Abstract
Background
People with heart failure and preserved ejection fraction (HFpEF) develop increases in left ventricular (LV) end-diastolic pressures (LVEDP) during exercise that contribute to dyspnea. In normal open-chest animal preparations, the pericardium restrains LV filling when central blood volume increases. We hypothesized that resection of the pericardium using a minimally-invasive epicardial approach would mitigate the increase in LVEDP that develops during volume loading in normal and diseased hearts with the chest intact.
Methods and Results
Invasive hemodynamic assessment was performed at baseline and following saline load before and after pericardial resection in normal canines with open (n=3) and closed chest (n=5) and in a pig model with features of human HFpEF with sternum intact (n=4). In closed-chest animals, pericardiotomy was performed using a novel subxiphoid procedure. In both experimental preparations of normal dogs, pericardiotomy blunted the increase in LVEDP with saline infusion, while enhancing the saline-mediated increase in LV end-diastolic volume (LVEDV). With chest intact in the pig model, percutaneous pericardial resection again blunted the increase in LVEDP secondary to volume expansion (+4±3 vs +13±5 mmHg, p=0.014) while enhancing the saline-mediated increase in LVEDV (+17±1 vs +10±2 ml, p=0.016).
Conclusions
This proof of concept study demonstrates that pericardial resection through a minimally-invasive percutaneous approach mitigates the elevation in LV filling pressures with volume loading in both normal animals and a pig model with diastolic dysfunction. Further study is warranted to determine whether this method is safe and produces similar acute and chronic hemodynamic benefits in people with HFpEF.
Subject Terms: Heart Failure, Hemodynamics, Pathophysiology
Keywords: Heart Failure, Hemodynamics, Diastolic Heart Failure, Pericardium
Half of patients with heart failure (HF) have preserved ejection fraction (HFpEF), and few effective treatments are available.1–3 HFpEF is mechanistically characterized by elevation in left ventricular (LV) filling pressures that contribute to symptoms of dyspnea and exercise intolerance.4–7 Filling pressures are elevated at rest in patients with more advanced HFpEF, and they become elevated when venous return to the heart increases during exercise in patients with earlier stage HFpEF.4, 8 Acute removal of the pericardium in animal models improves effective LV diastolic compliance even as myocardial properties remain unchanged, because the external constraining effect of the pericardium is eliminated.9–15 Indeed, studies in normal animals have demonstrated improvements in filling pressures, cardiac output reserve, and exercise capacity after pericardiectomy.16, 17
Based upon these observations, we reasoned that pericardial resection might constitute a novel treatment for people with HFpEF. However, typical patients with HFpEF are elderly and frail, making them poor candidates for conventional surgical pericardiectomy. Accordingly, we sought to develop and test a novel, minimally-invasive pericardial resection procedure that could be performed in the catheterization lab to remove the restraining effects of the pericardium without the need for open sternotomy. We hypothesized that removing this restraining effect of the pericardium using minimally-invasive methods would attenuate the increase in LV filling pressures accompanying states of increased venous return, while leaving muscle properties intact. To test this hypothesis, we assessed the hemodynamic effects of pericardial resection using our newly developed subxiphoid technique in normal dogs, and then in a hypertensive pig model with features of human HFpEF.
Methods
The goal of this study was to develop and test the hemodynamic effects of a new prototype device and technique designed to enable percutaneous resection of the anterior parietal pericardium. To test this new device and procedure, we evaluated the hemodynamic effects at baseline and with acute saline loading in (1) open chest normal dogs, and then in (2) a second group of closed chest normal dogs and a (3) a closed chest pig model with features of HFpEF. Experiments for (1) were performed with chest open while (2) and (3) were performed using a novel minimally-invasive, subxiphoid approach that we have developed.
Five bench tests were conducted in ex vivo porcine hearts using preliminary prototypes, which were further refined and re-prototyped for subsequent surgeries around the beating heart. These devices were designed to be deployed into a subxiphoid sheath enabling access to epicardial space for in vivo testing in large animals (Figure 1). Following prototype development, mongrel dogs weighing 30–40 kilograms underwent open chest (n=3) experiments. Following these experiments, another 5 normal dogs then underwent minimally invasive, percutaneous closed-chest procedures using the prototyped tools and technique.
