Abstract
As a personality trait, grit involves the tendency to strive to achieve long-term goals with continual passion and perseverance and plays an extremely crucial role in personal achievement. However, the neural mechanisms of grit remain largely unknown. In this study, we aimed to explore the association between grit and the fractional amplitude of low-frequency fluctuations (fALFF) in 217 healthy adolescent students using resting-state functional magnetic resonance imaging (RS-fMRI). We found that an individual’s grit was negatively related to the regional fALFF in the right dorsomedial prefrontal cortex (DMPFC), which is involved in self-regulation, planning, goal setting and maintenance, and counterfactual thinking for reflecting on past failures. The results persisted even after the effects of general intelligence and the ‘big five’ personality traits were adjusted for. More importantly, the fALFF of the right DMPFC played a mediating role in the association between grit and academic performance. Overall, these findings reveal regional fALFF as a neural basis of grit and highlight the right DMPFC as a neural link between grit and academic performance.
Keywords: grit, academic performance, resting-state fMRI, dorsomedial prefrontal cortex
Introduction
Why do some people achieve more than others? Searching for the secret to success is one major goal in the field of psychology. Many psychological attributes, such as general intelligence (Gottfredson, 1997) and conscientiousness (Tett et al., 1991), are consistently related to achievement. Over the past decade, a newly explored personality trait named ‘grit’ has been found to play an extremely crucial role in achievement. Duckworth et al. (2007) first introduced this personality construct, defining it at the trait level as passion and perseverance for long-term goals. Moreover, Duckworth and colleagues proposed that grit differs from several related personality traits such as conscientiousness and self-control (Duckworth et al., 2007; Duckworth and Gross, 2014). Specifically, grit involves maintaining both effort and passion toward long-term goals over months or even years, whereas the traits mentioned above do not necessarily involve such goals.
Numerous studies have shown that grit predicts achievement-related outcomes in many professional domains. For instance, many cross-sectional studies have revealed that grit is related to education level (Duckworth et al., 2007), academic achievement (Duckworth et al., 2007; Eskreis-Winkler et al., 2014; Strayhorn, 2014; Bowman et al., 2015; Wolters and Hussain, 2015), exercise adherence (Reed et al., 2013) and work engagement (Suzuki et al., 2015). Furthermore, a series of longitudinal studies have suggested that grit can predict subsequent academic performance in middle school students and undergraduates (Duckworth et al., 2007; Duckworth and Quinn, 2009; Bowman et al., 2015; Bazelais et al., 2016), final score and rank in the National Spelling Bee (Duckworth et al., 2007; Duckworth and Quinn, 2009; Duckworth et al., 2011), retention of the hard summer training at West Point (Duckworth et al., 2007; Maddi et al., 2012; Kelly et al., 2014) and teacher effectiveness (Duckworth et al., 2009; Robertson-Kraft and Duckworth, 2014). These findings indicate that grit plays a causal role in achievement-related outcomes.
Although the concept of grit has drawn considerable scientific and popular attention over the past several years (Tough, 2011), the neural processes underlying this trait remain largely unknown. According to our knowledge, only one recent study investigated the neural basis underlying grit, and this study found that grit was related to the resting-state functional connectivity (RSFC) between the ventral striatum and the prefrontal cortex (PFC), including the dorsomedial prefrontal cortex (DMPFC), dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) (Myers et al., 2016). Even so, a body of previous studies of grit-related constructs have suggested that cortical regions involved in grit may be part of the PFC. Specifically, grit shares features with high conscientiousness and low neuroticism in the ‘big five’ personality model (Duckworth et al., 2007; Duckworth and Quinn, 2009). Many studies have revealed that conscientiousness and neuroticism are mainly correlated with subregions of the PFC, such as the DMPFC and DLPFC (Wright et al., 2006; Wright et al., 2007; DeYoung et al., 2010; Kunisato et al., 2011; Forbes et al., 2014; Lu et al., 2014; Servaas et al., 2014). Broadly, grit is a subcomponent of the complex construct of self-regulation (McClelland et al., 2015), and mounting empirical evidence has indicated that the PFC is closely associated with self-regulation (Kelley et al., 2015). Furthermore, numerous lesion studies have suggested that patients with PFC damage have difficulty making plans and maintaining long-term goals (Shallice and Burgess, 1996; Kain and Perner, 2003; Szczepanski and Knight, 2014). Taken together, the findings consistently suggest that the PFC, which is related to goal-directed thought and behavior (Fuster, 1988; Stuss and Knight, 2013), task management and planning (Koechlin et al., 2000; Tanji et al., 2007), and cognitive control (Miller and Cohen, 2001; Ridderinkhof et al., 2004; Taren et al., 2011), may play an essential role in individual differences in grit.
In recent years, resting-state functional magnetic resonance imaging (RS-fMRI) has become a sound instrument for detecting brain activity reflected in low-frequency fluctuations (LFFs, Logothetis et al., 2001), which are associated with behavior performance (Fox and Raichle, 2007; Biswal, 2012). RS-fMRI can reliably predict individual differences of brain activities during task performance (Tavor et al., 2016). Due to its task-free condition and low cost, RS-fMRI confers a unique advantage in exploring the neural correlates underlying personality traits (Mar et al., 2013). Here, we employed the fractional amplitude of low-frequency fluctuations (fALFF) (Zou et al., 2008), which is a reliable and popular method to measure LFFs (Zuo et al., 2010). Prior studies have indicated that the fALFF reflects regional properties of intrinsic brain dynamics and is behaviorally relevant (Zou et al., 2008; Raichle, 2010). Many investigations have used the fALFF to detect neuropsychiatric disorders, including mild cognitive impairment (Han et al., 2011), substance abuse disorder (Orr et al., 2013), internet gaming disorder (Lin et al., 2015), schizophrenia (Hoptman et al., 2010) and major depressive disorder (Guo et al., 2013; Liu et al., 2013). Furthermore, the fALFF in healthy individuals has been correlated with individual differences in the ‘big five’ personality traits (Kunisato et al., 2011), empathy (Cox et al., 2012), inhibitory control (Hu et al., 2014), subjective well-being (Kong et al., 2015) and working memory (van Dam et al., 2015). Together, the findings suggest that fALFF analysis can be employed to uncover the neural mechanisms underlying human cognition, emotion, personality and social behavior. Based on the studies of grit reviewed above, we speculated that the fALFF in PFC regions (e.g. the DMPFC, DLPFC and ACC) might be correlated with individual differences in grit.
