Abstract
Background and Purpose
We have previously demonstrated that the local delivery of monocyte chemotactic protein-1 (MCP-1) via a MCP-1-releasing poly(lactic-co-glycolic acid) (PLGA) -coated coil promotes intra-aneurysmal tissue healing. In this study, we demonstrate that interleukin-6 (IL-6) and osteopontin (OPN) are downstream mediators in the MCP-1-mediated aneurysm healing pathway.
Methods
Murine carotid aneurysms were created in C57BL/6 mice. Drug-releasing coils (MCP-1, IL-6 and OPN) and control PLGA coils were created and then implanted into the aneurysms in order to evaluate their intra-aneurysmal healing capacity. In order to investigate the downstream mediators for aneurysm healing, blocking antibodies for IL-6 receptor and OPN were given to the mice implanted with the MCP-1-releasing coils. A histological analysis of both murine and human aneurysms was utilized to cross-validate the data.
Results
We observed increased expression of IL-6 in MCP-1-coil treated aneurysms and not in control-PLGA-only treated aneurysms. MCP-1-mediated intra-aneurysmal healing is inhibited in mice given blocking antibody to IL-6 receptor. MCP-1-mediated intra-aneurysmal healing is also inhibited by blocking antibody to OPN. The role of IL-6 in intra-aneurysmal healing is in recruiting of endothelial cells and fibroblasts. Local delivery of OPN to murine carotid aneurysms via OPN-releasing coil significantly promotes intra-aneurysmal healing, but IL-6-releasing coil does not, suggesting that IL-6 cannot promote aneurysm healing independent of MCP-1. In the MCP-1-mediated aneurysm healing, OPN expression is dependent on IL-6; inhibition of IL-6 receptor significantly inhibits OPN expression in MCP-1-mediated aneurysm healing.
Conclusions
Our findings suggest that IL-6 and OPN are key downstream mediators of MCP-1-mediated intra-aneurysmal healing.
Keywords: MCP-1, IL-6, Osteopontin, Intracranial Aneurysm, Aneurysm Healing
Subject Terms: Animal Models of Human Disease, Basic Science Research, Vascular Disease, Inflammation, Vascular Biology
Introduction
Cerebral aneurysms (CAs) occur in up to 5% of the population in the United States, and up to 7% of all strokes are caused by CA rupture 1–3, which is associated with up to 50% death or dependency. Histological analysis of completely cured CAs obtained at autopsy compared to incompletely cured CAs obtained at surgery demonstrate that intra-aneurysmal tissue repair with collagen, macrophages, neutrophils, smooth muscle cells, and endothelial cells is needed to achieve a cure for CAs 4. We have studied the mechanism of intra-aneurysmal tissue repair in a murine carotid aneurysm model 5 and previously demonstrated that local delivery of monocyte chemotactic protein-1 (MCP-1) via a poly(lactic-co-glycolic acid) (PLGA)-coated platinum coil that releases MCP-1 promotes intra-aneurysmal tissue healing 6. While MCP-1 and its role in tissue repair and remodeling have been studied in other disease models such as wound healing 7, 8 and myocardial infarction 9, CAs differ significantly in that the tissue healing must occur and be induced within an intraluminal intra-aneurysmal space with no native matrix upon which inflammatory cells can infiltrate and connective tissue can proliferate. Therefore, an intraluminal scaffold must be surgically introduced to initiate an inflammatory response and promote the tissue repair and remodeling cascade, which in this model, is the MCP-1-releasing coil.
We have demonstrated that intravascular MCP-1–mediated inflammatory tissue healing is MIP-1a and MIP-2 dependent. When MIP-1a or MIP-2 was blocked, MCP-1-mediated aneurysmal healing in our murine carotid aneurysm model was significantly reduced. Although we hypothesized the postulated pathway by MCP-1 in the inflammatory tissue healing cascade, the downstream mediators of MCP-1 in aneurysmal healing have not been clarified. Some studies showed that MCP-1 activates inflammatory cells followed by the recruitment of monocytes/macrophages which release a variety of cytokines. (29,37,26,47) Additional studies were needed to further define the pathway of MCP-1-mediated inflammatory aneurysmal tissue healing. The detailed mechanisms and the pathway of downstream mediators in this specific MCP-1-mediated aneurysm healing, however, remain unclear. In this study, we demonstrate that interleukin-6 (IL-6) and osteopontin (OPN) are downstream mediators in the MCP-1-mediated aneurysm healing pathway.
Methods
Animals
All animal procedures were performed under the approval of the University of Florida Animal Care and Use Committee and guidelines. In all experiments, female C57BL/6 mice (6–10 weeks old) were used (Charles River, Wilmington, Massachusetts, USA).
Human aneurysm specimens
The studies of human aneurysm specimens and control superficial temporal arteries (STAs) were performed under the approval of University of Florida Institutional Review Board (IRB). Patients signed informed IRB research consent before undergoing aneurysm surgery, and aneurysms and control STAs were collected at the time of surgery.
Drug-releasing coil
Drug (cytokine)-releasing coils were created as previously described 6. Briefly, bare platinum coils were dipped into 10 mg/mL of each protein (MCP-1, IL-6 and OPN, R&D Sytems, Minneapolis, MN) in 50:50 poly-DL-lactic glycolic acid (PLGA) and dichloromethane anhydrous with Mg(OH)2. Control PLGA-only coils were created by dipping bare platinum coils into an aqueous suspension of PBS in 50:50 PLGA and dichloromethane anhydrous without protein.
