Abstract
Motivation and attention constitute major determinants of human perception and action. Nonetheless, it remains a matter of debate whether motivation effects on the visual cortex depend on the spatial attention system, or rely on independent pathways. This study investigated the impact of motivation and spatial attention on the activity of the human primary and extrastriate visual cortex by employing a factorial manipulation of the two factors in a cued pattern discrimination task. During stimulus presentation, we recorded event-related potentials and pupillary responses. Motivational relevance increased the amplitudes of the C1 component at ∼70 ms after stimulus onset. This modulation occurred independently of spatial attention effects, which were evident at the P1 level. Furthermore, motivation and spatial attention had independent effects on preparatory activation as measured by the contingent negative variation; and pupil data showed increased activation in response to incentive targets. Taken together, these findings suggest independent pathways for the influence of motivation and spatial attention on the activity of the human visual cortex.
Keywords: motivation, reward, spatial attention, C1, ERP
Introduction
In the light of limited cognitive resources, human perception is highly selective in order to ensure the prioritization of relevant information. Two major questions arise from this fact: How are relevant stimuli selected, and what is deemed relevant for selective processing? Concerning the first question, extensive research has identified a fronto-parietal network that controls spatial and object-based attention allocation in service of current goals (Corbetta and Shulman, 2002; Ptak 2012). Key regions of this fronto-parietal attention network include the anterior cingulate cortex (ACC), frontal eye fields, and the posterior parietal cortex (PCC), especially the intraparietal sulcus (Nobre et al. 1997; Corbetta et al., 2008). This extended brain network biases the activity in sensory areas by amplification of relevant and suppression of irrelevant input, thus causing a sharpening in sensory representations (Desimone and Duncan, 1995; Kastner and Ungerleider, 2000; Martinez-Trujillo and Treue 2004). In a similar way, attention can be governed by other determinants, including memory-related, emotional and motivational processes (for reviews see Knudsen, 2007; Vuilleumier 2015). However, while research points to the existence of a common sensory pathway in the visual cortex (Serences and Saproo, 2010), the specific time courses of differential attention effects, as well as possible interdependences, remain to be fully understood.
With regard to ‘what’ is deemed relevant for selective processing, motivation and, in particular, the prospect of reward have long been identified as important determinants of human perception and cognition (for review, see Schultz, 2000; Wise, 2004). Similar to attention, reward leads to an amplification in the neural processing of input associated with it, and its effects are visible even in the absence of conscious processing (Seitz et al., 2009) or if it is no longer provided (Della Libera and Chelazzi, 2009; Anderson and Yantis, 2013; Failing and Theeuwes, 2014, 2015). Furthermore, motivation and reward were shown to impact learning and to engage memory mechanisms (for reviews, see Chelazzi et al., 2013; Pessoa, 2015; Bourgeois et al., 2016)
Given the essential importance of both attention and motivation in shaping human perception and action, it is remarkable that little is known about the interplay of the two factors. More specifically, evidence is equivocal as to whether motivation depends on (prior or concurrent) activation of the spatial attention network in order to bias perception, or whether it acts through independent pathways, thus bypassing the spatial attention system (cf. Pessoa and Engelmann, 2010; Mohanty and Sussman, 2013). Although the first alternative entails an integrative system that would likely be characterized by interactive effects of motivation and attention, the second one, assuming parallel mechanisms, would rather result in independent, additive effects (Pessoa and Engelmann, 2010).
Evidence for an integrative account was provided by neuroimaging studies which showed that incentive value increases the activity of the fronto-parietal attention network during visual attention tasks, most likely resulting in a joint influence on visual perception (Small et al., 2005; Engelmann et al., 2009; Weil et al., 2010; see also Stănişor et al., 2013). As a behavioral consequence, detection sensitivity increased as a function of incentive value (Small et al., 2005; Engelmann and Pessoa, 2007; Engelmann et al., 2009).
In contrast to these findings, mounting evidence suggests that motivation might not merely engage the spatial attention system in order to bias visual perception and cognition, but might rather act in an independent way. Using event-related potentials (ERPs), Baines et al. (2011) reported independent effects of motivation and spatial attention on ERP components indexing sensory analysis in the extrastriate visual cortex (P1) and subsequent attention-related components (N1 and P3). In a MEG study (Hopf et al. 2015), reward and feature-based attention evoked overlapping but additive effects on ventral extrastriate cortex activity, implying that the two factors can indeed target the same visual areas, but most likely through independent top-down mechanisms. In line with this result, neuroimaging data provide evidence that reward-related modulations in early visual cortex cannot merely be explained by engagement of spatial attention (Serences and Saproo, 2010; Arsenault et al., 2013).
This study aimed at investigating the interplay of reward prospect and spatial attention on visual perception, and in particular on the activity of the primary visual cortex (V1). Activity in this area of the occipital cortex was long believed to exclusively reflect bottom-up low-level analyses of visual input e.g. (Heinze et al., 1994; Clark and Hillyard, 1996), but is now known to be susceptible to top-down influences including both attention for reviews (Posner and Gilbert, 1999; see Gilbert and Sigman, 2007) and reward (Shuler and Bear, 2006; Serences, 2008; Serences and Saproo, 2010; Arsenault et al., 2013; Stănişor et al., 2013) in animals as well as humans. However, it is important to bear in mind that V1 activations reported in neuroimaging studies might reflect fast feedback from higher cortical areas rather than the initial wave of activation (e.g. Roelfsema et al., 1998; Martínez et al., 1999), which are indistinguishable in fMRI data due to their poor temporal resolution. Electrophysiological recordings, in contrast, provide sufficient temporal information to pinpoint the initial activation sweep in the primary visual cortex around 50 to 100 ms after stimulus presentation, as reflected in the C1 component of ERPs (Foxe and Simpson, 2002). Due to the retinotopic organization of V1, the C1 shows reversed polarity in response to stimuli presented in the upper versus the lower visual hemifield. In recent years, a number of top-down factors have been shown to affect the C1, including spatial and feature-based attention (Kelly et al., 2008; Karns and Knight, 2009; Proverbio et al., 2010), perceptual load (Rauss et al., 2009, 2012; Rossi and Pourtois, 2012), perceptual and aversive learning (Stolarova et al., 2006; Pourtois et al., 2008); and anxiety, mood state and emotional processing (Pourtois et al., 2004; Eldar et al., 2010; West et al., 2010; Rossi and Pourtois, 2012, 2014; Vanlessen et al., 2013, 2014). Importantly, however, to date it is unknown whether motivation can modulate the C1 component in human subjects. Furthermore, it is unclear whether such an effect might interact with or depend on visuo-spatial attention.