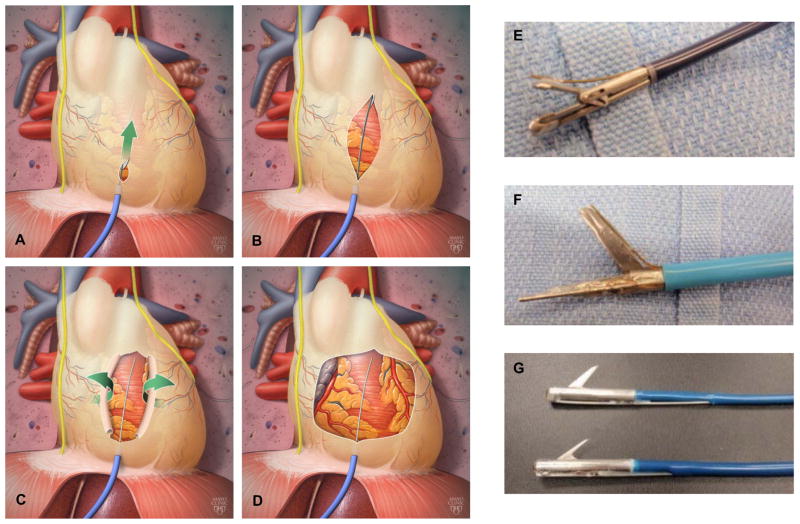
Figure 1.
[A–D] Illustration depicting release of pericardial restraint using the percutaneous sub-xiphoid approach. Prototypes built to date that can track over a guidewire and grasp the pericardium [E], deliver electrical energy to stimulate the phrenic nerve and cut the pericardium in a forward scissors-like motion [F], and cut the pericardium with an actuating reverse-cutting blade [G].
To test the effects of pericardial resection in diseased hearts, we employed a recently developed hypertensive, hypercholesterolemic pig model displaying typical features of HFpEF.18–20 In this model, domestic pigs are fed a high-fat diet (2% cholesterol and 15% lard by weight) for 6 weeks followed by surgical induction of renovascular hypertension by unilateral renal artery coiling. Animals then continue with high fat diet for an additional 10 weeks. This model has been shown to display increased LV mass, diastolic dysfunction, impaired coronary microvascular function, a pro-inflammatory milieu, and increased myocardial oxidative stress despite preserved EF,18–20 mirroring changes seen in human HFpEF.1–3
For all experiments, an intramuscular sedative was administered in fasted animals prior to obtaining vascular access. Animals were instrumented under general anesthesia using 1–3% isoflurane after appropriate induction with 10 mg/kg ketamine and 0.5 mg/kg diazepam. Body temperature was monitored and maintained with a dorsal water flow heating pad and solutions warmed to 41°. These studies were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Open chest surgical experiments
In the first stage of experiments, sternotomy was conducted, after which a puncture was made into the pericardium in order to gain epicardial access. The prototype cutting device was deployed in the epicardial space to allow slitting of the pericardium, taking care to avoid the phrenic nerves. Once slit, control of the slit margins was obtained at two sites, and the anterior pericardium was reflected away from the initial slit and then resected and removed.
Closed chest percutaneous surgery
Minimally invasive pericardial resection was then performed with chest intact in normal dogs, and subsequently in model pigs (Figure 1). Percutaneous epicardial access was obtained using a method similar to that described by Sosa et al.21–23 The devices were deployed in the epicardial space under fluoroscopic guidance and used to slit the pericardium in a scissor-like fashion, similar to the procedure performed in the open experiments. Damage to the phrenic nerve is avoided by use of a pacing electrode that stimulates diaphragmatic contraction when the cutting tool approaches the phrenic nerve. Following pericardial resection and after completion of all hemodynamic assessments, the sternum was opened to allow direct visualization of the heart, lungs, and resected pericardium, followed by animal sacrifice.
Hemodynamic Assessment
Intracardiac pressures were measured using fluid-filled catheters placed under fluoroscopic guidance in the LV, right atrium (RA), and pulmonary artery (PA). Left ventricular end diastolic pressure (LVEDP) was measured by visual inspection just before the rapid upstroke of LV pressure. Left ventriculography was performed in the right anterior oblique position to measure LV end diastolic volume (LVEDV), end systolic volume, and LVEF using a 6 Fr pigtail catheter. Angiographic data was recorded in digital format at 30 frames per second. LV volume was measured using the area-length method, where calculated volume (Vcalc) is determined from the silhouette area (A) and long axis length (L) by the following equation: Vcalc=8A2/3πL. Magnification correction was determined based upon a distance of 18 cm from table to LV cavity. Actual LV volume was then calculated using a regression formula (V = 0.81Vcalc + 1.9 ml) as validated by Kennedy et al.24 LV stroke volume (SV) was calculated as the difference between LVEDV and LV end systolic volume. Cardiac output was determined by the product of SV and heart rate (HR).
To examine diastolic LV reserve following pericardial modification, hemodynamics were then re-assessed immediately following rapid infusion of pre-warmed normal saline (500 ml over 3 minutes) to simulate the increase in central blood volume that accompanies physiologic stressors such as exercise. Saline infusion was performed before and after pericardial modification in all experiments. Because fluids redistribute following saline load, the same timing and order of assessment was used following bolus completion at both time points. Specifically, intracardiac and pulmonary pressures were assessed first, immediately after completion of the saline bolus, followed immediately by left ventriculography within 10–20 seconds of pressure records.
