Abstract
Key points
We tested perceived head‐on‐feet orientation and the direction of vestibular‐evoked balance responses in passively and actively held head‐turned postures.
The direction of vestibular‐evoked balance responses was not aligned with perceived head‐on‐feet orientation while maintaining prolonged passively held head‐turned postures. Furthermore, static visual cues of head‐on‐feet orientation did not update the estimate of head posture for the balance controller.
A prolonged actively held head‐turned posture did not elicit a rotation in the direction of the vestibular‐evoked balance response despite a significant rotation in perceived angular head posture.
It is proposed that conscious perception of head posture and the transformation of vestibular signals for standing balance relying on this head posture are not dependent on the same internal representation. Rather, the balance system may operate under its own sensorimotor principles, which are partly independent from perception.
Abstract
Vestibular signals used for balance control must be integrated with other sensorimotor cues to allow transformation of descending signals according to an internal representation of body configuration. We explored two alternative models of sensorimotor integration that propose (1) a single internal representation of head‐on‐feet orientation is responsible for perceived postural orientation and standing balance or (2) conscious perception and balance control are driven by separate internal representations. During three experiments, participants stood quietly while passively or actively maintaining a prolonged head‐turned posture (>10 min). Throughout the trials, participants intermittently reported their perceived head angular position, and subsequently electrical vestibular stimuli were delivered to elicit whole‐body balance responses. Visual recalibration of head‐on‐feet posture was used to determine whether static visual cues are used to update the internal representation of body configuration for perceived orientation and standing balance. All three experiments involved situations in which the vestibular‐evoked balance response was not orthogonal to perceived head‐on‐feet orientation, regardless of the visual information provided. For prolonged head‐turned postures, balance responses consistent with actual head‐on‐feet posture occurred only during the active condition. Our results indicate that conscious perception of head‐on‐feet posture and vestibular control of balance do not rely on the same internal representation, but instead treat sensorimotor cues in parallel and may arrive at different conclusions regarding head‐on‐feet posture. The balance system appears to bypass static visual cues of postural orientation and mainly use other sensorimotor signals of head‐on‐feet position to transform vestibular signals of head motion, a mechanism appropriate for most daily activities.
Keywords: EMG, posture, somatosensory systems, standing balance, vestibular system
Key points
We tested perceived head‐on‐feet orientation and the direction of vestibular‐evoked balance responses in passively and actively held head‐turned postures.
The direction of vestibular‐evoked balance responses was not aligned with perceived head‐on‐feet orientation while maintaining prolonged passively held head‐turned postures. Furthermore, static visual cues of head‐on‐feet orientation did not update the estimate of head posture for the balance controller.
A prolonged actively held head‐turned posture did not elicit a rotation in the direction of the vestibular‐evoked balance response despite a significant rotation in perceived angular head posture.
It is proposed that conscious perception of head posture and the transformation of vestibular signals for standing balance relying on this head posture are not dependent on the same internal representation. Rather, the balance system may operate under its own sensorimotor principles, which are partly independent from perception.
Abbreviations
- EMG
electromyography
- EVS
electrical vestibular stimulation
- RMS
root mean square
- SCM
sternocleidomastoid
Introduction
Located within the inner ear, the vestibular apparatus encodes the orientation and motion of the head with respect to the external world, providing the CNS with important cues that are relied upon for standing balance (Fitzpatrick & Day, 2004). Vestibular cues of motion are head‐referenced and require a coordinate transformation to be useful in the context of postural control. While standing, for instance, a sudden head acceleration can occur in any direction in three‐dimensional space, meaning that the CNS must consolidate the head motion with respect to whole‐body orientation. Multisensory integration of signals pertaining to head‐on‐feet posture is required to interpret and transform vestibular signals to elicit an appropriate whole‐body postural response. This spatial transformation of vestibular cues allows for functions spanning the perception of self‐motion and orientation to reflexes that are relied upon for gaze and standing balance (Mergner & Rosemeier, 1998; van der Kooij et al. 1999; Angelaki & Cullen, 2008; Forbes et al. 2016). Humans rely upon vestibular, visual and somatosensory inputs to construct a conscious perception of environmental surroundings and postural orientation (Fitzpatrick & McCloskey, 1994; Massion 1998; Bertholz & Viaud‐Delmon, 1999) and to organize balance control (Fitzpatrick et al. 1994; van der Kooij et al. 1999; Mergner et al. 2003). A broadly held view is that a common central integration of sensorimotor cues is responsible for the conscious perception of postural orientation and the functional transformation of sensorimotor signals for balance control (Popov et al. 1986; Gurfinkel et al. 1988, 1989; Massion 1998). In the present study, we re‐examined the classical work from Gurfinkel and colleagues (Popov et al. 1986; Gurfinkel et al. 1989) and evaluated whether a single internal representation of head‐on‐feet orientation drives conscious perception of head posture and the spatial transformation of vestibular signals for standing balance.
Spatial transformation of vestibular signals can be assessed using an isolated vestibular error while the balance system is engaged (Lund & Broberg, 1983; Britton et al. 1993; Pastor et al. 1993). Electrical vestibular stimulation (EVS) evokes a virtual signal of head rotation (Day & Fitzpatrick, 2005; Peters et al. 2015, 2016) that is interpreted by the CNS as an unexpected vestibular perturbation requiring a whole‐body compensatory postural response to maintain upright balance (Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997; Fitzpatrick & Day, 2004). This vestibular‐evoked response is indicative of the vestibular control of standing balance and has been used widely to assess how head‐referenced vestibular signals contribute to upright posture (Nashner & Wolfson, 1974; Lund & Broberg, 1983; Fitzpatrick et al. 1994; Dakin et al. 2007; Lee Son et al. 2008; Forbes et al. 2016). While standing, subjects can orientate their head in various positions with respect to their feet, meaning that the EVS‐evoked virtual head motion can be orientated in many directions with respect to the base of support. Fittingly, the vestibular‐evoked motor response is aligned interaurally with different head‐on‐feet postures (Lund & Broberg, 1983), demonstrating an orthogonal relationship with head yaw orientation (Lund & Broberg, 1983; Pastor et al. 1993; Mian & Day, 2009). This orthogonal relationship between the direction of the EVS‐evoked balance responses and head‐on‐feet orientation, however, has been suggested to be influenced by body stability (Mian & Day, 2014) and perceptual illusion of head movement (Popov et al. 1986; Gurfinkel et al. 1989).
A well‐accepted model may explain why the perception of head‐on‐feet orientation affects the spatial transformation of the vestibular control of balance. This model suggests that a single internal representation of body configuration (including head‐on‐feet orientation tested here), which integrates sensory and motor cues to form estimates of postural orientation, is used by the CNS to govern perception and balance control (Gurfinkel et al. 1988; Massion, 1994, 1998). Hence, the CNS creates a conscious perception of postural orientation and organizes balance responses from this single internal representation (Popov et al. 1986; Gurfinkel et al. 1988; Massion, 1998; Fig. 1 A). Support for this view comes from the experiments of Gurfinkel and colleagues (Popov et al. 1986; Gurfinkel et al. 1989). In the presence of head‐on‐feet perceptual illusions elicited through muscle vibration or prolonged head‐turned postures, the direction of the vestibular‐evoked balance responses was directed interaurally in relation to the perceived, instead of the actual, head‐on‐feet orientation (Popov et al. 1986; Gurfinkel et al. 1989). Recent work, however, has proposed an alternative model, suggesting that conscious perception and balance control rely, at least partially, on separate sensorimotor integration processes to derive distinct internal representations of whole‐body posture (Luu et al. 2012). For instance, perceived effort during standing is only one‐third of the actual torque applied to the support surface (Luu, 2010), and engagement of the vestibular control of balance occurs irrespective of conscious perception of self‐balancing (Luu et al. 2012). This second model leads to an alternative hypothesis that the internal representations of body orientation for perceptual and balance control may rely on different integrations of sensorimotor cues (Fig. 1 A).
Figure 1. Conceptual schematic representing the underlying theoretical framework related to perception and the vestibular control of balance.