Moreover, PFC regions have also been associated with measures related to academic performance. For example, using electroencephalography (EEG), Aguirre-Pérez et al. (2007) found that in working memory tasks, frontal energy in the delta and theta frequencies was higher in students with greater academic achievement than in students with lower academic achievement. Evidence from a longitudinal functional MRI (fMRI) study also showed that activation in frontal areas in an auditory narrative comprehension task could significantly predict students’ college preparedness test scores (Horowitz-Kraus et al., 2015). In addition, a recent voxel-based morphometry study revealed a positive relationship between the regional gray matter volume of the frontal lobe and academic performance in children and adolescents (Hair et al., 2015). Therefore, based on these findings and previous neural findings regarding grit, we speculated that the spontaneous activity of PFC regions may predict academic performance. Considering the central role of grit in academic performance, we also hypothesized that spontaneous activity in the PFC regions may mediate the influence of grit on academic performance.
To explore these questions, we first employed the 8-item Short Grit Scale (Grit-S, Duckworth and Quinn, 2009) to assess individual differences in grit between healthy adolescent students. Second, we correlated the participants' self-reported grit scores with regional fALFF to identify cortical areas that could be significantly associated with grit. Third, we conducted mediation analyses to confirm the mediating effect of these grit-related areas on the relationship between grit and academic performance. To obtain satisfactory statistical power for whole-brain analyses, we focused on a large sample of adolescent students within a narrow age range because it is hard to identify individual differences across a broad age range (Mackey et al., 2015).
Methods
Participants
The participants included 234 students [52.1% females; mean age = 18.60 years, standard deviation (s.d.) = 0.78 years]. The data were collected from a longitudinal project that aimed to investigate the determinants of health, well-being and academic performance among adolescents in Chengdu, China. All participants were new high school graduates who graduated in June 2015 from several local public high schools. Seventeen participants were excluded for one of two reasons: no academic performance scores available (n = 14) or abnormal brain structure (n = 3) (e.g. unusual cyst). Therefore, 217 students (53.0% females; mean age = 18.48 years, s.d. = 0.55 years) were included in subsequent data analysis. None of the remaining participants reported a history of psychiatric disorder or neurological illness, and all were right-handed based on self-report using the Edinburgh Handedness Inventory (Oldfield, 1971). The study was approved by the local research ethics committee of West China Hospital, Sichuan University. Prior to testing, we obtained written informed consent from all participants and their parents. The behavioral tests and MRI scans were conducted from June 2015 to September 2015.
Assessment of the trait grit
We used the Grit-S (Duckworth and Quinn, 2009) to measure individual differences in the trait grit. The scale includes two dimensions, each with four items: the consistency of interest, which reflects the maintenance of one’s interests over a very long time; and the perseverance of effort, which reflects the tendency to continue to put forth effort in the face of failure or adversity. The response options for each item range from 1 (not at all like me) to 5 (very much like me). The scale’s reliability and validity have been well established (Duckworth and Quinn, 2009). The Chinese version of the Grit-S shows satisfactory test-retest reliability (r = 0.78), internal reliability (Cronbach’s α = 0.80), and criterion-related validity related to conscientiousness, self-control and academic performance (Li et al., forthcoming). In this study, Cronbach’s α value for the Grit-S was 0.81, indicating adequate internal consistency.
To confirm that the previously reported two-factor structure with one higher-order factor of Grit-S score could be used with our data, we used AMOS software (Version 22.0) to conduct a confirmatory factor analysis (CFA). Four criteria were employed to assess the model’s goodness-of-fit: the non-normed fit index (NNFI), and the comparative fit index (CFI), with values above 0.95 representing a good fit; the standardized root mean square residual (SRMR) and the root mean square error of approximation (RMSEA), with values below 0.06 indicating an excellent fit (Hu and Bentler, 1999; Kline, 2015). The results suggested the two-factor model fit well to the data [χ2 (19) = 27.79, P < 0.001]. The indices of goodness-of-fit also revealed an excellent fit for the model (NNFI = 0.97, CFI = 0.98, SRMR = 0.035, RMSEA = 0.046). In summary, the CFA supported the two-factor structure of the Grit-S, which fits well with previous findings (Duckworth and Quinn, 2009). In the following analyses, we used only Grit-S total scores for two major reasons. First, the concept of grit was operationally defined as passion and perseverance for long-term goals, thus the total score might reflect a more comprehensive meaning of grit (Duckworth et al., 2007; Duckworth and Gross, 2014). Second, previous studies have shown high correlations between the total score and subscale scores and inter-factor correlations (Duckworth et al., 2007; Duckworth and Quinn, 2009).
Assessment of general intelligence
The Raven’s Advanced Progressive Matrix (RAPM) is a popular and sound psychometric test for general intelligence (Raven, 2000). Here, we used the RAPM as a confounding variable because the significant associations between grit, resting-state brain activity and academic performance may be caused by the indirect correlations between general intelligence, resting-state brain activity and academic performance (Gottfredson, 1997; Song et al., 2008). The RAPM consists of 36 nonverbal items, and participants are instructed to choose the missing part of a graphical matrix for each item (Raven, 2000). The intelligence score was computed by summing the number of correct test answers. In this study, Cronbach’s α value for the RAPM was 0.82, indicating adequate internal reliability.
Assessment of personality
Because the ‘big five’ personality traits have been found to be associated with grit (Duckworth et al., 2007; Duckworth and Quinn, 2009), academic performance (Poropat, 2009) and resting-state brain activity (Kunisato et al., 2011), we used the NEO Five-Factor Inventory (NEO-FFI) (Costa and McCrae, 1992) to control for the possible effect of personality on the relationships between grit, resting-state brain activity and academic performance. The questionnaire includes five subscales: neuroticism, extraversion, openness to experience, agreeableness and conscientiousness. Each subscale consists of 12 items that are rated on a 5-point Likert-type scale. The Chinese version of the NEO-FFI has acceptable internal reliability and criterion-related validity related to achievement motivation, learning approaches and thinking styles among Chinese students (Zhang and Huang, 2001; Zhang, 2003; Chen and Zhang, 2011). In this study, the NEO-FFI subscales showed adequate reliability, with Cronbach’s α values ranging between 0.71 and 0.81.