In order to investigate this question, we employed a factorial manipulation of motivation and spatial attention in a pattern discrimination task, allowing for conclusions about the functional locus of a possible interplay between these two factors. Colored cues concurrently indicated the hemifield of target presentation and whether participants would receive performance-based monetary incentives ( ‘incentive condition’; rewarded for correct responses and punished for incorrect responses), or not (‘neutral condition’). During stimulus presentation, we recorded high-density ERPs in order to investigate the temporal characteristics of sensory processing in the primary and extrastriate visual cortex (C1 and P1, respectively). In order to control for differential cue-related preparatory processes and cognitive effort as a function of attention and/or motivation, we additionally analyzed the contingent negative variation (CNV) (Grent-’t-Jong and Woldorff, 2007), behavioral measures, and pupil dilations (Sirois and Brisson, 2014).
Methods
Participants
Data were collected from 26 participants; the data sets of four participants had to be discarded due to excessive EEG artifacts (2) or poor task performance (2). The remaining participants (thirteen women) had a mean age of 22.5 years (s.d. = 3.4 years). All were right-handed, had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders according to self-report. Participation was compensated with course credit or 8 euro per hour; additionally, all participants received the amount of money they had earned during the task (mean final balance = 24.03 euro, s.d. = 12.08 euro).
Stimuli and task
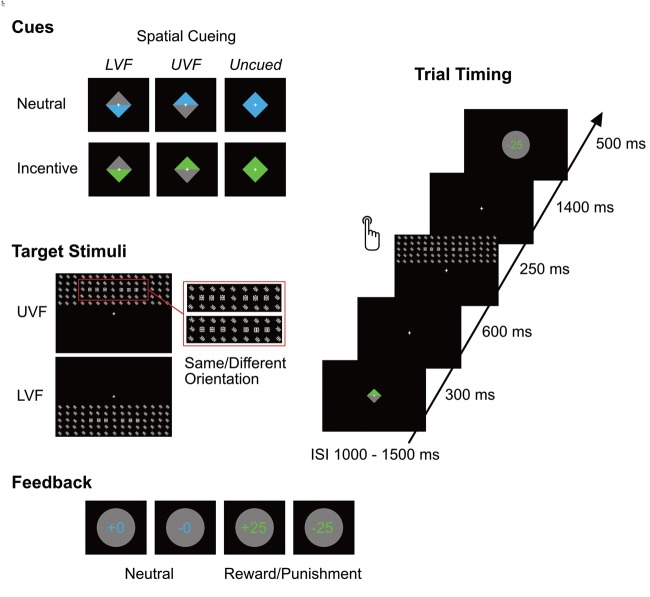
The task required participants to discriminate the orientation of symbols embedded in a large stimulus array presented in the upper or lower visual field (UVF/LVF), while maintaining central fixation; for an overview of task and experimental stimuli, see Figure 1. Stimulus arrays were preceded by a cue, which consisted of a diamond-shaped rectangle presented at the middle of the screen (1.8 * 1.8° of visual angle) for 300 ms. The cue provided twofold information: First, the color of the diamond conveyed information about the motivational relevance of an upcoming target. Blue cues indicated incentive trials, meaning that successful task performance would result in winning 25 cents, while incorrect performance would lead to a loss of 25 cents. Green cues predicted motivationally irrelevant (‘neutral’) trials, without monetary consequences of task performance. Colors were equiluminant; the assignment of colors to the conditions was counterbalanced. Second, the distribution of color in the diamond shape acted as a spatial cue, informing about the location of the upcoming target. If the upper half of the diamond-shaped cue was colored (and the remaining half was grey), the target would always appear in the UVF, whereas a colored lower half indicated target presentation in the LVF. Cues consisting of entirely colored diamonds did not provide any information on the target location (uncued condition). Cues were 100% valid, the ratio of cued/uncued and incentive/neutral trials was 50 percent. A small, white fixation cross (0.3*0.3° of visual angle) was shown continuously throughout the task at the center of the screen.
Fig. 1.
Stimuli and trial structure. Cues provided information about the motivational relevance and the spatial location (UVF/LVF) of the upcoming target stimulus. Target stimuli contained six target symbols, three on each side, which had the same or a different orientation. After giving their response, participants were informed about the accuracy of their response by a feedback stimulus, which also indicated the amount of money they had won or lost on incentive trials.
600 ms after the offset of the cue, a target stimulus was presented in the UVF or LVF for 250 ms. Target stimuli consisted of matrices of 5 × 15 of white symbols on a black background, which were tilted either to the right or to the left side (45° and 315°, respectively). In each matrix six target symbols (three per side) were embedded directly left and right of the vertical meridian. Target symbols were identical to the other items in the matrix, except that they were oriented either horizontally or vertically. In order to keep the target location unpredictable within the matrix across trials, target symbols varied in eccentricity. Participants had to indicate whether the target symbols on the left and right side had the same orientation (both horizontal or both vertical) or not (see Figure 1). Matrices spanned a visual angle of 28.1 * 7.5° and were presented in the UVF/LVF with equal frequency.
Participants were instructed to respond by pressing one of two buttons with their index fingers within 1500 ms after target onset. A feedback was shown in the middle of the screen 1650 ms after target onset for 500 ms, informing the participants about the accuracy of their response and resulting monetary gain/loss. Feedback stimuli consisted of grey disks in which the amount of money participants had either won or lost (‘−25’ or ‘+25’) was indicated. In neutral trials, the disks showed ‘+0’ for correct responses and ‘−0’ for incorrect responses in order to provide performance feedback even in the absence of monetary consequences. The inscription on the disk matched the cue colors. The inter-stimulus interval lasted 1250 ms on average, ranging from 1000 to 1500 ms (variable across trials).
