Abstract
Therapeutic alliance and perceived social support are important predictors of treatment response for post-traumatic stress disorder (PTSD). Intranasal oxytocin administration may enhance treatment response by increasing sensitivity for social reward and thereby therapeutic alliance and perceived social support. As a first step to investigate this therapeutical potential, we investigated whether intranasal oxytocin enhances neural sensitivity to social reward in PTSD patients. Male and female police officers with (n = 35) and without PTSD (n = 37) were included in a double-blind, randomized, placebo-controlled cross-over fMRI study. After intranasal oxytocin (40 IU) and placebo administration, a social incentive delay task was conducted to investigate neural responses during social reward and punishment anticipation and feedback. Under placebo, PTSD patients showed reduced left anterior insula (AI) responses to social rewards (i.e. happy faces) compared with controls. Oxytocin administration increased left AI responses during social reward in PTSD patients, such that PTSD patients no longer differed from controls under placebo. Furthermore, in PTSD patients, oxytocin increased responses to social reward in the right putamen. By normalizing abberant insula responses and increasing putamen responses to social reward, oxytocin administration may enhance sensitivity for social support and therapeutic alliance in PTSD patients. Future studies are needed to investigate clinical effects of oxytocin.
Keywords: PTSD, oxytocin, social, reward, insula, medication-enhanced psychotherapy (MEP)
Introduction
Although effective psychotherapy is available for post-traumatic stress disorder (PTSD), such as cognitive behavioral therapy and exposure therapy, about one-fifth of patients dropout of therapy (Imel et al., 2013). Additionally, in patients who do complete treatment, about one third still meet PTSD criteria after treatment (Bradley et al., 2005). It is therefore imperative to improve currently available treatments for PTSD patients. Medication-enhanced psychotherapy (MEP) is a possible strategy to increase psychotherapy response in PTSD (Dunlop et al., 2012). Administering pharmacological agents during psychotherapy can target specific factors that are known to affect treatment success in PTSD.
Therapeutic alliance, defined as the formation of a collaborative and affective bond between patient and therapist, is one of the most consistent predictors of psychotherapy outcome in psychiatric and PTSD patients (Martin et al., 2000; Ormhaug et al., 2015). Perceived social support, within and outside of the therapeutic setting, is also an important predictor of treatment success in PTSD (e.g. Thrasher et al., 2010). Unfortunately however, PTSD can lead to social withdrawal and reduced perceived social support (Kaniasty and Norris, 2008; Pietrzak et al., 2010). Thereby, PTSD patients are at increased risk of low therapeutic alliance and reduced treatment success (Charuvastra and Cloitre, 2008).
PTSD patients are less sensitive to positive social stimuli and show reduced motivation to approach socially rewarding stimuli, which may underlie feelings of social detachment, low perceived social support and reduced motivation for therapeutic alliance (Charuvastra and Cloitre, 2008; Nawijn et al., 2015). For example, PTSD patients reported less positive feelings, less approach and more avoidance behavior in response to happy faces and positive social autobiographical memories compared with controls (Frewen et al., 2010; Felmingham et al., 2014; Steuwe et al., 2014; Clausen et al., 2016). Also, reduced neural sensitivitiy to reward is observed in PTSD patients, in brain areas important for signaling rewarding and socially salient stimuli; when compared with controls, patients with PTSD showed reduced neural responses towards positive social stimuli (e.g. happy faces) in the striatum, medial prefrontal cortex (mPFC) and insula (Ehlers et al., 2006; Frewen et al., 2010; Aupperle et al., 2012; MacNamara et al., 2013; Felmingham et al., 2014; Moser et al., 2015). Striatal responses to reward were negatively correlated with anhedonic symptoms such as social detachment in PTSD patients (Elman et al., 2009; Felmingham et al., 2014).
Alongside reduced sensitivity to positive social stimuli, PTSD patients are more sensitive to negative stimuli (Stein and Paulus, 2009). A meta-analysis on neural responses to negative (non-trauma-related) stimuli, such as angry faces, found consistent increased neural responsiveness [e.g. amygdala and anterior insula (AI)] in PTSD patients compared with controls (Hayes et al., 2012). For example, female PTSD patients showed increased insula responses to videos of negative social scenes and during anticipation of negative pictures, but decreased insula responses to positive social videos and anticipation of positive pictures, compared with healthy women (Aupperle et al., 2012; Moser et al., 2015).
Together, reduced sensitivity to positive social stimuli and increased sensitivity for negative social stimuli may be involved in social withdrawal and anhedonia symptoms, and the repeatedly observed reduced perceived social support and reduced ability and motivation to form a therapeutic alliance in PTSD patients (Charuvastra and Cloitre, 2008). Pharmacological enhancement of social reward sensitivity may augment response to currently available psychotherapy, by facilitating social engagement in the therapeutic setting (Johansen and Krebs, 2009; Dunlop et al., 2012). The neuropeptide oxytocin is an interesting candidate for enhancing sensitivity for positive social stimuli (e.g. Bakermans-Kranenburg and van IJzendoorn, 2013). A meta-analysis showed that in healthy individuals oxytocin increases (in-group) trust (Van IJzendoorn and Bakermans-Kranenburg, 2012). Also, intranasal oxytocin increased social approach behavior, such as reduced social distance between participants and the experimenter (Preckel et al., 2014), increased feelings of social support during recall of negative memories (Cardoso et al., 2016), and increased eye contact with the experimenter (Auyeung et al., 2015). Furthermore, oxytocin increased the ability to benefit from social support during stressful situations (Heinrichs et al., 2003). In PTSD patients, oxytocin administration was found to increase compassion towards others (Palgi et al., 2016). On a neural level, oxytocin administration increased striatal, AI and mPFC responses to positive social stimuli, and other areas important in processing social reward in healthy individuals (Groppe et al., 2013; Scheele et al., 2013, 2014; Striepens et al., 2014). Together, these findings suggest that oxytocin has the potential to increase social reward sensitivity. This may benefit social interaction within psychotherapy, increasing the capacity to profit from social support and form a stable therapeutic alliance, thereby enhancing effectiveness of currently available PTSD psychotherapy (Olff et al., 2010; Quirin et al., 2014).
Furthermore, oxytocin administration is generally thought to decrease sensitivity to negative social stimuli or social punishment. Whereas intranasal oxytocin increased amygdala responses to happy faces, it decreased responses to angry faces in healthy subjects (Gamer et al., 2010). Also in generalized social anxiety disorder and borderline personality disorder, oxytocin reduced amygdala responses to angry and fearful faces (Labuschagne et al., 2010; Bertsch et al., 2013). However, several studies report that oxytocin may also increase sensitivity to negative social stimuli (e.g. Domes et al., 2010; Striepens et al., 2012). These discrepancies may depend on (clinical) characteristics and especially sex of the investigated populations (Bartz et al., 2011). For instance, in healthy men, oxytocin decreased amygdala responses to emotional faces (Domes et al., 2007), whereas in women oxytocin increased amygdala responses (Domes et al., 2010). To date, only a few studies have directly investigated sex differences in oxytocin effects within the same study. Two recent studies that directly compared males and females suggest that oxytocin enhances striatal responses to social cooperation in men, but has no effect or even reduces striatal responses to social cooperation in females (Feng et al., 2015; Rilling et al., 2014). Differential oxytocin effects have also been observed in patient-control studies, showing beneficial effects in patients with sub-optimal amygdala responses under placebo, but no effects in controls (e.g. Labuschagne et al., 2010; Bertsch et al., 2013). Furthermore, results of the first (pilot) MEP studies investigating oxytocin administration in addition to psychotherapy in patients with mood and anxiety disorders have been mixed, and included decreased social avoidance behavior in depressed males (MacDonald et al., 2013) but also nominally lower ratings of therapeutic alliance in patients with arachnaphobia (Acheson et al., 2015). Thus, to increase our understanding of the clinical and neurobiological effects of oxytocin administration in psychiatric populations, it is necessary to investigate different psychiatric populations, both male and female patients. As trauma-exposure without PTSD is also associated with altered reward processing (e.g. Pizzagalli 2014) as well as differential effects of oxytocin administration (e.g. Simeon et al., 2011), it is also important to control for potential effects of trauma-exposure.
