Abstract
Background
The fruit Terminalia belerica is a rich source of vitamins, acids, and nutraceuticals which have free radical scavenging activity. Thus, the ethanolic extract of fruit and its isolated compound (Tb-01) were intended to estimate antiplatelet and antioxidant activities.
Methods
The ethanolic extract was submitted to Si-gel CC and the compound was isolated. The compound Tb-01 was characterized as benzoyl-β-D-(4′→10″ geranilanoxy)-pyranosides on the basis of spectral data [ultra violet (UV), infrared (IR), 1H nuclear magnetic resonance (NMR), 13C NMR, and Mass Spectroscopy] and chemical analyses. The ethanolic extract and Tb-01 at different concentrations were in vitro screened for antiplatelet and antioxidant activity. The antiplatelet activity was carried out by using platelet rich plasma prepared by centrifugation of rabbit whole blood (containing 0.9% sodium citrate as anticoagulant) and antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl, reducing power, and nitric oxide anion scavenging activity models.
Results
The compound Tb-01 was an amorphous brownish powder, yield 0.64% (w/w), melting point 105–110 °C, Retardation factor/Retention Value (Rf value) at 0.42 in methanol:chloroform (20:80) solvent system, UV absorption maxima at 243 nm, and molecular peak [M + H]+ at 394.15 m/z. It was observed that the ethanolic extract and Tb-01 at different concentrations showed significant antiplatelet and antioxidant activity. The antioxidant activity, like scavenging of 1,1-diphenyl-2-picrylhydrazyl radicals, nitric oxide radical, and reductive power were found to be concentration-dependent and increased when increasing amounts of sample were used.
Conclusion
Mass spectra and 1H NMR confirmed the isolated compound structure which was supported by 13C NMR and IR spectra. Tb-01could be promising for future applications in the treatment of blood clots, pulmonary embolism, and other related diseases.
Keywords: antiplatelet activity, benzoyl-β-D glycoside, platelet rich plasma, spectroscopy, Terminalia belerica
1. Introduction
Blood platelets are the cell remains, which prevents excessive hemorrhage by forming blood clots.1 The blood clot is a mass of blood cells and blood components which are formed to prevent bleeding resulting from blood vessel injury. In this process, platelets in the blood become sultry and clump together at the site of injury. Clotting is the body's normal response to prevent a person from hemorrhaging to death.2 However, blood clot formation can be hazardous if it occurs well within blood vessels, or if not violated after due time. Serious diseases like heart attacks and pulmonary embolisms are connected with the unfortunate blood clot formation.3, 4 There are some synthetic blood thinners like aspirin and heparin present in the market; these synthetic drugs have many side effects like ulceration and gastrointestinal problems.5 Herbal products can be isolated and identified as potential antiplatelet medicines.6
Terminalia belerica (Roxb.) f. (Combretaceace) is cultivated in the east and northeast region of India, particularly in Assam, West Bengal, Uttar Pradesh, Bihar, Tamil Nadu, and Karnataka. The plant fruit is one of the main ingredients of the well-known preparation “Triphala”, which is used in for the treatment of the common cold, pharyngitis, constipation, headache, leucorrhoea, liver diseases, gastrointestinal complaints, and hair loss.7 It is a powerful adaptogenic plant fruit that nourishes the lungs, eyes, throat, voice, and hair.8 It expels stones or other kapha-type accumulations in the urinary, digestive, and respiratory tracts.9 It has powerful laxative and astringent properties, it purges the bowels and simultaneously tones the tissues of the digestive tract, and it also provides potency to the tissues of the sense organs.10 Pharmacological evaluations of the fruits have indicated them to have anthelmintic, antidiarrheal, antiseptic, astringent, expectorant, antidiabetic,11 rejuvenative, and hepatoprotective effects.12 The overall tonic potential of these fruits has been known for thousands of years in India and Asian countries. Phytochemical investigation of T. belerica fruits showed the presence of ellagic acid, ethyl gallate, galloyl glucose, lignans (termilignan), chebulagic acid, phyllemblin, β-sitosterol, glucoside (bellericanin), gallo-tannic acid, mannitol, glucose, fructose, rhamnose, coloring matter, resins, and greenish yellow oil.13 The fruit of this plant is a rich source of vitamins, acids and nutraceuticals which have free radical scavenging activity.14 Free radicals like nitric oxide (NO) and superoxide (O2−) are implicated in the pathophysiology of different ailments.15 The present paper describes the isolation and characterization of 4′-substituted benzoyl-β-D glycoside from the ethanolic extract of T. belerica fruit and it is investigated for antiplatelet and antioxidant activity.
