ABSTRACT
The gut microbiota is the assembly of microorganisms living in our intestine and their genomes are known as the microbiome. The correct composition and functionality of this microbiome is essential for maintaining a “healthy status.” Aging is related to changes in the gut microbiota which are frequently associated with physiological modifications of the gastrointestinal tract, as well as, to changes in dietary patterns, together with a concomitant decline in cognitive and immune function, all together contributing to frailty. Therefore, nutritional strategies directed at restoring the microbiota in the elderly have to be addressed from a global perspective, considering not only the microbiota but also other extra-intestinal targets of action. The present review aims at summarizing the current knowledge on intestinal microbiota alterations and other functions impaired in the elderly and to analyze tools for implementing nutritional strategies, through the use of probiotics, prebiotics or specific nutrients in order to counterbalance such alterations.
KEYWORDS: Clostridium difficile, diet, elderly, microbiota, microbiome, nutritional strategy, nutrient, probiotics, prebiotics
Introduction
During the past 2 centuries lifespan has been increasing by approximately 2 y per decade in developed countries. A quarter of European population will be older than 65 y by the middle of the present century.1 While this increase of life expectancy represents an extraordinary social and medical achievement, the healthcare costs associated with recurrent disease and disability that frequently occurs in the elderly, constitute an economic burden and a sustainability challenge for the current social structure of industrialized countries. The elderly population frequently suffers from an impairment of several biological functions that are associated with important changes of their intestinal microbiota. It is increasingly clear that nutrition contributes to shape the microbiota, which opens the possibility of interacting through diet with this microbial community inhabiting our body. This is especially important in those situations where other extra-intestinal disorders are also associated with microbiota alterations, as occurs in the elderly. Thus, advancing our knowledge on the interactions between nutrition and gut microbiota structure and function in older individuals could help to improve the general health status of this population through the design on individualized nutritional strategies.
The gut microbiome: Composition, functionality and evolution across the lifespan
The gut microbiota is the wide, complex and diverse collection of microorganisms living in our intestine, and includes bacteria, archaea, viruses and some unicellular eukaryotes that have co-evolved with humans in a commensal way. The gut microbiota represents the largest number and concentration of microorganisms in the human body, reaching levels of 1014 cells in the colon.2 This microbiota contains a vast genetic catalog, the so called “intestinal microbiome.” Overall almost 10 million different genes have been found in the human microbiome so far,3 representing an important genetic resource. A number of 1000 to 1500 bacterial species have been reported to be widespread in the human gut, each person harboring around 150 different species,4 which all together contain more genes than the human genome.4 The microbiome is usually recognized as our second genome5 and contributes extensively to our physiology and metabolism.4
There is an increasing interest by the research community to decipher the role of the microbiota in maintaining human health, and several projects have been launched with this aim across the globe.6 In the last few years, the development of high-throughput analytical tools and “meta-omics” technologies has allowed more detailed studies on the gut microbiome composition and functionality. Despite this progress, what really constitutes a “healthy” gut microbiota remains still unclear.
The microbial colonization of the gastrointestinal tract (GIT) starts immediately after birth, and this process determines to some extent the predisposition to develop diseases in early and later life.7,8 The colonizing microbiota community is initially unstable and suffers a succession phenomenon that involves a first colonization by facultative anaerobes, which creates a more reduced environment for the subsequent colonization by strict anaerobes.9 From an initial low diversity and complexity, the intestinal microbiota evolves until reaching a diverse, complex, and stable population about the age of 3 y.10,11 At the phylum taxonomic level, the dominant bacteria into healthy adults are Firmicutes, and Bacteroidetes, which constitute between 80–90% of the total microbiota, with representatives of Proteobacteria, Fusobacteria, Cyanobacteria, Verrucomicrobia and Actinobacteria phyla, among others often found at considerably lower levels.10 Furthermore, in spite of the high inter-individual variability, 3 specific microbial enterotypes, each one being dominated by a particular bacterial genus (Bacteroides, Prevotella or Ruminococcus), that appear to be independent of nationality, sex, age, or body mass index, have been proposed.12 These enterotypes have been associated with the long-term dietary pattern13 and are influenced by short-term diets.14,15 Nevertheless, the classification of human-associated bacteria in enterotypes is a controversial concept and some disparities have been found, due to the lack of consistency in the methodologies employed.16,17 The adult-like intestinal microbiota is regarded as relatively stable throughout adulthood although it is susceptible to variations owing to stress, antibiotics and as a consequence of diet and lifestyles.18 At senescence, an inverse process occurs that resembles a mirror image of the neonatal gut colonization and the microbiota becomes unstable again.
The intestinal microbiota at advanced age
Age-related changes in the gut microbiota are associated with physiological changes in the GIT, as well as in dietary patterns, with a concomitant decline in the normal function of the immune system that may contribute to increased risk of infection and frailty.19-21 The gut microbiota of elderly subjects is characterized by a reduced bacterial diversity, shifts in the dominant species, a decline in beneficial microorganisms, increase of facultative anaerobic bacteria and a decrease in the availability of total short chain fatty acids.11 More specifically, when comparing the microbiota of elderly with that of younger adults, lower levels of Firmicutes, mainly Clostridium cluster XIVa and Faecalibacterium prausnitzii, and Actinobacteria (mainly bifidobacteria), and increased populations of Proteobacteria have been found19-24 (Table 1). With regard to other relevant intestinal microbial populations such as the phylum Bacteriodetes, the results are more variable, with some studies reporting lower levels19,23-27 while others have indicated increases of this microbial group in elderly subjects.21 Similarly, variable results have also been observed for lactobacilli, with some studies reporting a reduction22,28 and others an increase in the levels of these microorganisms at older ages.24,27,29 It is still unclear whether this variability in the results obtained for certain microbial groups is related to actual population differences or to methodological issues, such as the different techniques used for determining microbial abundancies (Table 1).
Table 1.
