Abstract
The accumulation of lipofuscin in the cells of the retinal pigment epithelium (RPE) is thought to play an important role in the development and progression of degenerative diseases of the retina. The bulk of RPE lipofuscin originates in reactions of the rhodopsin chromophore, retinal, with components of the photoreceptor outer segment. The 11-cis retinal isomer is generated in the RPE and supplied to rod photoreceptor outer segments where it is incorporated as the chromophore of rhodopsin. It is photoisomerized during light detection to all-trans and subsequently released by photoactivated rhodopsin as all-trans retinal, which is removed through reduction to all-trans retinol in a reaction requiring metabolic input in the form of NADPH. Both 11-cis and all-trans retinal can form lipofuscin precursor fluorophores in rod photoreceptor outer segments. Increased accumulation of lipofuscin has been suggested to result from excess formation of lipofuscin precursors due to buildup of all-trans retinal released by light exposure. In connection with this suggestion, the Abca4 transporter protein, an outer segment protein defects in which result in recessive Stargardt disease, has been proposed to promote the removal of all-trans retinal by facilitating its availability for reduction. To examine this possibility, we have measured the outer segment levels of all-trans retinal, all-trans retinol, and of lipofuscin precursors after bleaching by imaging the fluorescence of single rod photoreceptors isolated from wild type and Abca4−/− mice. We found that all-trans retinol and all-trans retinal levels increased after bleaching in both wild type and Abca4−/− rods. At all times after bleaching, there was no significant difference in all-trans retinal levels between the two strains. All-trans retinol levels were not significantly different between the two strains at early times, but were lower in Abca4−/− rods at times longer than 20 min after bleaching. Bleaching in the presence of lower metabolic substrate concentrations resulted in higher all-trans retinal levels and increased formation of lipofuscin precursors in both wild type and Abca4−/− rods. The results show that conditions that result in buildup of all-trans retinal levels result in increased generation of lipofuscin precursors in both wild type and Abca4−/− rods. The results are consistent with the proposal that Abca4 facilitates the reduction of all-trans retinal to retinol; absence of Abca4 however does not appear to be associated with higher all-trans retinal levels compared to wild type.
Keywords: photoreceptor, retinal, retinol, NADPH, ABCA4, lipofuscin, fluorescence
1. INTRODUCTION
Lipofuscin, a complex mixture of fluorescent pigments, accumulates with age in the lysosomes of post-mitotic cells (Katz and Robison, 2002), including the retinal pigment epithelial (RPE) cells in the eye (Delori et al., 1995a; Delori et al., 2001; Feeney, 1978; Wing et al., 1978). Lipofuscin has a range of deleterious effects on cell and tissue function (Eldred and Lasky, 1993; Lakkaraju et al., 2007; Rozanowska et al., 1998; Sparrow et al., 2000; Vives-Bauza et al., 2008), and its accumulation is thought to play a role in degenerative diseases of the retina, including Age-related Macular Degeneration (AMD) (Sparrow and Boulton, 2005; Winkler et al., 1999) and Stargardt disease (Delori et al., 1995b; Weng et al., 1999). Although the exact composition of lipofuscin is not known, bis-retinoid compounds, which are derived from the condensation of two molecules of retinal with other cellular components (Sparrow et al., 2012), have been identified as major constituents of RPE lipofuscin. A2E is the best characterized bis-retinoid lipofuscin component to date (Ben-Shabat et al., 2002; Parish et al., 1998).
Lipofuscin fluorophores originate in the photoreceptor outer segments (Boyer et al., 2012; Katz et al., 1986), and, through the daily phagocytosis of the outer segments by the RPE, enter the RPE lysosomal compartment where they accumulate after additional processing. Reactions of the visual pigment chromophore play a pivotal role in lipofuscin formation: suppressing the generation of the visual pigment chromophore 11-cis retinal results in loss of most of RPE lipofuscin (Katz and Redmond, 2001). Rhodopsin, the visual pigment of rod photoreceptors, utilizes 11-cis retinal as the chromophore to detect light (Ebrey and Koutalos, 2001). Light detection is initiated by an active rhodopsin photointermediate generated through the photoisomerization of the 11-cis chromophore of rhodopsin to all-trans. Photoactivated rhodopsin triggers a cascade of reactions culminating to a change in membrane potential, which constitutes the light response. Photoisomerization destroys rhodopsin, which is then regenerated via a two-step process: first, all-trans retinal is released, leaving the apo-protein opsin, and, second, opsin binds freshly supplied 11-cis retinal and re-forms rhodopsin. The released all-trans retinal is reduced within the rod outer segment to all-trans retinol, which is then transported to the RPE where it is recycled to make 11-cis retinal (Lamb and Pugh, 2004; Saari, 2000; Tang et al., 2013). The reduction of all-trans retinal to retinol is catalyzed by the enzyme retinol dehydrogenase RDH8 (Chen et al., 2012; Maeda et al., 2005) and requires metabolic input in the form of NADPH (Adler et al., 2014; Futterman et al., 1970). 11-Cis and all-trans retinal can both generate lipofuscin precursorsin rod outer segments, as evidenced by measurements of single cell fluorescence (Boyer et al., 2012), as well as of bis-retinoids (Ben-Shabat et al., 2002; Liu et al., 2000; Mata et al., 2000; Quazi and Molday, 2014). In the absence of 11-cis retinal generation, and therefore absence of both 11-cis and all-trans retinal in rod outer segments, no bis-retinoid formation is detected in RPE or neural retina (Boyer et al., 2012; Wu et al., 2009). The residual fluorescence signal detected in RPE cells and in rod outer segments of Rpe65−/− mice is much smaller and has a different emission spectrum from that of lipofuscin that forms with intact 11-cis retinal generation. Thus, it appears that the bulk of RPE lipofuscin originates from reactions of 11-cis and all-trans retinal with outer segment components.