Murine Carotid Aneurysm and Coil Implant
Murine carotid aneurysms were created in C57BL/6 mice as previously described5, 6. Briefly, the right common carotid artery (RCCA) is exposed, and then, 10 Unit/mL of porcine pancreatic elastase solution (Worthington Biochemical Corp, Lakewood, NJ) in PBS is applied for 20 minutes. After elastase, the RCCA is occluded distally using a heat cauterization apparatus. The aneurysms are fully grown in 3 weeks. Fully mature murine carotid aneurysms were treated with drug-releasing or control PLGA-only coils as described previously6. Briefly, the RCCA is exposed and temporarily ligated by 3-0 suture proximal and distal. A 27G needle or micro surgical knife is used to make a small incision. A coil is inserted into the aneurysm after which the incision is closed using a heat cauterization apparatus. Animals were euthanized with 4% paraformaldehyde (PFA) in PBS by cardiac perfusion at 3 days, 1 week, 2 weeks or 3 weeks after the coil implant. For cytokine array analysis, animals were euthanized without 4% PFA. Animals were randomly assigned to the experimental group.
Cytokine and Receptor Blocking in vivo
IL-6 receptor blocking antibody (200ug/mL/animal, IP, Genentech), OPN blocking antibody (100ug/mL/animal, IP, R&D systems) or their control isotype matched antibodies were given to mice 2 days before the MCP1-releasing coil implant and every 48 hours for up to 3 weeks. Mice were randomly selected from the cage and received blocking antibodies or isotype-matched IgG control which were blindly labeled with numbers. The mouse surgeon and data collectors were blinded.
In Vitro Cell Assays
In vitro intercellular cell signaling assay was performed using macrophages (J774, ATCC) and fibroblasts (3T3, ATCC). Briefly, macrophages alone, fibroblasts alone or co-cultured macrophages and fibroblasts were cultured in 24 well plates and grown to 80% confluence followed by supplementing 10ng/mL of MCP-1 protein (R&D Systems) or control PBS. Forty-eight hours later, culture medium was collected and IL-6 levels were measured using an IL-6 ELISA kit (R&D Systems).
The recruitment effect of IL-6 on ECs (HUVEC, C-003-5C, Invitrogen), macrophages (J774, ATCC), SMCs (MOVAS, ATCC), and fibroblasts (3T3, ATCC) was studied in vitro by cell migration assay. Oris Pro Cell Migration Assay kit was used for cell migration assay. All cells were cultured with media supplemented with fetal bovine serum (FBS) and grown to 100% confluence followed by serum starvation for 12 hours in 96-well cell culture plates. The assay was started with 50 ng/mL of IL-6 protein or PBS supplemented in culture medium. All procedures followed the manufacturer’s instructions. Twenty-four hours after the assay started, cell migratory area was measured using Image Pro software.
Immunohistochemical Analysis
All human samples were fixed using 4% PFA solution after collection, and transferred to 70% ethanol solution for paraffin block embedding. All mouse samples were fixed in 4% PFA solution after collection, and transferred to 30% sucrose solution for OCT frozen embedding. The blocks were sectioned using a microtome or cryostat into 5 μm sections. Immunohistochemistry (IHC) was performed on mouse and human aneurysms and control specimens. To evaluate aneurysm formation, the following antibodies were used for IHC: mouse MCP-1, mouse IL-6, mouse OPN, human MCP-1, human IL-6 and human OPN.
For the human coiled aneurysms and the STA samples, the respective fluorescent intensities of the expressions of MCP-1, IL6, and OPN were analyzed. Samples were collected using systematic random sampling to ensure biasfree analysis. The tissues were sectioned into 5 μm sections through the use of a microtome. A total of five sections, with one collected every 100 μm (20 sections), were then used for analysis. The fluorescent intensity of their expression was analyzed using ImageJ software. IHC for temporal cascade analysis were performed. Coiled aneurysms were harvested at 3 days, 1 week, 2 weeks and 3 weeks after coil treatment implant (n=5 mice for each time point). All samples were fixed using a 4% PFA solution after collection and transferred to 30% sucrose solution for OCT frozen block embedding. Various stereological techniques were used to ensure biasfree analysis. Samples were collected using systematic random sampling. The tissues were cross-sectioned into 5 μm sections through the use of a cryostat from the distal part of the aneurysm. Once the blade made contact with the coil, the tissues were sectioned every 100 μm. A total of five sections, with one collected every 100 μm (20 sections), were then used for analysis. An immunohistochemistry (IHC) analysis was performed on the mouse aneurysms. In order to evaluate the temporal cascade of inflammatory cells in aneurysm, the following antibodies were used for the IHC analysis: anti-mouse IL-6, anti-mouse CD45, anti-mouse NIMP-R14 (neutrophil), anti-mouse F4/80, anti-mouse iNOS, anti-α smooth muscle actin (SMA), and anti-fibroblast-specific protein 1. Stereological counting rules were used for the cell counts. After the staining, five microscopic high resolution images per slides were obtained using a 20× objective lens by blinded observer. All images were not overlapped with other fields and all image files were blindly named. Cells were counted by two blinded observers using Image-Pro software (Media Cybernetics). For IL-6 and αSMA, the fluorescent intensity of their expression was analyzed using ImageJ software.