Statistical analysis
Data are reported as mean ± standard deviation when providing a description of the sample data. Hemodynamics for the preliminary open-chest experiment were compared using paired t-tests given their limited sample size (n=3). Hemodynamics for the closed chest experiments (dogs and HFpEF model pigs) were compared between states (baseline and saline) and before and after pericardial modification using a linear mixed model. A separate model was fit for each outcome, stratified by animal type. The fitted regression model consisted of a random intercept (blocking factor for each animal) and a fixed effect term for the experimental period. Further evaluation of the appropriateness of the model was conducted by visual inspection of the distribution of values by experimental condition. To test planned hypotheses, statistical contrasts of estimated means were used. Reported p-values are two-sided and have not been adjusted for multiple comparisons. P-values <0.05 were considered statistically significant. Analyses were performed using JMP 10.0.0 and SAS 9.4 (SAS Institute, Cary, NC).
Results
Effects of Complete Pericardiectomy with Open Chest
With the chest closed and pericardium intact in the first 3 animals, saline infusion increased LVEDP from 15±2 to 32±5 mmHg (p=0.026). Similar changes were observed with saline infusion with the chest open and pericardium intact, with LVEDP increasing from 15±2 to 33±4 mmHg (p=0.007). Open pericardiectomy had no effect on baseline LVEDP. However, the saline-mediated increase in LVEDP following full pericardiectomy with open chest was more than 50% lower as compared to pericardium intact (+7±6 vs +18±3 mmHg, p=0.07).
Effects of Anterior Pericardial Resection with Chest Intact in Normal Dogs
Given the salutary benefits of full pericardiectomy noted in preliminary open chest experiments, the effects of anterior pericardial resection via minimally invasive, subxiphoid pericardiotomy were then assessed with the chest intact in a different set of 5 acute canine studies using the prototyped tools developed in the bench and preliminary open chest experiments. Prior to pericardial modification with chest intact, rapid saline infusion increased biventricular filling pressures, pulmonary artery pressure, cardiac output, stroke volume and LVEDV dramatically, with an increase in LVEDP from 11 to 30 mmHg (p<0.001, Table 1). Following volume expansion, LVEDP returned to baseline values (p=0.92).
Table 1.
Effects of Pericardial Resection in the Dog.
| Pericardium Intact | Pericardium Incised | Incised vs Intact | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Saline | Delta 1* | Baseline | Saline | Delta 2* | Delta 3† | |||||||||||
| Mean | SD | Mean | SD | Est | 95% CI | p-value | Mean | SD | Mean | SD | Est | 95% CI | p-value | Est | 95% CI | p-value | |
| Primary Outcome Measure | |||||||||||||||||
| LVEDP (mmHg) | 11 | 2 | 30 | 6 | 19 | (14, 23) | <0.001 | 11 | 4 | 18 ** | 5 | 7 | (3, 12) | 0.002 | −11 | (−17, 5) | 0.001 |
| Secondary Outcome Measures | |||||||||||||||||
| LVEDV (ml) | 43 | 8 | 56 | 13 | 13 | (5, 20) | 0.005 | 44 | 5 | 66 # | 9 | 22 | (14, 30) | <0.001 | 10 | (−1, 20) | 0.077 |
| RA pressure (mmHg) | 11 | 3 | 16 | 3 | 6 | (3, 8) | <0.001 | 10 | 3 | 12 # | 3 | 2 | (−0, 5) | 0.062 | −3 | (−7, 0) | 0.062 |
| PA systolic pressure (mmHg) | 25 | 5 | 36 | 4 | 10 | (7, 14) | <0.001 | 27 | 6 | 32□ | 8 | 5 | (2, 9) | 0.003 | −5 | (−9, −1) | 0.029 |
| PA diastolic pressure (mmHg) | 11 | 1 | 20 | 6 | 9 | (4, 14) | 0.001 | 12 | 3 | 17 | 4 | 5 | (−0, 9) | 0.055 | −4 | (−11, 2) | 0.18 |
| Cardiac output (l/min) | 2.0 | 0.5 | 2.9 | 0.7 | 0.9 | (0.2, 1.8) | 0.047 | 2.5 | 0.5 | 3.2 | 0.8 | 0.6 | (−0.3, 1.5) | 0.145 | −0.3 | (−1.6, 1.0) | 0.63 |
| Stroke volume (ml) | 19 | 4 | 28 | 8 | 9 | (3, 14) | 0.008 | 24 | 6 | 34□ | 9 | 10 | (4, 15) | 0.004 | 1 | (−7, 9) | 0.77 |
| LVEF (%) | 51 | 15 | 57 | 17 | 6 | (−2, 14) | 0.127 | 59‡ | 12 | 55 | 11 | −4 | (−12, 4) | 0.30 | −10 | (−21, 1) | 0.077 |
| Heart Rate (bpm) | 105 | 8 | 107 | 10 | 3 | (−12, 17) | 0.70 | 106 | 19 | 96 | 12 | −10 | (−25, 5) | 0.158 | −13 | (−33, 8) | 0.20 |
Delta 1 and 2 are the model-based estimates (Est) of the change observed with the introduction of saline in the intact and incised pericardium conditions, respectively.
