Abstract
The ability to voluntarily regulate our emotional response to threatening and highly arousing stimuli by using cognitive reappraisal strategies is essential for our mental and physical well-being. This might be achieved by prefrontal brain regions (e.g. inferior frontal gyrus, IFG) down-regulating activity in the amygdala. It is unknown, to which degree effective connectivity within the emotion-regulation network is linked to individual differences in reappraisal skills. Using psychophysiological interaction analyses of functional magnetic resonance imaging data, we examined changes in inter-regional connectivity between the amygdala and IFG with other brain regions during reappraisal of emotional responses and used emotion regulation success as an explicit regressor. During down-regulation of emotion, reappraisal success correlated with effective connectivity between IFG with dorsolateral, dorsomedial and ventromedial prefrontal cortex (PFC). During up-regulation of emotion, effective coupling between IFG with anterior cingulate cortex, dorsomedial and ventromedial PFC as well as the amygdala correlated with reappraisal success. Activity in the amygdala covaried with activity in lateral and medial prefrontal regions during the up-regulation of emotion and correlated with reappraisal success. These results suggest that successful reappraisal is linked to changes in effective connectivity between two systems, prefrontal cognitive control regions and regions crucially involved in emotional evaluation.
Keywords: PPI, fMRI, ventrolateral prefrontal cortex, reappraisal
Introduction
The ability to regulate our emotions is essential for our mental and physical well-being (Gross and Muñoz, 1995; Gross et al., 2006; Eftekhari et al., 2009; Berking and Wupperman, 2012). Impairment in self-regulating emotions plays a role in psychological disorders such as anxiety, major depression and several personality disorders (Davidson, 2000; Amstadter, 2008; Cisler et al., 2010; Gruber et al., 2012; Krause-Utz et al., 2014). One well-studied emotion regulation strategy is reappraisal, which refers to the ability to cognitively change the appraisal of an emotional situation by altering its emotional impact (Gross and Thompson, 2007). Emotion regulation relies on basal cognitive functions such as working memory, attention, self-reflection, semantic memory and language (Ochsner and Gross, 2005; Zelazo and Cunningham, 2007; Phillips et al., 2008), which are represented in a widespread neural network (Kalisch, 2009; Ochsner et al., 2012; Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014; Morawetz et al., 2016b).
Previous research into emotion regulation has mainly used standard correlation analysis to investigate the association between activation in the cortical control network and the subcortical affective system and converged on a top-down model whereby neural responses to emotional stimuli in the amygdala and ventral striatum are down-regulated by prefrontal regions (Ochsner and Gross, 2005; Urry et al., 2006; Johnstone et al., 2007; Phillips et al., 2008; Wager et al., 2008; Ochsner et al., 2012). For example, using functional magnetic resonance imaging (fMRI), an increase in activation in the ventrolateral (Ochsner et al., 2002), orbitofrontal (Ochsner et al., 2004) and ventromedial prefrontal cortex (VMPFC) (Urry et al., 2006) has been found to correlate with decreased amygdala activity during emotion regulation. Only few studies, however, have directly measured changes in effective connectivity during reappraisal. These further yielded inconsistent results regarding patterns of connectivity as well as proposed directions in connectivity changes (Banks et al., 2007; Kanske et al., 2011; Payer et al., 2012; Sripada et al., 2014). One possible explanation for these inconsistencies is that connectivity patterns might directly relate to individuals’ reappraisal ability (i.e. regulation success), which has not been considered in most studies. Only two studies assessed emotion regulation success using behavioral self-report, but not in conjunction with effective connectivity. Using mediation analysis, one of these studies found that both ventrolateral prefrontal cortex (VLPFC) activity and reappraisal success were mediated by activity in the ventral striatum (Wager et al., 2008). The second study showed that activity in the left amygdala correlated with self-reported reappraisal success (Eippert et al., 2007).
We recently used dynamic causal modeling (DCM) to investigate effective connectivity during emotion regulation and demonstrated the importance of left IFG in emotion regulation (Morawetz et al., 2016c). In particular, bidirectional changes in connectivity strength between the IFG and dorsolateral prefrontal cortex (DLPFC) were found to be the neural substrate of a feedback mechanism mediating reappraisal. However, as amygdala activity could not be consistently observed in each participant—which is a prerequisite for regions to be included into the DCM model space (Friston et al., 2003; Lohmann et al., 2012)—the amygdala could not be included in the analysis (Morawetz et al., 2016c). Given that previous studies emphasized the importance of the amygdala, we aimed to expand on our findings by explicitly investigating how patterns of effective connectivity change during reappraisal in a broader network of brain regions involved in emotion regulation (including cortical and subcortical seed regions), and how these changes are linked to individuals’ reappraisal success. We hypothesized that the IFG is involved in linking the prefrontal control system to regions that are directly related to basal emotion processing (such as the amygdala) in order to orchestrate emotion regulatory processes. Specifically, this study examined within-subject effective connectivity (Friston et al., 1997; Friston, 2011) of the IFG and the amygdala and its association with reappraisal success using fMRI and psychophysiological interaction (PPI) analysis.
However, emotional responses not only involve changes in the brain, but also in autonomic physiology. Previous studies investigating spontaneous reactions to unpleasant pictures reported increased electrodermal activity (EDA) (Bradley et al., 2008). Furthermore, reappraisal processes have been demonstrated to modulate skin conductance responses (SCRs); i.e. EDA was higher when increasing and lower when decreasing compared with maintaining emotional responses thereby indicating a modulation of emotional arousal by reappraisal (Eippert et al., 2007; Urry et al., 2009; Morawetz et al., 2016b,c). Thus, we also recorded EDA to obtain a physiological control measure, which was expected to mirror self-reports of emotional state after emotion regulation.
Our participants were presented with highly arousing and aversive pictures while using cognitive reappraisal to increase, maintain, or decrease their emotional responses. Based on previous studies showing the involvement of the left amygdala and VLPFC in successful reappraisal (Eippert et al., 2007; Wager et al., 2008; Morawetz et al., 2016b), we hypothesized task-dependent inter-regional covariance between the IFG and the amygdala with other brain regions that are crucially involved in emotion regulation. In addition, we were interested in how effective coupling might be dependent on reappraisal success. Since previous results were inconsistent, and no study has used cortical seed regions or included up-regulation of emotion in the experimental design, hypotheses regarding the interaction of effective coupling and regulation skills were rather unspecific. However, there are neuroanatomical studies, which demonstrated interconnections within the prefrontal cortices (Barbas and Pandya, 1989, 1991; Barbas, 2000, 2009; Goulas et al., 2012; Yeterian et al., 2012) and reciprocal connections between the amygdala and PFC (Amaral and Price, 1984; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). Prior neuroimaging studies also demonstrated that VLPFC and DLPFC are intrinsically connected during resting state and effectively coupled during emotion regulation (e.g. Goulas et al., 2012; Morawetz et al., 2016c), and similar results have been obtained for amygdala and frontal regions (e.g. Banks et al., 2007; Kanske et al., 2011; Ray and Zald, 2011). Based on this, we expected that (i) enhanced connectivity within the prefrontal network including lateral (VLPFC, DLPFC) and medial PFC regions (ventromedial and dorsomedial PFC; VMPFC and DMPFC) would correlate with reappraisal success, and (ii) enhanced coupling between the amygdala and PFC would predict reappraisal success.
Material and methods
Subjects
Twenty-three right-handed subjects (mean age = 25.70 years, s.d. = 5.95 years; 12 female) participated in the study. Handedness was assessed with Edinburgh-Handedness Inventory (Oldfield, 1971), and eligibility was assessed with a general health questionnaire and fMRI safety screening form. Subjects had normal or corrected to normal vision, gave written, informed consent to participate in the study and had no history of neurological or psychiatric disease. The study was approved by the ethics committee of the German Psychological Society and carried out in accordance to the Declaration of Helsinki.
Stimuli
Stimuli consisted of 126 aversive and 42 neutral pictures from the International Affective Picture System (IAPS) (Bradley and Lang, 2007). During the fMRI experiment, images were presented in the centre of the screen with an 800 × 600 pixel display subtending 32° × 24° visual angle on dual display goggles (VisuaStim, MR Research, USA) using the stimulation software Presentation (Version 14.1, Neurobehavioral Systems, USA). Pictures subtended a 24° × 18° visual angle, presented against a black background.
Task
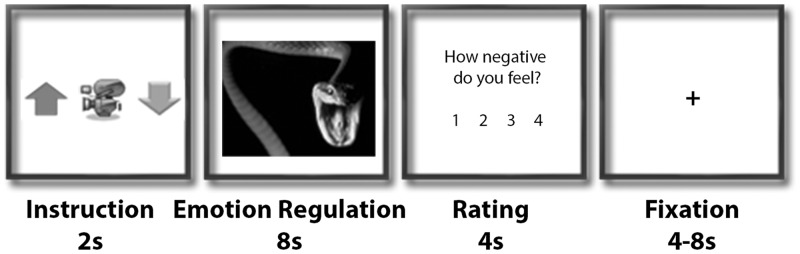
The task design was adapted from previous studies (Kim and Hamann, 2007; Morawetz et al., 2016b). Four task conditions were implemented (Figure 1): two reappraisal conditions (‘Increase, Decrease’) and two control conditions (‘Look-Neutral, Look-Negative’). (i) In the ‘Increase’ condition, subjects were asked to engage themselves with the depicted situation and to increase their sense of subjective closeness to the displayed events by e.g. imaging a close friend/family member in the situation depicted in the picture (Ochsner et al., 2004; Eippert et al., 2007; Urry et al., 2009). (ii) Conversely, in the ‘Decrease’ condition subjects were instructed to reduce the intensity of the negative emotion by distancing themselves from the image by becoming a detached observer e.g. through thinking that the depicted situation is not real. (iii) In the ‘Look’ condition, subjects were asked to view the neutral (‘Look-Neutral’) or negative (‘Look-Negative’) stimuli attentively and allow themselves to experience/feel any emotional responses, which these might elicit without trying to manipulate them. Subjects received a training session to practice the emotion reappraisal strategies before scanning.
Fig. 1.
Task Design. Each trial started with an instruction screen of 2 s, showing a cue indicating the experimental condition: Red arrow pointing upwards indicated Increase, video camera indicated Look and green arrow pointing downwards indicated Decrease. The instruction was followed by the presentation of an IAPS picture for 8 s, during which subjects were asked to either up-regulate (Increase), down-regulate (Decrease) their emotions or not modulate their emotions at all (Look-Neutral, Look-Negative). After this regulation phase, subjects rated their current emotional state on a scale from 1 to 4 within 4 s. Each trial ended with a jittered fixation phase of 4–8 s.
