Abstract
The AMP-activated protein kinase (AMPK) is activated by phosphorylation at Thr172, either by the tumor suppressor kinase LKB1 or by an alternate pathway involving the Ca2+/calmodulin-dependent kinase, CAMKK2. Increases in AMP:ATP and ADP:ATP ratios, signifying energy deficit, promote allosteric activation and net Thr172 phosphorylation mediated by LKB1, so that the LKB1-AMPK pathway acts as an energy sensor. Many tumor cells carry loss-of-function mutations in the STK11 gene encoding LKB1, but LKB1 re-expression in these cells causes cell cycle arrest. Therefore, it was investigated as to whether arrest by LKB1 is caused by activation of AMPK or of one of the AMPK-related kinases, which are also dependent on LKB1 but are not activated by CAMKK2. In three LKB1-null tumor cell lines, treatment with the Ca2+ ionophore A23187 caused a G1-arrest that correlated with AMPK activation and Thr172 phosphorylation. In G361 cells, expression of a truncated, CAMKK2 mutant also caused G1-arrest similar to that caused by expression of LKB1, while expression of a dominant negative AMPK mutant, or a double knockout of both AMPK-α subunits, also prevented the cell cycle arrest caused by A23187. These mechanistic findings confirm that AMPK activation triggers cell cycle arrest, and also suggest that the rapid proliferation of LKB1-null tumor cells is due to lack of the restraining influence of AMPK. However, cell cycle arrest can be restored by re-expressing LKB1 or a constitutively active CAMKK2, or by pharmacological agents that increase intracellular Ca2+ and thus activate endogenous CAMKK2.
Implications
Evidence here reveals that the rapid growth and proliferation of cancer cells lacking the tumor suppressor LKB1 is due to reduced activity of AMPK, and suggests a therapeutic approach by which this block might be circumvented.
Keywords: AMP-activated protein kinase, AMPK, cell cycle, calmodulin-dependent kinase kinase, CAMKK2
Introduction
The AMP-activated protein kinase (AMPK) is an energy sensor involved in regulating energy balance at both the cellular and the whole body levels (1–3). The kinase occurs as heterotrimeric complexes composed of a catalytic α subunit encoded by one of two genes (PRKAA1, PRKAA2) and regulatory β and γ subunits also encoded by multiple genes (PRKAB1-2; PRKAG1-3). All AMPK heterotrimers are activated by phosphorylation of a critical threonine residue (Thr172) within the α subunit kinase domain by upstream kinases (4). Metabolic stresses that either inhibit ATP synthesis or stimulate ATP consumption cause increases in the cellular ADP:ATP ratio, which are amplified by adenylate kinase into larger increases in AMP:ATP ratio (5). AMP, ADP and ATP bind antagonistically at up to three sites on the γ subunit (6–8), with binding of AMP causing conformational changes that have three effects, all antagonized by ATP (5): (i) promoting Thr172 phosphorylation; (ii) inhibiting Thr172 dephosphorylation; (iii) allosteric activation. Of these effects, the first two may also be triggered by ADP binding, but the third is specific to AMP (5, 9–11). The three effects of AMP/ADP provide a sensitive switch mechanism that produces a large activation of AMPK in response to small increases in the cellular AMP:ATP and/or ADP:ATP ratios. Activation of AMPK during energy stress then acts to restore energy homeostasis by stimulating catabolic pathways that generate ATP, while inhibiting ATP-consuming processes such as lipid, polysaccharide, ribosomal RNA and protein biosynthesis, and hence cell growth (1–3). Pharmacological activation of AMPK also triggers a G1 cell cycle arrest that has been proposed to be due to increased expression of one or more inhibitors of the G1-S phase transition, i.e. CDKN1A (p21waf1) and CDKN1B (p27kip1) (12–14).
The major upstream kinase phosphorylating Thr172 on AMPK was identified in 2003 as a complex containing the protein kinase LKB1 (STK11) (15–17), which had been previously identified genetically as a tumor suppressor (18). The finding that LKB1 activated a downstream kinase, AMPK, that inhibited cell growth and proliferation suggested that its tumor suppressor effects might be mediated by AMPK. However, LKB1 was subsequently shown also to act upstream of a family of twelve other AMPK-related kinases (ARKs) (19), introducing the possibility that one or more of the latter could mediate some of its tumor suppressor effects.
Many human tumor cell lines, such as HeLa, A549 and G361 cells (originally derived from cervical, lung and skin cancers, respectively) carry deletions or loss-of-function mutations in STK11, the gene encoding LKB1. STK11 also carries mono- or bi-allelic mutations in up to 30% of non-small cell lung cancers (20, 21), 20% of cervical cancers (22), and 10% of cutaneous melanomas (23). G361 cells proliferate rapidly, but re-expression of LKB1 causes a marked inhibition of proliferation (24) and cell cycle arrest in G1 phase (25). Although HeLa cells fail to express LKB1, Thr172 still becomes phosphorylated in response to treatment with the Ca2+ ionophore, A23187 (26), which led to the discovery that Ca2+- and calmodulin dependent protein kinase kinases (especially CAMKK2) act as alternate upstream kinases phosphorylating Thr172 (26–28). The CaMKK-AMPK pathway is triggered by a rise in cytosolic Ca2+ without any requirement for an increase in AMP, and is responsible for AMPK activation in response to many hormones and other extracellular agonists (29–32).
In this paper, we have addressed the question as to whether the inhibitory effects on cell proliferation following re-expression of LKB1 in LKB1-null tumor cell lines are mediated by AMPK, or by one or more of the ARKs. We have made use of previous findings that, while AMPK can be activated either by LKB1 or by CAMKK2, the ARKs are only phosphorylated and activated by LKB1 (33). Our results suggest that cell cycle arrest induced by Ca2+, or by expression of LKB1 or an activated CAMKK2 in these cells, is mediated entirely by AMPK rather than by an AMPK-related kinase. They also suggest that agonists that increase cytosolic Ca2+ might represent a novel therapy to arrest growth of tumors in which LKB1 has been inactivated.
Experimental
Materials
A23187, nocodazole, propidium iodide and sheep pre-immune immunoglobulin were from Sigma, and RNase from Qiagen.
Plasmids
Plasmids encoding GFP-LKB1, FLAG-STRADα (STRADA) and myc-MO25α (CAB39) were described previously (25, 34). To generate GFP-CAMKK2, PCR was performed with the following primers: 5′-CGCTCGAGCGGCTCATCATGTGTCTAGCCA-3′ and 5′-CGGGGTACCCCGCAAGAGCAGTTCCTCCTCCCC-3′ using a plasmid encoding human CAMKK2 (25) as template. The resulting PCR product was inserted into the XhoI-KpnI restriction sites of pEGFP-C2. The C-terminal truncation of CAMKK2 (CA-CAMKK2) was generated using site-directed mutagenesis to insert a stop codon after residue 471. The kinase inactive form (D330A) was generated using the Quikchange II site-directed mutagenesis kit (Stratagene). Positive clones were verified by DNA sequencing. DNA sequencing was performed using Applied Biosystems Big-Dye version 3.1 chemistry on an Applied Biosystems model 3730 automated capillary DNA sequencer.
Antibodies
Antibodies against the phosphorylated forms of AMPK (pT172) and Raptor (pS792), and against total CDKN1A, CDKN1B and Raptor, were from Cell Signaling, against actin and the FLAG epitope from Sigma-Aldrich, and against GFP from Roche. Antibodies against AMPK-α1 and -α2, the phosphorylated form of acetyl-CoA carboxylase (pACC), ACACA and CAMKK2 were described previously (26, 35). Secondary antibodies were from Li-Cor Biosciences.
Cell culture
All cell lines were from the European Collection of Cell Cultures (ECACC), and were re-validated by STR profiling (Public Health England, certificate dated 08/14/2015). G361 cells were cultured in McCoy’s 5A medium containing 1% (v/v) glutamine, 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin. A549 and HeLa cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin, and 1% (v/v) non-essential amino acids for A549 cells. G361 cells were transfected with the indicated GFP construct using Effectene transfection reagent (Qiagen) according to manufacturer’s instructions. Cells were cultured for a further 36 hr and were left untreated, or were treated with 10 µM A23187 as indicated. For dose response experiments, cells were treated with the indicated concentrations of A23187 for 20-22 hours.