To our knowledge, the effects of oxytocin administration on social reward have not yet been studied in PTSD patients, although this group of psychiatric patients is highly in need of improved treatment options. Therefore, we investigated the potential of a single intranasal oxytocin administration [40 international units (IUs)] to enhance neural sensitivity to social reward and potentially decrease sensitivity to social punishment in PTSD patients, by conducting a randomized within-subjects fMRI study in trauma-exposed male and female police officers with and without PTSD. Based on the above, we specifically focused on the striatum, amygdala and AI as regions of interest (ROI).
Methods
Participants and procedure
In total, 40 healthy trauma-exposed police officers (n = 20 males) and 40 police officers with PTSD (n = 21 males) were included. Participants were recruited through advertisements in magazines and on websites of the Dutch police. Additionally, PTSD participants were recruited through a diagnostic outpatient center for police personnel (Diemen, the Netherlands). Clinical interviews were administered to assess psychopathology in potentially eligible participants. The Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995) was used to assess current PTSD symptomatology. The Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) or Structured Clinical Interview for DSM–IV (SCID, for participants included at the diagnostic outpatient center) (First et al., 2002) was used to assess other axis 1 psychiatric disorders. For PTSD participants inclusion criteria were current PTSD diagnosis according to the DSM-IV and a CAPS score ≥45. Exclusion criteria for PTSD patients were current severe MDD (MDD with high suicide risk and/or psychotic symptoms), current alcohol/substance abuse, bipolar disorder or psychotic disorders. Because of high comorbidity between PTSD and MDD, comorbid mild to moderately severe major depressive disorder (MDD) was allowed in the PTSD group. Inclusion criteria for control participants were a CAPS score < 15 while having experienced at least one traumatic event according to the Life Events Checklist (Blake et al., 1995). Additional exclusion criteria for controls were lifetime PTSD or MDD or any current psychiatric disorder. Exclusion criteria for both groups were daily use of psychotropic medication (e.g. antidepressants), use of systemic glucocorticoids, MRI contraindications, severe medical conditions, history of neurological disorders, colorblindness and current pregnancy or breastfeeding (females). The study was approved by the Institutional Review Board of the Academic Medical Center, Amsterdam, the Netherlands. Written informed consent was provided by all participants before study participation.
Controls were matched to patients on age, years of service and educational level within the sex groups. After inclusion, participants were invited for two fMRI scanning sessions scheduled at least 3 days apart. Following a double-blind cross-over design, participants were randomized to receive either oxytocin on the first and placebo on the second session, or vice versa. Under supervision of the experimenter participants self-administered oxytocin (40 IU, Defiante Farmaceutica, S.A., Funchal Portugal) and placebo (0.8% NaCl solution) via nasal spray (10 puffs, 5 puffs per nostril). Additional self-report questionnaires were administered to assess trauma exposure in childhood [early trauma inventory (ETI) (Bremner et al., 2007)] and police work-related trauma (PLES) exposure [police life events scale (PLES) (Carlier and Gersons, 1992)].
Social incentive delay task
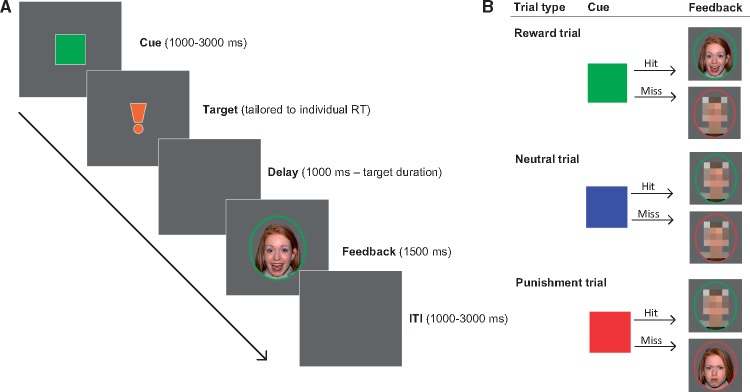
The social incentive delay (SID) task (Spreckelmeyer et al., 2009) was presented to participants during both fMRI sessions to assess neural responses to social reward and punishment. The SID task is a social version of the monetary incentive delay task (Knutson et al., 2000) and consists of a reaction time (RT) task in which participants are rewarded or punished with presentation of a happy face or an angry face, respectively. Participants received task instructions and a computerized practice session prior to each session. During each scan session, 27 trials were presented per trial type (reward, punishment, neutral). Each trial started with presentation of a colored cue indicating trial type (see Figure 1). Subsequently, a target was presented to which participants were to press a button as fast as possible. Responses within target presentation time were hits; omissions or responses outside of target presentation time were misses. Individual RTs were used to tailor the duration of target presentation to individual performance (long: mean response time + 400 ms; short: mean response time—150 ms). For reward and neutral trials, 66.7% of trials had long target durations resulting in about 66.7% hits, whereas 66.7% of punishment trials had short target durations resulting in about 66.7% misses. This resulted in sufficient reward hit feedback trials (±18) and sufficient punishment miss feedback trials (±18) for further analyses. After target presentation, feedback was presented. Reward trials hits resulted in social reward feedback (i.e. happy face), reward trial misses resulted in neutral feedback (i.e. scrambled face). Punishment trial misses resulted in social punishment feedback (i.e. angry face), punishment trial hits in neutral feedback. Neutral trial hits and misses both resulted in neutral feedback. Six males and six females were selected from the NimStim set of facial expressions (http://www.macbrain.org/resources.htm), of which both angry and happy expressions were used. Different sets of faces were used for the first and second fMRI session (task order counterbalanced). Trial type order was pseudo-randomized. RTs were assessed (milliseconds between start of target presentation and response). After each fMRI session, participants rated perceived rewardingness of the happy faces and perceived punishment by the angry faces on a 5-point Likert scale (0: Not at all–5: Very much), and participants were asked to indicate which intranasal drug treatment they thought they had received, and to indicate the underlying reason.
Fig. 1.
SID task. (A) Stimulus order per trial. Each trial started with presentation of an anticipation cue [green square for reward trials, blue for neutral trials, red for loss trials, see (B)]. Cue duration was jittered between 1000 and 3000 ms. Cues were followed by the target stimulus, for which duration was tailored to individual mean RT based on ten practice trials, to result in feasible target duration (individual aRT + 400 ms) or unfeasible target duration (individual aRT—150 ms). After a delay period with a duration of 1000 ms minus target duration, trial feedback was presented for 1500 ms, followed by a blank screen inter-trial interval, duration jittered between 1000 and 3000 ms. (B) Anticipation cues and feedback stimuli per trial type. Reward trials were signaled by a green square (reward anticipation), neutral trials by a blue square (neutral anticipation) and punishment trials by a red square (punishment anticipation). Reward feedback (i.e. happy faces) was shown in response to hits on reward trials, punishment feedback (i.e. angry faces) was shown in response to misses on punishment trials.