Procedure
The study was approved by the ethics committee of the Department of Psychology at the University of Göttingen, Germany. Participants received information about the experimental procedures and provided informed consent and demographic data prior to the start of the experiment. After EEG preparation, they were seated in a dimly lit and electrically shielded room in front of a 23’ monitor (refresh rate = 60 Hz) positioned at a distance of 57 cm. Participants placed their chin and forehead on a head rest in order to avoid movements and ensure correct tracking of eye movements. After pupil diameter calibration, participants received detailed instructions about the experimental task. The experiment consisted of 10 blocks of 48 stimuli each; after each block, participants were informed about their current balance. Prior to the 10 experimental blocks, participants performed one practice block in order to get familiarized with the task and the trial structure.
Data acquisition and pre-processing
The EEG was recorded continuously from 128 electrodes placed in an electrode cap (Biosemi Active Two) with a 512 Hz sampling rate. Online, signals were referenced to the CMS-DLR ground, which drives the average potential across all electrodes as close as possible to the amplifier zero. Electrode offsets were kept within a range of ±20 μV. Additional external electrodes were applied to the left and right mastoids and at the outer canthi and below both eyes in order to record horizontal and vertical electro-oculograms.
Offline, data processing was performed using BrainVision Analyzer (Brain Products GmbH, Munich, Germany). ERP data were first re-referenced to average reference in order to perform blink correction using Surrogate Multiple Source Eye Correction (Ille et al., 2002) as implemented in Besa (Brain Electric Source Analysis, MEGIS Software GmbH). Data were then re-referenced to average mastoids and bandpass filtered using a zero phase Butterworth filter ranging from 0.016 to 70 Hz with a slope of 12 db/octave. A Notch filter was additionally applied (bandwidth 50 Hz, 24 db/octave). Channels with poor signals were interpolated using spherical splines of order 4 (3.8% of channels).
For both cues and target stimuli, continuous data were segmented into epochs ranging from 100 ms before to 1000 ms after stimulus onset and referred to a 100 ms pre-stimulus baseline. Errors were discarded together with their corresponding cue segment. Epochs containing artifacts, i.e. activations exceeding ± 100 μV or voltage steps larger than 100 μV were discarded in a semiautomatic way. This led to rejection of 6.9% of trials for cue signals (no significant differences between conditions, all Fs < 1.03, all Ps > 0.321) and 7.2% for target signals (no significant differences between conditions, all Fs < 1). Target segments containing saccades towards peripherally presented targets were identified using eye position data (see below) and excluded from analyses along with their corresponding cue segments (1.1% of segments). Finally, segments were averaged per subject, hemifield, and experimental condition (motivation × cueing) separately for targets and cues.
Eye movements and pupil diameter were recorded binocularly using a desktop-mounted eyetracker (EyeLink 1000, SR Research) at a 500 Hz sampling rate. Before the start of the experiment, pupil diameter was calibrated with an artificial pupil in order to obtain absolute pupil diameter values. Recalibration of gaze position was performed at the start of each experimental block using a custom nine-point-grid spanning the area of (peripheral) stimulus presentation. Offline analyses of eye position data and pupil diameter were performed using Matlab. Eye movements towards the peripheral stimulus were identified if eye position deviated for more than 2° of visual angle from the central fixation (corresponding to the proximal edge of the matrix) at any point during the time window from 20 ms before to 400 ms after target onset; these segments were discarded from further analyses. For the analyses of pupil activity, pupil diameter was recalculated to absolute values. Blinks were interpolated and a moving average window of 40 ms was applied. Continuous data were segmented into epochs from 200 ms prior to cue onset to 900 ms after cue onset and from target onset to 1500 ms after target onset; both cue- and target segments were referred to a 200 ms pre-cue baseline. Segments containing artifacts were discarded; remaining segments were averaged per subject and experimental condition.
Data analyses
Behavioral data
Reaction times (RTs) and accuracy (in percent) were calculated separately for each experimental condition and analyzed with repeated-measures (rm) ANOVAs including the factors motivation (incentive, neutral), cueing (cued, uncued) and hemifield (UVF/LVF). In order to perform a manipulation check for the task, we performed further rm-ANOVAs including the vertical eccentricity of the target symbols as additional factor (five levels), assuming that RTs would increase and accuracy would decline with increasing distance from central fixation.
ERPs
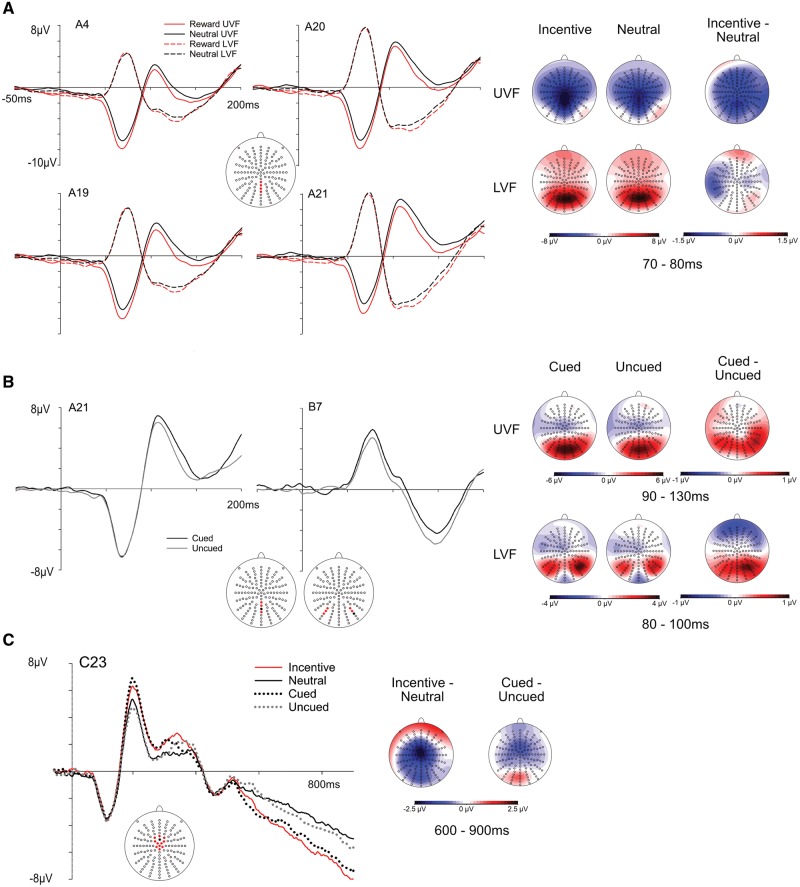