Pictures were presented in an event-related design (Figure 1). Each trial started with an instruction screen (2 s) showing a symbol indicating one of the experimental conditions (camera symbol: ‘Look’; red arrow pointing upwards: ‘Increase’; green arrow pointing downwards: ‘Decrease’). Subsequently, a picture was presented (8 s), followed by a rating of the current emotional state (4 s) (four-point Likert scale from 1 to 4; less negative to very negative). Subjects indicated their affect by pressing a button on a button fiber optic response pad (Cambridge Research Systems Ltd., England). Finally, a fixation cross presented in the centre of the screen (4–8 s) concluded the trial. One experimental run consisted of 28 trials (7 trials per condition). Each experimental session consisted of six runs (168 trials in total). Experimental conditions were randomized within runs.
Electrodermal activity
Previous research demonstrated that EDA is modulated by emotional arousal and emotion regulation (Urry et al., 2009). We recorded EDA using two cup electrodes with an internal impedance of 15 kΩ (7 mm) filled with isotonic paste and attached to the proximal phalanges of the index and middle fingers on the left hand. EDA was acquired at a sampling rate of 5000 Hz using an MR-compatible amplifier system (BrainAmp GSR-module, Brain Products, Gliching, Germany) and constant voltage electrode excitation. During the analysis, the data was down-sampled offline to 10 Hz. We then decomposed skin-conductance data into continuous tonic and phasic activity (Benedek and Kaernbach, 2010a) and averaged across trials within each condition applying an 8 s time window using Ledalab Version 3.3.1 (Benedek and Kaernbach, 2010b). SCRs were defined as a deflection of at least 0.01 µS occurring 1–8 s after stimulus onset. Only runs including more than 10% SCRs exceeding the above criterion were used for analysis. Values for phasic SCRs were extracted as the difference between a local minimum and the succeeding local maximum within the response window. Due to technical problems at recording, data from seven subjects could not be analyzed, leaving a total of n = 16 EDA data sets.
Analysis of self-report data
As the main focus of our study was successful emotion regulation, we calculated reappraisal success scores based on the affect ratings acquired after each trial. Overall reappraisal success was defined as either the mean decrease or mean increase in reported emotion when applying a cognitive reappraisal strategy (‘Increase’ and ‘Decrease’) relative to the mean affect ratings of the control condition (‘Look-Negative’), the latter representing the ‘natural’ emotional response to the stimuli. The ‘Look-Neutral’ condition served mainly as a break in the task design in which no emotions were elicited. Thus, reappraisal success scores for ‘Increase’ (‘Increase’ minus ‘Look-Negative’) and ‘Decrease’ (‘Decrease’ minus ‘Look-Negative’) for each participant were used as a predictor in the PPI analysis.
fMRI data acquisition
Whole brain functional and anatomical images were acquired using a 3.0 T Magnetom TrioTim MRI scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. A high-resolution 3D T1-weighted dataset was acquired for each subject (176 sagittal sections, 1 × 1 × 1 mm³; 256 × 256 data acquisition matrix). Functional images were acquired using a T2*-weighted, gradient-echo planar imaging pulse sequence recording 37 sections oriented parallel to the anterior and posterior commissure at an in-plane resolution of 3 × 3 × 3mm³ (interslice gap = 0; TE = 30 ms; TR = 2 s; FA = 90°; FoV = 192 × 192 mm2; 64 × 64 data acquisition matrix). For each experimental run 285 whole brain volumes were recorded.
fMRI data analysis
Data were analyzed using a random effects general linear model (GLM) as implemented in BrainVoyager QX 2.3.1 (Brain Innovation, Maastricht, The Netherlands). Pre-processing of fMRI data included 3D-motion-correction, temporal high pass filtering (three cycles/run), linear trend removal, slice scan time correction, spatial smoothing (Gaussian smoothing kernel, 8 mm full width half maximum), and transformation into Talairach space (Talairach and Tournoux, 1988).
Data were visualized and statistically thresholded using NeuroElf Version 0.9c (neuroelf.net). We utilized height and cluster size thresholding after establishing family wise error (FWE) thresholds using the alphasim procedure (Forman et al., 1995) at an initial significance level of P < 0.001 and an FWE cluster level correction of P < 0.05 (implemented in NeuroElf, http://neuroelf.net). AlphaSim was computed for each map separately so as to accurately estimate the inherent smoothness of each contrast, rather than to rely on the size of the Gaussian kernel used during pre-processing which can lead to errors in thresholding (Bennett et al., 2009). Extent thresholds for the contrasts of the univariate analysis were thresholded at 95 voxels and PPI maps were thresholded at 91 voxels.
Univariate analysis. Separate regressors in the GLM were specified for the instruction cue, rating, and the conditions during stimulus viewing. The regressors-of-interest were the four task conditions ‘Increase, Decrease, Look-Negative and Look-Neutral’. The ‘Look-Negative’ condition constituted the natural response to all images and was used as a control condition. As described below, emotion regulation contrasts were defined as ‘differences’ to this control condition.
In line with our a priori hypotheses, we used the left IFG and amygdala as regions of interest (ROIs). The left IFG and the amygdala were chosen as ROIs and seed regions for the PPI analyses on the basis of the following three criteria: (i) Prior evidence of their functional involvement in emotion regulation tasks: Several meta-analyses have implicated both regions in emotion regulation (Kalisch, 2009; Diekhof et al., 2011; Ochsner et al., 2012; Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014; Messina et al., 2015). Amygdala activity has been associated with emotion processing, emotional reactivity and the evaluation of emotional stimuli (Phan et al., 2002; Zald, 2003 ; Sergerie et al., 2008; Ray and Zald, 2012). The VLPFC is known to be implicated in emotion regulation processes such as in the semantic description of emotional states and in response selection/inhibition (Phillips et al., 2008; Ochsner et al., 2012; Ray and Zald, 2012). Furthermore, recent meta-analyses support the view that the VLPFC/IFG is involved in language and semantic processes during emotion regulation (Kohn et al., 2014; Messina et al., 2015). In line with these findings, one of our previous studies indicated a key role of the left IFG within the emotion regulation network (Morawetz et al., 2016a). (i) Prior evidence for an association between reappraisal success and brain activity in those regions: Increased activity in left IFG and the amygdala have been associated with reappraisal success (Eippert et al., 2007; Morawetz et al., 2016a,b; Wager et al., 2008). (ii) Evidence in our own fMRI study for task effects in those regions: Our whole-brain analysis revealed significant task-related effects of emotion regulation in the left IFG, but not within the amygdala. Thus, based on the contrast (‘Increase + Decrease > Look-Negative’), we functionally determined the left IFG, which was subsequently used as seed region (left IFG: −45, 26, 7; 5022 mm3), while the amygdala was defined purely anatomically using the Anatomy Toolbox (Eickhoff et al., 2005) (left amygdala: −18, −4, −16; 1546 mm3; right amygdala: 22, −3, −15, 1424 mm3). Furthermore, we performed an ROI analysis of the bilateral amygdala to demonstrate reappraisal-related activity (see ‘Results’ section and Supplementary Figure S1).
PPI analysis.To compare changes in effective coupling between different brain regions (physiological component) during reappraisal and passive looking (psychological component) we performed an analysis of PPI (Friston et al., 1997) using NeuroElf.
Our PPI analysis was based on the two ROI regions, the left IFG and the left amygdala (see above). Time-series from the left amygdala and left IFG were extracted as physiological seeds by averaging the neural activity within single-subject ROI masks, and the regulation contrasts (‘Increase > Look-Negative’) and (‘Decrease > Look-Negative’) were used as psychological context, to create the PPI term. This interaction term was entered into a voxelwise regression, with the covariates of amygdala and IFG raw time-series and all original regressors of the GLM model (Instruction, Rating, ‘Increase, Decrease, Look-Negative, Look-Neutral’). The resulting PPI parameter estimates denoted the change in strength of effective coupling between the seed regions and the remainder of the brain during reappraisal relative to ‘Look-Negative’ trials (see Supplementary Tables S1 and S2). To examine the extent to which individual differences in reappraisal ability predicted this connectivity, reappraisal success scores were entered as a covariate into a voxelwise regression, serving as a predictor of the PPI map.
Results
Behavioral results
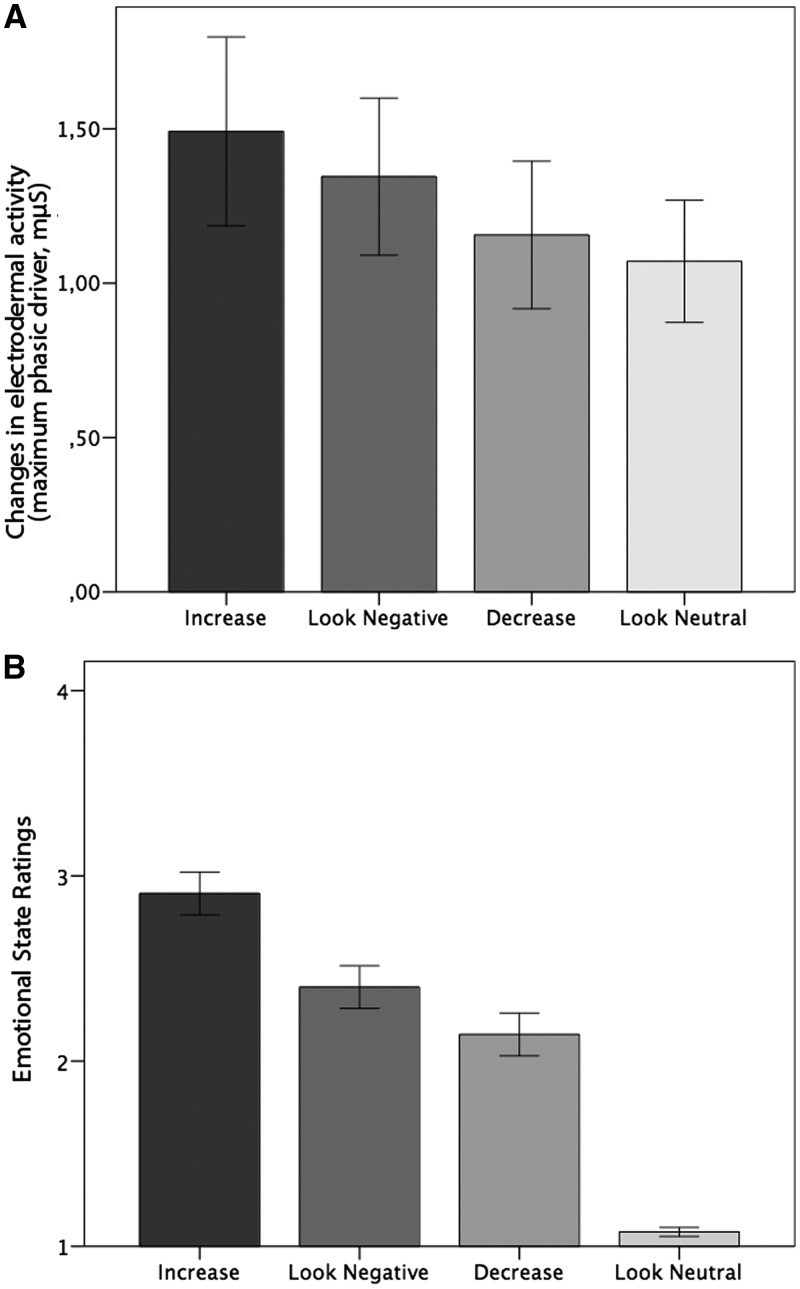
Emotion induction. Skin conductance data provided support for the success of the emotion induction (Figure 2A). We found a significant main effect of task [F(1,15) = 3.80, P < 0.01]. Post-hoc t-tests indicated that SCRs during ‘Increase’ were higher compared with ‘Decrease’ [t(15) = 2.62, P < 0.05] and ‘Look-Neutral’ [t(15) = 2.78, P < 0.05]. The SCR amplitudes were significantly higher during the ‘Look-Negative’ compared with the ‘Look-Neutral’ condition [t(15) = 2.35, P < 0.05].