Delta 3 is the difference in deltas between pericardium intact and pericardium incised.
represents p-values in the range of [0.01, 0.05) for comparison of the two baseline conditions.
represent p-values of [0.01, 0.05), [0.001, 0.01), and <0.001, respectively, for comparison of the two saline conditions.
Pericardial resection was then performed via percutaneous subxiphoid approach (Figure 1). As in the open chest experiments, hemodynamics were not statistically different following pericardial resection at baseline in these closed-chest experiments (Table 1). However, the increase in LV filling pressures in response to saline infusion with the chest closed was significantly attenuated by minimally-invasive pericardial modification, while the increase in LVEDV was enhanced (Table 1, Figures 2 and 3).
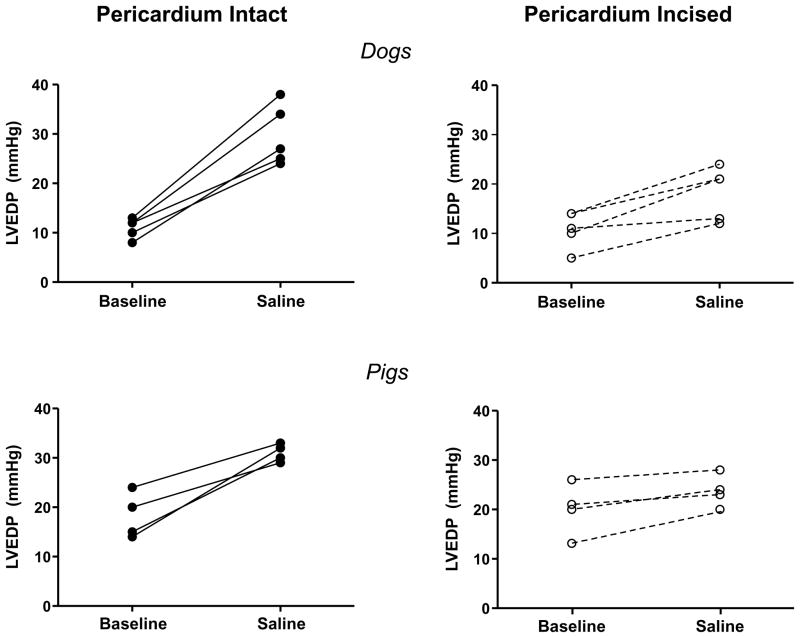
Figure 2.
Left ventricular end diastolic pressures (LVEDP) at rest and following saline load for all individual animals in chest intact experiments before (left panels, solid lines) and after pericardial resection (right panels, dotted lines).
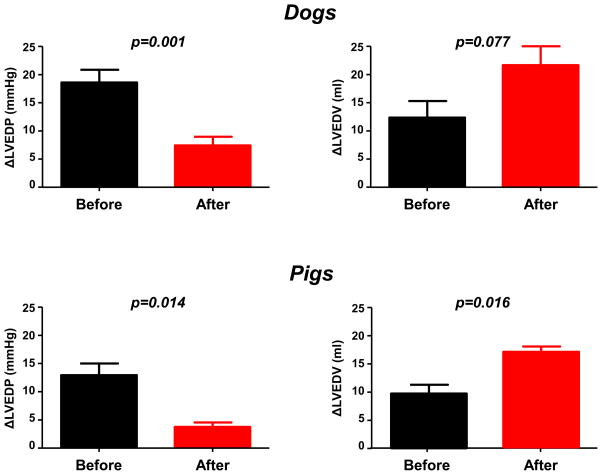
Figure 3.
Hemodynamic effects of volume loading before and after pericardial resection. With chest intact, minimally-invasive percutaneous pericardial resection significantly attenuated the increase in left ventricular end diastolic pressure (LVEDP) during rapid saline loading and enhanced the increase in left ventricular end diastolic volume (LVEDV), both in normal dogs (top panels) and in a pig model with features of human HFpEF (bottom panels). Error bars reflect SEM.
Effects of Anterior Pericardial Resection with Chest Intact in Pig Model
Next the effects of pericardial resection were tested in the swine model with features of HFpEF (n=4). These animals displayed high LVEDP (18±5 mmHg) and normal EF at baseline, consistent with human HFpEF (Table 2). Like the normal dogs, saline infusion led to marked increases in filling pressures, with increases in cardiac output and stroke volume in pigs according to the Frank-Starling mechanism. Following saline infusion with pericardium intact, hemodynamics returned to baseline values.
Table 2.