Assessment of academic performance
Academic performance was assessed using the standardized total score on the Chinese National College Entrance Examination (CNCEE); these scores were retrieved from the Chengdu Education Institute database. The CNCEE, which includes tests of four curriculum subjects (Chinese, English, Mathematics and Comprehensive Ability), was administered in June 2015.
RS-fMRI data acquisition
We collected the data using a 3.0 T MRI scanner (Siemens-Trio, Erlangen, Germany) that was located at West China Hospital of Sichuan University, Chengdu, China. During the scanning, we required the participants to remain awake with their eyes closed and to not intentionally think about anything. We used headphones and foam pads to reduce scanner noise and to restrict head motion. For each participant, the 480 s scanning time included 240 contiguous echo-planar imaging (EPI) volumes (TR/TE = 2000/30 ms, slices = 30, thickness = 5 mm, in-plane matrix = 64 × 64, FOV = 240 mm × 240 mm, flip angle = 90°, interslice gap = 0 mm, voxel size = 3.75 × 3.75 × 5 mm3). In addition, we collected T1-weighted anatomical images for spatial registration with 1 × 1 × 1 mm3 resolution (TR/TE/TI = 1900/2.26/900 ms, matrix size = 256 × 256, flip angle = 9°, 176 slices).
RS-fMRI data analysis
We preprocessed the data with the DPARSF toolbox (Yan and Zang, 2010) and functions of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/SPM8). First, we discarded the first 10 images to ensure the stability of the MRI signals during adaption of the participants. Second, we performed slice timing and head movement correction for the remaining 230 images; we excluded no data because the rotational and translational parameters never exceeded ±1.5° or ±1.5 mm. Third, we spatially normalized these images to the SPM8 EPI template with 3 × 3 × 3 mm3 resolution. Fourth, we used an 8 mm FWHM Gaussian kernel to smooth the resulting normalized data and then removed the linear trends. Finally, we regressed out the six head motion parameters to ensure that the LFF amplitude analysis was not affected by head motion (Satterthwaite et al., 2012). Moreover, we calculated the mean framewise displacement (FD) (Van Dijk et al., 2012) as a covariate in group-level analyses, although the FD was not correlated with grit (r = −0.07, P = 0.273).
We calculated the fALFF using the method developed by Zou et al. (2008) and Zuo et al. (2010). First, we used the Fourier transform to convert the time series of a specific voxel into the frequency domain and then obtained the power spectrum. Because the power of a given frequency is proportional to the square of the amplitude of this frequency component, we computed the square root at each frequency of the power spectrum and then obtained the mean square root across a low-frequency range (0.01–0.08 Hz) at each voxel. This mean square root was regarded as the ALFF (Zang et al., 2007; Wang et al., 2011). As a normalized score of the ALFF, the fALFF was calculated as a fraction of the sum of the amplitudes across the entire frequency range (0–0.25 Hz). Compared to the ALFF, the fALFF has been shown to be less susceptible to obvious pulsatile motion and nuisance signals (Zuo et al., 2010). Finally, to obtain the fALFF Z-score map for each participant, we subtracted the global mean fALFF and divided by the standard deviation.
Statistical analysis
To identify the neural substrates of grit, a whole-brain regression analysis was performed with REST toolbox 1.8 (Song et al., 2011). In this analysis, we included the voxel-wise fALFF value as the dependent variable, the Grit-S score as the covariate of interest, and age, gender and FD as confounding covariates. For multiple comparisons correction, we employed Monte Carlo simulation (Cox, 1996) using the AlphaSim program in REST. We set P < 0.05 for a corrected cluster with 10 000 iterations (voxel-wise: P < 0.001; at least 24 voxels, 648 mm3). The AlphaSim program has been widely employed in prior studies that analyzed fALFF data (e.g. Kunisato et al., 2011; Kong et al., 2015).
To determine whether the grit-related brain regions could reliably explain the association between grit and academic performance, we performed a mediation analysis with the Grit-S score as the predictor variable (X), the fALFF of particular brain regions as the mediator variable (M) and academic performance as the outcome variable (Y). The three-variable mediation model used standard conventions to test the significance of the following relationships (Preacher and Hayes, 2008): path a, indicating the relationship from X to M; path b, indicating the relationship from M to Y after controlling for X; path c (total effect), indicating the relationship from X to Y; and path c′ (direct effect) indicating the relationship from X to Y after controlling for M. The variable M is considered a mediator if the indirect effect (c − c′ or a × b) is significant. To examine the significance of the indirect effect, a bootstrapping test (5000 samplings) was used to generate 95% confidence intervals (CIs); a CI that does not include 0 suggests that the total effect between X and Y was mediated by M. The mediation analysis was conducted using the SPSS macro program (Preacher and Hayes, 2008).
Results
fALFF-Behavior correlation analysis
The descriptive statistics of all measures are presented in Table 1. According to the assumption of normality (Marcoulides and Hershberger, 1997), the skewness and kurtosis of the measures are satisfactory (ranges from −1 to +1). Because the Grit-S total score was highly correlated with the two Grit-S subscale scores (consistency of interest: r = 0.86, P < 0.001; perseverance of effort: r = 0.84, P < 0.001), we used only the Grit-S total score as the measure of grit in the following analyses.
Table 1.
Descriptive statistics of participant-level variables (N = 217)
| Variable | Mean | s.d. | Range | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Age | 18.48 | 0.55 | 16–20 | 0.49 | 1.67 |
| Grit | 25.55 | 4.30 | 16–38 | 0.16 | −0.57 |
| RAPM | 24.16 | 5.59 | 6–36 | −0.29 | 0.02 |
| NEO-FFI | |||||
| Neuroticism | 33.84 | 6.52 | 15–48 | −0.16 | −0.16 |
| Extraversion | 42.48 | 6.18 | 25–58 | −0.08 | −0.14 |
| Openness | 41.26 | 4.77 | 31–57 | 0.30 | 0.00 |
| Agreeableness | 42.04 | 3.62 | 31–52 | −0.07 | −0.05 |
| Conscientiousness | 39.49 | 5.83 | 22–55 | −0.02 | 0.30 |
| Academic performance | 520.49 | 73.28 | 301–645 | −0.56 | 0.01 |
Note: N = number; s.d. = standard deviation; RAPM, Raven’s Advanced Progressive Matrix; NEO-FFI, NEO Five-Factor Inventory.