C1 amplitudes were quantified by semiautomatic peak detection in the time window from 50 to 100 ms following target presentation in channels A4, A19, A20 and A21, corresponding to electrodes spanning from CPPz to POz along the midline, in accordance with previous literature (Rossi and Pourtois, 2014). For stimuli presented in the UVF, the C1 was identified as the most negative peak, whereas for LVF presentation, the C1 was scored as the most positive peak. So as to include data from both hemifields into one analysis, C1 amplitudes for UVF presentation were transformed to absolute values (see Kelly et al., 2008). In order to exclude that baseline differences (reflecting differences in CNV activations) would influence C1 results, we performed additional peak analyses using a baseline interval from stimulus onset to 50 ms after stimulus onset. P1 peaks to stimuli presented in the UVF corresponded to a typical P1m, often observed when stimuli occupy the periphery of the UVF (Fu et al., 2001; Handy et al., 2001). Its amplitude was quantified as the most positive deflection within 90 to 130 ms after target onset at electrodes A20–A23, corresponding to PPOz-POz-POOz and Oz. In order to account for differences in the preceding C1 component, P1 amplitudes were analyzed as absolute base-to-peak values by subtracting C1 amplitudes. The P1 in response to LVF stimuli showed a more lateralized topography lacking a pronounced peak, nesting in the offset slope of the preceding (positive) C1 component. Therefore, the P1 was identified by this shift in topographical distribution from midline to occipito-lateral locations (see Kelly et al., 2008), and scored as mean activation ranging from 80 to 100 ms at the lateralized occipital electrodes A8, A9, A10, B5, B6 and B7 (see Figure 2). In order to analyze preparatory processing of cue-related information, we analyzed the CNV in the time window from 600 to 900 ms after cue onset, corresponding to the 300 ms directly preceding target onset. The CNV was scored as mean amplitude on central electrodes A1, A2, B1, C1, C2, C11, C22, C23, C24, D1, D2, D15 (see Figure 2 for the location of electrodes). All target-related ERP components were analyzed using rm—ANOVAs including the factors motivation (incentive, neutral), cueing (cued, uncued) and hemifield (UVF/LVF). Analyses of cue-related CNV activity included only the factors motivation and cueing.
Fig. 2.
ERP grand mean waveforms and topographies. (A) C1 waveforms (left) and scalp topographies (right) for incentive and neutral trials presented in the UVF/LVF. The position of the electrodes is indicated by the red dots on the electrode layout. Scalp distributions refer to specified time intervals. (B) P1 waveforms (left) and scalp distributions (right) of the P1 in response to stimuli presented in the UVF (electrode A21) and in the LVF (electrode B7). Electrodes comprising the respective regions of interest are marked in red, depicted electrodes are indicated in black. (C) Grand mean waveforms in response to cue stimuli (left), depicting enhanced amplitudes of the CNV for incentive vs neutral and cued vs uncued trials. On the right, distributions of difference waves between incentive minus neutral and cued minus uncued conditions.
Pupil data
Pupil data were analyzed by means of rm-ANOVAs including the factors motivation and cueing. In order to account for differences in cue-related pupil activity attributable to both luminosity and cognitive processing of the cue, analyses included an additional cue/target factor (e.g. a time factor). This procedure allows for assessing target-related activity independently of cue-related activations. Using this procedure, relevant effects were expected in the form of interactions of experimental factors (motivation and cueing) and the cue/target factor, indicating a significant change over time. Cue-related activity was quantified as the mean activation from cue onset to target onset; target-related activity comprised the mean from target onset to 1500 ms after target onset. In order to provide further information about the time course of experimental effects, we calculated point-wise 95% within-subject confidence intervals (according to Cousineau 2005) from 200 ms prior to cue onset to 2500 ms after target onset.
Results
Behavior
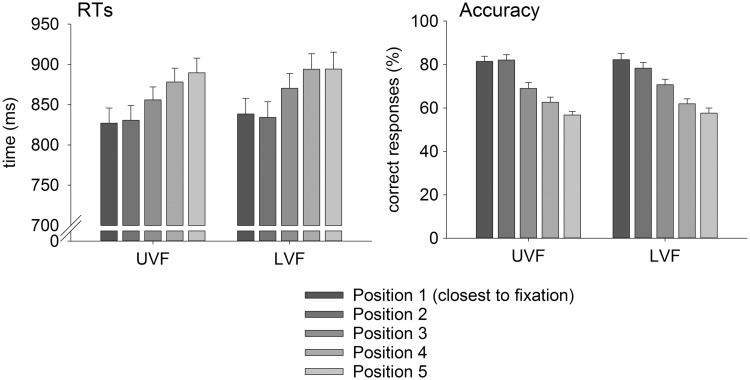
Results of the task manipulation check revealed that the vertical eccentricity of the target symbols affected both RTs and accuracy (see Figure 3). As expected, RTs increased with increasing distance from central fixation, F(4,84) = 23.30, P < 0.001, = 0.526, while accuracy decreased, F(4,84) = 83.97, P < 0.001, = 0.800.
Fig. 3.
Behavioral results. RTs and accuracy (means and SEs) for UVF, LVF and all eccentricities of target symbol presentation. Results show increasing RTs and decreasing accuracy with increasing distance of the target symbol from central fixation.
RT analyses revealed a main effect of cueing, F(1,21) = 9.69, P < 0.01, = 0.316, reflecting faster responses to cued in comparison to uncued trials (Table 1). Furthermore, participants reacted faster in response to stimuli presented in the upper than in the LVF, F(1,21) = 5.53, P < 0.05, = 0.208. The factor motivation did not influence RTs, F(1,21) = 1.36, P = 0.257, and there was no interaction between motivation and cueing, F(1,21) = 2.04, P = 0.168.
Table 1.