Effects of Pericardial Resection in the Pig
| Pericardium Intact | Pericardial Incised | Incised vs Intact | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Saline | Delta 1* | Baseline | Saline | Delta 2* | Delta 3† | |||||||||||
| Mean | SD | Mean | SD | Est | 95% CI | p-value | Mean | SD | Mean | SD | Est | 95% CI | p-value | Est | 95% CI | p-value | |
| Primary Outcome Measure | |||||||||||||||||
| LVEDP (mmHg) | 18 | 5 | 31 | 2 | 13 | (8, 17) | <0.001 | 20 | 6 | 24 # | 3 | 4 | (−0, 9) | 0.058 | −9 | (−15, −2) | 0.014 |
| Secondary Outcome Measures | |||||||||||||||||
| LVEDV (ml) | 35 | 8 | 46 | 5 | 10 | (7, 14) | <0.001 | 41‡ | 9 | 57 ** | 8 | 17 | (13, 20) | <0.001 | 6 | (2, 11) | 0.016 |
| RA pressure (mmHg) | 12 | 3 | 17 | 2 | 5 | (3, 6) | <0.001 | 12 | 3 | 14 # | 1 | 2 | (0, 3) | 0.032 | −3 | (−5, −1) | 0.020 |
| PA systolic pressure (mmHg) | 31 | 5 | 38 | 4 | 8 | (3, 12) | 0.006 | 30 | 7 | 34 | 5 | 5 | (−0, 9) | 0.062 | −3 | (−10, 4) | 0.34 |
| PA diastolic pressure (mmHg) | 18 | 4 | 26 | 3 | 8 | (4, 12) | 0.002 | 18 | 4 | 20□ | 1 | 2 | (−2, 6) | 0.29 | −6 | (−11, 0) | 0.056 |
| Cardiac output (l/min) | 1.9 | 0.5 | 2.7 | 0.5 | 0.9 | (0.5, 1.2) | <0.001 | 2.3‡ | 0.7 | 3.3 # | 0.4 | 1.0 | (0.6, 1.4) | <0.001 | 0.1 | (−0.4, 0.6) | 0.66 |
| Stroke volume (ml) | 21 | 5 | 31 | 4 | 9 | (3, 16) | 0.011 | 27 | 8 | 39□ | 6 | 12 | (6, 19) | 0.002 | 3 | (−6, 12) | 0.48 |
| LVEF (%) | 61 | 9 | 67 | 2 | 7 | (−8, 21) | 0.34 | 66 | 16 | 68 | 8 | 3 | (−12, 17) | 0.71 | −4 | (−25, 17) | 0.67 |
| Heart Rate (bpm) | 87 | 7 | 89 | 5 | 2 | (−9, 13) | 0.68 | 89 | 8 | 86 | 9 | −3 | (−13, 8) | 0.61 | −5 | (−20, 11) | 0.52 |
Delta 1 and 2 are the model-based estimates (Est) of the change observed with the introduction of saline in the intact and incised pericardium conditions, respectively.
Delta 3 is the difference in deltas between pericardium intact and pericardium incised.
represents p-values in the range of [0.01, 0.05) for comparison of the two baseline conditions.
represent p-values of [0.01, 0.05), [0.001, 0.01), and <0.001, respectively, for comparison of the two saline conditions.
Percutaneous anterior pericardial resection did not alter biventricular filling pressures at baseline in the pig model, but it did increase LVEDV and SV (Table 2). Similar to the normal dogs, the saline-mediated increase in LVEDP was markedly attenuated following anterior pericardial resection, with greater increase in LVEDV from volume loading with chest intact (Table 2, Figures 2 and 3).
Gross Pathology and Safety
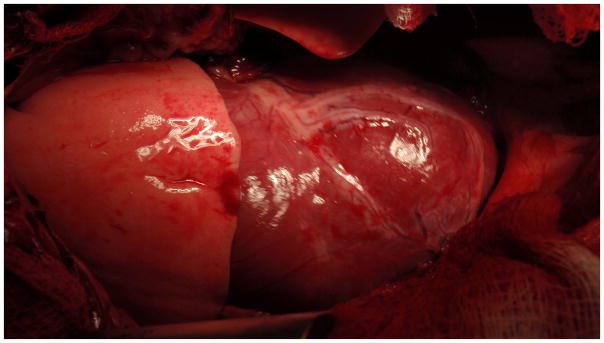
There were no acute cardiac complications from the open or minimally-invasive pericardial resections, with no tamponade developing and no damage to the phrenic nerves. One animal developed a laceration of the left lung caused by the pericardial cutting tool noted at the time of sacrifice. The hemodynamic improvements following subxiphoid pericardial modification were coupled with wide resection of the pericardium noted on gross pathology, with little to no residual coverage of the anterior surface of the heart observed in all animals. Figure 4 displays typical gross results following conclusion of the experiments and opening of the chest.
Figure 4.
Photograph from a representative animal taken after closed-chest experiments were been completed and the sternum was opened prior to sacrifice, showing that the epicardial surface of the heart is unrestrained and lack of pericardial coverage following minimally-invasive percutaneous pericardial resection in a normal canine.
