Abstract
Background
The phase III COntinuous or INtermittent (COIN) trial failed to demonstrate a benefit in overall survival of cetuximab in combination with chemotherapy for patients with metastatic colorectal cancer (mCRC). High derived neutrophil to lymphocyte ratio (dNLR) has been shown to be prognostic in patients with mCRC. The aim of this analysis is to evaluate dNLR as a predictive biomarker of the survival according to RAS and BRAF mutations status within the COIN trial.
Methods
A post-hoc exploratory analysis of the COIN trial arms A and B was performed. All patients with available white blood cell (WBC) and neutrophil data were analysed. The dNLR was calculated using a formula which has previously demonstrated predictive power in cancer patients: dNLR=ANC/(WBC-ANC). A high dNLR was defined as a value of ≥2.22. dNLR was correlated with clinical outcomes using Kaplan-Meier and cox regression analysis.
Results
A total of 1603 patients were assigned to the oxaliplatin based chemotherapy (arm A, N=815) or oxaliplatin based chemotherapy plus cetuximab (arm B, N=815) arms. There was a strong association between dNLR level and overall survival using Kaplan-Meier analysis. In all mutation groups, dNLR<2.2 was associated with better overall survival (OS) compared to dNLR≥2.2. Median OS in patients with wild type disease (dNLR<2.2 vs dNLR≥2.2) was 22.8 vs 13.1 months (HR 1.33); 16.9 vs 11.8 months (HR 1.36) in patients with RAS mutant tumours; and 12.6 vs 6.8 (HR 1.67) in patients with BRAF mutant tumours.
In patients with dNLR<2.2, the median OS was 19.2 months in arm A compared to 18.0 months in arm B (HR 1.11). Among patients with dNLR≥2.2, the median OS was 13.0 months in arm A compared to 13.1 months in arm B (HR of 0.96).
Conclusion
dNLR is strongly prognostic for survival in all mutations groups. dNLR does not predict for benefit from the addition of cetuximab.
Keywords: Colorectal cancer, neutrophil lymphocyte ratio, Cetuximab, RAS, BRAF
Background
The Continuous or Intermittent (COIN) phase III randomised study demonstrated a prognostic effect of BRAF, KRAS, and NRAS mutations on the outcome of patients with advanced colorectal cancer. However, benefit of additional cetuximab treatment to oxaliplatin based chemotherapy in first line treatment of these patients was not proved. [1] Comparable studies have demonstrated mixed response outcome data for patients with RAS wild-type tumours in the context of chemotherapy combinations with epidermal growth factor receptor (EGFR) inhibitors. [2–5] To further clarify sub-group sensitivity to EGFR inhibition prospective testing is needed. [6–7]
The tumour microenvironment and the inflammatory response have been shown to play a vital role in cancer development. Measurable serum parameters of C-reactive protein, neutrophil/lymphocyte ratio (NLR) and platelet-lymphocyte ratio have been associated with poor outcomes in patients with colorectal cancer. [8–10] NLR is a marker of host inflammation and may reflect cytokine activation and therefore be a surrogate marker of more aggressive disease. A recently reported meta-analysis of 100 studies comprising 40559 patients with various solid tumours, found that NLR >4 was associated with poorer OS (HR 1.81; 95% CI = 1.67 to 1.97; p < 0.001). This effect was observed in all of the disease sites, subgroups and stages. [11] Within this meta-analysis, 6 prospective studies, contained a total of 1817 patients with mCRC.
The COIN trial did not collect lymphocyte count data, however the derived NLR (dNLR) has been shown to possess similar prognostic value. [12] In a previous analysis of the COIN trial we have determined that dNLR is predictive of survival when administering intermittent versus continuous treatment. [13] In this study, we examined dNLR as a prognostic factor and assessed its’ predictive power regarding the potential benefit of EGFR inhibition, particularly in the RAS and BRAF populations.