The significant lipofuscin fluorescence and bis-retinoid levels measured in dark-reared wild type and Abca4−/− animals (Boyer et al., 2012; Lenis, 2016; Ueda et al., 2016) suggest that 11-cis retinal is a major contributor to lipofuscin generation. The extent of all-trans retinal contribution is not as clear, especially in view of the observation that lipofuscin levels in dark-reared animals are at least the same or even higher than those in cyclic-light-reared ones (Boyer et al., 2012; Ueda et al., 2016). It is important to note however, that photodegradation of bis-retinoids lowers lipofuscin levels in cyclic-light-reared mice (Ueda et al., 2016), blunting the observable contribution of all-trans retinal. Focusing on the reactions in rod outer segments and the origins of lipofuscin, the degree to which all-trans retinal contributes to lipofuscin generation will depend on the pathways that facilitate its removal after its release from photoactivated rhodopsin.
The removal of all-trans retinal has been proposed to be facilitated by the ABCA4 transporter protein, defects in which are associated with recessive Stargardt disease. Defects in ABCA4 also result in increased accumulation of lipofuscin in the RPE (Boyer et al., 2012; Weng et al., 1999), as well as increased levels of lipofuscin precursors in rod photoreceptor outer segments (Boyer et al., 2012). In terms of function, ABCA4 is known to catalyze the translocation of phosphatidylethanolamine and its Schiff bases with 11-cis and all-trans retinal, from the lumen to the cytosolic side of the rod outer segment membrane disks (Quazi et al., 2012). Thus, loss of ABCA4 function would result in trapping of all-trans retinal in the disk lumen, preventing its access to RDH8 and reduction to all-trans retinol. The build-up of all-trans retinal would result in increased formation of lipofuscin precursors in the rod outer segments followed by increased deposition of lipofuscin in the RPE of Abca4−/− mice.
Because of the cytotoxicity of lipofuscin, conditions that can promote its formation, such as the lack of the Abca4 transporter protein or the buildup of all-trans retinal, have received a lot of attention. Previous studies have relied on biochemical measurements, which cannot distinguish between the all-trans retinal that has been released from photoactivated rhodopsin and the one that is still covalently bound to it (in the form of the photointermediates metarhodopsin II and III) – in biochemical measurements all of the all-trans retinal is extracted and quantified, not just the released. Here, we have used fluorescence imaging to measure all-trans retinol, all-trans retinal, and lipofuscin precursors in single rod photoreceptors isolated from the retinas of wild type Sv/129 and Abca4−/− mice. We have used these measurements to compare the appearance and removal of the all-trans retinal generated by light in wild type and Abca4−/− rods. We have also examined whether the increased accumulation of all-trans retinal brought about by limited availability of metabolic substrate results in increased formation of lipofuscin precursor fluorophores.
2. MATERIALS AND METHODS
2.1 Animals
Wild type Sv/129 and Abca4−/− transgenic mice originated from established colonies at the Medical University of South Carolina. Sv/129 mice were originally obtained from Harlan Laboratories (Indianapolis, IN); breeding pairs of Abca4−/− animals were generous gifts of Dr. G.H. Travis. The background strain of the Abca4−/− animals was Sv/129. All animal procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and followed National Institutes of Health guidelines for care and use of Laboratory animals. Animals were reared in cyclic light, 12-h light cycle (06:00–18:00); light intensity at cage level during the light cycle was 130 – 170 lux, measured with a light meter (LM631; Meterman Test Tools, Everett, WA).
2.2 Fluorescence imaging of isolated rods
For experiments, 2–3 months old animals were dark-adapted overnight and sacrificed under dim red light. Single isolated rod photoreceptor cells were obtained and imaged at 37 °C in physiological solution on a Zeiss Axiovert 100 epifluorescence microscope, as described previously (Chen et al., 2009). Both metabolically intact rods (with attached ellipsoids) and metabolically compromised broken off rod outer segments (ROS) were used (Chen and Koutalos, 2010). The physiological solutions used for experiments contained different concentrations of glucose or glutamine as metabolic substrates. For all-trans retinal and all-trans retinol measurements, fluorescence was excited by 340 and 380 nm light with emission collected >420 nm (Chen et al., 2012). For lipofuscin precursor measurements, fluorescence was excited with 490 nm light and emission collected >515 nm (Boyer et al., 2012). Data were collected and analyzed using Slidebook (Intelligent Imaging Innovations, Denver, CO).
2.3 Separation of the fluorescence signals of all-trans retinol and all-trans retinal
It is not straightforward experimentally to separate the fluorescence signals of all-trans retinol and all-trans retinal by using different excitation filters. With glass optics, 340 nm is the lowest wavelength that can be used, which is only the isosbestic point for retinal (absorption λmax~380 nm) and retinol (absorption λmax~325 nm) (Chen et al., 2012). Because of this constraint, fluorescence measurements were carried out with 340 and 380 nm excitation light (emission >420 nm), providing the fluorescence intensities Fex-340 and Fex-380, respectively. The fluorescence signals of retinol and retinal both contribute to Fex-340 and Fex-380. If Fex-λ is the rod outer segment fluorescence excited with light of wavelength λ (λ = 340 or 380 nm), and Fex-λ(ROL) and Fex-λ(RAL) the contributions of retinol and retinal fluorescence, respectively, then
| (1) |
The contributions of retinol and retinal are proportional to the respective extinction coefficients e-λ(ROL) and e-λ(RAL) at wavelength λ, the fluorescence quantum yields Q(ROL) and Q(RAL) (which do not depend on wavelength), and their outer segment concentrations [ROL] and [RAL]. That is,
| (2a) |
| (2b) |
To estimate the contributions Fex-λ(ROL) and Fex-λ(RAL) of retinol and retinal to the overall outer segment fluorescence signal Fex-λ, it is necessary to know the relative fluorescence quantum yield Q(ROL)/Q(RAL). This however is not known with sufficient accuracy (Chen et al., 2012), hence in general it is not possible to separate the contributions of retinol and retinal using the measurements of Fex-340 and Fex-380. It is only in special cases where the contributions can be separated by simplifying Eq. 1.