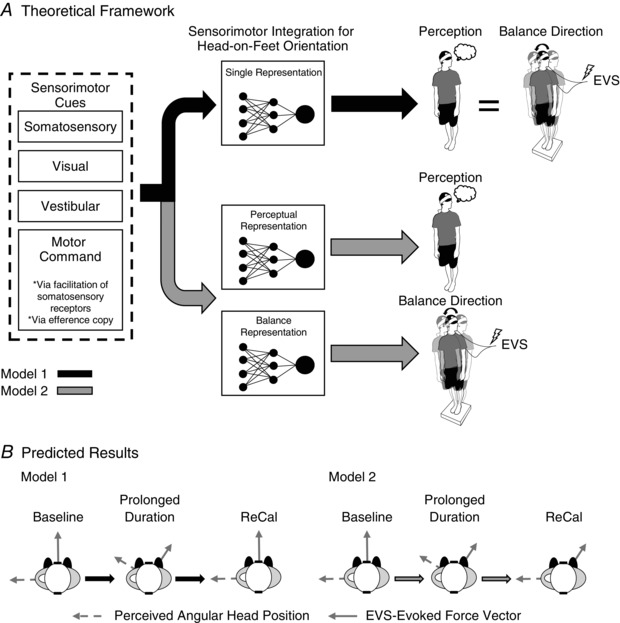
A, two distinct models that represent the conceptual flow of how sensorimotor cues are used to generate perception of head‐on‐feet orientation and transformation of vestibular signals for the control of standing balance. Model 1 (thick black arrows) states that both conscious perception and the spatial transformation of vestibular signals for balance control share a single internal representation, whereas model 2 (thick grey arrows) depicts sensorimotor cues forming two distinct internal representations of head‐on‐feet orientation that govern conscious perception and the vestibular control of balance separately. The shaded black circles and thin black lines depict a conceptual schematic of the convergence of sensorimotor neurons to form the internal representation of body configuration. The curved thin black arrow represents the direction of the balance response. B, the possible expected results for perceived angular head position and the direction of the electrical vestibular stimulation (EVS)‐evoked balance response. For model 1, we expect a perception of head yaw rotation (thin dashed grey arrow) would rotate analogously with the EVS‐evoked balance vector direction (thin solid grey arrow) during the prolonged head‐turned posture and this relationship would return to an interaural direction following visual re‐calibration (ReCal) of head‐on‐feet posture. Conversely, if the directions of perception of head orientation and the EVS‐evoked balance vector rely upon independent internal representations of head‐on‐feet orientation (model 2), visual ReCal of head‐on‐feet posture would affect perception of head yaw orientation without influencing the direction of the EVS‐evoked balance vector.
The purpose of the present study was to explore these two models and determine whether a common, single internal representation of head‐on‐feet orientation is responsible for both conscious perception and spatial transformation of vestibular signals for standing. We designed three experiments in which human participants stood quietly while maintaining a head posture (through neck yaw rotation) facing over the left shoulder for a prolonged duration (>10 min). The first experiment was designed to reproduce the findings of Gurfinkel et al. (1989). Throughout the trials, we intermittently asked participants to report perceived head angular posture and subsequently delivered EVS to elicit whole‐body balance responses. While maintaining a prolonged head‐turned posture with the eyes closed, humans typically experience an illusion of head‐return, i.e. a perceived head angular positional rotation towards an anatomical position (Gurfinkel et al. 1992; Ivanenko & Grasso, 1997; Guerraz et al. 2006). Visual re‐calibration (i.e. periods with and without vision) of head‐on‐feet posture was manipulated to assess the relationship between perceived head orientation and the direction of vestibular‐evoked balance responses. If both conscious perception and spatial transformation of vestibular balance control depend on a single internal representation of body orientation (model 1), we hypothesized that direction of the vestibular‐evoked balance response would be orthogonal (interaural) to the perceived head‐on‐feet posture. Specifically, we expected the vestibular‐evoked balance vector to rotate in accordance with the perceived rotation of head yaw, both before (when the actual and perceived head orientation differ) and after (when the actual and perceived head orientation agree) visual recalibration (Fig. 1 B). Conversely, if conscious perception and balance control rely, at least partially, on separate sensorimotor integration processes to derive distinct internal representations of head‐on‐feet posture (model 2), we hypothesized that the direction of the vestibular‐evoked balance vector would be, under certain circumstances, non‐orthogonal to the perceived head‐on‐feet posture. Specifically, we expected the vestibular‐evoked balance vector to rotate in accordance with the perceived rotation of head yaw during a prolonged head‐turned posture. After visual recalibration, however, we expected the perception of head‐on‐feet posture to align with actual head orientation while the vestibular‐evoked balance vector would not be orthogonal to the perceived (and actual) head yaw orientation (Fig. 1 B). Our results indicate that conscious perception of postural orientation and the spatial transformation of the vestibular‐evoked balance response do not rely on the same internal representation, but instead treat sensorimotor cues in parallel and may therefore arrive at different conclusions regarding head‐on‐feet posture. After establishing this, we further explored the role of static visual cues and neck motor commands in organizing the balance and perceptual internal representations of head‐on‐feet orientation by asking participants to keep their eyes open or actively hold a head‐turned posture.
Methods
Participants
Sixteen (15 males) healthy participants (27.3 ± 5.9 years; 176.4 ± 7.9 cm; 74.4 ± 12.0 kg) with no known history of neurological or vestibular dysfunction took part in three separate experiments (7 in Experiment 1; 7 in Experiment 2; 10 in Experiment 3). Prior to participation, the experimental protocol was explained and each participant granted oral and written informed consent. The study's procedures were conducted in accordance with the Declaration of Helsinki, and the University of British Columbia Clinical Research Ethics Board for human subjects granted approval.
Vestibular stimulation and data collection
Binaural bipolar EVS was delivered to the participants via carbon rubber electrodes (9 cm2) coated in Spectra 360 electrode gel (Parker Laborotories, Fairfield, NJ, USA) that were secured over the mastoid processes with durapore tape (3M Innovations, St. Paul, MN, USA) and an elastic headband. The electrical vestibular stimuli were generated on a PC computer using LabVIEW software (National Instruments, Austin, TX, USA) and sent to an isolated constant current unit (STMISOLA, Biopac Systems Inc., Goleta, CA, USA) via a multifunction data acquisition board (PXI‐6289, National Instruments). The EVS signal consisted of a filtered white noise scaled to a specific amplitude [i.e. stochastic vestibular stimulation (Dakin et al. 2007; Mian & Day, 2009)]. Participants were exposed to 90 s EVS trials with a bandwidth of 0–20 Hz and a peak to peak amplitude of ±4.0 mA [root mean square (RMS) = 1.7 mA] for Experiments 1 and 3 and ±4.5 mA for Experiment 2 (RMS = 1.9 mA).
For the purposes of this study, we utilized stochastic vestibular stimuli to elicit whole‐body postural responses because data collection can occur more quickly and the whole‐body postural response exhibits a greater signal‐to‐noise ratio than traditional galvanic vestibular stimulation (Dakin et al. 2007; Mian and Day, 2009). The time domain measures estimated using such stimuli (cumulant density function – see data analysis below) represents a normalized cross‐covariance between the EVS signal and the motor response, and are similar to the averaged response of square‐wave galvanic vestibular stimulation (Dakin et al. 2007).
To determine the direction of the vestibular‐evoked balance response, time domain measures of correlation between the EVS signal (input) and the horizontal ground reaction forces acting on the body (motor output) were used (Fig. 2 B). This net vestibular‐evoked balance response vector, represented by the ground reaction forces acting on the body, captures the contribution of all muscles involved in the whole‐body response to the vestibular error signal (Pastor et al. 1993; Mian & Day 2009). We recorded and amplified (×50) anteroposterior (Fy) and mediolateral (Fx) shear forces with a force plate (Bertec 4060‐80, Bertec Corp., Columbus, OH, USA). Forces and vestibular stimuli were digitized and sampled at 2048 Hz with a data acquisition board (PXI‐6289; National Instruments) using custom LabVIEW software (National Instruments). Surface electromyography (EMG) was collected from the right sternocleidomastoid (SCM) to estimate neck muscle activity during the prolonged head‐turned posture. Prior to electrode placement, the surface of the skin was cleaned with an alcohol swab and abraded with gel (Nu‐Prep, Weaver and Company, Aurora, CO, USA). Self‐adhesive Ag‐AgCl surface electrodes (Blue Sensor M, Ambu A/S, Ballerup, Denmark) were positioned over the belly of the muscle in a bipolar configuration, in line with the muscle fibres with an inter‐electrode distance of 2 cm centre‐to‐centre. A ground electrode was placed over the anterior surface of the sternal end of the right clavicle. Surface EMG signals were amplified (×1000, Neurolog, Digitimer Ltd, Welwyn Garden City, UK) and band‐pass filtered (30–1000 Hz). Owing to the need for a prolonged continuous recording session, a separate data acquisition board (Micro 1401, Cambridge Electronic Design, Cambridge, UK) was used for EMG data collection. However, based on limitations of the analog‐to‐digital converter, the sampling rate was set at 2017 Hz.
Figure 2. Experimental set‐up, exemplar unprocessed data and experimental timeline.
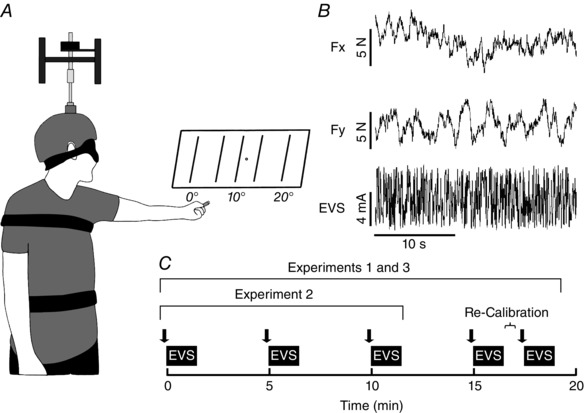
A, a tracing from the experimental set‐up in which the participant is indicating their perceived angular head position in space. B, exemplar data from one subject for Experiment 1 (Passive) at baseline (0 min) while being subjected to electrical vestibular stimulation (EVS). The top tracing represents mediolateral forces (Fx) and the middle tracing depicts the anteroposterior forces (Fy) acting upon the body, whereas the bottom tracing portrays the EVS signal. C, a chronological timeline for the experimental trials made to scale. Filled squares represent the 90 s EVS every 5 min, and the filled arrows depict when the participant indicated his or her perceived angular head position. During Experiments 1 and 3, a visual re‐calibration period of 30 s was performed after the termination of the fourth EVS bout in which the eyes were opened without any active contractions of the participant's neck muscles. The re‐calibration period was followed immediately by another bout of EVS.