Main studies employing molecular techniques to analyze the gut microbial composition in elderly. nF, number females; nM, number males; NSAID, non-steroidal anti-inflammatory drugs; Ref, reference; U, unknown;
| Subjects, country, age (nF, nM) | Gut microbiota alterations identified | Molecular technique | Ref. |
|---|---|---|---|
| • Healthy elderly, Japan, 74-94y (5F,1M) | ↓Clostridium cluster XIVa | 16S DNA libraries, T-RFLP | 25 |
| • Healthy adults (HY), United Kingdom, 19-35y, (9F, 3M) • Healthy elderly (HE), United Kingdom 67-75y (6F) • Elderly hospitalized receiving antibiotics (AE), United Kingdom 73-101y (2F,8M) |
↓Bacteroides, Bifidobacteria, species diversity (HE, AE) ↑Fusobacteria, Clostridia, Propionibacteria AEvsHE, HY ↑Enterobacteriaceae (HE, AE) |
16S rRNA qPCR | 30 |
| • Adult group (A): France (F), Germany (G), Italy (I), Sweden (S) 20-50y (85, U) • Elderly group (E): France (F), Germany (G), Italy (I), Sweden (S)>60y (145, U) |
↑Enterobacteria (EF, EI, EG, ES) ↓Bacteroides (EI), ↑Bacteroides (EG, ES) ↑Clostridium cluster XIVa (EG), ↓Clostridium cluster XIVa, F. prausnitzii (EI) ↑F. prausnitzii (EF, EG, ES) |
16S rRNA FISH | 35 |
| • Infant group, U, 3weeks-10 months (21, U) • Adult group, U, 25-45y (21,U) • Elderly group, U, 70-90y (20, U) |
↓Firmicutes/ Bacteroidetes ratio in elderly vs adults ↑Escherichia coli in elderly vs adults |
16S rRNA qPCR | 108 |
| • Institutionalized elderly, Austria, 78-94y (9F,8M) • Young volunteers, Austria, 18-31y (9F,8M) |
↑Bacteroides in elderly ↓Bifidobacterium, Clostridium cluster IV, total bacteria and Clostridium cluster IV diversity in elderly |
16S rRNA qPCR, PCR-DGGE, | 109 |
| • Young adults (Y), Italy, 25-40y (9F,11M) • Elderly: Group E, Italy, 63-76y (11F,11M)+ offspring of centenarians (Group F), Italy, 59-78y (11F,11M) • Centenarians (C). Italy, 99-104y (20F, 1M) |
↓Clostridium cluster XIVa (C) ↓F. prausnitzii and relatives, species diversity (C) ↑Eubacterium limosum and relatives, Proteobacteria, Bacilli (C) |
HITChip, 16S rRNA qPCR | 23 |
| • NSAID elderly, Finland, 77–85y (6F,3M) • No NSAID elderly, Finland 70–83y (6F,3M) • Young adults, Finland, 21–39y (5F,9M) |
↓Ruminococcus, Roseburia, Coprobacillus, Dialister in No NSAID elderly vs adults ↑Lactobacillus, Streptococcus, Bacteroides in No NSAID elderly vs adults ↑Lactobacillus, Collinsella in No NSAID elderly vs NSAID elderly |
% G+C content, 16S rDNA sequencing | 29 |
| • Healthy elderly, Ireland, >65y (161, U) • Healthy adult, Ireland, 28-46y (9,U) |
Bacteroidetes dominate Irish elderly ↑Clostridium cluster IV (Faecalibacterium, Sporobacter, Ruminococcus) in elderly |
16S rRNA gene sequencing | 19 |
| • Institutionalized elderly, Spain, 77–95y (7M, 31F) • Middle-Aged adults, Spain, 57–67y (11M, 27F) |
↓Faecalibacterium, Bacteroides, Clostridium cluster XIVa in elderly ↑Lactobacillus group in elderly |
16S rRNA qPCR | 24 |
| • Group RC, China, 100-108y (5F,3M) • Group RE, China, 85-99y (5F,3M) • Group CE , China, 80-92y (4F, 4M) |
↑Roseburia, Escherichia in centenarians ↓Lactobacillus, Faecalibacterium, Parabacteroides, Butyricimonas, Caprococcus, Megamonas, Mitsuokella, Sutterela, Akkermansia in centenarians |
16S rRNA gene sequencing | 110 |
| • Young adult (Y), Italy, 22-48y (8F, 7M) • Young elderly (E), Italy, 65-75y (7F,8M) • Supercentenarians (S), Italy, 105-109y (18F, 6M) • Centenarians (C), Italy, 99-104y(14F , 1M) |
↓ by age: Bacteroidaceae, Lachnospiraceae, Ruminococcaceae Coprococcus, Roseburia, Faecalibacterium: (-) correlation with age Oscillospira, Odoribacter, Butyricimonas: (+) correlation with age ↑by age: Eggerthela, Akkemansia, Anaerotruncus, Synergistaceae, Bilophila, Christensenellaceae ↓Bifidobacterium E, C but ↑S |
16S rRNA gene sequencing | 111 |
| • Healthy volunteers, Japan, 0-104y (213F,158M): elderly volunteers, Japan >60y (53F, 36M) |
↓by age: Actinobacteria ↑ Bacteroidetes, Betaproteobacteria, Deltaproteobacteria in elderly ↑Porphyromonas, Treponema, Fusobacterium, Pseudoramibacter in elderly |
16S rRNA gene sequencing, qPCR | 21 |
As expected from the differences on microbiota observed between elderly and younger adults and different dietary patterns between the groups of age, the production of bacterial metabolites is also altered at old-age. The levels of short chain fatty acids (SCFA), the main bacterial metabolites in the colon, are lower in elderly and the ratios among the different fecal SCFA also vary with respect to healthy younger adults.20,24 These changes in the production of bacterial metabolites have been suggested to be related with variations in the colonic bacterial metabolism, which shifts from the predominantly saccharolytic metabolism normally observed in adults toward a predominantly putrefactive metabolism.30
It is important to highlight that a change in the gut microbiota may not necessarily mean a detrimental health effect. Functional redundancy is known to exist in the gut microbiota and, thus, not all replaces in microbial composition are going be translated into a functional deficiency. However, in the case of elderly subjects, an altered microbiota has been repeatedly reported to be associated with frailty.19,31 In this way, some microorganisms, such as F. prausnitzii, and a reduced microbial diversity, have been associated negatively, whereas other microorganisms were associated positively with frailty. Therefore, there is indication of potential detrimental effects of the microbiota changes associated with aging. It is also important to consider that the above mentioned shifts in the gut microbiota do not initiate at a defined age, and they are rather a gradual process,32 likely related with the physiological decline of the individual. Moreover, not surprisingly, life-style and diet have been found as the most important drivers for these microbiota changes20 pointing out at the need to promote healthy dietary habits among the elderly population.
There are a limited number of studies in the literature that describe the composition of the microbiota of elderly individuals, which restricts our ability to establish cause and effects relationships. This is especially relevant in this human population since aging is related with dietary changes and a subsequent increase in the risk of malnutrition, as well as, with changes in the immune system.24,33,34 All these alterations considered together, may explain the higher susceptibility of elderly people to disease. Besides, differences among elderly from different geographical locations have been also observed.35
Clostridium difficile-associated diarrhea
Clostridium difficile (C. difficile) is an obligate anaerobic, gram-positive and spore-forming bacterium that is often present in the large intestine of healthy adults.36,37 However, it is able to proliferate and produce toxins and disease in a susceptible host, when there is an event that causes a disruption of the intestinal microbiota.38 C. difficile spores are resistant to high temperatures, ultraviolet light and harsh chemicals, and can survive for long periods of time in hospitals and health care facilities, thus favoring its dissemination.39,40 Furthermore, spores are also resistant to antibiotics and can persist in the GIT and potentially contribute to recurrent disease following treatment against the vegetative form.39
C. difficile infection (CDI) is the most common cause of nosocomial diarrhea in the industrialized world.41 It generally affects elderly hospitalized patients who have received a broad-spectrum antimicrobial treatment.42 The incidence, severity and mortality of CDI have significantly increased over the last decade. These epidemiologic changes in CDI incidence have been partially explained by the emergence of hyper-virulent strains, such as C. difficile BI/NAP1/027;40 infection by this strain in patients between 60 and 90 y of age has been related to an increased probability of CDI-related death.38
The most important risk factors for the development of CDI, mortality and recurrence of the disease are advanced age (≥65 years of age) and exposure to antibiotics. The disproportionate burden of CDI in elderly individuals may be related to the increase of exposure to healthcare environments, the raise of comorbidities, use of drugs including antibiotics, and age-related changes in the immune system and the intestinal microbiota of the host.41 Moreover, antibiotic exposure alters the intestinal microbiota, which facilitates the overgrowth of C. difficile. The most commonly antibiotics associated to CDI are clindamycin, aminopenicilins, second- and third-generation cephalosporins and fluoroquinolones.37
The main mechanism of virulence in C. difficile is related to the production of 2 enterotoxins, TcdA and TcdB, which cause the intestinal injury and activate an inflammatory cell response. Therefore, CDI is a toxin-mediated disease of the colon with clinical manifestations ranging from asymptomatic colonization or mild, self-limited diarrhea, to fulminant pseudomembranous colitis, toxic megacolon, colonic perforation, sepsis, shock and death.43 It is well known that, both the characteristics of C. difficile strain and the host´s immune response, influence CDI severity, recurrence risk and mortality.40
Vancomycin and metronidazole have been used as effective treatment for CDI for over 30 y. However, a high CDI recurrence rates in patients who respond to initial treatment with these 2 antibiotics has been observed. For this reason, new therapeutic strategies are now under investigation for the prevention and treatment of CDI.44
In spite of ethical concerns and practical issues that should be solved and adequately managed, fecal microbiota transplantation is currently emerging as a highly effective therapy for intestinal multidrug resistant pathogens, with particular efficacy against recurrent CDI.45 This therapy aims to restore normal gut microbiota by instillation of donor stool into the gastrointestinal tract of patients with CDI.44 However, concerns of donor infection transmission to patients has limited its use. In spite of this, a recent systematic and meta-analysis indicates that adverse events after fecal microbiota transplantation such as autoimmune disease and infectious disease was not significant in a follow up of 90 d after transplantation, whereas authors identified old age of patients (>65 years) as a risk factor for the primary cure failure and early recurrence.46 Beyond this, it should be possible to isolate a fecal cocktail of defined bacterial multi-species and/or design synthetic mixtures that could be administered for correcting intestinal dysbiosis, thus avoiding the risk of transmission of potentially harmful microorganisms from donors to recipients.47
Physiological and immune status in the elderly
It is important to differentiate between those physiological, organic and structural changes occurring as a result of the advance of time and those derived from pathological processes. In this context, the term “senescence” refers mainly to non-pathological biological and physiological processes strictly dependent of age, while aging, a more generic term, would refer to changes, physiological and pathological, associated to the passage of time.48,49 Age-related changes include a physiological general decline that involves all organs and functions, such as glomerular filtration rate, maximum heart rate, vital capacity or immune response.
