Abstract
Gases are sensed by lung cells and can activate specific intracellular signalling pathways, and thus have physiological and pathophysiological effects. Carbon dioxide (CO2), a primary product of oxidative metabolism, can be sensed by eukaryotic cells eliciting specific responses via recently identified signalling pathways. However, the physiological and pathophysiological effects of high CO2 (hypercapnia) on the lungs and specific lung cells, which are the primary site of CO2 elimination, are incompletely understood. In this review, we provide a physiological and mechanistic perspective on the effects of hypercapnia on the lungs and discuss the recent understanding of CO2 modulation of the alveolar epithelial function (lung oedema clearance), epithelial cell repair, innate immunity and airway function.
Keywords: alveolar epithelial function, airway function, carbon dioxide, hypercapnia, injury and repair, innate immunity and host defense
Abbreviations
- ALI
acute lung injury
- AMPK
AMP kinase
- AQP
aquaporin
- ARDS
acute respiratory distress syndrome
- cAMP
3′,5′‐cyclic adenosine monophosphate
- CAMKK‐β
Ca2+/calmodulin‐dependent protein kinase kinase‐β
- COPD
chronic obstructive pulmonary disease
- ERK
extracellular signal‐regulated kinase
- IL‐6
interleukin‐6; IDH2, isocitrate dehydrogenase‐2
- JNK
c‐Jun‐N‐terminal kinase
- PAL
prolonged air leak; PKA‐Iα, protein kinase A‐Iα
- PKC‐ζ
protein kinase C‐ζ
- sAC
soluble adenylyl cyclase
- TNF
tumour necrosis factor
Introduction
Carbon dioxide (CO2) is a primary product of oxidative metabolism. The physiological levels of CO2 in exhaled breath of mammals are significantly higher than the room air (∼5% vs. ∼0.04%) (Monastersky, 2013; Cummins et al. 2014) and inextricably linked to physiological conditions. In humans, elevated CO2 (hypercapnia) can occur as a consequence of lung diseases when inadequate gas exchange takes place (Vadasz et al. 2012 b). Despite the fact that the lung is the primary site of CO2 elimination, the effects of hypercapnia have been argued and contradictory data have been reported. Here, we review recent advances in our understanding of the effects of hypercapnia on the lung.
CO2 transport and sensing in the lung
CO2 is a small non‐polar molecule thought to traverse biological cell membranes via passive diffusion, depending upon the transmembrane concentration gradient of CO2 and the lipid/water partition behaviour of the gas (Missner & Pohl, 2009). However, this view has been challenged with the discovery of the effect of cholesterol on CO2 permeability and of protein channels used by CO2 to cross membranes, aquaporins (AQPs) (Verkman, 2007; Musa‐Aziz et al. 2009) and rhesus proteins (Endeward et al. 2008). Functionally, high permeability for CO2 seems to be exhibited by AQP1, AQP4‐M23, AQP5 and AQP6 (Musa‐Aziz et al. 2009). Several AQPs are expressed in the lung: AQP1 in microvascular endothelia, AQP3 and AQP4 in airway epithelia, and AQP5 in type I alveolar epithelial cells and a subset of airway epithelial cells (Verkman, 2007). Once inside the cell, CO2 very rapidly equilibrates with its hydrated form, H2CO3, which in turn rapidly dissociates into H+ and HCO3 − catalysed by carbonic anhydrases (Casey et al. 2010). Cellular enzymes and chemical reactions are sensitive to pH, and cells actively transport H+ and HCO3 − across their cell membrane to maintain intracellular pH (Casey et al. 2010). Cells appear to sense CO2 via different mechanisms: soluble adenylyl cyclase senses CO2/HCO3 −, generating the second messenger 3′,5′‐cyclic adenosine monophosphate (cAMP), which is a key signalling molecule affecting a range of processes (Kamenetsky et al. 2006; Lecuona et al. 2013). Transmembrane adenylyl cyclases have also been described to play a role in CO2 sensing in the carotid body (Holmes et al. 2015). Connexin 26 hemichannels, causally linked to respiratory chemosensitivity, respond to an increase in CO2 and are an important conduit for the CO2‐dependent ATP release (Meigh et al. 2013). Receptor protein tyrosine phosphatase‐γ, which has an extracellular ligand binding domain 40% identical to the catalytic domain of carbonic anhydrases, is an extracellular CO2/HCO3 − sensor critical for pH homeostasis (Zhou et al. 2016). The role of transmembrane adenylyl cyclases, connexin 26 and receptor protein tyrosine phosphatase‐γ in CO2 sensing in the lung has not yet been established.