Cytokine Array Analysis
Protein was extracted from aneurysm samples using RIPA lysis and extraction buffer with proteinase inhibitor. Cytokine array was performed with a RayBio Mouse Cytokine Antibody Array Kit (RayBiotech, Norcross, GA).
Statistical Analysis
Continuous responses are summarized by means, standard deviations, and 95% confidence intervals as well as by medians and ranges. Because of small sample sizes and possible non-normal distribution of responses for continuous variables, the nonparametric Mann-Whitney U test was used to detect shifts in the distribution of responses in experimental groups relative to control groups for single comparison. A two-way ANOVA model was used to compare mean transformed responses between IgG control and Anti-IL6 treated animal groups observed at 3 or 4 different time points. Separate groups of animals were observed at each time point. Animal group, observation day, and the interaction between animal group and observation day were modeled as fixed effects.
Results
MCP-1 induces IL-6 expression in macrophages and fibroblasts co-culture
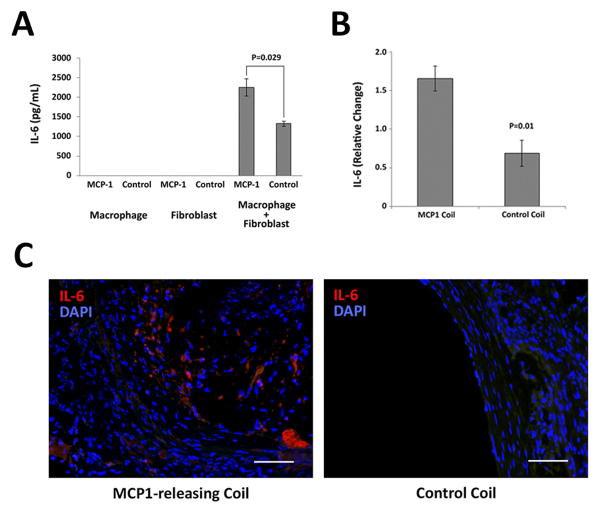
Intercellular cell signaling assays were performed to determine downstream mediators of MCP-1 and the role of cell-cell interactions. We co-cultured lipopolysaccharide (LPS)-activated macrophages with fibroblasts and exposed them to their culture media containing MCP-1 or control PBS. Expression of IL-6 by ELISA was not detected in the culture medium of macrophages alone nor fibroblasts alone, however, secretion of IL-6 by macrophages-fibroblasts co-culture was significantly upregulated. MCP-1 compared to control PBS demonstrated significantly increased secretion of IL-6 in culture medium (P=0.029) (Figure 1A).
Figure 1. MCP-1 induces IL-6 expression both in vitro and in vivo.
A) In vitro intercellular cell signaling assays were performed with macrophages alone, fibroblasts alone or macrophages-fibroblasts co-culture exposed to MCP-1 or control PBS. Significantly increased secretion of IL-6 was observed in macrophage-fibroblast co-cultured medium which are stimulated by MCP-1 (2250.0 +/− 219.9 mg/mL) compared with control PBS exposed co-cultured medium (1322.5 +/− 63.0 mg/mL). IL-6 secretion was not detected in single-cultured cells medium. (n=8 each group) B) Three weeks after coil implant, the coiled aneurysms were harvested. Cytokine array analysis of MCP-1-releasing coil treated murine carotid aneurysms (n=5) reveal significantly higher expression of IL-6 (1.7 +/− 0.2) compared to PLGA-control-coil-treated aneurysms (n=5) (0.7 +/− 0.2) (P=0.01). C) Immunofluorescent staining of representative sections of aneurysms implanted with MCP-1–releasing coil and PLGA control coil. Scale bar = 50μm.
IL-6 is expressed in MCP-1-treated murine aneurysms and “healed” human coiled aneurysms
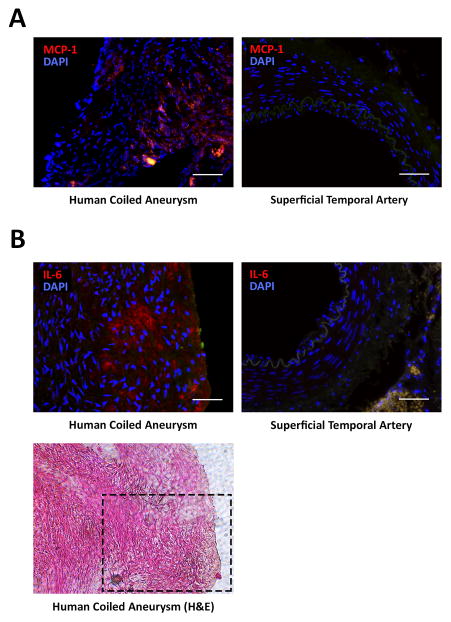
Cytokine array analysis was performed on MCP-1-coil-treated murine carotid aneurysms compared to control PLGA-only-coil treated murine carotid aneurysms 3 weeks after coil treatment, and demonstrated significantly increased IL-6 expression in the MCP-1-treated aneurysms compared to PLGA-only-treated aneurysms (P=0.01) (Figure 1B). Immunofluorescent staining demonstrates IL-6 expression in the intra-aneurysmal tissue ingrowth (n=5) (Figure 1C). Human cerebral aneurysms that have been previously coiled (n=2) demonstrate MCP-1 and IL-6 expression in the tissue ingrowth of the coiled part of the aneurysm, but not in human superficial temporal artery (n=5) (Figure 2, Supplemental Figure I, and Supplemental Table I).