One of the most recognized effects of aging is the decline in immune function, or more precisely the age-associated immune deregulation,50 that includes a decrease in the proliferative response to mitogens, low activity of natural killer (NK) cells,51,52 a progressive involution of the thymus together with lower number and reduced response of immune T cells,53 and increased levels of pro-inflammatory cytokines.24,33,34 This imbalance, commonly called “immunosenescence”, may contribute to explain the high susceptibility of elderly people to disease.
Recently, a link between immune aging and some neurological disorders associated with senescence has been proposed, suggesting that microglia, the innate immune cells of the brain, could undergo this process.54 In turn, senescence affects per se neurological functions and neuronal plasticity55 which leads frequently to cognitive impairment and, in extreme cases, to brain diseases such as dementia or Alzheimer, affecting the independence and quality of life.56
With advancing age, it becomes more difficult for the organism to maintain homeostasis, especially under stress conditions.49,57 Multiple changes take place at different levels of the GIT: a decrease in saliva production (xerostomy) and in gastric acid secretion, a lower absorption of nutrients such as iron and vitamin B12,58 slower gastrointestinal motor function and food transit, and a reduced chemical food digestion and nutrient absorption.59 As age increases there is a natural trend to decrease food intake, resulting in the anorexia of aging.60 Some authors hypothesize that this lack of appetite could be the result of a physiological adaptation to the decrease in calorie requirements, as a consequence of the changes in body composition and reduction of physical activity.61 Multiple factors could explain the reduction in the appetite in seniors: among homeostatic factors, there is a clear deregulation in the secretion and response to some of principal hormones related with the control of food intake such as cholecystokinin, ghrelin or insulin.59 This dysregulation together with a decrease in smell and taste perception, modifies the appetence and food preferences, increasing the risk of malnutrition. Within non-homeostatic factors, life-style factors such as social isolation, low incomes, geographical location, social support, illness, the use of multiple drugs, or depression, could compromise food intake and the quality of life in the elderly.62 Also, the presence of oral problems and xerostomy modifies the foods choice; especially those foods with hard and sticky consistency tend to be avoided, leading to changes in dietary patterns.
Concerning body composition, the proportion of fat mass in human body increases significantly over time, as a consequence of the decrease in lean mass and bone mass, phenomenon known as “sarcopenia.”63 This is related with a decrease in the plasma concentration of albumin, a biochemical risk factor for cardiovascular disease and mortality,64,65 and with a reduction in the total body water content.66 Also, the relative increase in body fat reduces the energy requirements as a consequence of the decline in the basal metabolic rate. However, maintaining a nutrient-dense diet is of critical importance since the consumption of nutrients below the recommendations is associated with higher risk of mortality,61 whereas an over-consumption is associated with higher risk of obesity and related diseases.
Nutritional status in the elderly
It has been traditionally considered that a healthy diet requires an appropriate balance of energy, macro- and micronutrients, and water. While maintaining correct dietary habits is important for individuals of all ages, this is particularly relevant for older adults, who as stated above are more vulnerable to malnutrition. The nutritional needs of the elderly are not substantially different from those of younger adults with similar anthropometric and physiological characteristics, and caloric expenditure.24 However, the efficiency of nutrient absorption may be impaired, which, together with common chewing difficulties and loss of appetite, may alter the nutritional status of seniors.24
Due to the lack of standard tools for the nutritional assessment of elderly people, there is no consensus in the prevalence of malnutrition, and results from different studies are difficult to compare. The percentage of malnourished people is variable among countries, but, it is generally well accepted that this proportion is higher in hospitalized elderly than in institutionalized ones, followed by the free living population.67 From macronutrients, the third National Health and Nutrition Examination Survey (NHANES III 1991–1994) NHANES revealed that approximately 30% of people over 50 y do not meet the Recommended Dietary Allowances (RDA) for protein,68 one of the main factors limiting muscle synthesis in the elderly.69,70 Another nutrient for which intake falls short of the recommended intake is dietary fiber, important for maintaining intestinal health and protecting against cardiovascular disease.71 Also, in almost every dietary survey conducted over the past few decades, older adults have inadequate intakes of iron, vitamin B6, vitamin B12, folic acid and vitamin D.72
Iron deficiency is common for different reasons: an inadequate intake of heme iron (present in animal products), a reduced protein intake, the high presence in the diet of components that reduce iron absorption as phytates or oxalates, and some gastrointestinal disturbances frequent in old age (gastritis, intestinal atrophy, ulcers or other digestive disorders).73 Data from NHANES revealed a prevalence of anemia in 20% of subjects older than 85 years, associated with functional decline and other adverse outcomes.74 Also, low levels of vitamin D are frequent in seniors and especially in those subjects with low sun exposure, this being associated with a higher risk of fractures and osteoporosis.75 The association of B-group vitamins with neurological disorders has been recently discussed.76 The role of other nutrients at this age remains to be elucidated.
It is well-known that an adequate nutritional status is of great importance for maintaining proper immune system functionality and preventing frailty in the elderly, but the components in foods that improve immune functions in elderly are still far from fully understood.77 Protein-calorie malnutrition is one of the main causes of immune deficiency in elderly.78 An inadequate protein intake may lead to a decrease in the amino acids, compromising the immune system functioning.79 In addition, monounsaturated fatty acids, β-carotene and vitamins A, B6, C, and D, and bioactive compounds have been linked to a better immune response.80-82
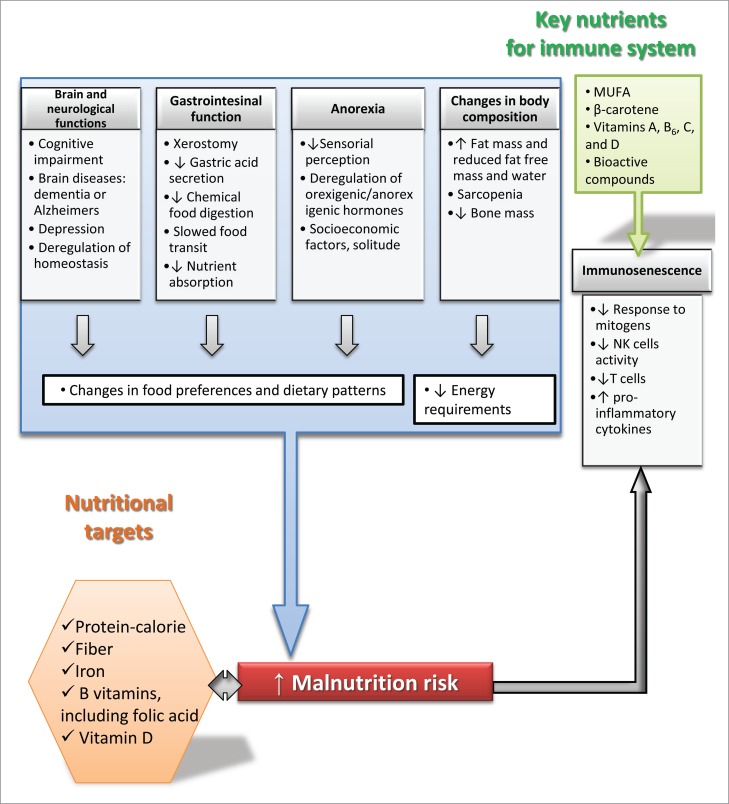
Thus, the correction or improvement of all these age-related changes constitutes a target for the development of nutritional intervention strategies focused to this population group (Fig. 1).