Construction of G361 cells expressing dominant negative AMPK mutant
G361 cells were transfected using Effectene (Qiagen) with pFRT/lacZeo (Life Technologies). Media containing 100 µg/ml zeocin was added 48hr after transfection, and media were replaced every 3 days until individual foci could be selected and expanded. Integration of a single Flp Recombinase Target (FRT) site was verified using Southern blotting, and the FRT line expressing the highest level of β-galactosidase was selected.
DNAs encoding FLAG-tagged human AMPK-α2 (PRKAA2), both wild type and D157A mutant, were amplified and cloned into the FlpIn™ pcDNA5 FRT vector (Life Technologies). The resulting plasmid and DNA encoding FLP recombinase (Life Technologies), at a ratio of 1:9, were transfected using Effectene (Qiagen) into the G361 FRT cells cultured without zeocin. Fresh medium was added to the cells 24 hr after transfection, and medium containing 50 µg/ml hygromycin B added 48 hr after transfection. Medium was replaced every 3 days until foci could be identified, and individual foci were then selected and expanded. The expression of AMPK-α2 was confirmed by Western blotting using anti-FLAG antibodies.
Construction of G361 cells with double knockout of AMPK-α1 and –α2
Knockout of AMPK-α1 and -α2 (PRKAA1 and PRKAA2) in G361 cells was carried out using the CRISPR-Cas9 method (36). Using the database of potential CRISPR sites provided targeting oligonucleotides were chosen and a cloning cacc tag added, as follows: (i) caccGAGTCTGCGCATGGCGCTGC (targets α1 amino acids 1-4, exon1); (ii) caccGAAGATCGGCCACTACATTC (targets α1 amino acids 23-29, exon1); (iii) caccGAGGCCGCGCGCGCCGAAGA (targets α2 intron and ATG start, exon1); (iv) caccGAAGCAGAAGCACGACGGGC (targets α2 intron and ATG start, exon1). These oligonucleotides were annealed to their complements containing the cloning tag aaac, and inserted into the back-to-back BbsI restriction sites of pSpCas9(BB)-2A-Puro (PX459) and pSpCas9(BB)-2A-GFP (PX458) (Addgene plasmids #48139 and #48138). G361 cells (≈1.5 x 106 per well of a 6 well plate) were transfected with 2.5 µg of plasmid DNA, consisting of equal amounts of GFP vector containing oligonucleotides 1 and 2, and Puro vector containing oligonucleotides 3 and 4, for α1 and α2 respectively. After 24 hr, cells were trypsinized and plated into 150 mm plates containing DMEM medium supplemented with 10% fetal calf serum (FCS) and 0.4 mg/ml puromycin. The medium was changed to DMEM plus 10% FCS after two days, and colonies were allowed to form over the course of 7 days. Individual colonies were harvested and amplified before screening for the presence of AMPK-α1 or -α2 by Western blotting. Cells that proved negative for AMPK-α expression were further analysed by immunoprecipitation and kinase assay to confirm the absence of AMPK catalytic activity.
Kinase assays
Endogenous AMPK from G361, A549 and HeLa cells were assayed as described previously (37, 38) using the AMARA peptide (39) as substrate.
Immunoprecipitation of CAMKK2
G361, A549 or HeLa cell lysates (0.75 mg) were incubated at 4°C for 2 hr on a roller mixer with 5 μl of Protein G-Sepharose conjugated to 5 μg of CAMKK2 antibodies or pre-immune sheep IgG. After extensive washing, the immunoprecipitates were heated in SDS-PAGE sample buffer, resolved by SDS-PAGE and blots probed with the indicated antibodies.
Cell cycle analysis
Cell cycle analysis of G361 cells transfected with the indicated GFP construct was performed as described (25). For calcium ionophore experiments G361, A549 and HeLa cells were treated with the indicated concentration of A23187 for 20-22 hours followed by treatment with 70 ng/ml (G361, A549) or 30 ng/ml (HeLa) nocodazole for 18 hr. Cells were fixed, stained and analyzed as described (25).
Flow cytometry
Cells were harvested in 1% paraformaldehyde, transferred into FACS tubes and incubated at 37 °C for 15 min. They were then washed in phosphate-buffered saline (PBS) and fixed in 70% ethanol for 10 min before further washing in PBS containing 1% FBS. Cell number was adjusted to ≈1 x 106 and the cells were incubated with anti-p21 –Alexa647 (1:100 dilution) in PBS-FBS for 30 min. The cells were then washed in 2 ml PBS-FBS and resuspended in 0.5 ml PBS-FBS containing 10 μg/ml 4',6-diamidino-2-phenylindole (DAPI).
Other analytical procedures
SDS-PAGE was performed using precast Bis-Tris 4–12% gradient polyacrylamide gels in the MOPS buffer system (Invitrogen). Proteins were transferred to nitrocellulose membranes (BioRad) using the Xcell II Blot Module (Invitrogen). Membranes were blocked for 1 hr in Tris-buffered saline (TBS) containing 5% (w/v) non-fat dried skimmed milk. The membranes were probed with appropriate antibody (0.1–1 µg/ml) in TBS-Tween and 2% (w/v) non-fat dried skimmed milk. Detection was performed using secondary antibody (1 μg/ml) coupled to IR 680 or IR 800 dye, and the membranes were scanned using the Li-Cor Odyssey IR imager. Protein concentrations were determined by Coomassie Blue binding with bovine serum albumin as standard (40).
Statistical analysis
Significances of differences were estimated with GraphPad Prism 6 for Mac OSX, using 1-way or 2-way ANOVA as appropriate, and (unless stated otherwise) Sidak’s multiple comparison test. Numbers of replicates (n) refer to biological replicates, i.e. the number of independent cell cultures analyzed.
Results
CAMKK2 is expressed in G361 cells and Ca2+ ionophore caused a cell cycle arrest
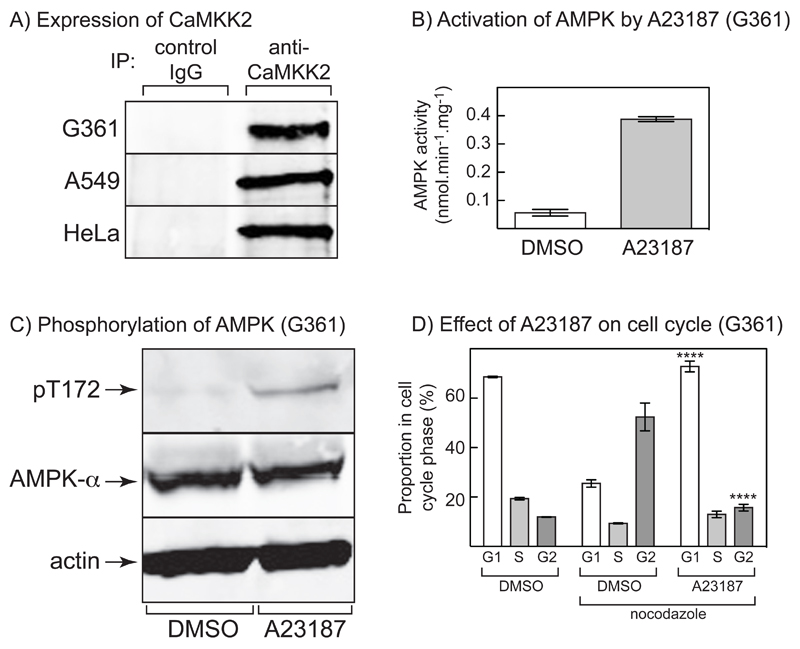
We first assessed whether CAMKK2 is expressed in the LKB1-deficient G361 and A549 cells, as already shown for HeLa cells (26). Utilizing an antipeptide antibody, the signals obtained by Western blotting in total cell lysates were indistinct, but clear bands with the expected mobility (corresponding to the expected mass of 65 kDa) were obtained by Western blotting of proteins immunoprecipitated from all three cell lines using the same antibody, but not control immunoglobulin (Fig. 1A). To test whether activation of AMPK via the CAMKK2 pathway in cells lacking LKB1 would cause a cell cycle arrest, we treated G361 cells for 20 hr with the Ca2+ ionophore A23187. This caused a 7-fold increase in AMPK activity that was associated with increased phosphorylation of Thr172 (Fig. 1B, 1C). Next, proliferating G361 cells were fixed, stained with propidium iodide, and analysed by flow cytometry to assess cell cycle distribution (Fig. 1D). The majority of unsynchronized cells (69%) were in G1 phase, with only small proportions (19% and 12%) in S and G2/M. To assess whether the Ca2+ ionophore caused a G1 arrest, cells were treated with nocodazole so that most cells that had already traversed the G1/S boundary would arrest at the subsequent G2/M transition. As expected, after nocodazole treatment a much larger proportion of the cells (52 ± 3%, mean ± SEM, n = 3) had a G2/M DNA content, with only 25 ± 1% (n = 3) in G1 phase. However, if the cells were treated with A23187 prior to addition of nocodazole, a much higher proportion of cells (73 ± 1%, n = 3) remained in G1 and a lower proportion (16 ± 1%, n = 3) in G2/M. This demonstrates that the Ca2+ ionophore was causing a G1 cell cycle arrest, concomitant with AMPK activation.