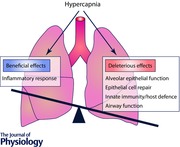
Beneficial effect of hypercapnia/hypercapnic acidosis
The use of lower tidal volumes as a method of protective ventilation in patients with acute respiratory distress syndrome (ARDS) has been documented to show a significant reduction in mortality rates (ARDS Team Network, 2000). This protective ventilation leads to hypercapnia and the associated drop in pH resulting in hypercapnic acidosis. From studies spanning the last 30 years, hypercapnia, especially hypercapnic acidosis, has been associated with improvement in the outcome of patients with acute lung injury (ALI)/ARDS and the concepts of ‘permissive’ and even ‘therapeutic’ hypercapnia have been proposed in treating these patients (Hickling et al. 1994; Contreras et al. 2015). The protective effects of hypercapnic acidosis in preclinical models are mediated through effects on the host immune system, with key effects mediated through inhibition of the NF‐κB pathway, a pivotal transcriptional activator in inflammation, injury and repair (Contreras et al. 2015). On the other hand, hypercapnia‐mediated NF‐κB inhibition may also explain several deleterious effects, including delayed epithelial wound healing and decreased bacterial killing (Wang et al. 2010).
Deleterious effects of hypercapnia/hypercapnic acidosis
There have been also studies on the harmful effects of hypercapnia as described below.
Alveolar epithelial function
One of the most extensively investigated effects of hypercapnia focused on the high CO2 effects on alveolar epithelial function and particularly on the clearance of lung oedema. Disruption of the alveolo‐capillary barrier, which is a hallmark of ARDS, results in accumulation of alveolar oedema, which results in impaired gas exchange. The Na+,K+‐ATPase plays a key role in the active transport of Na+ and K+ across membranes, thus, maintaining cellular ion homeostasis and favouring water reabsorption by generating an ion gradient (Sznajder et al. 2002). Reduction of lung oedema clearance is associated with the endocytosis of the Na+,K+‐ATPase from the plasma membrane of alveolar epithelial cells, which leads to decreased Na+,K+‐ATPase activity (Lecuona et al. 2007). Exposure of lungs, in an isolated rodent lung model ex vivo, to hypercapnia leads to impaired alveolar fluid reabsorption, independently of extra‐ and intracellular acidosis (Briva et al. 2007; Vadasz et al. 2008, 2012 a). The mechanism of impaired alveolar fluid in hypercapnia involves activation of protein kinase C (PKC)‐ζ, which directly phosphorylates the Na+,K+‐ATPase α1‐subunit at the Ser‐18 residue, leading to endocytosis of the Na+,K+‐ATPase (Briva et al. 2007; Vadasz et al. 2008). The activation of PKC‐ζ is regulated by AMP kinase (AMPK) via Ca2+/calmodulin‐dependent protein kinase kinase‐β (CAMKK‐β) and extracellular signal‐regulated kinase (ERK) (Vadasz et al. 2008; Welch et al. 2010). The endocytosis of the Na+,K+‐ATPase by hypercapnia is also regulated by c‐Jun‐N‐terminal kinase (JNK) via an AMPK‐PKC‐ζ signalling (Vadasz et al. 2012 a). However, JNK does not phosphorylate the Na+,K+‐ATPase, but promotes the phosphorylation of LMO7b, which regulates the actin cytoskeleton in epithelial cells, followed by its colocalization and interaction with the Na+,K+‐ATPase and several components of the clathrin‐dependent endocytic machinery (Dada et al. 2015). The protein kinase A (PKA)‐Iα has also been reported to play a role in the Na+,K+‐ATPase endocytosis during hypercapnia. Namely, a novel pathway was proposed whereby hypercapnia via a CO2/HCO3 −‐sensitive soluble adenylyl cyclase (sAC) increases the production of cAMP, activates PKA‐Iα and in turn, the phosphorylation of the actin cytoskeleton component α‐adducin, culminating in the Na+,K+‐ATPase endocytosis from the cell plasma membrane (Lecuona et al. 2013). Taken together, these reports suggest that hypercapnia has deleterious effects on the alveolar epithelial function by impairing the resolution of lung oedema via a pH‐independent mechanism that involves the endocytosis of the Na+,K+‐ATPase (Fig. 1).
Figure 1. Hypercapnia impairs alveolar fluid reabsorption.