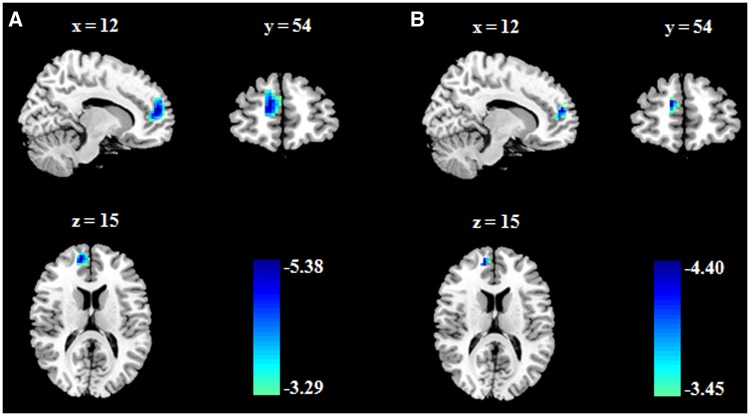
To uncover the neural basis underlying grit, we conducted a whole-brain regression analysis. After controlling for age, gender and FD, grit was significantly and negatively related to the fALFF in a cluster that included the right DMPFC (BA10; MNI coordinates: 12, 54, 15; r = −0.35; Z = −5.38; P < 0.001; cluster size = 3591 mm3) (Table 2 and Figure 1A). We obtained no other significant associations.
Table 2.
Summary of brain regions related to grit
| Brain region | BA | Side | MNI coordinates |
Peak Z score | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controlling for age, gender and FD | |||||||
| DMPFC | 10 | R | 12 | 54 | 15 | −5.38 | 3591 |
| Controlling for age, gender, FD, RAPM and NEO-FFI | |||||||
| DMPFC | 10 | R | 12 | 54 | 15 | −4.40 | 1026 |
Note: We set the threshold at P < 0.05 at the cluster level (combined with voxel-wise P < 0.001; at least 24 voxels, 648 mm3). DMPFC, dorsomedial prefrontal cortex; BA, Brodmann’s area; L = left, R = right; MNI = Montreal Neurological Institute; FD, framewise displacement; RAPM, Raven’s Advanced Progressive Matrix; NEO-FFI, NEO Five-Factor Inventory.
Fig. 1.
Brain regions associated with grit. (A) After controlling for age, gender and FD, grit was negatively associated with the fALFF in the right dorsomedial prefrontal cortex (DMPFC). (B) Grit was still associated with the fALFF in the right DMPFC, even after general intelligence and the ‘big five’ personality traits, as well as age, gender and FD were adjusted for.
To test the specificity of this correlation, we excluded confounding factors, including the RAPM and NEO-FFI dimensions. Behaviorally, we found no significant association between grit and RAPM (r = 0.04, P = 0.536). Grit was associated with conscientiousness (r = 0.56, P < 0.001), neuroticism (r = −0.37, P < 0.001), extraversion (r = 0.17, P < 0.05), and agreeableness (r = 0.27, P < 0.001) but not with openness to experience (r = 0.03, P = 0.624). These results fit well with prior findings (Duckworth et al., 2007; Duckworth and Quinn, 2009). Then, we generated an additional model to test the specificity of the association between grit and the fALFF in the right DMPFC, with the RAPM, NEO-FFI dimensions, age, gender and FD as confounding variables. The association remained significant even after these variables were controlled for (right DMPFC; BA10; MNI coordinates: 12, 54, 15; r = −0.29; Z = −4.40; P < 0.001; cluster size = 1026 mm3) (Table 2 and Figure 1B).
Mediation analysis
After identifying the neural basis of grit, we further explored whether the region identified through the initial analysis acted as a link between grit and academic performance. First, we replicated the significant correlation between grit and academic performance (r = 0.27, P < 0.001). Importantly, grit explained additional variance in academic performance (△R2 = 5.6%, P < 0.001) beyond that explained by age, gender and FD. Second, we tested whether the region associated with grit was related to academic performance. The results indicated that the association between the fALFF in the right DMPFC and academic performance was significant (r = −0.26, P < 0.001). Furthermore, the fALFF in the right DMPFC explained additional variance in academic performance (△R2 = 5.3%, P < 0.001) beyond that explained by age, gender and FD.
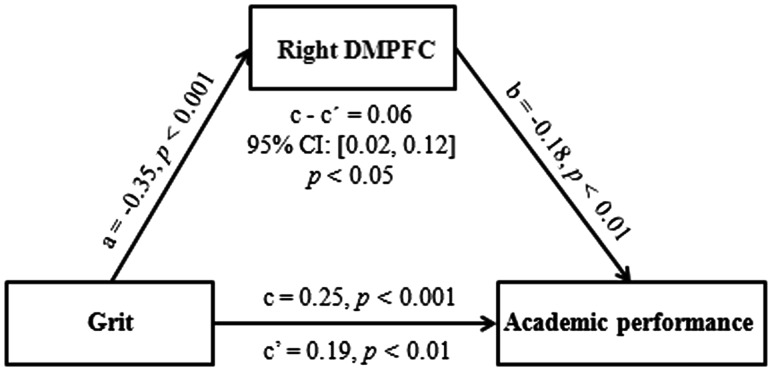
To examine whether the fALFF in the right DMPFC could mediate the influence of grit on academic performance, we carried out a mediation analysis with age, gender and FD as confounding variables. The results revealed that the right DMPFC played a mediating role in the association between grit and academic performance [95% CI = (0.02, 0.12), P < 0.05; Figure 2]. Moreover, we examined the mediating effect of the brain region identified from the secondary fALFF-behavior analysis in which age, gender, FD, RAPM and NEO-FFI dimensions were adjusted for. After controlling for the confounding factors, the smaller right DMPFC still mediated the effect of grit on academic performance [95% CI = (0.02, 0.11), P < 0.05]. Together, our findings indicate that the right DMPFC partially mediates the influence of grit on academic performance.
Fig. 2.
The right dorsomedial prefrontal cortex (DMPFC) mediates the effect of grit on academic performance. The presented panel is the path diagram of the mediation analysis showing that grit affects academic performance through the fALFF of the DMPFC. All standard regression coefficients are significant (a, b, c and c′), and the indirect effect (c − c′ or a × b) is significant. Age, gender and FD are controlled for in the analysis.