2. Methods
2.1. Collection and authentication of plant specimen
The mature fruits of T. belerica were procured in July 2013 from a local market in Lucknow, Uttar Pradesh, India and authenticated by Dr. Tarique Husain Botanist, at the National Botanical Research Institute, Lucknow. A voucher specimen No-95482 was retained in this laboratory for further reference.
2.2. Extraction and isolation
The fruits were dried in shade under a hot air blower and powdered. The fruit powder (200 g) was defatted with petroleum ether and extracted with ethanol using Soxhlet apparatus. The ethanolic extract was then concentrated and dried to yield 21.63% w/w. The ethanolic extract (25 g) was submitted to SiO2 gel CC eluting petroleum ether, chloroform, and methanol to afford 46 fractions. The compound was isolated from fractions 18–23 (chloroform:methanol 80:20) and subjected to thin layer chromatography using the solvent system chloroform:methanol (8:2) which showed a single spot at Rf value of 0.42 detected by spraying with anisaldehyde sulfuric acid. This fraction was collected and dried to get 160 mg of amorphous brownish powder with a melting point of 105–110 °C which was coded as Tb-01.16
2.3. Instrumentation and chemicals
UV spectra scanned in methanol on a Lambda Bio 20 spectrophotometer (Shimadzu-U, Singapore). IR spectra were recorded in KBr pellets on a Win IR FTS 135 instrument (Biorad, Philadelphia, PA, USA). 1H nuclear magnetic resonance (NMR) (300 MHz) and 13C NMR (75 MHz) spectra were recorded on a Brucker spectrometer (Brucker, Billerica, MA, USA) in Deuterated methanol (MeOD) with Tetramethyl silane (TMS) as internal standard. The Mass Spectroscopy were measured on a JEOL-AccuTOF machine, Ambala, Haryana, India equipped with a Direct Analysis in Real Time (DART) ion source and helium was used as a gas of collision. Melting points were determined on Perfit melting point apparatus, Ambala, Haryana, India. Silica gel (60–120 mesh, Qualigens, Mumbai, India) was used for column chromatography. Silica gel G (Qualigens) was used for analytical thin layer chromatography. Spots were visualized by exposure to iodine vapors, a UV lamp (254 nm), and by spraying with anisaldehyde sulfuric acid reagents. 1,1-Diphenyl-2-picrylhydrazyl (DPPH, Sigma-Aldrich, Bangalore, Karnataka, India). The solvents for isolation were obtained from Merck Mumbai, India.
2.4. Blood specimen
Whole blood was drawn from healthy rabbits of both sexes aged 20 weeks of the race Cunistar and New Zealander, with an average mass of 2.2 kg. The required volume of blood was taken into each of the microcentrifuge tubes. The experimental protocol was approved by the Institutional Ethical Committee (approval number IU/Pharm/Ph.D./CPCSEA/10/03) following the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) which complies with international norms of the Indian National Science Academy.
2.5. Antiplatelet activity
Platelet rich plasma (PRP) was prepared by centrifugation of rabbit whole blood (containing 0.9% sodium citrate as anticoagulant) at 252 g for 15 minutes. The concentration of PRP was adjusted to optical density 1.0 at λ 400 nm by adding normal saline and 1 mL of PRP was taken in each of nine tubes. Tubes 2–9 contained different concentrations (1.0 μg/mL, 2.0 μg/mL, 3.0 μg/mL, 4.0 μg/mL, 5.0 μg/mL, 6.0 μg/mL, 7.0 μg/mL, and 8.0 μg/mL, respectively) of isolated compound (Tb-01), ethanolic extract, and aspirin, and Tube 1 contained normal saline solution as a blank. The final volume of each tube was adjusted up to 2 mL by adding normal saline solution. All of these tubes were incubated at 37 °C for 3 minutes and then 0.2 mL of collagen 2 μg/mL was added in to each tube. Aggregation was induced under continuous stirring at 112 g for 3 minutes. The aggregation was monitored under a spectrophotometer at λ 400 nm.17, 18
2.6. Antioxidant activity
The radical scavenging activities of the ethanolic fruit extracts and Tb-01 against DPPH were determined. An aliquot of (0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL, and 1.2 mg/mL) of extract and Tb-01 was mixed in a test tube containing 3 mL of methanol and 0.5 mL of 1 mM DPPH. Ascorbic acid was used as the antioxidant standard at the same concentrations and a blank solution was prepared containing the same amount of methanol and DPPH. The reaction mixture was incubated at 37 °C for 30 minutes, and absorbance of the chromophore formed was read using a UV spectrophotometer at 517 nm.19 The radical scavenging activity was calculated using the following equation:
where Ac is the absorbance of control and At is the absorbance of the test sample.