Figure 1. Expression of CAMKK2 in LKB1-deficient tumor cells, and AMPK activation and cell cycle arrest in G361 melanoma cells.
(A) Extracts of G361, A549 and HeLa cells were immunoprecipitated with anti-CAMKK2 antibody or control immunoglobulin (IgG), and the precipitates analyzed by Western blotting using the same anti-CAMKK2 antibody. (B) AMPK activity in immunoprecipitates from G361 cells treated with 0.3 μM A23187 for 20 hr; results are mean ± SD, n = 2. (C) Western blotting using anti-pT172, anti-AMPK-α and anti-actin antibodies in extracts of G361 cells treated with A23187 as in (B). (D) G361 cells were treated with vehicle (DMSO) or A23187 (0.3 μM) for 20 hr with (where indicated) nocodazole (70 ng.ml-1) added for a further 18 hrs. The cells were then fixed, stained with propidium iodide and DNA content analyzed by flow cytometry. Bars show the percentages of cells with G1, S and G2/M phase DNA content (mean ± SD, n = 3); significant differences from DMSO control (with nocodazole) by 2-way ANOVA are shown, ****p<0.0001. Similar results were obtained in three identical experiments.
Ca2+ ionophore also caused cell cycle arrest in A549 and HeLa cells
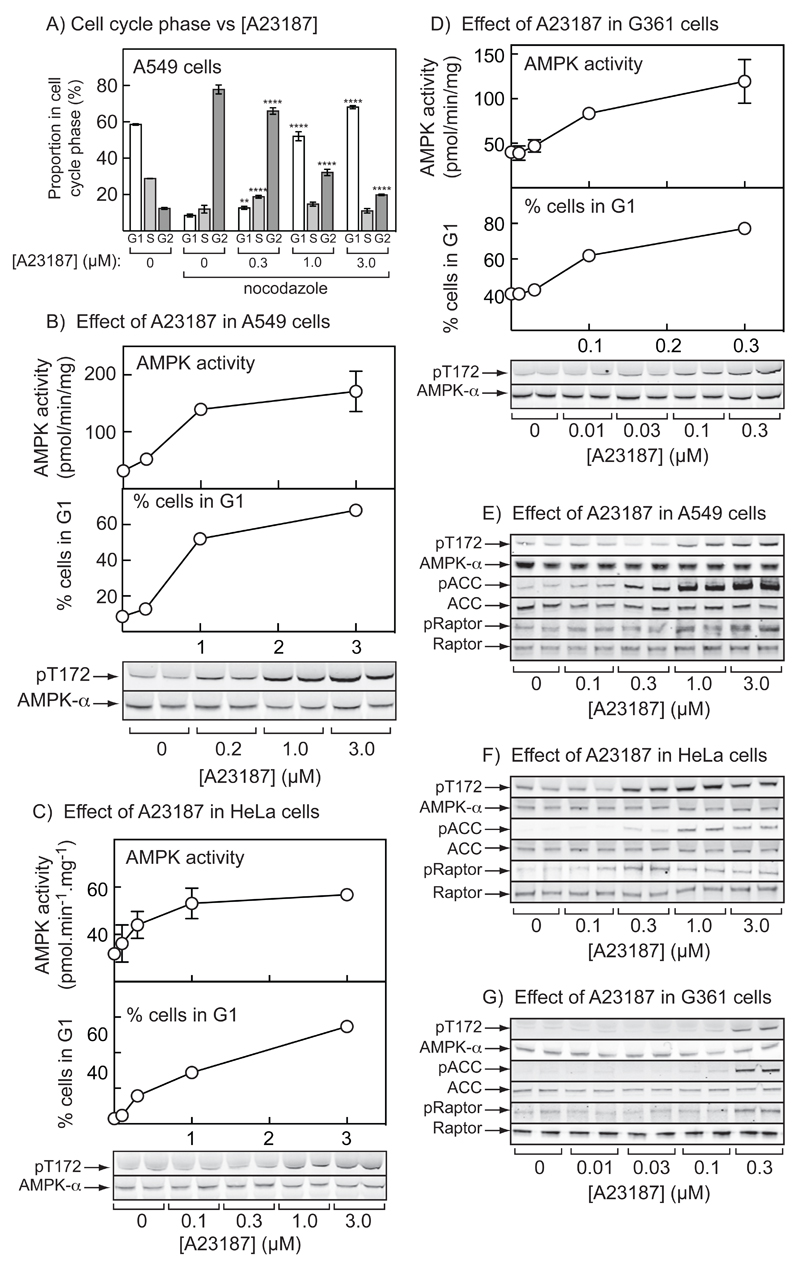
We next tested whether A23187 would cause cell cycle arrest in two other LKB1-null cell lines, i.e. A549 and HeLa cells. Cell viability measured by MTT assay was unaffected by identical incubations of G361 or A549 cells with A23187, although there was a small loss of viability in HeLa cells at the highest concentration (viability with 3 µM A23187 = 74 ± 1% of control without A23187 (P<0.001); mean ± SEM, n = 4). Concentrations of A23187 from 0.3 to 3 µM caused increasing G1 cell cycle arrest in A549 cells (Fig. 2A). With all three LKB1-null cell lines, there was a good correlation between activation and Thr172 phosphorylation of AMPK and G1 arrest (Figs. 2B-2D), as well as phosphorylation of Thr172 and the downstream targets ACC (S79) and Raptor (S792) (Figs, 2E-2G), at different concentrations of the ionophore, despite small differences in the sensitivity of the cells to the ionophore (note different scales on the abscissae of Figs. 2B/C and 2D).
Figure 2. Effect of A23187 on AMPK activation and cell cycle arrest in A549, HeLa and G361 cells.
(A) A549 cells were treated with the indicated concentration of A23187 or vehicle (DMSO) for 20 hr with (where indicated) nocodazole (70 ng.ml-1) added for a further 18 hrs. The cells were then fixed, stained with propidium iodide and DNA content analyzed by flow cytometry. Bars show the percentages of cells with G1, S and G2/M phase DNA content (mean ± SD, n = 3); significant differences in the % of cells in the same cell cycle phase compared with DMSO control by 2-way ANOVA are shown: **p<0.01, ****p<0.0001. Similar results were obtained in three identical experiments. (B) AMPK activity measured in immunoprecipitates (top), percentage of cells in G1 phase (middle) (both mean ± SEM (n = 3)), and Thr172 phosphorylation (bottom) in A549 cells treated with various concentrations of A23187 for 20 hr;. (C) As (B), but in HeLa cells. (D) As (B), but in G361 cells (note different scale on x axis). (E) Phosphorylation of AMPK (Thr172), ACC (Ser79) and Raptor (Ser792) in A549 cells incubated as in (B). (F) As (E), but in HeLa cells incubated as in (C). (G) As (E), but in G361 cells incubated as in (D).