Methods
The phase III COIN trial was undertaken by the Medical Research Council Clinical Trials Unit and was overseen by an independent trial steering committee. The trial was approved by national research ethics committees in the UK and Ireland and both the Medicines and Healthcare Regulatory Agency and Irish Medicines Board. The trial design and eligibility criteria have been reported previously. [1]
COIN trial’s primary objective was to assess the effect of the addition of EGFR-targeted monocloncal antibody (cetuximab) to continuous oxaliplatin and fluoropyrimidine combination chemotherapy on survival. Shortly after COIN completed recruitment, external evidence showed that anti-EGFR antibodies were unlikely to benefit mCRC patients whose tumours carry KRAS mutations. [14]
Treatment allocation was non-blinded and randomly assigned (1:1) to the control arm of continuous oxaliplatin based (oxaliplatin plus capecitabine or oxaliplatin plus fluorouracil and folinic acid) chemotherapy (arm A) or continuous chemotherapy plus cetuximab (arm B). The treatment was continued until progression of disease, development of cumulative toxicities or patient choice. [1]
We have performed a post-hoc exploratory analysis of the prognostic and predictive power of dNLR in the COIN trial arms A and B. All patients with available white blood cell (WBC) and neutrophil data were analysed. Unfortunately, lymphocyte data was not collected at patient entry to the COIN trial.
Derived Neutrophil/lymphocyte ratio (dNLR) calculation
WBC and absolute neutrophil count (ANC) were collected on all patients at enrolment to the COIN trial. dNLR was calculated using this formula - dNLR=ANC/(WBC-ANC). [8,12]
Statistical methods
All statistical analyses were performed by the Cancer Research UK and University College London Cancer Trials Centre. Stats version 12.1 was used to analyse data.
A high dNLR was defined as ≥2.2. dNLR was correlated with clinical outcomes including overall survival (OS), progression-free survival (PFS) and objective response rate (ORR). Kaplan-Meier survival curves were generated based on dNLR. Comparison between groups was performed using cox-regression analysis adjusted for treatment, age, sex, tumour status (resected, unresected, or local recurrence), primary site (colon, rectum, rectosigmoid junction, multiple growths), liver-only metastases (yes vs no), number of metastatic sites (0, 1, 2, ≥3), platelets (<400,000 vs ≥400,000 µL), alkaline phosphatase (<300 vs ≥ 300 U/L). Prognostic value was assessed with ROC analysis, using one year survival as the outcome, and reporting the estimate of AUC. [13]