2.7. Nitric oxide anion scavenging activity
An aliquot 1 mL of extract, Tb-01, and ascorbic acid (0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL, and 1.2 mg/mL in methanol) was mixed in 1 mL of sodium nitroprusside (10 mM) in phosphate buffered saline and incubated at room temperature for 180 minutes. The reaction does not have test sample but equivalent amount of methanol served as control. After the incubation period, 0.5 mL of Greiss reagent (1% sulfanilamide, 2% phosphoric acid, and 0.1% naphthylenediamine dihydrochloride) was added. Ascorbic acid was used as a positive control and absorbance of the chromophore formed was read by a UV spectrophotometer at 546 nm.15
2.8. Reducing power
The ethanolic extract, Tb-01 and gallic acid at concentrations of 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL, and 1.2 mg/mL in distilled water were mixed with 2.5 mL phosphate buffer (0.2 mM, pH 6.6) and 1% of 2.5 mL potassium ferricyanide. The mixture was incubated at 45 °C for 30 minutes. Subsequently, 2.5 mL of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged for 10 minutes at 700 g. The upper layer of the solution (1.5 mL) was mixed with 1.5 mL distilled water and FeCl3 (0.3 mL, 0.1%) and the absorbance was measured at 700 nm using a UV-VIS spectrophotometer.20
2.9. Statistical analysis
The experiment results are expressed as the mean ± standard deviation and IC50 values were calculated by linear regression analysis for antioxidant activity. One-way analysis of variance followed by Dunnett test was performed by using Graph Pad Prism 2.01 (Graph Pad Software, Inc., San Diego, CA, USA) for antiplatelet activity and a value of p < 0.05 and p < 0.01 were considered as statistically significant.21
3. Results
3.1. Structural elucidation of isolated compound (Tb-01)
Amorphous brownish powder; yield 0.64% (w/w); melting point 105–110 °C; Rf 0.42 (CHCl3:MeOH, 80:20); UV-visible λmax nm (CH3OH):243; IR υmax (KBr):OH (str) 3401; Ali C-H (str) 2926; C=C 1525; C-O (pyran) 1646; OH (bend) 1215; C-C (str) 758 cm-1; 1H-NMR (300 MHz, MeOD) and 13C-NMR (75 MHz, MeOD) (spectral data: Table 1); MS DART m/z (Direct Analysis in Real Time intense): 394.15 [M]+ (C22H34O6), 237.06,141.14 and 162.13.
Table 1.
1H and 13C-nuclear magnetic resonance (NMR) spectral values of 4′-substituted benzoyl-β-D glycoside (Tb-01).