Fig. 2.
(A) SCRs during scanning. Mean changes in EDA (μS) as a function of task conditions. (B) Emotional state ratings as a function of task conditions. After each emotion regulation phase subjects rated their current emotional state on a scale from 1 (not negative) to 4 (very negative). Error bars represent standard errors.
Emotion regulation. A significant main effect of task was found [F(1,22) = 50.92, P < 0.001] (Figure 2B). Post-hoc t-tests revealed significantly more negative emotional state ratings for ‘Increase’ compared with ‘Decrease [t(22) = 9.25, P < 0.001], as well as for ‘Increase’ compared with ‘Look-Negative’ [t(22) = 6.13, P < 0.001] and ‘Look-Neutral’ [t(22) = 16.24, P < 0.001’. ‘Look-Negative’ also resulted in more negative emotional state ratings compared with ‘Decrease’ [t(22) = 3.98, P < 0.01] and compared with ‘Look-Neutral’ [t(22) = 12.20, P < 0.001]. ‘Decrease’ was associated with higher emotional state ratings as ‘Look-Neutral’ [t(22) = 9.88, P < 0.001]. These results confirm that emotion regulation was successful.
fMRI data
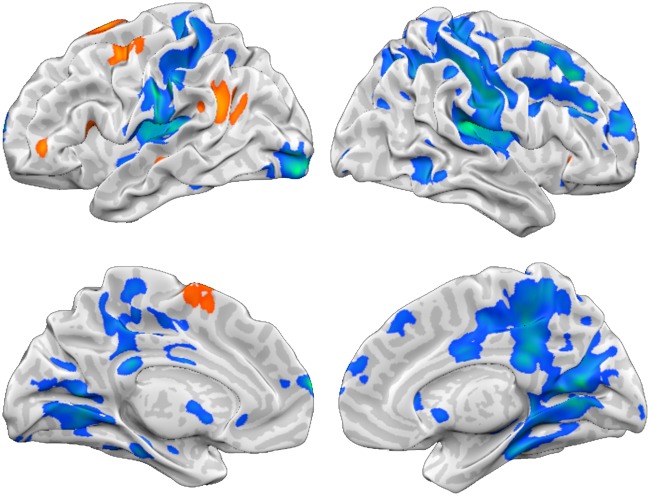
General effects of reappraisal. First, we tested for reappraisal effects independent of reappraisal goals by contrasting [‘Increase + Decrease > Look-Negative’] (Figure 3, Table 1). Reappraisal was associated with increased responses in prefrontal regions including superior frontal (SFG), medial frontal (medFG), middle frontal (MFG) and IFG as well as superior temporal gyrus (STG).
Fig. 3.
Reappraisal-related activation. Reappraisal (Increase + Decrease) vs Look-Negative (P < 0.05 FEW corrected). Orange-yellow indicates stronger activity for Increase and Decrease. Blue-green indicates enhanced activity for Look-Negative.
Table 1.
Effects of reappraisal
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | Voxels | t-value |
| ‘Increase+Decrease>Look Negative’ | |||||||
| Superior Frontal Gyrus* | 6 | LH | −15 | 11 | 70 | 197 | 4.11 |
| SFG | 6 | LH | −15 | 11 | 70 | LM | 4.11 |
| medFG | 6 | LH | −9 | 2 | 61 | LM | 2.96 |
| STG | 39 | LH | −45 | −52 | 28 | 234 | 3.96 |
| MFG | 6 | LH | −39 | −1 | 64 | 162 | 3.93 |
| IFG | 13 | LH | −45 | 26 | 7 | 186 | 3.75 |
| IFG | 13 | LH | −45 | 26 | 7 | LM | 3.75 |
| ‘Look Negative > Increase + Decrease’ | |||||||
| medFG | 10 | LH | −6 | 65 | 16 | 11306 | 6.10 |
| Insula | 13 | LH | −45 | −13 | 7 | LM | 5.95 |
| Post-central gyrus | 2 | LH | −57 | −22 | 31 | LM | 5.61 |
| Transverse temporal gyrus | 41 | LH | −42 | −31 | 13 | LM | 5.29 |
| SFG | 9 | RH | 42 | 35 | 25 | LM | 5.27 |
| Posterior cingulate | 30 | RH | 12 | −61 | 10 | LM | 5.25 |
| MFG | 8 | RH | 24 | 11 | 40 | LM | 5.25 |
| Post-central gyrus | 40 | LH | −63 | −19 | 19 | LM | 5.23 |
| Insula | 13 | LH | −27 | −28 | 16 | LM | 5.19 |
| Insula | 13 | RH | 33 | −22 | 19 | LM | 5.06 |
| Precuneus | 7 | RH | 24 | −61 | 34 | LM | 4.98 |
| Post-central gyrus | 2 | RH | 51 | −28 | 40 | LM | 4.96 |
| medFG | 8 | RH | 18 | 29 | 43 | LM | 4.95 |
| Pre-central gyrus | 6 | RH | 48 | −10 | 22 | LM | 4.84 |
| Inferior parietal lobule | 40 | RH | 36 | −34 | 46 | LM | 4.79 |
| Inferior parietal lobule | 40 | RH | 54 | −28 | 22 | LM | 4.51 |
| MFG | 6 | RH | 27 | 14 | 61 | LM | 4.32 |
| Lingual gyrus | 19 | LH | −15 | −61 | 4 | LM | 4.29 |
| Lentiform nucleus | RH | 33 | −13 | 4 | LM | 4.29 | |
| medFG | 6 | RH | 15 | −13 | 49 | LM | 4.15 |
| Paracentral lobule | 5 | RH | 12 | −37 | 46 | LM | 4.15 |
| SFG | 10 | RH | 27 | 53 | 16 | LM | 4.09 |
| Cingulate gyrus | 31 | RH | 3 | −37 | 31 | LM | 4.08 |
| Paracentral lobule | 4 | RH | 9 | −37 | 58 | LM | 3.83 |
| SFG | 10 | RH | 9 | 68 | 13 | LM | 3.82 |
| Post-central gyrus | 2 | LH | −24 | −34 | 67 | LM | 3.80 |
| Cingulate gyrus | 24 | LH | 0 | −4 | 31 | LM | 3.73 |
| Inferior temporal gyrus | 20 | RH | 54 | −46 | −11 | LM | 3.68 |
| Caudate | LH | −6 | 8 | −2 | LM | 3.55 | |
| Superior parietal lobule | 7 | LH | −27 | −52 | 43 | LM | 3.53 |
| Precuneus | 31 | LH | −24 | −67 | 22 | LM | 3.50 |
| Lentiform nucleus | RH | 24 | −1 | −2 | LM | 3.46 | |
| Post-central gyrus | 3 | RH | 30 | −31 | 61 | LM | 3.37 |
| Inferior parietal lobule | 40 | LH | −42 | −37 | 40 | LM | 3.36 |
| Post-central gyrus | 43 | RH | 66 | −13 | 16 | LM | 3.31 |
| medFG | 9 | RH | 15 | 41 | 19 | LM | 3.29 |
| Parahippocampal gyrus | 28 | RH | 27 | −22 | −5 | LM | 3.28 |
| Sub−gyral | 40 | LH | −21 | −34 | 55 | LM | 3.26 |
| Fusiform gyrus | 37 | LH | −51 | −43 | −17 | LM | 3.22 |
| Cingulate gyrus | 24 | RH | 9 | −13 | 40 | LM | 3.21 |
| Pre-central gyrus | 9 | RH | 45 | 17 | 34 | LM | 3.21 |
| Insula | 13 | LH | −39 | −1 | −8 | LM | 3.20 |
| Fusiform gyrus | 37 | LH | −39 | −58 | −8 | LM | 3.16 |
| Middle temporal gyrus | 19 | RH | 33 | −61 | 10 | LM | 3.16 |
| Caudate | RH | 15 | 23 | 7 | LM | 3.06 | |
| Middle temporal gyrus | 21 | RH | 66 | −7 | −2 | LM | 3.01 |
| Caudate | RH | 9 | 11 | −2 | LM | 3.00 | |
| STG | 22 | LH | −66 | −19 | 1 | LM | 2.96 |
| Cingulate gyrus | 31 | LH | −15 | −25 | 34 | LM | 2.91 |
| medFG | 6 | LH | −9 | −16 | 46 | LM | 2.89 |
| medFG | 9 | RH | 6 | 50 | 25 | LM | 2.87 |
| Cingulate gyrus | 24 | LH | −12 | −7 | 46 | LM | 2.61 |
| Cuneus | 18 | LH | −18 | −79 | 22 | LM | 2.60 |
| medFG | 10 | LH | −9 | 47 | 13 | LM | 2.47 |
| Precuneus | 7 | RH | 6 | −58 | 37 | LM | 2.46 |
| Middle occipital gyrus | 19 | RH | 30 | −79 | 13 | LM | 2.32 |
| Thalamus | RH | 15 | −22 | 4 | LM | 2.28 | |
| Inferior occipital gyrus | 18 | LH | −33 | −97 | −11 | 379 | 5.57 |
| Parahippocampal gyrus | 36 | LH | −33 | −28 | −17 | 131 | 4.22 |
LM, local maxima.
The contrast ‘Decrease’ compared with ‘Look-Negative’ revealed increased activity in left IFG, SFG, and STG (Table 2). The contrast between ‘Increase’ and ‘Look-Negative’ revealed enhanced responses in left MFG, SFG, IFG, STG and pre-central gyrus (Table 3).
Table 2.
