Abstract
Key points
We found that extracellular Ca2+, but not other divalent cations (Mg2+ and Ba2+) or intracellular Ca2+, is involved in heat‐evoked activation of green anole (ga) TRPA1.
Heat‐evoked activation of chicken (ch) and rat snake (rs) TRPA1 does not depend solely on extracellular Ca2+.
Neutralization of acidic amino acids on the outer surface of TRPA1 by extracellular Ca2+ is important for heat‐evoked large activation of gaTRPA1, chTRPA1 and rsTRPA1.
Abstract
Transient receptor potential ankyrin 1 (TRPA1) is a homotetrameric non‐selective cation‐permeable channel that has six transmembrane domains and cytoplasmic N‐ and C‐termini. The N‐terminus is characterized by an unusually large number of ankyrin repeats. Although the 3‐dimensional structure of human TRPA1 has been determined, and TRPA1 channels from insects to birds are known to be activated by heat stimulus, the mechanism for temperature‐dependent TRPA1 activation is unclear. We previously reported that extracellular Ca2+, but not intracellular Ca2+, plays an important role in heat‐evoked TRPA1 activation in green anole lizards (gaTRPA1). Here we focus on extracellular Ca2+‐dependent heat sensitivity of gaTRPA1 by comparing gaTRPA1 with heat‐activated TRPA1 channels from rat snake (rsTRPA1) and chicken (chTRPA1). In the absence of extracellular Ca2+, rsTRPA1 and chTRPA1 are activated by heat and generate small inward currents. A comparison of extracellular amino acids in TRPA1 identified three negatively charged amino acid residues (glutamate and aspartate) near the outer pore vestibule that are involved in heat‐evoked TRPA1 activation in the presence of extracellular Ca2+. These results suggest that neutralization of acidic amino acids by extracellular Ca2+ is important for heat‐evoked activation of gaTRPA1, chTRPA1, and rsTRPA1, which could clarify mechanisms of heat‐evoked channel activation.
Keywords: calcium, heat, mutation, TRPA1
Key points
We found that extracellular Ca2+, but not other divalent cations (Mg2+ and Ba2+) or intracellular Ca2+, is involved in heat‐evoked activation of green anole (ga) TRPA1.
Heat‐evoked activation of chicken (ch) and rat snake (rs) TRPA1 does not depend solely on extracellular Ca2+.
Neutralization of acidic amino acids on the outer surface of TRPA1 by extracellular Ca2+ is important for heat‐evoked large activation of gaTRPA1, chTRPA1 and rsTRPA1.
Abbreviations
- AITC
allyl isothiocyanate
- chTRPA1
chicken TRPA1
- gaTRPA1
green anole TRPA1
- rsTRPA1
rat snake TRPA1
- TRPA1
transient receptor potential ankyrin 1
Introduction
Thermosensation is essential for survival and adaptation. The ability to sense ambient temperature mainly relies on sensory neurons (trigeminal and dorsal root ganglia) that project axonal processes to both peripheral termini (the outer layer of the skin) and central termini (spinal cord) (Woolf & Ma, 2007). In the last two decades, transient receptor potential (TRP) ion channels have been identified as molecular sensors for many different ambient stimuli, including temperature. The temperature sensitivity of TRP ion channel family members varies. TRP vanilloid (TRPV) 1–4 (Caterina et al. 1997, 1999; Guler et al. 2002; Peier et al. 2002 b; Smith et al. 2002; Xu et al. 2002) and TRP melastatin (TRPM) 2–5 (Talavera et al. 2005; Togashi et al. 2006; Vriens et al. 2011) are activated by hot and warm temperatures. Meanwhile, TRPM8 and TRP canonical (TRPC) 5 in mouse and humans are activated by cold stimulus (McKemy et al. 2002; Peier et al. 2002 a; Zimmermann et al. 2011).
TRPA1 is primarily expressed in dorsal root ganglia (DRG) as well as trigeminal ganglia (TG) and nodose ganglia in mammals, where it was reported to respond to noxious cold and some pungent chemicals (Story et al. 2003; Bautista et al. 2005). TRPA1 is a homotetrameric non‐selective cation permeable channel with six transmembrane (TM) domains (Gaudet, 2008; Paulsen et al. 2015) that functions as a polymodal receptor to detect various noxious compounds (Bandell et al. 2004; Macpherson et al. 2005; Koo et al. 2007; Iwasaki et al. 2009; Okumura et al. 2010) and stimuli such as mechanical stimulus (Kwan et al. 2006), intracellular alkalization (Fujita et al. 2008) and hyperosmotic stress (Zhang et al. 2008). However, the response of TRPA1 to temperature shows species‐specific differences. Several studies showed that mouse TRPA1 is a thermosensitive TRP channel that is activated by noxious cold both in vitro (Story et al. 2003) and in vivo (Karashima et al. 2009), while other studies found no cold activation of TRPA1 (Jordt et al. 2004; Zurborg et al. 2007). A comparison of mouse and Drosophila TRPA1s showed opposing TRPA1 thermosensitivity (Viswanath et al. 2003), suggesting that these two TRPA1s are not functional orthologues. Moreover, TRPA1 expressed by different snake species (Gracheva et al. 2010), fly (Kang et al. 2012), frog, lizard and chicken (Saito et al. 2012, 2014; Kurganov et al. 2014) are activated by heat, but not cold. TRPA1 activity in snakes (Cordero‐Morales et al. 2011) and Drosophila (Kang et al. 2012) is thought to be modulated at the N‐terminus, which is believed to be involved in thermosensation. On the other hand, it has been suggested that all thermosensitive TRP channels have a U‐shaped activation profile (Clapham & Miller, 2011), which would explain the finding that several heat‐activated TRP channels also show activation in response to cold. Several groups have provided experimental evidence for a U‐shaped thermosensitive response of human TRPA1 (hTRPA1) (Moparthi et al. 2014, 2016), while other studies reported that hTRPA1 is not a thermosensitive channel (Chen et al. 2013). Meanwhile, some groups have proposed allosteric gating and coupling models to explain temperature‐evoked activation of TRPA1 channels (Salazar et al. 2011; Voets, 2012; Jara‐Oseguera & Islas, 2013; Qin, 2013). Thus, the mechanisms that mediate heat‐ or cold‐evoked activation of TRPA1 channel activity remain unclear.