| Position of proton | Nature of proton |
δH (ppm) |
δC (ppm) | |
|---|---|---|---|---|
| α | β | |||
| C1 | C | – | – | 146.49 |
| C2 | CH | 7.52 (m) | – | 139.69 |
| C3 | CH | 7.08 (m) | – | 123.48 |
| C4 | CH | 6.98 (m) | – | 110.43 |
| C5 | CH | 7.05 (m) | – | 118.37 |
| C6 | CH | 7.46 (m) | – | 122.06 |
| C7 | C=O | – | – | 170.51 |
| C1′ | CH | 4.67 (d, J = 7.2 Hz) | – | 90.31 |
| C2′ | CH | 4.38 (m) | – | 75.08 |
| C3′ | CH | 3.74 (m) | – | 69.84 |
| C4′ | CH | 4.04 (m) | – | 73.97 |
| C5′ | CH2 | 3.56 (d, J = 4.8 Hz) | 3.52 (d, J = 5.7 Hz) | 64.51 |
| C1″ | CH3 | 0.86 (t, J = 6.5 Hz) | – | 13.18 |
| C2″ | CH2 | 1.19 (m) | 1.15 (m) | 22.03 |
| C3″ | CH2 | 2.58 (m) | 2.59 (m) | 46.34 |
| C4″ | CH2 | 2.50 (m) | 2.53 (m) | 30.16 |
| C5″ | CH2 | 2.56 (m) | 2.48 (m) | 36.27 |
| C6″ | CH2 | 2.11 (m) | 2.07 (m) | 38.89 |
| C7″ | CH | 2.90 (m) | – | 42.18 |
| C8″ | CH3 | 1.27 (d, J = 5.4 Hz) | – | 15.07 |
| C9″ | CH3 | 1.37 (d, J = 6.4 Hz) | – | 17.38 |
| C10″ | CH2 | 3.31 (d, J = 6.1 Hz) | 3.34 (d, J = 6.1 Hz) | 62.65 |
3.2. Antiplatelet activity testing
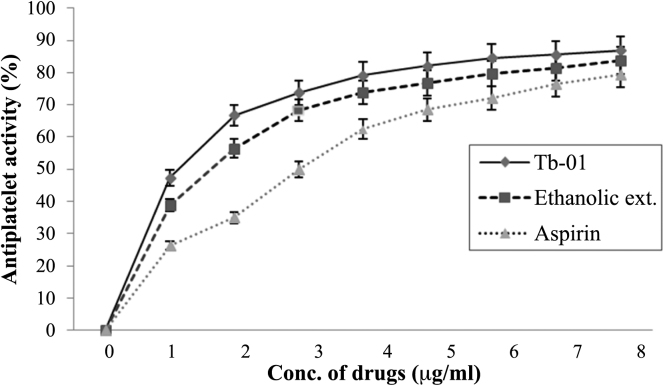
T. belerica showed very good antiplatelet activity in both Tb-01 and ethanolic extracts. It was observed that both the Tb-01 and ethanolic extract, at different concentrations, showed significant antiplatelet activity when compared with the control group. The maximum antiplatelet activity was observed between the concentrations 5–8 μg/mL (p < 0.01). The ethanolic extract showed less potency than Tb-01 and both were more potent than the aspirin (Fig. 1).
Fig. 1.
Comparative antiplatelet activity of Tb-01, ethanolic extracts, and aspirin at different concentrations. Each value expressed percentage of antiplatelet activity at different increasing concentrations.
3.3. Antioxidant activity
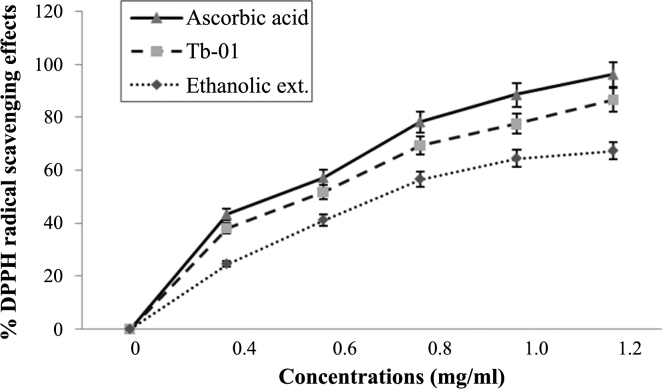
The free radical scavenging capacity of Tb-01, ethanolic extract, and ascorbic acid against DPPH radical was evaluated and they showed a dose-dependent radical scavenging activity. The drugs showed maximum inhibition at 1.2 mg/mL. The calculated IC50 value of Tb-01, extract, and ascorbic acid were 0.62 ± 0.06 mg/mL, 0.76 ± 0.12 mg/mL, and 0.48 ± 0.08 mg/mL, respectively (Fig. 2).
Fig. 2.
Dose-dependent scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals by ascorbic acid, Tb-01, and ethanolic extract. The data represent the percentage scavenging of DPPH radicals. Each value represents mean ± standard deviation (n = 4).