Effects of down-regulation of emotion
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | Voxels | t-value |
| ‘Decrease>Look Negative’ | |||||||
| IFG | 45 | LH | −45 | 23 | 10 | 164 | 3.94 |
| IFG | 45 | LH | −45 | 38 | 1 | LM | 2.71 |
| SFG | 6 | LH | −12 | 11 | 67 | 145 | 3.79 |
| SFG | 6 | LH | −9 | −1 | 73 | LM | 2.65 |
| STG | 22 | LH | −51 | −55 | 19 | 161 | 3.61 |
| ‘Look Negative >Decrease’ | |||||||
| medFG | 10 | LH | −6 | 65 | 16 | 12716 | 7.35 |
| Inferior occipital gyrus | 18 | LH | −33 | −97 | −11 | LM | 6.13 |
| Insula | 13 | RH | 33 | −25 | 19 | LM | 6.03 |
| Pre-central gyrus | 6 | RH | 48 | −10 | 25 | LM | 5.82 |
| post-central gyrus | 40 | LH | −63 | −22 | 19 | LM | 5.70 |
| Inferior parietal lobule | 40 | RH | 57 | −28 | 22 | LM | 5.48 |
| Caudate | LH | −9 | 20 | 13 | LM | 5.46 | |
| Post-central gyrus | 2 | LH | −57 | −22 | 31 | LM | 5.23 |
| Insula | 13 | LH | −30 | −31 | 16 | LM | 5.19 |
| Post-central gyrus | 2 | RH | 36 | −25 | 31 | LM | 5.11 |
| Insula | 41 | LH | −45 | −25 | 16 | LM | 4.86 |
| Post-central gyrus | 2 | LH | −24 | −37 | 67 | LM | 4.71 |
| Cuneus | 23 | RH | 15 | −70 | 10 | LM | 4.57 |
| Posterior cingulate | 30 | LH | −12 | −61 | 13 | LM | 4.56 |
| Cingulate gyrus | 31 | LH | −21 | −40 | 37 | LM | 4.51 |
| Caudate | LH | −3 | 11 | 7 | LM | 4.50 | |
| Cingulate gyrus | 32 | RH | 18 | 20 | 31 | LM | 4.47 |
| Caudate | RH | 36 | −34 | −5 | LM | 4.40 | |
| Cingulate gyrus | 24 | RH | 18 | 8 | 28 | LM | 4.29 |
| Posterior cingulated | 30 | LH | −21 | −52 | 10 | LM | 4.29 |
| medFG | 6 | LH | −12 | −7 | 49 | LM | 4.19 |
| Posterior cingulate | 30 | RH | 30 | −61 | 10 | LM | 4.16 |
| Caudate | RH | 15 | 26 | 7 | LM | 4.15 | |
| Parahippocampal gyrus | 27 | RH | 9 | −37 | 4 | LM | 4.12 |
| Precuneus | 7 | RH | 24 | −61 | 34 | LM | 4.09 |
| MFG | 8 | RH | 24 | 20 | 43 | LM | 4.07 |
| Insula | 13 | LH | −45 | −7 | 10 | LM | 4.05 |
| Paracentral lobule | 5 | LH | −6 | −37 | 49 | LM | 4.02 |
| Cingulate gyrus | 31 | LH | −15 | −25 | 37 | LM | 4.01 |
| Inferior parietal lobule | 40 | RH | 36 | −34 | 46 | LM | 3.97 |
| SFG | 9 | RH | 39 | 35 | 25 | LM | 3.97 |
| medFG | 6 | RH | 12 | −13 | 49 | LM | 3.89 |
| Post-central gyrus | 2 | RH | 51 | −25 | 40 | LM | 3.88 |
| Parahippocampal gyrus | 36 | LH | −36 | −28 | −17 | LM | 3.88 |
| Post-central gyrus | 5 | RH | 27 | −40 | 64 | LM | 3.88 |
| Cingulate gyrus | 31 | RH | 15 | −22 | 37 | LM | 3.88 |
| Parahippocampal gyrus | 35 | LH | −24 | −25 | −23 | LM | 3.84 |
| Insula | 13 | LH | −39 | −1 | −8 | LM | 3.81 |
| Cingulate gyrus | 31 | LH | 0 | −43 | 34 | LM | 3.79 |
| Post-central gyrus | 3 | LH | −9 | −34 | 64 | LM | 3.74 |
| Paracentral lobule | 4 | RH | 9 | −37 | 58 | LM | 3.72 |
| Anterior cingulate | 32 | LH | −3 | 47 | 1 | LM | 3.71 |
| Precuneus | 7 | RH | 9 | −34 | 43 | LM | 3.71 |
| Post-central gyrus | 7 | RH | 6 | −52 | 67 | LM | 3.66 |
| Pre-central gyrus | 4 | RH | 18 | −28 | 64 | LM | 3.66 |
| Parahippocampal gyrus | 30 | RH | 21 | −49 | 10 | LM | 3.66 |
| medFG | 10 | RH | 15 | 47 | 13 | LM | 3.55 |
| Cuneus | 18 | RH | 15 | −100 | −2 | LM | 3.51 |
| Inferior Parietal Lobule | 40 | LH | −45 | −34 | 37 | LM | 3.48 |
| Precuneus | 19 | LH | −30 | −67 | 31 | LM | 3.46 |
| Cingulate Gyrus | 32 | RH | 24 | 8 | 43 | LM | 3.46 |
| Cingulate Gyrus | 31 | RH | 27 | −49 | 25 | LM | 3.43 |
| Middle Occipital Gyrus | 18 | LH | −27 | −79 | 1 | LM | 3.41 |
| Caudate | RH | 33 | −43 | 10 | LM | 3.39 | |
| Posterior cingulate | 29 | RH | 3 | −52 | 13 | LM | 3.38 |
| Lentiform nucleus | RH | 21 | −1 | −2 | LM | 3.37 | |
| Pre-central gyrus | 6 | LH | −57 | −1 | 10 | LM | 3.23 |
| Middle occipital gyrus | 18 | RH | 33 | −94 | 1 | LM | 3.17 |
| Lingual gyrus | 17 | RH | 12 | −97 | −14 | LM | 3.13 |
| Cingulate gyrus | 24 | LH | 0 | −4 | 31 | LM | 3.06 |
| Cingulate gyrus | 24 | LH | −15 | 5 | 31 | LM | 2.95 |
| Caudate | LH | −24 | −10 | 25 | LM | 2.91 | |
| Cuneus | 18 | RH | 3 | −85 | 19 | LM | 2.83 |
| Inferior occipital gyrus | 17 | RH | 27 | −100 | −11 | LM | 2.69 |
| medFG | 9 | RH | 6 | 47 | 28 | LM | 2.69 |
| Fusiform gyrus | 19 | RH | 27 | −61 | −8 | LM | 2.65 |
| MFG | 6 | RH | 27 | 14 | 61 | LM | 2.62 |
| Precuneus | 7 | LH | −6 | −64 | 64 | LM | 2.60 |
| MFG | 10 | RH | 48 | 50 | 13 | LM | 2.57 |
| Precuneus | 7 | RH | 27 | −58 | 49 | LM | 2.54 |
| Parahippocampal gyrus | 28 | RH | 15 | −10 | −11 | LM | 2.53 |
| Parahippocampal gyrus | 19 | RH | 24 | −52 | −5 | LM | 2.49 |
| fusiform gyrus | 19 | LH | −24 | −64 | −8 | LM | 2.46 |
| Precuneus | 7 | RH | 12 | −67 | 46 | LM | 2.44 |
| Cuneus | 18 | LH | −21 | −79 | 22 | LM | 2.27 |
LM, local maxima.
Table 3.
Effect of up-regulation of emotion
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | Voxels | t-value |
| ‘Increase>Look Negative’ | |||||||
| STG | 13 | LH | −42 | −46 | 25 | 234 | 4.66 |
| MFG | 6 | LH | −36 | −1 | 61 | 351 | 4.15 |
| SFG | 6 | LH | −6 | 11 | 73 | LM | 3.74 |
| SFG | 6 | LH | −6 | −4 | 64 | LM | 3.09 |
| IFG | 45 | LH | −47 | 37 | 4 | 175 | 3.41 |
| IFG | 45 | LH | −49 | 22 | 11 | LM | 2.85 |
| IFG | 44 | LH | −47 | 4 | 14 | LM | 2.46 |
| Insula | 13 | LH | −35 | 0 | 14 | LM | 2.36 |
| ‘Look Negative>Increase’ | |||||||
| Post-central gyrus | 1 | RH | 66 | −16 | 28 | 6174 | 7.02 |
| medFG | 8 | RH | 15 | 29 | 43 | LM | 5.98 |
| Cingulate gyrus | 32 | RH | 24 | 8 | 40 | LM | 5.01 |
| Precuneus | 19 | RH | 30 | −61 | 40 | LM | 4.94 |
| Post-central gyrus | 3 | RH | 33 | −34 | 46 | LM | 4.61 |
| MFG | 6 | RH | 24 | 11 | 61 | LM | 4.59 |
| Lentiform nucleus | RH | 33 | −13 | 4 | LM | 4.48 | |
| Posterior cingulate | 30 | RH | 12 | −61 | 10 | LM | 4.48 |
| Post-central gyrus | 2 | RH | 51 | −25 | 40 | LM | 4.36 |
| MFG | 46 | RH | 42 | 35 | 22 | LM | 4.24 |
| Pre-central gyrus | 9 | RH | 45 | 17 | 34 | LM | 4.23 |
| Insula | 13 | RH | 30 | −22 | 19 | LM | 4.02 |
| Lingual gyrus | 19 | LH | −15 | −58 | −2 | LM | 4.01 |
| Insula | 13 | RH | 39 | −28 | 13 | LM | 3.96 |
| Cingulate gyrus | 31 | RH | 3 | −37 | 31 | LM | 3.83 |
| Inferior temporal gyrus | 20 | RH | 51 | −49 | −11 | LM | 3.78 |
| Paracentral lobule | 5 | RH | 9 | −37 | 46 | LM | 3.61 |
| Cingulate gyrus | 24 | RH | 3 | −1 | 31 | LM | 3.56 |
| Lingual gyrus | 19 | RH | 15 | −46 | −2 | LM | 3.52 |
| STG | 41 | RH | 54 | −19 | 10 | LM | 3.50 |
| Parahippocampal gyrus | 36 | RH | 33 | −28 | −23 | LM | 3.43 |
| Paracentral lobule | 5 | LH | 0 | −43 | 61 | LM | 3.36 |
| Post-central gyrus | 3 | RH | 33 | −31 | 61 | LM | 3.15 |
| Inferior parietal lobule | 40 | RH | 57 | −46 | 49 | LM | 2.79 |
| medFG | 6 | LH | −6 | −19 | 49 | LM | 2.73 |
| Middle occipital gyrus | 37 | RH | 39 | −61 | 4 | LM | 2.26 |
| STG | 22 | LH | −48 | −13 | 7 | 1253 | 5.26 |
| Post-central gyrus | 2 | LH | −60 | −22 | 34 | LM | 4.70 |
| Post-central gyrus | 40 | LH | −63 | −19 | 19 | LM | 4.43 |
| STG | 41 | LH | −42 | −34 | 13 | LM | 4.20 |
| Insula | 13 | LH | −33 | −19 | 19 | LM | 3.85 |
| Inferior parietal lobule | 40 | LH | −48 | −28 | 46 | LM | 3.59 |
| STG | 22 | LH | −66 | −19 | 1 | LM | 3.33 |
| Superior parietal lobule | 7 | LH | −27 | −55 | 46 | LM | 3.32 |
| IFG | 9 | LH | −63 | 8 | 31 | LM | 2.84 |
| Post-central gyrus | 5 | LH | −30 | −40 | 58 | LM | 2.66 |
| Post-central gyrus | 2 | LH | −24 | −34 | 67 | LM | 2.64 |
| MFG | 10 | RH | 27 | 62 | 22 | LM | 4.04 |
| MFG | 10 | RH | 27 | 62 | 22 | LM | 4.04 |
| SFG | 10 | RH | 9 | 68 | 13 | LM | 3.61 |
| SFG | 10 | LH | −9 | 65 | 16 | LM | 3.37 |
| SFG | 10 | RH | 3 | 65 | 28 | LM | 2.52 |
| medFG | 9 | RH | 6 | 50 | 28 | LM | 2.48 |
| Parahippocampal gyrus | 36 | LH | −33 | −28 | −17 | 303 | 4.02 |
| Fusiform gyrus | 37 | LH | −42 | −58 | −8 | LM | 3.61 |
| Fusiform gyrus | 37 | LH | −48 | −46 | −14 | LM | 3.08 |
| Middle occipital gyrus | 19 | LH | −57 | −67 | −5 | LM | 2.99 |
| Middle temporal gyrus | 21 | LH | −66 | −55 | 4 | LM | 2.77 |
| STG | 22 | LH | −63 | −61 | 16 | LM | 2.75 |
| Middle occipital gyrus | 19 | LH | −51 | −82 | 10 | 250 | 3.72 |
| Fusiform gyrus | 18 | LH | −27 | −97 | −17 | LM | 3.61 |
| STG | 38 | RH | 48 | 17 | −20 | 102 | 3.31 |
| IFG | 47 | RH | 36 | 20 | −14 | LM | 3.23 |
LM, local maxima.