One special case obtains under physiological metabolic conditions for metabolically intact rods, that is, in the presence of 5 mM glucose as metabolic substrate, when >70–80% of the all-trans retinal released after bleaching is converted to all-trans retinol. Under these conditions, because e-340(ROL) ≈ e-340(RAL), Q(ROL)/Q(RAL) ≥ 5, and [ROL]/[RAL] ≥ 3–4, all-trans retinal contributes less than 10% of the fluorescence excited by 340 nm light. So,
| (3) |
that is, Fex-340, the fluorescence excited by 340 nm light, is mostly due to all-trans retinol and can be used as a measure of its level. On the other hand, Fex-380, the fluorescence excited by 380 nm light, contains significant contributions by both retinol and retinal, even in this case. These contributions can however be separated using Eq. 3 and the fluorescence intensity ratio
| (4) |
the value of which has been determined experimentally previously (Adler et al., 2014; Chen et al., 2012). From Eq. 1,
| (5) |
Substituting from Eqs. 3 and 4, and rewriting Eq. 3, we obtain
| (6a) |
| (6b) |
Eqs. 6a and 6b provide separate measures of the retinal and retinol levels. It should be emphasized that these equations hold only under the specific conditions of metabolically intact rods in the presence of 5 mM glucose.
2.4 Statistical analysis
Error bars denote SEM. Linear regression was used to test for statistical significance.
3. RESULTS
3.1 Levels of all-trans retinol and all-trans retinal after bleaching in single isolated rods from wild type and Abca4−/− mice
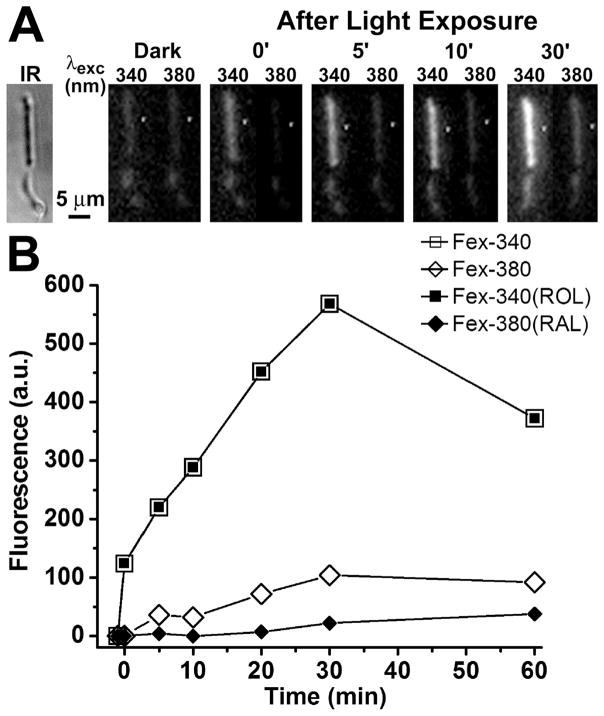
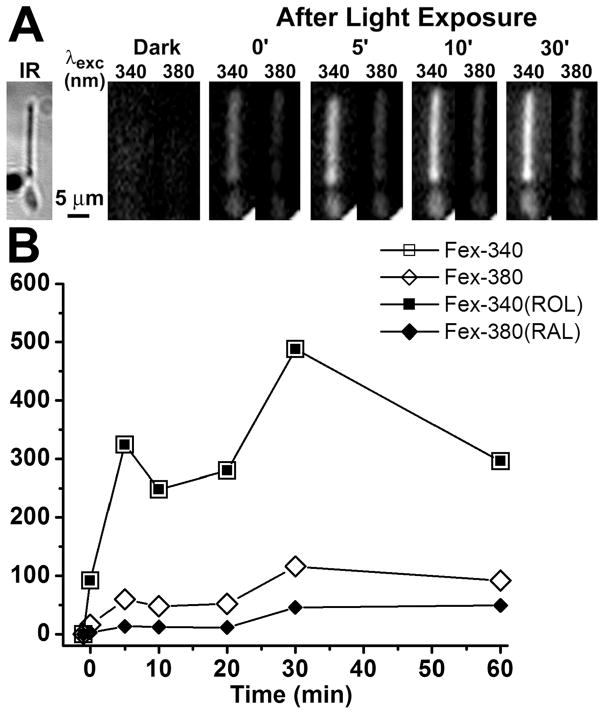
The outer segment fluorescence of a dark-adapted rod photoreceptor excited by UV light increases after bleaching. This increase in fluorescence is due to the all-trans retinal and all-trans retinol generated by bleaching. In a metabolically intact wild type rod photoreceptor with access to non-limiting metabolic substrate concentrations, the intensity of the fluorescence excited by 340 nm light, Fex-340, is much higher than that excited by 380 nm, Fex-380 (Fig. 1A). The high Fex-340/Fex-380 ratio reflects the conversion of the greater part of the all-trans retinal released after bleaching to all-trans retinol, a conversion that is made possible by the generation of the requisite quantities of NADPH. Fig. 1B shows the values of the rod outer segment fluorescence excited by 340 and 380 nm, Fex-340 and Fex-380, measured at different times after bleaching. From the values of Fex-340 and Fex-380, the fluorescence signals due to all-trans retinal, Fex-380(RAL), and all-trans retinol, Fex-340(ROL), were calculated using Eqs. 6a and 6b, for each time point after bleaching. Fig. 2 shows that a metabolically intact Abca4−/− rod photoreceptor also converts the greater part of the all-trans retinal released after bleaching to all-trans retinol. Fig. 3 compares the fluorescence signals due to the all-trans retinol and all-trans retinal generated after bleaching in Sv/129 (n = 12) and Abca4−/− (n = 13) rods. There was no detectable difference in the all-trans retinal levels between wild type (○) and Abca4−/− (●) rods at all times after bleaching. There was also no difference in the all-trans retinol levels at early times (up to 10 min) after bleaching. At later times however (longer than 20 min), all-trans retinol levels were consistently lower in Abca4−/− (▲) rods compared to wild type (△), although this reduction in levels was statistically significant only at 20 min (p = 0.028; Student’s t-test).