3.4. Nitric oxide anion scavenging activity
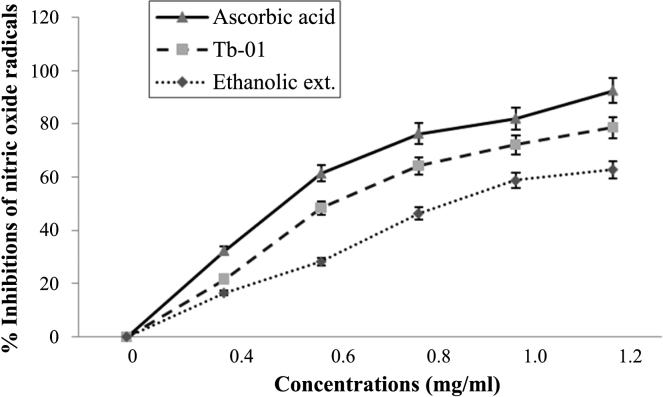
Fig. 3 shows the ability of Tb-01, ethanolic extract, and ascorbic acid to quench NO• radicals in dose-dependent manner and maximum inhibition was obtained at 1.20 mg/mL. The IC50 values of Tb-01, ethanolic extract, and ascorbic acid were 0.069 ± 0.014 mg/mL, 0.082 ± 0.018 mg/mL, and 0.058 ± 0.012 mg/mL, respectively.
Fig. 3.
Nitric oxide radical scavenging activity of ascorbic acid, Tb-01, and ethanolic extract at different concentration. The data represent the percentage nitric oxide inhibition. Each value represents mean ± standard deviation (n = 4).
3.5. Reducing power
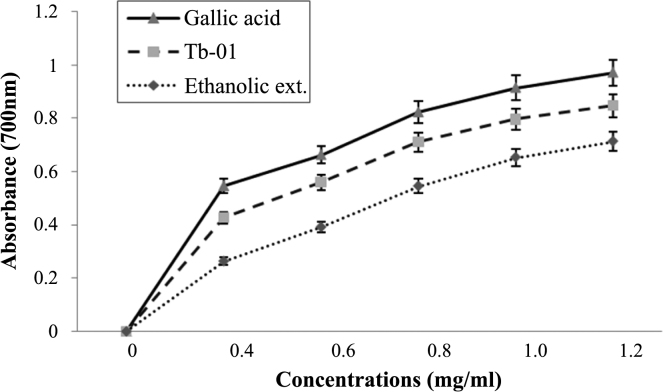
Fig. 4 shows the reductive capabilities of the different concentrations of Tb-01, ethanolic extract, and gallic acid. The reducing power of tested drugs was increased as the concentrations increased. At the highest dose of 1.2 mg/mL, the absorbance of Tb-01, ethanolic extract, and gallic acid were 0.848, 0.714, and 0.973, respectively.
Fig. 4.
The reductive ability of gallic acid, Tb-01 and ethanolic extract at different concentration. The absorbance (A700) was plotted against concentration of sample. Each value represents mean ± standard deviation (n = 4).
4. Discussion
The compound Tb-01 was obtained as an amorphous brownish powder from the chloroform-methanol (80:20) eluent. It gave positive Fehling's test for glycosides and showed IR absorption band for hydroxyl groups (3,401/cm), unsaturation C=C aromatic group (1,525/cm), benzoyl C-O group (1,646/cm), and aliphatic chain (758/cm). Absorption band 3401/cm arises from polymeric structures of hydrogen in hydroxyl groups and another strong band appears around 2,926/cm assigned to the linear vibration of hydrogen in C-H groups of the compound. Vibration of a C-O linkage has been shown to produce an absorption band at 1,646/cm, supporting the presence of a benzoyl group in the compound.22
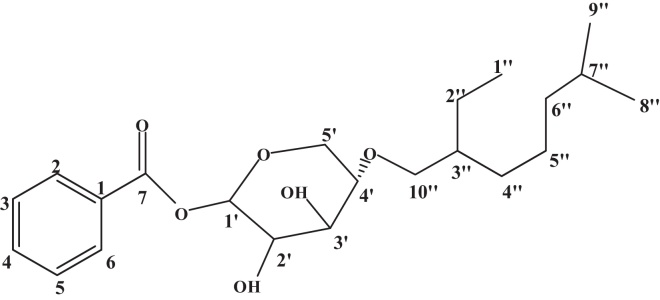
On the basis of mass and 13C NMR spectra, the radical molecular ion of Tb-01 found at m/z 394.15 [M]+ was consistent to the molecular formula C22H34O6. The 1H NMR spectrum of Tb-01 showed one single proton doublet at δH 4.67 (J = 7.2 Hz) assigned to anomeric proton H-1′ of sugar attached to the benzoyl group and other sugar methine protons from δH 4.38 to 3.74. The multiplet signal at δH 4.04 ppm indicates the presence of methine proton of sugar at C-4′ and coupled with H-5α and H-5β. The methine proton (CH) which is in a highly deshielded environment, gives multiplet signals at δH 7.52, 7.08, 6.98, 7.05, and 7.46 which were accounted to the presence of benzoyl groups. The gem dimethyl protons of the geranyl group give a triplet signal at δH 0.88 (J = 6.5 Hz) and other two methyl protons showed doublet at δH 1.27 (d, J = 5.4 Hz) and 1.37 (d, J = 5.4 Hz). The 13C NMR spectrum exhibited aglycone ester carbonyl carbon at δC 170.51 (C-7), aromatic carbons at δC 1-6 146.49, 139.69, 123.48, 110.43, 118.37, and 122.06, one anomeric sugar carbon at δC 90.31 (C-1′) and other sugar carbons at δC 90.31– 64.51. The methyl carbon (CH3) give signals at δC 13.18, 15.07, and 17.38.23 The compound shows a typical fragmentation pattern with molecular ions and a few fragment ions. MS of Tb-01 exhibited major ion fragments generated at m/z 394.15 (C22H34O6), 237.06 (C12H14O5 glucose esterified with benzene), 141.14 [C10H22 (2,6-dimethyloctane) aglycon geranyl] and 162.13 (C6H11O5 pyran). On the basis of these results, the structure of Tb-01 was characterized as benzoyl-β-D-(4′→10″ geranilanoxy)-xylopyranosides (Fig. 5).
Fig. 5.
Benzoyl-β-D-(4′→10″ geranilanoxy)-pyranosides.
The present study of antiplatelet activity of T. belerica was carried out by the UV spectrophotometric method. Collagen was taken as platelet aggregation inducer. The positive (aspirin) and negative (Normal Saline) controls clearly revealed the appropriateness of the methods under investigation. These results show that in vitro, the Tb-01 and ethanol extract inhibit collagen-induced platelet aggregation in a concentration dependent manner. Therefore, they have power to prevent thrombotic or cardiovascular disease. The principle of the method is when an agonist (collagen) was added in PRP, the platelets aggregated and settled down, and hence absorbed less light, so the transmission increased; this was then detected by the photocell (Table 2). The working concentration of collagen was taken as 2 μg/mL and 0.2 mL of collagen was used for each tube, as this was the optimized amount and referred from published work. Comparative antiplatelet activities of Tb-01, ethanolic extracts, and aspirin at different concentrations are shown in Fig. 1.
Table 2.
Antiplatelet activity of Tb-01 and ethanolic extract of Terminalia belerica fruit
| Test tube No. | Conc. of drugs (μg/mL) | Optical density |
||
|---|---|---|---|---|
| Tb-01 | Ethanolic ext. | Aspirin | ||
| 1. | 0.0 | 0.121 ± 0.023 | 0.121 ± 0.023 | 0.121 ± 0.023 |
| 2. | 1.0 | 0.232 ± 0.017 | 0.198 ± 0.014 | 0.164 ± 0.023 |
| 3. | 2.0 | 0.363 ± 0.028* | 0.278 ± 0.017 | 0.186 ± 0.028 |
| 4. | 3.0 | 0.461 ± 0.025* | 0.381 ± 0.023* | 0.242 ± 0.019 |
| 5. | 4.0 | 0.582 ± 0.017* | 0.464 ± 0.014* | 0.322 ± 0.024* |
| 6. | 5.0 | 0.676 ± 0.028** | 0.521 ± 0.017** | 0.384 ± 0.026* |
| 7. | 6.0 | 0.781 ± 0.019** | 0.596 ± 0.023** | 0.434 ± 0.017* |
| 8. | 7.0 | 0.834 ± 0.016** | 0.651 ± 0.019** | 0.514 ± 0.024** |
| 9. | 8.0 | 0.917 ± 0.026** | 0.728 ± 0.021** | 0.587 ± 0.018** |
Data were expressed as mean ± standard deviation (n = 3). One-way analysis of variance was performed followed by Dunnett test.