Univariate ROI analyses. Based on our a priori hypotheses, we performed ROI analyses on bilateral amygdala by calculating the mean signal change across all voxels within the ROI (Supplementary Figure S1). This analysis was conducted to provide evidence of activity related to emotion regulation i.e. either differential activity between ‘Increase’ and ‘Decrease’, or between ‘Decrease’ and ‘Look-Negative/Look-Neutral’, or ‘Increase’ and ‘Look-Negative/Look-Neutral’, supporting the applicability of the following PPI analysis in these regions.
A main task effect was observed in the left amygdala [F(1,22) = 3.08, P < 0.05], but not within the right amygdala. Post-hoc tests revealed that responses in the left amygdala significantly differed between the ‘Increase’ and ‘Decrease’ condition [t(22) = 2.72, P < 0.05]. Thus, the left amygdala was used as seed region in the subsequent PPI analyses.
Effective connectivity analysis. We applied a seed-based connectivity analysis to examine changes in effective connectivity during emotion regulation relative to the ‘Look-Negative’ condition with respect to reappraisal skills (ability to successfully regulate emotions, as measured by the reappraisal success scores). PPI analyses were performed on two seed regions, left IFG and left amygdala. For this, we determined task-dependent effective interaction based on the contrast ‘Increase’ or ‘Decrease’ vs control condition (‘Increase > Look-Negative’ and ‘Decrease > Look-Negative’) using the individual mean reappraisal success scores as predictor of the PPI map, meaning that the better subjects could regulate their emotions, the stronger was the effective coupling between the seed regions and the remainder of the brain. A positive correlation means that the greater the task-dependent change in effective connectivity between these regions and the seed region, the more effectively participants regulated their emotions during the ‘Increase’ or ‘Decrease’ condition. Contrarily, a negative correlation means that a greater task-dependent change in effective connectivity between these regions and the seed region was associated with less effective emotion regulation during the ‘Increase’ or ‘Decrease’ condition.
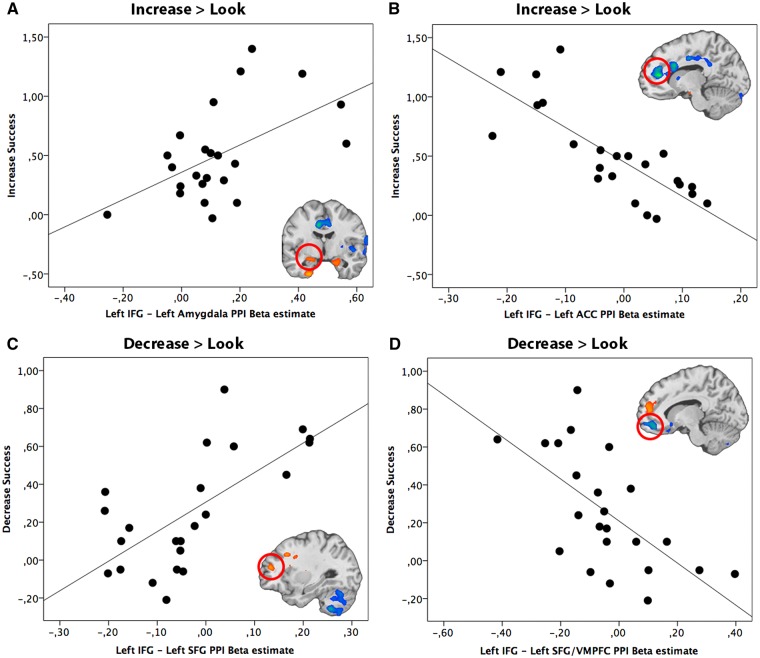
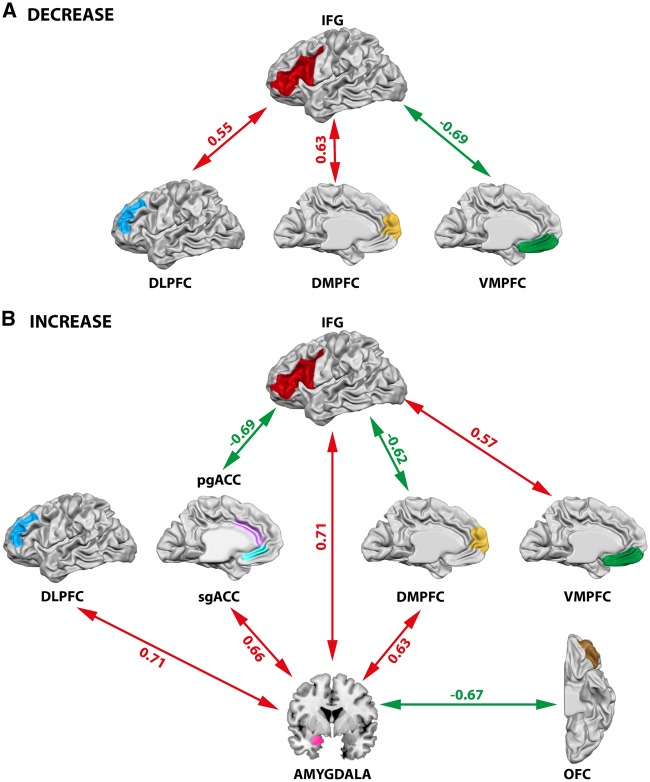
Left IFG seed region. During the up-regulation of emotion we found a positive correlation between ‘Increase’ success and the effective coupling of the left IFG (seed region) and left STG, VMPFC and bilateral amygdalae (Table 4; Figure 4A illustrates one of these positive correlations, using the success-dependent connectivity between IFG as seed region and the left amygdala). We also observed a negative correlation between ‘Increase’ success and activity of the left IFG that was coupled with pregenual anterior cingulate cortex (pgACC), DMPFC, caudate, right insula, left IPL, right MFG, right STG and right IFG (Table 4). Figure 4B illustrates the negative correlation between ‘Increase’ success and connectivity between left IFG and ACC.
Table 4.
Task-related PPI analysis with success scores as covariate for left IFG seed region
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | Voxels | t-value |
| ‘Decrease > Look Negative with Decrease success as covariate’ | |||||||
| Middle temporal gyrus | 21 | RH | 39 | −4 | −23 | 173 | 0.70 |
| Middle temporal gyrus | 21 | RH | 39 | −4 | −23 | LM | 0.70 |
| STG | 22 | RH | 51 | −16 | −8 | LM | 0.61 |
| STG | 38 | RH | 42 | 5 | −11 | LM | 0.59 |
| STG | 38 | RH | 57 | 14 | −11 | LM | 0.54 |
| SFG/DMPFC | 10 | LH | −27 | 44 | 16 | 148 | 0.63 |
| SFG | 10 | LH | −27 | 44 | 16 | LM | 0.63 |
| medFG | 10 | LH | −9 | 50 | 10 | LM | 0.61 |
| IFG/VMPFC | 11 | RH | 18 | 35 | −17 | 155 | −0.69 |
| IFG | 11 | RH | 18 | 35 | −17 | LM | −0.69 |
| IFG | 47 | RH | 21 | 23 | −17 | LM | −0.62 |
| SFG | 11 | LH | −12 | 44 | −14 | 100 | −0.62 |
| Orbital Gyrus | 47 | LH | −15 | 20 | −23 | 95 | −0.59 |
| Orbital gyrus | 47 | LH | −15 | 20 | −23 | LM | −0.59 |
| Subcallosal gyrus | 25 | LH | −3 | 8 | −14 | LM | −0.56 |
| Lentiform nucleus/putamen | LH | −21 | 5 | 16 | 171 | 0.68 | |
| Lentiform nucleus/putamen | LH | −21 | 5 | 16 | LM | 0.68 | |
| MFG | 8 | LH | −30 | 20 | 37 | LM | 0.65 |
| MFG/DLPFC | 9 | LH | −42 | 14 | 37 | LM | 0.55 |
| Pre-central Gyrus/DLPFC | 9 | LH | −33 | 5 | 34 | LM | 0.52 |
| ‘Increase>Look Negative with Increase success as covariate’ | |||||||
| Uncus | 20 | LH | −24 | −7 | −41 | 184 | 0.66 |
| STG | 38 | LH | −30 | 8 | −20 | LM | 0.59 |
| Parahippocampal gyrus (amygdala) | LH | −24 | −7 | −17 | LM | 0.52 | |
| Parahippocampal gyrus (amygdala) | 34 | RH | 27 | 5 | −14 | 138 | 0.62 |
| IFG/VMPFC | 47 | RH | 26 | 17 | −11 | LM | 0.57 |
| Anterior cingulate | 32 | LH | −12 | 38 | 10 | 1146 | −0.83 |
| Cingulate gyrus | 24 | LH | −9 | 14 | 25 | LM | −0.72 |
| Cingulate gyrus | 24 | LH | −6 | 5 | 25 | LM | −0.67 |
| medFG/DMPFC | 10 | RH | 15 | 53 | 13 | LM | −0.70 |
| medFG/DMPFC | 10 | LH | 0 | 56 | 16 | LM | −0.54 |
| Anterior cingulate/DMPFC | 32 | RH | 12 | 35 | 16 | LM | −0.62 |
| Cingulate gyrus/DMPFC | 32 | RH | 9 | 20 | 34 | LM | −0.54 |
| Caudate | LH | −6 | 23 | 7 | LM | −0.70 | |
| Insula | 13 | RH | 33 | 29 | 10 | LM | −0.52 |
| Caudate | LH | −12 | 8 | 4 | LM | −0.52 | |
| Fusiform gyrus | 18 | LH | −24 | −100 | −14 | 151 | −0.69 |
| Cingulate gyrus | 24 | LH | −9 | −7 | 37 | 679 | −0.69 |
| Cingulate gyrus | 31 | RH | 6 | −34 | 34 | LM | −0.66 |
| Cingulate gyrus | 31 | RH | 9 | −22 | 40 | LM | −0.62 |
| Cingulate gyrus | 24 | RH | 9 | −4 | 37 | LM | −0.60 |
| Cingulate gyrus | 31 | LH | −18 | −46 | 25 | LM | −0.60 |
| Cingulate gyrus | 31 | RH | 24 | −22 | 40 | LM | −0.59 |
| medFG | 6 | RH | 9 | −16 | 58 | LM | −0.58 |
| Cingulate gyrus | 31 | LH | −18 | −28 | 37 | LM | −0.57 |
| Inferior parietal lobule | 40 | LH | −30 | −37 | 37 | LM | −0.56 |
| Precuneus | 31 | RH | 9 | −46 | 34 | LM | −0.54 |
| Paracentral lobule | 6 | LH | −6 | −25 | 52 | LM | −0.53 |
| Paracentral lobule | 5 | RH | 21 | −34 | 49 | LM | −0.50 |
| MFG | 47 | RH | 51 | 41 | −8 | 119 | −0.67 |
| STG | 38 | RH | 54 | 20 | −14 | LM | −0.67 |
| IFG | 46 | RH | 51 | 41 | 13 | LM | −0.59 |
| Cuneus | 19 | RH | 30 | −88 | 28 | 239 | −0.65 |
| Cuneus | 19 | RH | 12 | −91 | 25 | LM | −0.59 |
| Pre-central gyrus | 6 | RH | 57 | 2 | 37 | 177 | −0.65 |
| Middle temporal gyrus | 21 | RH | 66 | −4 | −5 | LM | −0.62 |
| STG | 42 | RH | 69 | −10 | 7 | LM | −0.58 |
| IFG | 44 | RH | 57 | 8 | 16 | LM | −0.56 |
| STG | 22 | RH | 60 | 2 | 4 | LM | −0.53 |
| STG | 22 | RH | 48 | −10 | −2 | LM | −0.54 |
LM, local maxima.