General experimental set‐up
Participants stood barefoot on a force plate with the feet parallel at a ∼ 5 cm inter‐malleoli distance while their arms were relaxed at the sides. The participants faced a semicircular panel that consisted of large vertical markings and numbers providing a scale (in degrees) for an indication of the direction the participant was facing in combination with his or her perceived head yaw posture (Fig. 2 A). Binaural bipolar EVS activates vestibular afferents from both the semicircular canal and otolithic end organs, resulting in specific head motion vector signals from each afferent (Fitzpatrick & Day, 2004). The net summation leads to a virtual head motion that is largely rotational (Fitzpatrick & Day, 2004; Day & Fitzpatrick, 2005; Peters et al. 2015, 2016). In the present study, we were not interested in exploring the origin (i.e. contribution from the various vestibular afferents, descending pathways) of the vestibular‐evoked balance response. Rather we focused our questions on the direction of the balance control's motor output. Therefore, we carefully controlled head‐on‐feet posture. In all experiments the head‐turned posture was maintained passively (Experiments 1 and 2) or actively (Experiment 3) to the left through neck rotation and pitched such that Reid's plane (inferior orbital margin to the external acoustic meatus) was maintained ∼ 19 deg up from horizontal. This orientation parallels the EVS‐evoked rotational vector (see Fitzpatrick & Day, 2004) with horizontal, maximizing the balance response to EVS (Lund & Broberg, 1983; Cathers et al. 2005; Mian & Day, 2009). To control yaw rotation of the trunk and head we used a custom‐designed set‐up. A rigid steel rod attached to a series of braces strapped around the participants’ chest and waist via inelastic Velcro straps was used to control the trunk yaw rotation. The steel rod was situated behind the participants and its shaft could telescope during movement, allowing typical trunk sway. In Experiments 1 and 2, participants also wore a helmet (Pro‐Tec, San Diego, CA, USA) that was fixed in yaw and passively held the head in the prolonged head‐turned posture, but was capable of moving in all other degrees of freedom. This was achieved using two universal joints and a telescoping shaft (Forbes et al. 2013). Despite restricted yaw rotation of the head and trunk, participants could sway freely in pitch and roll while attached to the device (Fig. 2 A; Gurfinkel et al. 2006). Owing to the trunk bracing system, it is important to note that most participants could not comfortably rotate the head to 90 deg. In such cases, the head was turned as close to 90 deg as possible without causing discomfort (mean head position for all experiments = ∼6 deg from 90 deg or ∼ 84 deg from anatomical position). To set head angular (yaw) position, the experimenter aligned a laser with the nose of the participant to the desired head position on the semicircular panel. This allowed a measure of actual head‐turned posture and was set as the target position.
Experimental procedures
Prior to data collection, the experimenter guided the participants’ head slowly from 0 deg (facing forward parallel with the feet) to the head‐turned target position (∼84 deg left) for the passively held posture (Experiments 1 and 2). For Experiment 3, participants rotated their head slowly, under verbal direction from the experimenter, to the actively held head‐turned posture. The experimenter noted the corresponding angle on the semicircular panel and this point became the participants’ baseline value for the head‐turned posture. Throughout the experiments, participants stood relaxed with either their eyes closed (Experiments 1 and 3) or open (Experiment 2) while holding a laser pointer in their left hand. When instructed, participants pointed the laser ‘to where they felt their nose was pointing in space’. The experimenter emphasized the pointing action to be achieved with a straight arm in a slow and controlled motion (Fig. 2 A). The experiments began with the participants indicating their perceived head‐turned posture followed by the simultaneous commencement of a running timer and first EVS trial (providing baseline values for both perception and the vestibular‐evoked balance response). Thereafter, head‐turned posture perception with a subsequent EVS trial were performed every 5 min (Fig. 2 C). Experiments 1 and 3 consisted of five trials of EVS, including a final EVS trial following a ∼ 60 s period in which the eyes were open for 30 s to provide a re‐calibration (see below), whereas for Experiment 2 only three trials of EVS were delivered.
Experiment 1: passive head‐turned with eyes closed
Experiment 1 assessed whether a single internal representation of head‐on‐feet posture subserves the conscious perception of body orientation and the transformation of the vestibular signals for the control of balance. During this experiment, participants were asked to keep their neck muscles as relaxed as possible during the head‐turned posture to maintain a passive state. Prior to data collection, the helmet was locked at the desired yaw orientation to prevent axial rotation of the head on the torso. All participants were blindfolded and told the helmet may slowly rotate their head clockwise towards a neutral position (although the locking mechanism prevented this possibility). These procedures were used to maximize the elicited perceptual illusion of head return (Ivanenko & Grasso, 1997; Guerraz et al. 2006). The experiment consisted of participants indicating their perceived head‐turned posture and multiple trials of EVS as described above. Following the EVS trial at 15 min, the blindfold was removed and participants were asked to open their eyes for 30 s and focus on where their head was facing in space while remaining relaxed and not actively contracting their neck muscles. This period of visual re‐calibration allowed the participants to update visually their head‐on‐feet posture and realize it did not move for the duration of the experiment. Finally, subjects were blindfolded again, asked for their perception of the head‐turned posture and a final EVS trial was performed (Fig. 2 C). If, following visual re‐calibration of head position, the direction of the vestibular‐evoked balance vector is orthogonal (interaural) to the perceived (and actual) head‐on‐feet orientation, additional support for the model proposing a single internal representation of head‐on‐feet posture will be provided (Fig. 1). Any alternative result, such that the direction of the vestibular‐evoked balance responses is non‐orthogonal to perceived head‐on‐feet orientation, would lend support to the alternative model (Fig. 1).
Experiment 2: passive head‐turned with eyes open
Experiment 2 was designed to determine the spatial transformation of the vestibular‐evoked balance response when the perception of head angular position remained constant. The helmet set‐up from Experiment 1 was used to maintain a passive head‐turned posture. During this experiment, the participants were instructed that their head would not move and they maintained visual cues of head‐on‐feet posture by keeping the eyes open throughout the experiment. To ensure a sufficient EVS‐evoked balance response with the eyes open, a stimulus amplitude of ± 4.5 mA was used for this experiment. The procedures for Experiment 2 resembled those of Experiment 1, except the standing protocol was terminated immediately following the 10 min EVS trial (Fig. 2 C). Findings from our first experiment (see Results below) revealed that visual recalibration can update the perception of head‐on‐feet orientation but not the direction of the vestibular‐evoked force vector. For Experiment 2, we examined if the balance system uses static visual cues to represent head‐on‐feet orientation. If visual cues are not used by the balance system to update its internal representation of static head‐on‐feet posture, we hypothesized that the direction of the vestibular‐evoked balance responses would rotate clockwise (i.e. in the same direction as during the head‐return illusion in Experiment 1), despite the veridical perceived head‐on‐feet orientation.
Experiment 3: active head‐turned with eyes closed
The final experiment was conducted to evaluate the effect of an active prolonged head‐turned posture on perception and the transformation of the vestibular‐evoked balance response. Here, an active motor command from the neck muscles was required to maintain the head‐turned posture and thus the helmet set‐up was excluded from this experiment. An adjustable headband with a laser affixed was secured around the head to indicate the participant's head posture. Prior to experimentation, the investigator guided the participant's head to the appropriate position, and marked a target on the semicircular panel that aligned with the nose. As the head was unrestrained, the experimenter monitored head position throughout the experimental protocol with the laser and provided constant verbal feedback to ensure the appropriate head position. A maximum of ±2 deg of yaw head motion around the target position was permitted and the value was recorded manually with the corresponding perceived head‐turned posture. Once participants closed the eyes and achieved the head‐turned posture, they replicated the procedures of Experiment 1. For Experiment 3, we examined if the perceptual and balance systems benefit from motor cues to update their respective internal representation of head‐on‐feet orientation. If motor signals contribute to the internal representations of head‐on‐feet orientation, we hypothesized that an actively held head‐turned posture would minimize the rotation observed in perception of angular head position and the direction of the vestibular‐evoked balance responses compared with passively held head‐turned postures.