Results
1,630 of 2,445 patients in the COIN trial were randomised to arm A (chemotherapy) and arm B (chemotherapy plus cetuximab). Our total cohort was 1,603 patients (accounting for 98.3% of the total study population), excluding 9 patients with no WBC and ANC data and 18 patients with other missing data. The median value of dNLR was 2.2; baseline characteristics within each dNLR group are shown in table 1.
Table 1.
Demographic characteristics of patients
| dNLR < 2.2 (N=811) | dNLR >= 2.2 (N=792) | |
|---|---|---|
| Treatment | ||
| A - Standard chemotherapy | 391 (48.2) | 408 (51.5) |
| B – Cetuximab | 420 (51.8) | 384 (48.5) |
| Age; median (range) | 64.3 (22-82) | 63.6 (25-87) |
| Sex | ||
| Male | 543 (67.0) | 507 (64.0) |
| Female | 268 (33.0) | 285 (36.0) |
| WHO Performance Status | ||
| 0 | 422 (52.0) | 312 (39.4) |
| 1 | 350 (43.2) | 400 (50.5) |
| 2 | 39 (4.8) | 80 (10.1) |
| Status of primary tumour | ||
| Resected | 502 (61.9) | 350 (44.2) |
| Local recurrence | 43 (5.3) | 43 (5.4) |
| Unresected/unresectable | 266 (32.8) | 399 (50.4) |
| Number of metastatic sites | ||
| 0 | 4 (0.5) | 9 (1.1) |
| 1 | 304 (37.5) | 274 (34.6) |
| 2 | 310 (38.2) | 314 (39.6) |
| 3+ | 193 (23.8) | 195 (24.6) |
| Liver-only metastases | ||
| No | 628 (77.4) | 614 (77.5) |
| Yes | 183 (22.6) | 178 (22.5) |
| Platelet count > 400,000 µL | ||
| Normal | 633 (78.1) | 470 (59.3) |
| Elevated | 178 (21.9) | 322 (40.7) |
| CEA cutoff at 100 U/L | ||
| Low | 408 (66.1) | 319 (51.0) |
| High | 209 (33.9) | 307 (49.0) |
| Alkaline cutoff at 300 U/L | ||
| Normal | 726 (89.5) | 624 (78.8) |
| High | 85 (10.5) | 168 (21.2) |
| All wild-type mutations | ||
| Any mutation | 360 (54.7) | 328 (54.2) |
| All wild-type | 298 (45.3) | 277 (45.8) |
dNLR as a prognostic marker
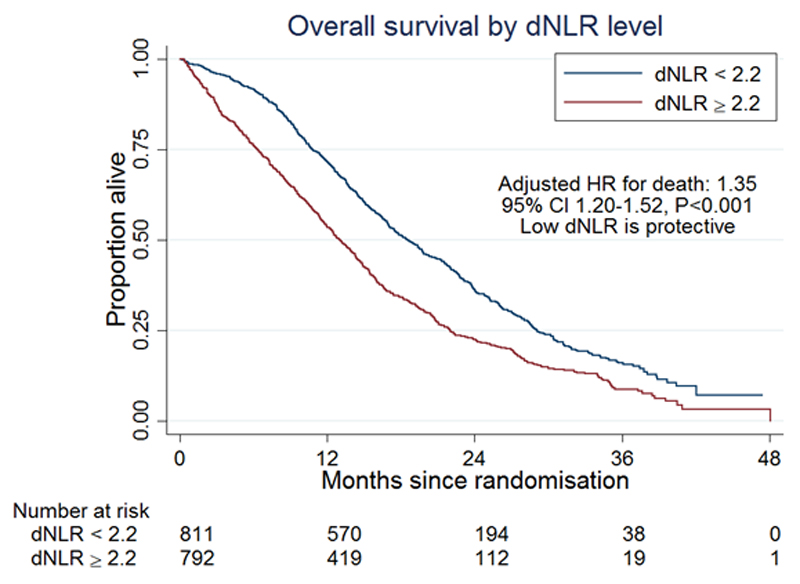
There was a strong association between dNLR level and outcome. We found that patients with dNLR ≥ 2.2 had a hazard ratio (HR) of 1.35 (95% CI 1.20-1.52; p<0.001) for OS (figure 1) and 1.25 (95% CI 1.13-1.40; p<0.001) for PFS.
Figure 1.
Kaplan–Meier curves for overall survival according to dNLR.
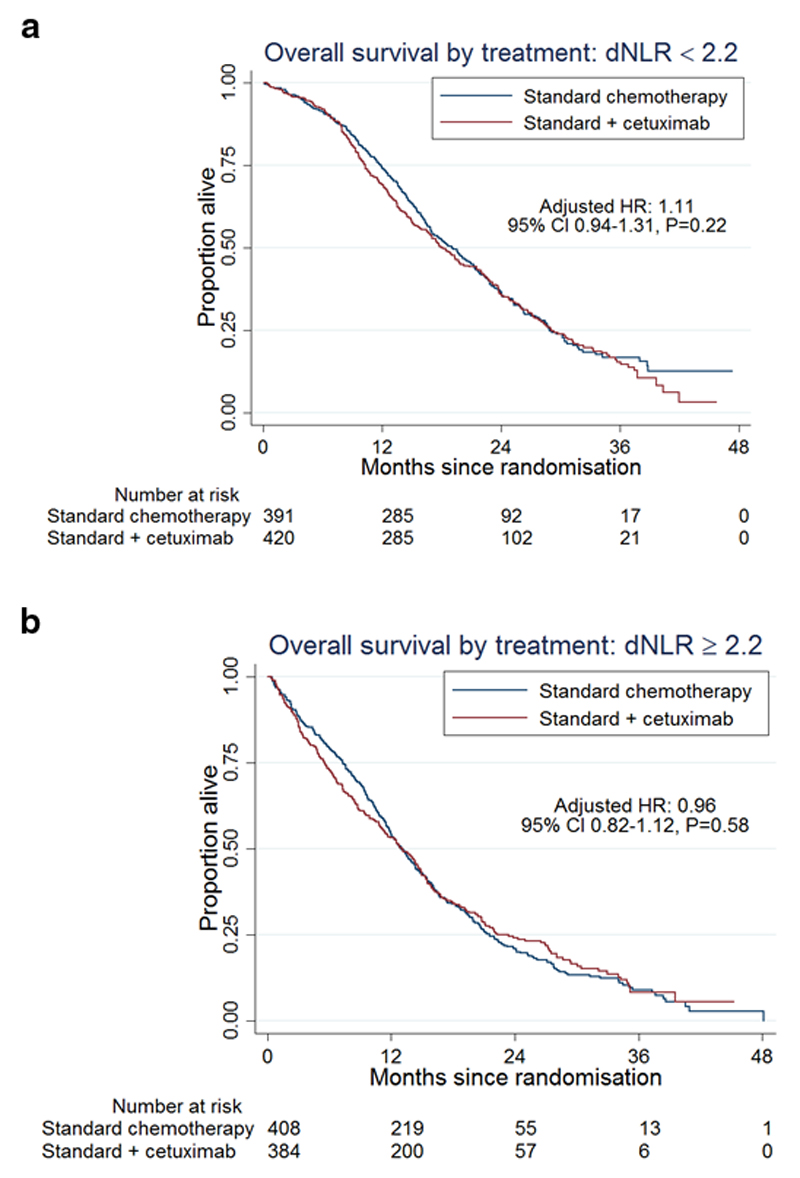
In patients with dNLR<2.2, the median overall survival was 19.2 months in arm A and 18.0 months in arm B - HR 1.11 (figure 2a). Among patients with dNLR≥2.2, the median overall survival was 13.0 months in arm A compared to 13.1 months in arm B- HR 0.96 (figure2b). A differential treatment effect between the two dNLR groups was not seen (p = 0.21).