Fig. 4.
Relationship between reappraisal success scores (Increase Success) and PPI beta estimate of IFG coupling with (A) left amygdala and (B) left ACC during the upregulation of emotion (Increase > Look). Relationship between reappraisal success (Decrease Success) scores and PPI beta estimate of IFG coupling with (C) left (SFG/DMPFC) and (D) left (SFG/VMPFC) during the down-regulation of emotion (Decrease > Look). The scatter plot is solely for illustrative purposes (e.g. to show the absence of outliers), and is not used for statistical inference.
The results further indicated significant positive effective coupling between the left IFG (seed region) and DLPFC, DMPFC, right MTG and STG during down-regulation of emotion depending on reappraisal success (Table 4). Figure 4C illustrates the positive correlation between effective connectivity beta estimates for the left IFG and SFG and ‘Decrease’ success. In contrast, a negative correlation between effective connectivity and ‘Decrease’ success was found between left IFG (seed region) and right IFG, left SFG/VMPFC and left orbital gyrus (Table 4). Figure 4D illustrates the negative correlation between effective connectivity beta estimates for the left IFG (and left SFG/VMPFC) and ‘Decrease’ success.
Left amygdala seed region. The PPI analysis based on the left amygdala as seed region demonstrated a positive correlation between ‘Increase’ success and the coupling with a number of regions such as left IFG, left MFG, left SFG, subgenual ACC (sgACC), left STG, left insula, left IPL, left MTG and left parahippocampal gyrus (Table 5). Figure 5A illustrates the positive correlation between successful up-regulation of emotion and effective coupling of the left amygdala (seed region) with left IFG. Effective connectivity between the left amygdala (seed region) and the right SFG/OFC was negatively correlated with ‘Increase’ success, illustrated in Figure 5B and reported in Table 5.
Table 5.
Task-related PPI analysis with success scores as covariate for left amygdala seed region
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | BA | Side | x | y | z | Voxels | t-value |
| ‘Decrease>Look Negative with Decrease success as covariate’ | |||||||
| Lingual Gyrus | 18 | RH | 6 | −88 | −11 | 447 | 0.71 |
| Middle occipital gyrus | 19 | RH | 30 | −91 | 16 | LM | 0.67 |
| Inferior occipital gyrus | 17 | RH | 18 | −91 | −5 | LM | 0.61 |
| Fusiform gyrus | 19 | RH | 24 | −82 | −14 | LM | 0.60 |
| Inferior occipital gyrus | 18 | LH | −30 | −91 | −2 | 293 | 0.63 |
| Fusiform gyrus | 19 | LH | −39 | −76 | −11 | LM | 0.63 |
| Lingual gyrus | 18 | LH | −18 | −97 | −11 | LM | 0.62 |
| Fusiform gyrus | 37 | RH | 36 | −43 | −11 | 99 | 0.63 |
| Parahippocampal gyrus | 36 | RH | 36 | −34 | −14 | LM | 0.56 |
| Precuneus | 7 | RH | 30 | −46 | 52 | 98 | −0.60 |
| Precuneus | 7 | RH | 15 | −61 | 49 | LM | −0.54 |
| ‘Increase>Look Negative with Increase success as covariate’ | |||||||
| IFG/VLPFC | 47 | LH | −33 | 17 | −11 | 1542 | 0.71 |
| STG | 22 | LH | −45 | −10 | −5 | LM | 0.67 |
| Insula | 13 | LH | −39 | −25 | 16 | LM | 0.64 |
| IFG | 47 | LH | −30 | 32 | −2 | LM | 0.64 |
| Inferior parietal lobule | 40 | LH | −48 | −31 | 22 | LM | 0.63 |
| Pre-central gyrus | 4 | LH | −51 | −10 | 25 | LM | 0.62 |
| Middle temporal gyrus | 19 | LH | −39 | −61 | 13 | LM | 0.62 |
| Insula | 13 | LH | −33 | 26 | 13 | LM | 0.60 |
| Post-central gyrus | 2 | LH | −36 | −25 | 28 | LM | 0.60 |
| Inferior temporal gyrus | 21 | LH | −60 | −13 | −17 | LM | 0.59 |
| Post-central gyrus | 43 | LH | −63 | −10 | 16 | LM | 0.55 |
| Caudate | LH | −30 | −37 | 4 | LM | 0.55 | |
| Parahippocampal gyrus | 36 | LH | −24 | −37 | −8 | LM | 0.52 |
| Lentiform nucleus | LH | −27 | 2 | 10 | LM | 0.51 | |
| STG | 22 | LH | −57 | −43 | 13 | LM | 0.50 |
| STG | 39 | LH | −39 | −58 | 31 | LM | 0.45 |
| MFG/DLPFC | 9 | LH | −42 | 11 | 34 | 498 | 0.71 |
| MFG | 6 | LH | −24 | 8 | 43 | LM | 0.65 |
| medFG | 8 | RH | 12 | 38 | 40 | LM | 0.64 |
| SFG | 8 | LH | −9 | 41 | 43 | LM | 0.63 |
| medFG | 8 | LH | −12 | 26 | 46 | LM | 0.60 |
| medFG | 6 | LH | −15 | 29 | 37 | LM | 0.60 |
| Anterior cingulate | 32 | RH | 12 | 38 | 25 | LM | 0.58 |
| Anterior cingulate | 32 | LH | −3 | 38 | 19 | LM | 0.52 |
| Middle temporal gyrus | 21 | RH | 63 | −40 | −14 | 1255 | 0.67 |
| Lentiform nucleus | RH | 33 | −16 | 1 | LM | 0.65 | |
| Middle temporal gyrus | 21 | RH | 60 | −22 | −8 | LM | 0.63 |
| Insula | 13 | RH | 45 | −16 | 22 | LM | 0.61 |
| Middle temporal gyrus | 21 | RH | 57 | −40 | 1 | LM | 0.61 |
| Thalamus | RH | 21 | −34 | 1 | LM | 0.59 | |
| Midbrainred nucleus | LH | 0 | −16 | −2 | LM | 0.57 | |
| STG | 22 | RH | 48 | −1 | 1 | LM | 0.57 |
| STG | RH | 66 | −25 | 1 | LM | 0.57 | |
| STG | 22 | RH | 66 | −40 | 10 | LM | 0.56 |
| Middle temporal gyrus | 37 | RH | 42 | −58 | 4 | LM | 0.56 |
| Transverse temporal gyrus | RH | 57 | −13 | 10 | LM | 0.55 | |
| Midbrainred nucleus | LH | 0 | −25 | −8 | LM | 0.55 | |
| STG | 22 | RH | 48 | −22 | −11 | LM | 0.54 |
| Post-central gyrus | 2 | RH | 60 | −22 | 34 | LM | 0.54 |
| STG | 42 | RH | 66 | −25 | 16 | LM | 0.51 |
| STG | 39 | RH | 45 | −52 | 19 | LM | 0.51 |
| Anterior cingulate | 24 | RH | 3 | 35 | 7 | 133 | 0.66 |
| medFG/DMPFC | 10 | LH | −12 | 50 | 7 | LM | 0.63 |
| MFG/DMPFC | 10 | LH | −24 | 50 | 4 | LM | 0.58 |
| Post-central gyrus | 3 | LH | −18 | −37 | 70 | 165 | 0.65 |
| Post-central gyrus | 1 | LH | −33 | −34 | 64 | LM | 0.62 |
| Post-central gyrus | 2 | LH | −42 | −28 | 52 | LM | 0.52 |
| Posterior cingulate | 23 | RH | 3 | −34 | 16 | 244 | 0.56 |
| Cingulate gyrus | 31 | LH | −15 | −49 | 19 | LM | 0.56 |
| Posterior cingulate | 30 | LH | −6 | −52 | 13 | LM | 0.55 |
| SFG/OFC | 11 | RH | 27 | 53 | −20 | 117 | −0.67 |
| SFG/OFC | 11 | RH | 18 | 53 | −17 | LM | −0.63 |
LM, local maxima.
Fig. 5.