Figure 1.
Measurements of fluorescence of all-trans retinol and all-trans retinal in a metabolically intact rod photoreceptor isolated from a dark-adapted Sv/129 wild type mouse (2 month old). The fluorescence signals due to retinol and retinal can be separated by exciting rod outer segment fluorescence with 340 and 380 nm light and collecting emission >420 nm. (A) Infrared (IR) and fluorescence images of the cell before (Dark) and at different times after bleaching; to facilitate comparisons, the fluorescence images of the cell are shown with the same intensity scaling. Bleaching was carried out with long-wavelength (>530 nm) light for 1 min. (B) Intensities of the outer segment fluorescence excited by 340 and 380 nm light, and the fluorescence signals due to outer segment all-trans retinol, Fex-340(ROL), and all-trans retinal, Fex-380(RAL), at different times after bleaching. All-trans retinol is responsible for all of the fluorescence excited by 340 nm. Bleaching was carried out between t = −1 and t = 0 min. Experiment at 37 °C.
Figure 2.
Measurements of fluorescence of all-trans retinol and all-trans retinal in a metabolically intact rod photoreceptor isolated from a dark-adapted Abca4−/− mouse (2 month old). (A) Infrared (IR) and fluorescence images of the cell before (Dark) and at different times after bleaching; to facilitate comparisons, the fluorescence images of the cell are shown with the same intensity scaling. Bleaching was carried out with long-wavelength (>530 nm) light for 1 min. (B) Intensities of the outer segment fluorescence excited by 340 and 380 nm light, and the fluorescence signals due to outer segment all-trans retinol, Fex-340(ROL), and all-trans retinal, Fex-380(RAL), at different times after bleaching. All-trans retinol is responsible for all of the fluorescence excited by 340 nm. Bleaching was carried out between t = −1 and t = 0 min. Experiment at 37 °C.
Figure 3.
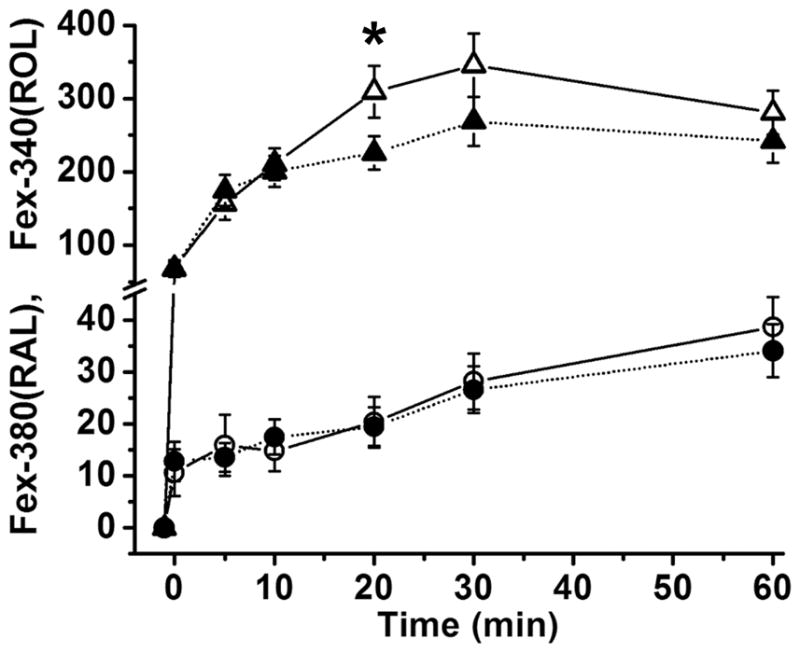
The kinetics of all-trans retinal and all-trans retinol in metabolically intact rod photoreceptors isolated from dark-adapted Sv/129 wild type and Abca4−/− mice. All-trans retinol and all-trans retinal levels were measured from their contributions, Fex-340(ROL) and Fex-380(RAL), to the fluorescence excited by 340 and 380 nm, as shown in Figs. 1 and 2. Dark-adapted isolated cells were bleached with long-wavelength (>530 nm) light for 1 min (between t = −1 and t = 0 min). Fluorescence due to all-trans retinol was the same for Sv/129 wild type (△, n = 12) and Abca4−/− cells (▲, n = 13) at early times after bleaching, but was significantly lower in Abca4−/− cells at 20 min. The fluorescence due to all-trans retinal measured simultaneously was the same for Sv/129 wild type (○) and Abca4−/− (●) rods at all times after bleaching. Mice were 2 months old. Error bars represent standard errors. Experiments at 37 °C.