To confirm that the results observed were replicable and reliable, we randomly divided our sample into two groups and determined whether both groups showed the same results. In group 1 (N = 108; 55 females; mean age = 18.55, s.d. = 0.58), grit was correlated with academic performance (r = 0.24, P < 0.01) and the DMPFC (r = −0.37, P < 0.001); academic performance was associated with the DMPFC (r = −0.25, P < 0.01); further mediation analyses showed that the DMPFC mediated the association of grit with academic performance [95% CI = (0.01, 0.10), P < 0.05]. In group 2 (N = 109; 60 females; mean age = 18.42, s.d. = 0.52), grit was correlated with academic performance (r = 0.26, P < 0.01) and the DMPFC (r = −0.31, P < 0.001); academic performance was associated with the DMPFC (r = −0.24, P < 0.01); further mediation analyses showed that the DMPFC mediated the association of grit with academic performance [95% CI = (0.01, 0.09), P < 0.05]. Age, gender and FD were controlled for in these analyses. Therefore, the results observed were replicable and reliable.
Discussion
The present study examined the neural basis of grit and the relationship of the neural basis with academic performance in a sample of healthy adolescent students. Two main findings were revealed. First, a higher Grit-S score was associated with lower fALFF in the right DMPFC, identifying a functional neural mechanism of grit. Furthermore, after adjusting for the RAPM and NEO-FFI dimensions, the fALFF in the right DMPFC continued to be associated with grit, suggesting this measure is specific to grit. Second, the fALFF in the right DMPFC mediated the influence of grit on academic performance. Moreover, after controlling for the RAPM and NEO-FFI dimensions, the mediating effect of the right DMPFC was still significant, indicating the specificity of this effect. In summary, our findings provided initial evidence of the neural correlates underlying grit and identified the right DMPFC as a neural link between grit and academic performance.
Confirming our first prediction, we found that the fALFF in the right DMPFC could significantly predict individual differences in grit. The negative correlation between grit and spontaneous brain activity fits with several studies demonstrating higher (f)ALFF of the DMPFC in patients with self-regulation-related disorders such as attention deficit hyperactivity disorder (Li et al., 2014), substance use disorder (Orr et al., 2013), anxiety disorder (Qiu et al., 2015) and major depressive disorder (Guo et al., 2013; Liu et al., 2013). Higher fALFFs of the DMPFC in low-grit individuals and patients with self-regulation failures might reflect a compensatory mechanism to offset structural or functional defects (Yang et al., 2011; Bing et al.,2013; Orr et al., 2013), a larger effort to inhibit subcortical brain activities (Jiao et al., 2011; Liu et al., 2013), or an increased cortical modulation of neural activity (Xu et al., 2014; Zhou et al., 2014). This finding is also consistent with the findings in healthy individuals who showed higher DMPFC spontaneous activity and higher trait anxiety (Tian et al., 2016). Behaviorally, lower grit scores have been found to be related to higher scores in impulsiveness (Moshier et al., 2016), substance use behaviors (Guerrero et al., 2016), anxiety (Sheridan et al., 2015) and depression (Kleiman et al., 2013; Anestis and Selby, 2015). Because this study investigated only the neural correlates of grit in the resting-state, future studies may explore this issue in the task-state and compare the results using different measures of the brain.
In addition, many studies have consistently reported that the DMPFC is involved in various components of self-regulation, such as impulsiveness (Cho et al., 2013; Davis et al., 2013; Muhlert and Lawrence, 2015), emotional regulation (Buhle et al., 2014), cognitive control (Venkatraman et al., 2009; Taren et al., 2011) and delay discounting (Carter et al., 2010; Cho et al., 2013; Scheres et al., 2013). Moreover, evidence from lesion studies have shown that DMPFC dysfunction leads to an inability to make plans, to set and maintain goals, and to comprehend the complex intentions of others (Kain and Perner, 2003; Szczepanski and Knight, 2014). Furthermore, the DMPFC has also been found to be associated with counterfactual thinking for reflecting on the past or predicting the future (Barbey et al., 2009). Evidence from a recent behavioral study has also shown that reflecting on past failures can improve participants’ grit scores and that this improvement results in improved behavioral outcomes (DiMenichi and Richmond, 2015). Thus, the activity of the DMPFC might correlate with grit through its association with counterfactual thinking for reflecting on past failures. Because the DMPFC is known as a high associative center in the frontal cortex (Eickhoff et al., 2016), the finding of a relationship between grit and DMPFC spontaneous activity might reflect its role in self-regulation, planning, the pursuit of goals and counterfactual thinking for reasoning about the past failures.
Confirming our second prediction, the current study suggested that the fALFF in the right DMPFC partially mediated the effect of grit on academic performance. As many prior behavioral studies have shown, grit is stably associated with academic performance, even after controlling for demographic factors and other predictors (Duckworth et al., 2007; Duckworth and Quinn, 2009; Eskreis-Winkler et al., 2014; Strayhorn, 2014; Bowman et al., 2015; Wolters and Hussain, 2015; Bazelais et al., 2016). This observation is consistent with our finding that grit explained additional variance in academic performance after controlling for age, gender, general intelligence and the ‘big five’ personality traits. Therefore, grit is likely a crucial personality strength for cultivating students’ academic performance. In addition, the finding that the fALFF in the right DMPFC correlates with academic performance fits well with several previous studies that reported associations between the DMPFC and measures of academic performance (Springer et al., 2005; Bonnet et al., 2009; Hair et al., 2015). For example, Springer et al. (2005) found that in older adults, education level was positively related to DMPFC activity during a memory task, whereas in young adults, education level was negatively related to DMPFC activity. Given the youth and narrow age range of our sample, the negative correlation revealed in the present study is unsurprising. Considering the relationship of the right DMPFC with grit, the right DMPFC might serves as a neural link that can explain the common variance between grit and academic performance.
There are several limitations to this study. First, the participants were recruited from a population of healthy Chinese adolescents. Although the narrow age range of the participants confers the advantage of sufficient statistical power for whole-brain analyses, it may limit the generalizability of our findings. Therefore, future studies are needed to validate our findings among more diverse populations such as adults, the elderly and individuals with mental disorders. Second, considering the cross-sectional design, we could not come to a causal conclusion about the relationship between grit, fALFF and academic performance. Longitudinal studies may help determine the causal associations between these variables. Third, the assessment of grit relied on a self-reported questionnaire, which was vulnerable to subjectivity, although it was selected due to its favorable psychometric properties. Using multiple methods for measurement might diminish the effect of response bias. Finally, the academic performance was evaluated only on a single exam, which might not sufficiently reflect an individual’s real-world achievement and might be biased by factors that were not controlled for in this study. Future investigations using the scores on exams across time are required to confirm our findings.