Expression of constitutively active CAMKK2 in G361 cells caused cell cycle arrest
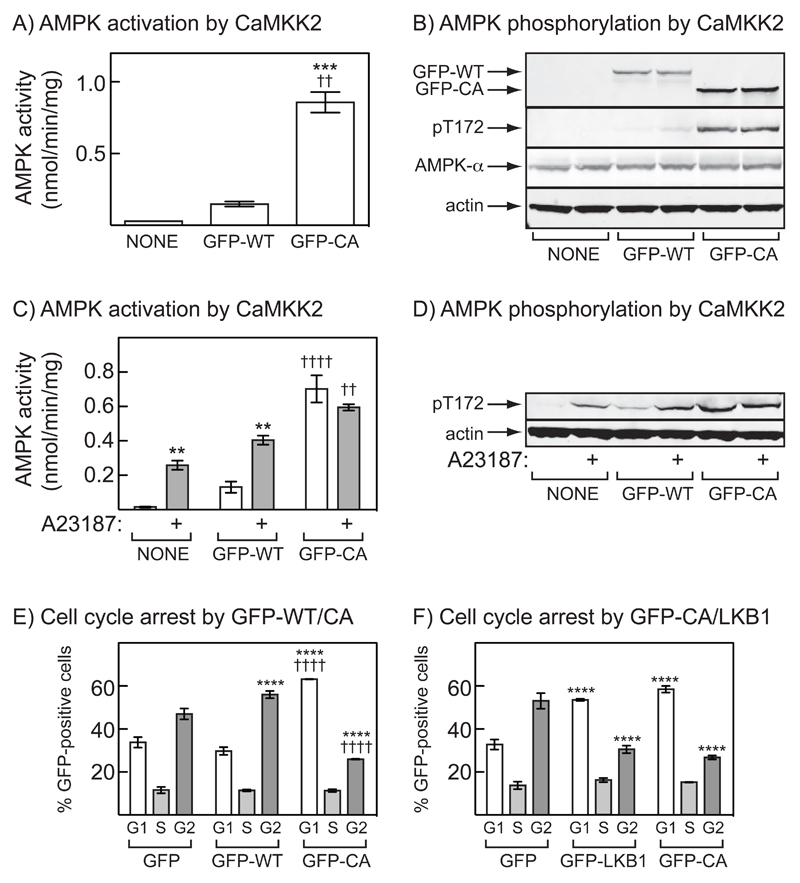
The results in Figs. 1 and 2 showed that activation of AMPK by the CAMKK2 pathway correlated with cell cycle arrest in three different tumor cell lines that lacked LKB1, but did not prove a causal relationship. To test whether activation of CAMKK2 was sufficient for cell cycle arrest in G361 cells, we expressed a constitutively active form. Rat CAMKK1 truncated at residue 434 (which removes the calmodulin-binding region and an overlapping auto-inhibitory sequence) was constitutively active and Ca2+/calmodulin-independent (41). We therefore expressed in G361 cells either full length human CAMKK2 (1-588) or a truncated form (1-471; residue 471 aligns with residue 434 in rat CAMKK1). Both were expressed with GFP fused at their N-termini, with the full length and the truncated, constitutively active forms being referred to below as GFP-WT and GFP-CA respectively. Figs. 3A/3B shows that expression of GFP-WT in G361 cells caused a modest activation and Thr172 phosphorylation of AMPK, while expression of GFP-CA caused much larger effects. With GFP-WT, there were modest increases in both basal and A23187-stimulated activation and Thr172 phosphorylation of AMPK. By contrast, when cells were transfected with GFP-CA, there was a large increase in the basal activity and Thr172 phosphorylation of AMPK, which was no longer stimulated by A23187 (Fig. 3C/D). This confirms that the GFP-CA construct is constitutively active and Ca2+-independent.
Figure 3. Effect of expression of constitutively active CAMKK2 on AMPK activation and the cell cycle in G361 cells.
(A) Cells were left untransfected, or were transfected with DNAs encoding GFP fused to full length (1-588, GFP-WT), or truncated (1-471, GFP-CA) CAMKK2. After 36 hr, AMPK activity was measured in immunoprecipitates; significant differences by 1-way ANOVA compared with untransfected control (***p<0.001) or GFP-WT (††p<0.01) are shown. (B) Analysis by Western blotting of extracts of untransfected cells and cells transfected with DNAs encoding GFP-WT or GFP-CA. Extracts were made after 36 hr and antibodies used were anti-GFP (top panel), anti-pt172, anti-AMPK-α or anti-actin. Results show samples from duplicate cell incubations: similar results were obtained in three independent experiments. (C) AMPK activity in immunoprecipitates from cells 36 hr after transfection with DNAs encoding GFP-WT or GFP-CA, or untransfected controls. Cells were then incubated with or without 10 μM A23187 for 1 hr; results are mean ± S.D. (n = 2); significant effects by two way ANOVA compared with vehicle controls, **p<0.01, or untransfected controls (††††p<0.0001, ††p<0.01) are shown. (D) Analysis by Western blotting of extracts of the same cells shown in (C). (E) Percentage of cells in G1, S or G2/M for cells transfected with DNAs encoding GFP, GFP-WT or GFP-CA (mean ± S.D., n = 3). After 36 hr, cells were treated with nocodazole (70 ng.ml-1) and 18 hrs later were fixed, stained with propidium iodide and the DNA content analyzed by flow cytometry. The flow cytometer was set-up to analyze only cells expressing GFP. Significant differences between the percentage of that cell cycle phase compared with GFP alone (****p<0.0001) or between GFP-WT and GFP-CA (††††p<0.0001) are shown (2-way ANOVA). Similar results were obtained in three identical experiments. (F) As (E), except that cells were transfected with DNAs encoding GFP, GFP-CA, or GFP-LKB1 plus STRADA and CAB39. Similar results were obtained in two identical experiments.
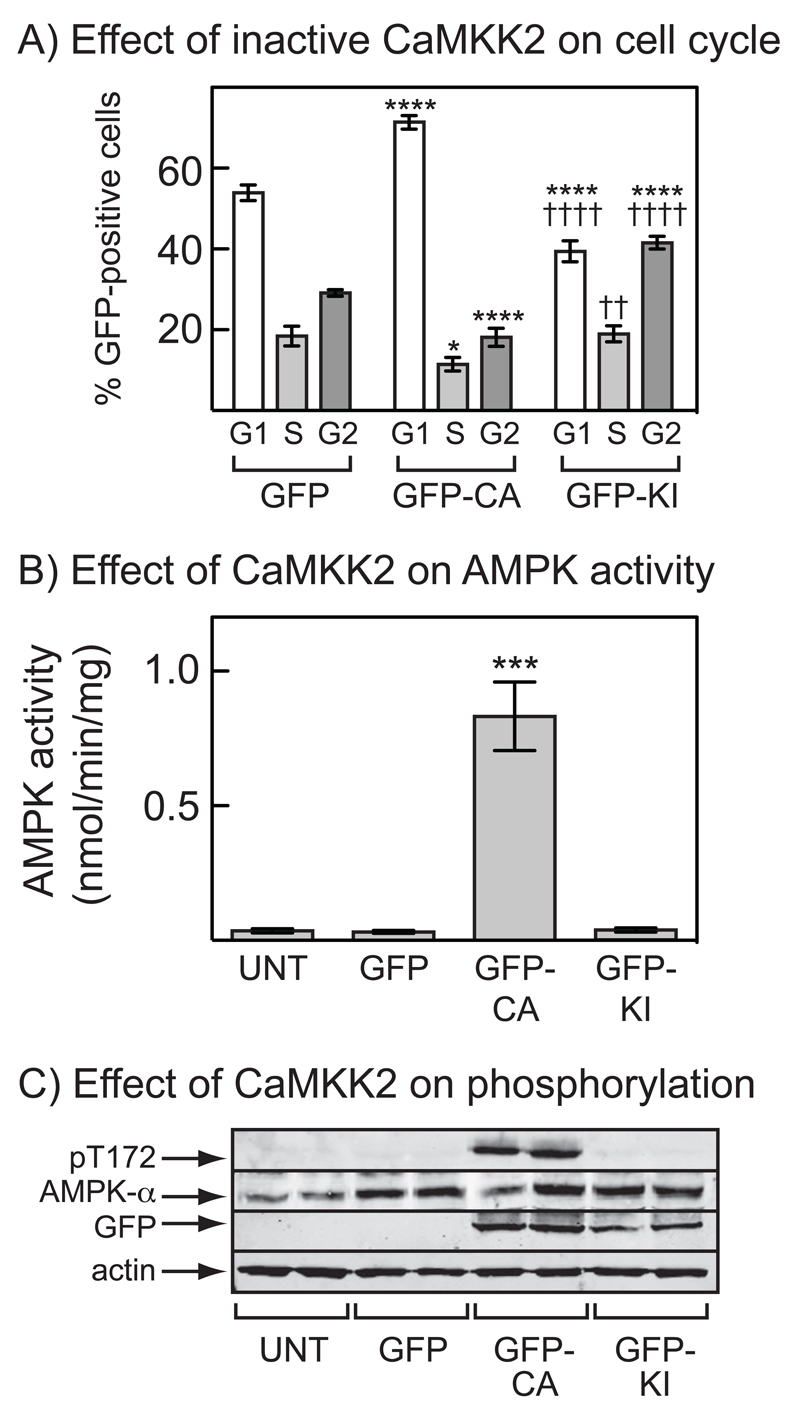
We next assessed the effect of expression of GFP-WT or GFP-CA on the cell cycle in nocodazole-treated G361 cells. Because the transfection efficiency was quite low, the flow cytometer was set up to perform analysis of DNA content only in GFP-expressing cells, with cells expressing GFP alone as controls. Fig. 3E shows that expression of GFP-WT (in the absence of a Ca2+ ionophore) had only marginal effects on the cell cycle (if anything, there was a small increase in G2 content), whereas expression of GFP-CA caused a marked G1 arrest. The G1 arrest obtained by expression of GFP-CA was very similar to that produced by expression of a GFP:LKB1 fusion (Fig. 3F). The effect of GFP-CA was also dependent on kinase activity, because when the experiment was repeated with a kinase-inactive mutant of GFP-CA (D330A, termed GFP-KI), a G1 arrest was not observed (in fact, there were fewer cells in G1 and more in G2; Fig. 4A). As expected, only the expression of the active GFP-CA form was associated with activation and Thr172 phosphorylation of AMPK (Fig. 4B, 4C).