Nutritional strategies for restoring a balanced composition and functionality of the microbiota in the elderly
The higher susceptibility to disease in the elderly population, frequently related with malnutrition, impairment of the intestinal microbiota functionality and a pro-inflammatory status of the immune system, provide a rationale for developing nutritional strategies and functional foods aiming at microbiota and immune modulation in this population group. Prior to designing nutritional interventions for specific human populations, it is necessary to identify the specific action targets with those precise groups. The microbiota of healthy subjects is considered the reference for comparison, in order to restore a balanced microbiota. At the beginning of life, for newborns, the microbiota of full-term vaginally-delivered breast-fed babies is considered as the gold standard of a healthy microbiota. However, the identification of human populations bearing a “healthy microbiota” becomes less evident as life progresses. The human groups of reference must have had a socio-economic status the most similar as possible to the population to be studied. This is especially important in the elderly, where the prolonged exposure to different environments could make it difficult to identify variations specifically due to aging.20,24 In this case, the microbiota of a population of younger healthy adults, sharing similar characteristics in terms of diet, geographical location, social habits, historical past etc. with the elderly population under study, could be considered the healthy microbiota of reference.11,24
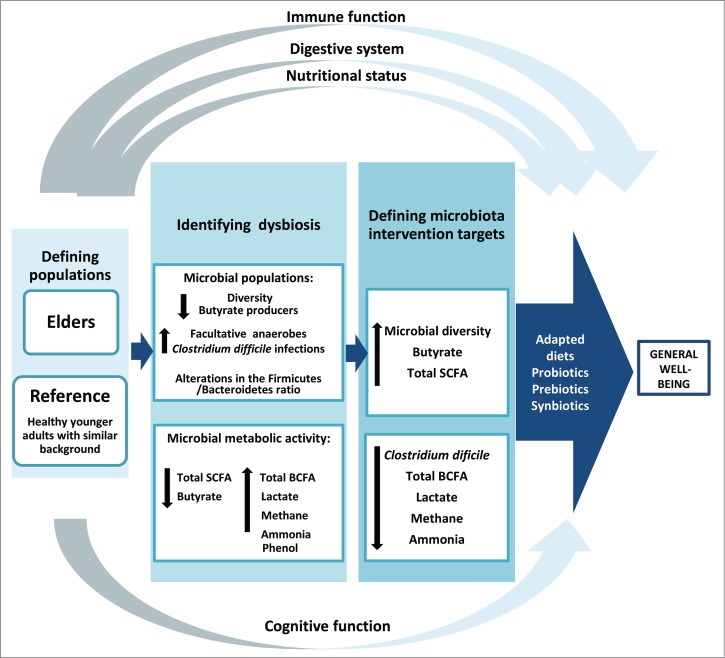
Intervention targets on the intestinal microbiota and the immune system may differ among elderly groups from different environments or geographical locations. This is especially relevant as differential effects of probiotics in different population groups have been previously demonstrated83 suggesting that the targets on the gut microbiota may also be different depending on location. Loss of the community-associated microbiota, defined as the group of microorganisms shared by the individuals from a given social group, has been correlated with increased frailty.19,31,84 The reduced microbial diversity, low levels of butyrate producing bacteria, decreased levels and imbalanced proportions of SCFA, the incidence of CDI, and higher levels of lactate, methane and branched chain fatty acids (valeric, isovaleric, isobutyric, and caproic acids) are generally considered as relevant targets for intervention.19,24,30,37,85-88 As mentioned previously, changes in the intestinal microbiota composition and functionality in the elderly are usually accompanied by alterations in the physiology and function of the digestive tract, and by nutritional deficiencies related with lower intakes of specific nutrients that are important for maintaining the immune and gastrointestinal functions.89 Additionally, the actual nutritional needs of the older population can differ from those of the middle-age.90 Therefore, nutritional strategies in the elderly should be addressed from a holistic perspective, considering the intestinal microbiota, the immune system as well as nutritional deficiencies and needs as a whole (Fig. 2).
Figure 1.
Schematic representation of physiological, nutritional and immune targets of intervention generally identified for elderly people. MUFA: mono-unsaturated fatty acids; NK: natural killer cells.
Figure 2.
General strategy proposed for designing nutritional interventions in the elderly. The intestinal microbiota and its functionality have been considered the central axis, clustering around them the other intestinal and extra-intestinal possible targets. SCFA: short chain fatty acids; BCFA; branched chain fatty acids.
In general, the moderate enhancement of the colonic fermentation of dietary fiber could be considered as beneficial in the elderly. This will strengthen the intestinal barrier against pathogens, increasing the intestinal motility and helping to reduce the underlying pro-inflammatory status (immunosenescence), thus contributing to the general well-being. Of particular relevance is the enhancement of the intestinal butyrate production through the promotion of microbial interactions by cross-feeding mechanisms involving members of the intestinal microbiota able to produce butyrate (Blautia coccoides and Clostridium leptum groups, among the most relevant).91,92
The design of adapted diets and the specific use of selected probiotics, prebiotics or synbiotics (combined use of probiotics and prebiotics) are relevant nutritional strategies for improving the intestinal homeostasis. Prebiotics are “selectively fermented ingredients resulting in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefits upon host health.”93 Most prebiotics are complex carbohydrates present in whole grains, fruits and vegetables or are produced industrially. These compounds are undigested and unabsorbed in the small intestine and reach the colon where their selective fermentation promotes changes in the composition and metabolic activity of the intestinal microbiota. Probiotics are defined as “live microorganisms, which when administered in adequate amounts confer health benefit to the host.”94 The use and scope of the term probiotic has been recently discussed by an expert panel from the International Scientific Association for Probiotics and Prebiotics.95 Probiotics may inhibit enteric pathogens, interact with the intestinal microbiota and modulate the immune system directly or through the modification of the gut microbiota. The most commonly used probiotics in foods are bifidobacteria and lactobacilli species. Some in vitro studies confirm the participation of potentially probiotic Bifidobacterium strains in cross-feeding interactions with intestinal microorganisms producing butyrate in the presence of prebiotic substrates, as inulin-type fructans and fructo-oligosaccharides.96-100
Regarding CDI, in a recent study Vincent et al.101 observed differences in the microbiota between elderly developing infection and those colonized by the microorganism but remaining healthy. Moreover, the authors identified potentially protective microorganisms. These sort of data suggest both, microbial markers for an increased risk of infection as well as targets of microbiota modulation for disease prevention in the elderly. To this regard, potentially probiotic strains and prebiotic substrates have been selected on the basis of their ability to counterbalance the specific microbiota alterations associated to old-age.102 Recent in vitro studies also demonstrated the ability of some B. longum and B. breve strains to inactivate the toxins released by C. difficile and to partially inhibit the growth of this pathogen, also reducing its toxicity in the presence of some prebiotic substrates.103,104
There is a growing interest of the food industry in the elderly population. Despite this, there is a scarcity of intervention studies demonstrating the effectiveness of specific prebiotic substrates for the elderly population, and no specific probiotic products are available in the market for old people. Several intervention studies with commercial strains from Lactobacillus and Bifidobacterium genera have been performed in the elderly population during the last 10 y. Only few of them were made with the aim of testing the effect of probiotics on the gut microbiota composition (Table 2) but mainly to assess the efficacy of these strains in gastrointestinal disorders associated with age, such as constipation, and diarrhea due to antibiotics consumption or associated with CDI.105 Some of these probiotics have shown to be efficient in promoting the increase of bifidobacteria or other microorganisms such as lactobacilli and enterococci, or to cause a decrease of certain opportunistic pathogens (i.e. C. difficile, Enterococcus faecium, Clostridium perfringens or Campylobacter) and enterobacteria. Regarding the prebiotic approach, the use of galacto-oligossaccharides has been successful in correcting microbiota changes and for improving the immunological state associated with the elderly106,107 (Table 2), however intervention studies with alternative prebiotics (pectin, xilo-oligosaccharides, malto-oligosaccharides, etc) are lacking. Synbiotics have been also recently tested to improve age-related changes in the gut microbiota, with some positive effects on specific intestinal microbial populations (Table 2). In spite of this, very few studies have addressed the influence on the metabolic functionality of the intestinal microbiota in elders (i.e., through the production of SCFA or organic acids); in addition, the low number of individuals recruited and the different specific geographical locations of individuals participating in interventions, mainly in the case of prebiotics and synbiotics, prevents the formulation of firm conclusions on the efficacy of these functional dietary ingredients in the elderly population. Therefore, there is still a need for a joint effort by clinicians and scientists to establish standardized, reproducible and comparable intervention protocols that would enable investigation into the efficacy of probiotics, prebiotics and adapted diets in different groups of the elderly population.