Discussion
The normal pericardium restrains ventricular filling, contributing to the elevation in intracardiac pressures that develop during conditions of increased venous return such as exercise. Patients with HFpEF characteristically develop marked increases in filling pressures with exercise or volume loading owing to diastolic dysfunction.4–7 We show here for the first time in normal canines and pigs with hemodynamic features of HFpEF that pericardial resection using a novel, minimally-invasive approach substantially mitigates the increase in filling pressures associated with volume loading. Importantly, the procedure and its hemodynamic benefits are seen with the chest intact and with limited anterior pericardial resection, suggesting that full pericardiectomy is not required to observe salutary effects on filling pressures. While saline infusion does not fully recapitulate the hemodynamic loading seen with exercise,7 the current results suggest that anterior pericardial resection, which can be performed using this new procedure without the need for open sternotomy in the catheterization laboratory, might be an effective treatment for human HFpEF.
Trials in patients with HFpEF have failed to identify an effective medical treatment, possibly due to heterogeneity in the underlying causes.1–3 Despite this heterogeneity, pathologic elevation in cardiac filling pressures during exercise is common to all patients4–7 and represents a viable therapeutic target that may improve clinical status, regardless of the underlying etiology.
Traditional approaches to reduce filling pressures have relied upon drugs.1–3 However, a substantial proportion of the pressure within the LV during diastole is attributable to external restraining effects exerted by the pericardium and right heart.9, 25 Acute removal of the pericardium in open-chest, normal animal preparations results in a downward/rightward shift in the diastolic pressure-volume relationship.9–15 This effect becomes greater when venous return is augmented. Indeed, above an LVEDP of 9 mmHg, 65% of any further increase in LV diastolic pressure is caused by pericardial restraint rather than muscle properties alone.13 In human HFpEF patients, the LV diastolic pressure volume relationship shifts upward during exercise in HFpEF, consistent with an increase in pericardial restraint as venous return increases.5 These observations support the idea that removal of pericardial restraint may improve exercise hemodynamics in HFpEF.
Previous animal studies tested pericardiectomy with the chest open and in normal hearts only.9–15 The current data show for the first time that even with the chest intact and in animals with features of human HFpEF, the pericardium restrains filling, since acute disruption of pericardial restraint substantially abrogated the increase in LVEDP during volume loading (Figures 2 and 3). Also in contrast to prior studies, we show that these salutary hemodynamic effects can be achieved with limited anterior pericardial resection, without the need for complete open pericardiectomy.
Importantly, the lowering of filling pressures with saline infusion observed in the porcine model in the current study suggests that pericardial resection may be an effective treatment even when the dominant cause of congestion is due to changes in viscoelastic properties of the LV. This model has been shown to display several features of human HFpEF, including elevation in LVEDP, hypertension, oxidative stress, and coronary microvascular dysfunction.18–20, 26–29 However, we did not perform experiments to demonstrate that these animals had diastolic dysfunction or true clinical heart failure, and further study is warranted in this regard.
The improvement in filling pressures in patients with myocardial disease may be subtle, and we do not expect that this approach would normalize elevated LVEDP that is principally caused by diastolic dysfunction. However, even modest benefits could be clinically meaningful and may enable greater benefit from other therapies such as exercise training. The fact that pericardial resection can be performed in a single procedure, without the need for daily pharmacotherapy, is also a potential benefit.
In addition to acute experiments, two chronic studies performed in different species (dogs and swine) have demonstrated improvements in the exercise-induced augmentation in stroke volume, cardiac output, and maximal exercise capacity following pericardiectomy in normal animals.16, 17 This benefit was related to a greater ability to utilize the Frank-Starling mechanism to enhance cardiac output.17 Pericardial resection in animals and humans is associated with mild, balanced increases in LV dimension and mass.17, 30 This modest increase in chamber size, if confirmed in chronic animal studies and in humans, would be expected to improve forward stroke volume and cardiac output, which limit exercise capacity in many patients with HFpEF.6
However, implementation of conventional surgical approaches would be problematic for people with HFpEF, most of whom are elderly and frail, making open heart surgery a less palatable option. The novel part of this study was not the demonstration of lowered filling pressures with pericardial resection seen in previous open chest studies evaluating normal animals, but rather the observations that these salutary effects can be seen following resection of the anterior pericardium alone, without full pericardiectomy, with the chest intact, using a new device prototype and procedure that may eventually enable application to patients a minimally invasive, epicardial approach. With appropriate further development and human studies, this could potentially be performed in the clinical catheterization laboratory without the risk and long recovery time associated with conventional cardiac surgery.
Further study is clearly required to determine if the salutary effects of percutaneous pericardial modification observed in the current study will be sustained over time, and whether the procedure is safe and translates to similar hemodynamic benefits in people with HFpEF. If there is residual pericardial restraint over one side of the heart but not the other, this could alter the coupling between left and right heart pressures and volume. The restraining effect of the pericardium may prevent RV dilation and maintenance of RV function in the setting of pulmonary hypertension, and resection may not be advised in these patients. Finally, the potential effects or pericardial resection remain unclear in HFpEF patients with increased heart volume.31 If the pericardium dilates more than the heart in these circumstances, there could be less potential to derive benefit from resection. Alternatively, if heart size increases more than the pericardium dilates, this could increase ventricular interaction and make patients more likely to derive benefit.