Ca2+ acts as a permeant ion and an intracellular modulator of various TRP channels. The importance of intracellular and extracellular Ca2+ for activation, as well as potentiation and inactivation, of TRPA1 has been reported (Jordt et al. 2004; Doerner et al. 2007; Zurborg et al. 2007; Wang et al. 2008; Karashima et al. 2009). However, whether extracellular Ca2+ plays a role in temperature‐dependent gating of TRPA1 channels is unclear. Recently, we reported that heat‐induced activation of green anole TRPA1 (gaTRPA1) requires extracellular Ca2+ (Kurganov et al. 2014). In this study, we identified several negatively charged amino acids near the opening of the ion channel pore that are necessary for heat‐evoked activation of TRPA1, chicken TRPA1 (chTRPA1) and rat snake TRPA1 (rsTRPA1).
Methods
DNA
The entire coding regions of TRPA1 from green anole and chicken were previously cloned into pcDNA3.1 (Invitrogen Corp, Carlsbad, CA, USA) (Saito et al. 2012, 2014). Rat snake TRPA1 was a kind gift from David Julius (Gracheva et al. 2010) and was subcloned into the pcDNA3.1 vector for HEK293 cell expression.
Cell culture
Human embryonic kidney‐derived 293T (HEK 293T) cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's Medium (WAKO Pure Chemical Industries, Osaka, Japan) containing 10% fetal bovine serum (Biowest SAS, Nuaillé, France), 100 units ml−1 penicillin (Invitrogen Corp.), 100 μg ml−1 streptomycin (Invitrogen Corp.), and 2 mm GlutaMAX (Invitrogen Corp.). For patch‐clamp recordings, 1 μg green anole, chicken, or rat snake TRPA1 in pcDNA3.1 and 0.1 μg pGreen Lantern 1 cDNA were transfected to HEK293T cells cultured in 35 mm dishes using Lipofectamine Plus Reagent (Invitrogen Corp.). After incubating for 3–4 h, the cells were reseeded on coverslips and further incubated at 33°C in 5% CO2. Patch‐clamp recordings were performed 1 day after transfection.
Chemicals
Allyl isothiocyanate (AITC) was purchased from Kanto Chemical (Tokyo, Japan) and HC‐030031 was purchased from Sigma‐Aldrich (St. Louis, MO, USA). AITC was dissolved in methanol for stock solutions (1 m and 100 mm), and HC‐030031 was dissolved in DMSO as a stock solution (10 mm) and diluted to the final concentration using bath solution.
Construction of mutant TRPA1 channels
TRPA1 point mutants were constructed according to the procedures described in the QuikChange site‐directed mutagenesis kit (Agilent Technologies inc, Santa Clara, CA, USA) with minor modifications. Point mutations were introduced by PCR using the gaTRPA1–pcDNA3.1, chTRPA1–pcDNA3.1 and rsTRPA1–pcDNA3.1 as templates with oligonucleotide primers (Table 1) containing the intended mutations. The amplified PCR products were transformed in Escherichia coli, and pcDNA3.1 vectors containing TRPA1 were then purified using standard procedures. The entire TRPA1 coding sequences were determined to confirm that only the intended mutations were introduced.
Table 1.
Primer sets for making green anole, chicken and rat snake TRPA1 glutamate to glutamine, glutamine to glutamate, aspartate to asparagine and glutamate to lysine mutations (Small letters indicate mutated nucleotides)
| Mutation | Sense primer (5′→3′) | Antisense primer (5′→3′) | |
|---|---|---|---|
| Green anole TRPA1 |
|
|
|
| Chicken TRPA1 |
|
|
|
| Rat snake TRPA1 |
|
|
|
Electrophysiology
For whole‐cell experiments, the experimental solutions were (1) bath solution: 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm Hepes and 10 mm glucose at pH 7.4 adjusted with NaOH (for Ca2+‐free experiments 5 mm EGTA was added instead of 2 mm CaCl2); (2) pipette solution: 140 mm KCl, 5 mm EGTA and 10 mm Hepes at pH 7.4 adjusted with KOH. In some experiments, the free calcium concentration of the pipette solution was maintained at 1 μm as calculated with the MAXC program (Stanford University). Data from whole‐cell voltage‐clamp recordings were acquired at 10 kHz throughout the experiments and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Axon Instruments, Foster City, CA, USA). The membrane potential was clamped at −60 mV.
All experiments were performed at 25°C unless otherwise stated. Heat stimulation was induced by increasing the bath temperature using a pre‐heated solution warmed in an inline heater (1°C s−1, with a maximum of 62°C). The temperature was monitored using a thermocouple (TC‐344; Warner Instruments, Hamden, CT, USA) placed within 100 μm of the patch‐clamped cell. The heat stimulation was stopped immediately upon confirming that TRPA1 currents are inactivated or desensitized. Temperature profiles and Arrhenius plots for the data from whole‐cell voltage‐clamp recordings were calculated using Origin software (OriginLab, Northampton, MA, USA). Temperature sensitivity has been explained by a mechanism based on a fine balance of large changes in enthalpy and entropy (Voets et al. 2004). Alternatively, temperature gating could be driven by changes in heat capacity (Clapham & Miller, 2011). Temperature directionality could also be determined by the degree of allosteric coupling of a unidirectional temperature sensor to the channel gate (Jara‐Oseguera & Islas, 2013). Although the above mechanisms of temperature activation differ substantially, we believe that thermal sensitivity (Q 10) is still a reliable marker to some extent. The absolute current values were plotted on a log scale against the reciprocal of the absolute temperature (T) (Arrhenius plot), and the temperature threshold for channel activation was determined by the temperature that caused a change in the slope. For current density analysis of TRPA1 channels, the peak currents induced by heat stimulation were measured and presented as pA pF−1.
Statistical analysis
Data in all figures are shown as means ± standard error of the mean (S.E.M.). Statistical analysis was performed by Student's t test or analysis of variance (ANOVA) followed by a two‐tailed multiple t test with Bonferroni correction using Origin 8.5 software. Probability values (P) of <0.05 were considered statistically significant.