The current study explored the neural processes underlying grit and the relationship of these processes with academic performance. We found that spontaneous activity in the right DMPFC was related to individual differences in grit. This finding persisted even after the effects of general intelligence and the ‘big five’ personality traits were controlled for. Briefly, our study demonstrated a unique functional neural mechanism underlying grit. Furthermore, our research indicates that the spontaneous activity of the right DMPFC acts as a neural link between grit and academic performance. Finally, our findings may have educational implications in that grit-related behavioral (e.g. Gamel, 2014) and neural training programs (e.g. Tang et al., 2013) can be developed to improve students’ grit-related skills, which can help them achieve better academic-related outcomes.
Acknowledgments
This study was supported by the National Natural Science Foundation (Grant Nos. 81621003, 81271565, 31470047, 81220108013, 81227002 and 81030027), National Key Technologies R&D Program (Program No. 2012BAI01B03) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT Grant No. IRT1272) of China. Drs. Meiyun Wang and Qiyong Gong contributed equally to playing the role of corresponding author. Dr. Gong would also like to acknowledge the support from his Changjiang Scholar Professorship Award (Award No. T2014190) of China and American CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA.
Conflict of interest: None declared.
References
- Aguirre-Pérez D.M., Otero-Ojeda G.A., Pliego-Rivero F.B., Ferreira-Martinez A.A. (2007). Relationship of working memory and EEG to academic performance: a study among high school students. International Journal of Neuroscience, 117(6), 869–82. [DOI] [PubMed] [Google Scholar]
- Anestis M.D., Selby E.A. (2015). Grit and perseverance in suicidal behavior and non-suicidal self-injury. Death Studies, 39(1–5), 211–8. [DOI] [PubMed] [Google Scholar]
- Barbey A.K., Krueger F., Grafman J. (2009). Structured event complexes in the medial prefrontal cortex support counterfactual representations for future planning. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 364(1521), 1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazelais P., Lemay D.J., Doleck T. (2016). How does grit impact college students’ academic achievement in science? European Journal of Science and Mathematics Education, 4(1), 33–43. [Google Scholar]
- Bing X., Ming-Guo Q., Ye Z., et al. (2013). Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Research, 1490, 225–32. [DOI] [PubMed] [Google Scholar]
- Biswal B.B. (2012). Resting state fMRI: a personal history. Neuroimage, 62(2), 938–44. [DOI] [PubMed] [Google Scholar]
- Bonnet M.C., Dilharreguy B., Allard M., Deloire M.S.A., Petry K.G., Brochet B. (2009). Differential cerebellar and cortical involvement according to various attentional load: role of educational level. Human Brain Mapping, 30(4), 1133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman N.A., Hill P.L., Denson N., Bronkema R. (2015). Keep on truckin' or stay the course? Exploring grit dimensions as differential predictors of educational achievement, satisfaction, and intentions. Social Psychological and Personality Science, 6(6), 639–45. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Meyer J.R., Huettel S.A. (2010). Functional neuroimaging of intertemporal choice models: a review. Journal of Neuroscience, Psychology, and Economics, 3(1), 27–45. [Google Scholar]
- Chen C., Zhang L.F. (2011). Temperament, personality and achievement goals among Chinese adolescent students. Educational Psychology, 31(3), 339–59. [Google Scholar]
- Cho S.S., Pellecchia G., Aminian K., et al. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26(3), 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. (1992). Normal personality assessment in clinical practice: the NEO Personality Inventory. Psychological Assessment, 4(1), 5–13. [Google Scholar]
- Cox C.L., Uddin L.Q., Di Martino A., Castellanos F.X., Milham M.P., Kelly C. (2012). The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7(6), 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Davis F.C., Knodt A.R., Sporns O., et al. (2013). Impulsivity and the modular organization of resting-State neural networks. Cerebral Cortex, 23(6), 1444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung C.G., Hirsh J.B., Shane M.S., Papademetris X., Rajeevan N., Gray J.R. (2010). Testing predictions from personality neuroscience: brain structure and the big five. Psychological Science, 21(6), 820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMenichi B.C., Richmond L.L. (2015). Reflecting on past failures leads to increased perseverance and sustained attention. Journal of Cognitive Psychology, 27(2), 180–93. [Google Scholar]
- Duckworth A., Gross J.J. (2014). Self-control and grit: related but separable determinants of success. Current Directions in Psychological Science, 23(5), 319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A.L., Kirby T.A., Tsukayama E., Berstein H., Ericsson K.A. (2011). Deliberate practice spells success: why grittier competitors triumph at the National Spelling Bee. Social Psychological and Personality Science, 2(2), 174–81. [Google Scholar]
- Duckworth A.L., Peterson C., Matthews M.D., Kelly D.R. (2007). Grit: perseverance and passion for long-term goals. Journal of Personality and Social Psychology, 92(6), 1087–101. [DOI] [PubMed] [Google Scholar]
- Duckworth A.L., Quinn P.D. (2009). Development and validation of the Short Grit Scale (Grit-S). Journal of Personality Assessment, 91(2), 166–74. [DOI] [PubMed] [Google Scholar]
- Duckworth A.L., Quinn P.D., Seligman M.E.P. (2009). Positive predictors of teacher effectiveness. Journal of Positive Psychology, 4(6), 540–7. [Google Scholar]
- Eickhoff S.B., Laird A.R., Fox P.T., Bzdok D., Hensel L. (2016). Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex, 26(1), 304–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskreis-Winkler L., Shulman E.P., Beal S.A., Duckworth A.L. (2014). The grit effect: predicting retention in the military, the workplace, school and marriage. Frontiers in Psychology, 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C.E., Poore J.C., Krueger F., Barbey A.K., Solomon J., Grafman J. (2014). The role of executive function and the dorsolateral prefrontal cortex in the expression of neuroticism and conscientiousness. Social Neuroscience, 9(2), 139–51. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. (1988). The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Raven Press. [Google Scholar]
- Gamel M. (2014). Impact of Character Development and Empowerment Program on Grit and Resilience Growth in Early and Middle Adolescents Dissertations, Theses and Capstone Projects. Paper 646.Available: http://digitalcommons.kennesaw.edu/etd/646 [accessed December 1, 2014].