Table 2.
Intervention studies showing the impact of probiotics, prebiotics or synbiotics on the gut microbiota in elderly people. The general treatment performed (probiotics, prebiotics or synbiotics) is indicated with uppercase bold letters. D, day; w, week; m, month; y, year.
| Subjects, country, age (n) | Dietary intervention (period) | Main gut microbiota changes | Reference |
|---|---|---|---|
| PROBIOTICS | |||
| Healthy elderly, Finland, 76-92y (66) | Randomized, double blinded placebo controlled study: fermented oat drink with Bifidobacterium longum 46 and B. longum 2C (BIF) vs non fermented placebo oat drink (6 m) | BIF:↑bifidobacteria, Bifidobacterium catenulatum, Bifidobacterium bifidum, Bifidobacterium breve | 112 |
| Healthy elderly, Italy, 71-78y (32) | Randomized double-blinded placebo-controlled study: biscuit with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 (PRO) vs placebo biscuit (1 m) | PRO:↓ opportunistic pathogens: Clostridium cluster XI, Clostridium difficile and Clostridium perfringens, Enterococcus faecium and Campylobacter | 113 |
| Healthy elderly, Italy, France, Germany, 65-85y (62) | Open label, randomized, multicentre study: RISTOMED diet alone (RD) vs RD + probiotic VSL#3 (VSL) (8 w) | VSL:↑ bifidobacteria. | 114 |
| Healthy elderly (>60y) (80) | Randomized double-blinded placebo-controlled study: reconstituted skimmed milk with Bifidobacterium lactis HN019 at low dose (L), B. lactis medium dose (M), B. lactis high dose (H) vs placebo (4w and 2w wash out ) |
L, M, H:↑ bifidobacteria, lactobacilli, enterococci L, M, H:↓ enterobacteria |
115 |
| PREBIOTICS | |||
| Healthy elderly, England, (64-79y) (44) |
Randomized, double blinded placebo controlled, cross over: galacto-oligosaccharides mixture (BGOS) vs placebo (10w and 4w wash out ) |
BGOS:↑ bifidobacteria, lactobacilli, Clostridia Cluster XIVa ↓bacteroides, Clostridium histolyticum group, Escherichia coli, and Desulfovibrio spp |
116 |
| Healthy elderly, England, (>50y) (37) |
Randomized, double blinded placebo controlled, cross over: juice containing galacto-oligosaccharides (GOS) vs placebo juice (3w and 3w wash out ) | GOS:↑ bifidobacteria | 107 |
| Healthy elderly, England, (65-80y) (40) |
Randomized, double blinded placebo controlled, cross over: galacto-oligosaccharides mixture(BGOS) vs placebo (10w and 4w wash out ) |
BGOS:↑ bifidobacteria, bacteroides ↑ lactic acid |
106 |
| SYNBIOTICS | |||
| Healthy elderly, Scotland, (>62 y) (18) |
Double blinded placebo controlled: mixture of Bifidobacterium bifidum BB-02 + Bifidobacterium lactis BL-01 + inulin (BB+BL+IN) vs placebo (5w and 3w wash out ) | BB+BL+IN:↑ bifidobacteria, B. bifidum, lactobacilli | 117 |
| Healthy elderly, Finland, (>65y) (51) | Double blinded placebo controlled: mixture of Lactobacillus acidophilus NCFM+ lactitol (LB+L) vs placebo (4w and 3w wash out ) | LB+L:↑ bifidobacteria, lactobacilli | 118 |
| Healthy elderly, Scotland, (65-90y) (43) | Randomized, double blinded placebo controlled, cross over: mixture of Bifidobacterium longum + inulin (BL+IN) vs placebo (4w and 4 w washout) |
BL+IN:↑ bifidobacteria, phyla Actinobacteria and Firmicutes ↑butyrate ↓Proteobacteria |
119 |
Conclusions
Over the past 5 years, advancements in high throughput technologies and the associated reduction in costs has enabled investigations into the composition and functionality of complex microbial species across the lifespan. More studies are needed in elderly populations, particularly those with the most advanced ages (>90 years of age), to better understand how the microbiota shifts overtime and how it is associated with dietary changes and associated comorbidities. Understanding the mechanisms underlying the beneficial actions of prebiotics, prebiotics, as well as interactions between specific nutrients and the microbiota, is needed to provide the scientific support for the rational design of specific food products for the elderly. Ideally, it would be possible to assess the disease risk of specific susceptible subpopulations and to design cost-effective personalized treatments and products.
Abbreviations
- CDI
Clostridium difficile infection
- GIT
Gastrointestinal Tract
- NHANES
National Health and Nutrition Examination Survey
- NK
Natural Killer
- RDA
Recommended Dietary Allowances
- SCFA
Short Chain Fatty Acids
- TcdA
Toxin A produced by Clostridium difficile
- TcdB
Toxin B produced by Clostridium difficile
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Authors receive financial support from Plan Estatal de I+D (Spanish Ministry of Economy and Competitiveness) through the project AGL2013-43770-R, and from “Plan Regional de Investigación” of Principado de Asturias through the Grant GRUPIN14-043. Both projects receive cofounding from European Union FEDER funds. N. Salazar benefits from a post-doctoral “Clarín” contract (Marie Curie European Co-Fund Program) linked to the grant ACB14-08 co-funded by the Marie Curie European CoFund Program.