Figure 2.
Kaplan–Meier curves for overall survival according to treatment, in low (figure2a) and high (figure2b) dNLR.
The AUC for dNLR was 63.9% (95% CI 61.1-66.7). Dichotomising the data at the median value of dNLR (2.2) resulted in a true detection rate of 57.7% and a false positive rate of 38.5% for one year survival.
RAS/RAF mutations
RAS and BRAF mutation status was available for 1,263 (78.8%) patients. Of these, 575 (45.1%) were RAS/RAF wild type; 587 (46.5%) were RAS mutated and 101 (8.0%) were BRAF mutated. There was clear evidence of an association between low dNLR and improved overall survival in each of these four groups (table 2). No evidence for a beneficial effect of additional cetuximab was demonstrated in any group of patients.
Table 2.
Median survival by dNLR
| Median survival time (months) | |||
|---|---|---|---|
| dNLR < 2.2 | dNLR ≥ 2.2 | HR (95% CI) | |
| All patients | 18.6 | 13.1 | 1.35 (1.20-1.52) |
| All wild type | 22.8 | 16.6 | 1.33 (1.07-1.64) |
| KRAS wild type | 21.3 | 14.7 | 1.32 (1.10-1.59) |
| BRAF mutant | 12.6 | 6.8 | 1.67 (1.02-2.75) |
| RAS mutant | 16.9 | 11.8 | 1.36 (1.12-1.65) |
Discussion
Inflammation is well reported to contribute to tumour formation and is now a recognised hallmark of cancer. It is known that the tumour microenvironment can attract, educate and control invading leukocytes to promote angiogenesis, viability, motility and invasion. [16] The stroma around solid cancers has been compared with a poorly healing wound and its' associated chronic inflammation. [17] The association of high NLR with worse survival is more pronounced in metastatic than localised disease and therefore may reflect greater tumour burden or a more prolonged chronic inflammatory process. [11] It is uncertain why NLR is more strongly associated with outcome than neutrophil or lymphocyte counts alone. This biological mechanism requires further investigation. Neutrophils may act as tumour-promoting leukocytes through TGF-β, IL-10 and regulatory T-cells induced signal pathways and circulating neutrophils can also secrete the vascular endothelial growth factor (VEGF), resulting in higher levels of VEGF in the tumours. [18] High NLR may also represent a relatively depleted lymphocyte count, potentially impairing the host immune response to malignancy and therefore negatively impact outcomes.
There is also evidence that RAS mutations influence the host immune response. KRAS and NRAS are critical components of intracellular signalling. Functional specificity of mutated RAS isoforms has been demonstrated and the role of mutant proteins in onset and progression of disease continues to be investigated. [19,20] NRAS activation has been shown to suppress stress-induced apoptosis in human colorectal cancer cells lines and therefore contribute to colorectal cancer development. Mouse models also indicated that NRAS mutations enhance colon cancer development in the context of inflammation. [20]
Recently, retrospective analysis investigated the relationship of NLR with molecular alterations (KRAS/NRAS/BRAF/PIK3CA/CIMP) and circulating cytokines. [21] High NLR was associated with a poor prognosis in metastatic colorectal cancer, independent of the common molecular alterations. Similarly, in our study, the correlation between dNLR and survival was seen in all mutation groups. These results were consequently not predictive of benefit from the addition of cetuximab in any particular mutation group. Although modest, our results have shown a numerically poorer survival for patients with dNLR≥2.2 treated with additional cetuximab compared to those treated with chemotherapy alone. The BRAF mutated cohort was relatively small in number and underpowered. This data should therefore be interpreted with caution as this limits the ability to differentiate between prognostic and predictive value of dNLR in the context of chemotherapy with cetuximab. A meta-analysis with similar BRAF mutated cohorts may be of value.