Limitations
Experiments were performed in a small number of healthy dogs and in a porcine model with features of HFpEF. While we visually observed that the anterior pericardium was removed following the experiments in all animals, we cannot ascertain how much of the observed reduction in LVEDP with saline load was due to removal of pericardial restraint over the LV, decrease in RV restraint mediated across the septum (ventricular interaction), or both. However, either or both mechanisms would be expected to decrease pulmonary capillary hydrostatic pressures and reduce dyspnea and long-term risk for developing pulmonary hypertension and right heart failure. Hemodynamics decayed toward baseline fairly quickly following saline load, but intracardiac pressure assessments, ventriculography and CO measurements were performed rapidly and in the exact same time sequence following saline infusion before and after pericardiectomy. Each animal served as its own control, as the effects of the saline load were transient and hemodynamics returned to baseline levels prior to recording the post-pericardiectomy basal parameters. Central venous pressures were somewhat elevated at rest in the animals, and this could influence the hemodynamic response to volume manipulation leading to greater effect of saline loading because of the nonlinear pressure-volume relationship in both ventricles. While one could envision a progressively hypervolemic state from serial volume infusions before and after pericardiectomy, this would only be expected to bias our results toward the null, as progressive saline infusions would lead to greater hypervolemia and distention of the heart to the steeper portions of its 4-chamber pressure-volume relationship. This study examined acute effects only, and it remains unknown whether these benefits will be sustained over time, or whether untoward effects of pericardial resection such as excessive dilatation, fibrosis with constriction, or herniation might occur. While the data were consistent in normal animals and a porcine model with features of human HFpEF, it remains unclear if similar beneficial effects on hemodynamics would extend to human HFpEF, and this will require further study in early phase trials. Saline loading does not recapitulate all of the physiologic changes of exercise, so we cannot conclude from these data that pericardial resection would also improve exercise LVEDP.7 HFpEF represents a complex and heterogenous disorder, and while elevation filling pressures represent an important therapeutic target, reducing these pressures may not lead to symptomatic benefit in all patients, particularly in those whose symptoms are related to abnormalities in the periphery.32
Conclusions
Pericardial resection performed with the chest intact using a minimally invasive, subxiphoid approach improves left ventricular diastolic reserve during saline loading in both normal dogs and in a porcine model with features of HFpEF. Further study is required to determine whether these beneficial acute effects are sustained chronically in animals, and whether similar acute and chronic benefits may be translatable to human HFpEF.
Supplementary Material
Clinical Perspective.
People with heart failure and preserved ejection fraction (HFpEF) develop marked elevation in cardiac filling pressures that contribute to symptoms of dyspnea, particularly during stresses associated with increased venous return to the heart, such as exercise. The pericardium contributes to this increase in cardiac filling pressures that occurs with high venous return, as it restrains further ventricular filling. Here we demonstrate that percutaneous resection of the anterior pericardium, performed in a minimally-invasive subxiphoid procedure, attenuates the rise in left ventricular filling pressures during volume loading in normal dogs and in a hypertensive pig model with features of human HFpEF. In addition to reducing filling pressures, improvements in left ventricular volume with saline loading were also observed; suggesting improvement in Frank Starling Reserve. This proof of concept study shows for the first time that resection of the anterior pericardium, which can be accomplished through a minimally invasive approach, without the need for sternotomy, may be a viable option to improve hemodynamic reserve in HFpEF. Further study is required testing this concept in chronic animal studies and in humans.
Acknowledgments
Funding Sources: This research was funded by the Earl Wood Career Development Award in Cardiovascular Research (to BAB). BAB is also supported by RO1 HL128526 and U10 HL110262. CVD was supported by an NIH T32 training grant (HL007111)
Footnotes
Disclosures: BAB, SJA and VM have a provisional patent (#61/798,382) for the tools and approach for the minimally-invasive pericardial modification procedure.