Figure 4. Cell cycle arrest and AMPK activation in G361 cells requires the kinase activity of CaMKKβ.
(A) Percentage of cells in G1, S or G2/M for cells transfected with DNAs encoding GFP, GFP-CA or GFP-KI (mean ± SD, n = 3). After 36 hr, cells were treated with nocodazole (70 ng.ml-1) and 18 hrs later were fixed, stained with propidium iodide and the DNA content analyzed by flow cytometry. The cytometer was set-up to analyze only cells expressing GFP. Significant differences by 2-way ANOVA compared with values for the same cell cycle phase with GFP alone (*p<0.05, ****p<0.001) or between GFP-CA and GFP-KI (††p<0.01, ††††p<0.0001) are shown. Similar results were obtained in two identical experiments. (B) AMPK activity measured in immunoprecipitates from untransfected cells or cells transfected with DNAs encoding GFP, GFP-CA or GFP-KI. Results are mean ± S.D. (n = 2); significant differences compared with untransfected cells are shown: ***p<0.001 (1-way ANOVA). (C) Analysis by Western blotting of extracts of the cells shown in (B) (n = 2).
AMPK is necessary for cell cycle arrest induced by A23187 or expression of constitutively-active CaMKK2 in G361 cells
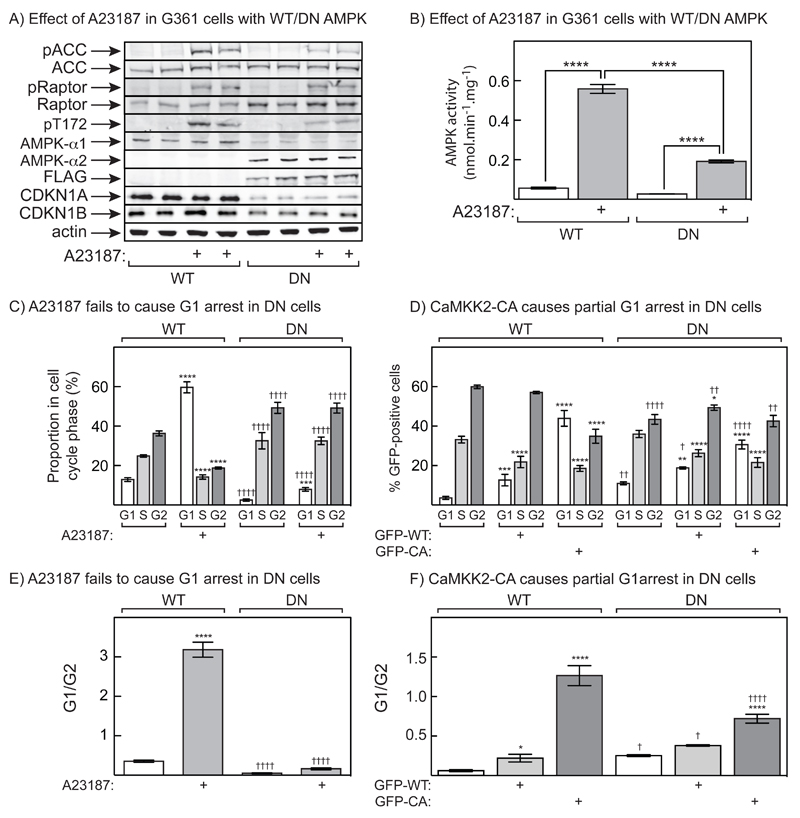
The Ca2+ ionophore A23187 might be causing activation of many Ca2+-dependent enzymes other than CAMKK2. To confirm that AMPK was necessary for the cell cycle arrest induced by A23187, we first generated a line of G361 cells harbouring a single Flp recombinase target (FRT) site, which were then used to generate a line stably expressing a FLAG-tagged, kinase-inactive (D157A) AMPK-α2 (PRKAA2) mutant from the FRT site. By competing for binding to the available β and γ subunits with the endogenous AMPK-α subunits, which in G361 cells are primarily α1 (PRKAA1), the inactive α2 was expected to act as a dominant negative mutant (the cells will be referred to as DN cells). Consistent with this, the DN cells expressed recombinant FLAG-α2, detected either using anti-α2 or anti-FLAG antibodies, and the level of expression of endogenous α1 decreased. The activation and Thr172 phosphorylation of AMPK, and the phosphorylation of its downstream target acetyl-CoA carboxylase (ACACA), were also reduced, although not completely eliminated, after A23187 treatment of the DN cells compared to the WT parental cells (Fig. 5A/B). Surprisingly, the phosphorylation of the AMPK target Raptor at S692 in response to A23187 did not appear to be reduced in the DN cells. However, its expression had increased 2-fold in the DN cells (an interesting result that will be addressed in the Discussion), so that the degree of phosphorylation of Raptor would indeed have been around 50% lower (Fig. 5A). Interestingly, the DN cells grew more rapidly than the WT cells (data not shown), and this was associated with a clear reduction in the expression of CDKN1A, with a smaller reduction in expression of CDKN1B (Fig. 5A); by densitometry, the expression of CDKN1A went down by 85% (n = 8, P<0.0001 by Student’s t test), and of CDKN1B by 39% (n = 8, P<0.01). When WT cells were treated with A23187 followed by nocodazole, the Ca2+ ionophore could be seen to cause a large increase in the proportion of cells in G1, and a concomitant reduction of the proportion in G2 (Fig. 5C), consistent with the expected G1 cell cycle arrest. However, even though AMPK activation and phosphorylation of ACC and Raptor was only partially abrogated in the DN cells, the cell cycle arrest caused by A23187 was completely eliminated (Fig. 5C). This could be seen particularly clearly by expressing the results as the G1:G2 ratio (Fig. 5E).
Figure 5. Cell cycle arrest induced by A23187, or expression of constitutively active CAMKK2, in G361 cells is AMPK-dependent.