References
- [1].McLeod M, Breen L, Hamilton DL, Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology 2016; 17:497-510; PMID:26791164; http://dx.doi.org/ 10.1007/s10522-015-9631-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 2001; 292:1115-8; PMID:11352068; http://dx.doi.org/ 10.1126/science.1058709 [DOI] [PubMed] [Google Scholar]
- [3].Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, et al.. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 2014; 32:834-41; PMID:24997786; http://dx.doi.org/ 10.1038/nbt.2942 [DOI] [PubMed] [Google Scholar]
- [4].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al.. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59-65; PMID:20203603; http://dx.doi.org/ 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, Patel S, Cutting M, Madden T, Hamilton H, et al.. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J 2013; 27:1012-22; PMID:23165986; http://dx.doi.org/ 10.1096/fj.12-220806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jeffery IB, O'Toole PW. Diet-microbiota interactions and their implications for healthy living. Nutrients 2013; 5:234-52; PMID:23344252; http://dx.doi.org/ 10.3390/nu5010234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 2012; 79:763-72; PMID:22126419; http://dx.doi.org/ 10.1111/j.1574-6941.2011.01261.x [DOI] [PubMed] [Google Scholar]
- [8].Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et al.. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 2015; 26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003; 91:48-55; PMID:14599042 [DOI] [PubMed] [Google Scholar]
- [10].Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al.. Human gut microbiome viewed across age and geography. Nature 2012; 486:222-7; PMID:22699611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salazar N, Arboleya S, Valdés L, Stanton C, Ross P, Ruiz L, Gueimonde M, de los Reyes-Gavilán CG. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front Genet 2014; 5:406; PMID:25484891; http://dx.doi.org/ 10.3389/fgene.2014.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al.. Enterotypes of the human gut microbiome. Nature 2011; 473:174-80; PMID:21508958; http://dx.doi.org/ 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al.. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334:105-8; PMID:21885731; http://dx.doi.org/ 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI. Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl Environ Microbiol 2014; 80:1142-9; PMID:24296500; http://dx.doi.org/ 10.1128/AEM.03549-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559-63; PMID:24336217; http://dx.doi.org/ 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One 2012; 7:e34242; PMID:22719824; http://dx.doi.org/ 10.1371/journal.pone.0034242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, Huttenhower C, Ley RE. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol 2013; 9:e1002863; PMID:23326225; http://dx.doi.org/ 10.1371/journal.pcbi.1002863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci 2015; 16:7493-519; PMID:25849657; http://dx.doi.org/ 10.3390/ijms16047493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al.. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011; 108 Suppl 1:4586-91; PMID:20571116; http://dx.doi.org/ 10.1073/pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al.. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488:178-84; PMID:22797518 [DOI] [PubMed] [Google Scholar]
- [21].Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 2016; 16:90; PMID:27220822; http://dx.doi.org/ 10.1186/s12866-016-0708-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001; 48:198-205; PMID:11156640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, et al.. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 2010; 5:e10667; PMID:20498852; http://dx.doi.org/ 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salazar N, López P, Valdés L, Margolles A, Suárez A, Patterson AM, Cuervo A, de los Reyes-Gavilán CG, Ruas-Madiedo P, González S, et al.. Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J Am Coll Nutr 2013; 32:399-406; PMID:24606713; http://dx.doi.org/ 10.1080/07315724.2013.827047 [DOI] [PubMed] [Google Scholar]
- [25].Hayashi H, Sakamoto M, Kitahara M, Benno Y. Molecular analysis of fecal microbiota in elderly individuals using 16S rDNA library and T-RFLP. Microbiol Immunol 2003; 47:557-70; PMID:14524616; http://dx.doi.org/ 10.1111/j.1348-0421.2003.tb03418.x [DOI] [PubMed] [Google Scholar]
- [26].Bartosch S, Fite A, Macfarlane GT, McMurdo ME. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 2004; 70:3575-81; PMID:15184159; http://dx.doi.org/ 10.1128/AEM.70.6.3575-3581.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res Rev 2010; 9:107-16; PMID:19874918; http://dx.doi.org/ 10.1016/j.arr.2009.10.004 [DOI] [PubMed] [Google Scholar]
- [28].Woodmansey EJ. Intestinal bacteria and ageing. J Appl Microbiol 2007; 102:1178-86; PMID:17448153; http://dx.doi.org/ 10.1111/j.1365-2672.2007.03400.x [DOI] [PubMed] [Google Scholar]
- [29].Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr 2010; 103:227-34; ; http://dx.doi.org/ 10.1017/S0007114509991553 [DOI] [PubMed] [Google Scholar]
- [30].Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol 2004; 70:6113-22; PMID:15466557; http://dx.doi.org/ 10.1128/AEM.70.10.6113-6122.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O'Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med 2016; 8:8; PMID:26822992; http://dx.doi.org/ 10.1186/s13073-016-0262-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O'Toole PW, Jeffery IB. Gut microbiota and aging. Science 2015; 350:1214-5; PMID:26785481; http://dx.doi.org/ 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- [33].Candore G, Caruso C, Jirillo E, Magrone T, Vasto S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr Pharm Des 2010; 16:584-96; PMID:20388068; http://dx.doi.org/ 10.2174/138161210790883868 [DOI] [PubMed] [Google Scholar]
- [34].Vallejo AN. Immunological hurdles of ageing: indispensable research of the human model. Ageing Res Rev 2011; 10:315-8; PMID:21315185; http://dx.doi.org/ 10.1016/j.arr.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Müeller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, et al.. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 2006; 72:1027-33; PMID:16461645; http://dx.doi.org/ 10.1128/AEM.72.2.1027-1033.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Faris B, Blackmore A, Haboubi N. Review of medical and surgical management of Clostridium difficile infection. Tech Coloproctol 2010; 14:97-105; PMID:20454824; http://dx.doi.org/ 10.1007/s10151-010-0574-3 [DOI] [PubMed] [Google Scholar]
- [37].Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatric Med 2014; 30:79; PMID:24267604; http://dx.doi.org/ 10.1016/j.cger.2013.10.008 [DOI] [PubMed] [Google Scholar]
- [38].Rodriguez C, Taminiau B, Van Broeck J, Delmée M, Daube G. Clostridium difficile infection and intestinal microbiota interactions. Microb Pathog 2015; 89:201-9; PMID:26549493; http://dx.doi.org/ 10.1016/j.micpath.2015.10.018 [DOI] [PubMed] [Google Scholar]
- [39].Heinlen L, Ballard JD. Clostridium difficile infection. Am J Med Sci 2010; 340:247-52; PMID:20697257; http://dx.doi.org/ 10.1097/MAJ.0b013e3181e939d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martin JS, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol 2016; 13:206-16; PMID:26956066; http://dx.doi.org/ 10.1038/nrgastro.2016.25 [DOI] [PubMed] [Google Scholar]
- [41].Mizusawa M, Doron S, Gorbach S. Clostridium difficile diarrhea in the elderly: Current issues and management options. Drugs Aging 2015; 32:639-47; PMID:26233437; http://dx.doi.org/ 10.1007/s40266-015-0289-2 [DOI] [PubMed] [Google Scholar]
- [42].Yuille S, Mackay WG, Morrison DJ, Tedford MC. Optimising gut colonisation resistance against Clostridium difficile infection. Eur J Clin Microbiol Infect Dis 2015; 34:2161-6; PMID:26354525; http://dx.doi.org/ 10.1007/s10096-015-2479-6 [DOI] [PubMed] [Google Scholar]