Behavioral analyses
Analyses were conducted with SPSS20 (IBM statistics, Chicago, USA). Demographic and behavioral variables were checked for deviations from normality. Variables were log-transformed when necessary and outliers (>3 SD) excluded. Differences between PTSD and control groups in demographic characteristics were tested separately in males and females, with independent t-tests for continuous variables, Mann-Whitney U-tests for continuous non-normally distributed variables and χ2-tests for categorical variables.
Mean accuracy on feasible trials under placebo and oxytocin were investigated per trial type (reward, neutral, punishment) to check for sufficient behavioral engagement in the task. Accuracy had a non-normal distribution and was therefore compared between groups and drug sessions with Mann-Whitney U-tests and Wilcoxon signed-rank tests, respectively. Group and sex effects on RTs, relative reaction times [(RRTs), i.e. RTs during reward and punishment trials relative to neutral trials, to control for individual differences in behavioral/motor response speed] and subjective ratings under placebo were tested with univariate ANOVA’s, with factors group (PTSD, control) and sex (male, female). Subsequently, drug effects were investigated with repeated-measures ANOVA’s, with factors group, sex and drug (oxytocin, placebo). Within the PTSD and control group separately, correlations were assessed between CAPS symptom severity (PTSD group only), subjective ratings, neural responses in the placebo condition, and with the extent of the oxytocin effect (delta oxytocin-placebo).
Image acquisition
A Philips Achieva 3T MR scanner with a 32-channel head-coil (Philips Medical Systems, Best, the Netherlands) was used for scanning. Functional images were acquired using an echo-planar imaging sequence [Field of view (FOV): 240 × 240 mm; flip angle: 76°; echo-time (TE): 27.63 ms; repetition time (TR): 2 s; voxel size: 3 mm3; acquisition matrix size (AMS): 80; 37 slices]. Anatomical images were acquired with a high-resolution FAST MP-RAGE sequence (FOV: 240 × 188 mm; flip angle: 8°; TE: 3.8 ms; TR: 8.2 s; voxel size:1 mm3; AMS: 240; 220 slices).
Imaging analyses
Imaging data statistical analyses were done with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were realigned, slice-timing corrected, co-registered with structural images, segmented, normalized to MNI space, resampled to 2 mm3 voxels and smoothed with an 8 mm full-width half maximum Gaussian kernel. Three subjects were excluded due to incomplete scanning data, two subjects were excluded due to scanning artifacts and three due to excessive movement (>6 mm/degrees). At first level, nine event types were modeled and convolved with a hemodynamic response function at stimulus onset: social reward, neutral and punishment anticipation, and social reward, neutral and punishment hit feedback, and social reward, neutral and punishment miss feedback. Six realignment parameters were included to control for movement. A high-pass filter (1/128 Hz) was applied. The following contrast images were calculated at first level and taken to second level analyses: reward anticipation vs neutral anticipation; punishment anticipation vs neutral anticipation; social reward hit feedback vs neutral hit feedback; social punishment miss feedback vs neutral miss feedback. At second level, we first tested effects of task, and effects of between-subject factors group (PTSD, control) and sex (male, female) under placebo, in separate models for trial types (reward and punishment) and task phases (anticipation and feedback), using the full-factorial design for univariate ANOVAs in SPM. General task effects were tested by assessing overall activation across groups and sexes. Also, main and interaction effects of group and sex were investigated under placebo. Then, for both reward and punishment anticipation and feedback separately, oxytocin effects were investigated using the flexible-factorial design for repeated-measured ANOVAs (Gläscher and Gitelman, 2008), including between-subject factors group (PTSD, control), sex (male, female) and within-subject factor drug (oxytocin, placebo). Based on previous (social) reward processing studies (Rademacher et al., 2010; Liu et al., 2011), the striatum (i.e. caudate and putamen combined), amygdala and AI were selected as ROI. Bilateral anatomical ROIs were created using the Wakeforest University (WFU) pickatlas toolbox implemented in SPM (http://fmri.wfubmc.edu/software/pickatlas). AI ROI was created by selecting the anterior part of the WFU pickatlas anatomical insula, i.e. all voxels with y ≥ 1 (Harsay et al., 2012). All imaging analyses were run within these a priori determined ROIs. As exploratory analyses, these analyses were run on whole brain level as well. Significant interaction effects between group, sex and/or drug observed in the voxel-wise analyses were further investigated with post hoc testing. t-tests were performed on beta-weights, extracted from a 5mm sphere around the peak-voxel of significant interaction effects with Marsbar (http://marsbar.sourceforge.net/); paired sample t-tests to test for effects of drug; independent-sample t-tests to test for effects of group and sex. In SPM, P-values were corrected for multiple comparisons using family-wise-error rate correction (PFWE); in SPSS, P-values for post hoc tests were corrected with false discovery rate (FDR) correction (Benjamini and Hochberg, 1995).
Results
Participant characteristics
In total, 37 healthy control participants (n = 19 males) and 35 PTSD participants (n = 21 males) were included in the final analyses (see Table 1) (see methods for exclusions). There were no significant differences between PTSD patients and controls in age, years of service, educational level or hormonal contraceptive use. However, PTSD males reported more types of traumatic events during childhood compared with male controls, as assessed with the ETI (t(30.39) = −2.047, P = 0.049). PTSD females reported exposure to nominally less different types of PLES events compared with female controls, as assessed with the PLES [t(30) = 1.849, P = 0.074]. This group-difference in exposure to work-related traumatic events in females was no longer significant when controlling for years of police service (P > 0.1). As expected, PTSD patients had a significantly higher CAPS scores compared with controls [males: t(24.25) = −17.685, P < 0.001; females: t(17.19) = −20.778, P < 0.001]. In addition, eight PTSD patients (23%) fulfilled diagnostic criteria for current MDD, based on clinical interviews (MINI/SCID).
Table 1.
Participant characteristics
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Control (n = 19) | PTSD (n = 21) | P-value | Control (n = 18) | PTSD (n = 14) | P-value | |
| Age (years) | 41.11 (10.86) | 42.29 (9.83) | 0.720 | 38.06 (9.08) | 38.21 (9.85) | 0.963 |
| Educational level | ||||||
| Low | 0 (0%) | 0 (0%) | 0.874 | 0 (0%) | 0 (0%) | 0.370 |
| Medium | 15 (79%) | 17 (81%) | 17 (94%) | 14 (100%) | ||
| High | 4 (21%) | 4 (19%) | 1 (6%) | 0 (0%) | ||
| Years of police service | 18.08 (10.21) | 19.07 (14.93) | 0.819 | 17.83 (9.56) | 15.21 (10.81) | 0.473 |
| PLES | 20.32 (6.57) | 22.50 (5.95) | 0.242 | 19.44 (7.68) | 13.64 (10.09) | 0.074# |
| Childhood trauma (ETI) | 3.79 (2.32) | 6.10 (4.55) | 0.049* | 4.22 (5.02) | 5.50 (5.43) | 0.456 |
| PTSD symptom severity (CAPS) | 4.58 (4.89) | 68.05 (15.62) | 0.000** | 4.67 (4.80) | 67.79 (10.55) | 0.000** |
| Major depressive disorder comorbidity, n(%) | 0 (0%) | 4 (19%) | 0.045* | 0 (0%) | 4 (29%) | 0.015* |
| Contraceptive use (%) | ||||||
| None | 8 (44%) | 6 (43%) | 0.910 | |||
| Hormonal contraceptive | 8 (44%) | 7 (50%) | ||||
| Menopausal | 2 (11%) | 1 (7%) | ||||
Group differences between patients and controls were tested with independent-sample t-tests [age, years of service, PLES (females), ETI, CAPS], Mann-Whitney U-tests [PLES (males)] or χ2-tests [education, MDD, contraceptive use (females)]. t-tests of years of police service in males, and ETI in females were based on log-transformed data to reach a normal distribution. All means and SDs in table are based on untransformed data. **P-value < 0.001; *P-value < 0.050; #P-value < 0.100. PLES, police life events scale (Carlier and Gersons, 1992); ETI, early trauma inventory—short version (Bremner et al., 2007); CAPS, Clinician Administered PTSD Scale (Blake et al., 1995); PTSD, post-traumatic stress disorder.