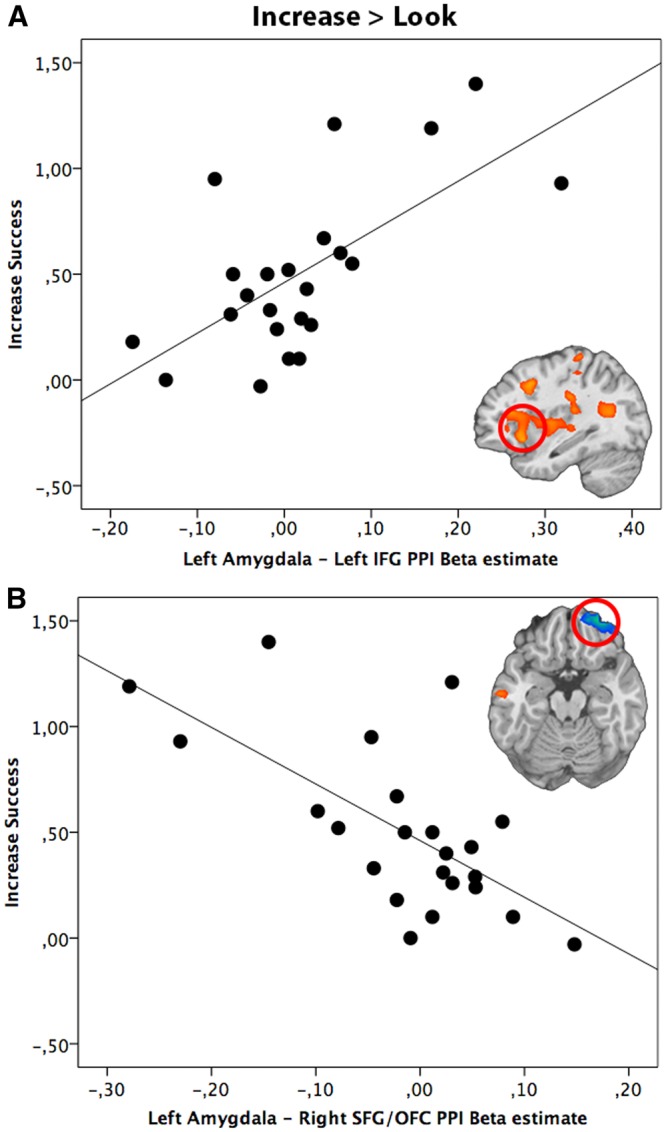
Relationship between reappraisal success scores (Increase Success) and PPI beta estimate of left amygdala coupling with (A) left IFG and (B) right SFG (SFG/OFC) during the up-regulation of emotion (Increase > Look). The scatter plot is solely for illustrative purposes (e.g. to show the absence of outliers), and is not used for statistical inference.
The activity in the left amygdala was positively correlated with activity in the bilateral fusiform gyri, right parahippocampal gyrus and occipital cortex and ‘Decrease’ success (Table 5). We also found a negative correlation between ‘Decrease’ success and the effective coupling between the left amygdala and the right precuneus.
In summary, we found that the down-regulation of emotion was associated with increased effective connectivity in a prefrontal network, including the left VLPFC, DLPFC and DMPFC, depending on reappraisal success (Figure 6A). Furthermore, decreased coupling between left IFG and VMPFC during the down-regulation of emotion was correlated with greater reappraisal success. Up-regulation of emotion was based on increased effective connectivity between the left IFG and VMPFC and correlated positively with reappraisal success (Figure 6B). A negative correlation between reappraisal success and connectivity of the left IFG with the DMPFC and pgACC during the up-regulation of emotion was observed. Moreover, increased effective coupling between the IFG with the amygdala correlating positively with reappraisal success was found for the ‘Increase’ condition. The amygdala was further coactivated with the sgACC, DLPFC and DMPFC and this coupling was predicted by higher reappraisal success when up-regulating emotions. In contrast, there was a negative relationship between reappraisal success during the up-regulation of emotional responses and the coupling between the amygdala with the OFC. These results indicate that emotion regulation was based on amygdala-prefrontal interactions that were tightly linked to the ability to successfully regulate emotions.
Fig. 6.
Illustration of the results of the PPI-analyses with focus on prefrontal-subcortical interactions. Red arrows indicate a positive correlation between the effective coupling of two regions and reappraisal success, while green arrows indicate a negative correlation between the effective coupling of two regions and reappraisal success. (A) Prefrontal network observed during the down-regulation (Decrease) of emotion. (B) Amygdalaprefrontal network observed during the up-regulation (Increase) of emotion. IFG, inferior frontal gyrus; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; VMPFC, ventromedial prefrontal cortex; pgACC, pregenual anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; OFC, orbitofrontal cortex. t-values for each connection are reported.
Discussion
Previous studies on reappraisal indicated that VLPFC, in particular the IFG, and the amygdala serve different key functional roles in the reappraisal process (Eippert et al., 2007; Wager et al., 2008). However, the effective coupling between VLPFC and amygdala with other regions of the brain during reappraisal and their association with reappraisal success is unclear. The present work constitutes the first investigation into this question by explicitly including reappraisal success as a regressor in the connectivity model.
In our reappraisal task, subjects had to either up- or down-regulate their emotions in response to aversive pictures. The effect of emotion regulation was demonstrated on the behavioral, psychophysiological and neural level. First, during emotion regulation subjects rated their emotional state as either increased or decreased according to the reappraisal condition compared with the control condition. Second, SCRs were increased during the up-regulation compared with the down-regulation of emotion (Eippert et al., 2007; Urry et al., 2009; Morawetz et al., 2016b,c). Third, in accord with previous findings, reappraisal compared with the control condition was associated with enhanced responses in VLPFC, temporal and parietal regions as well as the amygdala (Urry et al., 2006; Eippert et al., 2007; Kim and Hamann, 2007; Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014; Morawetz et al., 2016c). We therefore conclude that our paradigm was suited to investigate the effect of emotion regulation on connectivity and its relation to reappraisal success.
Prefrontal network underlying the down-regulation of emotion
No study to date has used the IFG as seed region for a connectivity analysis in the context of emotion regulation. This is surprising since the left IFG has been implicated in response selection and inhibition, language, social cognition and inner speech during the emotion regulation process (Ochsner et al., 2012; Kohn et al., 2014; Messina et al., 2015). Here we found that increased effective coupling between the left IFG and dorsal prefrontal regions (DMPFC and DLPFC) was associated with reappraisal success during the down-regulation of emotional responses. In a previous study, we found that emotion regulation was mediated by directional changes of connection strength between the IFG and DLPFC (Morawetz et al., 2016c). We now demonstrate that the ability to successfully regulate ones emotions was predicted by an enhanced effective connectivity between those two prefrontal regions. This indicates that the suggested feedback mechanism between DLPFC and IFG during emotion regulation (Morawetz et al., 2016c) might be more prominent in individuals possessing higher reappraisal skills. Within this frontal network, DMPFC appears to play a critical role in emotion regulation as it has repeatedly been shown to be involved in various aspects of reflective emotional processing (Phan et al., 2002). This region has been suggested to be implicated in the control of emotional behaviors and to relay internal state information to the DLPFC and VLPFC via a feed-forward mechanism (Phillips et al., 2008).
Interestingly, we found a negative correlation between IFG-VMPFC coupling and down-regulation success, while the reverse pattern was found for the up-regulation of emotion. These findings are closely aligned with the view that VMPFC plays a key role in conceptually driven affect (Roy et al., 2012). Reappraisal as a conceptually-driven form of emotion regulation, i.e. with the goal to generate positive (‘Decrease’) or negative (‘Increase’) conceptual frames, has previously been linked to VMPFC activity (Diekhof et al., 2011). Previous imaging studies also implicated the VMPFC in the positive and negative valuation of stimuli in a context- and goal-dependent manner (Hare et al., 2009; Hutcherson et al., 2012). Our results show that the successful reframing of negative stimuli in a positive direction (i.e. decreasing emotions to feel less negative affect) was related to less effective coupling between IFG and VMPFC. In contrast, the successful reframing of aversive pictures in a negative direction (i.e. increasing emotions to feel more negative affect) was associated with enhanced effective connectivity between IFG and VMPFC. These findings indicate that the up-regulation of emotion might be more cognitively demanding which could lead to the stronger subsequent recruitment of the VMPFC. In the opposite scenario, it might be easier to develop appropriate positive concepts to down-regulate emotions, and this process might therefore be associated with less effective coupling between IFG and VMPFC.
Of note, the observed network is based on the IFG as seed region because this prefrontal region has been directly linked to reappraisal success in previous studies (see ‘Materials and methods’ section). However, it might be worthwhile to use other prefrontal regions as seed regions if there is an indication that this region is related to successful emotion regulation. In the present study we used a hypothesis-driven analysis approach and only considered brain regions for which clear evidence for a direct link to emotion regulation success was given.
Amygdala-prefrontal network underlying the up-regulation of emotion
Consistent with previous findings (Banks et al., 2007) we found that increased connectivity between the left amygdala and OFC was associated with the experience of less negative affect. In addition, we extended previous studies (Banks et al., 2007; Kanske et al., 2011; Sripada et al., 2014) by showing that several other prefrontal regions (VLPFC, DLPFC, DMPFC and sgACC) demonstrated increased coupling with the amygdala with increasing success in up-regulating emotions.
The reappraisal-modulated coactivation of amygdala and IFG with ACC with respect to regulating negative affect is in line with prior neuroimaging results that implicate the ACC in emotion assessment, emotion related learning, and active, voluntary emotion regulation (Ochsner and Gross, 2005; Phillips et al., 2008; Wager et al., 2008; Etkin et al., 2011; Ochsner et al., 2012; Stevens et al., 2012). We observed a differential connectivity profile for the ACC during the up-regulation of emotion: Increased coupling between sgACC and amygdala predicted increased reappraisal success, while decreased coupling between pgACC and IFG predicted less reappraisal success. The increased coupling between the IFG and pgACC and its negative association with reappraisal success might represent a form of successful emotional conflict resolution (Beauregard et al., 2001; Etkin et al., 2006): when participants were unable to up-regulate their negative emotions, this might have led to less emotional conflict and thus less effective coupling between IFG and pgACC. In support, the sgACC has been found to be activated when participants were asked to attend to core affective feelings and rate their feelings (Lindquist et al., 2012). Consistent with this view, reappraisal-related sgACC activation has been interpreted to signify self-relevant processing or focus on one’s feelings (Kross et al., 2009; Urry et al., 2009). This implies that the observed positive association between reappraisal success and increased coupling between the amygdala with sgACC might indicate heightened monitoring of internal emotional states (Abler et al., 2008).
Our findings suggest that the successful down-regulation of emotions predicted increased coupling of prefrontal regions, while the successful up-regulation of emotional experience was related to increased coupling of amygdala-prefrontal interactions. The discrepancy to previous studies (Urry et al., 2006; Banks et al., 2007; Kanske et al., 2011) reporting amygdala-frontal coupling during the down-regulation of emotion might be explained by individual differences in reappraisal success. To test this hypothesis, we performed the same PPI analysis with the left amygdala as seed region ‘without’ using reappraisal success as covariate. Consistent with the notion that frontal cortex exerts a top-down inhibitory effect on the amygdala (Ochsner et al., 2002; Quirk and Beer, 2006; Urry et al., 2006; Johnstone et al., 2007; Phillips et al., 2008; Wager et al., 2008), we found that increased activity in bilateral VLPFC was associated with decreased activity in the left amygdala during the down-regulation of negative affect. In contrast, an increase in response in the left VLPFC was associated with an increase in signal change in the amygdala during the up-regulation of emotions (see Supplementary Table S1). This supports the idea that individual differences in reappraisal success might affect amygdala-frontal coupling during the down-regulation of emotion.