Data analysis
To determine the direction of the vestibular‐evoked balance response, we calculated a force vector by employing a time domain measure of correlation between the EVS signal and the motor output (ground reaction forces) (Dakin et al. 2007; Mian and Day 2009, 2010), known as cumulant density. Cumulant density is equivalent to a cross‐covariance and can be interpreted as an associative measure between two signals (Dakin et al. 2007; Reynolds, 2010). Prior to analysis, the DC offset was removed from the anteroposterior and mediolateral forces. The cumulant density estimates were derived using an archive of MATLAB (MathWorks, Natick, MA, USA) functions based on multivariate Fourier analysis (NeuroSpec, http://www.neurospec.org) as described by Halliday et al. (1995). Each 90 s data record (one EVS trial) was sectioned into 2 s segments with a frequency resolution of 0.5 Hz. Cumulant density estimates were calculated by transforming the cross‐spectra between the EVS signal and the ground reaction forces acting on the body into the time domain. The amplitudes of the cumulant density function were normalized by the product of the vector norms of the EVS signal (input) and the ground reaction forces (output) signal (Dakin et al. 2010). This transforms the cumulant density estimate into values that are bounded between −1 and 1. Uncorrelated signals will have an expected value of zero and values deviating significantly from zero represent a correlation between the two signals with a distinct time lag. A positive value represents an association between the EVS signal and force signals of the same polarity; whereas negative values indicate an association between signals of opposite polarities. For our specific experiment, an anode right (cathode left) current represents a positive vestibular signal whereby a positive cumulant density function estimate indicates the anode right (cathode left) configuration eliciting forces applied to the body that are directed anteriorly (Fy) or leftward (Fx).
The cumulant density estimate produces a biphasic motor response consisting of two distinct peaks of short and medium latencies (Dakin et al. 2007; Reynolds, 2010; Dalton et al. 2014). For the purposes of this study, we are interested in the medium latency response as it represents a whole‐body postural reaction to a vestibular error signal (Mian & Day, 2009, 2014; Mian et al. 2010). Therefore, the direction of the whole‐body response (medium latency) will grant information related to the spatial transformation of the vestibular signals for the control of balance. First, we determined that all trials exhibited a significant vestibular‐evoked response using 95% confidence intervals constructed for each EVS trial. We assessed, for each participant on a trial‐by‐trial basis, when the peak amplitude of the medium latency exceeded the derived confidence intervals. If a significant response was not evoked for any trial, data from the corresponding experimental testing session would have been discarded, but this did not occur. Figure 3 represents exemplar cumulant density estimates for EVS–whole‐body forces for an EVS trial of one subject for Experiment 1.
Figure 3. Quantification of vestibular‐evoked response direction and exemplar vestibular‐evoked force responses.
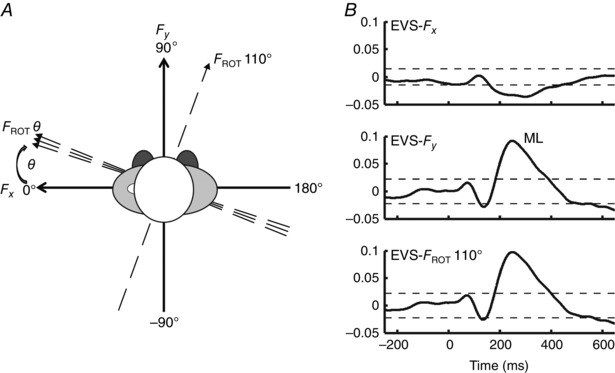
A, an aerial view of the experimental set‐up for all testing trials whereby the head was rotated leftward ∼90 deg in yaw. This head position was maintained for the duration of the trial either passively (Experiments 1 and 2) or actively (Experiment 3). The coordinate system (black orthogonal lines) pertains to directions of the vestibular‐evoked force vector and perceived head position. Further, the dashed arrows represent the incrementally rotating axis (Rotθ), in which the maximum vestibular‐evoked response was detected. This axis was rotated ±180 deg from a starting direction equal to Fx. The axis at 110 deg coincides with the maximum amplitude response angle for one subject at the 10 min mark of Experiment 1. In B, the cumulant density functions are displayed for the electrical vestibular stimulation (EVS)‐Fx, EVS‐Fy and EVS‐maximum response amplitude force direction (Rot 110 deg). The horizontal dashed lines represent the 95% confidence intervals.
The medium latency response to a vestibular signal is primarily interaural (Mian & Day, 2009). To quantify the direction of the maximal EVS‐evoked whole‐body force response with respect to the orientation of the force plate, we implemented the methods of Mian & Day (2009) (Fig. 3). In 1 deg increments, an axis (Rotθ) was rotated ±180 deg from a starting direction equal to Fx (Fig. 3). At each degree of rotation (θ), the time series depicting the force component acting along the rotating axis was calculated as follows:
where s is the sample. After this transformation of the force coordinate system, the cumulant density function was quantified between the EVS signal and F ROT θ. At each rotation transformation of θ (in 1 deg increments), the peak amplitude of the medium latency EVS–whole‐body force cumulant density estimate (time range 200–500 ms) was calculated and plotted as a function of θ. The θ that resulted in the greatest peak medium latency amplitude was considered the direction of the vestibular‐evoked force vector (Fig. 3).
For the SCM surface EMG, a 60 s epoch was taken prior to an EVS trial and the RMS amplitude was calculated. Owing to proximity, stimulation artifacts from the EVS site prevented the estimation of neck EMG signal amplitude during the EVS trials for data analysis. The RMS amplitude of each 60 s epoch was normalized to the initial 10 s of the EMG signal of each experimental session.
For statistical purposes, data were analysed using SPSS version 22 (IBM, Chicago, IL, USA). For all experiments, the dependent variables of actual head angular position (deg), perceived head angular position (deg), the direction of the vestibular‐evoked balance response (deg) and RMS EMG amplitude (%) were analysed using a one‐way ANOVA with repeated measures for the independent variable of time [Experiments 1 and 3 (Baseline, 5 min, 10 min, 15 min, Re‐Calibration) and Experiment 2 (Baseline, 5 min, 10 min)]. When main effects were detected, a Holm–Bonferroni post hoc analysis was performed to determine where the statistical differences occurred. Effect sizes were calculated using the partial eta‐squared (ηp2) method to explore the strength of apparent statistical effects. To determine whether the passive prolonged head‐turned condition evoked a greater perceived illusion and rotation in the direction of the vestibular‐evoked balance response than the active condition, the change in perceived head‐turned posture and EVS‐evoked force vector at 15 min from baseline for Experiment 1 were respectively compared with those of Experiment 3 with unpaired t‐tests (effect size was estimated using a Cohen's d). The alpha level was set at 0.05 for all experiments. For the purposes of our study we chose linear statistics over a circular statistical test owing to our repeated measures design and the angular data being concentrated within a small arc of the circle and not crossing the ±180 deg discontinuity. Descriptive data reported in the text are reported as means ± standard deviations (SDs) and values reported in figures are means ± standard errors of the mean (SEMs).
Results
Whole‐body responses to the electrical vestibular stimuli were observed in all subjects. In the following subsections, exemplar data are provided for a single subject (Fig. 4) and group values are provided for statistical analyses.
Figure 4. Representative data from an individual participant.
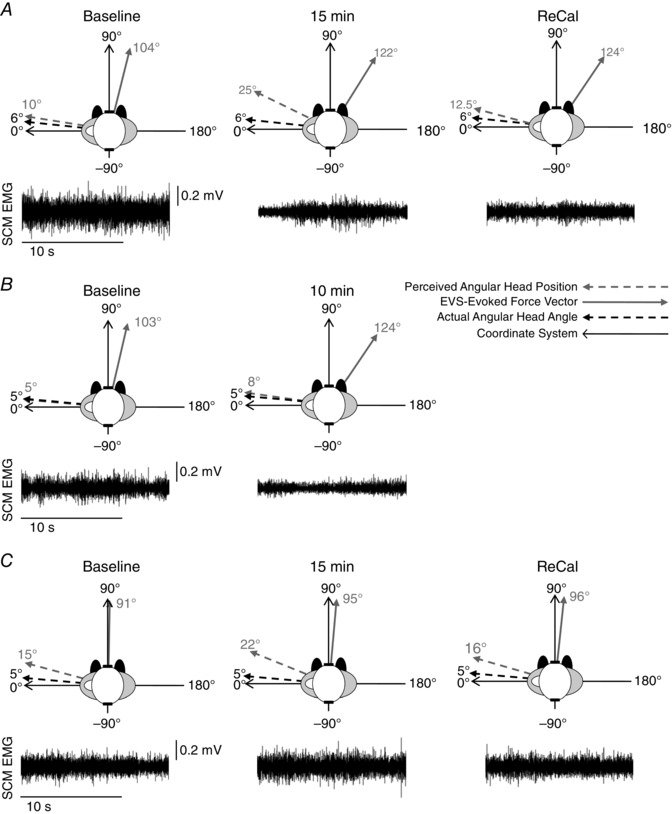
A, the direction of the electrical vestibular stimulation (EVS)‐evoked force vector direction (solid grey arrow), perceived angular head position (dashed grey arrow), actual angular head position (dashed black arrow) and unprocessed sternocleidomastoid electromyography (SCM EMG) at baseline, at 15 min and following visual re‐calibration (ReCal) for one subject during Experiment 1; B and C, the same exemplar data as Experiment 1 for Experiments 2 and 3, respectively. For Experiment 1, the direction of the vestibular‐evoked balance response rotated clockwise by 18 deg within 15 min and remained rotated following ReCal and the SCM EMG amplitude decreased by 15 min from baseline and remained lower following ReCal. However, the perceived angular head position rotated clockwise by 15 deg at 15 min, but returned to the baseline value following ReCal while actual angular head position did not change throughout the experiment. Actual and perceived angular head position remained stable throughout Experiment 2, but the vestibular‐evoked balance response rotated clockwise by 21 deg by 10 min along with a reduction in surface SCM EMG. For Experiment 3, the vestibular‐evoked balance response, surface SCM EMG amplitude and actual angular head position remained constant over time, whereas the perceived angular head position only rotated clockwise up to 7 deg.