(A) G361 cells stably expressing a kinase-inactive (D157A) mutant of AMPK-α2 (DN), or control G361 cells (WT), were treated with A23187 (0.3 μM, 24 hr) and duplicate cell lysates analysed by Western blotting using the indicated antibodies. (B) AMPK activity was assessed in immunoprecipitates made with pan-α antibody. Significant differences by 2-way ANOVA are indicated (****p<0.0001). (C) Cells treated with A23187 (0.3 μM) for 24 hr and then nocodazole (70 ng.mL-1) for 24 hr were fixed, stained with propidium iodide and the proportions of cells in G1, S and G2 assessed by flow cytometry. Significant differences by 2-way ANOVA from controls without A23187: ***p<0.001, ****p<0.0001; significant differences between the same treatments in WT and DN cells: ††††p<0.0001. (D) Cell cycle arrest by transient expression of wild type or constitutively active CAMKK2 (GFP-WT or GFP-CA) is reduced in DN cells. DNAs encoding GFP-WT, GFP-CA or GFP alone (control) was transfected into G361 cells for 24 hr, and the cells then treated with nocodazole for a further 24 hr; cell cycle analysis was then performed only on transfected cells, i.e. those expressing GFP. Significant differences by 2-way ANOVA from GFP controls: **p<0.01, ***p<0.001, ****p<0.0001; significant differences between the same treatments in WT and DN cells: †p<0.05, ††p<0.01, ††††p<0.0001. (E) Same data as (C), but analysing G1:G2 ratio; statistical tests as in (C). (F) Same data as (D), but analysing G1:G2 ratio; statistical tests as in (D).
We also examined the effects of expressing the GFP-tagged full length CAMKK2 (GFP-WT) or the truncated, activated mutant (GFP-CA) in the WT and DN cells, using GFP alone as a control. In the WT cells, expression of GFP-WT caused a modest increase in the proportion of cells in G1, while the GFP-CA mutant caused a much larger increase in cells arrested in G1 and a concomitant decrease in G2. These effects were reduced, although not completely abolished, in the DN cells (Fig. 5D), most clearly seen by expressing the results as the G1:G2 ratio (Fig. 5F).
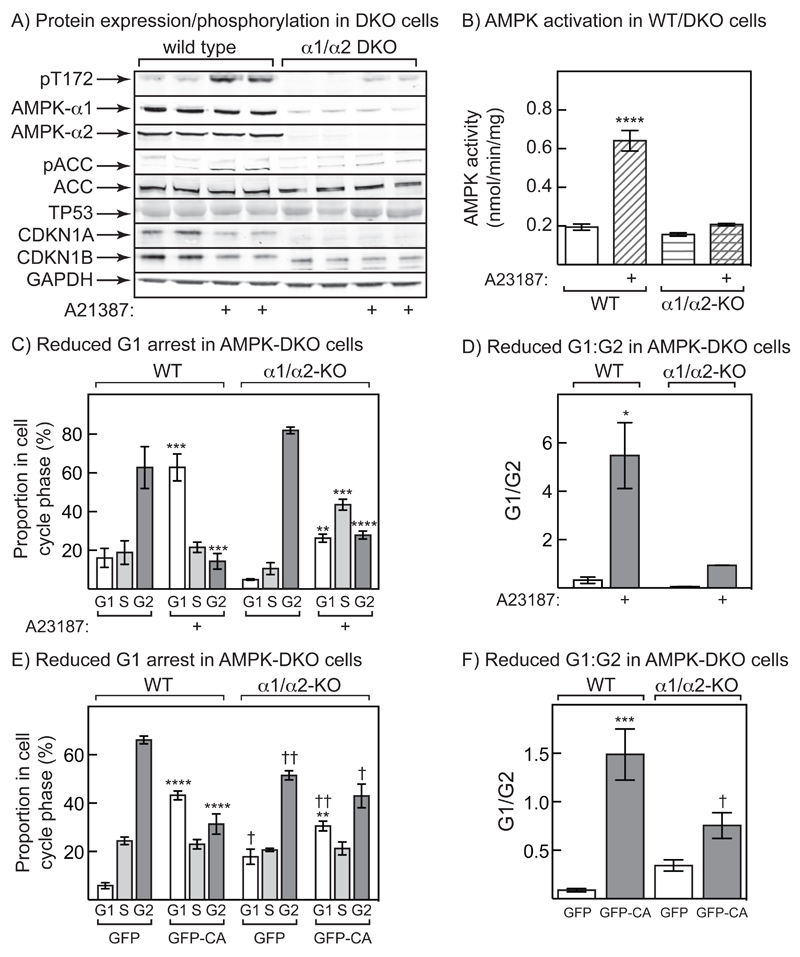
To confirm the results obtained by expressing the dominant negative mutant, we also made a double knockout of both PRKAA1 and PRKAA2 (α1/α2 DKO) in G361 cells using the CRISPR/Cas9 system. For reasons that remain unclear, a slight trace of AMPK-α1 expression, and increased phosphorylation of AMPK and ACC in response to A23187, remained evident in the DKO cells (Fig. 6A). Correlating with Thr172 phosphorylation, there was also a greatly reduced AMPK activation in response to A23187, although some basal activity was measurable (Fig. 6B). As in the DN cells, expression of CDKN1A was clearly reduced in the DKO cells, while the expression of CDKN1B was also slightly reduced; there also appeared to be a mobility shift in CDKN1B that might indicate reduced phosphorylation (Fig. 6A). In the wild type cells, pre-treatment with A23187 prior to addition of nocodazole caused a switch from the cells being predominantly in G2 to G1 phase, with no effect on the proportion in S phase. This G1 arrest was largely, although not completely, prevented in the α1/α2 DKO cells (Fig. 6C), most clearly seen by expressing the results as the G1:G2 ratio (Fig. 6D). We also expressed the GFP-CA mutant, or GFP alone as a control, in wild type or DKO G361 cells, and analysed effects on the cell cycle in cells expressing GFP. Expression of GFP-CA caused a marked arrest in G1 phase as before, and this effect was reduced but not abolished (similar to the effects of A23187) in DKO cells (Fig. 6E); this was clearly seen by expressing the results as the G1:G2 ratio (Fig. 6F).
Figure 6. Cell cycle arrest by A23187 in G361 cells is AMPK-dependent.
(A) Expression and phosphorylation of proteins in wild type (WT) and α1/α2 double knockout (DKO) cells derived from G361 cells using the CRISPR/Cas9 system. Phosphorylation of AMPK, ACC and Raptor, and expression of AMPK-α1, -α2, ACC, Raptor, TP53, CDKN1A and CDKN1B, was assessed in cells treated with and without 0.3 μM A23187 for 24 hr. (B) AMPK activity in WT and DKO cells treated with and without 0.3 μM A23187 for 24 hr. Significant differences between A23187- and vehicle-treated samples, determined by 2-way ANOVA, are indicated: ****p<0.0001. (C) Cell cycle analysis in cells treated with 0.3 μM A23187 for 24 hr, then with nocodazole for a further 24 hr. The proportion of cells in each cell cycle phase was determined by flow cytometry of cells stained with propidium iodide. Significant differences in each cell cycle phase between A23187- and vehicle-treated samples, determined by 2-way ANOVA, are indicated: **p<0.01, ***p<0.001, ****p<0.0001. (D) Results from the experiment shown in (C) but expressed as ratios of G1:G2 phases. Significant differences between A23187- and vehicle-treated samples, determined by 2-way ANOVA, are indicated: p<0.05. (E) Cell cycle analysis in WT or DKO cells transfected with DNA encoding GFP or GFP-CA.for 24hr, then with nocodazole for 24 hr. Significant differences in each cell cycle phase between GFP and GFP-CA-transfected samples (**p<0.01; ****p<0.0001), and between WT and DKO samples (†p<0.05, ††p<0.01) are indicated. (F) Same data as (E), but analysing G1:G2 ratios. Significant differences between GFP and GFP-CA-transfected samples (***p<0.001), and between WT and DKO samples (†p<0.05) are indicated.