Means and standard errors of RTs and accuracy in the pattern recognition task, separately for UVF/LVF presentations
| Incentive |
Neutral |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cued |
Uncued |
Cued |
Uncued |
|||||
| UVF | LVF | UVF | LVF | UVF | LVF | UVF | LVF | |
| RT [ms] | 818.8 (30.2) | 828.1 (30.4) | 830.4 (23.7) | 852.9 (27.9) | 810.9 (30.9) | 811.8 (30.9) | 837.8 (23.8) | 842.6 (25.5) |
| Accuracy [%] | 68.6 (2.3) | 70.3 (2.6) | 68.5 (2.4) | 69.0 (2.7) | 70.0 (2.7) | 67.4 (2.4) | 66.8 (2.1) | 67.9 (2.0) |
The accuracy of participants’ responses was not influenced by motivation, cueing, or their interaction, all Fs(1,21) < 1.86, Ps > 0.187.
C1
Analyses revealed a main effect of motivation, F(1,21) = 6.11, P < 0.05, = 0.225, reflecting larger C1 amplitudes for incentive trials as compared with neutral trials (Figure 2). This result was replicated in an auxiliary analysis applying a 0–50 ms baseline in order to account for pre-target differences during the CNV interval, F(1,21) = 6.33, P < 0.05, = 0.232. The amplitudes of the C1 were not affected by the factor cueing, F(1,21) < 1, and there was no interaction between motivation and cueing, F(1,21) = 1.23, P = 0.278. As evident from Figure 2, motivation effects on the C1 seemed restricted to the UVF, although the interaction of motivation and hemifield did not reach significance, F(1,21) = 2.79, P = 0.110. We performed additional planned post-tests (Bonferroni-corrected for multiple comparisons) of C1 amplitudes within each hemifield, which revealed that the effect of motivation was indeed limited to the UVF, F(1,21) = 6.84, P < 0.05, while it was absent for the LVF, F(1,21) < 1.
P1
P1 amplitudes were enhanced for cued trials in comparison to uncued trials, both for stimuli presented in the UVF, F(1,21) = 4.78, P < 0.05, = 0.186, and in the LVF, F(1,21) = 6.40, P < 0.05, = 0.234. For both hemifields, P1 activity was not influenced by the factor motivation or by the interaction of motivation and cueing, all Fs(1,21) < 1.
CNV
The CNV was affected by both motivation and cueing, but did not show an interaction effect between these two factors. Its amplitude was larger, i.e. more negative, for incentive as compared with neutral trials, F(1,21) = 21.54, P < 0.001, = 0.506, and for cued in comparison to uncued trials, F(1,21) = 9.61, P < 0.01, = 0.314; there was no interaction between motivation and cueing, F(1,21) < 1.
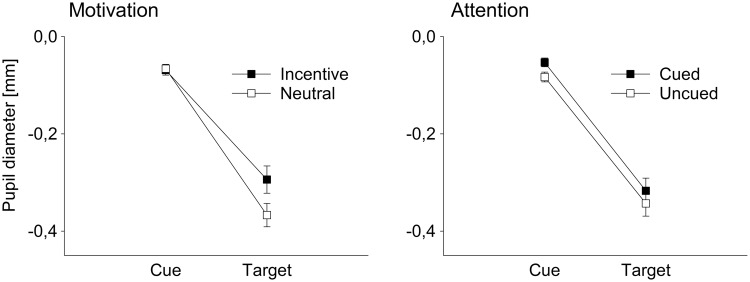
Pupil activity
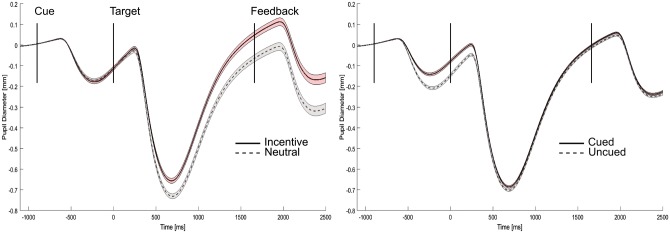
Analyses of mean pupil diameter revealed a significant interaction of the cue/target factor and motivation, F(1,21) = 66.51, P < 0.001, = 0.760, reflecting larger pupillary dilations for incentive than for neutral trials selectively during target processing (see Figure 4). The factor cueing did not show a significant interaction with cue/target activity, F(1,21) = 1.08, P = 0.311, showing that there was no effect of cueing on target activity beyond cue-related differences. Finally, there was no three-way interaction between the cue/target factor, motivation and cueing, F(1,21) = 1.53, P = 0.230. Analyses of point-wise 95% CIs revealed the onset of motivation effects at around 270 ms after target onset, whereas effects of spatial attention were limited to the cueing interval (Figure 5).
Fig. 4.
Cue-related and target-related pupillary responses. Left side: Results show larger pupil diameter for incentive trials compared with neutral trials during target processing, irrespective of cue-related responses. Right side: Cueing does not influence target-related pupillary responses above cue-related differences. Note that the general decrease in pupil size during target processing is due to the increased luminance of the stimulus arrays.
Fig. 5.
Time course of pupillary responses. Mean pupil diameter for incentive vs neutral trials (left side) and cued vs uncued trials (right side), including point-wise within-subject 95% CIs.
Discussion
This study investigated the impact of reward prospect and spatial attention on the activity of the human primary (V1) and extrastriate visual cortex using ERPs and a factorial design. For the first time, we show that motivational relevance can increase the first wave of activation in human V1 (i.e. the C1 component) within 100 ms after stimulus onset. Importantly, this early striate modulation was not co-occurring or interacting with effects of spatial attention, which were evident only at a later processing stage, at the level of the P1 generated in the extrastriate visual cortex.
Increased activity in V1 for reward was previously reported in a number of fMRI studies (Serences, 2008; e.g. Serences and Saproo, 2010). However, unlike ERPs, fMRI data cannot distinguish between the initial activation sweep in V1 per se, and later, re-entrant processing (Martínez et al., 1999) due to its sluggish temporal resolution. Therefore, the present results provide the first evidence for a direct impact of motivational relevance on the activity of the human primary visual cortex to neutral visual stimuli. As such, they contribute to the growing understanding that early V1 activity in humans, as captured by the C1, does not reflect purely bottom-up low-level visual processing, but instead, is susceptible to top-down modulations (Rauss et al., 2011), in this case with a focus on the actual and trial-dependent motivational relevance of the stimulus. Notably, motivation effects on the C1 only occurred in response to stimuli presented in the UVF. This finding is compatible with a study showing that effects of perceptual learning on C1 activity were limited to UVF stimuli (Pourtois et al., 2008), possibly due to its reduced contrast sensitivity, which might leave room for further tuning in visual texture processing (Talgar and Carrasco, 2002).