Experiment 1: passive head‐turned posture with eyes closed
To determine whether the conscious perception of head posture and the transformation of vestibular signals for standing rely on a common internal representation of body configuration, participants stood quietly with the head rotated passively (facing over the left shoulder) for a prolonged period (>15 min) with vision removed. In this posture, the representative participant perceived his head returning towards a neutral posture, but his actual head position did not change (Fig. 4). This illusion of head rotation was accompanied by a rotation of the vestibular‐evoked whole‐body force response in the same direction while SCM EMG amplitude decreased. If this illusion of head yaw rotation and the spatial transformation of vestibular balance control depended on a single internal representation, the vestibular‐evoked balance vector should return to an interaural direction following visual re‐calibration of head‐on‐feet posture (remaining orthogonal with perception), but it did not in this representative subject (Fig. 4 A; Experiment 1).
Results from all subjects confirmed these observations. For the duration of the experiment, actual head position remained at 6 ± 3°. The subjects’ perception of their head position, however, rotated over time (F = 12.32, P < 0.001, ηp2 = 0.67), such that they thought their heads rotated up to 16 deg from baseline by 15 min (Holm–Bonferroni: P < 0.01). Their perceived head position shifted back to baseline values following visual re‐calibration (Holm–Bonferroni: P = 0.15, Fig. 5 A and B). Correspondingly, the direction of the EVS‐evoked force vector also rotated (F = 6.17, P = 0.001, ηp2 = 0.51) clockwise by 16 deg within 15 min compared to baseline (Holm–Bonferroni: P < 0.01, Fig. 5 A and B). After participants opened their eyes to re‐orientate perceived head angular position, the direction of the vestibular‐evoked balance vector remained rotated by 19 deg compared to baseline (Holm–Bonferroni: P < 0.01). Therefore, the direction of the vestibular‐evoked balance response was not orthogonal to the perceived head angular position following visual re‐calibration. Surface EMG amplitude of the SCM decreased (F = 8.43, P = 0.008, ηp2 = 0.58) by 48% within 5 min and remained depressed until the end of the standing task compared with baseline (Holm–Bonferroni: P < 0.01, Fig. 5 C).
Figure 5. Experiment 1: passive condition with eyes closed.
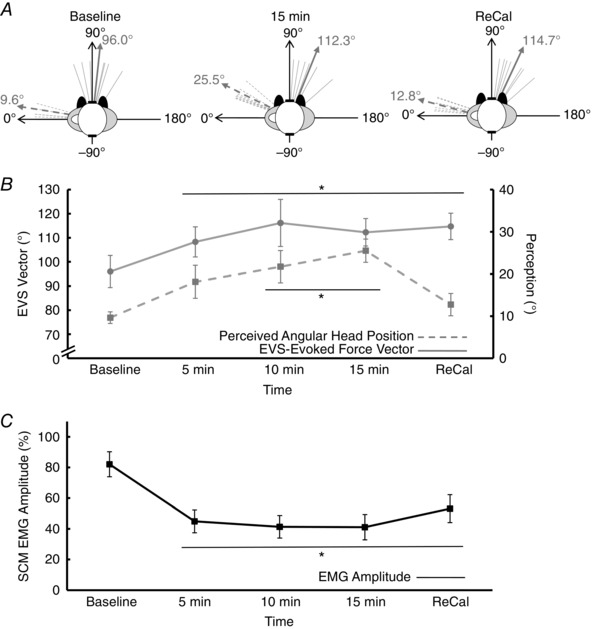
A, the direction of the electrical vestibular stimulation (EVS)‐evoked force vector (thin grey solid lines) and the perceived angular position of the head (thin grey dashed lines) for each individual participant. The thick solid grey line represents the group mean direction of the EVS‐evoked force vector, whereas the thick grey dashed line represents the group mean perceived angular head position. Data are provided for baseline, 15 min and following visual re‐calibration (ReCal). B, the changes in the direction of the EVS‐evoked force vector (grey circles) and perceived angular head position (grey squares) over time. The EVS‐evoked force vector rotated clockwise by ∼16 deg within 15 min and remained shifted even following visual re‐calibration. The perceived angular head position rotated ∼ 16 deg clockwise by 15 min, but regressed to baseline values following visual re‐calibration. C, a change in the amplitude of the sternocleidomastoid surface electromyogram (EMG) over time whereby EMG amplitude decreased by ∼ 45% at 5 min and remained depressed thereafter. Values are means ± SEM. *Significant difference from baseline.
Experiment 2: passive head‐turned posture with eyes open
In Experiment 2, participants used visual cues to update the internal representation of their head‐on‐feet posture while maintaining the same head‐turned posture (passively) as Experiment 1. If visual cues contribute to the perceptual representation of static head‐on‐feet orientation but not to the balance estimate, maintaining visual cues throughout the passively held head‐turned posture would eliminate the illusion of ‘head‐return’ but would not prevent a clockwise rotation in the direction of the vestibular‐evoked balance response. The representative subject's perception of head posture remained constant throughout the task, but the vestibular‐evoked balance vector rotated clockwise in conjunction with a decrease in SCM EMG amplitude (Fig. 4 B, Experiment 2).
The group data confirmed that subjects perceived their head angular position to remain unchanged throughout the experiment at 5 ± 3 deg (F = 0.15, P = 0.76, ηp2 = 0.02). This perceived head position corresponded with the actual head angular position (5 ± 3 deg, Fig. 6 A and B). However, the vestibular‐evoked balance response direction deviated from perception and rotated clockwise. The direction of the EVS‐evoked force vector rotated (F = 5.15, P = 0.02, ηp2 = 0.46) by 21 deg at 10 min compared with baseline (Holm–Bonferroni: P = 0.01), despite continuous visual updating of spatial orientation of head‐on‐feet posture (Fig. 6 A and B). Surface EMG amplitude from the SCM (F = 4.55, P = 0.03, ηp2 = 0.43) exhibited a 26% decrease by 10 min compared with baseline (Holm–Bonferroni: P = 0.01, Fig. 6 C).
Figure 6. Experiment 2: passive condition with eyes open.
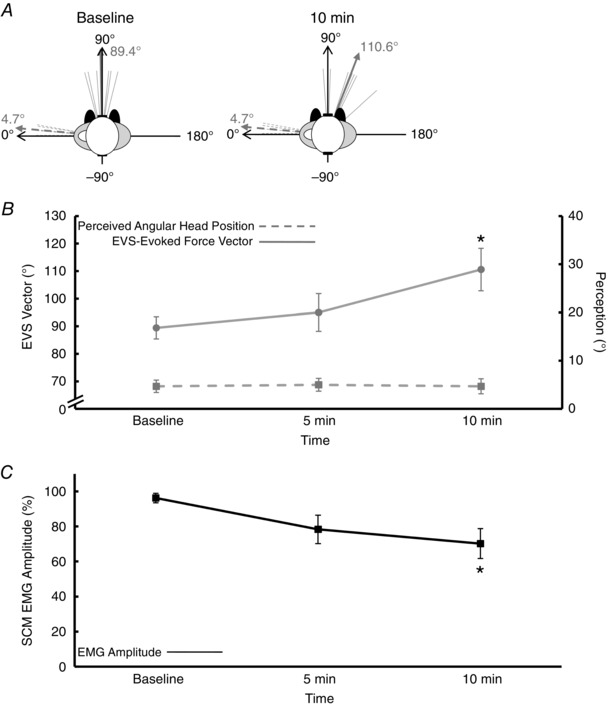
A, the electrical vestibular stimulation (EVS)‐evoked force vector (thin grey solid lines) and the perceived position of the head (thin grey dashed lines) for each individual participant. The thick solid grey line represents the group mean EVS‐evoked force vector, whereas the thick grey dashed line represents the group mean perceived head position. Data are provided for baseline and 10 min. B, changes in the EVS‐evoked force vector (grey circles) and perceived head perception (grey squares) over time. The EVS‐evoked force vector rotated ∼ 21 deg clockwise by 10 min, but the perceived angular head position did not change over time. C, a change over time for the amplitude of the sternocleidomastoid surface electromyogram (EMG) whereby EMG amplitude decreased by ∼ 26% at 10 min. Values are means ± SEM. *Significant difference from baseline.
Experiment 3: active head‐turned posture with eyes closed
To determine whether an actively held head‐turned posture would minimize the perception of head return and play a role in updating the representation of head‐on‐feet posture necessary for the transformation of vestibular signals for the control of standing balance, participants stood quietly in the prolonged posture, but actively held their head while blindfolded. During the active condition, the representative participant's perception rotated slightly, but not to the same extent as Experiment 1; whereas the vestibular‐evoked force vector and neck motor output (SCM EMG) remained constant throughout (Fig. 4 C; Experiment 3).