Behavioral analyses
Reaction times. Under placebo, there was a significant main effect of sex on reward trial RT [F(1,68) = 11.295, P = 0.001], men being faster than women. PTSD status did not affect reward RT. Under placebo, there was a significant main effect of group on punishment trial RT [F(1,68) = 4.398, P = 0.040], controls being faster than PTSD patients; and a significant main effect of sex [F(1,68) = 4.902, P = 0.030], men being faster than women. The effects of PTSD and sex on punishment RT were also significant after controlling for drug order. However, reward and punishment RRTs were not affected by group or sex, suggesting that the differences in RT are due to general differences in response speed, and not specific to motivational trials (reward/punishment). Oxytocin did not significantly affect RT or RRT.
Accuracy.All participants mastered the task. As task difficulty was adjusted to individual performance, accuracy showed very limited variance. See Supplementary Table S1 for average accuracy on feasible trials per group per trial per session and supplemental information for analyses of group- and drug effects on accuracy.
Subjective ratings. Under placebo, there was no effect of group or sex on reward ratings. There was a significant effect of group on punishment ratings under placebo [F(1,65) = 11.928, P = 0.001], PTSD patients rated the angry faces as significantly more punishing than controls. The effect of group on punishment ratings was still significant after correcting for drug order. Sex did not affect punishment ratings. Oxytocin did not significantly affect subjective ratings of reward or punishment.
See Supplementary Materials for analyses concerning subjective treatment awareness.
Neuroimaging
SID anticipation phase. Under placebo, on whole-brain level, social reward anticipation significantly activated the bilateral occipital pole, bilateral hippocampus and amygdala (Supplementary Table S3). PTSD status and sex had no significant main or interaction effects on neural responses during the SID reward anticipation phase under placebo, whole-brain or ROI. No significant main or interaction effects of oxytocin on neural responses during social reward anticipation were observed, whole-brain or ROI. Punishment anticipation under placebo yielded no significantly activated voxels on whole-brain level. Effects of group, sex and oxytocin were therefore not further investigated.
SID feedback phase
Task effects.Under placebo, on whole-brain level, presentation of social reward feedback significantly activated the bilateral fusiform gyrus and amygdala, and the right inferior frontal gyrus and middle temporal gyrus (Supplementary Table S4). Punishment feedback presentation yielded significant activation in the bilateral occipital lobes and fusiform gyrus, the right amygdala and middle temporal gyrus (Supplementary Table S4).
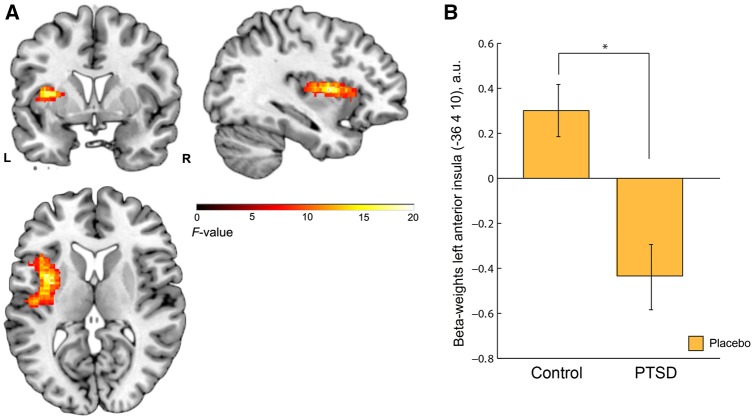
Group effects. Under placebo, ROI analyses revealed a significant main effect of group in the left AI during reward feedback [xyz = −36,4,10, F(1,68) = 18.11, Z = 3.83, PFWE = 0.024, k = 44, Figure 2], PTSD patients showing significantly lower left AI responses compared with controls. No other significant main or interaction effects of group or sex on reward or punishment feedback were observed under placebo, neither whole-brain nor within ROIs.
Fig. 2.
Main effect of group during feedback of social reward (vs neutral) under placebo in the AI ROI. (A) Left AI [xyz = −36,4,10, F(1,68) = 18.11, PFWE = 0.024, k = 44]; Cluster is overlaid on a single-subject anatomical scan (SPM template), whole brain threshold set at P < 0.01 uncorrected for display purposes. (B) Extracted beta-weights (arbitrary units, a.u.) from 5 mm sphere around peak voxel (xyz = −36,4,10) showing a main effect of group. PTSD patients had significantly lower AI responses to social reward feedback (vs neutral) compared with controls. Error bars indicate standard error of the mean. *P < 0.05.
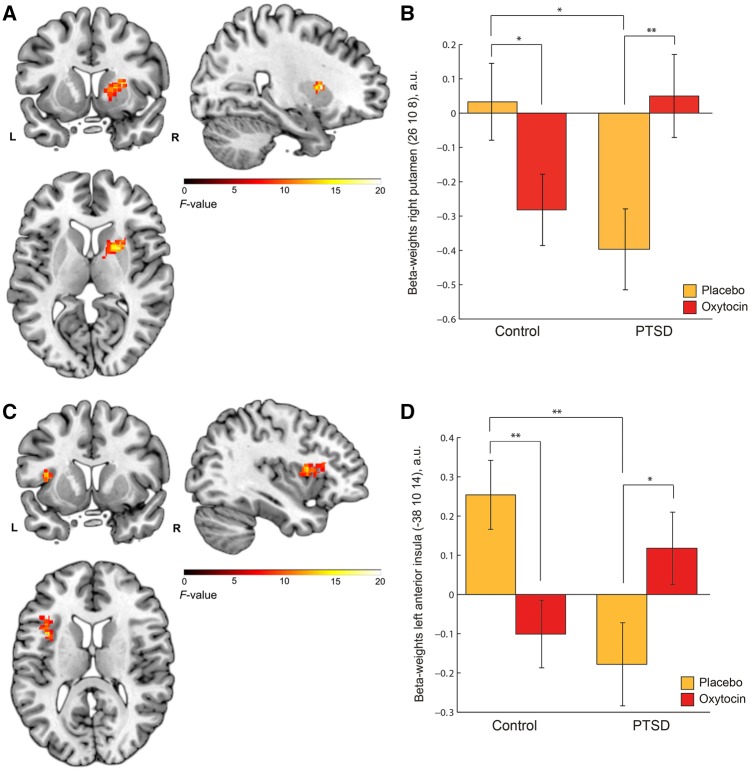
Oxytocin effects. When looking at effects of intranasal oxytocin, during reward feedback a significant drug by group interaction was observed in the right putamen within the striatum ROI [xyz = 26,10,8, F(1,68) = 19.18, Z = 3.93, PFWE = 0.047, k = 29, Figure 3A]. Additionally, a nominally significant drug by group interaction was observed in the left AI within the AI ROI [xyz = −38,10,14, F(1,68) = 15.37, z = 3.53, PFWE = 0.067, k = 9, Figure 3C], in the same location as the significant main effect of group was observed under placebo. Post hoc t-tests (see Supplementary Table S5) revealed that in PTSD patients, oxytocin significantly increased reward responses compared to placebo within the right putamen [t(34) = −2.927, P = 0.006] and left AI [t(34) = −2.234, P = 0.032], whereas in controls, oxytocin significantly decreased reward responses compared with placebo in the putamen [t(36) = 2.207, P = 0.034] and AI [t(36) = 2.879, P = 0.007] (Figure 3B and D). These effects remained significant after FDR correction. After oxytocin, PTSD patients showed similar putamen and AI responses to reward feedback as controls, both under placebo (P > 0.1) and oxytocin (P > 0.1). Oxytocin had no other significant main or interaction effects on reward or punishment feedback, whole-brain or within ROI’s.