Given the temporal precedence of motivational relevance over spatial attention effects reported here, the current data provide support for the hypothesis that the impact of reward motivation on visual cortex activity does not strictly depend on spatial attention mechanisms (e.g. Serences and Saproo, 2010; but see Stănişor et al., 2013 for interactions of motivation and feature-based attention). As a consequence, motivational effects seem to be spatially unspecific rather than limited to relevant sections of the visual field. In line with our findings, previous fMRI studies reported that V1 activity was exclusively modulated by incentive value, despite interactions of motivation and attention in the fronto-parietal attention network (Engelmann et al. 2009; see also Krebs et al., 2012). Similarly, ERP and MEG effects of motivation and attention in the visual cortex were previously shown to be mostly independent and non-overlapping, although interactive effects occurred at later, higher-order processing stages (Baines et al., 2011; see also Hopf et al., 2015). Therefore, it seems that motivation effects on neural activity in the visual cortex can precede and occur independently of spatial attention, even in case of interactions within the fronto-parietal attention network. Rather, reward-related cortical and subcortical brain areas might play a causal role in regulating the primary visual cortex in expectation of motivationally relevant stimuli. Indications for the existence of a hierarchical neural architecture for effects of motivation and attention comes from a study showing reward timing representations in V1 neurons of rats (Shuler and Bear, 2006). Likely candidate regions for the translation of motivational value to the visual cortex include the amygdala (Amaral et al., 2003; Pourtois et al., 2013) and the orbitofrontal cortex (Kringelbach and Rolls, 2004). Furthermore, research suggests the involvement of dopaminergic pathways in this process (Arsenault et al., 2013), both by direct connections and indirectly via the striatum and frontal and parietal cortices (for a discussion, see Serences and Saproo, 2010), and locus coeruleus- norepinephrine activity (Markovic et al., 2014). As an alternative to a hierarchical organization, it was suggested that both motivation and spatial attention might contribute to a shared priority map (Ptak, 2012; Chelazzi et al., 2014) which might then influence perception; however, the current design does not allow for a differentiation between the two options.
When interpreting the present results, it is important to bear in mind that the modulation of V1 activity reported here does not solely reflect reward-related processing, but motivational relevance in a broader sense. This is due to the design of this study where, on incentive trials, participants would not only win money for correct performance, but would also lose money in case of errors. Therefore, incentive trials reflect both reward approach and punishment avoidance. Previous research suggested the involvement of dissociable brain regions underlying these two factors: Although the prospect of winning was primarily reflected in the orbitofrontal cortex, the prospect of losing money was reflected in the dorsal ACC (Small et al., 2005; Shackman et al., 2011). Clearly, future research needs to determine the role of the two types of incentive motivation in tuning the activity of the visual cortex.
A second consideration concerns the nature of the motivational effect reported in this study. Due to the cueing paradigm, motivational relevance was conveyed by the cue, rather than by the target stimulus itself (for a review of different paradigms, see Chelazzi et al., 2013). As such, the current design does not allow for conclusions whether the effect of motivation is target-specific or reflects a general boost in activation, since it did not include a non-target condition. Future research is needed to determine whether the motivational relevance of a feature embedded in a target stimulus, rather than in a cue, is able to influence the activity of the primary visual cortex (i.e. in terms of tuning), and whether such an effect could be influenced by directing spatial attention towards a relevant location.
Effects of spatial attention occurred first after around 100 ms after stimulus onset, increasing the amplitudes of the P1; this effect did not interact or co-occur with motivation effects. Although the finding of increased P1 amplitudes is in line with previous results obtained in a feature-based reward paradigm (Hickey et al., 2010), other studies reported interactions of reward and attention at the level of the P1 component (MacLean and Giesbrecht, 2015) and even in V1 activity (Stănişor et al., 2013). This mixed pattern of results suggests that effects and interactions of attention and motivation within the visual cortex might strongly depend upon specific task parameters and the way attention and motivational relevance are eventually operationalized. Apart from different experimental designs (conveying motivational relevance either by a cue or by a target feature), variations of attention include not only feature-based and spatial attention, but, in the latter case, also comparisons of attended vs unattended locations (in case of valid/invalid cues) and attention vs no-attention conditions. Since attention is known to sharpen sensory representations by both amplifying relevant (i.e. attended) and suppressing irrelevant (unattended) information (Martinez-Trujillo and Treue, 2004), these different operationalizations might well influence the specific pattern of results and interactions found in a given study. For example, spatial attention was previously shown to impact the C1 amplitude in a comparison of attended vs unattended locations (Kelly et al., 2008), but did not show an effect in this study, where attended locations were compared with uncued locations (which are characterized by a diffuse attentional focus, rather than by being unattended per se).1
By now, there is growing consensus that the influence of motivation critically depends upon task parameters and demands (Padmala and Pessoa, 2010). For example, RTs in response to motivationally relevant stimuli were previously shown to be faster in tasks that included a RT cutoff (Small et al., 2005; Baines et al., 2011). In this study, however, there was no change in RT speed since participants did not need to reach a time criterion for receiving reward. However, despite a lack of behavioral effects of motivation, pupil dilations were increased during incentive trials as compared with neutral trials, suggesting increased cognitive effort in the incentive condition. A recent study demonstrated that luminance-related increases in pupil diameter cause decreases of C1 amplitudes (Bombeke et al., 2016). Thus, the present finding of increased pupillary responses in combination with increased C1 amplitudes in response to incentive trials provides evidence for a true top-down modulation of V1 irrespective of a possible confound of pupil size. Additionally, and in line with previous literature (Schevernels et al., 2014; van den Berg et al. 2014), cue-related preparatory processes as indexed by the CNV were strongly increased preceding incentive vs. neutral targets, as well as for spatial attention, showing that participants actively used the information provided by the cue in order to prepare for the upcoming trial. However, despite this evidence for increased effort and preparation, behavioral accuracy remained unaffected by both motivation and spatial attention. This finding is most likely due to the nature of stimuli and task, which was optimized for eliciting large C1 responses and required participants to maintain broad peripheral attention in order to detect and compare the target symbols on both sides of the matrix. As such, task performance might not have profited from focused attention or increased effort, and future research should aim to replicate the present findings with more established paradigms of spatial attention. However, although we cannot exclude that participants did not make use of the motivational cue, we believe that the current findings rather indicate that increased preparation (CNV) and heightened activation in the visual cortex (C1) were not reflected at the behavioral level, probably due to the nature of the task.