*p < 0.05 and **p < 0.01 compared with resting platelets (control).
1 mL of diluted platelet rich plasma and 0.2 mL of collagen (2 μg/mL) were taken in each tube.
In this study, the isolated glycoside Tb-01 may be hydrolyzed to benzoic acid and geraniol, which have similar activity to salicylates (aspirin). The effect of aspirin on platelet function is to inactivate a key platelet enzyme cyclooxygenase by diffusing into the cyclooxygenase channel in the membrane to the catalytic site for the enzyme. Aspirin has a vital role in the prevention of thromboembolic problems from atherosclerotic disease.24 In addition, we further investigated whether this efficacy of Tb-01 in vitro is implemented in in vivo function.
Free radicals have been involved in many disease conditions, the important ones being NO, superoxide, hydroxyl, and peroxyl radical. In the present study, Tb-01 and ethanolic extract of T. belerica fruits were investigated for antioxidant activity based on the response obtained in in vitro models. DPPH is one of the free radicals used for testing the preliminary radical scavenging activity. Both Tb-01 and ethanolic extract of fruit showed a good antiradical activity by scavenging DPPH radicals (Fig. 2).
NO exhibits abundant physiological properties and is implicated in several pathological states.15 It is produced in various cells including neurons, endothelial cells, and neutrophils. The contact of NO with other radicals leads to the creation of more hazardous radicals such as peroxy nitrite anion. Tb-01 and the ethanolic extract showed dose-dependent NO scavenging activity (Fig. 3).
The reducing capacity of a compound provides a significant indicator of its potential antioxidant activity. The reductive capabilities of Tb-01 and ethanolic extract were compared with gallic acid (Fig. 4). For the measurements of the reductive capacity, we investigated the Fe3+ to Fe2+ transformation in the presence of Tb-01 and the ethanolic extract.
A new (Tb-01) 4′-substituted β-D glycoside (benzoyl-β-D-(4′→10″ geranilanoxy)–xylopyranosides) has been isolated from the ethanolic extract of T. belerica fruits. In antiplatelet assays using freshly isolated rabbits platelets, the Tb-01 and ethanolic extracts showed significant inhibition of collagen-induced platelet aggregation. We propose that the inhibitory effects of Tb-01 and ethanolic extract on collagen-induced platelet aggregation might inhibit collagen-stimulated platelet aggregation without any toxicity. The DPPH, NO radical scavenging, and reducing capacity of Tb-01 and ethanolic extracts were evaluated, which might be helpful in preventing or slowing the progress of various oxidative stress related diseases, and could be a promising strategy in many cardiovascular diseases such as atherosclerosis, myocardial infarction, and thrombosis.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
One of the authors is thankful to Dr. Arsad Hussain, Faculty of Pharmacy, Integral University, Lucknow, Uttar Pradesh (India) for providing necessary facilities for the research. The authors are thankful to the Central Drug Research Institute, Lucknow and Professor (Dr.) Mohammad Ali, Jamia Hamdard for characterization and interpretation of isolated compound.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imr.2016.08.001.
Appendix A. Supplementary data
References
- 1.Campbell N.A. 8th ed. Pearson Education; London: 2008. Biology; p. 912. [Google Scholar]
- 2.Elliot W.H., Elliot D.C. 3rd ed. Oxford University Press; Oxford, UK: 2005. Biochemistry and Molecular Biology. [Google Scholar]
- 3.Anthony M.R. University of Zululand; 2011. In vitro anti-platelet aggregation activity of the extracts of Protorhus longifolia. [MSc thesis] [Google Scholar]
- 4.Gwala P.E. University of Zululand; 2011. The anti-platelet aggregation activity of Rapaneamelanophloeos –A Zulu medicinal plant. [MSc thesis] [Google Scholar]
- 5.Sanchez-Lamar A., Fiore M., Cundari E., Ricordy R., Cozzi R., De Salvia R. Phyllanthus orbicularis aqueous extract cytotoxic, genotoxic and antimutagenic effects in the CHO cell line. Toxic App Pharm. 1999;161:231–239. doi: 10.1006/taap.1999.8814. [DOI] [PubMed] [Google Scholar]
- 6.Meshram G., Patil B., Datta S., Metangale G. Effect of Epigallocatechin gallate isolated from Terminalia belerica fruit rind on glucoamylase activity in vitro. J Appl Pharm Sci. 2011;1:115–117. [Google Scholar]
- 7.Amritpal S.S. Science Publishers;; 2011. Herbalism phytochemistry and ethnopharmacology; pp. 357–361. [Google Scholar]
- 8.Nadkarni K.M. Vol. 1. Published by Ramdas Bhatkal for Popular Prakashan Pvt. Ltd.; Mumbai: 2002. pp. 202–1205. (Indian meteria medica). [Google Scholar]
- 9.The Ayurvedic Pharmacopoeia of India. Govt. of India, Ministry of Health and Family Welfare, Part-I. 1st ed., Vol. 3. New Delhi: The Controller of Publications, Dept. of ISM and H; 2001, Part-1, 1, p. 252.