Results
Extracellular Ca2+‐dependent heat activation of gaTRPA1
We recently found that whole‐cell and inside‐out single‐channel recordings in HEK293T cells expressing gaTRPA1 showed both heat and AITC‐evoked current activation. We also found that temperature‐evoked gaTRPA1 currents were not observed in the absence of extracellular Ca2+, although these cells did respond to AITC stimulation (Kurganov et al. 2014). To explore how extracellular Ca2+ is involved in the heat‐evoked activation of gaTRPA1, we first examined gaTRPA1 channel activation upon heat stimulation using a high concentration (1 μm) of intracellular free Ca2+ in the pipette solution because intracellular Ca2+ is known to affect TRPA1 channel function (Jordt et al. 2004; Doerner et al. 2007; Zurborg et al. 2007). In the whole‐cell configuration, negligible inward currents were observed at −60 mV following heat stimulation (from 25°C to over 45°C) with 1 μm intracellular Ca2+ (Fig. 1 A) or without intracellular Ca2+ (Fig. 1 C) in the absence of extracellular Ca2+, yet gaTRPA1 channels responded to AITC (400 μm) with large inward currents. This activation is known to occur through covalent modification of cytosolic cysteine residues of TRPA1 (Macpherson et al. 2005; Hinman et al. 2006) and was inhibited by the TRPA1‐specific antagonist HC‐030031 (50 μm) (Fig. 1 A). Next, we tested whether other divalent cations are involved in heat‐evoked activation of gaTRPA1. The absence of extracellular Mg2+ did not affect the heat‐evoked activation of gaTRPA1 (Fig. 1 B). Substitution of extracellular Ca2+ with another divalent cation, Mg2+ or Ba2+, did not evoke gaTRPA1 currents upon heat stimulation, but AITC‐evoked currents were induced (Fig. 1 C and D), suggesting that heat‐evoked activation of gaTRPA1 channel is solely dependent on extracellular Ca2+. To gain more information on the dependency of TRPA1 heat activation on extracellular Ca2+, we recorded channel activity in the presence of increasing extracellular Ca2+ concentrations (0 to 2 mm) under heat stimulation. The current densities of the heat‐evoked gaTRPA1 currents increased with increasing extracellular Ca2+ concentrations (Fig. 1 E and F). However, the temperature thresholds for heat‐evoked gaTRPA1 activation did not differ between 1 and 2 mm extracellular Ca2+ (34.6 ± 1.5 °C, n = 5 and 35.8 ± 0.5 °C, n = 6, respectively) (Fig. 1 G and H). These results suggest that extracellular, but not intracellular, Ca2+ ions are important for heat activation of gaTRPA1.
Figure 1. Heat‐evoked gaTRPA1 currents in HEK293T cells in the absence and presence of extracellular Ca2+ .
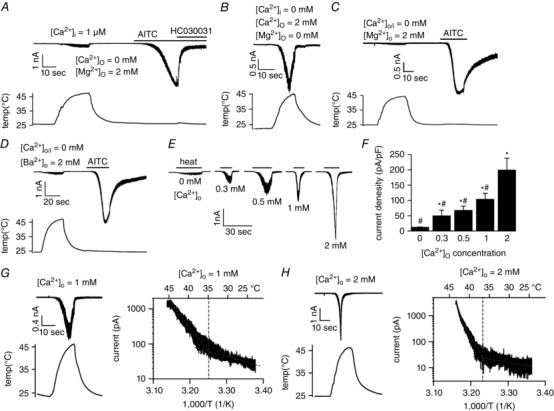
A, representative current (upper) and temperature (lower) traces in response to heat or AITC (400 μm) stimulation in the absence of extracellular Ca2+ in HEK293T cells expressing gaTRPA1. HC030031 (50 μm) was added after AITC stimulation. The pipette solution included 1 μm intracellular free Ca2+. B, representative current (upper) and temperature (lower) traces in the absence of extracellular Mg2+ with extracellular Ca2+ (2 mm) in HEK293T cells expressing gaTRPA1. C, representative current (upper) and temperature (lower) traces for heat and AITC (400 μm) stimulation in the absence of extracellular and intracellular Ca2+. D, representative current (upper) and temperature (lower) traces in response to heat or AITC (400 μm) simulation in the presence of extracellular Ba2+ (2 mm) and absence of extracellular Ca2+ in HEK293T cells expressing gaTRPA1. E, representative gaTRPA1‐mediated heat‐evoked whole‐cell current traces. Lines above the traces show heat stimulation duration. Extracellular Ca2+ concentrations ([Ca2+]o) are shown below the traces. F, heat‐evoked gaTRPA1 current densities at different extracellular Ca2+ concentrations. G and H, representative current (upper) and temperature (lower) traces for gaTRPA1 at 1 mm (G, left) and 2 mm (H, left) extracellular Ca2+, and Arrhenius plots of currents elicited by heat stimulation from 25°C at 1 mm (G, right) and 2 mm (H, right) extracellular Ca2+. Data represent means plus/minus S.E.M. (n = 5–8). * P < 0.05 vs. 0 mm; # P < 0.05 vs. 2 mm.
Extracellular Ca2+ is not essential for heat‐evoked activation of chicken and rat snake TRPA1
To find the residue(s) responsible for heat‐evoked gaTRPA1 activation in the presence of extracellular Ca2+, we examined heat‐evoked currents of TRPA1 from different species. Based on the finding that chTRPA1 and rsTRPA1 expressed in Xenopus oocytes are activated by heat (Gracheva et al. 2010; Saito et al. 2014), we examined whether heat‐evoked activation of chTRPA1 and rsTRPA1 in HEK293T cells requires extracellular Ca2+. We first compared chTRPA1 and rsTRPA1 activation in the presence and absence of extracellular Ca2+ in whole‐cell configurations at −60 mV. Similar to gaTRPA1 (Fig. 1 B and E), we observed large inward currents for both chTRPA1 (Fig. 2 A) and rsTRPA1 (Fig. 2 E) in the presence of extracellular Ca2+ which were inactivated/desensitized even during heat stimulation. Interestingly, small but significant inward currents were elicited upon heat stimulation even in the absence of extracellular Ca2+ for chTRPA1 (Fig. 2 B) and rsTRPA1 channels (Fig. 2 F), suggesting that heat‐evoked TRPA1 activation in these species does not depend solely on extracellular Ca2+. Although the heat‐evoked currents were smaller in the absence of extracellular Ca2+, temperature thresholds for heat‐evoked activation of chTRPA1 and rsTRPA1 determined by constructing Arrhenius plots were unchanged in the presence of extracellular Ca2+ (chTRPA1, 39.3 ± 0.5°C, n = 5 and 39.6 ± 1.2°C, n = 6, Fig. 2 C and D; and rsTRPA1, 37.4 ± 0.9°C, n = 6 and 39 ± 0.6°C, n = 6, Fig. 2 G and H).