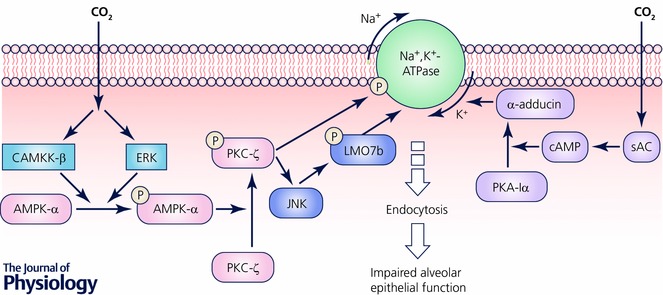
Reduction of lung edema clearance is associated with the endocytosis of the Na+,K+‐ATPase from the plasma membrane of alveolar epithelial cells, which leads to decreased Na+,K+‐ATPase activity. During hypercapnia protein kinase C (PKC)‐ζ directly phosphorylates the Na+,K+‐ATPase α1‐subunit at Ser 18 residue, leading to endocytosis of the Na+,K+‐ATPase. The activation of PKC‐ζ is regulated by AMP kinase (AMPK) via Ca2+/calmodulin‐dependent protein kinase kinase‐β (CAMKK‐β) and extracellular signal‐regulated kinase (ERK). The endocytosis of the Na+,K+‐ATPase by hypercapnia is also regulated by c‐Jun‐N‐Terminal Kinase (JNK) via an AMPK‐PKC‐ζ signaling. JNK promotes the phosphorylation of LMO7b, which regulates the actin cytoskeleton in epithelial cells, followed by its colocalization and interaction with the Na+,K+‐ATPase and several components of the clathrin‐dependent endocytic machinery. The protein kinase A (PKA)‐Iα also plays a role in the Na+,K+‐ATPase endocytosis during hypercapnia. Namely, hypercapnia via a CO2/HCO3 −‐sensitive soluble adenylyl cyclase (sAC) increases the production of cAMP, activates PKA‐Iα and in turn, the phosphorylation of the actin cytoskeleton component α‐adducin, culminating in the Na+,K+‐ATPase endocytosis from the cell plasma membrane.
Alveolar epithelial repair
Alveolar epithelial repair is critical for patients to recover from lung injury with the repair process involving cell proliferation and migration (Berthiaume et al. 1999). Hypercapnia, independently of extracellular acidosis, has been shown to impair proliferation of alveolar epithelial cells (Vohwinkel et al. 2011). The decreased cell proliferation was due to hypercapnia‐mediated mitochondrial dysfunction, resulting from hypercapnia‐induced miR‐183, which down‐regulates the tricarboxylic acid (TCA) cycle enzyme isocitrate dehydrogenase‐2 (IDH2) (Fig. 2). In a different model, hypercapnic acidosis was shown to impair plasma membrane wound resealing in ventilator‐injured lungs (Doerr et al. 2005). In line with this observation, hypercapnic acidosis has been shown to decrease alveolar epithelial wound repair via reduced NF‐κB activation (O'Toole et al. 2009) (Fig. 2). A recent report demonstrated that miR‐183 inhibits NF‐κB by directly targeting its 3′‐untranslated region (Sha et al. 2014). In addition, miR‐183 is known to negatively regulate cell migration in cancer cells (Lowery et al. 2010; Zhu et al. 2012). These observations raise the possibility that miR‐183 may play an important role in alveolar epithelial cell migration as well as proliferation in hypercapnic conditions. Recently, a clinical study reported an association between hypercapnia and prolonged air leaks (PALs) in patients after thoracic surgery. PAL is an important cause of morbidity and mortality after lung resection (Okereke et al. 2005). Intrapleural hypercapnia was associated with delayed resolution of PAL in patients after lobectomy, and reducing pleural CO2 levels was associated with faster resolution of air leaks (Bharat et al. 2016). Collectively, hypercapnia appears to impair alveolar epithelial cell proliferation and migration, which is deleterious to alveolar epithelial repair.
Figure 2. Hypercapnia inhibits alveolar epithelial repair.
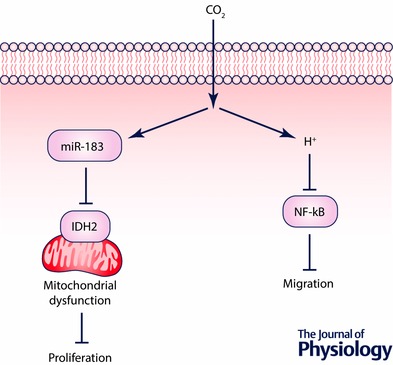
Hypercapnia inhibits proliferation of alveolar epithelial cells due to mitochondrial dysfunction resulting from hypercapnia‐induced miR‐183 which down‐regulates the TCA cycle enzyme isocitrate dehydrogenase‐2 (IDH2). Hypercapnic acidosis impairs alveolar epithelial cell migration by the NF‐κB‐dependent mechanism.
Innate immunity and host defence
Although previous studies have reported that patients with lung injury may have benefited from permissive hypercapnia (Hickling et al. 1994; