
| Clinical measures | ||
| BDI-II | 13.47 (7.16) | .90 |
| STAI-T | 49.68 (4.19) | .85 |
| LSAS | 74.26 (16.20) | .84 |
Note: Education level was assessed according to the numbers of years of education completed after starting primary school. Cronbach’s alphas were computed over the data of the current sample.
BDI-II, Beck Depression Inventory; STAIT-T, Spielberger State-Trait Anxiety Inventory-Trait; LSAS, Liebowitz Social Anxiety Scale.
Measures
Questionnaires
Participants were screened via the self-report version of the LSAS (Liebowitz,1987). They also completed the Spielberger Trait Anxiety Inventory (STAI-T; Spielberger et al., 1983) and the Beck Depression Inventory (BDI-II; Beck et al., 1996). The LSAS is a 24-item scale that measures anxiety and avoidance of social interactions and performance situations. The STAI-T is a 20-item self-report questionnaire assessing anxiety proneness. The BDI-II is a 21-item self-report measure of symptoms of depression. We used the validated French versions of these scales (LSAS, Heeren et al., 2012b; BDI-II, Beck et al., 1996; STAI-T, Bruchon-Schweitzer and Paulhan, 1993).
Measure of attentional bias
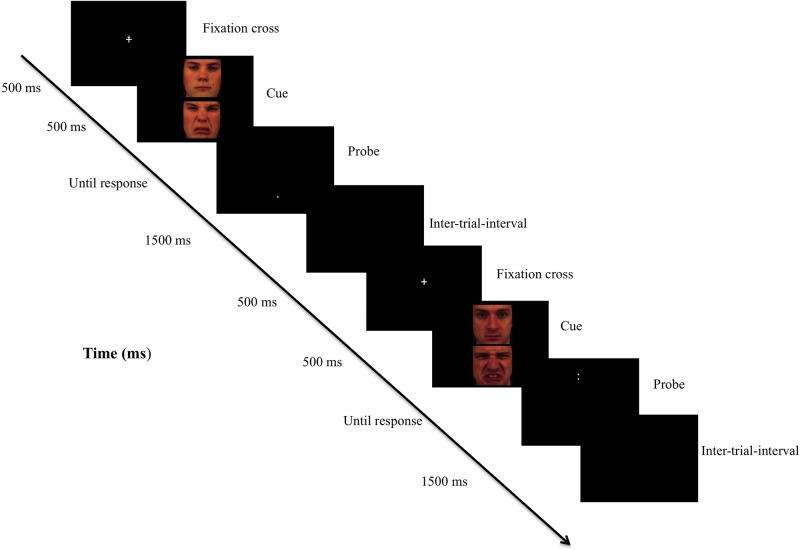
To assess AB, we used a probe discrimination task modeled on the dot-probe detection task (MacLeod et al., 1986). The task was administered twice, once during the anodal tDCS and once during the sham stimulation. The task consisted of 320 trials delivered in one block. Each trial began with a central fixation cross which appeared on the screen for 500 ms. Immediately following the disappearance of the cross, a pair of faces appeared on the screen for 500 ms. One face appeared on the top of center screen, whereas the other face appeared on the bottom of center screen. Each pair of faces displayed neutral-disgust facial expressions. Immediately following their disappearance, a probe appeared in the location previously occupied by one of the two faces. Figure 2 shows an illustration of the trials. Participants were asked to indicate whether the probe was a dot (i.e. ‘.’) or a colon (i.e. ‘:’) by pressing a corresponding button using the right hand as quickly and accurately as possible. They were also instructed to look at the fixation cross at the start of each trial. The probe remained on screen until a response was given. The inter-trial interval was 1500 ms. There were an equal number of trials for each type of stimuli location (top or down), probe location (top or down) and probe type (‘.’ or ‘:’). We used an equal number of trials in each condition as a function of these parameters (i.e. 320 trials = 40 face-pairs × 2 face positions × 2 cue types × 2 cue positions). Each of the 320 trials appeared in a different random order for each participant and each type of stimulation (anodal tDCS versus sham). Stimuli consisted of 40 different face pairs (20 male, 20 female), each pair displaying neutral-disgust facial expressions, randomly selected from a validated version (Goeleven et al., 2008) of the Karolinska Directed Emotional Faces (Lundqvist et al., 1998), which is a standardized set of emotional faces. Faces were standardized for size (326 × 329 pixels). The task was programmed and presented using E-Prime 2 Professional® (Psychology Software Tools, Pittsburgh, PA, USA). The distance between participant’s eyes and the screen was around 50 cm, and the target stimuli subtended a visual angle of about 4° in the horizontal field.
Transcranial direct current stimulation
Direct electrical current was applied by a saline-soaked pair of surface sponge rubber electrodes (35 cm2) and delivered by a battery-driven stimulator (Neuroconn, GmbH, Ilmenau, Germany). We used a sham-controlled within-subject design in which all participants serve as their own control, a design that substantially increases statistical power. To stimulate the left dlPFC, the anode electrode was vertically positioned centered over the F3 according to the 10–20 international system for electroencephalogram electrode placement. The reference electrode was placed vertically at the ipsilateral arm (Cogiamanian et al., 2007; Priori et al., 2008). During the first 30 s of stimulation, the current was ramped up to 2 mA and then delivered constantly for 25 min. At the end of the stimulation, the current was ramped down to 0 mA over 30 s. For sham stimulation, the position of the electrodes was exactly the same as during anodal stimulation; however, the current was ramped down after 30 s. This procedure is commonly used by tDCS researchers and has been found to be an optimal way to provide the initial sensation of stimulation without the subsequent effects on cortical excitability (Nitsche et al., 2008; Ohn et al., 2008). Predefined codes assigned to either sham or real stimulation were used to start the stimulator and thus allowed for a double-blind study design. Anodal stimulation, or sham stimulation, respectively, started 5 min before the beginning of the probe discrimination task and was delivered for a further 20 min. Thus, the probe discrimination task was performed parallel to the stimulation. To avoid carry-over effect from the previous stimulation, the second stimulation was carried out after an exact 48 h-interval. The order of the anodal and sham stimulation was counterbalanced across participants.
Procedure
This study comprised three sessions. Within the first session, participants were administered the questionnaires and underwent the structured medical and psychiatric interviews by a trained clinician. Then, the two stimulations sessions were conducted. At the beginning of each session, electrodes were soaked in saline solution and placed on the participant’s scalp using the electrode montage depicted above. Following 5 min of stimulation (anodal or sham tDCS), participants started with the probe discrimination task. Participants were asked to perform the task as quickly and accurately as possible. The order of the two stimulation conditions was randomly counterbalanced across participants (i.e. 10 participants received the anodal stimulation first, 9 participants received the sham stimulation first). Further detail on tDCS compliance can be found in the Supplemental Materials. Each session was administrated individually in a dimly lit and quiet room. All participants provided their written informed consent. The study was approved by the local ethical committee and conducted according to the Declaration of Helsinki. Participants were debriefed at the end of the experiment and received compensation (25 euros).
Data preparation
We addressed outliers and errors in the probe discrimination tasks as follows. First, trials with incorrect responses were excluded [3.90% of all the trials during sham; 3.85% of all the trials during anodal tDCS; these rates did not significantly differ between anodal tDCS and sham, t(18) = 1.52, P = 0.17]. Second, RTs lower than 200 ms or greater than 2000 ms were removed from analyses [less than 0.05% of all the trials for both sham and anodal tDCS and no significant difference between anodal tDCS and sham, t(18) = 1.48, P = 0.21].
Data analytic plan
To assess AB, we calculated a bias score for each participant at each session by subtracting the mean latencies when the probe appeared in the same location as the threatening stimuli from the mean latency when the probe and the threatening stimuli appeared at different locations. Hence, positive bias scores represent attention bias toward social threat, and negative bias scores represent bias away from threat, or equivalently, bias toward neutral faces. This bias score is the most frequently used index to determine AB from a probe discrimination task procedure (MacLeod et al., 1986; Mogg et al., 2004).
Then, to investigate the impact of tDCS on AB, we first computed a paired t-test to compare bias scores during sham and anodal stimulation. Following McGough and Faraone (2009), we also computed the percentage of tDCS responders (i.e. the percentage of individuals who have a bias score below the mean of the sham bias-score during the anodal stimulation).
To follow-up these effects, we further examined the impact of the anodal tDCS on the two types of trials involved in the computation of the bias score index, which are the probes presented in the vicinity of the neutral and threat cues, respectively. We performed a 2 (Stimulation: anodal versus sham) × 2 (Probe Location: vicinity of threat cue versus vicinity of neutral cue) repeated analysis of variance (ANOVA) with repeated measurement on the two factors and latencies as dependent variable.
Finally, although it confirms the clinical status of our sample, the participants exhibited mild to moderate BDI-II and STAI-T scores. Consequently, we examined whether tDCS-induced improvement in AB (i.e. anodal tDCS scores minus sham score), AB during sham stimulation, and AB during anodal tDCS did correlate with the severity of social anxiety symptoms (i.e. LSAS scores at baseline), depressive symptoms at baseline (i.e. BDI scores), trait-anxiety scores at baseline (i.e. STAI-T scores) and demographic variables (i.e. years of education, age) using Pearson product-moment correlations.
All statistical analyses were performed using R (R Core Team, 2015). The significance level was set at an alpha level of 0.05. Effect sizes are reported in the form of partial eta-squared () for ANOVA and Cohen’s d using the formula for paired comparison (i.e. mean pairs difference divided by the pooled SD).
Results
Presence of attentional bias
Consistent with previous studies (Bantin et al., 2016), participants did exhibit an AB for threat, as indexed by a bias score significantly greater than zero (i.e. zero signifying an absence of AB towards disgust faces) during the sham condition, t(18) = 2.11, P = 0.04, d = 0.48). Especially, latencies when the probes appeared in the vicinity of social-threat cues were significantly shorter than latencies when the probes appeared in the vicinity of the non-threat cues, t(18) = 2.11, P = 0.04, d = 0.48. Data are shown in Table 2.
Table 2.
Mean latencies (in milliseconds) as function of probe nature and tDCS condition (SD)
| Sham stimulation | Anodal tDCS | |
|---|---|---|
| Probes in the vicinity of the social-threat cues | 505.60 (66.37) | 494.67 (65.66) |
| Probes in the vicinity of the non-threat cues | 510.89 (68.89) | 488.89 (69.26) |
Notes: Whereas participants displayed significantly shorter latencies to discriminate the identity of probes presented in the vicinity of the threat cues as compared to probes presented in the vicinity of neutral cues during the sham stimulation, they displayed the reverse pattern of latencies during the anodal tDCS, i.e. significantly shorter latencies to discriminate the identity of probes presented in the vicinity of the neutral cues relative to probes presented in the vicinity of threat cues.
Change in attentional bias
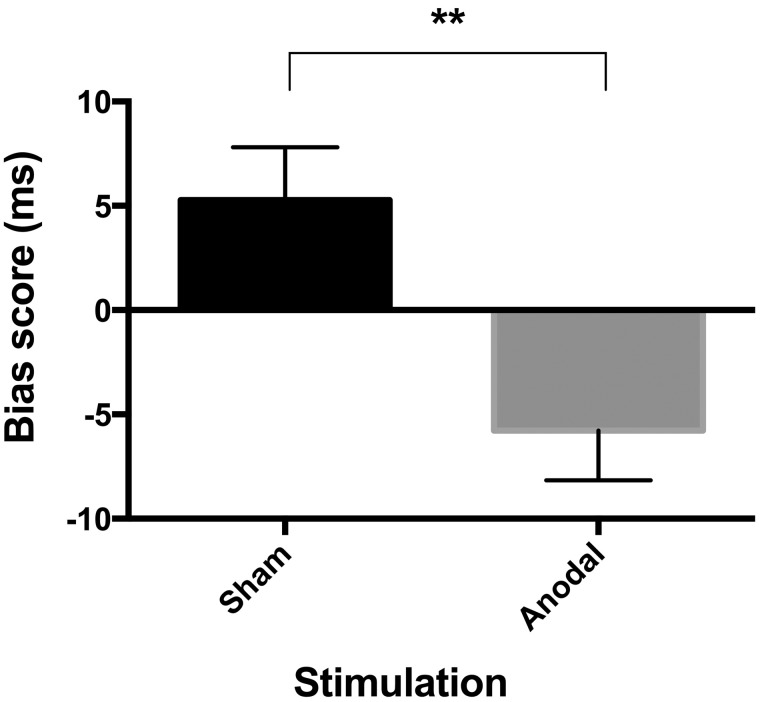
As shown in Figure 3, individuals with SAD exhibited a significant reduction in bias score during the anodal tDCS relative to the sham stimulation, t(18) = 3.20, P = 0.009, d = 0.71. Highlighting the consistence of our findings across the participants, 79% of the participants exhibited a bias score lower than the mean sham-related score during anodal tDCS. Moreover, as shown in Figure 2, although the bias score was significantly greater than zero during the sham condition, it became significantly smaller than zero—signifying AB towards non-threat faces—during the anodal tDCS, t(18) = −2.42, P = 0.02, d = 0.56.
Fig. 3.
Change in Attentional Bias for Threat as a Function of tDCS condition. Note: Scores for the attention bias scores (ms) as a function of tDCS condition. Error bars represents standard errors of the mean. **P < 0.01.
Fig. 2.
Illustration of the probe discrimination task. Note: Each trial began with a central fixation cross which appeared on the screen for 500 ms. Immediately following the disappearance of the cross, a pair of faces appeared on the screen for 500 ms. One face appeared on the top of center screen, whereas the other face appeared on the bottom of center screen. Each pair of faces displayed neutral-disgust facial expressions. Immediately following their disappearance, a probe appeared in the location previously occupied by one of the two faces. The inter-trial interval was 1500 ms. Participants were asked to indicate whether the probe was a dot (i.e. ‘.’) or a colon (i.e. ‘:’) by pressing a corresponding button using the right hand as quickly and accurately as possible. They were also instructed to look at the fixation cross at the start of each trial. The probe remained on screen until a response was given.
The 2 (Stimulation) × 2 (Probe Location) ANOVA revealed a significant two-way Stimulation × Probe Location interaction, F(1,18) = 8.38, P = 0.01, η2p = 0.32, implying that the stimulation did interact with the valence of the material vacating its location to the probe. Neither the main effect of Stimulation, F(1,18) = 1.34, P = 0.26, η2p = 0.07, nor the main effect of Probe Location, F(1,18) = 0.12, P = 0.73, η2p = 0.01, were significant. As shown in Table 2, the pattern of latencies suggests that anodal tDCS is associated to a significant reduction of the bias score via changes in the latencies to discriminate the identity of probes presented in the vicinity of the neutral cues. Indeed, whereas participants displayed shorter latencies to discriminate the identity of probes presented in the vicinity of the threat cues as compared to probes presented in the vicinity of neutral cues during the sham stimulation [t(18) = 2.11, P = 0.04, d = 0.48], they displayed the reverse pattern of latencies during the anodal tDCS, i.e. shorter latencies to discriminate the identity of probes presented in the vicinity of the neutral cues relative to probes presented in the vicinity of threat cues [t(18)= 2.43, P = 0.03, d = 0.56].
Complementary analysis
None of the correlations between AB-related indices and baseline self-report measurements were significant [all absolute values of r(19) < 0.24, all P-values > 0.31]. As participant’s characteristics at baseline, such as anxiety level, may moderate the modification of AB for threat (Vanderhasselt et al., 2011), we also examined whether tDCS responders did differ on baseline data. However, none of the independent t-tests comparing responders to non-responders were significant [all t-values < 0.1.42; all P-values > 0.18]. Moreover, we did not find any potential stimulation-order effect or gender-effect vis-à-vis the task-related material (see Supplemental Materials).
Discussion
We sought to investigate the influence of left dlPFC activation on AB for threat among individuals with a DSM-5 diagnosis of SAD. Consistent with our prediction as well as with earlier rTMS results in healthy female volunteers (De Raedt et al., 2010), the application of anodal tDCS over the left dlPFC does reduce AB for threat among female individuals with SAD, with 79% of the participants demonstrating a significant reduction of AB for threat during the anodal tDCS.
Because anodal tDCS over the left dlPFC reduced AB for threat, our results are the first to confirm previous claims that this region influences the intensity of AB for threat among individuals with SAD. These findings are clearly in line with Bishop’s hypothesis (Bishop et al., 2004; Bishop, 2009) that AB can be conceptualized as a failure to recruit the dlPFC when processing task-irrelevant threatening material. This notion mainly derived from neuroimaging data implicating the left dlPFC in the ongoing maintenance of top-down attention control to support task-required performance in the presence of threat-related distractors (Luks et al., 2007; Bishop, 2009; Peers et al., 2013). Moreover, as dlPFC activation has often been considered as a proxy of top-down attention control (Ochsner and Gross, 2005), our data also extend previous knowledge that this latter moderates AB for threat in SAD (Gorlin and Teachman, 2015; Taylor et al., 2016). For instance, Taylor et al. (2016) reported that SAD individuals with lower attention control exhibited stronger AB in comparison to those with higher attention control exhibiting smaller AB.
By untangling the differential impact of anodal tDCS over the two types of trials involved in the computation of the bias score index, the observation of shorter latencies to discriminate the identity of probes that appeared in the vicinity of non-threat stimuli takes us one step closer to elucidating the reduction of AB for threat observed during the anodal tDCS. Given the previous observation that anodal tDCS combined with ABM reduces AB via an increased attentional selectivity for non-threat cues among non-anxious control participants (Clarke et al., 2014b), the observation that anodal tDCS promotes attentional selectivity for non-threat cues does not come as a surprise. Furthermore, this observation also lends some support to the hypothesis that a genuine reduction of AB for threat among individuals with SAD should be directly compensated by an increased attentional selectivity for non-threat cues. Indeed, as argued by cognitive theorists of SAD (Heimberg et al., 2010; Morrison and Heimberg, 2013; Wong and Rapee, 2016), AB for threat may interfere with the ability to process external non-threat cues that disconfirm the negative beliefs of people with SAD when they encounter socially challenging situations. This way, anodal tDCS may help foster attentional selectivity for external non-threat cues in SAD.
Moreover, because dlPFC may initiate control over emotions by inhibition of the amygdala (Pessoa, 2008, 2010; Aupperle and Paulus, 2010), this boost of attentional selectivity for external non-threat cues may also be attributable to a tightening of the hyperactivity of the amygdala during anodal tDCS. As suggested by Bishop’s model of anxiety (Bishop et al., 2004; Bishop, 2009), AB for threat might result from a failure to recruit regulatory PFC regions that are mandatory to downregulate amygdala activation when conflicting demands are made on attention, such as when two salient stimuli compete for processing resources. A wealth of data has demonstrated that the amygdala and its functionally related subcortical limbic regions are involved in early threat detection mechanisms (Davis & Whalen, 2001; Öhman, 2005). Because individuals with SAD exhibit heightened amygdala activation in response to social threat (Stein et al., 2002; Phan et al., 2006), the tDCS-induced boost within dlPFC might have triggered the shrinkage of this amygdala-based early threat detection mechanism. In turn, individuals with SAD may no longer exhibit difficulty filtering out task-irrelevant threatening distractors. Next step would thus be to examine how anodal tDCS foster PFC-amygdala connectivity by replicating the present experiment during fMRI.
Our findings yield several important therapeutic implications. Although the present study still remains a mandatory proof-of-concept prior to consider any potential Phase II/III clinical trials, the observation of a genuine alteration of AB for threat during anodal tDCS supports the idea that tDCS per se may be an effective tool to mitigate AB for threat via the promotion of attentional selectivity for non-threat cues. As AB for threat may interfere with the ability to process external non-threat cues that disconfirm the negative beliefs of people with SAD when they encounter socially challenging situations, turning on the ability to process such external non-threat cues via the promotion of the left dlPFC activation may create a snowballing cascade of patholytic social encounters that foster elimination of SAD. Indeed, failure to disconfirm these beliefs may impede anxiety reduction, which, in turn motivates avoidance of social situations and worsens anxiety or at least prevents it from extinguishing (Heimberg et al., 2010; Heeren and McNally, 2016). Remarkably, reducing AB for threat via ABM increases attentional selectivity for non-threat cues, which, in turn, improves SAD symptoms among individuals with SAD (Heeren et al., 2012c). Consequently, the critical next step will thus be to examine whether AB reduction through repeated sessions of anodal tDCS can de facto interrupt this vicious cycle and, in turn, foster beneficial cascade of downstream benefits.
In follow-up research several issues require further consideration. First, we did not collect post-stimulation data. Although enhancement of brain activity after a single session of anodal tDCS tends to return to baseline after approximately 90 min (Nitsche and Paulus, 2001; De Berker et al., 2013), long-lasting cognitive improvements have already been evidenced in studies including repeated sessions of tDCS (Dockery et al., 2009). Yet, the persistent reduction of psychopathological symptoms usually requires repeated tDCS sessions (Tortella et al., 2015; Remue et al., 2016). Future studies should thus examine the persistence of the present findings. Second, the present study only included female participants. Given the observation of gender differences in the neural processing of emotional facial expressions (Lithari et al., 2010; Gardener et al., 2013), uncertainty still abounds regarding the generalization of the present findings to men with SAD. Yet, most of the previous studies using neuromodulation techniques to modify AB for threat were restricted to female samples (De Raedt et al., 2010; Vanderhasselt et al., 2011). Third, although our decision to target the left dlPFC relied on previous rTMS and tDCS studies (De Raedt et al., 2010; Clarke et al., 2014b; Heeren et al., 2015b), vlPFC has been also associated to AB for threat (Hartley and Phelps, 2010; Fox and Pine, 2012). Consequently, one cannot exclude that our tDCS-induced effects may stem from excitatory projections from the dlPFC to the vlPFC. Yet, several reviews suggested that left dlPFC does constitute an optimal target for therapeutic uses of tDCS in clinical samples (De Raedt et al., 2015; Tortella et al., 2015). Future studies should thus further delineate the respective role of ventral and dorsal compartments of PFC in the modification of AB, by inhibiting versus stimulating these brain areas separately during the probe discrimination task. In-depth exploration of the brain connectivity alterations following anodal tDCS may also be done. Finally, we were unable to differentiate tDCS-responders from non-responders. Yet the study has only four non-responders. As understanding who profits from anodal tDCS is decisive before it can be reliably applied in larger Phase II/III randomized trials, future studies must clarify the variables that differentiate responders from non-responders.
Supplementary Material
Acknowledgements
The authors are also thankful to Laura Genon and Charlotte Coussement for their help with data collection.
Funding
This research was supported by a Grant (F.R.S.-FNRS #FC78142) from the Belgian National Fund for Scientific Research (awarded to Alexandre Heeren), a Grant (FWO08/PDO/168) from the Research Foundation Flanders (awarded to Marie-Anne Vanderhasselt), and a Grant (BOF16/GOA/017) for a Concerted Research Action of Ghent University (awarded to Rudi De Raedt). Pierre Maurage is funded, as a research associate, by the Belgian National Fund for Scientific Research (F.R.S.-FNRS). This work also received the support from the Belgian Foundation for Vocation (Vocatio) and the WBI World Excellence Grant (sub/2015/228106243177), both awarded to Alexandre Heeren. This work was also supported by the Ghent University Multidisciplinary Research Partnership ‘The integrative neuroscience of behavioral control’. These foundations did not exert any editorial direction or censorship on any part of this manuscript.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Adolphs R., Tranel D., Damasio H., Damasio A.R. (1995). Fear and the human amygdala. Journal of Neuroscience, 15, 5879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Amir N., Taylor C.T., Donohue M.C. (2011). Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical Psychology, 79, 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Paulus M.P. (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues in Clinical Neuroscience, 12, 517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantin T., Stevens S., Gerlash A.L., Hermann C. (2016). What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. Journal of Behaviour Therapy and Experimental Psychiatry, 50, 40–51. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Beck Depression Inventory Manual, 2nd edn. San Antonio, TX: Psychological Corporation. French adaptation, Paris, France: Editions du Centre de Psychologie Appliquée. [Google Scholar]
- Bishop S.J. (2008). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129, 141–52. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12, 92–8. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Brett M., Lawrence A.D. (2004). Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience, 7, 184–8. [DOI] [PubMed] [Google Scholar]
- Bouvard M., Cottraux J. (2010). Protocoles Et Échelles D’évaluation En Psychiatrie Et Psychologie,5th edn.Paris, France: Masson. [Google Scholar]
- Britton J.C., Suway J.G., Clementi M.A., Fox N.A., Pine D.S., Bar-Haim Y. (2014). Neural changes with attention bias modification for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience, 10, 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Holmes E.A., Murphy S.E., Goodwin G.M., Harmer C.J. (2010). Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry, 67, 919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchon-Schweitzer M., Paulhan I. (1993). Adaptation Francophone De L’inventaire D’anxiété Trait-Etat (Forme Y) De Spielberger. Paris, France: Editions du Centre Psychologie Appliquée. [Google Scholar]
- Clarke P.J.F., Browning M., Hammond G., Notebaert L., MacLeod C. (2014b). The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biological Psychiatry, 76, 946–52. [DOI] [PubMed] [Google Scholar]
- Clarke P.J.F., Notebaert L., MacLeod C. (2014a). Absence of evidence or evidence of absence: reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry, 14, 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F., Marceglia S., Ardolino G., Barbieri S., Priori A. (2007). Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. European Journal of Neuroscience, 26, 242–9. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- De Berker A.O., Bikson M., Bestmann S. (2013). Predicting the behavioral impact of transcranial direct current stimulation: issues and limitations. Frontiers in Human Neuroscience, 7, 613.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoncker J., Brunoni A.R., Baeken C., Vanderhasselt M.A. (2016). A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimulation,9, 501–17. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Leyman L., Baeken C., et al. (2010). Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biological Psychology, 85, 487–95. [DOI] [PubMed] [Google Scholar]
- De Raedt R., Vanderhasselt M.A., Baeken C. (2015). Neurostimulation as an intervention for treatment resistant depression: from research on mechanisms towards targeted neurocognitive strategies. Clinical Psychology Review, 41, 61–9. [DOI] [PubMed] [Google Scholar]
- Dockery C.A., Hueckel-Weng R., Birbaumer N., Plewnia C. (2009). Enhancement of planning ability by transcranial direct current stimulation. Journal of Neuroscience, 29, 7271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Pine D.S. (2012). Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener E.K.T., Carr A.R., MacGregor A., Felmingham K.L. (2013). Sex differences and emotion regulation: an event-related potential study. PLoS One, 8(10), e73475.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E., De Raedt R., Leyman L., Verschuere B. (2008). The Karolinska directed emotional faces: a validation study. Cognition and Emotion, 22, 1094–118. [Google Scholar]
- Gorlin E.I., Teachman B.A. (2015). Inhibitory control as a moderator of threat-related interference biases in social anxiety. Cognition and Emotion, 29, 723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton B., MacLeod C. (2016). Engaging with the wrong people: the basis of selective attention to negative faces in social anxiety. Clinical Psychological Sciences, 3, 1. [Google Scholar]
- Hartley C.A., Phelps E.A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology, 35, 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C., Wilson K.A., Lagle K., Kraemer H.C., Killen J.D., Taylor C.B. (2008). The developmental psychopathology of social anxiety in adolescents. Depression and Anxiety, 25, 200–6. [DOI] [PubMed] [Google Scholar]
- Heeren A., Baeken C., Vanderhasselt M.A., Philippot P., De Raedt R. (2015b). Impact of anodal and cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex during attention bias modification: an eye-tracking study. PLoS One, 10(4), e0124182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A., De Raedt R., Koster E.H.W., Philippot P. (2013). The (neuro)cognitive mechanisms behind attention bias modification in anxiety: proposals based on theoretical accounts of attentional bias. Frontiers in Human Neuroscience, 7, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A., Maurage P., Rossignol M., et al. (2012b). The self-report version of the Liebowitz social anxiety scale: psychometric properties of the French version. Canadian Journal of Behavioural Science, 44, 99–107. [Google Scholar]
- Heeren A., McNally R.J. (2016). An integrative network approach to social anxiety disorder: the complex dynamic interplay among attentional bias for threat, attentional control, and symptoms. Journal of Anxiety Disorders, 42, 95–104. [DOI] [PubMed] [Google Scholar]
- Heeren A., Mogoaşe C., Philippot P., McNally R.J. (2015a). Attention bias modification for social anxiety: a systematic review and meta-analysis. Clinical Psychology Review, 40, 76–90. [DOI] [PubMed] [Google Scholar]
- Heeren A., Peschard V., Philippot P. (2012a). The causal role of attentional bias to threat cues in social anxiety: a test on a cyber-ostracism task. Cognitive Therapy and Research, 36, 512–21. [Google Scholar]
- Heeren A., Reese H.E., McNally R.J., Philippot P. (2012c). Attention training toward and away from treat in social phobia: effects on behavioural, subjective, and physiological measures of anxiety. Behaviour Research and Therapy, 50, 30–9. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Brozovich F.A., Rapee R.M. (2010). A cognitive model of social anxiety disorder: update and extension In: Hofmann S.G., DiBartolo P.M., editors. Social Anxiety: Clinical, Developmental, and Social Perspectives, 2nd edn, pp. 395–422.New York: Academic Press. [Google Scholar]
- Hirsch C.R., Clark D.M. (2004). Information-processing bias in social phobia. Clinical Psychology Review, 24, 799–825. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Weiller E., Bonora L.I., Amorin P., Lépine J.P. (1998). French Adaptation of the Mini International Neuropsychiatric Interview (MINI 5.0.0.). Internal report, Unité INSERM 302. Paris, France: Hôpital de la Salpétrière. [Google Scholar]
- Leyman L., De Raedt R., Vanderhasselt M.A., Baeken C. (2009). Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychological Medicine, 39, 1019–28. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–73. [DOI] [PubMed] [Google Scholar]
- Linetzky M., Pergamin-Hight L., Pine D.S., Bar-Haim Y. (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety, 32, 383–91. [DOI] [PubMed] [Google Scholar]
- Lithari C., Frantzidis C.A., Papadelis C., Vivas A.B., Klados M.A., et al. (2010). Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topography, 23, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks T.L., Simpson G.V., Dale C.L., Hough M.G. (2007). Preparatory allocation of attention and adjustments in conflict processing. Neuroimage, 35, 949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Öhman A. (1998). The Karolinska Directed Emotional Faces (KDEF). Karolinska Hospital, Stockholm: Department of Neurosciences. [Google Scholar]
- MacLeod C., Mathews A., Tata P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95, 15–20. [DOI] [PubMed] [Google Scholar]
- MacLeod C., Rutherford E., Campbell L., Ebsworthy G., Holker L. (2002). Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology, 111, 107–23. [PubMed] [Google Scholar]