References
- 1.Sharma K, Kass DA. Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 8.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 9.Slinker BK, Ditchey RV, Bell SP, LeWinter MM. Right heart pressure does not equal pericardial pressure in the potassium chloride-arrested canine heart in situ. Circulation. 1987;76:357–362. doi: 10.1161/01.cir.76.2.357. [DOI] [PubMed] [Google Scholar]
- 10.Shirato K, Shabetai R, Bhargava V, Franklin D, Ross J., Jr Alteration of the left ventricular diastolic pressure-segment length relation produced by the pericardium. Effects of cardiac distension and after load reduction in conscious dogs. Circulation. 1978;57:1191–1198. doi: 10.1161/01.cir.57.6.1191. [DOI] [PubMed] [Google Scholar]
- 11.Glantz SA, Misbach GA, Moores WY, Mathey DG, Lekven J, Stowe DF, Parmley WW, Tyberg JV. The pericardium substantially affects the left ventricular diastolic pressure-volume relationship in the dog. Circ Res. 1978;42:433–441. doi: 10.1161/01.res.42.3.433. [DOI] [PubMed] [Google Scholar]
- 12.LeWinter MM, Pavelec R. Influence of the pericardium on left ventricular end-diastolic pressure-segment relations during early and later stages of experimental chronic volume overload in dogs. Circ Res. 1982;50:501–509. doi: 10.1161/01.res.50.4.501. [DOI] [PubMed] [Google Scholar]
- 13.Applegate RJ, Santamore WP, Klopfenstein HS, Little WC. External pressure of undisturbed left ventricle. Am J Physiol. 1990;258:H1079–1086. doi: 10.1152/ajpheart.1990.258.4.H1079. [DOI] [PubMed] [Google Scholar]
- 14.Applegate RJ, Johnston WE, Vinten-Johansen J, Klopfenstein HS, Little WC. Restraining effect of intact pericardium during acute volume loading. Am J Physiol. 1992;262:H1725–1733. doi: 10.1152/ajpheart.1992.262.6.H1725. [DOI] [PubMed] [Google Scholar]
- 15.Assanelli D, Lew WY, Shabetai R, LeWinter MM. Influence of the pericardium on right and left ventricular filling in the dog. J Appl Physiol (1985) 1987;63:1025–1032. doi: 10.1152/jappl.1987.63.3.1025. [DOI] [PubMed] [Google Scholar]
- 16.Stray-Gundersen J, Musch TI, Haidet GC, Swain DP, Ordway GA, Mitchell JH. The effect of pericardiectomy on maximal oxygen consumption and maximal cardiac output in untrained dogs. Circ Res. 1986;58:523–530. doi: 10.1161/01.res.58.4.523. [DOI] [PubMed] [Google Scholar]
- 17.Hammond HK, White FC, Bhargava V, Shabetai R. Heart size and maximal cardiac output are limited by the pericardium. Am J Physiol. 1992;263:H1675–1681. doi: 10.1152/ajpheart.1992.263.6.H1675. [DOI] [PubMed] [Google Scholar]
- 18.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens. 2014;32:154–165. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eirin A, Zhu XY, Ferguson CM, Riester SM, van Wijnen AJ, Lerman A, Lerman LO. Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem Cell Res Ther. 2015;6:7. doi: 10.1186/scrt541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbieta-Caceres VH, Zhu XY, Jordan KL, Tang H, Textor K, Lerman A, Lerman LO. Selective improvement in renal function preserved remote myocardial microvascular integrity and architecture in experimental renovascular disease. Atherosclerosis. 2012;221:350–358. doi: 10.1016/j.atherosclerosis.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophyisol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 22.Lachman N, Syed FF, Habib A, Kapa S, Bisco SE, Venkatachalam KL, Asirvatham SJ. Correlative anatomy for the electrophysiologist, part i: The pericardial space, oblique sinus, transverse sinus. J Cardiovasc Electrophysiol. 2010;21:1421–1426. doi: 10.1111/j.1540-8167.2010.01872.x. [DOI] [PubMed] [Google Scholar]
- 23.Syed F, Lachman N, Christensen K, Mears JA, Buescher TL, Cha YM, Friedman PA, Munger TM, Asirvatham SJ. The pericardial space: Obtaining access and approach to fluoroscopic anatomy. Card Electrophysiol Clin. 2010;2:9–23. doi: 10.1016/j.ccep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy JW, Trenholme SE, Kasser IS. Left ventricular volume and mass from single-plane cineangiocardiogram. A comparison of anteroposterior and right anterior oblique methods. Am Heart J. 1970;80:343–352. doi: 10.1016/0002-8703(70)90099-2. [DOI] [PubMed] [Google Scholar]
- 25.Dauterman K, Pak PH, Maughan WL, Nussbacher A, Arie S, Liu CP, Kass DA. Contribution of external forces to left ventricular diastolic pressure. Implications for the clinical use of the starling law. Ann Intern Med. 1995;122:737–742. doi: 10.7326/0003-4819-122-10-199505150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammed SF, Majure DT, Redfield MM. Zooming in on the microvasculature in heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e003272. doi: 10.1161/CIRCHEARTFAILURE.116.003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail. 2016;9:e002562. doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 30.Tischler MD, Rowan M, LeWinter MM. Increased left ventricular mass after thoracotomy and pericardiotomy. A role for relief of pericardial constraint? Circulation. 1993;87:1921–1927. doi: 10.1161/01.cir.87.6.1921. [DOI] [PubMed] [Google Scholar]
- 31.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 32.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: More than a heart problem. J Geriatr Cardiol. 2015;12:294–304. doi: 10.11909/j.issn.1671-5411.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.