- [43].Shields K, Araujo-Castillo RV, Theethira TG, Alonso CD, Kelly CP. Recurrent Clostridium difficile infection: From colonization to cure. Anaerobe 2015; 34:59-73; PMID:25930686; http://dx.doi.org/ 10.1016/j.anaerobe.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol 2016; 13:150-60; PMID:26860266; http://dx.doi.org/ 10.1038/nrgastro.2015.220 [DOI] [PubMed] [Google Scholar]
- [45].Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis (Lond) 2016; 48:587-92; PMID:27194400; http://dx.doi.org/ 10.1080/23744235.2016.1177199 [DOI] [PubMed] [Google Scholar]
- [46].Li YT, Cai HF, Wang ZH, Xu J, Fang JY. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther 2016; 43:445-57; PMID:26662643; http://dx.doi.org/ 10.1111/apt.13492 [DOI] [PubMed] [Google Scholar]
- [47].Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013; 1:3; PMID:24467987; http://dx.doi.org/ 10.1186/2049-2618-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rowe JW. The new gerontology. Science 1997; 278:367; PMID:9381128; http://dx.doi.org/ 10.1126/science.278.5337.367 [DOI] [PubMed] [Google Scholar]
- [49].Troen BR. The biology of aging. Mt Sinai J Med 2003; 70:3-22; PMID:12516005 [PubMed] [Google Scholar]
- [50].Mazari L, Lesourd BM. Nutritional influences on immune response in healthy aged persons. Mech Ageing Dev 1998; 104:25-40; PMID:9751430; http://dx.doi.org/ 10.1016/S0047-6374(98)00047-5 [DOI] [PubMed] [Google Scholar]
- [51].Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, Deng Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol 2007; 42:719-32; PMID:17368996; http://dx.doi.org/ 10.1016/j.exger.2007.01.009 [DOI] [PubMed] [Google Scholar]
- [52].Candore G, Balistreri CR, Colonna-Romano G, Grimaldi MP, Lio D, Listi F, Scola L, Vasto S, Caruso C. Immunosenescence and anti-immunosenescence therapies: the case of probiotics. Rejuvenation Res 2008; 11:425-32; PMID:18442326; http://dx.doi.org/ 10.1089/rej.2008.0662 [DOI] [PubMed] [Google Scholar]
- [53].Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede JL. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999; 15:116-22; PMID:9990575; http://dx.doi.org/ 10.1016/S0899-9007(98)00171-3 [DOI] [PubMed] [Google Scholar]
- [54].Deleidi M, Jaggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci 2015; 9:172; PMID:26089771; http://dx.doi.org/ 10.3389/fnins.2015.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature–What does it tell us? J Nutr Health Aging 2006; 10:466-85; discussion 85-7; PMID:17183419 [PubMed] [Google Scholar]
- [56].Anderton BH. Ageing of the brain. Mech Ageing Dev 2002; 123:811-7; PMID:11869738; http://dx.doi.org/ 10.1016/S0047-6374(01)00426-2 [DOI] [PubMed] [Google Scholar]
- [57].Ritz P. Physiology of aging with respect to gastrointestinal, circulatory and immune system changes and their significance for energy and protein metabolism. Eur J Clin Nutr 2000; 54 Suppl 3:S21-5; PMID:11041071; http://dx.doi.org/ 10.1038/sj.ejcn.1601021 [DOI] [PubMed] [Google Scholar]
- [58].Culross B. Gerontology update, Nutrition:meeting the needs of the elderly. ARN Network 2008 [Google Scholar]
- [59].Remond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron MA, Dos Santos CN, Walther B, Bordoni A, Dupont D, et al.. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015; 6:13858-98; PMID:26091351; http://dx.doi.org/ 10.18632/oncotarget.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Volkert D. Malnutrition in older adults - urgent need for action: a plea for improving the nutritional situation of older adults. Gerontology 2013; 59:328-33; PMID:23406648; http://dx.doi.org/ 10.1159/000346142 [DOI] [PubMed] [Google Scholar]
- [61].Amarya S, Sinhg H, Sabharwalh M. Changes during ageind and their association with malnutrition. J Clin Gerontol Geriatr 2015; 6:78-84; http://dx.doi.org/ 10.1016/j.jcgg.2015.05.003 [DOI] [Google Scholar]
- [62].Soderstrom L, Thors Adolfsson E, Rosenblad A, Frid H, Saletti A, Bergkvist L. Mealtime habits and meal provision are associated with malnutrition among elderly patients admitted to hospital. Clin Nutr 2013; 32:281-8; PMID:22898590; http://dx.doi.org/ 10.1016/j.clnu.2012.07.013 [DOI] [PubMed] [Google Scholar]
- [63].Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr 2000; 54 Suppl 3:S48-53; http://dx.doi.org/ 10.1038/sj.ejcn.1601025 [DOI] [PubMed] [Google Scholar]
- [64].Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22:415-21; PMID:12880610; http://dx.doi.org/ 10.1016/S0261-5614(03)00098-0 [DOI] [PubMed] [Google Scholar]
- [65].Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marin-Ciancas F, Malafarina V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015; 81:17-27; PMID:25782627; http://dx.doi.org/ 10.1016/j.maturitas.2015.02.009 [DOI] [PubMed] [Google Scholar]
- [66].Tabloski P. Nutrition and aging In: Tabloski P, ed. Gerontological Nursing. Up Saddle, NJ: Prentice Hall, 2006; 110-46 [Google Scholar]
- [67].Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony PS, Charlton KE, Maggio M, et al.. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010; 58:1734-8; PMID:20863332; http://dx.doi.org/ 10.1111/j.1532-5415.2010.03016.x [DOI] [PubMed] [Google Scholar]
- [68].Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008; 87:1554S-7S; PMID:18469286 [DOI] [PubMed] [Google Scholar]
- [69].Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008; 87:150-5; PMID:18175749 [DOI] [PubMed] [Google Scholar]
- [70].Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008; 11:693-700; PMID:18827572; http://dx.doi.org/ 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mackowiak K, Torlinska-Walkowiak N, Torlinska B. Dietary fibre as an important constituent of the diet. Postepy Hig Med Dosw (Online) 2016; 70:104-9; PMID:26943307; http://dx.doi.org/ 10.5604/17322693.1195842 [DOI] [PubMed] [Google Scholar]
- [72].Pray LA. Institute of Medicine (US). Planning Committee for Food Supply and Aging Populations, National Academies Press (US) Providing healthy and safe foods as we age: workshop summary. Washington, DC: National Academies Press, 2010 [PubMed] [Google Scholar]
- [73].Dao MC, Meydani SN. Micronutrient status, immune response and infectious disease in elderly of less developed countries. Sight Life Mag 2009; 3:6-15; PMID:22540112 [PMC free article] [PubMed] [Google Scholar]
- [74].Rohrig G. Anemia in the frail, elderly patient. Clin Interv Aging 2016; 11:319-26; PMID:27051279; http://dx.doi.org/ 10.2147/CIA.S90727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001; 22:477-501; PMID:11493580; http://dx.doi.org/ 10.1210/edrv.22.4.0437 [DOI] [PubMed] [Google Scholar]
- [76].Miles LM, Mills K, Clarke R, Dangour AD. Is there an association of vitamin B12 status with neurological function in older people? A systematic review. Br J Nutr 2015; 114:503-8; PMID:26202329; http://dx.doi.org/ 10.1017/S0007114515002226 [DOI] [PubMed] [Google Scholar]
- [77].Artaza-Artabe I, Sáez-López P, Sánchez-Hernández N, Fernández-Gutierrez N, Malafarina V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016; 93:89-99; PMID:27125943 [DOI] [PubMed] [Google Scholar]
- [78].Delafuente JC. Nutrients and immune responses. Rheum Dis Clin North Am 1991; 17:203-12; PMID:1907394 [PubMed] [Google Scholar]
- [79].Dasgupta M, Sharkey JR, Wu G. Inadequate intakes of indispensable amino acids among homebound older adults. J Nutr Elder 2005; 24:85-99; PMID:15911526; http://dx.doi.org/ 10.1300/J052v24n03_07 [DOI] [PubMed] [Google Scholar]
- [80].Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol 2002; 71:16-32; PMID:11781377 [PubMed] [Google Scholar]
- [81].Camina-Martín MA, de Mateo-Silleras B, Malafarina V, López-Mongil R, Nino-Martin V, López-Trigo JA, Redondo-del-Rio MP. Nutritional status assessment in geriatrics: Consensus declaration by the Spanish Society of Geriatrics and Gerontology Nutrition Work Group. Maturitas 2015; 81:414-9; http://dx.doi.org/ 10.1016/j.maturitas.2015.04.018 [DOI] [PubMed] [Google Scholar]
- [82].Gupta C, Prakash D. Nutraceuticals for geriatrics. J Tradit Complement Med 2015; 5:5-14; PMID:26151003; http://dx.doi.org/ 10.1016/j.jtcme.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, Schmidt I, Jahreis G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy 2008; 38:93-102; PMID:18028460 [DOI] [PubMed] [Google Scholar]