Cell cycle arrest induced by expression of GFP-CAMKK2 or GFP-LKB1 was accompanied by increased expression of CDKN1A but not CDKN1B
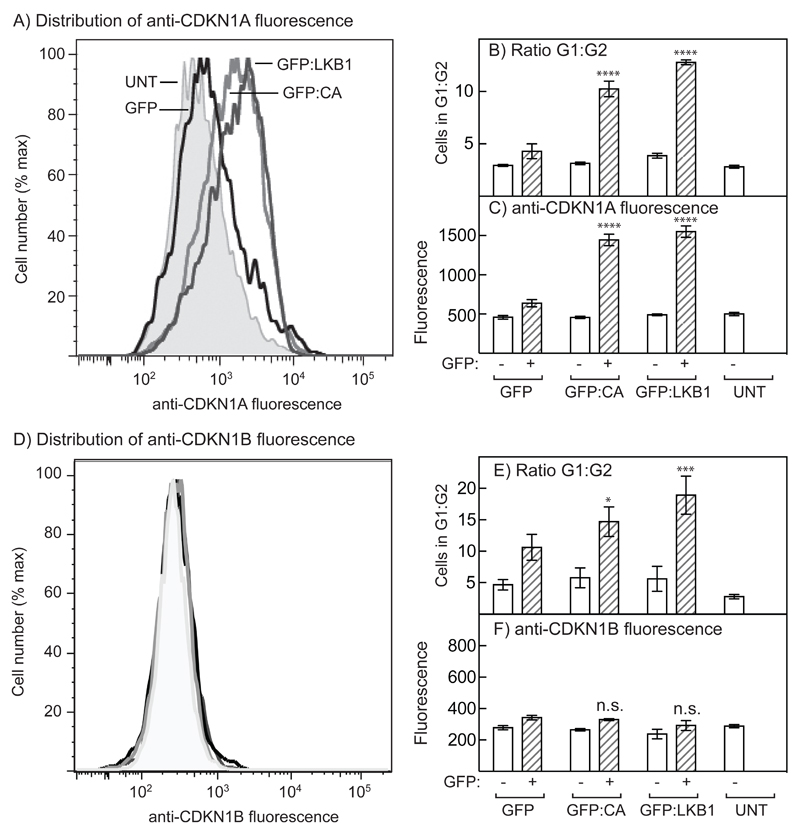
In both the DN (Fig. 5A) and DKO (Fig. 6A) G361 cells there were large decreases in expression of the cyclin-dependent kinase inhibitor, CDKN1A, and smaller decreases in the expression of CDKN1B. However, we did not detect any increases in expression in response to acute treatment with A23187 in the wild type cells, perhaps because we were studying unsynchronised cells and these cyclin-dependent kinase inhibitors are expressed in a cell cycle-dependent manner. To increase the sensitivity of our analysis, we used flow cytometry. We expressed either GFP-LKB1 or the constitutively active GFP-CA mutant of CAMKK2 in G361 cells, using both cells expressing GFP alone and untransfected cells as controls. The expression of CDKN1A by flow cytometry in cells expressing GFP alone was not significantly different from that in untransfected cells, but was clearly higher in cells expressing GFP-CA or GFP-LKB1 (Fig. 7A). As already shown in Fig. 3F, expression of GFP-CA or GFP-LKB1 caused large increases in G1:G2 ratio compared with the GFP control. We sorted the GFP-expressing or untransfected cells after each treatment, and assessed their expression of CDKN1A by flow cytometry. There was a striking correlation between the G1:G2 ratio and the expression of CDKN1A in cells transfected with DNAs encoding GFP, GFP-CA or GFP-LKB1, although the cell cycle distribution and CDKN1A expression was unaffected by any of the treatments in untransfected cells (Fig. 7B/7C).
Figure 7. AMPK activation in G361 cells induces increased expression of CDKN1A but not CDKN1B, and cell cycle arrest.
G361 cells were transiently transfected with DNAs encoding GFP, GFP-LKB1 or GFP-CA. (A) Distribution of CDKN1A expression using anti-CDKN1A antibody in untransfected cells (UNT) and in cells expressing GFP, GFP-LKB1 or GFP-CA. (B) Ratios of G1:G2 phases in cells not expressing GFP (open bars), or in cells from the same dish expressing GFP, GFP-LKB1 or GFP-CA (hatched bars); significant differences by 2-way ANOVA from controls expressing GFP alone are shown: ****p<0.0001 (n = 4). (C) Expression of CDKN1A by flow cytometry in the same cells analysed in (B); significant differences by 2-way ANOVA from controls expressing GFP alone are shown: ****p<0.0001 (n = 4). (D) Distribution of CDKN1B expression using anti-CDKN1B antibody in untransfected cells (UNT) and in cells expressing GFP, GFP-LKB1 or GFP-CA; the grayscale coding is as in 1A and there are no significant differences. (E) Ratios of G1:G2 phases in untransfected cells (open bars), or in cells from the same dish expressing GFP, GFP-LKB1 or GFP-CA (hatched bars); significant differences by 2-way ANOVA from controls expressing GFP alone are shown: *p<0.05, ***p<0.001 (n = 4). (F) Expression of CDKN1B by flow cytometry in the same cells analysed in (E); there were no significant differences (n = 4).
We repeated the experiment but analysed expression of CDKN1B. Unlike CDKN1A, CDKN1B was not elevated in cells expressing GFP-CA or GFP-LKB1 relative to GFP or untransfected controls. However, cell cycle arrest still took place, as seen by increases in the G1:G2 ratio in cells expressing GFP-CA or GFP-LKB1 relative to controls. We also attempted to analyse the expression of TP53, but the level of expression appeared to be too low to be detectable using this methodology.
Discussion
Our results show that, even in LKB1-null tumor cells, activation of AMPK is sufficient for cell cycle arrest, and necessary for the arrest induced by Ca2+-elevating agents. Inhibition of cell proliferation and G1 arrest in response to AMPK activation has been demonstrated previously in cells expressing LKB1 (12–14). However, those studies relied on the use of the pharmacological activator 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), which can have AMP-independent “off-target” effects (42), or on over-expression of activated AMPK, which might also lead to non-physiological effects. Our results show that the G1 arrest (25) and consequent inhibition of proliferation (24) caused by the re-expression of LKB1 in LKB1-null tumor cells is mediated by AMPK and not by any of the AMPK-related kinases (ARKs). We have previously shown that the ARKs that have detectable activity in HeLa cells, i.e. SIK1, SIK2, SIK3, NUAK2, MARK1, MARK2/3 and MARK4, were (unlike AMPK) not activated in the cells by treatment with the Ca2+ ionophore A23187, or by over-expression of CAMKK2, although all were activated by re-expression of LKB1 (33). BRSK1 and BRSK2 are two brain-specific ARKs not detectable in HeLa cells, but neither of them are activated by CAMKK2 in cell-free assays (33). We initially made use of this lack of effect of the Ca2+-CAMKK pathway on ARKs to assess whether the G1 arrest brought about by expression of LKB1 in G361 cells was caused by activation of AMPK or one or more of the ARKs. We found that increasing intracellular Ca2+ using A23187 in any of three cell lines lacking LKB1 led to a G1 arrest. Although the three cell lines varied somewhat in their sensitivity to A23187, in every case there was a good correlation between the degree of AMPK activation, phosphorylation of known downstream targets of AMPK (ACC and Raptor), and G1 arrest.
Although the results obtained with A23187 are consistent with the cell cycle arrest being caused by activation of CAMKK2 and AMPK, the Ca2+ ionophore would also activate other Ca2+-dependent intracellular processes, and we wished to use a more specific method to activate CAMKK2. This was achieved in G361 cells by expressing a truncated form of CAMKK2 that lacks the regulatory region and is thus constitutively active. As expected, expression of the GFP-tagged constitutively active kinase (GFP-CA) in G361 cells caused an increase in activity and Thr172 phosphorylation of endogenous AMPK that was Ca2+-independent, in contrast to expression of full length CAMKK2 (GFP-WT), where a large increase in AMPK activity and phosphorylation was only seen when a Ca2+ ionophore was added. Expression of GFP-CA caused a G1 cell cycle arrest identical to that caused by expression of GFP-LKB1, whereas expression of GFP-WT had no effect compared to a GFP control (Fig. 3E/F). The G1 arrest caused by expression of the truncated CAMKK2 mutant was also dependent on its kinase activity, because the effect was lost when an inactive mutant was expressed (Fig. 4).
Although these results showed that treatment of any of the three LKB1-null cell lines with A23187, or expression of constitutively active CAMKK2 in G361 cells, was sufficient to cause a G1 arrest similar to that observed on re-expression of LKB1, they did not prove that AMPK was necessary for these effects. To address this, we initially generated G361 cells stably expressing a dominant negative AMPK-α2 mutant. This reduced the expression of the endogenous AMPK-α1 (the predominant isoform expressed in G361 cells) and caused a 60-70% decrease in total AMPK activation and Thr172 phosphorylation after A23187 treatment. Even though down-regulation was incomplete, the G1 arrest caused by A23187 in the parental cells was almost completely abrogated. By contrast, the G1 arrest caused by expression of constitutively active CAMKK2 (GFP-CA) was only partially prevented. The latter approach causes a larger activation and Thr172 phosphorylation of AMPK (Fig. 3A/B), and this may have left sufficient AMPK function to cause a partial cell cycle arrest, even though the active AMPK in the cells was reduced by 60-70%.