Figure 2. Heat‐evoked currents of chTRPA1 and rsTRPA1 expressed in HEK293T cells in the presence and absence of extracellular Ca2+ .
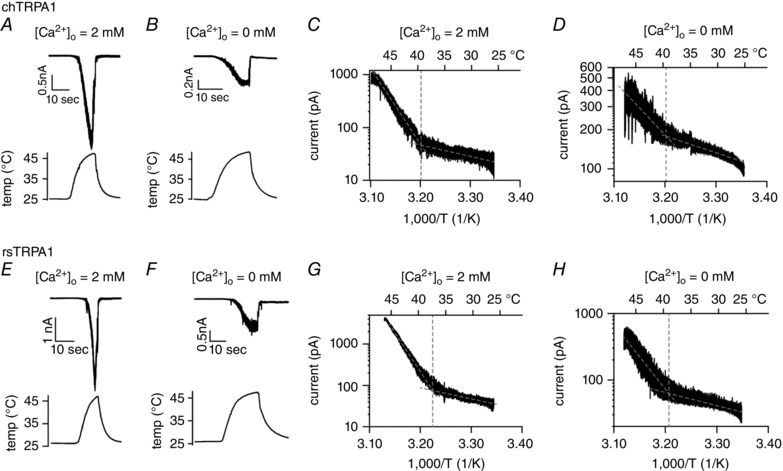
A and B , representative current (upper) and temperature (lower) traces for chTRPA1 in the presence (A) and absence (B) of extracellular Ca2+. C and D , Arrhenius plots of currents elicited by heat stimulation from 25°C in the presence (C) and absence (D) of extracellular Ca2+. The average temperature thresholds for heat‐evoked activation were 39.3 ± 0.5°C, (n = 5) and 39.6 ± 1.2°C (n = 6) in the presence and absence, respectively, of extracellular Ca2+. E and F , representative current (upper) and temperature (lower) traces for rsTRPA1 in the presence (E) and absence (F) of extracellular Ca2+. G and H, Arrhenius plots of currents elicited by heat stimulation from 25°C in the presence (G) and absence (H) of extracellular Ca2+. Temperature thresholds for heat‐evoked activation were 37.4 ± 0.9°C (n = 6) and 39 ± 0.6°C (n = 6) in the presence and absence, respectively, of extracellular Ca2+.
A single mutation within the pore vestibule participates in gaTRPA1 heat activation
Given the findings that gaTRPA1 heat activation requires extracellular, but not intracellular Ca2+ and that heat‐evoked activation of chTRPA1 and rsTRPA1 occurred even in the absence of extracellular Ca2+, we compared the TRPA1 amino acid sequences for the three species (Fig. 3 A). Because extracellular Ca2+ is required for channel activation following heat stimulation, we hypothesized that negatively charged amino acids located on the extracellular face of gaTRPA1, but not chTRPA1 or rsTRPA1, are involved in this heat‐evoked activation. Through an amino acid alignment, we found only one negatively charged glutamate at position 894 of gaTRPA1, whereas the equivalent residues in chTRPA1 (897) and rsTRPA1 (895) had neutral glutamines (Fig. 3 A in red). To understand the location of gaTRPA1 Glu894 in greater detail, we performed homology modelling of gaTRPA1 based on the cryo‐EM structure of human TRPA1 (Paulsen et al. 2015). The resulting model suggests that Glu894 is exposed to the extracellular side of the gaTRPA1 channel at the pore vestibule (Fig. 3 B). As such, we constructed a gaTRPA1 channel with Glu894 mutated to Gln (E894Q) and analysed the channel activity of this mutant expressed in HEK293T cells following heat and chemical stimulations. E894Q exhibited a large inward current upon heat application in the presence of extracellular Ca2+, and a small but significant inward current was observed even in the absence of extracellular Ca2+ (Fig. 3 C and D), suggesting that Glu894 of gaTRPA1 is involved in the heat‐evoked activation of gaTRPA1 in the presence of extracellular Ca2+. Although the sizes of the heat‐evoked currents for E894Q differed in the presence and absence of extracellular Ca2+, AITC‐evoked currents were similar among wild‐type (WT) TRPA1 (420.2 ± 50.6 pA pF−1, n = 6) in the presence of extracellular Ca2+ and TRPA1 E894Q in the presence (425.3 ± 67.9 pA pF−1, n = 5) and absence (415.2 ± 81.5 pA pF−1, n = 6) of extracellular Ca2+ (Fig. 3 E). Moreover, changing Glu894 to positively charged Lys (E894K) conferred heat‐evoked current activation in the presence (219.4 ± 26.5 pA pF−1, n = 5) and absence (87.7 ± 15.3 pA pF−1, n = 6) of extracellular Ca2+ (Fig. 3 F and G), which was similar to that seen for E894Q, suggesting that neutralization of the negative charge is important for heat‐evoked activation of gaTRPA1 in the absence of extracellular Ca2+. To further confirm the importance of the negative charge at position 894 of gaTRPA1 for heat‐evoked activation in the presence of extracellular Ca2+, we mutated Gln897 in chTRPA1 and Gln895 in rsTRPA1 to Glu. Interestingly, both Q897E (chTRPA1) and Q895E (rsTRPA1) mutants showed significantly smaller inward currents upon heat stimulation in the absence of extracellular Ca2+ (20.9 ± 6.3 pA pF−1, n = 4 and 50.8 ± 11.9 pA pF−1, n = 5, respectively) than in WT channels (Fig. 3 I, J, L and M), while Q897E (chTRPA1) and Q895E (rsTRPA1) mutants showed large inward currents upon heat stimulation in the presence of extracellular Ca2+ (97.1 ± 16.5 pA pF−1, n = 5 and 335.7 ± 22.2 pA pF−1, n = 5, respectively) (Fig. 3 H, J, K and M), similar to that seen for WT channels. These data suggest that more negative charges on the surface increase Ca2+ sensitivity of the channels to heat stimulation as in gaTRPA1. Together, these results indicate that AITC‐induced activation of gaTRPA1 is different from activation induced by temperature and that a negatively charged amino acid is important for heat‐evoked activation of TRPA1 channels in the presence of extracellular Ca2+.