- Gottfredson L.S. (1997). Why g matters: the complexity of everyday life. Intelligence, 24(1), 79–132. [Google Scholar]
- Guerrero L.R., Dudovitz R., Chung P.J., Dosanjh K.K., Wong M.D. (2016). Grit: a potential protective factor against substance use and other risk behaviors among Latino adolescents. Academic Pediatrics, 16, 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.B., Liu F., Zhang J., et al. (2013). Dissociation of regional activity in the default mode network in first-episode, drug-naive major depressive disorder at rest. Journal of Affective Disorders, 151(3), 1097–101. [DOI] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. (2015). Association of child poverty, brain development, and academic achievement. JAMA Pediatrics, 169(9), 822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Wang J.H., Zhao Z.L., et al. (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage, 55(1), 287–95. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X.N., Butler P.D., et al. (2010). Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophrenia Research, 117(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T., Eaton K., Farah R., Hajinazarian A., Vannest J., Holland S.K. (2015). Predicting better performance on a college preparedness test from narrative comprehension at the age of 6 years: an fMRI study. Brain Research, 1629, 54–62. [DOI] [PubMed] [Google Scholar]
- Hu L.T., Bentler P.M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling-a Multidisciplinary Journal, 6(1), 1–55. [Google Scholar]
- Hu S., Chao H.H.A., Zhang S., Ide J.S., Li C.S.R. (2014). Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Structure and Function, 219(3), 983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Q., Ding J., Lu G., et al. (2011). Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLoS One, 6(9), e25159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain W., Perner J. (2003). Do children with ADHD not need their frontal lobes for theory of mind? A review of brain imaging and neuropsychological studies In: Bruner M., Ribbert H., Schiefenhovel W., editors. The Social Brain: Evolution and Pathology, 197–230. Chichester, UK: Wiley. [Google Scholar]
- Kelley W.M., Wagner D.D., Heatherton T.F. (2015). In search of a human self-regulation system. Annual Review of Neuroscience, 38, 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D.R., Matthews M.D., Bartone P.T. (2014). Grit and hardiness as predictors of performance among West Point cadets. Military Psychology, 26(4), 327–42. [Google Scholar]
- Kleiman E.M., Adams L.M., Kashdan T.B., Riskind J.H. (2013). Gratitude and grit indirectly reduce risk of suicidal ideations by enhancing meaning in life: evidence for a mediated moderation model. Journal of Research in Personality, 47(5), 539–46. [Google Scholar]
- Kline R.B. (2015). Principles and Practice of Structural Equation Modeling, 2nd edn New York: Guilford. [Google Scholar]
- Koechlin E., Corrado G., Pietrini P., Grafman J. (2000). Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proceedings of the National Academy of Sciences of the United States of America, 97(13), 7651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Hu S.Y., Wang X., Song Y.Y., Liu J. (2015). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. Neuroimage, 107, 136–45. [DOI] [PubMed] [Google Scholar]
- Kunisato Y., Okamoto Y., Okada G., et al. (2011). Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neuroscience Letters, 492(2), 109–13. [DOI] [PubMed] [Google Scholar]
- Li F., He N., Li Y.Y., et al. (2014). Intrinsic brain abnormalities in attention deficit hyperactivity disorder: a resting-state functional MR imaging study. Radiology, 272(2), 514–23. [DOI] [PubMed] [Google Scholar]
- Li J., Zhao Y., Kong F., Du S., Yang S., Wang S. (Forthcoming). Psychometric assessment of the Short Grit Scale among Chinese adolescents. Journal of Psychoeducational Assessment. [Google Scholar]
- Lin X., Jia X.Z., Zang Y.F., Dong G.H. (2015). Frequency-dependent changes in the amplitude of low-frequency fluctuations in internet gaming disorder. Frontiers in Psychology, 6, 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.H., Ma X., Wu X., et al. (2013). Resting-state brain activity in major depressive disorder patients and their siblings. Journal of Affective Disorders, 149(1–3), 299–306. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Pauls J., Augath M., Trinath T., Oeltermann A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843), 150–7. [DOI] [PubMed] [Google Scholar]
- Lu F.M., Huo Y.J., Li M.L., et al. (2014). Relationship between personality and gray matter volume in healthy young adults: a voxel-based morphometric study. Plos One, 9(2), e88763.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey A.P., Finn A.S., Leonard J.A., et al. (2015). Neuroanatomical correlates of the income-achievement gap. Psychological Science, 26(6), 925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi S.R., Matthews M.D., Kelly D.R., Villarreal B., White M. (2012). The role of hardiness and grit in predicting performance and retention of USMA cadets. Military Psychology, 24(1), 19–28. [Google Scholar]
- Mar R.A., Spreng R.N., DeYoung C.G. (2013). How to produce personality neuroscience research with high statistical power and low additional cost. Cognitive, Affective, & Behavioral Neuroscience, 13(3), 674–85. [DOI] [PubMed] [Google Scholar]
- Marcoulides G.A., Hershberger S.L. (1997). Multivariate Statistical Methods: A First Course. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- McClelland M.M., John Geldhof G., Cameron C.E., Wanless S.B. (2015). Development and self-regulation In: Overton W.F., Molenaar P.C., editors. Theory and Method, Vol. 1: Handbook of Child Psychology and Developmental Science, 7th edn, pp. 523–65. Hoboken, NJ: Wiley. [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Moshier S.J., Szuhany K.L., Hearon B.A., Smits J.A.J., Otto M.W. (2016). Anxiety sensitivity uniquely predicts exercise behaviors in young adults seeking to increase physical activity. Behavior Modification, 40(1–2), 178–98. [DOI] [PubMed] [Google Scholar]
- Muhlert N., Lawrence A.D. (2015). Brain structure correlates of emotion-based rash impulsivity. Neuroimage, 115, 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C.A., Wang C., Black J.M., Bugescu N., Hoeft F. (2016). The matter of motivation: striatal resting-state connectivity is dissociable between grit and growth mindset. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Orr C., Morioka R., Behan B., et al. (2013). Altered resting-state connectivity in adolescent cannabis users. American Journal of Drug and Alcohol Abuse, 39(6), 372–81. [DOI] [PubMed] [Google Scholar]
- Poropat A.E. (2009). A meta-analysis of the five-factor model of personality and academic performance. Psychological Bulletin, 135(2), 322–38. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–91. [DOI] [PubMed] [Google Scholar]