For all participants, the experimenter did not permit the head‐turned posture to rotate more than ±2 deg from baseline by guiding the subject with verbal cues. Perceived head angular position (F = 3.25, P = 0.02, ηp2 = 0.27) rotated clockwise 6 deg by 5 min and remained at that perceived position thereafter compared with baseline (Holm–Bonferroni: P < 0.05, Fig. 7 A and B), while actual head position did not change throughout (5.9 ± 4.0 deg). The direction of the EVS‐evoked whole‐body force vector, however, did not significantly change over time (F = 1.21, P = 0.32, ηp2 = 0.12) throughout maintenance of the active head‐turned posture (Fig. 7 A and B). Surface SCM EMG amplitude (F = 2.14, P = 0.10, ηp2 = 0.19) also remained constant for the duration of this experiment (Fig. 7 C). The clockwise shift in perceived head angular position at 15 min was significantly greater for the passively held (15.9 ± 6.7 deg) than for the actively held head‐turned posture (6.2 ± 7.7 deg, t = 2.70, P = 0.02, d = 1.35, Figs 5 B and 7 B). Rotation in the EVS‐evoked whole‐body force vector at 15 min was also greater for the passively held (16.3 ± 10.2 deg) than for the actively held head‐turned posture (−4.8 ± 17.9 deg, t = 2.80, P = 0.01, d = 1.45, Figs 5 B and 7 B).
Figure 7. Experiment 3: active condition with eyes closed.
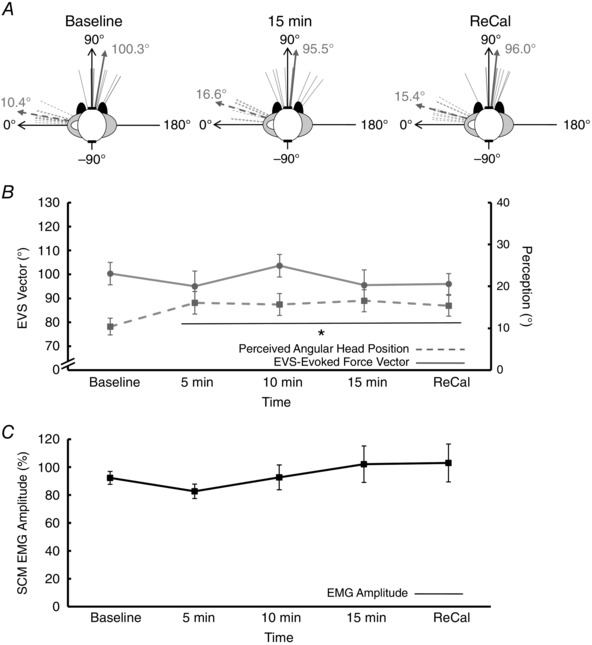
A, the electrical vestibular stimulation (EVS)‐evoked force vector (thin grey solid lines) and the perceived position of the head (thin grey dashed lines) for each individual participant. The thick solid grey line represents the group mean EVS‐evoked force vector, whereas the thick grey dashed line represents the group mean perceived head position. Data are provided for baseline, 15 min and following visual re‐calibration (ReCal). B, mean data for the EVS‐evoked force vector (grey circles) and perceived angular head position (grey squares) over time. The EVS‐evoked force vector was unaltered over time despite a minor clockwise rotation of ∼ 6 deg in perceived angular head position by 5 min, where it remained throughout the protocol. C, mean amplitude of the sternocleidomastoid surface electromyogram (EMG) whereby EMG amplitude was unaltered over time. Values are means ± SEM. *Significant difference from baseline.
Discussion
We tested two alternative models of sensorimotor integration, one proposing that a single internal representation of head‐on‐feet orientation is responsible for perceived head posture and the vestibular control of standing balance, and a second model proposing that conscious perception and control of balance are driven from separate processes (Fig. 1). Our findings support the latter model, which emphasizes that vestibular signals of head motion are spatially transformed for whole‐body balance control, at least partially, independent from conscious perception. In Experiment 1, we observed that both the vestibular‐evoked balance response direction and perception of head‐on‐feet posture are aligned orthogonally as previously reported (Popov et al. 1986; Gurfinkel et al. 1989). The perception of head yaw rotation and the direction of the vestibular‐evoked whole‐body response rotated by more than 16 deg by 15 min following baseline. However, following visual re‐calibration of head‐on‐feet orientation, the perception of head yaw posture returned to baseline values whereas the direction of the vestibular‐evoked whole‐body response remained rotated by 19 deg. Even when allowing vision to update continuously the orientation of head‐on‐feet posture (Experiment 2), the direction of the vestibular‐evoked whole‐body response rotated by more than 20 deg. Although static visual cues can reliably update perception of head‐on‐feet posture, they are not sufficient to transform the direction of vestibular‐evoked balance responses, suggesting a predominant role of other sensorimotor signals in this spatial transformation.
Conscious perception and standing balance representation of head posture
In all three of our experiments, situations arose in which the vestibular‐evoked balance response was not aligned with perceived head‐on‐feet orientation. When the eyes were closed, participants made perceptual reports indicating that their head was rotating from a head‐turned posture towards anatomical orientation (Gurfinkel et al. 1992; Ivanenko & Grasso, 1997). After the eyes were opened, the perceived head‐on‐feet orientation was updated immediately (Fig. 5 A and B). In contrast, the direction of the vestibular‐evoked balance vector was not influenced by the perceived head posture if a visual re‐calibration was performed or when the eyes remained open. This mismatch between perception of head‐on‐feet posture and the direction of the vestibular‐evoked balance responses indicates that the two processes do not depend on one another. Rather, we propose they process common sensory and motor signals separately and thus may arrive at different conclusions of head‐on‐feet estimates depending on the relative importance given to each source of information signalling head‐on‐feet orientation.
Previous discussions on postural control suggest that the CNS updates an internal representation of the body's current configuration (termed ‘body schema’) with available sensory and motor signals (Popov et al. 1986; Gurfinkel et al. 1988, 1989; Massion, 1998). From this single internal representation (Gurfinkel et al. 1988; Massion, 1998), the conscious perception of posture and vestibular control of balance are believed to be updated and organized. Our results do not support this model, showing a clear discrepancy between perceived head‐on‐feet posture and the spatial transformation of sensory (vestibular) signals for the balance system. We propose that this is indicative of the vestibular control of standing balance operating under its own principles (Héroux et al. 2015), with minimal access to or influence from perceptual processing. Experimental evidence provides support for this model of standing balance. When voluntarily replicating the lower limb activity perceived during stance, humans produce one‐third of their quiet standing torque levels (Luu, 2010). Correspondingly, corticomuscular coherence associated with stance is one‐third that of a torque‐equivalent voluntary contraction (Luu, 2010). Luu (2010) suggested that a substantial amount of the motor drive used for standing does not arise from the cortex and is therefore not accessible for conscious perception. Furthermore, when humans control a robotic balance simulator, vestibular‐evoked motor responses are only present in the muscles directly influencing balance control, regardless of whether the participant perceives they are balancing their body in space (Luu et al. 2012). Therefore, the CNS can use sensorimotor signals to determine whether the body is actively engaged in a balancing task and consequently modulate vestibular‐driven postural control, at least partially, independent of cognitive perception (Luu et al. 2012). Similar dissociations have been reported for the perception of whole‐body motion and vestibulo‐ocular reflexes during seated (Merfeld et al. 2005 a, b ) and upright (Pettorossi et al. 2013) postures. We propose that the distinct internal representations of head‐on‐feet orientation for balance and perception may arise due to divergent multisensory and motor cue combinations, a process thought to be task‐ and context‐dependent (van Beers et al. 1999, 2002).
The role of sensorimotor signals in the balance system's representation of head‐on‐feet posture
To transform vestibular signals of head motion appropriately for whole‐body balance control, the CNS must consolidate how the head is orientated with respect to the body and feet. Changes to head‐on‐feet orientation can occur with rotations at various body segments (e.g. neck, trunk, hips) and sensory receptors associated with these segments inform the CNS of postural orientation. In our study, head yaw rotation was performed at the neck because signals arising from the neck provide information regarding head position relative to the body (Pettorossi & Schieppati, 2014), making it a strong candidate for the spatial transformations exploited here. Therefore, we focus our discussion on neck sensorimotor signals. Here, we created an unfamiliar context for the participants, in which a prolonged head‐turned posture was maintained through an external device (passive conditions: Experiments 1 and 2). Regardless of how head posture is maintained, the balance system may use what it expects to be reliable neck somatosensory and motor information in order to spatially transform vestibular cues for standing balance. The prolonged passively held head‐turned postures were associated with vestibular‐evoked balance response directions that rotated clockwise over time and remained rotated even in the presence of visually correct cues of spatial orientation. We propose that sensory and motor (SCM EMG) signals of neck origin decayed over time, resulting in the observed spatial rotation. In contrast, the actively held posture exhibited spatially stable balance responses irrespective of conscious perception, which is at odds with previous work (Gurfinkel et al. 1989). In this case, sensory signals originating from the neck and maintained motor command (see SCM EMG results) contributed to the establishment of an accurate balance representation of head‐on‐feet posture.