Furthermore, amygdala-frontal coupling might also be affected by reappraisal training. Recent findings indicate that reappraisal training, i.e. increasing reappraisal success, not only results in reductions in self-reported negative affect over time (Denny and Ochsner, 2014), but also leads to long-lasting effects on the amygdala response (Denny et al., 2015). In line with these findings, previous studies demonstrated that neurofeedback training leads to an increase in top-down connectivity from the DMPFC onto the amygdala (Koush et al., 2015) as well as in bottom-up connectivity from the amygdala to the VMPFC (Paret et al., 2016). These findings indicate that the amygdala-frontal coupling underlying successful emotion regulation can be modulated in a self-organized, endogenous fashion. Furthermore, the coupling within cortico-limbic circuits can also be influenced pharmacologically by targeting the serotonergic system, which has been implicated in a wide range of psychiatric diseases, in particular in anxiety disorders and depression. Serotonin has been linked to several aspects of emotional information processing (Merens et al., 2007) and thus might be implicated in amygdala-frontal coupling. Indeed, a recent study demonstrated that effective connectivity within the prefrontal-amygdala network can be modulated by (S)-citalopram, a selective serotonin reuptake inhibitor, during emotional face processing (Sladky et al., 2015). Hence, the proposed emotion regulation network could not only be modulated on a behavioral, but also on a neuropharmacological level.
Conclusions
Our findings have several implications, first by providing further evidence for the importance of frontal brain regions to regulate emotions. Furthermore, these findings add to prior neuroimaging studies by showing that up- and down-regulation of emotions are based on different coupling between prefrontal regions and the amygdala. Increased coupling within a prefrontal network was related to success in down-regulation of emotions, and increased coupling between the amygdala and the prefrontal network was related to success in the up-regulation of emotions. They additionally suggest that there is a direct link between effective connectivity underlying emotion regulation and the success in controlling emotions thereby highlighting the role of individual differences. In relation to conceptual models of emotion regulation (Ochsner et al., 2012), the findings demonstrate that successful reappraisal might rely on a more direct interaction between a system involved in the control of emotions based on frontal regions and a system implicated in generating emotions involving emotion-related regions such as the amygdala. Finally, given that psychiatric disorders have been found to be associated with affective instability and emotion dysregulation (Phillips et al., 2003), our findings might inform future studies investigating whether specific connectivity deficits could underlie emotion regulation deficits in mental disorders, such as anxiety, mood and personality disorders.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
References
- Abler B., Hofer C., Viviani R. (2008). Habitual emotion regulation strategies and baseline brain perfusion. Neuroreport, 19, 21–4. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology, 230, 465–96. [DOI] [PubMed] [Google Scholar]
- Amstadter A. (2008). Emotion regulation and anxiety disorders. Journal of Anxiety Disorders, 22, 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience 2, 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. (2000). Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin 52, 319–30. [DOI] [PubMed] [Google Scholar]
- Barbas H. 2009. Prefrontal cortex: structure and anatomy In: Squire L., editor. Encyclopedia of Neuroscience, pp. 909–18. Oxford: Academic Press. [Google Scholar]
- Barbas H., Pandya D.N. (1989). Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative. Neurology, 286, 353–75. [DOI] [PubMed] [Google Scholar]
- Barbas H., Pandya N.. 1991. Patterns of connections of the prefrontal cortex in the rhesus monkey associated with cortical architecture In: Levin H., Eisenberg H., Benton A., editors. In Frontal Lobe Function and Dysfunction, pp. 35–58. New York: Oxford University Press. [Google Scholar]
- Beauregard M., Lévesque J., Bourgouin P. (2001). Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience, 21, RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Kaernbach C. (2010a). Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology, 47, 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M., Kaernbach C. (2010b). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.M., Wolford G.L., Miller M.B. (2009). The principled control of false positives in neuroimaging. Social Cognitive and Affective Neuroscience, 4, 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M., Wupperman P. (2012). Emotion regulation and mental health: recent findings, current challenges, and future directions. Current Opinion in Psychiatry, 25, 128–34. [DOI] [PubMed] [Google Scholar]
- Bradley M., Miccoli L., Escrig M., Lang P. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45, 602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J.. 2007. The International Affective Picture System (IAPS) in the study of emotion and attention In: Coan J.A., Allen J.J., editors. Handbook of Emotion Elicitation and Assessment, Series in Affective Science, pp. 29–46. New York: Oxford University Press. [Google Scholar]
- Buhle J.T., Silvers J. a., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Olatunji B.O., Feldner M.T., Forsyth J.P. (2010). Emotion Regulation and the Anxiety Disorders: An Integrative Review. Journal of. Psychopathology and Behavioral Assessment, 32, 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. (2000). Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science, 289, 591–4. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Inhoff M.C., Zerubavel N., Davachi L., Ochsner K.N. (2015). Getting over it: long-lasting effects of emotion regulation on amygdala response. Psychological Science, 26, 1377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B.T., Ochsner K.N. (2014). Behavioral effects of longitudinal training in cognitive reappraisal. Emotion, 14, 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58, 275–85. [DOI] [PubMed] [Google Scholar]
- Eftekhari A., Zoellner L.A., Vigil S.A. (2009). Patterns of emotion regulation and psychopathology. Anxiety Stress Coping, 22, 571–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H.. et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage, 25, 1325–35. [DOI] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., Erb M., Birbaumer N., Anders S. (2007). Regulation of emotional responses elicited by threat-related stimuli. Humand Brain Mapping, 28, 409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin a., Egner T., Peraza D., Kandel E., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine, 33, 636–47. [DOI] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–11. [DOI] [PubMed] [Google Scholar]
- Friston K., Buechel C., Fink G., Morris J., Rolls E., Dolan R. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Friston K.J. (2011). Functional and effective connectivity: a review. Brain Connect, 1, 13–36. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. (2003). Dynamic causal modelling. Neuroimage, 19, 1273–302. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115, 1261–79. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34, 905–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas A., Stiers P., Uylings H.B.M. (2012). Unravelling the Intrinsic Functional Organization of the Human Lateral Frontal Cortex: A Parcellation Scheme Based on Resting State fMRI. Journal of. Neuroscience, 32, 10238–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., Muñoz R.F. (1995). Emotion regulation and mental health. Clinical Psychology: Sciecne and Practice, 2, 151–64. [Google Scholar]
- Gross J.J., Richards J.M., John O.P.. 2006. Emotion regulation in everyday life In: Snyder D.K., Simpson J.A., Hughes J.N., editors. Emotion Regulation in Couples and Families: Pathways to Dysfunction and Health, pp. 13–35. Washington, DC: American Psychological Association. [Google Scholar]
- Gross J.J., Thompson R.A.. 2007. Emotion regulation: conceptual foundations In: Gross J.J., editor. Handbook of Emotion Regulation, pp. 3–24. New York: Guilford Press. [Google Scholar]
- Gruber J., Harvey A.G., Gross J.J. (2012). When trying is not enough: emotion regulation and the effort-success gap in bipolar disorder. Emotion, 12, 997–1003. [DOI] [PubMed] [Google Scholar]
- Hare T. a., Camerer C.F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–8. [DOI] [PubMed] [Google Scholar]
- Hutcherson C. a., Plassmann H., Gross J.J., Rangel A. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. Journal of Neuroscience, 32, 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience 27, 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews, 33, 1215–26. [DOI] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Bongers A., Wessa M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–88. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitvie Neuroscience, 19, 776–98. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird a. R., Fox P.T., Habel U. (2014). Neural network of cognitive emotion regulation - an ALE meta-analysis and MACM analysis. Neuroimage, 87, 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y., Meskaldji D.E., Pichon S.. et al. (2015). Learning control over emotion networks through connectivity-based neurofeedback. Cerebral Cortex, 17. doi:10.1093/cercor/bhv311. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A., Winter D., Niedtfeld I., Schmahl C. (2014). The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports, 16, 438.. [DOI] [PubMed] [Google Scholar]
- Kross E., Davidson M., Weber J., Ochsner K. (2009). Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry, 65, 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K. a., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. Behavioral and. Brain Sciences, 35, 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., Erfurth K., Müller K., Turner R. (2012). Critical comments on dynamic causal modelling. Neuroimage, 59, 2322–9. [DOI] [PubMed] [Google Scholar]
- Merens W., Willem Van der Does A.J., Spinhoven P. (2007). The effects of serotonin manipulations on emotional information processing and mood. Journal of Affective Disorders, 103, 43–62. [DOI] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., Viviani R. (2015). Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology, 6, 974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C., Alexandrowicz R.W., Heekeren H.R.. 2016. Successful emotion regulation is predicted by amygdala activity and aspects of personality: A latent variable approach. Emotion November 7. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Jacobs A.M., Heekeren H.R. (2016b). Neural representation of emotion regulation goals. Human Brain Mapping 37, 600–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Kirilina E., Heekeren H.R. (2016c). Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cerebral Cortex, 26, 1923–37. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fmri study of the cognitive regulation of emotion. J. Cognitive Neuroscience, 14, 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Science, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. doi:10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Paret C., Ruf M., Gerchen M.F., et al. (2016). FMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. Neuroimage, 125, 182–8. [DOI] [PubMed] [Google Scholar]
- Payer D.E., Baicy K., Lieberman M.D., London E.D. (2012). Overlapping neural substrates between intentional and incidental down-regulation of negative emotions. Emotion, 12, 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 16, 331–48. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. (2003). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry, 54, 515–28. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 829–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16, 723–7. [DOI] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews, 36, 479–501. doi:10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Science, 16, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32, 811–30. [DOI] [PubMed] [Google Scholar]
- Sladky R., Spies M., Hoffmann A., et al. (2015). (S)-citalopram influences amygdala modulation in healthy subjects: a randomized placebo-controlled double-blind fMRI study using dynamic causal modeling. Neuroimage, 108, 243–50. [DOI] [PubMed] [Google Scholar]
- Sripada C., Angstadt M., Kessler D., et al. (2014). Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage, 89, 110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens F.L., Hurley R.A., Taber K.H. (2012). Anterior cingulate cortex: unique role in cognition and emotion. Journal ofClinical Neuroscience, 23, 2–6. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P.. 1988. Co-Planar Stereotaxic Atlas of the Human Brain (Thieme Classics). Stuttgart: Thieme. [Google Scholar]
- Urry H.L., Reekum V., Marije C., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Davidson R.J. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage, 47, 852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian E.H., Pandya D.N., Tomaiuolo F., Petrides M. (2012). The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex, 48, 58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H. (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41, 88–123. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D., Cunningham W.A.. 2007. Executive function: Mechanisms underlying emotion regulation In: Gross J., editor. Handbook of Emotion Regulation, pp. 135–158. New York: Guilford. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.