Finally, it should be mentioned that pupillary responses might reflect arousal-related processing rather than increased cognitive effort (Sirois and Brisson, 2014). Nevertheless, it appears unlikely that unspecific arousal might account for our C1 modulation given that previous ERP studies failed to evidence a clear link between physiological arousal and changes in C1 amplitude (Rossi and Pourtois, 2014).
This study employed a cued pattern recognition task including factorial manipulations of motivation and spatial attention. The C1 component, indexing activity within the primary visual cortex with a peak at around 70 ms after stimulus onset, was increased in response to motivationally relevant as compared with irrelevant (neutral) trials. This boost in early V1 activity occurred independently of spatial attention, which increased the activity of the P1 component at around 100 ms after stimulus onset. These findings point to the existence of a neural architecture supporting the amplification of motivationally relevant input from the first steps in the visual cortex and independently of top-down spatial attention.
Funding
The authors want to thank the U4 network for the generous financial support. G.P. is supported by the Belgian Science Policy, Interuniversity Attraction Poles program (P7/11), by a Concerted Research Action Grant from Ghent University and by the FWO (Research Foundation Flanders). A.S. and M.B. are supported by the German Research Foundation (DFG; Grant no. SCHA1848/1-1). V.R. is supported by the Special Research 60 Funds at Ghent University (Grant no. BOF13/PDO/095).
Conflict of interest. None declared.
Footnotes
Since Kelly and colleagues (2008) used an individualized scoring procedure for quantifying C1 amplitudes, it cannot be excluded that the lack of attentional effect in the current study is due to these differences in data analyses. However, this would imply the assumption of differential distributions of C1 effects for motivation and spatial attention, which would be hard to reconcile with its assumed neural origin.
References
- Amaral D.G., Behniea H., Kelly J.L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118, 1099–120. [DOI] [PubMed] [Google Scholar]
- Anderson B.A., Yantis S. (2013). Persistence of value-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 39, 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault J.T., Nelissen K., Jarraya B., et al. (2013). Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron 77, 1174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines S., Ruz M., Rao A., et al. (2011). Modulation of neural activity by motivational and spatial biases. Neuropsychologia, 49, 2489–97. [DOI] [PubMed] [Google Scholar]
- Bombeke K., Duthoo W., Mueller S.C., et al. (2016). Pupil size directly modulates the feedforward response in human primary visual cortex independently of attention. Neuroimage, 127, 67–73. [DOI] [PubMed] [Google Scholar]
- Bourgeois A., Chelazzi L., Vuilleumier P. (2016). How motivation and reward learning modulate selective attention. Progress in Brain Research 229, 325–42. [DOI] [PubMed] [Google Scholar]
- Chelazzi L., Eštočinová J., Calletti R., et al. (2014). Altering spatial priority maps via reward-based learning. Journal of Neuroscience, 34, 8594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L., Perlato A., Santandrea E., et al. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–62. [DOI] [PubMed] [Google Scholar]
- Clark V.P., Hillyard S.A. (1996). Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience, 8, 387–402. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58, 306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Cousineau D. (2005). Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology, 1, 42–5. [Google Scholar]
- Della Libera C., Chelazzi L. (2009). Learning to attend and to ignore is a matter of gains and losses. Psychological Science, 20, 778–84. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Eldar S., Yankelevitch R.R., Lamy D., et al. (2010). Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology, 85, 252–7. [DOI] [PubMed] [Google Scholar]
- Engelmann J.B., Damaraju E., Padmala S., et al. (2009). Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.B., Pessoa L. (2007). Motivation sharpens exogenous spatial attention. Emotion, 7, 668–74. [DOI] [PubMed] [Google Scholar]
- Failing M.F., Theeuwes J. (2014). Exogenous visual orienting by reward. Journal of Vision, 14, 1–9. [DOI] [PubMed] [Google Scholar]
- Failing M.F., Theeuwes J. (2015). Nonspatial attentional capture by previously rewarded scene semantics. Vision Cognition, 23, 82–104. [Google Scholar]
- Foxe J.J., Simpson G.V. (2002). Flow of activation from V1 to frontal cortex in humans: a framework for defining “early” visual processing. Experimental Brain Research, 142, 139–50. [DOI] [PubMed] [Google Scholar]
- Fu S., Fan S., Chen L., et al. (2001). The attentional effects of peripheral cueing as revealed by two event-related potential studies. Clinical Neurophysiology, 112, 172–85. [DOI] [PubMed] [Google Scholar]
- Gilbert C.D., Sigman M. (2007). Brain States: Top-Down Influences in Sensory Processing. Neuron, 54, 677–96. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T., Woldorff M.G. (2007). Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biology, 5, 0114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy T.C., Soltani M., Mangun G.R. (2001). Perceptual load and visuocortical processing: event-related potentials reveal sensory-level selection. Psychological Science, 12, 213–8. [DOI] [PubMed] [Google Scholar]
- Heinze H.J., Mangun G.R., Burchert W., et al. (1994). Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature, 372, 543–6. [DOI] [PubMed] [Google Scholar]
- Hickey C., Chelazzi L., Theeuwes J. (2010). Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience, 30, 11096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf J.M., Schoenfeld M.A., Buschschulte A., et al. (2015). The modulatory impact of reward and attention on global feature selection in human visual cortex. Vision Cogntion, 23, 229–48. [Google Scholar]
- Ille N., Berg P., Scherg M. (2002). Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology, 19, 113–24. [DOI] [PubMed] [Google Scholar]
- Karns C.M., Knight R.T. (2009). Intermodal auditory, visual, and tactile attention modulates early stages of neural processing. Journal of Cognitive Neuroscience, 21, 669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–41. [DOI] [PubMed] [Google Scholar]
- Kelly S.P., Gomez-Ramirez M., Foxe J.J. (2008). Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral Cortex, 18, 2629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E.I. (2007). Fundamental Components of Attention. Annual Review of Neuroscience, 30, 57–78. [DOI] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Roberts K.C., et al. (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22, 607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M., Rolls E.T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–72. [DOI] [PubMed] [Google Scholar]