- [84].Jeffery IB, Lynch DB, O'Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J 2016; 10:170-82; PMID:26090993; http://dx.doi.org/ 10.1038/ismej.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 2013; 69:11-20; PMID:23079287; http://dx.doi.org/ 10.1016/j.phrs.2012.10.005 [DOI] [PubMed] [Google Scholar]
- [86].Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol 2014; 29C:38-42; http://dx.doi.org/ 10.1016/j.coi.2014.03.008 [DOI] [PubMed] [Google Scholar]
- [87].Milani C, Ticinesi A, Gerritsen J, Nouvenne A, Lugli GA, Mancabelli L, Turroni F, Duranti S, Mangifesta M, Viappiani A, et al.. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep 2016; 6:25945; PMID:27166072; http://dx.doi.org/ 10.1038/srep25945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].O'Sullivan O, Coakley M, Lakshminarayanan B, Conde S, Claesson MJ, Cusack S, Fitzgerald AP, O'Toole PW, Stanton C, Ross RP. Alterations in intestinal microbiota of elderly Irish subjects post-antibiotic therapy. J Antimicrob Chemother 2013; 68:214-21; PMID:22949626; http://dx.doi.org/ 10.1093/jac/dks348 [DOI] [PubMed] [Google Scholar]
- [89].González S, López P, Margolles A, Suárez A, Patterson AM, Cuervo A, de los Reyes-Gavilán CG, Gueimonde M. Fatty acids intake and immune parameters in the elderly. Nutr Hosp 2013; 28:474-8 [DOI] [PubMed] [Google Scholar]
- [90].McCarty MF, DiNicolantonio JJ. An increased need for dietary cysteine in support of glutathione synthesis may underlie the increased risk for mortality associated with low protein intake in the elderly. Age (Dordr) 2015; 37:96; PMID:26362762; http://dx.doi.org/ 10.1007/s11357-015-9823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009; 294:1-8; PMID:19222573; http://dx.doi.org/ 10.1111/j.1574-6968.2009.01514.x [DOI] [PubMed] [Google Scholar]
- [92].Rios-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016; 7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al.. Prebiotic effects: metabolic and health benefits. Br J Nutr 2010; 104 Suppl 2:S1-63; PMID:20920376; http://dx.doi.org/ 10.1017/S0007114510003363 [DOI] [PubMed] [Google Scholar]
- [94].FAO/WHO Probiotics in Food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition paper 2006; 85 [Google Scholar]
- [95].Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al.. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11:506-14; PMID:24912386; http://dx.doi.org/ 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- [96].Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr 2004; 91:915-23; PMID:15182395; http://dx.doi.org/ 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- [97].Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 2006; 72:3593-9; PMID:16672507; http://dx.doi.org/ 10.1128/AEM.72.5.3593-3599.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rios-Covián D, Gueimonde M, Duncan SH, Flint HJ, de Los Reyes-Gavilán CG. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett 2015; 362(21). PMID:26420851; http://dx.doi.org/27233082 10.1093/femsle/fnv176 [DOI] [PubMed] [Google Scholar]
- [99].Moens F, Weckx S, De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int J Food Microbiol 2016; 231:76-85; PMID:27233082; http://dx.doi.org/ 10.1016/j.ijfoodmicro.2016.05.015 [DOI] [PubMed] [Google Scholar]
- [100].Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 2006; 72:7835-41; PMID:17056678; http://dx.doi.org/ 10.1128/AEM.01296-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vincent C, Miller MA, Edens TJ, Mehrotra S, Dewar K, Manges AR. Bloom and bust: intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 2016; 4:12; PMID:26975510; http://dx.doi.org/ 10.1186/s40168-016-0156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Valdés L, Salazar N, González S, Arboleya S, Rios-Covián D, Genovés S, Ramón D, de los Reyes-Gavilán C, Ruas-Madiedo P, Gueimonde M. Selection of potential probiotic bifidobacteria and prebiotics for elderly by in vitro faecal batch cultures. Eur Food Res Technol 2016. in press; http://dx.doi.org/ 10.1007/s00217-016-2732-y [DOI] [Google Scholar]
- [103].Valdés-Varela L, Alonso-Guervos M, García-Suarez O, Gueimonde M, Ruas-Madiedo P. Screening of Bifidobacteria and Lactobacilli able to antagonize the cytotoxic effect of Clostridium difficile upon intestinal epithelial HT29 Monolayer. Front Microbiol 2016; 7:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Valdés-Varela L, Hernández-Barranco AM, Ruas-Madiedo P, Gueimonde M. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front Microbiol 2016; 7:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rondanelli M, Giacosa A, Faliva MA, Perna S, Allieri F, Castellazzi AM. Review on microbiota and effectiveness of probiotics use in older. World J Clin Cases 2015; 3:156-62; PMID:25685762; http://dx.doi.org/ 10.12998/wjcc.v3.i2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, Gibson GR. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr 2015; 114:586-95; PMID:26218845; http://dx.doi.org/ 10.1017/S0007114515001889 [DOI] [PubMed] [Google Scholar]
- [107].Walton GE, van den Heuvel EG, Kosters MH, Rastall RA, Tuohy KM, Gibson GR. A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br J Nutr 2012; 107:1466-75; PMID:21910949; http://dx.doi.org/ 10.1017/S0007114511004697 [DOI] [PubMed] [Google Scholar]
- [108].Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 2009; 9:123; PMID:19508720; http://dx.doi.org/ 10.1186/1471-2180-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zwielehner J, Liszt K, Handschur M, Lassl C, Lapin A, Haslberger AG. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol 2009; 44:440-6; PMID:19376217; http://dx.doi.org/ 10.1016/j.exger.2009.04.002 [DOI] [PubMed] [Google Scholar]
- [110].Wang F, Yu T, Huang G, Cai D, Liang X, Su H, Zhu Z, Li D, Yang Y, Shen P, et al.. Gut microbiota community and its assembly associated with age and diet in Chinese Centenarians. J Microbiol Biotechnol 2015; 25:1195-204; PMID:25839332; http://dx.doi.org/ 10.4014/jmb.1410.10014 [DOI] [PubMed] [Google Scholar]
- [111].Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al.. Gut Microbiota and Extreme Longevity. Curr Biol 2016; 26:1480-5; PMID:27185560; http://dx.doi.org/ 10.1016/j.cub.2016.04.016 [DOI] [PubMed] [Google Scholar]
- [112].Lahtinen SJ, Tammela L, Korpela J, Parhiala R, Ahokoski H, Mykkanen H, Salminen SJ. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Dordr) 2009; 31:59-66; PMID:19234769; http://dx.doi.org/ 10.1007/s11357-008-9081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rampelli S, Candela M, Severgnini M, Biagi E, Turroni S, Roselli M, Carnevali P, Donini L, Brigidi P. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J Nutr Health Aging 2013; 17:166-72; PMID:23364497; http://dx.doi.org/ 10.1007/s12603-012-0372-x [DOI] [PubMed] [Google Scholar]
- [114].Valentini L, Pinto A, Bourdel-Marchasson I, Ostan R, Brigidi P, Turroni S, Hrelia S, Hrelia P, Bereswill S, Fischer A, et al.. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota - The “RISTOMED project:” Randomized controlled trial in healthy older people. Clin Nutr 2015; 34:593-602; PMID:25453395; http://dx.doi.org/ 10.1016/j.clnu.2014.09.023 [DOI] [PubMed] [Google Scholar]
- [115].Ahmed M, Prasad J, Gill H, Stevenson L, Gopal P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J Nutr Health Aging 2007; 11:26-31; PMID:17315077 [PubMed] [Google Scholar]
- [116].Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008; 88:1438-46; PMID:18996881 [DOI] [PubMed] [Google Scholar]
- [117].Bartosch S, Woodmansey EJ, Paterson JC, McMurdo ME, Macfarlane GT. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin Infect Dis 2005; 40:28-37; PMID:15614689; http://dx.doi.org/ 10.1086/426027 [DOI] [PubMed] [Google Scholar]
- [118].Björklund M, Ouwehand AC, Forssten SD, Nikkila J, Tiihonen K, Rautonen N, Lahtinen SJ. Gut microbiota of healthy elderly NSAID users is selectively modified with the administration of Lactobacillus acidophilus NCFM and lactitol. Age (Dordr) 2012; 34:987-99; PMID:21853265; http://dx.doi.org/ 10.1007/s11357-011-9294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther 2013; 38:804-16; PMID:23957631; http://dx.doi.org/ 10.1111/apt.12453 [DOI] [PubMed] [Google Scholar]