Figure 2. MCP-1 and IL-6 expression in “healed” human coiled cerebral aneurysms.
Immunofluorescent staining of human cerebral aneurysms that have been previously coiled demonstrate A) MCP-1 and B) IL-6 expression in the tissue ingrowth of the coiled part of the aneurysm. Scale bar = 50μm. H&E staining of an adjacent section of a coiled aneurysm demonstrates cell orientation in the healed part of tissue (bottom panel). Black dashed line: the area where immunofluorescence was performed for IL-6.
IL-6 induces migration of endothelial cells and fibroblasts and not smooth muscle cells or macrophages
Healed human cerebral aneurysms are characterized by inflammatory cells: fibroblasts, smooth muscle cells, endothelial cells, and macrophages 4. IL-6 chemotaxis of fibroblasts, smooth muscle cells, endothelial cells, and macrophages was studied in in vitro cell migration assays with IL-6 and control serum-free medium. Cell migration assays demonstrate that IL-6 has a recruitment effect on the migration of endothelial cells (245086.1 +/− 9266.3 pixel vs 322835.4 vs 10720.9 pixel, P=0.01) and fibroblasts (103259.5 +/− 10307.8 pixel vs 144382.4 +/− 3741.7 pixel, P=0.01) (Figure 3A). However, there were no effects on migration of SMCs and macrophages.
Figure 3. IL-6 induces in vitro migration of endothelial cells and fibroblasts.
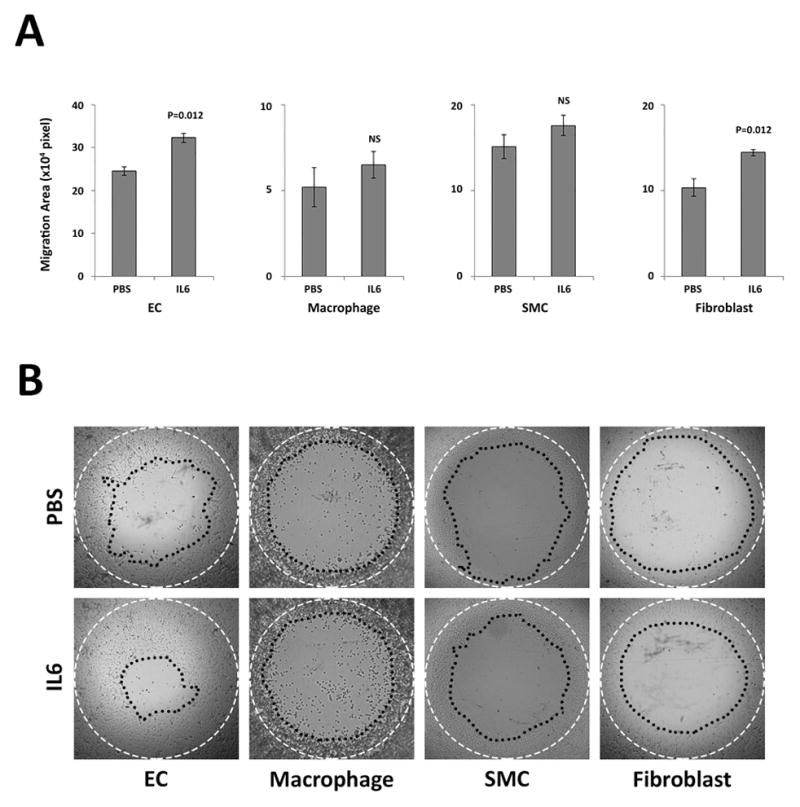
IL-6 chemotaxis for endothelial cells (ECs), macrophages, smooth muscle cells (SMCs) and fibroblasts was tested by Oris Pro Cell Migration and Invasion assay kit with 50 ng/mL of IL-6 protein or without protein (PBS) (n=8 each group). A) After the assay was performed, the migratory cells were measured using Image Pro software. The assay revealed that IL-6 induces ECs and fibroblast migration but not macrophages and SMCs. B) Representative images of migration assays in each group. White dashed line: pre-migration area (Diameter=2mm). Black dotted line: post-migration area.
Temporal cascade of MCP-1-IL-6-mediated intra-aneurysmal healing
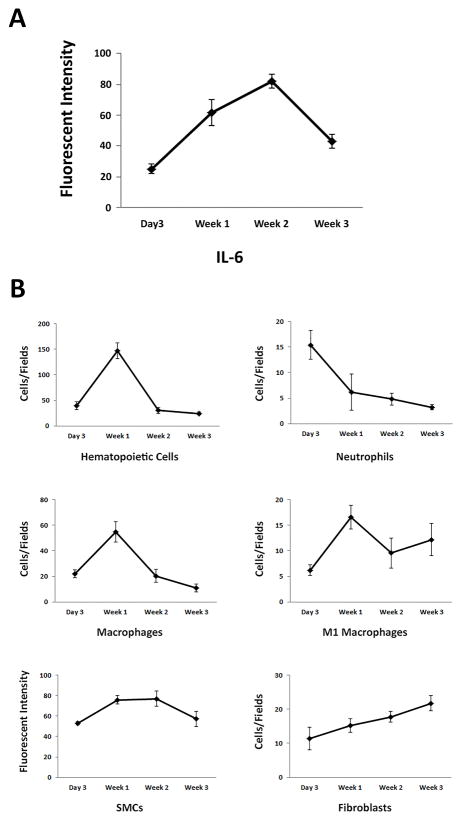
To define the temporal cascade of MCP-1-IL-6 mediated intra-aneurysmal healing, MCP-1-coil treated murine carotid aneurysms were harvested at 3 days, 1 week, 2 weeks, and 3 weeks after coil treatment, and immunofluorescent analysis was performed. IL-6 expression appeared at 3 days, reached highest levels at 2 weeks, and decreased after. CD45 positive hematopoietic cells, including neutrophils and macrophages, peaked at 1 week and then decreased after. Fibroblasts peaked at 3 weeks, and smooth muscle cells peaked at 2 weeks (Figure 4).