Fig. 3.
Oxytocin effects on neural responses in the right putamen and left AI during social reward feedback (group by drug interaction effect) (A) Right putamen [xyz = 26,10,8, F(1,68) = 19.18, Z = 3.93, PFWE = 0.047, k = 29]; (B) Extracted beta-weights (a.u.) from 5 mm sphere around peak voxel (xyz = 26,10,8) of the right putamen showing a group by drug interaction in controls and PTSD patients; (C) Left AI [xyz = −38,10,14, F(1,68) = 15.37, z = 3.53, PFWE = 0.067, k = 9]; (D) Extracted beta-weights (a.u.) from 5 mm sphere around peak voxel (xyz = −38,10,14) of the left AI showing a group by drug interaction in controls and PTSD patients. For AI group differences under placebo, see Figure 2. Clusters are overlaid on a single-subject anatomical scan (SPM template), whole brain threshold set at P < 0.01 uncorrected for display purposes. Error bars indicate standard error of the mean. *P < 0.05, **P < 0.01. All significant results survived FDR correction.
PTSD symptoms, subjective ratings and neural responses
Within the PTSD group, total CAPS symptom severity score was not significantly correlated with subjective ratings of social reward or punishment under placebo or oxytocin effects on social reward ratings, nor with AI responses during social reward and punishment feedback under placebo or oxytocin effects on AI responses (all P > 0.1). Total CAPS symptoms were significantly negatively correlated with putamen responses during reward feedback under placebo (r = −0.421, P = 0.012), but not to putamen responses to punishment feedback under placebo. Oxytocin effects on putamen responses during reward and punishment feedback were not related to CAPS symptoms. Oxytocin effect on subjective punishment ratings (delta OT-PL) was significantly negatively correlated to CAPS score (r = −0.464, P = 0.008), such that higher CAPS scores were related to a stronger dampening of punishment ratings after oxytocin compared with placebo.
We also investigated whether subjective responses to reward feedback were correlated to putamen and insula responses to reward feedback. Under placebo, subjective ratings of social reward were significantly positively correlated with insula responses during social reward feedback in PTSD patients (r = 0.354, p = 0.047), but not in controls (P > 0.1). Subjective ratings of social reward under placebo were not significantly correlated with putamen responses during placebo social reward feedback in PTSD patients or controls (P > 0.1). After oxytocin administration, subjective reward ratings were not significantly correlated to putamen or insula responses during reward feedback in PTSD patients or controls (P > 0.1). Also, oxytocin effects on subjective ratings (delta OT-PL) were not significantly correlated to oxytocin effects on insula responses (delta OT-PL) in patients or controls (P > 0.1).
Discussion
We investigated whether a single intranasal oxytocin administration affected neural responses during social reward and punishment processing in PTSD patients and trauma-exposed controls, focusing on AI, striatum and amygdala. Under placebo, we observed significantly lower responses in the left AI of PTSD patients during social reward feedback compared with controls. Oxytocin administration significantly increased the otherwise hypoactive left AI responses to social reward feedback observed in PTSD patients to a level similar to controls under placebo. Furthermore, oxytocin significantly increased right putamen responses to reward feedback in PTSD patients.
The observed deviations in AI responses to positive stimuli (i.e. happy faces) in PTSD patients replicate previous findings of decreased insula responses to social and non-social positive stimuli in PTSD patients (Aupperle et al., 2012; Moser et al., 2015). Furthermore, PTSD symptom severity was negatively correlated with putamen responses to reward feedback under placebo. Our findings under placebo thus support the notion that the salience and reward networks, in which the AI and striatum are central nodes, are hypoactive towards positive stimuli in PTSD (Stein and Paulus, 2009). Previous findings of hyperactive neural responses to negative stimuli in PTSD patients (Paulus and Stein, 2006; Aupperle et al., 2012; Mazza et al., 2012; Sripada et al., 2012; Moser et al., 2015) were not replicated however, although on a behavioral level, PTSD patients in our study rated social punishment (i.e. angry faces) as more punishing than controls. The left AI plays an important role in detecting and subsequently guiding attention towards salient stimuli in the environment (Seeley et al., 2007; Uddin, 2014). Furthermore, AI response magnitude reflects the degree of subjective salience an individual attributes to a particular stimulus (Uddin, 2014). The putamen is involved in motivational behavior, associative learning and evaluation of reward and punishment feedback (Liu et al., 2011). Regarding our findings, this indicates that PTSD patients attributed less salience and/or motivational value to social reward than healthy traumatized controls.
When testing effects of oxytocin, we observed a nominally significant group by drug interaction in the AI: A single intranasal oxytocin administration normalized the aberrant insula responses to social reward in PTSD patients to a level comparable to controls under placebo. This replicates previous findings of increased insula responses after oxytocin towards various stimuli in a meta-analysis in healthy individuals (Wigton et al., 2015), and in studies looking specifically at responses to positive social stimuli in healthy individuals (Domes et al., 2010; Rilling et al., 2012; Groppe et al., 2013; Scheele et al., 2013, 2014; Striepens et al., 2014) and PTSD patients (Nawijn et al., 2016). Furthermore, a significant group by drug interaction was observed in the striatum: Under placebo, PTSD patients showed reduced putamen responses to reward feedback compared with controls. Oxytocin increased putamen responses towards reward feedback in patients, such that they no longer differed from controls under placebo. This replicates previous findings of oxytocin effects on striatal responses to positive stimuli (Rilling et al., 2012; Groppe et al., 2013; Scheele et al., 2013; Feng et al., 2015; Hu et al., 2015; Scheele et al., 2016; Nawijn et al., 2016). Seeing the role of the putamen in motivation and reward feedback evaluation (Liu et al., 2011), oxytocin may thus facilitate reward evaluation, social reinforcement learning and goal-directed behavior, as was observed in previous studies in healthy individuals (Groppe et al., 2013; Hu et al., 2015; Scheele et al., 2016).
Previously, oxytocin administration increased SID accuracy in participants with low hit rates under placebo (Groppe et al., 2013). However, accuracy levels in our sample were already relatively high under placebo in all participants, leaving little room for improvement by oxytocin. Oxytocin did increase accuracy levels on feasible punishment trials in PTSD patients from 95 to 99%. Although the clinical significance of this increase in accuracy seems negligible, it is possible that stronger behavioral effects on the SID task would have been observed if a ceiling-effect had not been reached in all groups and trial types (reward, neutral, punishment).
Oxytocin did not affect subjective ratings of social reward in our study in a similar fashion as neural responses—possibly because subjective ratings were assessed in retrospect (±2 h after nasal spray administration). However, in previous studies in healthy individuals oxytocin-induced increases in striatal and AI responses were paralleled by (concurrent) ratings of increased pleasantness of touch and attractiveness of faces (Scheele et al., 2013, 2014, 2016; Striepens et al., 2014). Also, the fact that under placebo, insula responses to social reward feedback were positively correlated with subjective ratings of reward in patients, fits with the interpretation that stronger insula responses are associated with higher reward sensitivity. Thus, by increasing putamen and AI responses to positive social reward, oxytocin administration may restore a decreased reward sensitivity towards positive affective stimuli in PTSD.