3.2 Lipofuscin precursor formation after bleaching in single isolated rods from wild type and Abca4−/− mice
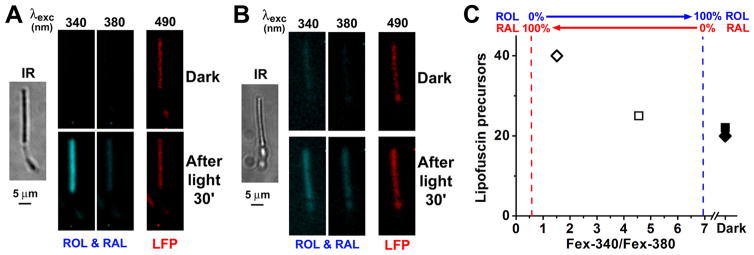
The outer segments of rod photoreceptors from wild type and Abca4−/− mice contain fluorophores that originate from reactions of 11-cis and all-trans retinal, and their fluorescence emission spectrum (490 nm light excitation) is similar to that of lipofuscin. We refer to them as lipofuscin precursors and their fluorescence is measured by exciting with 490 nm light and collecting the emission >515 nm (Boyer et al., 2012). At the same time, after bleaching a dark-adapted rod the extent of the conversion of the all-trans retinal released from photoactivated rhodopsin to all-trans retinol can be measured from the Fex-340/Fex-380 fluorescence ratio. Then, the Fex-340/Fex-380 ratio can be used as a measure of the accumulation of all-trans retinal after bleaching: the lower the ratio, the higher the accumulation of all-trans retinal. Thus, by measuring these different fluorescence signals, we can examine whether accumulation of all-trans retinal results in increased formation of lipofuscin precursors. For metabolically intact rod photoreceptors in the presence of 5 mM glucose, the lipofuscin precursor fluorescence at 30 min after light exposure (Fig. 4A and Fig. 4C, □) is similar to that in the dark-adapted cell (Fig. 4A and Fig. 4C, ■), in agreement with previous work (Boyer et al., 2012)). Under these conditions, there is also limited accumulation of all-trans retinal released from photoactivated rhodopsin: it is quantitatively reduced to all-trans retinol, as denoted by the high value of the Fex-340/Fex-380 ratio (Fig. 4A and Fig. 4C, □), in agreement with previous work (Adler et al., 2014)). In the absence of metabolic substrate, at 30 min after light exposure the Fex-340/Fex-380 ratio was lower (Fig. 4B and Fig. 4C, ⋄), indicating the accumulation of all-trans retinal; at the same time, lipofuscin precursor fluorescence (Fig. 4B and Fig. 4C, ⋄) increased substantially compared to the dark-adapted cell (Fig. 4B and Fig. 4C, ◆). Low concentrations of metabolic substrate have previously been shown to result in accumulation of all-trans retinal (Adler et al., 2014). Measuring the fluorescence of lipofuscin precursors in the same cells before and after bleaching, provided the relation between the accumulation of all-trans retinal and the generation of lipofuscin precursors (Fig. 5). In wild type Sv/129 rods, limiting the availability of metabolic substrate increased the accumulation of all-trans retinal, and at the same time increased the levels of lipofuscin precursors after bleaching (Fig. 5, ○; a, 5 mM glucose, n = 9 cells; b, 0.5 mM glutamine, n = 7; c, 50 μM glucose, n = 17; d, 50 μM glutamine, n = 16; e, 0 substrate, n =12; f, broken-off rod outer segments, n =11).. The basal levels of lipofuscin precursor fluorescence in dark-adapted rods, before bleaching, did not vary significantly across the different conditions and were averaged for all wild type rods (Fig. 5, ●, n = 72 cells). Similar results were obtained in Abca4−/− rods, (Fig. 5, △; a, 5 mM glucose, n = 9 cells; b, 0.5 mM glutamine, n = 8; c, 50 μM glucose, n = 8; d, 50 μM glutamine, n = 9; e, 0 substrate, n =9; f, broken-off rod outer segments, n =7). The basal levels of lipofuscin precursor fluorescence in dark-adapted Abca4−/− rods, before bleaching, did not vary significantly across the different conditions and were averaged (Fig. 5, ▲, n = 50 cells); as expected, they were higher than the basal levels in wild type rods. Thus, after light exposure, lipofuscin precursor fluorescence increased with increased accumulation of all-trans retinal in rods from both Sv/129 wild type (Fig. 5, ○; p < 0.001, linear regression) and Abca4−/− mice (Fig. 5, △; p < 0.002, linear regression).
Figure 4.
Measurement of the fluorescence of all-trans retinol, all-trans retinal, and of lipofuscin precursors in metabolically intact rod photoreceptors isolated from dark-adapted Sv/129 wild type mice. Fluorescence signals due to retinol and retinal (ROL & RAL) were obtained by exciting rod outer segment fluorescence with 340 and 380 nm light and collecting emission >420 nm. Fluorescence signals due to lipofuscin precursors (LFP) were obtained with 490 nm excitation and collecting emission >515 nm. IR, infrared images of the cells; to facilitate comparisons, the fluorescence images of the cells before (Dark) and at 30 min after bleaching are shown with the same intensity scaling. Bleaching was carried out with long-wavelength (>530 nm) light for 1 min. (A) 5 mM glucose present as metabolic substrate. (B) No metabolic substrate present. (C) Relationship between lipofuscin precursor levels and all-trans retinal accumulation in the two cells. All-trans retinal accumulation was measured as the Fex-340/Fex-380 ratio. The value Fex-340/Fex-380 = 0.55 corresponds to 100% retinal-0% retinol; Fex-340/Fex-380 = 6.95 corresponds to 0% retinal-100% retinol. Initial levels of lipofuscin precursors were similar in the dark-adapted cells (5 mM glucose, ■; 0 glucose,◆). 30 min after bleaching, in 5 mM glucose, there was quantitative conversion of all-trans retinal to all-trans retinol and virtually no increase in the level of lipofuscin precursors (□); in 0 glucose, there was accumulation of all-trans retinal and a substantial increase in the level of lipofuscin precursors (◇). Experiments at 37 °C.
Figure 5.