- 10.Vaidyaratnam P.S. Vol. 5. Published by Orient Longman Private Ltd.; Chennai: 2004. pp. 258–262. (Varier's, Indian medicinal plants). [Google Scholar]
- 11.Latha P.C.R., Daisy P. Influence of Terminalia belerica fruits extract on biochemical parameters, in streptozotocin diabetic rats. Int J Pharmacol. 2010;06:89–96. [Google Scholar]
- 12.Shukla S., Jadon A., Bhadauria M. Protective effect of Terminalia belerica Roxb, and gallic acid against carbon tetra chloride induced damage in albino rats. J Ethnopharmacol. 2006;109:214–218. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi R.P., Mehrotra B.N. Vol. 1. Central Drug Research Institute (CDRI), Lucknow and National Institute of Communication and Information Resources; New Delhi: 2004. p. 406. (Compendium of medicinal plants). [Google Scholar]
- 14.Alam B. Antioxidant, antimicrobial and toxicity studies of the different fractions of fruits of Terminalia belerica Roxb. Glob. J Pharmacol. 2011;5:07–17. [Google Scholar]
- 15.Arif M., Fareed S., Hussain S. Estimation of antioxidant activity of microwave assisted extraction of total phenolics and flavonoids contents of the fruit Spondias mangifera. Asian J Trad Med. 2011;6:146–155. [Google Scholar]
- 16.Arif M., Fareed S., Hussain T., Ali M. Adaptogenic activity of lanostane triterpenoid isolated from Carissa carandas fruit against physically and chemically challenged experimental mice. Pharmacog J. 2013;5:216–220. [Google Scholar]
- 17.Andrea C., Fabio M.P., Pasquale P., Luisa L., Giacomo F., Pier P. Vitamin E potentiates the antiplatelet activity of aspirin in collagen-stimulated platelets. Haematol. 2002;87:420–426. [PubMed] [Google Scholar]
- 18.Aburjai T., Hudaib M., Antiplatelet antibacterial and antifungal activities of Achillea falcate extracts and evaluation of volatile oil composition. Pharmacog Mag. 2006;2:191–198. [Google Scholar]
- 19.Sachan N.K., Arif M., Zaman K., Kumar Y. Anti-inflammatory, analgesic and antioxidant potential of the stem bark of Spondias mangifera Willd. Arch Biol Sci Belgrade. 2011;63:413–419. [Google Scholar]
- 20.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 21.Brown A.M. A new software for carrying out one-way ANOVA post hoc tests. Comp Meth Prog Biomed. 2005;79:89–95. doi: 10.1016/j.cmpb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Arif M., Fareed S., Rahman M.A. Stress relaxant and antioxidant activities of acid glycoside from Spondias mangifera fruit against physically and chemically challenged albino mice. J Pharm Bioallied Sci. 2016;8:58–63. doi: 10.4103/0975-7406.171685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid H., Kaur G., Abdullah T., Ali M., Athar M., Alam S.M. Two new compounds from the Galls of Qiiercus infectoria with nitric oxide and superoxide inhibiting ability. Pharm Biol. 2005;43:317–323. doi: 10.1080/13880200590951711. [DOI] [PubMed] [Google Scholar]
- 24.Burch J.W., Stanford N., Majerus P.W. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest. 1978;61:314–319. doi: 10.1172/JCI108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.