Notably, whereas oxytocin increased putamen and AI reward sensitivity in PTSD patients, it decreased putamen and AI reward sensitivity in controls. This is at odds with previous findings of oxytocin increasing putamen and AI responses in healthy individuals, as discussed above (meta-analysis Rilling et al., 2012; Groppe et al., 2013; Scheele et al., 2013, 2014, 2016; Feng et al., 2015; Striepens et al., 2014; Hu et al., 2015; Wigton et al., 2015; Nawijn et al., 2016). However, differential effects of oxytocin administration depending on clinical status have been observed before. Several groups have reported that oxytocin administration mitigated aberrant amygdala connectivity and activity towards angry or fearful faces in psychiatric patients, but had no or even opposite effects in controls (e.g. Labuschagne et al., 2010; Bertsch et al., 2013; Dodhia et al., 2014; Gorka et al., 2015). Authors of previous studies showing differential oxytocin effects have suggested an inverted U-shaped response curve for effects of intranasal oxytocin (Feng et al., 2015; Rilling et al., 2014). They suggest that oxytocin administration has beneficial effects in individuals with suboptimal functioning, potentially due to low endogenous oxytocin levels, but has no or even detrimental effects in individuals with optimal functioning (Feng et al., 2015; Rilling et al., 2014). Our observed differential effects of oxytocin administration on putamen and AI responses to social reward feedback in controls and patients can be explained from this viewpoint, as these groups differed in neural functioning under placebo. Interestingly, within the males of this same group of participants we recently reported reduced endogenous salivary oxytocin levels in PTSD patients compared with controls (Frijling et al., 2015). It is therefore possible that dysregulated endogenous oxytocin levels underlie these reduced neural responses to social reward in PTSD patients. Indeed, endogenous oxytocin levels have been positively correlated to striatal responses to positive stimuli (Rilling et al., 2012; Scheele et al., 2016). The relatively high dosage of oxytocin used in the current study (40 IU) may explain the different findings in our healthy controls compared with previous studies in healthy individuals. One of the few dose-response studies of oxytocin to date reported that 24 IU had beneficial effects on cortisol reactivity to physical exercise in healthy controls, but 48 IU had no effect (Cardoso et al., 2013), suggesting an inverted-U-shaped dose-response curve. Thus, whereas a dosage of 24 IU, as was used in most previous studies in healthy individuals (meta-analysis Scheele et al., 2013, 2014, Striepens et al., 2014; Wigton et al., 2015), may still have beneficial effects in healthy individuals, 40 IU may have overstimulated oxytocin system in our control group. This further substantiates the notion that oxytocin administration may only have beneficial effects in individuals with suboptimal (oxytocin system) functioning, but that the relatively high dosage of oxytocin may have overstimulated the already optimal functioning controls.
Contrary to our hypothesis, we did not observe oxytocin effects in the amygdala. Although oxytocin effects on amygdala responses towards emotional faces have often been reported in literature, the meta-analysis of Wigton et al., (2015) did not report an overall amygdala effect but only an increase in insula responses after oxytocin. Oxytocin may only robustly decrease amygdala responses towards implicitly-shown emotional faces, whereas findings are less consistent in response to explicitly-shown faces (Wigton et al., 2015). In the same sample as the current study, oxytocin was found to dampen amygdala reactivity in PTSD patients in response to emotional faces (happy/neutral and angry/fearful) in an explicit emotional face-matching task (Koch et al., 2016). Although the stimuli in the SID task were comparable (i.e. positive and negative emotional faces), these were presented within a different context (i.e. as reinforcers in a motivational task vs explicit emotion recognition task). This suggests that oxytocin effects may differ depending on context, as has been suggested before (Landgraf and Neumann, 2004; Bartz et al., 2011; Bethlehem et al., 2013; Wigton et al., 2015), even within the same individuals.
Although sex has also been suggested as a potential moderator of oxytocin administration effects, we did not see any sex-dependent oxytocin effects on neural responsivity, opposing previous findings in healthy individuals (Feng et al., 2015; Rilling et al., 2014). In the previous studies, oxytocin increased striatal and AI responses to social cooperation in men, but had no effect or even decreased striatal and AI responses in women. Following the concept of the inverted-U-shaped response curve, effects of oxytocin administration depend on baseline functioning. Consequently it follows that when sex-differences are present under placebo, as in the studies by Rilling and colleagues, oxytocin administration may have sex-dependent effects (Feng et al., 2015; Rilling et al., 2014). If however no sex-differences are present under placebo, as observed in our study, intranasal oxytocin may have similar effects in men and women.
Taken together, our and previous findings fit with the notion that neural effects of oxytocin administration depend on individual factors such as patient status, sex or other individual characteristics, but only if these factors are related to differences in baseline functioning (Labuschagne et al., 2010; Bartz et al., 2011; Bakermans-Kranenburg and van I Jzendoorn, 2013; Olff et al., 2013; Feng et al., 2015; Rilling et al., 2014).
We did not observe effects of oxytocin administration on reactivity in response to social punishment feedback in patients or controls. Thus, our results do not match with previous findings of oxytocin-induced changes in sensitivity to negative social stimuli (e.g. Riem et al., 2011; Bos et al., 2015), nor with a general increase in social salience processing independent of valence, as was suggested by a recent meta-analysis of studies in healthy individuals (Wigton et al., 2015).
Our findings provide insight in the neurobiological mechanisms underlying the previously observed effects of oxytocin on social approach behavior (Harari-Dahan and Bernstein, 2014; Preckel et al., 2014; Auyeung et al., 2015), trust (Bakermans-Kranenburg and van I Jzendoorn 2013) and social support (Heinrichs et al., 2003; Cardoso et al., 2016). More importantly however, our findings specifically support the hypothesis that intranasal oxytocin administration can enhance neural sensitivity to social reward in PTSD patients, which could potentially increase treatment response by applying oxytocin in addition to psychotherapy (MEP). By increasing neural responses to social reward, oxytocin administration may (temporarily) restore a decreased sensitivity towards positive affective stimuli seen in PTSD patients (e.g. Mazza et al., 2012) and enhance the focus towards socially rewarding stimuli. By enhancing neural sensitivity for social reward, oxytocin may facilitate the initiation and maintenance of a therapeutic alliance as well as the sensitivity for social support in a treatment setting when applied as MEP (Olff et al., 2010; Quirin et al., 2014). This in turn can improve treatment response in PTSD patients and reduce dropout rates. However, given the neuroimaging nature of our study we can only speculate about the clinical inferences of our results, which need to be replicated and extended in a more clinical setting. Although neural effects were observed, these were not paralleled by behavioral or subjective effects, possibly due to ceiling effects in behavioral measures (accuracy) or questionnaire administration outside of the suspected active oxytocin window (subjective ratings). Future clinical studies should investigate whether oxytocin administration prior to treatment can indeed enhance sensitivity to positive social interaction in PTSD patients within a treatment setting, and subsequently benefit therapeutic alliance and perceived social support. Such clinical studies are crucial, especially seeing recent clinical (pilot) studies in mood- and anxiety disorders, showing mixed effects of combining oxytocin administration with psychotherapy (MacDonald et al., 2013; Acheson et al., 2015). As the currently published clinical studies incorporating oxytocin administration had fairly small samples, larger randomized controlled trials are needed to further investigate the potential of oxytocin in MEP. The hypothesized inverted U-shaped response curve of oxytocin administration based on our and previous findings further emphasizes the importance of dosage in relation to endogenous oxytocin system functioning. The few studies that investigated different dosages of oxytocin have found dose-dependent effects, with low dosages (24 IU) resulting in beneficial effect on e.g. positive ratings of social affiliation memory and cortisol stress responses, but high dosages (48 IU) having no effects in healthy individuals (Cardoso et al., 2013, 2014). Animal studies suggest similar dose-dependent effects (e.g. Bielsky and Young, 2004; Chini et al., 2014; Peters et al., 2014). Therefore, future (clinical) studies should focus on the potential influence of individual characteristics that relate to baseline (oxytocin) system functioning and oxytocin effects (e.g. sex, psychopathology), in combination with dosage effects (Macdonald and Feifel, 2013; Chini et al., 2014).