Why would positional signals arising from the neck – observed mostly in the passive case – change during the prolonged head‐turns performed in this study? Adaptation of afferent discharge can occur over sustained muscle contractions for primary muscle spindles (Cheney & Preston, 1976; Macefield et al. 1991) and golgi tendon organs (Gregory & Proske, 1979), as well as slowly adapting cutaneous afferents for a maintained skin deformation (Iggo & Muir, 1969). These adaptations can occur for passive and active contractions, although the process is less clear for muscle spindles owing to the influence of fusimotor drive. However, the contrast between the passive and active results reported here warrant further consideration of how signals of head position may have differed. In the passive head‐turned posture, SCM muscle fibres opposite to the head turn relaxed throughout the protocol (as suggested by the decrease in EMG), probably increasing their length over time, biasing the golgi tendon organs and muscle spindles firing. On the other hand, the actively held head posture resulted in a constantly active SCM muscle tension (constant EMG activity at a similar muscle fibre length and velocity), providing more robust positional signals from the muscle spindles and golgi tendon organs. The observed differences between passively and actively held head postures can be further explained by the motor influence on sensory inflow (von Holst & Mittelstaedt, 1950). Sensory signals associated with self‐generated actions are gated by a copy of the motor command (‘efference copy’ or ‘corollary discharge’), a process thought to contribute to position sense (Walsh et al. 2013). Here, the centrally generated motor commands to the SCM decreased for the passive condition but remained constant for the active one, probably resulting in a greater decay in the resulting signals of head position for the passively held compared to the actively held head posture. The peripheral sensor adaptation and central processes explaining the current observations are not mutually exclusive. For example, Luu et al. (2011) proposed that the efference copy mechanism may include passage through the central and peripheral pathways. We propose the more robust neck sensorimotor cues for the actively held compared to the passively held head posture led to the maintenance of the interaural vestibular‐evoked balance responses over prolonged periods (15 min) and to the relatively small shifts in the perception of head posture. Such representations of head posture may be partly implemented in the central vestibular system. Neurons within the vestibular nuclei code differently for active and passive head rotations (Roy & Cullen 2001; Cullen & Roy 2004), a mechanism thought to depend on neck afferent activity. Thus, differences in the actively and passively held head‐turned postures owing to different neck sensory and cortical signals projecting onto the vestibular nuclei (Wilson et al. 1999; Gdowski & McCrea, 2000) may subsequently influence the spatial transformation of the vestibular signals for balance control.
Why vision does not influence the balance system's representation of static head‐on‐feet posture
In the present study, the observation that perception of head‐on‐feet posture was recalibrated by vision is not surprising as optic cues provide accurate spatial information to determine head‐facing direction. These visual cues can be presumably used similarly by the balance system, as proposed previously (Gurfinkel et al. 1989). Visual information has been shown to modify the timing and amplitude of the vestibular‐evoked response (Fitzpatrick et al. 1994; Day et al. 1997; Day & Guerraz, 2007; Jessop & McFadyen, 2008) and plays a role in standing balance (Day et al. 1993). However, unlike perception of head orientation, visual cues of static head‐on‐feet posture did not influence the spatial transformation of vestibular‐evoked balance responses. This was evident in Experiments 1 and 2, where visual (re‐)calibration of head‐on‐feet posture did not reinstate or maintain interaural vestibular‐balance responses. Sensory (visual, vestibular, somatosensory) and motor systems can convey information that accurately reflects aspects of the body's posture. The perceptual system utilizes information from different modalities to establish an internal representation of head‐on‐feet posture, as observed during eyes open and closed periods of this study. On the other hand, the balance system does not appear to incorporate static cues from vision into its own internal representation of head‐on‐feet yaw orientation. Physiologically, the separate internal representations of head‐on‐feet posture for balance and perception may be linked to the frequency bandwidth of visual, vestibular, somatosensory and motor signals relevant for the combination of positional cues, a mechanism that has been proposed for the integration of vestibular signals (Mergner & Rosemeier, 1998; Laurens & Angelaki, 2011). It is important to emphasize here that passively holding a head posture for a prolonged period of time is not a situation that is frequently encountered and solved by the balance system. Therein, however, lies the main advantage of our experimental approach: by probing the spatial transformation of vestibular signals under unfamiliar conditions, we revealed an important physiological process. The vestibular control of balance is spatially transformed independently from static visual cues, and instead relies primarily on sensorimotor information from the neck, at least for a prolonged static head‐on‐feet posture.
For most circumstances associated with daily living, we will not experience passively held head‐on‐feet postures and the associated decay (return to neutral) in the expected representation of head orientation with respect to the actual head posture. Through repeated experiences, our balance system has learned to approximate head‐on‐feet posture using neck somatosensory information combined with the output of forward sensory models (Wolpert et al., 1995) to transform vestibular signals of head motion. The approximation of head‐on‐feet position derived from neck sensorimotor signals provides an adequate estimate for most activities of daily living, even for prolonged actively held head postures that are unlikely to be performed frequently. By bypassing the multimodal fusion of signals from all potential sensory and motor sources to perform an optimal estimation of head‐on‐feet orientation (state estimate), an approximation of head‐on‐feet posture reduces computational cost for the balance system. The disadvantage of this incomplete state estimate is that the balance system can be biased (e.g. during passively held head postures) even though we are aware of our actual head‐on‐feet posture (Experiments 1 and 2) as it does not appear possible for static visual cues to overwrite the balance representation of head‐on‐feet posture. This estimate of head‐on‐feet orientation leading to context‐dependent sensorimotor errors (Rasman & Blouin, 2016) is reminiscent of that reported for limb localization tasks. Tsay et al. (2016) demonstrated that conditioning of muscle spindle signals induces predictable biases in the localization of elbow joint position during limb matching but not during pointing. Although we propose that visual cues of static postural orientation are insufficient to update the balance system's representation of head‐on‐feet orientation, it is unclear if vision can play a role in transforming the direction of the vestibular‐evoked balance response under different scenarios. For example, the potential transformation of vestibular cues for balance control based on dynamic visual stimuli (with different frequency bandwidths) such as those associated with the illusion of vection (Pavard et al. 1976) remains to be determined.
Possible confounds
When assessing a state‐dependent process, such as the directional properties of vestibular‐driven balance control, it is important to consider the different factors that can influence spatial transformations. Mian & Day (2014) demonstrated that the direction of vestibular‐evoked responses is influenced by the anisotropic states of plane stability and, hence, reported a violation of the craniocentricity principle, despite consistent head postures. In the present study, we used a yaw‐locked helmet for passively held head‐on‐feet postures and it is possible that this helmet device creates a certain level of anisotropic stability between the frontal and sagittal planes. However, if this factor was solely responsible for observed rotations in the direction of the balance response, the vector would not be expected to change over time while a constant passively held head‐on‐feet posture was maintained. Furthermore, Mian & Day (2014) observed that anisotropic plane stability only influences the transformation of balance control when vestibular signals have sagittal and frontal components, such as when the head is orientated 45 deg with respect to the feet. In our experiment, the subjects’ head was positioned ∼90 deg over their left shoulder, and therefore a stability‐dependent phenomenon is unlikely.
Conclusions
The present study demonstrated that the spatial transformation of a vestibular error signal eliciting a balance response is not dependent on the same internal representation of head‐on‐feet posture that drives conscious perception. Hence, our brain can maintain two distinct internal representations of head‐on‐feet orientation, one for the balance system and another one for the perceptual system. The spatial transformation of the vestibular control of balance is driven by somatosensory and centrally generated motor command signals associated with the body's segments. It is largely unaffected by accurate static visual cues that can re‐calibrate our conscious perception of head‐on‐feet orientation.
Additional information
Competing interests
The authors disclose that there are no competing interests.
Author contributions
All experiments were conducted at the Sensorimotor Physiology Lab at the University of British Columbia, Vancouver, British Columbia. B.H.D., B.G.R., J‐S.B. and J.T.I. were involved in the conception and design of the work. B.H.D., B.G.R. and J‐S.B. were involved in the acquisition, analysis and interpretation of the data for the work. B.H.D., B.G.R., J‐S.B. and J.T.I. were involved in drafting or revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada (J‐S.B. and J.T.I.). B.H.D. received postdoctoral funding from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research and B.G.R. received a graduate scholarship from NSERC.
Gouvernement du Canada │ Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada) NSERC PGS, NSERC PGS, 183666‐12, 356026–2013. Canadian Institutes of Health Research 201202MFE‐276226‐197622. Michael Smith Foundation for Health Research ST‐PDF_02910(11‐1)BM.
Acknowledgements
We thank all of the participants involved in the study and Eli Edwards for technical support and data collection.
Linked articles This article is highlighted by a Perspective by Reynolds. To read this Perspective, visit http://dx.doi.org/10.1113/JP273874.