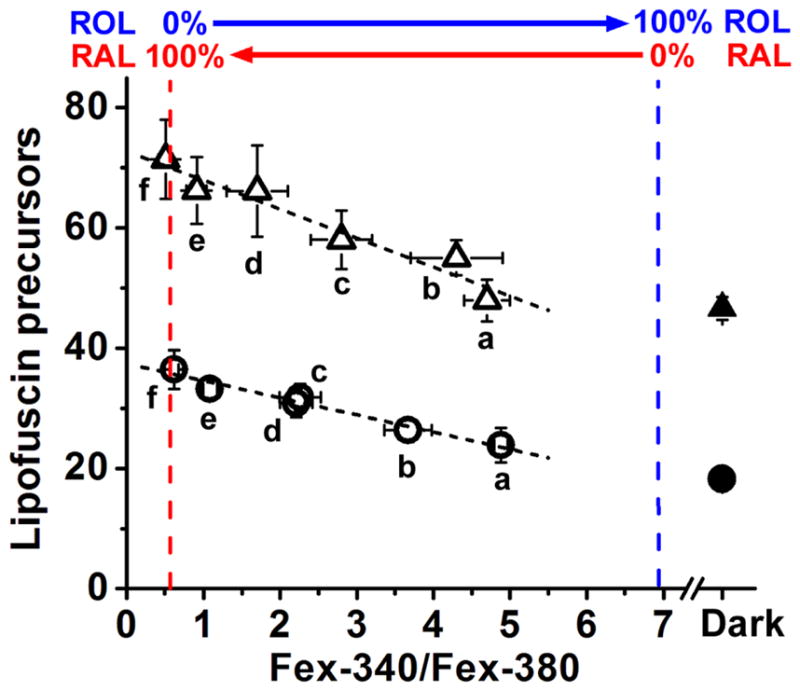
Increased levels of all-trans retinal after bleaching result in increased formation of lipofuscin precursors in rod photoreceptors isolated from Sv/129 (○, ●; n = 72 cells total) and Abca4−/− (△, ▲; n = 50 cells total) mice. Lipofuscin precursor fluorescence was measured in isolated dark-adapted rods (●, ▲); subsequently, the cells were bleached with long-wavelength (>530 nm) light for 1 min and rod outer segment fluorescence signals from all-trans retinol, all-trans retinal and lipofuscin precursors measured at 30 min after bleaching (○, △). Retinol and retinal fluorescence were obtained by exciting with 340 and 380 nm light and collecting emission >420 nm. Lipofuscin precursor fluorescence was obtained with 490 nm excitation and collecting emission >515 nm. All-trans retinal accumulation was measured as the Fex-340/Fex-380 ratio. The value Fex-340/Fex-380 = 0.55 corresponds to 100% retinal-0% retinol; Fex-340/Fex-380 = 6.95 corresponds to 0% retinal-100% retinol. Different levels of all-trans retinal were brought about by using conditions that suppress the supply of NADPH to the rod outer segment. Conditions were: a, 5 mM glucose; b, 0.5 mM glutamine; c, 50 μM glucose; d, 50 μM glutamine; e, no substrate; f, broken-off rod outer segments. Numbers of cells were: for wild type; a, n = 9; b, n = 7; c, n = 17; d, n = 16; e, n =12; f, n =11; for Abca4−/−; a, n = 9 cells; b, n = 8; c, n = 8; d, n = 9; e, n =9; f, n =7. Experiments at 37 °C. Error bars denote standard errors. The dashed lines through the data points represent linear regression lines.
4. DISCUSSION
We have used fluorescence imaging of single photoreceptor cells to measure the levels of all-trans retinol, all-trans retinal, and lipofuscin precursors in real time in a living cell and at physiological concentrations. Such single cell fluorescence measurements have important advantages as well as limitations. One important advantage is that of measuring the all-trans retinal that has been released from photoactivated rhodopsin, while biochemical measurements measure the total all-trans retinal, including the one that is still covalently bound in the form of the photointermediates metarhodopsin II and III, or as a Schiff base with phosphatidylethanolamine. One important limitation of fluorescence measurements is that because the measuring light bleaches the photopigment, meaningful measurements can be carried out only following a full bleach. Another important limitation is that the fluorescence signal does not by itself uniquely identify the fluorophores. This particular limitation has been addressed in previous experiments that included measurements of emission spectra, which support the assignment of the UV-excited fluorescence signals to all-trans retinol and retinal (Chen et al., 2012) and the 490 nm-excited signal to retinal-derived lipofuscin precursors (Boyer et al., 2012). The fluorescence signals excited by 340 and 380 nm, Fex-340 and Fex-380, as well as their intensity ratio, have been used before to partially separate the signals from all-trans retinal and all-trans retinol and estimate the conversion of the former to the latter (Adler et al., 2014; Chen et al., 2012). Using this assay, experiments with Rdh8−/− and Rdh12−/− mice showed that lack of the Rdh8 or Rdh12 dehydrogenase enzymes suppresses the conversion of all-trans retinal to all-trans retinol in the compartment that lacks a dehydrogenase (Chen et al., 2012). In metabolically intact rods and in the absence of metabolic limitations, the signal due to all-trans retinol dominates the fluorescence measured with 340 and 360 nm excitation and therefore can be measured reliably. Moreover, because all of all-trans retinol in the rod outer segment is virtually free (Wu et al., 2006) the pool giving rise to the fluorescence signal is the same with the one measured biochemically. Comparison of fluorescence and biochemical measurements of all-trans retinol shows that they are in good agreement ((Blakeley et al., 2011; Chen et al., 2012), see also (Adler et al., 2014)). However, due to the reasons given above, such comparison between fluorescence and biochemical measurements is not possible for all-trans retinal. In addition, and in contrast to all-trans retinol, there is no fluorescence signal that is dominated by all-trans retinal, which necessitates calculating its signal through a subtraction procedure (Eq. 6a). This introduces a level of uncertainty in the fluorescence measurement of all-trans retinal. In contrast to all-trans retinol and all-trans retinal, the chemical identity of the lipofuscin precursor fluorophores is unclear. They are likely to be bis-retinoids, but all-trans retinal Schiff bases may contribute to the fluorescence signal as well. Direct comparisons with biochemical experiments are lacking, with an important difference being that precursors form rapidly in cells, while bis-retinoids form very slowly in vitro.