Figure 4. Temporal cascade of inflammatory cells during MCP-1-IL-6-mediated intra-aneurysmal healing in mice.
Immunofluorescent staining and analysis on 3-day, 1-week, 2-week and 3-week murine MCP-1-releasing coil treated aneurysm tissues were performed. Five fields A) IL-6 expression peaks 2 weeks after coil treatment. B) Hematopoietic cells including macrophages appear as early as 3 days and peak 1 week after coil treatment. Neutrophils peak at day 3 and decrease after. SMCs peak 1 week after coil treatment, but number of fibroblasts increases and peaks at 3 weeks.
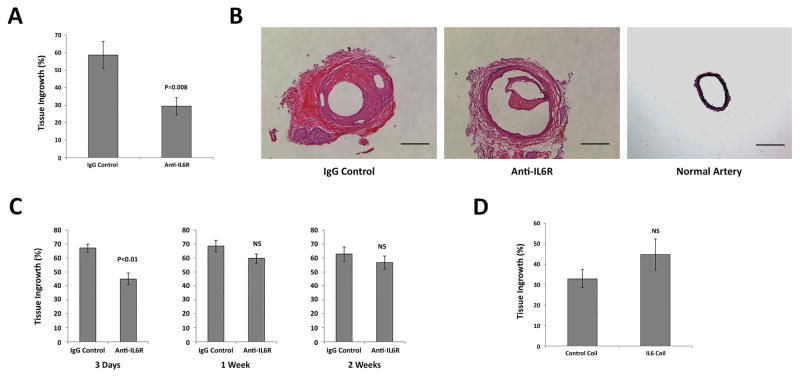
IL-6 receptor blockade inhibits MCP-1-mediated intra-aneurysmal healing
Mice harboring carotid aneurysms treated with MCP-1–releasing coils were administered an antibody antagonist of IL-6 receptor (IL-6R) or IgG control. Inhibition of IL-6R by antibody significantly reduced MCP-1 mediated aneurysm tissue healing compared with control (58.6 +/− 7.9 % vs 29.3 +/− 4.9 %, P=0.008) (Figure 5A and B). To investigate the temporal sequence of when IL-6 is necessary during MCP-1-mediated intra-aneurysmal healing, IL-6R was blocked at different time points (3 days, 1 week and 2 weeks after MCP-1-coil treatment). There was significantly decreased tissue ingrowth when IL-6R was blocked 3 days after coil implant (67.1 +/− 2.8 % vs 44.9 +/− 4.3 %, P<0.01) (Figure 5C). When IL-6R was blocked at both 1 and 2 weeks following the coil implant, however, the effect was noticeably smaller. Although there was no statistically significant difference at these later time periods, some biological inhibitory effects were noted. These results suggest that IL-6 has a more significant role in the earlier stages of MCP-1-mediated aneurysm healing as opposed to healing occurring after 1 to 2 weeks.
Figure 5. MCP-1-mediated aneurysm healing is IL-6 dependent.
C57BL/6 mice harboring aneurysms implanted with MCP-1-releasing coils were administered an antibody antagonist of IL-6 receptor (anti-IL-6R; 200μg/mL/injection, n=10 each) or IgG control (n=10 each). A) IL-6R blocked aneurysms had significantly reduced tissue ingrowth compared with IgG control. B) Hematoxylin and eosin staining of representative sections of MCP-1-releasing coil implanted aneurysms with anti-IL-6R, IgG control or normal artery. Scale bar = 200μm. C) Temporal sequence of IL-6R blockade revealed that IL-6 has significant role in MCP-1-mediated aneurysm healing between 3 days and 1 week after coil implant. (n=10 each) D) C57BL/6 mice harboring aneurysms implanted with IL-6-releasing coils did not improve intra-aneurysmal tissue healing (n=15 each).
IL-6 does not promote aneurysm healing independent of MCP-1
Murine carotid aneurysms were treated with IL-6 releasing coils or PLGA-only coils and no significant difference in intra-aneurysmal tissue healing was seen. These results suggest that MCP-1-mediated inflammatory aneurysm healing is IL-6 dependent, but IL-6 does not promote aneurysm healing independent of MCP-1 (Figure 5D).