Figure 3. Location of amino acid residues in gaTRPA1 that are required for heat‐evoked activation.
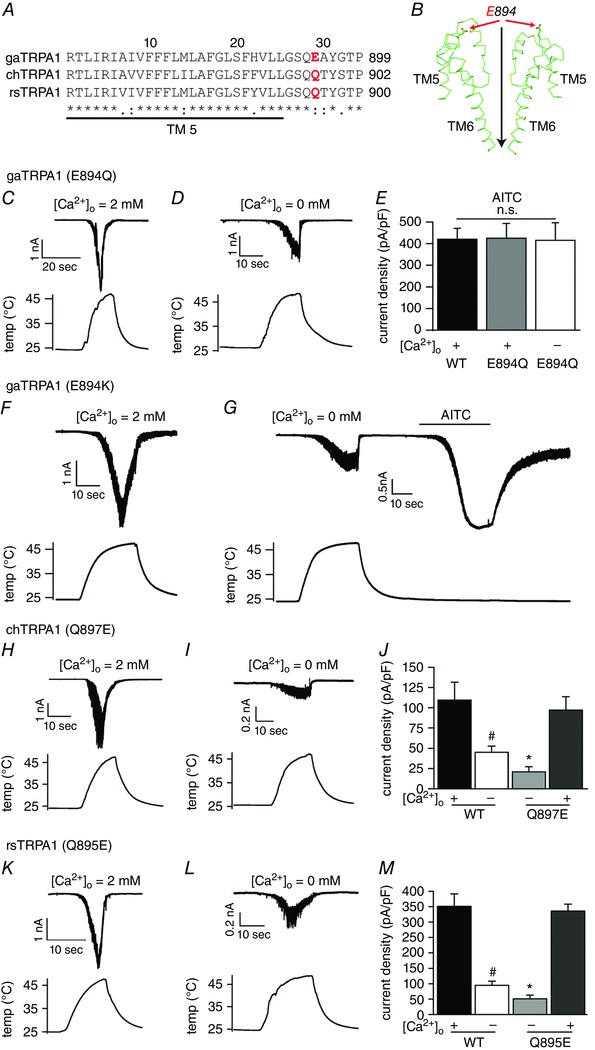
A, alignment of TM5 amino acids in gaTRPA1, chTRPA1 and rsTRPA1. The location of amino acids that differ from gaTRPA1 (E894) is indicated in red. Numbering is based on the gaTRPA1 sequence. *, : and . indicate identical, similar and different, respectively. B, a side view of the homology model structure for two gaTRPA1 subunits based on hTRPA1. Red and black arrow indicates the E894 side chain and direction of ion flow, respectively. C and D, representative current (upper) and temperature (lower) traces for gaTRPA1 (E894Q) in the presence (C) and absence (D) of extracellular Ca2+. E, comparison of AITC‐evoked currents for wild‐type (WT; black) and E894Q (grey and white) gaTRPA1 in the presence and absence of extracellular Ca2+. F and G, representative current (upper) and temperature (lower) traces for heat and AITC (400 μm) stimulation in the presence (F) and absence (G) of extracellular and intracellular Ca2+ in HEK293T cells expressing gaTRPA1 mutant (E894K). H, I, K and L, representative current (upper) and temperature (lower) traces for chTRPA1 (Q897E) (H, I) and rsTRPA1 (Q895E) (K, L) in the presence (H, K) and absence (I, L) of extracellular Ca2+. J and M, comparison of heat‐evoked currents for wild type (black and white) and Q to E mutants (light and dark grey) of chTRPA1 (J) and rsTRPA1 (M) in the presence (+) and absence (−) of extracellular Ca2+. Data represent the means ± S.E.M. (n = 4–6). n.s., no significance; * P < 0.05 vs. WT (+ and −), Q897E and Q895E (+); # P < 0.05 vs. WT (+), Q897E and Q895E (+).
Conserved mutations near the extracellular face of pore helix 2 are needed for large activation by heat
Since the gaTRPA1 E894Q mutant is similar to chTRPA1 and rsTRPA1 in that heat‐evoked currents are smaller in the absence of extracellular Ca2+ than in the presence of extracellular Ca2+ (Figs 2 and 3), we hypothesized that negatively charged amino acids at the extracellular side of the channel could be conserved across all three species (gaTRPA1, chTRPA1 and rsTRPA1). Further amino acid sequence alignment identified two candidate amino acids, Glu and Asp, before (green) and within (cyan) the pore helix 2 (Fig. 4 A). To neutralize these charged amino acids, we mutated the Glu and Asp to Gln and Asn, respectively, and made single, double and triple mutants of the channels. As mentioned above (Fig. 3 D), gaTRPA1 (E894Q) exhibited significantly larger heat‐evoked currents at −60 mV in the absence of extracellular Ca2+ than wild‐type gaTRPA1 (97.8 ± 14.3 pA pF−1, n = 6 vs. 11.9 ± 1.2 pA pF−1, n = 5) (Fig. 4 C). We therefore made two double mutations (E894Q–D918N and E894Q–E922Q) and one triple mutation (E894Q–D918N–E922Q) in gaTRPA1. Although the two double mutations showed heat‐evoked currents that were similar in size to the single mutation E894Q (E894Q–D918N, green, 125.6 ± 40.9 pA pF−1, n = 6; E894Q–E922Q, cyan, 120.5 ± 38.7 pA pF−1, n = 5) in the absence of extracellular Ca2+, the triple mutant showed large inward currents upon heat stimulation in the absence (197.2 ± 41.1 pA pF−1, n = 5, navy) and presence (200.4 ± 45.2 pA pF−1, n = 6, pink) of extracellular Ca2+, and the currents were similar in size to those of wild‐type gaTRPA1 currents in the presence of extracellular Ca2+ (199.1 ± 39.1 pA pF−1, n = 5, black) (Fig. 4 B and C). Unlike gaTRPA1, in the absence of extracellular Ca2+, chTRPA1 and rsTRPA1 channels were activated by heat with small inward currents (Fig. 2 B and F). Thus, we made two single point mutants in chTRPA1 and rsTRPA1 (D921N and D925N for chTRPA1; E919Q and E923Q for rsTRPA1), as well as double mutants (D921N–D925N for chTRPA1; E919Q–E923Q for rsTRPA1) and examined the channel activity of these mutants after heat stimulation in the absence or presence of extracellular Ca2+. Single point mutations in chTRPA1 (D921N and D925N) had inward currents (D921N, green, 48.6 ± 14 pA pF−1, n = 6; D925N, cyan, 44.8 ± 10.4 pA pF−1, n = 6) that were similar to wild type (white, 38.5 ± 7.6 pA pF−1, n = 6) (Fig. 4 D and E). A double chTRPA1 mutant (D921N/D925N) showed large inward currents (104 ± 16 pA pF−1, n = 6, navy), which were not affected by the presence of extracellular Ca2+ (113 ± 26 pA pF−1, n = 6, pink) and were similar to wild‐type chTRPA1 currents in the presence of extracellular Ca2+ (109.3 ± 22.2 pA pF−1, n = 6, black). For rsTRPA1, the single mutant E919Q (141.6 ± 37.5 pA pF−1, n = 5, green) had similar inward currents in response to heat stimulation compared to wild type (95.1 ± 25.4 pA pF−1, n = 6, white), whereas the E923Q single mutant showed significantly larger heat‐evoked currents (189.9 ± 24.9 pA pF−1, n = 5, cyan) than wild type (Fig. 4 F and G). Meanwhile, the E919Q–E923Q rsTRPA1 double mutant showed large inward currents (298.4 ± 56.5 pA pF−1, n = 5, navy) that were not affected by extracellular Ca2+ (330.2 ± 51.4 pA pF−1, n = 5, pink) and were similar to the wild‐type rsTRPA1 currents in the presence of extracellular Ca2+ (340.3 ± 40.9 pA pF−1, n = 7, black); this pattern was similar to that of chTRPA1. These results suggest that neutralization of the two charged amino acids by extracellular Ca2+ is important for a heat‐evoked large activation of gaTRPA1, chTRPA1, and rsTRPA1.