- MacLean M.H., Giesbrecht B. (2015). Neural evidence reveals the rapid effects of reward history on selective attention. Brain Research, 1606, 86–94. [DOI] [PubMed] [Google Scholar]
- Markovic J., Anderson A.K., Todd R.M. (2014). Tuning to the significant: neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research, 259, 229–41. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J.C., Treue S. (2004). Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology, 14, 744–51. [DOI] [PubMed] [Google Scholar]
- Martínez A., Anllo-Vento L., Sereno M.I., et al. (1999). Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience, 2, 364–9. [DOI] [PubMed] [Google Scholar]
- Mohanty A., Sussman T.J. (2013). Top-down modulation of attention by emotion. Frontiers in Human Neuroscience, 7, 102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre A., Sebestyen G., Gitelman D., et al. (1997). Functional localization of the system for visuospatial attention using positron emission tomography. Brain, 120, 515–33. [DOI] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. (2010). Interactions between cognition and motivation during response inhibition. Neuropsychologia, 48, 558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2015). Multiple influences of reward on perception and attention. Vision Cognition, 23, 272–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Gilbert C.D. (1999). Attention and primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 96, 2585–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Grandjean D., Sander D., et al. (2004). Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex, 14, 619–33. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Rauss K., Vuilleumier P., et al. (2008). Effects of perceptual learning on primary visual cortex activity in humans. Vision Research, 48, 55–62. [DOI] [PubMed] [Google Scholar]
- Pourtois G., Schettino A., Vuilleumier P. (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92, 492–512. [DOI] [PubMed] [Google Scholar]
- Proverbio A.M., Del Zotto M., Zani A. (2010). Electrical neuroimaging evidence that spatial frequency-based selective attention affects V1 activity as early as 40-60 ms in humans. BMC Neuroscience, 11, 59.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. (2012). The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscience, 18, 502–15. [DOI] [PubMed] [Google Scholar]
- Rauss K., Pourtois G., Vuilleumier P., et al. (2009). Attentional load modifies early activity in human primary visual cortex. Human Brain Mapping, 30, 1723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss K., Pourtois G., Vuilleumier P., et al. (2012). Effects of attentional load on early visual processing depend on stimulus timing. Hum Brain Mapping, 33, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss K., Schwartz S., Pourtois G. (2011). Top-down effects on early visual processing in humans: A predictive coding framework. Neuroscience and Biobehavioral Reviews, 35, 1237–53. [DOI] [PubMed] [Google Scholar]
- Roelfsema P.R., Lamme V.A., Spekreijse H. (1998). Object-based attention in the primary visual cortex of the macaque monkey. Nature, 395, 376–81. [DOI] [PubMed] [Google Scholar]
- Rossi V., Pourtois G. (2012). State-dependent attention modulation of human primary visual cortex: A high density ERP study. Neuroimage, 60, 2365–78. [DOI] [PubMed] [Google Scholar]
- Rossi V., Pourtois G. (2014). Electrical neuroimaging reveals content-specific effects of threat in primary visual cortex and fronto-parietal attentional networks. Neuroimage, 98, 11–22. [DOI] [PubMed] [Google Scholar]
- Schevernels H., Krebs R.M., Santens P., et al. (2014). Task preparation processes related to reward prediction precede those related to task-difficulty expectation. Neuroimage, 84, 639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1, 199–207. [DOI] [PubMed] [Google Scholar]
- Seitz A.R., Kim D., Watanabe T. (2009). Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron, 61, 700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences J.T., Saproo S. (2010). Population response profiles in early visual cortex are biased in favor of more valuable stimuli. Journal of Neurophysiology, 104, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences J.T. (2008). Value-based modulations in human visual cortex. Neuron, 60, 1169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., et al. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler M.G., Bear M.F. (2006). Reward timing in the primary visual cortex. Science (80-), 311, 1606–9. [DOI] [PubMed] [Google Scholar]
- Sirois S., Brisson J. (2014). Pupillometry. Wiley Interdiscip Rev Cogn Sci 5, 679–92. [DOI] [PubMed] [Google Scholar]
- Small D.M., Gitelman D., Simmons K., et al. (2005). Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex, 15, 1855–65. [DOI] [PubMed] [Google Scholar]
- Stănişor L., van der Togt C., Pennartz CM. a., et al. (2013). A unified selection signal for attention and reward in primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 110, 9136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarova M., Keil A., Moratti S. (2006). Modulation of the C1 visual event-related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cerebral Cortex, 16, 876–87. [DOI] [PubMed] [Google Scholar]
- Talgar C.P., Carrasco M. (2002). Vertical meridian asymmetry in spatial resolution: visual and attentional factors. Psychonomic Bulletin and Review, 9, 714–22. [DOI] [PubMed] [Google Scholar]
- van den Berg B., Krebs R.M., Lorist M.M., et al. (2014). Utilization of reward-prospect enhances preparatory attention and reduces stimulus conflict. Cognitive, Affective, and Behavioral Neuroscience, 14, 561–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlessen N., Rossi V., De Raedt R., et al. (2013). Positive emotion broadens attention focus through decreased position-specific spatial encoding in early visual cortex: Evidence from ERPs. Cognitive, Affective, and Behavioral Neuroscience, 60–79. [DOI] [PubMed] [Google Scholar]
- Vanlessen N., Rossi V., De Raedt R., et al. (2014). Feeling happy enhances early spatial encoding of peripheral information automatically: electrophysiological time-course and neural sources. Cognitive, Affective, and Behavioral Neuroscience, 951–69. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2015). Affective and motivational control of vision. Current Opinion in Neurology, 28, 29–35. [DOI] [PubMed] [Google Scholar]
- Weil R.S., Furl N., Ruff C.C., et al. (2010). Rewarding feedback after correct visual discriminations has both general and specific influences on visual cortex. Journal of Neurophysiology, 104, 1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G.L., Anderson A.A.K., Ferber S., et al. (2010). Electrophysiological evidence for biased competition in V1 favoring motivationally significant stimuli. Journal Vision, 9, 464. [Google Scholar]
- Wise R.A. (2004). Dopamine, learning and motivation. Nature Reviews Neuroscience, 5, 483–94. [DOI] [PubMed] [Google Scholar]