OPN is a downstream mediator in the MCP-1-IL-6 pathway of intra-aneurysmal tissue healing
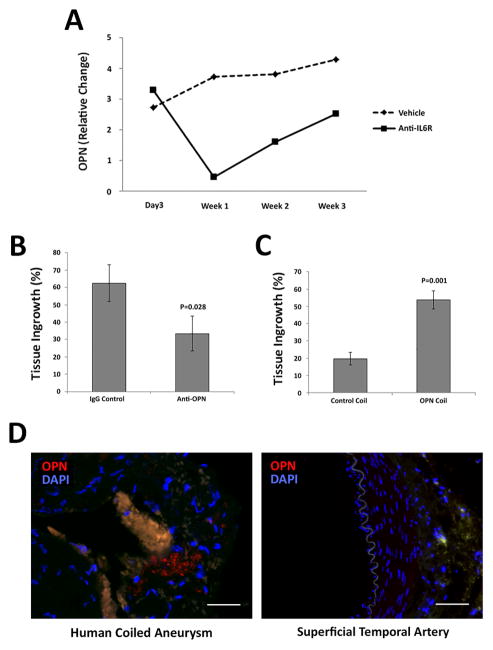
To identify downstream mediators of the MCP-1-IL-6 aneurysm healing pathway, cytokine array analysis was performed on MCP-1-coil treated murine carotid aneurysms from mice administered anti-IL-6R or control IgG. OPN expression was significantly decreased at one week after IL-6R blockade (P=0.001) (Figure 6A). The role of OPN in the MCP-1-IL-6 pathway was demonstrated by OPN blockade in MCP-1-coil treated mice. MCP-1-coil-treated murine carotid aneurysms from OPN-blocked mice have significantly decreased intra-aneurysmal tissue ingrowth compared to control IgG-treated mice (62.5 +/− 10.7 % vs 33.3 +/− 10.1 %, P=0.028) (Figure 6B).
Figure 6. MCP-1-IL-6-mediated aneurysm healing is OPN dependent.
A) Cytokine array analysis on 3-days, 1-week, 2-week and 3-week murine MCP-1-releasing coil treated with IL-6R blocked aneurysm tissues was performed. IL-6R blockade decreased expression of OPN in MCP-1-releasing coil treated aneurysm (n=5 each). B) MCP-1-coil-treated murine aneurysms from mice administered an antibody antagonist of OPN (100μg/mL/injection) (n=10) had significantly reduced intra-aneurysmal tissue ingrowth compared to the control group (n=10). C) OPN-releasing coil treated aneurysms (n=15) have significantly increased aneurysm healing compared to PLGA-control-coil-implanted aneurysm (n=15) (P=0.001). D) Immunofluorescent staining of human “healed” aneurysms express OPN in the tissue ingrowth of the coiled part of the aneurysm. Scale bar = 50μm.
OPN promotes aneurysm healing independent of MCP-1
Murine carotid aneurysms were treated with OPN-releasing coils or PLGA-only coils and OPN-releasing coil induced significantly greater aneurysmal tissue healing compared to the PLGA control coil (19.7 +/− 3.7 % vs 53.7 +/− 5.2 %, P=0.001) (Figure 6C). This suggests that OPN promotes intra-aneurysmal healing independent of MCP-1. Human cerebral aneurysms that have been previously coiled demonstrate OPN expression in the tissue ingrowth of the coiled part of the aneurysms (n=2) (Figure 6D, Supplemental Figure I, and Supplemental Table I).
Discussion
Cerebral aneurysms (CAs) occur in up to 5 % of the general population which is equal to up to 15 million people in the US1–3. CA ruptures cause subarachnoid hemorrhages (SAHs), which comprise up to 7 % of all strokes10 and result in 50% death or dependency. CAs can be treated endovascularly 11–14, and after endovascular treatment, some CAs are “healed” 15–19. However, not all CAs can be treated completely or recanalization of CAs can occur due to defective in filling by coil or healed tissue 12. The mechanisms of aneurysm healing after endovascular coiling are believed to be similar to other models of wound healing.
The earliest stage of the repair response is dominated by the inflammatory phase. During the inflammation and cell proliferation process of tissue healing, inflammatory cells and other types of cells secret chemokines such as monocyte chemotactic protein 1 (MCP-1) 20 to recruit adaptive immune cells to the site of healing. In addition, a number of cytokines, such as IL-6, have been primarily characterized as mediators of inflammatory and/or immunomodulatory reactions 21.
MCP-1, IL-6 and OPN in tissue healing
Monocyte chemotactic protein-1 (MCP-1) is known as a mediator that recruits monocytes in several inflammation models and diseases22, 23. It is also found in high levels in the early phase of wound repair 24, 25. MCP-1 is secreted by mononuclear cells and various vascular cells such as endothelial cells and vascular smooth muscle cells 26, 27. The other cytokine which has a crucial role in wound repair and healing is IL-6. IL-6 deficiency has been show to delay and reduce wound healing28, 29, which is caused by lack of MMP and TIMP modulation by IL-6 30. IL-6 expresses extracellularly according to our results (Figure 1C and Figure 2B). IL-6 is known to be expressed by a variety of cell types, but the most important sources are macrophages and monocytes at inflammatory sites. Since we have confirmed monocyte/macrophage infiltration in our MCP-1 mediated aneurysm healing model, it can be seen that they are the main source of IL-6 in the healed aneurysmal tissues. IL-6 has also been shown to have a role in the growth and differentiation of numerous cell types 31. Recent studies suggest IL-6 is involved in leukocyte trafficking and controlling the transition from innate to acquired immune responses 32. MCP-1 and IL-6 have been thought to work independently, but recent studies have shown that they are co-dependent 33–35. IL-6 has been shown to be a strong MCP-1 inducer in peripheral blood mononuclear cells 36–38. Interestingly, on the other hand, MCP-1 is able to induce IL-6 release by human epithelial cells 33. These observations suggest that MCP-1 and IL-6 can stimulate each other and are dependent on the environment and stage of tissue healing. We did not see significant tissue healing by IL-6-releasing coil independent of MCP-1. This may be because IL-6 may not recruit a sufficient number of monocytes to the site of the aneurysm. IL-6 is an essential cytokine and has a key role in tissue healing, however, other chemotactic cytokines, specifically MCP-1, need to be present first at the site of injury. OPN is expressed and secreted by cells in a variety of tissues, including vascular tissues as well as in activated macrophages and lymphocytes. OPN expression is observed in both pathological and pathophysiological conditions, and its synthesis can be induced in SMCs in various diseases 39. Similar to our results in IL-6 expression, OPN in both our murine model and human samples was observed as an extracellular-expressing cytokine, and we were not able to identify which cell types were expressing it. OPN is known as a secreted chemokine-like protein and an intracellular signaling complex which regulates cell adhesion, migration and bone regeneration40–42. Although the detailed mechanism is not clear yet, it has been shown that OPN expression in fibroblasts leads to wound healing and fibrosis 43. OPN works as both a pro-inflammatory and anti-inflammatory cytokine during injury and tissue remodeling. OPN has a key role in chemotaxis for monocytes/macrophages by supporting their adhesion as a pro-inflammatory cytokine 44. Another key role of OPN is the recruitment and regulation of fibroblasts. In OPN-null mice, the healed wound showed disorganization and a reduced number of collagen fibers 45, 46.