All-trans and 11-cis retinal have both been shown to generate lipofuscin precursor fluorophores and bis-retinoids when added to rod outer segments and outer segment membranes (Ben-Shabat et al., 2002; Boyer et al., 2012; Quazi and Molday, 2014). In rod outer segments in situ, lipofuscin precursors could be generated from the all-trans retinal released by photoactivated rhodopsin following light absorption, or from the 11-cis retinal provided by the RPE for the regeneration of opsin to rhodopsin. Abca4 has been shown to facilitate the translocation of both all-trans and 11-cis retinal, in the form of their Schiff bases with phosphatidylethanolamine, N-retinylidene-phosphatidylethanolamine (NRPE), from the lumen to the cytosolic side of disk membranes. On the cytosolic side of disk membranes, both all-trans and 11-cis retinal can be cleared by Rdh8, the rod outer segment retinol dedydrogenase enzyme. Although Rdh8 is specific for the all-trans isomer of retinal, phosphatidylethanolamine catalyzes the isomerization of 11-cis retinal to all-trans, converting 11-cis retinal into a substrate for Rdh8 as well (Quazi and Molday, 2014). On this basis, Abca4 has been proposed to allow for the faster clearance of any excess all-trans or 11-cis retinal. This mechanism can readily explain the higher levels of lipofuscin precursors present in the outer segments of rod photoreceptors from Abca4−/− mice, and the large accumulation of lipofuscin and bis-retinoids in the RPE of Abca4−/− mice and of Stargardt patients with defects in ABCA4.
This same mechanism would also predict that, following light exposure, higher levels of all-trans retinal would accumulate in Abca4−/− rod outer segments compared to wild type. This prediction is not borne out by the experiments shown in Fig. 3, which find no difference in the all-trans retinal levels between wild type and Abca4−/− rods. Fig. 3 does show somewhat lower levels of all-trans retinol in Abca4−/− rods at later times after bleaching. These results are in good agreement with our previous biochemical and fluorescence measurements of all-trans retinol formation kinetics in the retinas of Abca4−/− and wild type mice (Blakeley et al., 2011). They are however, only in partial agreement with the original report that, following light exposure, there is an elevation of all-trans retinal and a decline in all-trans retinol in Abca4−/− retinas compared to wild type (Weng et al., 1999). A factor complicating a direct comparison between the different studies is that in the original report the mice were anesthetized. A possible explanation that could reconcile the present experiments with those in the original report is that the elevated all-trans retinal levels found in the Abca4−/− originate solely from NRPE, that is, from its Schiff base with phosphatidylethanolamine. This would also explain the reduction in the all-trans retinol levels observed in both studies. Following the proposal in the initial study (Weng et al., 1999), this explanation posits that some of the all-trans retinal released by photoactivated rhodopsin forms a relatively stable NRPE in the disk lumen. This NRPE is not in rapid equilibrium with free all-trans retinal, it is trapped in the disk lumen, and can initiate bis-retinoid formation. In wild type rods, Abca4 translocates NRPE from the lumen to the cytosolic side of the disk, where the NRPE is hydrolyzed and all-trans retinal is reduced by Rdh8 – this process eliminates the trapped NRPE and limits bis-retinoid formation. In the absence of Abca4, NRPE is trapped in the disk lumen, giving rise to higher levels of total extracted all-trans retinal. Lack of Abca4 does not affect all-trans retinol formation at early times, but makes some difference at later times (Fig. 3), as some of the released all-trans retinal that has not been reduced ends up trapped as NRPE in the lumen. Lack of Abca4 also does not affect the levels of free all-trans retinal, which is at equilibrium with all-trans retinol but not with NRPE. The portion of all-trans retinal handled by Abca4 is that which is trapped as NRPE in the lumen of the disk. Judging from the reduction in all-trans retinol levels in Abca4−/− rods at later times (Fig. 3), this portion appears to be no more than 20–30%, in agreement with previous estimates (Lamb and Pugh, 2004). Thus, any accumulation of all-trans NRPE in Abca4−/− rods, although it can lead to excess formation of bis-retinoids, is not associated with accumulation of free all-trans retinal. However, such an excess formation of bis-retinoids in metabolically intact Abca4−/− rods was not detected in the experiments shown in Fig. 5, as the levels of lipofuscin precursor fluorophores following bleaching in 5 mM glucose are not significantly increased compared to those in the dark-adapted state (see also (Boyer et al., 2012)). A possible explanation is that this increase in lipofuscin precursor levels is below the resolution of the measurements.
The same argument, applied in the case of 11-cis retinal, can also account for the higher levels of lipofuscin fluorescence and bis-retinoids found in dark-reared Abca4−/− mice compared to wild type. Although 11-cis retinal is a poor substrate for Rdh8, rod outer segment lipids catalyze its isomerization to all-trans, allowing its reduction (Quazi and Molday, 2014). However, any 11-cis retinal that flows into the rod outer segment and does not end up binding to opsin, may be trapped in the disk lumen as a Schiff base with phosphatidylethanolamine and initiate bis-retinoid formation. Abca4 will translocate the 11-cis retinal Schiff base from the lumen to the cytosolic side of the disk, facilitating the eventual removal of 11-cis retinal (Quazi and Molday, 2014). Lack of Abca4 will result in the trapping of the 11-cis retinal Schiff base, diminished clearance of 11-cis retinal, and higher accumulation of lipofuscin compared to wild type.