References
- Angelaki DE, Cullen KE (2008). Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31, 125–150. [DOI] [PubMed] [Google Scholar]
- Bertholz A, Viaud‐Delmon I (1999). Multisensory integration in spatial orientation. Curr Opin Neurobiol 9, 708–712. [DOI] [PubMed] [Google Scholar]
- Britton T, Day B, Brown P, Rothwell J, Thompson P, Marsden C (1993). Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94, 143–151. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day B, Fitzpatrick R (2005). Otolith and canal reflexes in human standing. J Physiol 563, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Preston JB (1976). Classification of fusimotor fibers in the primate. J Neurophysiol 39, 9–19. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE (2004). Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91, 1919–1933. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Blouin J‐S, Allen MD, Rice CL, Inglis JT (2014). The altered vestibular‐evoked myogenic and whole‐body postural responses in old men during standing. Exp Gerontol 60, 120–128. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Son GM, Inglis JT, Blouin JS (2007). Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol 583, 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin J‐S (2010). Frequency‐specific modulation of vestibular‐evoked sway responses in humans. J Neurophysiol 103, 1048–1056. [DOI] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC (2005). Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol 567, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Guerraz M (2007). Feedforward versus feedback modulation of human vestibular‐evoked balance responses by visual self‐motion information. J Physiol 582, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Séverac Cauquil A, Bartolomei L, Pastor MA, Lyon IN (1997). Human body‐segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol 500, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Steiger MJ, Thompson PD, Marsden CD (1993). Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J Physiol 469, 479–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL (2004). Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96, 2301–2316. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, McCloskey DI (1994). Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478, 173–186, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC (1994). Task‐dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes PA, Dakin CJ, Vardy AN, Happee R, Siegmund GP, Schoutten AC, Blouin JS (2013). Frequency response of vestibular reflexes in neck, back, and lower limb muscles. J Neurophysiol 110, 1869–1881. [DOI] [PubMed] [Google Scholar]
- Forbes PA, Luu BL, Van der Loos HF, Croft E, Inglis JT, Blouin JS (2016). Transformation of vestibular signals for the control of standing in humans. J Neurosci 36, 11510–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA (2000). Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp Brain Res 135, 511–526. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Proske U (1979). The responses of Golgi tendon organs to stimulation of different combinations of motor units. J Physiol 295, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerraz M, Navarro J, Ferrero F, Cremieux J, Blouin J (2006). Perceived versus actual head‐on‐trunk orientation during arm movement control. Exp Brain Res 172, 221–229. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R (2006). Postural tone in the body axis of healthy humans. J Neurophysiol 96, 2678–2687. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Levik Yu. S, Popov KE, Smetanin BN (1988). Body scheme in the control of postural activity In Stance and Motion: Facts and Concepts, ed. Gurfinkel VS, Ioffe´ ME, Massion J. & Roll JP, pp. 185–193. Plenum Press, New York. [Google Scholar]
- Gurfinkel VS, Popov KE, Smetanin BN, Shlykov VY (1989). Changes in the direction of vestibulomotor response in the course of adaptation to protracted static head turning in man. Neurophysiology 21, 159–164. [PubMed] [Google Scholar]
- Gurfinkel VS, Lebedev MA, Levik (1992). What about the so‐called neck reflexes in humans? In The Head‐Neck Sensory Motor System, ed. Berthoz A, Vidal PP. & Graf W, pp. 543–547. Oxford University Press, New York. [Google Scholar]
- Halliday D, Rosenberg J, Amjad A, Breeze P, Conway, B , Farmer, F (1995). A framework for the analysis of mixed time series/point process data – theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64, 237–278. [DOI] [PubMed] [Google Scholar]
- Héroux ME, Law TCY, Fitzpatrick RC, Blouin JS (2015). Cross‐modal calibration of vestibular afference for human balance. PLoS ONE 10, e0124532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR (1969). The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200, 763–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R (1997). Integration of somatosensory and vestibular inputs in perceiving the direction of passive whole‐body motion. Brain Res Cogn Brain Res 5, 323–327. [DOI] [PubMed] [Google Scholar]
- Jessop D, McFadyen BJ (2008). The regulation of vestibular afferent information during monocular vision while standing. Neurosci Lett 441, 253–256. [DOI] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE (2011). The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Son GM, Blouin J‐S, Inglis JT (2008). Short‐duration galvanic vestibular stimulation evokes prolonged balance responses. J Appl Physiol 105, 1210–1217. [DOI] [PubMed] [Google Scholar]
- Lund S, Broberg C (1983). Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand 117, 307–309. [DOI] [PubMed] [Google Scholar]
- Luu BL (2010). Perception, perfusion and posture. Doctoral thesis. University of New South Wales, Sydney. [Google Scholar]
- Luu BL, Day BL, Cole JD, Fitzpatrick RC (2011). The fusimotor and reafferent origin of the sense of force and weight. J Physiol 589, 3135–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu BL, Inglis JT, Huryn TP, Van der Loos HF, Croft EA, Blouin JS (2012). Human standing is modified by an unconscious integration of congruent sensory and motor signals. J Physiol 590, 5783–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Hagbarth K‐E, Gorman R, Gandevia SC, Burke D (1991). Decline in spindle support to α‐motoneurones during sustained voluntary contractions. J Physiol 440, 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J (1994). Postural control system. Curr Opin Neurobiol 4, 877–887. [DOI] [PubMed] [Google Scholar]
- Massion J (1998). Postural control systems in developmental perspective. Neurosci Biobehav Rev 22, 465–472. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna‐Poulin C, Black FO, Wood S (2005. a). Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol 94, 186–198. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna‐Poulin C, Black FO, Wood S (2005. b). Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt and translation. J Neurophysiol 94, 199–205. [DOI] [PubMed] [Google Scholar]
- Mergner T, Maurer C, Peterka RJ (2003). A multisensory posture control model of human upright stance. Prog Brain Res 142, 189–201. [DOI] [PubMed] [Google Scholar]
- Mergner T, Rosemeier T (1998). Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions – a conceptual model. Brain Res Rev 28, 118–135. [DOI] [PubMed] [Google Scholar]
- Mian OS, Dakin CJ, Blouin J‐S, Fitzpatrick RC, Day BL (2010). Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol 588, 4441–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Day BL (2009). Determining the direction of vestibular‐evoked balance responses using stochastic vestibular stimulation. J Physiol 587, 2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Day BL (2014). Violation of the craniocentric principle for vestibularly evoked balance responses under conditions of anisotropic stability. J Neurosci 34, 7696–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P (1974). Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res 67, 255–268. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Day BL, Marsden CD (1993). Vestibular induced postural responses in Parkinson's disease. Brain 116, 1177–1190. [DOI] [PubMed] [Google Scholar]
- Pavard B, Bertholz A, Lestienne F (1976). Role of peripheral vision in linear motion detection (linear vection). Le travail humain: a bilingual and multi‐disciplinary. J Hum Factors 39, 115–138. [Google Scholar]
- Peters RM, Blouin J‐S, Dalton BH, Inglis JT (2016). Older adults demonstrate superior vestibular perception for virtual rotations. Exp Gerontol 82, 50–57. [DOI] [PubMed] [Google Scholar]
- Peters RM, Rasman BG, Inglis JT, Blouin J‐S (2015). Gain and phase of perceived virtual rotation evoked by electrical vestibular stimuli. J Neurophysiol 114, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorossi VE, Schieppati M (2014). Neck proprioception shapes the body orientation and perception of motion. Front Hum Neurosci 8, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorossi VE, Panichi R, Botti FM, Kyriakareli A, Ferraresi A, Faralli M, Schieppati M, Bronstein AM (2013). Prolonged asymmetric vestibular stimulation induces opposite, long‐term effects on self‐motion perception and ocular responses. J Physiol 591, 1907–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov KE, Smetanin BN, Gurfinkel VS, Kudinova MP & Shylkov VY (1986). Spatial perception and vestibulomotor responses in man. Neurophysiol 18, 548–554. [PubMed] [Google Scholar]
- Rasman BR, Blouin J‐S (2016). Context‐dependent use of muscle spindles for human position sense. J Physiol 594, 801–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF (2010). The effect of voluntary sway control on the early and late components of the vestibular‐evoked postural response. Exp Brain Res 201, 133–139. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE (2001). Selective processing of vestibular reafference during self‐generated head motion. J Neurosci 21, 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay AJ, Giummarra MJ, Allen TJ, Proske U (2016). The sensory origin of human position sense. J Physiol 594, 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers RJ, Sitting AC, van der Gon JJD (1999). Integration of proprioceptive and visual position‐information: an experimentally supported model. J Neurophysiol 81, 1355–1364. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Wolpert DM, Haggard P (2002). When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 12, 834–837. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, Jacobs R, Koopman B, Grootenboer H (1999). A multisensory integration model of human stance control. Biol Cybern 80, 299–308. [DOI] [PubMed] [Google Scholar]
- von Holst E & Mittelstaedt H (1950). Das reafferenzprinzip. Naturwissenschaften 37, 1109–1116. [Google Scholar]
- Walsh LD, Proske U, Allen TJ, Gandevia SC (2013). The contribution of motor commands to position sense differs between elbow and wrist. J Physiol 591, 6103–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Zarzecki P, Schor RH, Isu N, Rose PK, Sato H, Thomson DB, Umezaki T (1999). Cortical influences on the vestibular nuclei of the cat. Exp Brain Res 125, 1–13. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI (1995). An internal model for sensorimotor integration. Science 269, 1880–1882. [DOI] [PubMed] [Google Scholar]