Figure 4. Heat‐evoked activation of wild‐type and mutant TRPA1 channels from three species.
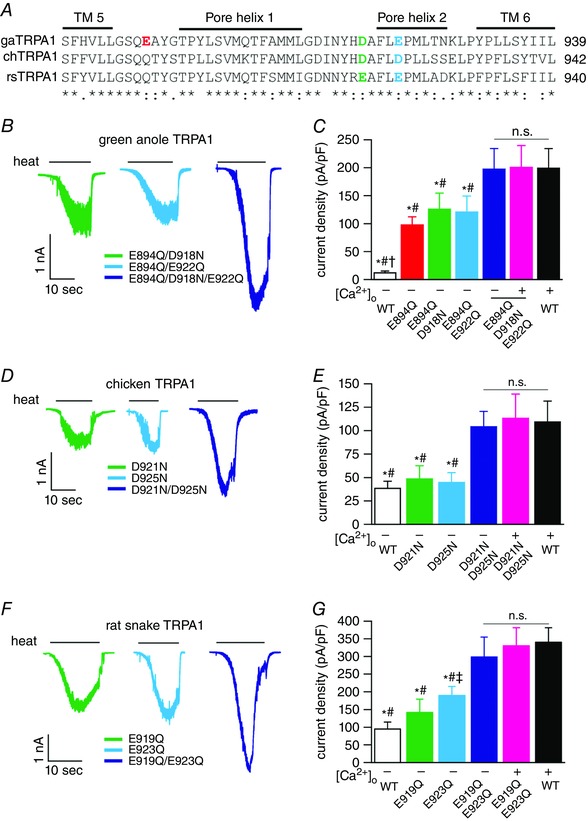
A, amino acid sequence alignment of gaTRPA1, chTRPA1, and rsTRPA1. The locations of TM5, pore helix 1, pore helix 2, and TM6 are indicated by lines above the alignment. The negatively charged amino acids examined are shown in red (gaTRPA1), green and cyan (all three species). Numbering on the right of alignment is based on gaTRPA1. *, : and . indicate identical, similar and different, respectively. B, D and F, representative heat‐evoked whole‐cell current traces recorded at −60 mV for gaTRPA1 (B), chTRPA1 (D), and rsTRPA1 (F) having the indicated mutations. C, E and G, comparison of heat‐evoked activation of wild‐type and mutated gaTRPA1 channels (C), chTRPA1 (E) and rsTRPA1 (G) in the presence (+) and absence (−) of extracellular Ca2+. Data represent the means ± S.E.M. (n = 5–7). n.s., no significance. * P < 0.05 vs. WT (+). # P < 0.05 vs. E894Q/D918N/E922Q (−,+); D921N/D925N (−,+); E919Q/E923Q (−,+). † P < 0.05 vs. E894Q; E894Q/D918N; E894Q/E922Q (−). ‡ P < 0.05 vs. WT (−).