Some limitations of this study must be mentioned. The participants in our study were highly trauma-exposed police officers, resulting in a homogeneous but also specific sample. This has the benefit of controlling for potentially confounding effects of trauma-exposure on neurobiology of reward functioning, but also decreases the generalizability of our findings towards other PTSD patients groups (e.g. civilian trauma). Also, it is possible that group differences are (in part) related to resilience of the control group instead of PTSD status. Furthermore, although we are one of the first to study oxytocin effects in male and female participants simultaneously, we could not control for potential effects of menstrual cycle in our female participants. Although this will likely have evened out between treatment sessions, menstrual cycle phase may have influenced effects of oxytocin (Macdonald, 2012). Some methodological issues must also be mentioned. We used a saline solution as placebo. Although subjective awareness of received treatment during the second scan session was (trend) significantly above chance level, the vast majority of participants indicated this was due to noticeable effects on psychological functioning, and not smell or taste. Also, it must be noted that the observed group by drug interaction in the insula was only marginally significant with a P-value of 0.067. Therefore, replication of our findings is warranted to confirm the current results.
In conclusion, by increasing neural sensitivity to social reward, a single intranasal oxytocin administration may alter salience processing of social reward in both male and female patients with PTSD. This could potentially enhance sensitivity for social support and therapeutic alliance, which in turn may positively affect treatment response and recovery from PTSD symptoms. This is one of the first studies investigating the effects of oxytocin in PTSD patients and our findings are a promising first step in investigating the therapeutic potential of intranasal oxytocin in patients with PTSD. Future clinical studies are needed to investigate whether our findings translate to a clinical setting, and to further substantiate the potential benefits of oxytocin administration in MEP to enhance the efficacy of currently available psychotherapy.
Funding
This research was supported by grants from ZonMw, the Netherlands organization for Health Research and Development (grant no. 91210041) and the Academic Medical Center Research Council (grant No. 110614) and is registered in the Netherlands Trial Registry (project name: ‘The effect of oxytocin on brain processes in PTSD’, registry number: NTR3516, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3516).
Supplementary Material
Acknowledgements
The authors would like to thank all participants for their contribution to this study, and Renée Hutter, Gré Westerveld, Marthe Hoofwijk and colleagues of the PDC police outpatient clinic and Bart Timmermans for their assistance in participant recruitment and data collection.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Acheson D.T., Feifel D., Kamenski M., et al. (2015). Intranasal oxytocin administration prior to exposure therapy for arachnophobia impedes treatment response. Depress Anxiety, 32, 400–7. [DOI] [PubMed] [Google Scholar]
- Aupperle R.L., Allard C.B., Grimes E.M., et al. (2012). Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry, 69, 360–71. [DOI] [PubMed] [Google Scholar]
- Auyeung B., Lombardo M.V., Heinrichs M., et al. (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translation of Psychiatry, 5, e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van I Jzendoorn M.H. (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translation of Psychiatry, 3, e258.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., et al. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Science, 15, 301–9. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistics Socials Series B (methodological), 57(1), 289–300. [Google Scholar]
- Bertsch K., Schmidinger I., Neumann I.D., et al. (2013). Reduced plasma oxytocin levels in female patients with borderline personality disorder. Hormone Behavior, 63, 424–9. [DOI] [PubMed] [Google Scholar]
- Bethlehem R.A., van Honk J., Auyeung B., et al. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasaloxytocin fMRI studies. Psychoneuroendocrinology, 38, 962–74. [DOI] [PubMed] [Google Scholar]
- Bielsky I.F., Young L.J. (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides, 25, 1565–74. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., et al. (1995). The development of a Clinician-Administered PTSD Scale. Jourrnal of Traumatic Stress, 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Bos P.A., Montoya E.R., Hermans E.J., et al. (2015). Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. Neuroimage, 113, 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., et al. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162, 214–27. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Bolus R., Mayer E.A. (2007). Psychometric properties of the Early Trauma Inventory-Self Report. Journal of Nervous and Mental Disorders, 195, 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C., Ellenbogen M. a., Orlando M.A., et al. (2013). Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology, 38, 399–407. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Orlando M.A., Brown C.A., et al. (2014). Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Society for Neuroscience, 9, 186–95. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Valkanas H., Serravalle L., et al. (2016). Oxytocin and social context moderate social support seeking in women during negative memory recall. Psychoneuroendocrinology, 70, 63–9. [DOI] [PubMed] [Google Scholar]
- Carlier I.V., Gersons B.P. (1992). Development of a scale for traumatic incidents in police officers. Psychiatric Fenn, 23, 59–70. [Google Scholar]
- Charuvastra A., Cloitre M. (2008). Social bonds and posttraumatic stress disorder. Annual Review of Psychology, 59, 301–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B., Leonzino M., Braida D., et al. (2014). Learning about oxytocin: pharmacologic and behavioral issues. Biological Psychiatry, 76, 360–6. [DOI] [PubMed] [Google Scholar]
- Clausen A., Youngren W., Sisante J., et al. (2016). Combat PTSD and implicit behavioral tendencies for positive affective stimuli: a brief report. Frontiers in Psychology, 7, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodhia S., Hosanagar A., Fitzgerald D.A., et al. (2014). Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology, 39, 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Gläscher J., et al. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62, 1187–90. [DOI] [PubMed] [Google Scholar]
- Domes G., Lischke A., Berger C., et al. (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology, 35, 83–93. [DOI] [PubMed] [Google Scholar]
- Dunlop B.W., Mansson E., Gerardi M. (2012). Pharmacological innovations for posttraumatic stress disorder and medication- enhanced psychotherapy. Current Pharmaceutical Design, 18, 5645–58. [DOI] [PubMed] [Google Scholar]
- Ehlers C.L., Hurst S., Phillips E., et al. (2006). Electrophysiological responses to affective stimuli in American Indians experiencing trauma with and without PTSD. Annals of the New York Academy of Sciencces, 1071, 125–36. [DOI] [PubMed] [Google Scholar]
- Elman I., Lowen S., Frederick B., et al. (2009). Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biological Psychiatry, 66, 1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K.L., Falconer E.M., Williams L., et al. (2014). Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One, 9, e103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Hackett P.D., DeMarco A.C., et al. (2015). Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behavior, 9, 754–64. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., et al. (2002). Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Washington, DC: American Psychiatric Publishing Inc. [Google Scholar]
- Frewen P.A., Dozois D.J.A., Neufeld R.W.J., et al. (2010). Social emotions and emotional valence during imagery in women with PTSD: Affective and neural correlates. Psychological Trauma: Theory, Research, Practice, and Policy 2, 145–57. [Google Scholar]
- Frijling J.L., van Zuiden M., Nawijn L., et al. (2015). Salivary oxytocin and vasopressin levels in police officers with and without post-traumatic stress disorder. Journal of Neuroendocrinology, 27, 743–51. [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Büchel C. (2010). Different amygdala subregions mediate valence- related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America, 18, 9400–5. DOI: 10.1073/pnas.1000985107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J.P., Gitelman D. (2008). Contrast weights in flexible factorial design with multiple groups of subjects. Unpublished tutorial. Available: http://www.jiscmail.ac.uk/cgi-bin/webadmin?A2=ind0803&L=SPM&P=R16629 (July, 2016).