Using the CRISPR/Cas9 system, we also generated G361 cells with a double knockout of AMPK-α1 and –α2 (PRKAA1/PRKAA2). Surprisingly, although the knockout of α2 appeared to be complete, a reduced but still significant level of α1 expression and Thr172 phosphorylation was observed (Fig. 6A). G361 cells are generally triploid, and it is possible that one allele remained functional to provide a low level of α1 expression. Indeed, cells in which all alleles of PRKAA1/PRKAA2 had been disrupted may have been selected against, because some AMPK function appears to be required for normal completion of mitosis and cytokinesis. In proliferating human cells, AMPK phosphorylated at Thr172 is found at specific locations during mitosis, such as at centrosomes in prophase and metaphase, at the mid-body of the spindle in anaphase, and at the cleavage furrow during cytokinesis. In these cases, Thr172 phosphorylation appears to be independent of LKB1, so may be catalysed by CaMKK2 (43–45). AMPK also appears to be required for progression through cytokinesis for phosphorylation of PPP1R12C, a regulatory subunit that targets protein phosphatase-1 to myosin regulatory light chain (46).
Despite the lack of complete ablation of PRKAA1 in our double knockout cells, the cell cycle arrest caused by treatment with A23187 was almost completely prevented, similar to the results obtained with the DN cells. Taken together, these results suggest that a threshold of AMPK activation has to be exceeded for it to cause G1 phase arrest. The lower level of AMPK in our DN and DKO lines of G361 cells, while perhaps sufficient to allow completion of mitosis and cytokinesis, may have been insufficient to cause G1 arrest.
What is the mechanism for the cell cycle arrest triggered by AMPK? In mouse embryo fibroblasts, AMPK activation using AICAR was associated with phosphorylation of TP53 at Ser-18 and consequent up-regulation of its expression, and enhanced expression of the G1 cyclin-dependent kinase inhibitor CDKN1A (13). AMPK activation in mouse embryo fibroblasts using AICAR was also reported to be associated with increased phosphorylation of CDKN1B at Thr198 and consequent stabilization of that CDK inhibitor (47). We observed that CDKN1A was down-regulated in both the DN and DKO G361 cells, suggesting that basal AMPK activity increases the expression of that CDK inhibitor. We also observed that expression of CDKN1A, but not CDKN1B, was 2- to 3-fold higher in the cells that expressed GFP-tagged LKB1 (GFP-LKB1) or the truncated, constitutively active CAMKK2 (GFP-CA), compared with control cells expressing GFP alone, or untransfected cells, correlating with increases in the ratio of cells in G1:G2 (Fig. 7A-C). However, we did not observe any changes in expression of CDKN1A when the cells were treated with the Ca2+ ionophore A23187 (Fig. 5A). Thus, the mechanism by which AMPK activation causes G1 arrest may involve more than a simple increase in expression of CDKN1A. Unfortunately, attempts to examine the phosphorylation at Ser18 on TP53 by Western blotting did not yield clear results in G361 cells.
Another interesting finding to emerge from our studies of the DN G361 cells was that the expression of Raptor appeared to increase about 2-fold, indicating that basal AMPK activity represses Raptor expression. Thus, the rapid growth of these cells that have reduced levels of AMPK may be due not only to decreased expression of CDKN1A, but also to increased expression of Raptor, an essential component of the mTORC1 complex.
In summary, our results suggest that the ability of LKB1 to trigger cell cycle arrest and inhibition of growth when it is re-expressed in LKB1-deficient tumor cells is entirely mediated by AMPK activation. This reinforces the idea that the AMPK system provides an “energy checkpoint” that only allows progression from G1 to S phase and hence DNA replication (a process that is costly in energy) if sufficient ATP is available (13). Moreover, they reveal that cell cycle arrest can still occur, even in tumor cells lacking LKB1, via agents that increase intracellular Ca2+ and thus activate the alternate CAMKK2-AMPK pathway. This has possible implications for therapy in cases of human cancers in which expression of LKB1 is deficient. However, the role of AMPK in cancer is complex, because there is evidence that the cytostatic effects of AMPK activation might protect tumors from cell death induced by stresses such as oxygen and nutrient starvation (48–50).
Supplementary Material
Acknowledgments
Financial support: DGH and DVC were supported by a Programme Grant (C37030/A15101) from Cancer Research UK, and DGH, FAR and AG by a Senior Investigator Award (097726) from the Wellcome Trust. SF was supported by a Wellcome Trust Studentship and GJG by a studentship from AstraZeneca. The laboratory was also partly funded by the pharmaceutical companies supporting the Division of Signal Transduction Therapy at Dundee (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen Pharmaceutica, Merck Serono and Pfizer).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Hardie DG. Molecular Pathways: Is AMPK a friend or a foe in cancer? Clin Cancer Res. 2015;21:3836–40. doi: 10.1158/1078-0432.CCR-14-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–52. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2014;33C:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–87. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 5.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–66. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–84. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, et al. Structural basis of AMPK regulation by small molecule activators. Nature Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Wang J, Zhang YY, Yan SF, Neumann D, Schlattner U, et al. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat Struct Mol Biol. 2012;19:716–8. doi: 10.1038/nsmb.2319. [DOI] [PubMed] [Google Scholar]
- 9.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–3. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–5. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 11.Ross FA, Jensen TE, Hardie DG. Differential regulation by AMP and ADP of AMPK complexes containing different gamma subunit isoforms. Biochem J. 2016;473:189–99. doi: 10.1042/BJ20150910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5'-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-d- ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 13.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Rattan R, Giri S, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-{beta}-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 15.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRADa/b and MO25a/b are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 19.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 21.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 22.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Monahan KB, Pfefferle AD, Shimamura T, Sorrentino J, Chan KT, et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell. 2012;21:751–64. doi: 10.1016/j.ccr.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–82. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 25.Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem. 2009;284:77–84. doi: 10.1074/jbc.M806152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 29.Thornton C, Sardini A, Carling D. Muscarinic receptor activation of AMP-activated protein kinase inhibits orexigenic neuropeptide mRNA expression. J Biol Chem. 2008 doi: 10.1074/jbc.M708987200. [DOI] [PubMed] [Google Scholar]
- 30.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, et al. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–12. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 32.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex - synergistic effects of Ca2+ and AMP. Biochem J. 2010;426:109–18. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, et al. MO25a/b interact with STRADa/b enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–14. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods A, Salt I, Scott J, Hardie DG, Carling D. The a1 and a2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–51. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 36.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–75. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- 38.Towler MC, Fogarty S, Hawley SA, Pan DA, Martin D, Morrice NA, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008;416:1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 39.Dale S, Wilson WA, Edelman AM, Hardie DG. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 1995;361:191–5. doi: 10.1016/0014-5793(95)00172-6. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.Tokumitsu H, Muramatsu M, Ikura M, Kobayashi R. Regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase. J Biol Chem. 2000;275:20090–5. doi: 10.1074/jbc.M002193200. [DOI] [PubMed] [Google Scholar]
- 42.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell cycle. 2011;10:1295–302. doi: 10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8:2385–98. doi: 10.4161/cc.8.15.9082. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Martin A, Lopez-Bonet E, Oliveras-Ferraros C, Perez-Martinez MC, Bernado L, Menendez JA. Mitotic kinase dynamics of the active form of AMPK (phospho-AMPKalpha(Thr172)) in human cancer cells. Cell Cycle. 2009;8:788–91. doi: 10.4161/cc.8.5.7787. [DOI] [PubMed] [Google Scholar]
- 46.Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–92. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 48.Zadra G, Batista JL, Loda M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol Cancer Res. 2015;13:1059–72. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in twelve flavour. FEBS J. 2016 doi: 10.1111/febs.13698. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monteverde T, Muthalagu N, Port J, Murphy DJ. Evidence of cancer promoting roles for AMPK and related kinases. FEBS J. 2015;282:4658–71. doi: 10.1111/febs.13534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.