Discussion
Our findings showed that heat‐evoked activation of gaTRPA1 is dependent on extracellular Ca2+ and requires Ca2+ binding to negatively charged amino acids near the extracellular face of the channel pore. Together, extracellular Ca2+ binding and heat could activate the TRPA1 channel, whereas the absence or substitution of other divalent cations for extracellular Ca2+ abrogated heat‐evoked currents (Fig. 1 A–D). The inability of intracellular Ca2+ to activate TRPA1 following heat stimulation in our experiments could support our hypothesis that extracellular Ca2+ is involved only in heat‐evoked activation of gaTRPA1, as opposed to previous reports showing that intracellular Ca2+ is important for TRPA1 activation (Jordt et al. 2004; Doerner et al. 2007; Zurborg et al. 2007), as well as potentiation/activation and secondary inactivation after Ca2+ influx through the TRPA1 channel following chemical stimulation (Nagata et al. 2005; Karashima et al. 2008; Wang et al. 2008). As we previously reported, the gaTRPA1 channel kinetics were quite different between AITC‐ and heat‐mediated stimulation (Kurganov et al. 2014), suggesting that the gating mechanisms differed between the two stimuli. This result could indicate that the requirement for intracellular Ca2+ also differs in the different stimulation. Meanwhile, heat‐evoked activation of chTRPA1 and rsTRPA1 was observed both in the absence and presence of extracellular Ca2+, although the inward currents were significantly smaller in the absence of extracellular Ca2+ (Figs 2 and 4). An alignment of gaTRPA1, chTRPA1, and rsTRPA1 amino acid sequences showed that the residues corresponding to Glu894 in gaTRPA1, 897 and 895, in chTRPA1 and rsTRPA1, respectively, were both Gln (Fig. 3 A). Mutation of Glu894 to Gln in the gaTRPA1 channel led to a small, but significant heat‐evoked activation even in the absence of extracellular Ca2+, whereas currents for the chTRPA1 (Q897E) and rsTRPA1 (Q895E) mutants were reduced in the absence of extracellular Ca2+ (Figs 3 and 4). Moreover, neutralization of acidic amino acids in TRPA1 that are common to all three species may mimic the effect of extracellular Ca2+, since TRPA1 with Gln showed activation of large heat‐evoked inward currents even in the absence of extracellular Ca2+, and the current sizes were comparable to those seen for wild‐type channels in the presence of extracellular Ca2+ (Fig. 4). Thus, binding of extracellular Ca2+ to acidic amino acids is likely to be important for heat‐evoked activation of TRPA1 expressed in green anole, chicken and rat snake. As shown in this study and in previous studies (Gracheva et al. 2010; Kurganov et al. 2014; Saito et al. 2014), the activation thresholds of TRPA1 from the three species are between 36°C and 40°C, suggesting that these TRPA1 channels would not be activated at 25°C, the temperature at which we performed patch‐clamp recordings. However, as we reported previously (Kurganov et al. 2014), there is a synergism between temperature and chemical agonists such as AITC, which indicates that in the presence of other stimulations, temperatures lower than the threshold can theoretically activate TRPA1 channels. Indeed, in an earlier study (Fujita et al. 2008), we observed single‐channel opening of human TRPA1 in HEK293T cells at 25°C in the absence of a possible agonist, suggesting that TRPA1 channels can be open under specific conditions.
Atomic level structures of TRPV1, a capsaicin‐ and heat‐activated TRP channel, have recently been determined by single‐particle analysis with cryo‐EM under conditions wherein the purified protein was in complex with amphipathic polymers or in nanodiscs (Caterina et al. 1997; Cao et al. 2013; Liao et al. 2013; Gao et al. 2016). The structural data suggest that the presence of phosphatidylinositides supports a closed state of TRPV1 and removal of endogenous phosphatidylinositides upon heat stimulation could contribute to channel gating and further cation influx through the TRPV1 pore (Gao et al. 2016). Thus, we propose a new model for extracellular Ca2+‐dependent heat‐evoked activation of TRPA1 wherein binding of extracellular Ca2+ to acidic amino acids near the extracellular side of the TRPA1 channel contributes to channel gating and cation influx upon heat stimulation (Fig. 5). Support for this model is provided by the involvement of Glu at position 920 of human TRPA1 in collecting cations into the mouth of the pore and changing the surface potential by ∼16 mV (Christensen et al. 2016). Our model is also supported by the channel gating and ion permeation of the highly Ca2+‐selective channel TRPV6, which involves extracellular cation binding to the channel (Saotome et al. 2016). Although the Ca2+ sensitivity and permeability of TRPV6 (>100) (Yue et al. 2001) and TRPA1 (>5) (Karashima et al. 2010) differ, the overall locations of the acidic amino acids in both channels are similar, suggesting that a comparison of the characteristics of the two channels could clarify the significance of Ca2+ binding in heat‐evoked gating of TRPA1 channels.
Figure 5. Possible external Ca2+ binding sites on the gaTRPA1 channel.
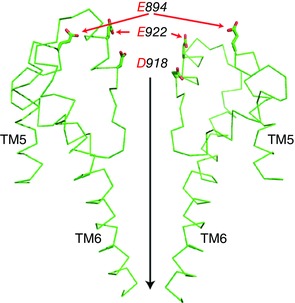
A ribbon representation showing a side view of the transmembrane domains of two opposing gaTRPA1 model subunits based on hTRPA1 is shown. The side chains of mutated amino acids are indicated by red arrows and the black downward vertical arrow indicates the direction of ion flow.
Since extracellular Ca2+ dependency has not been clearly shown in heat‐activated TRPA1 channels from various species (Hamada et al. 2008; Gracheva et al. 2010; Kang et al. 2012; Saito et al. 2012, 2014), we emphasize that, in addition to potentiation/activation or secondary inactivation (Nagata et al. 2005; Karashima et al. 2008; Wang et al. 2008) by intracellular Ca2+, binding of extracellular Ca2+ to acidic amino acids in the TRPA1 channel vestibule is important for heat‐evoked channel gating. Ca2+ concentrations vary in extracellular spaces during development (Brown et al. 1995) and proliferation of various cells such as skin keratinocytes (Pillai et al. 1990), although TRPA1 is not strongly expressed in epithelial cells. In the sensory neurons of vertebrates where TRPA1 is well expressed, physiological extracellular Ca2+ concentrations generally fluctuate between 1.8 and 2 mm, but do not fall below 1 mm. Thus, the physiological significance of the extracellular Ca2+ for heat‐evoked activation of TRPA1 from several species is not known. Nonetheless, the dependence of TRPA1 heat‐induced activation on extracellular Ca2+ in some species could be involved in physiological phenomena that are affected by extracellular Ca2+ levels.
In summary, we have identified important negatively charged amino acid residues located at the outer vestibule of the gaTRPA1 channel that play an important role in heat‐induced channel activation, but do not participate in responses to other stimuli such as AITC. Moreover, the observation that several acidic amino acids in TRPA1 from three species (green anole, chicken and rat snake) contribute to large channel activity following heat stimuli suggests that allosteric coupling may be involved in this type of channel activation.
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
E.K. and M.T. designed the experiments and wrote the manuscript. E.K. and C.T.S. performed the experiments. E.K., C.T.S., S.S. and M.T. discussed and interpreted the data. M.T. supervised this work. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (No. 15H02501 to M.T.), a Grant‐in‐Aid for Scientific Research on Innovative Areas ‘Integrative understanding of biological phenomena with temperature as a key theme’ (No. 15H05928 to M.T.) and CPIS of SOKENDAI.
Acknowledgements
We thank Drs Y. Kubo (NIPS), M. Nishida (NIPS) and T. Nakagawa (Kyoto University) for their helpful comments.