CRC patients with elevated NLR have been characterised by aggressive biology and distinctive expression profile of cytokines involved in angiogenesis, inflammation and regulation of the epidermal growth factor axis. In the retrospective analysis, elevated NLR was >5. [21] There is ongoing statistical uncertainty with respect to the cut-off of elevated NLR and the subsequent interpretation of NLR as a prognostic and predictive biomarker. Our analysis confirms the prognostic value of dNLR in advanced colorectal cancer. We have demonstrated a strong association between dNLR with OS and PFS. dNLR is therefore moderately prognostic for one-year survival in the COIN trial. This was independent of the treatment allocation arm.
Conclusion
Our study gives further support for the use of dNLR as a readily available, inexpensive biomarker for prediction of survival in MCRC. We have demonstrated that in the randomised phase III COIN trial, dNLR was a reliable prognostic marker in patients with mCRC that received first line oxaliplatin based chemotherapy with or without additional cetuximab. dNLR was strongly prognostic for survival in all mutations groups especially in patients with BRAF mutant tumours. dNLR was not predictive of benefit from cetuximab.
Acknowledgments
None
Funding
TG is a fellow of the European Society of Medical Oncology. SN was supported by CRUK grant C444/A15953 to the UCL CRUK trials centre. JB is partly supported by the UCLH/UCL Biomedical Research Centre.
List of abbreviations
- ANC
absolute neutrophil count
- AUC
area under curve
- CI
confidence interval
- CRC
colorectal cancer
- dNLR
derived neutrophil to lymphocyte ratio
- EGFR
epidermal growth factor receptor
- HR
hazard ratio
- IL2
interleukin 2
- mCRC
metastatic colorectal cancer
- MRC
Medical Research Council
- NLR
Neutophil to lymphocyte ratio
- OS
overall survival
- PFS
progression free survival
- ROC
receiver operating characteristic
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- WBC
white blood cells count
Footnotes
Availability of data and materials
Data and materials are available from the corresponding authors upon request.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2014. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Lenz H-J, Köhne C-H, et al. Fluorouracil, Leucovorin, and Irinotecan Plus Cetuximab Treatment and RAS Mutations in Colorectal Cancer. Journal of Clinical Oncology. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 3.Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. New England Journal of Medicine. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 4.Tveit KM, Guren T, Glimelius B, et al. Phase III Trial of Cetuximab With Continuous or Intermittent Fluorouracil, Leucovorin, and Oxaliplatin (Nordic FLOX) Versus FLOX Alone in First-Line Treatment of Metastatic Colorectal Cancer: The NORDIC-VII Study. Journal of Clinical Oncology. 2012;30:1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 5.Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. The Lancet Oncology. 2013;14:749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent-Puig P, Bridgewater JA, Primrose JN, et al. Mir-31-3p as a predictive biomarker of cetuximab effects in a post hoc analysis of new EPOC phase III trial. ASCO Meeting Abstracts. 2014;32:3523. [Google Scholar]
- 7.Seligmann JF, Elliott F, Richman S, et al. Combined epiregulin (EREG) and amphiregulin (AREG) expression levels as a biomarker of prognosis and panitumumab benefit in RAS-wt advanced colorectal cancer (aCRC) ASCO Meeting Abstracts. 2014;32:3520. [Google Scholar]
- 8.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. British journal of cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of Surgical Oncology. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Liu G, Bao Q, et al. The Baseline Ratio of Neutrophils to Lymphocytes is Associated with Patient Prognosis in Rectal Carcinoma. J Gastrointest Canc. 2010;41:116–120. doi: 10.1007/s12029-009-9125-4. [DOI] [PubMed] [Google Scholar]
- 11.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2014;10:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 12.Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? International Journal of Clinical Oncology. 2014:1–12. doi: 10.1007/s10147-014-0672-8. [DOI] [PubMed] [Google Scholar]
- 13.Grenader T, Nash S, Adams R, et al. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: a post hoc analysis of the MRC COIN study. Br J Cancer. 2016;114:612–615. doi: 10.1038/bjc.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 15.Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. The Lancet Oncology. 2011;12:642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:5247–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 19.Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin Cancer Res. 2015;21:5469–5479. doi: 10.1158/1078-0432.CCR-15-0526. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Velho S, Vakiani E, et al. Mutant N-RAS protects colorectal cancer cells from stress-induced apoptosis and contributes to cancer development and progression. Cancer Discov. 2013;3:294–307. doi: 10.1158/2159-8290.CD-12-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]