We observed that previously coiled human healed aneurysms express IL-6 and OPN. Bavinzski et al4 showed that after the endovascular coiling treatment in human CAs, successful healing occurs in some cases. In our mouse model, however, we have not seen successful healing by PLGA-only coil implantation. This may be why we observed IL-6 and OPN expression in only MCP1-releasing coil implanted murine aneurysms.
MCP-1-mediated aneurysm healing is associated with IL-6 and OPN expression
Intra-aneurysmal tissue healing is the desired outcome of brain aneurysm therapies. We have demonstrated that MCP-1 has an inducing effect on the migration of inflammatory cells, which have key roles in the inflammatory tissue healing response6. In the present study we demonstrate that IL-6 and OPN may have key roles in MCP-1-mediated aneurysm healing. OPN has been reported to correlate with the expressions of IL-6 47, 48. OPN can be induced in monocytes/macrophages after stimulation by IL-6 and other cytokines49. On the other hand, a study shows that OPN could upregulate the expression of IL-650. These observations could explain the change of OPN expression by blocking IL-6 in aneurysm healing. Based on our studies, local delivery of MCP-1 via an MCP-1-releasing coil to aneurysms, may cause the following intra-aneurysm healing/tissue ingrowth cascade: 1) MCP-1 initiates the induction of monocytes/macrophage and other inflammatory cells’ recruitment and migration; 2) MCP-1 upregulates IL-6 expression in inflammatory cells and epithelial cells; 3) IL-6 induces OPN expression and secretion in macrophages and other inflammatory cells; and 4) OPN recruit monocytes/macrophages and fibroblasts, and acts as both pro- and anti-inflammatory cytokine. This suggests that the MCP-1-mediated healing pathway is regulated by IL-6 and OPN. Our result also suggests that OPN can work independently as an aneurysm healing cytokine.
Summary
Here we demonstrate that intra-aneurysmal MCP-1–mediated inflammatory tissue healing is dependent on IL-6 and OPN. When IL-6 or OPN was blocked, the tissue in-growth was significantly decreased. Correlation between MCP-1 and IL-6 in tissue healing has been shown, however, the pathway by which MCP-1 is dependent on OPN has not been shown and therefore requires further study.
Limitations
In this study we used a murine carotid aneurysm model. The carotid artery and cerebral artery have clear histological differences, and therefore their respective responses to injury may differ. Coil-implanting experiments for research regarding CAs in animals have been replicated by other research groups using the carotid artery, as it is not possible to implant coils in cerebral arteries. Also, murine immunological response to injury and healing differ from that of humans. Our results may not be directly translated into treatment for human aneurysms, but the concept of the MCP-1-mediated aneurysm repair cascade is similar to that of cerebral aneurysm healing in humans.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from NIH (R01-NS083673 to B.L. Hoh) and Brain Aneurysm Foundation research grants to K. Hosaka.
Footnotes
Disclosures
None.