- Manson K.N.T., Carlbring P., Frick A., et al. (2013). Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Research Neuroimaging, 214, 229–37. [DOI] [PubMed] [Google Scholar]
- McGough J.J., Faraone S.V. (2009). Estimating the size of treatment effects: moving beyond p values. Psychiatry, 6, 21–9. [PMC free article] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45, 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K., Philippot P., Bradley B.P. (2004). Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology, 113, 160–5. [DOI] [PubMed] [Google Scholar]
- Mogoaşe C., David D., Koster E.H.W. (2014). Clinical efficacy of attentional bias procedures: an updated meta-analysis. Journal of Clinical Psychology, 70, 1133–57. [DOI] [PubMed] [Google Scholar]
- Morrison A.S., Heimberg R.G. (2013). Social anxiety and social anxiety disorder. Annual Review of Clinical Psychology, 9, 249–74. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., et al. (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry, 163, 1091–7. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorders. Archives of General Psychiatry, 65, 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M.A., Cohen L.G., Wasserman E.M., et al. (2008). Transcranial direct current stimulation: state of the art 2008. Brain Stimulation, 1(3), 206–23. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57, 1899–901. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Öhman A. (2005). The role of the amygdala in human fear. Automatic detection of threat. Psychoneuroendocrinology, 30, 953–8. [DOI] [PubMed] [Google Scholar]
- Ohn S.H., Park C.I., Yoo W.K., et al. (2008). Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport, 19, 43–7. [DOI] [PubMed] [Google Scholar]
- Peers P.V., Simons J.S., Lawrence A.D. (2013). Prefrontal control of attention to threat. Frontiers in Human Neuroscience, 7, 24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9, 148–58. [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2010). Emergent processes in cognitive-emotional interactions. Dialogues in Clinical Neurosciences, 12, 433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry, 59, 424–9. [DOI] [PubMed] [Google Scholar]
- Priori A., Mameli F., Cogiamanian F., et al. (2008). Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cerebral Cortex, 18, 451–5. [DOI] [PubMed] [Google Scholar]
- Rapee R.M., Heimberg R.G. (1997). A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35, 741–56. [DOI] [PubMed] [Google Scholar]
- Rapee R.M., MacLeod C., Carpenter L., et al. (2013). Integrating cognitive bias modification into a standard cognitive behavioural treatment package for social phobia: a randomized controlled trial. Behaviour Research and Therapy, 51, 207–15. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available: http://www.R-project.org/. [Google Scholar]
- Remue J., Baeken C., De Raedt R. (2016). Does a single neuromodulation session really affect mood in healthy individual? A systematic review. Neuropsychologia, 85, 184–98. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stein M.B., Goldin P.R., Sareen J., Zorilla L.T., Brown G.G. (2002). Increased amygdala activation to angry faces and contemptuous faces in generalized social phobia. Archives of General Psychiatry, 59, 1027–34. [DOI] [PubMed] [Google Scholar]
- Taylor C.T., Aupperle R.L., Flagan T., et al. (2014). Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience, 9, 1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.T., Cross K., Amir N. (2016). Attentional control moderates the relationship between social anxiety symptoms and attentional disengagement from threatening information. Journal of Behavior Therapy & Experimental Psychiatry, 50, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortella G., Casati R., Aparicio L.V.M., Mantovani A., Senço N., et al. (2015). Transcranial direct current stimulation in psychiatric disorders. World Journal of Psychiatry, 5, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Baeken C., Hendricks M., De Raedt R. (2011). The effects of high frequency rTMS on negative attentional bias are influenced by baseline state anxiety. Neuropsychologia, 49, 1824–30. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–94. [DOI] [PubMed] [Google Scholar]
- Wong Q.J.J., Rapee R.M. (2016). The aetiology and maintenance of social anxiety disorder: a synthesis of complimentary theoretical models and formulation of a new integrated model. Journal of Affective Disorders, 203, 84–100. [DOI] [PubMed] [Google Scholar]
- Xu X., Schneier F., Heimberg R.G., et al. (2012). Gender differences in social anxiety disorder: results from the national epidemiologic sample on alcohol and relations conditions. Journal of Anxiety Disorders, 26, 12–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.