References
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ & Patapoutian A (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE & Zygmunt PM (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Vassilev PM & Hebert SC (1995). Calcium ions as extracellular messengers. Cell 83, 679–682. [DOI] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y & Julius D (2013). TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ & Julius D (1999). A capsaicin‐receptor homologue with a high threshold for noxious heat. Nature 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD & Julius D (1997). The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B & Kim D (2013). Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4, 2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AP, Akyuz N & Corey DP (2016). The outer pore and selectivity filter of TRPA1. PLoS One 11, e0166167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE & Miller C (2011). A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc Natl Acad Sci USA 108, 19492–19497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero‐Morales JF, Gracheva EO & Julius D (2011). Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci USA 108, E1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H & Wetzel CH (2007). Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282, 13180–13189. [DOI] [PubMed] [Google Scholar]
- Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T & Tominaga M (2008). Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118, 4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Cao E, Julius D & Cheng Y (2016). TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet R (2008). TRP channels entering the structural era. J Physiol 586, 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero‐Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS & Julius D (2010). Molecular basis of infrared detection by snakes. Nature 464, 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M & Caterina M (2002). Heat‐evoked activation of the ion channel, TRPV4. J Neurosci 22, 6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ & Garrity PA (2008). An internal thermal sensor controlling temperature preference in Drosophila . Nature 454, 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM & Julius D (2006). TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Tanabe M, Kayama Y, Abe M, Kashio M, Koizumi K, Okumura Y, Morimitsu Y, Tominaga M, Ozawa Y & Watanabe T (2009). Miogadial and miogatrial with alpha,beta‐unsaturated 1,4‐dialdehyde moieties–novel and potent TRPA1 agonists. Life Sci 85, 60–69. [DOI] [PubMed] [Google Scholar]
- Jara‐Oseguera A & Islas LD (2013). The role of allosteric coupling on thermal activation of thermo‐TRP channels. Biophys J 104, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID & Julius D (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA & Garrity PA (2012). Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila . Nature 481, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T & Nilius B (2008). Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5‐biphosphate manipulators. Pflugers Arch 457, 77–89. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Talavera K, Janssens A, Voets T & Nilius B (2010). Agonist‐induced changes in Ca2+ permeation through the nociceptor cation channel TRPA1. Biophys J 98, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B & Voets T (2009). TRPA1 acts as a cold sensor in vitro and in vivo . Proc Natl Acad Sci USA 106, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JY, Jang Y, Cho H, Lee CH, Jang KH, Chang YH, Shin J & Oh U (2007). Hydroxy‐alpha‐sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci 26, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Kurganov E, Zhou Y, Saito S & Tominaga M (2014). Heat and AITC activate green anole TRPA1 in a membrane‐delimited manner. Pflugers Arch 466, 1873–1884. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ & Corey DP (2006). TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair‐cell transduction. Neuron 50, 277–289. [DOI] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D & Cheng Y (2013). Structure of the TRPV1 ion channel determined by electron cryo‐microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM & Julius D (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S & Patapoutian A (2005). The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15, 929–934. [DOI] [PubMed] [Google Scholar]
- Moparthi L, Kichko TI, Eberhardt M, Hogestatt ED, Kjellbom P, Johanson U, Reeh PW, Leffler A, Filipovic MR & Zygmunt PM (2016). Human TRPA1 is a heat sensor displaying intrinsic U‐shaped thermosensitivity. Sci Rep 6, 28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, Johanson U & Zygmunt PM (2014). Human TRPA1 is intrinsically cold‐ and chemosensitive with and without its N‐terminal ankyrin repeat domain. Proc Natl Acad Sci USA 111, 16901–16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G & Garcia‐Anoveros J (2005). Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25, 4052–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura Y, Narukawa M, Iwasaki Y, Ishikawa A, Matsuda H, Yoshikawa M & Watanabe T (2010). Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem 74, 1068–1072. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y & Julius D (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S & Patapoutian A (2002. a). A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S & Patapoutian A (2002. b). A heat‐sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049. [DOI] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Mancianti ML, Cline P & Hincenbergs M (1990). Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol 143, 294–302. [DOI] [PubMed] [Google Scholar]
- Qin F (2013). Demystifying thermal channels: driving a channel both forwards and backwards with a single gear? Biophys J 104, 2118–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Banzawa N, Fukuta N, Saito CT, Takahashi K, Imagawa T, Ohta T & Tominaga M (2014). Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol Biol Evol 31, 708–722. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T & Tominaga M (2012). Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem 287, 30743–30754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Moldenhauer H & Baez‐Nieto D (2011). Could an allosteric gating model explain the role of TRPA1 in cold hypersensitivity? J Neurosci 31, 5554–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome K, Singh AK, Yelshanskaya MV & Sobolevsky AI (2016). Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P & Davis JB (2002). TRPV3 is a temperature‐sensitive vanilloid receptor‐like protein. Nature 418, 186–190. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S & Patapoutian A (2003). ANKTM1, a TRP‐like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF & Nilius B (2005). Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025. [DOI] [PubMed] [Google Scholar]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y & Tominaga M (2006). TRPM2 activation by cyclic ADP‐ribose at body temperature is involved in insulin secretion. EMBO J 25, 1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A & Jegla T (2003). Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823. [DOI] [PubMed] [Google Scholar]
- Voets T (2012). Quantifying and modeling the temperature‐dependent gating of TRP channels. Rev Physiol Biochem Pharmacol 162, 91–119. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V & Nilius B (2004). The principle of temperature‐dependent gating in cold‐ and heat‐sensitive TRP channels. Nature 430, 748–754. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B & Voets T (2011). TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD & Liman ER (2008). The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283, 32691–32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ & Ma Q (2007). Nociceptors – noxious stimulus detectors. Neuron 55, 353–364. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos‐Santiago I, Xie Y, DiStefano PS, Curtis R & Clapham DE (2002). TRPV3 is a calcium‐permeable temperature‐sensitive cation channel. Nature 418, 181–186. [DOI] [PubMed] [Google Scholar]
- Yue L, Peng JB, Hediger MA & Clapham DE (2001). CaT1 manifests the pore properties of the calcium‐release‐activated calcium channel. Nature 410, 705–709. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Chen J, Faltynek CR, Moreland RB & Neelands TR (2008). Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci 27, 605–611. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A & Clapham DE (2011). Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold‐transducer in the peripheral nervous system. Proc Natl Acad Sci USA 108, 18114–18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O & Heppenstall PA (2007). Direct activation of the ion channel TRPA1 by Ca2+ . Nat Neurosci 10, 277–279. [DOI] [PubMed] [Google Scholar]