References
- 1.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 2.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 3.Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 2008;29:1761–1767. doi: 10.3174/ajnr.A1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavinzski G, Talazoglu V, Killer M, Richling B, Gruber A, Gross CE, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 5.Hoh BL, Velat GJ, Wilmer EN, Hosaka K, Fisher RC, Scott EW. A novel murine elastase saccular aneurysm model for studying bone marrow progenitor-derived cell-mediated processes in aneurysm formation. Neurosurgery. 2010;66:544–550. doi: 10.1227/01.NEU.0000365616.46414.2B. discussion 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoh BL, Hosaka K, Downes DP, Nowicki KW, Fernandez CE, Batich CD, et al. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1alpha and macrophage inflammatory protein-2-dependent pathway. Circulation. 2011;124:2243–2252. doi: 10.1161/CIRCULATIONAHA.111.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. Wound healing in mip-1alpha(−/−) and mcp-1(−/−) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, et al. Pro-inflammatory chemokine ccl2 (mcp-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9:e91574. doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 10.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 11.Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: Outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 12.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 13.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 14.Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 15.Kallmes DF, Borland MK, Cloft HJ, Altes TA, Dion JE, Jensen ME, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology. 1998;206:237–243. doi: 10.1148/radiology.206.1.9423678. [DOI] [PubMed] [Google Scholar]
- 16.Venne D, Raymond J, Allas S, Roy D, Leclerc G, Boushira M, et al. Healing of experimental aneurysms. Ii: Platelet extracts can increase the thickness of the neointima at the neck of treated aneurysms. J Neuroradiol. 1999;26:92–100. [PubMed] [Google Scholar]
- 17.Kallmes DF, Altes TA, Vincent DA, Cloft HJ, Do HM, Jensen ME. Experimental side-wall aneurysms: A natural history study. Neuroradiology. 1999;41:338–341. doi: 10.1007/s002340050760. [DOI] [PubMed] [Google Scholar]
- 18.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke. 1999;30:1657–1664. doi: 10.1161/01.str.30.8.1657. [DOI] [PubMed] [Google Scholar]
- 19.Feng L, Vinuela F, Murayama Y. Healing of intracranial aneurysms with bioactive coils. Neurosurg Clin N Am. 2005;16:487–499. v–vi. doi: 10.1016/j.nec.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of ccr2 by endothelial cells : Implications for mcp-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 21.Luger TA, Schwarz T. The role of cytokines and neuroendocrine hormones in cutaneous immunity and inflammation. Allergy. 1995;50:292–302. doi: 10.1111/j.1398-9995.1995.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 22.Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995;146:1287–1301. [PMC free article] [PubMed] [Google Scholar]
- 23.Strieter RM, Standiford TJ, Huffnagle GB, Colletti LM, Lukacs NW, Kunkel SL. “The good, the bad, and the ugly.” The role of chemokines in models of human disease. J Immunol. 1996;156:3583–3586. [PubMed] [Google Scholar]
- 24.Gibran NS, Ferguson M, Heimbach DM, Isik FF. Monocyte chemoattractant protein-1 mrna expression in the human burn wound. J Surg Res. 1997;70:1–6. doi: 10.1006/jsre.1997.5017. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines il-8, groalpha, mcp-1, ip-10, and mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kooten C, van der Linde X, Woltman AM, van Es LA, Daha MR. Synergistic effect of interleukin-1 and cd40l on the activation of human renal tubular epithelial cells. Kidney Int. 1999;56:41–51. doi: 10.1046/j.1523-1755.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 27.Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, Gerritsen AF, Bruijn JA, Daha MR, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 28.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of il-6 in the skin wound-healing process as evidenced by delayed wound healing in il-6-deficient mice. J Leukoc Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 29.McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of il-6 versus il-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 30.Gallucci RM, Sugawara T, Yucesoy B, Berryann K, Simeonova PP, Matheson JM, et al. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- 31.Sehgal PB. Interleukin-6: Molecular pathophysiology. J Invest Dermatol. 1990;94:2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T. Interleukin-6: From basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 33.Viedt C, Dechend R, Fei J, Hansch GM, Kreuzer J, Orth SR. Mcp-1 induces inflammatory activation of human tubular epithelial cells: Involvement of the transcription factors, nuclear factor-kappab and activating protein-1. J Am Soc Nephrol. 2002;13:1534–1547. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- 34.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, et al. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino M, Scuderi F, Provenzano C, Scheller J, Rose-John S, Bartoccioni E. Il-6 regulates mcp-1, icam-1 and il-6 expression in human myoblasts. J Neuroimmunol. 2008;196:41–48. doi: 10.1016/j.jneuroim.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the u937 cell line. Blood. 1998;91:258–265. [PubMed] [Google Scholar]
- 37.Arendt BK, Velazquez-Dones A, Tschumper RC, Howell KG, Ansell SM, Witzig TE, et al. Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia. 2002;16:2142–2147. doi: 10.1038/sj.leu.2402714. [DOI] [PubMed] [Google Scholar]
- 38.Klouche M, Rose-John S, Schmiedt W, Bhakdi S. Enzymatically degraded, nonoxidized ldl induces human vascular smooth muscle cell activation, foam cell transformation, and proliferation. Circulation. 2000;101:1799–1805. doi: 10.1161/01.cir.101.15.1799. [DOI] [PubMed] [Google Scholar]
- 39.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 40.El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: Knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Regan AW, Chupp GL, Lowry JA, Goetschkes M, Mulligan N, Berman JS. Osteopontin is associated with t cells in sarcoid granulomas and has t cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 45.Weber CE, Li NY, Wai PY, Kuo PC. Epithelial-mesenchymal transition, tgf-beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33:311–318. doi: 10.1097/BCR.0b013e318240541e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Take Y, Nakata K, Hashimoto J, Tsuboi H, Nishimoto N, Ochi T, et al. Specifically modified osteopontin in rheumatoid arthritis fibroblast-like synoviocytes supports interaction with b cells and enhances production of interleukin-6. Arthritis Rheum. 2009;60:3591–3601. doi: 10.1002/art.25020. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Abe M, Hiasa M, Oda A, Amou H, Nakano A, et al. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: A role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007;13:816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa D, Stone JF, Takata Y, Blaschke F, Chu VH, Towler DA, et al. Liver × receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ Res. 2005;96:e59–67. doi: 10.1161/01.RES.0000163630.86796.17. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Gao SG, Zhang FJ, Luo W, Xue JX, Lei GH. Effects of osteopontin on the expression of il-6 and il-8 inflammatory factors in human knee osteoarthritis chondrocytes. Eur Rev Med Pharmacol Sci. 2014;18:3580–3586. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.