- Gorka S.M., Fitzgerald D.A., Labuschagne I., et al. (2015). Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology, 40, 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe S.E., Gossen A., Rademacher L., et al. (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological Psychiatry, 74, 172–9. [DOI] [PubMed] [Google Scholar]
- Harari-Dahan O., Bernstein A. (2014). A general approach - avoidance hypothesis of Oxytocin: accounting for social and non-social effects of oxytocin. Neurosci Biobehavioral Reviews, 47C, 506–19. [DOI] [PubMed] [Google Scholar]
- Harsay H.A., Spaan M., Wijnen J.G., et al. (2012). Error awareness and salience processing in the oddball task: shared neural mechanisms. Frontiers in Human Neuroscience, 6, 246–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders, 2, 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., et al. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54, 1389–98. [DOI] [PubMed] [Google Scholar]
- Hu J., Qi S., Becker B., et al. (2015). Oxytocin selectively facilitates learning with social feedback and increases activity and functional connectivity in emotional memory and reward processing regions. Human Brain and Mapping, 36, 2132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn M.H., Bakermans-Kranenburg M.J. (2012). A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology, 37, 438–43. [DOI] [PubMed] [Google Scholar]
- Imel Z.E., Laska K., Jakupcak M., et al. (2013). Meta-analysis of dropout in treatments for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 81, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P., Krebs T.S. (2009). How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. Journal of Psychopharmacology, 23, 389–91. [DOI] [PubMed] [Google Scholar]
- Kaniasty K., Norris F.H. (2008). Longitudinal linkages between perceived social support and posttraumatic stress symptoms: sequential roles of social causation and social selection. Journal of Traumatic Stress, 21, 274–81. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp a., Kaiser E., et al. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12, 20–7. [DOI] [PubMed] [Google Scholar]
- Koch S.B., van Zuiden M., Nawijn L. et al. (2016). Intranasal Oxytocin Administration Dampens Amygdala Reactivity towards Emotional Faces in Male and Female PTSD Patients. Neuropsychopharmacology, 41, 1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology, 35, 2403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. (2004). Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology, 25, 150–76. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., et al. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience Biobehavioral Reviews, 35, 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald K., Feifel D. (2013). Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Frontiers in Neuroscience, 7, 35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K., MacDonald T.M., Brüne M., et al. (2013). Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology, 38, 2831–43. [DOI] [PubMed] [Google Scholar]
- Macdonald K.S. (2012). Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Frontiers in Neuroscience, 6, 194.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A., Post D., Kennedy A.E., et al. (2013). Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biological Psychology, 94, 441–9. [DOI] [PubMed] [Google Scholar]
- Martin D.J., Garske J.P., Davis M.K. (2000). Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. Journal of Consulting and Clinical Psychology, 68, 438–50. [PubMed] [Google Scholar]
- Mazza M., Catalucci A., Mariano M., et al. (2012). Neural correlates of automatic perceptual sensitivity to facial affect in posttraumatic stress disorder subjects who survived L’Aquila eartquake of April 6, 2009. Brain Imaging and Behavior, 6, 374–86. [DOI] [PubMed] [Google Scholar]
- Moser D.A., Aue T., Suardi F., et al. (2015). Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Social Cognitive and Affective Neuroscience, 10, 645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L., van Zuiden M., Frijling J.L., et al. (2015). Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neuroscience Biobehavioral Reviews, 51C, 189–204. [DOI] [PubMed] [Google Scholar]
- Nawijn L., van Zuiden M., Koch S.B., et al. (2016). Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology, 66, 228–3. [DOI] [PubMed] [Google Scholar]
- Olff M., Frijling J.L., Kubzansky L.D., et al. (2013). The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38, 1883–94. [DOI] [PubMed] [Google Scholar]
- Olff M., Langeland W., Witteveen A., et al. (2010). A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectrum, 15, 522–30. [DOI] [PubMed] [Google Scholar]
- Ormhaug S.M., Shirk S.R., Wentzel-Larsen T. (2015). Therapist and client perspectives on the alliance in the treatment of traumatized adolescents. European Journal of Psychotraumatology, 6, 27705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palgi S., Klein E., Shamay-Tsoory S.G. (2016). Oxytocin improves compassion toward women among patients with PTSD. Psychoneuroendocrinology, 64, 143–9. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. (2006). An insular view of anxiety. Biological Psychiatry, 60, 383–7. [DOI] [PubMed] [Google Scholar]
- Peters S., Slattery D.A., Uschold-Schmidt N., et al. (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–36. [DOI] [PubMed] [Google Scholar]
- Pietrzak R.H., Goldstein M.B., Malley J.C., et al. (2010). Structure of posttraumatic stress disorder symptoms and psychosocial functioning in Veterans of Operations Enduring Freedom and Iraqi Freedom. Psychiatry Research, 178, 323–9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel K., Scheele D., Kendrick K.M., et al. (2014). Oxytocin facilitates social approach behavior in women. Frontiers in Behavioral Neuroscience, 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M., Carter C.S., Bode R.C., et al. (2014). The role of oxytocin and alexithymia in the therapeutic process. Frontiers in Psychology, 5, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L., Krach S., Kohls G., et al. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage, 49, 3276–85. [DOI] [PubMed] [Google Scholar]
- Riem M.M.E., Bakermans-Kranenburg M.J., Pieper S., et al. (2011). Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry, 70, 291–7. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., et al. (2012). Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology, 37, 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Demarco A.C., Hackett P.D., et al. (2014). Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology, 39, 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Kendrick K.M., Khouri C., et al. (2014). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology, 39, 2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Wille A., Kendrick K.M., et al. (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences of the United States of America, 110, 20308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Plota J., Stoffel-Wagner B., et al. (2016). Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Social Cognitive and Affective Neuroscience, 11(5),767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27, 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D., Lecrubier Y., Sheehan K., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 2), 22–33. quiz 34–57. [PubMed] [Google Scholar]
- Simeon D., Bartz J., Hamilton H., et al. (2011). Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology, 36, 1418–21. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., et al. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4, 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., et al. (2012). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74, 904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Paulus M.P. (2009). Imbalance of approach and avoidance: the yin and yang of anxiety disorders. Biological Psychiatry, 66, 1072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C., Daniels J.K., Frewen P. a., et al. (2014). Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Social Cognitive and Affective Neuroscience 9, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N., Matusch A., Kendrick K.M., et al. (2014). Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology, 39, 74–87. [DOI] [PubMed] [Google Scholar]
- Striepens N., Scheele D., Kendrick K.M., et al. (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings of the National Academy of Sciences of the United States of America, 109, 18144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher S., Power M., Morant N., et al. (2010). Social support moderates outcome in a randomized controlled trial of exposure therapy and (or) cognitive restructuring for chronic posttraumatic stress disorder. Canadian Journal of Psychiatry, 55, 187–90. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. (2014). Salience processing and insular cortical function and dysfunction. Nature Review of Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Wigton R., Radua J., Allen P., et al. (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. Journal of Psychiatry Neuroscience, 40, E1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.