- Qiu C.J., Feng Y., Meng Y.J., et al. (2015). Analysis of altered baseline brain activity in drug-naive adult patients with social anxiety disorder using resting-state functional MRI. Psychiatry Investigation, 12(3), 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. (2010). Two views of brain function. Trends in Cognitive Science, 14(4), 180–90. [DOI] [PubMed] [Google Scholar]
- Raven J. (2000). The Raven's progressive matrices: change and stability over culture and time. Cognitive Psychology, 41(1), 1–48. [DOI] [PubMed] [Google Scholar]
- Reed J., Pritschet B.L., Cutton D.M. (2013). Grit, conscientiousness, and the transtheoretical model of change for exercise behavior. Journal of Health Psychology, 18(5), 612–9. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P.M., Segalowitz S.J., Carter C.S. (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–40. [DOI] [PubMed] [Google Scholar]
- Robertson-Kraft C., Duckworth A.L. (2014). True grit: trait-level perseverance and passion for long-term goals predicts effectiveness and retention among novice teachers. Teachers College Record, 116(3), http://www.tcrecord.org/Content.asp?ContentId=17352. [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., et al. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage, 60(1), 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A., de Water E., Mies G.W. (2013). The neural correlates of temporal reward discounting. Wiley Interdisciplinary Reviews-Cognitive Science, 4(5), 523–45. [DOI] [PubMed] [Google Scholar]
- Servaas M.N., Riese H., Ormel J., Aleman A. (2014). The neural correlates of worry in association with individual differences in neuroticism. Human Brain Mapping, 35(9), 4303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T., Burgess P. (1996). The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society B-Biological Sciences, 351(1346), 1405–11. [DOI] [PubMed] [Google Scholar]
- Sheridan Z., Boman P., Mergler A., Furlong M.J. (2015). Examining well-being, anxiety, and self-deception in university students. Cogent Psychology, 2(1), 993850–10. [Google Scholar]
- Song M., Zhou Y., Li J., et al. (2008). Brain spontaneous functional connectivity and intelligence. Neuroimage, 41(3), 1168–76. [DOI] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M.V., McIntosh A.R., Winocur G., Grady C.L. (2005). The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology, 19(2), 181–92. [DOI] [PubMed] [Google Scholar]
- Strayhorn T.L. (2014). What role does grit play in the academic success of black male collegians at predominantly White institutions? Journal of African American Studies, 18(1), 1–10. [Google Scholar]
- Stuss D.T., Knight R.T. (2013). Principles of Frontal Lobe Function. New York: Oxford University Press. [Google Scholar]
- Suzuki Y., Tamesue D., Asahi K., Ishikawa Y. (2015). Grit and work engagement: a cross-sectional study. PLoS One, 10(9), e0137501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski S.M., Knight R.T. (2014). Insights into human behavior from lesions to the prefrontal cortex. Neuron, 83(5), 1002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Y., Tang R.X., Posner M.I. (2013). Brief meditation training induces smoking reduction. Proceedings of the National Academy of Sciences of the United States of America, 110(34), 13971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J., Shima K., Mushiake H. (2007). Concept-based behavioral planning and the lateral prefrontal cortex. Trends in Cognitive Sciences, 11(12), 528–34. [DOI] [PubMed] [Google Scholar]
- Taren A.A., Venkatraman V., Huettel S.A. (2011). A parallel functional topography between medial and lateral prefrontal cortex: evidence and implications for cognitive control. Journal of Neuroscience, 31(13), 5026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I., Parker Jones O., Mars R.B., Smith S.M., Behrens T.E., Jbabdi S. (2016). Task-free MRI predicts individual differences in brain activity during task performance. Science, 352(6282), 216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett R.P., Jackson D.N., Rothstein M. (1991). Personality measures as predictors of job performance: a meta-analytic review. Personnel Psychology, 44(4), 703–42. [Google Scholar]
- Tian X., Wei D.T., Du X., et al. (2016). Assessment of trait anxiety and prediction of changes in state anxiety using functional brain imaging: a test-retest study. NeuroImage, 133, 408–16. [DOI] [PubMed] [Google Scholar]
- Tough P. (2011). What if the secret to success is failure. New York Times. [Google Scholar]
- van Dam W.O., Decker S.L., Durbin J.S., Vendemia J.M.C., Desai R.H. (2015). Resting state signatures of domain and demand-specific working memory performance. Neuroimage, 118, 174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V., Rosati A.G., Taren A.A., Huettel S.A. (2009). Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. Journal of Neuroscience, 29(42), 13158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yan C., Zhao C., et al. (2011). Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer's disease: a resting-state functional MRI study. Human Brain Mapping, 32(10), 1720–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters C.A., Hussain M. (2015). Investigating grit and its relations with college students’ self-regulated learning and academic achievement. Metacognition and Learning, 10(3), 293–311. [Google Scholar]
- Wright C.I., Feczko E., Dickerson B., Williams D. (2007). Neuroanatomical correlates of personality in the elderly. Neuroimage, 35(1), 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C.I., Williams D., Feczko E., et al. (2006). Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex, 16(12), 1809–19. [DOI] [PubMed] [Google Scholar]
- Xu K., Liu H., Li H.H., et al. (2014). Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. Journal of Affective Disorders, 152, 237–42. [DOI] [PubMed] [Google Scholar]
- Yan C., Zang Y. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wu Q.Z., Guo L.T., et al. (2011). Abnormal spontaneous brain activity in medication-naive ADHD children: a resting state fMRI study. Neuroscience Letters, 502(2), 89–93. [DOI] [PubMed] [Google Scholar]
- Zang Y.F., He Y., Zhu C.Z., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Development, 29(2), 83–91. [DOI] [PubMed] [Google Scholar]
- Zhang L.F. (2003). Does the big five predict learning approaches? Personality and Individual Differences, 34(8), 1431–46. [Google Scholar]
- Zhang L.F., Huang J.F. (2001). Thinking styles and the five-factor model of personality. European Journal of Personality, 15(6), 465–76. [Google Scholar]
- Zhou Y.X., Lui Y.W., Zuo X.N., et al. (2014). Characterization of thalamo-cortical association using amplitude and connectivity of functional MRI in mild traumatic brain injury. Journal of Magnetic Resonance Imaging, 39(6), 1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.H., Zhu C.Z., Yang Y.H., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C., et al. (2010). The oscillating brain: complex and reliable. Neuroimage, 49(2), 1432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]