It has previously been shown that in metabolically intact rod photoreceptors from wild type mice, and in the presence of adequate metabolic substrate, the all-trans retinal released from photoactivated rhodopsin is quantitatively converted to all-trans retinol (Chen and Koutalos, 2010; Chen et al., 2012), and there is no significant increase in lipofuscin precursor levels (Boyer et al., 2012). On the other hand, in broken-off rod outer segments, which have no access to NADPH and cannot reduce all-trans retinal (Chen et al., 2012), there is a significant increase in lipofuscin precursor levels following light exposure (Boyer et al., 2012). The experiments shown in Fig. 5 extend these results to intermediate levels of all-trans retinal accumulation. This was achieved by supplying the cells with low metabolic substrate concentrations and in this way suppressing the generation of NADPH to different extents. For both wild type and Abca4−/− rods, higher levels of all-trans retinal are associated with higher formation of lipofuscin precursors. For rod photoreceptors in situ, this would suggest that perturbations in metabolic activity during light exposure would result in increased formation of lipofuscin. The results cannot exclude the possibility that some lipofuscin precursors form from all-trans retinal even in the presence of adequate metabolic substrate, at levels that are not resolved by the present measurements. Indeed, and as discussed above, in intact Abca4−/− rods in the presence of 5 mM glucose, the results are consistent with some increase in the levels of lipofuscin precursors after bleaching originating from all-trans retinal trapped in the form of NRPE; but this increase is not detected in the experiments shown in Fig. 5. It is important to emphasize that the increased formation of lipofuscin precursors shown in Fig. 5 is due to the inadequate supply of NADPH brought about by the metabolic perturbations, which result in the accumulation of all-trans retinal. The higher levels of lipofuscin precursors found in Abca4−/− rods compared to wild type (and seen clearly in Fig. 5) do not originate from lack of NADPH and are not associated with any accumulation of all-trans retinal.
CONCLUSIONS
The present work shows that lack of Abca4 does not affect the formation of all-trans retinol at early times after light exposure; at later times however, all-trans retinol levels are slightly lower in Abca4−/− rods, consistent with some of the all-trans retinal being unavailable for reduction. Following light exposure, there is no accumulation of all-trans retinal in Abca4−/− rods compared to wild type, likely because all-trans retinal is trapped as a Schiff base or forms bis-retinoids. In both Abca4−/− and wild type rods, exposure to low metabolic substrate concentrations results in accumulation of all-trans retinal, which is associated with formation of higher levels of lipofuscin precursors.
Highlights.
Fluorescence imaging of retinal, retinol, and lipofuscin precursors in single rods
Accumulation of light-generated free all-trans retinal is unaffected by lack of Abca4
Metabolic limitations result in all-trans retinal accumulation
Metabolic limitations lead to increased formation of lipofuscin precursors
Acknowledgments
Supported by NEI grant EY014850 (Y.K.) and by an unrestricted award to the Department of Ophthalmology at Medical University of South Carolina from Research to Prevent Blindness, Inc. This work was conducted in a facility constructed with support from National Institutes of Health Grant C06 RR015455 from the Extramural Research Facilities Program of NCRR. The funding sources had no role: in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Author contributions: LA and CC carried out the experiments; LA, CC, and YK analyzed the results; LA and YK designed the experiments and wrote the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler L, 4th, Chen C, Koutalos Y. Mitochondria contribute to NADPH generation in mouse rod photoreceptors. J Biol Chem. 2014;289:1519–28. doi: 10.1074/jbc.M113.511295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat S, Parish CA, Vollmer HR, Itagaki Y, Fishkin N, Nakanishi K, Sparrow JR. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J Biol Chem. 2002;277:7183–90. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- Blakeley LR, Chen C, Chen CK, Chen J, Crouch RK, Travis GH, Koutalos Y. Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest Ophthalmol Vis Sci. 2011;52:3483–91. doi: 10.1167/iovs.10-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal. J Biol Chem. 2012;287:22276–86. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Blakeley LR, Koutalos Y. Formation of all-trans retinol after visual pigment bleaching in mouse photoreceptors. Invest Ophthalmol Vis Sci. 2009;50:3589–95. doi: 10.1167/iovs.08-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Koutalos Y. Rapid formation of all-trans retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem Photobiol Sci. 2010;9:1475–9. doi: 10.1039/c0pp00124d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Thompson DA, Koutalos Y. Reduction of all-trans-retinal in vertebrate rod photoreceptors requires the combined action of RDH8 and RDH12. J Biol Chem. 2012;287:24662–70. doi: 10.1074/jbc.M112.354514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995a;36:718–29. [PubMed] [Google Scholar]
- Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–66. [PubMed] [Google Scholar]
- Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt’s disease--Fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995b;36:2327–31. [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–6. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Futterman S, Hendrickson A, Bishop PE, Rollins MH, Vacano E. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J Neurochem. 1970;17:149–56. doi: 10.1111/j.1471-4159.1970.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Katz ML, Drea CM, Eldred GE, Hess HH, Robison WG., Jr Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res. 1986;43:561–73. doi: 10.1016/s0014-4835(86)80023-9. [DOI] [PubMed] [Google Scholar]
- Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3023–30. [PubMed] [Google Scholar]
- Katz ML, Robison WG., Jr What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr. 2002;34:169–84. doi: 10.1016/s0167-4943(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–31. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–80. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lenis TL, Jiang Z, Sarfare S, Le A, Eddington S, Bok D, Molday RS, Nusinowitz S, Redmond M, Travis GH, Radu RA. Elucidating the role of Retinal Pigment Epithelium (RPE)-specific ABCA4 in Stargardt disease. ARVO Meeting Abstracts. 2016;57:3177. [Google Scholar]
- Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275:29354–60. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–32. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–9. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci U S A. 1998;95:14609–13. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quazi F, Lenevich S, Molday RS. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat Commun. 2012;3:925. doi: 10.1038/ncomms1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quazi F, Molday RS. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc Natl Acad Sci U S A. 2014;111:5024–9. doi: 10.1073/pnas.1400780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24:1107–12. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–48. [PubMed] [Google Scholar]
- Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–35. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–9. [PubMed] [Google Scholar]
- Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res. 2013;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Zhao J, Kim HJ, Sparrow JR. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc Natl Acad Sci U S A. 2016;113:6904–9. doi: 10.1073/pnas.1524774113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Anand M, Shirazi AK, Magrane J, Gao J, Vollmer-Snarr HR, Manfredi G, Finnemann SC. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–80. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–7. [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Chen C, Koutalos Y. All-trans retinol in rod photoreceptor outer segments moves unrestrictedly by passive diffusion. Biophys J. 2006;91:4678–89. doi: 10.1529/biophysj.106.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem. 2009;284:20155–66. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]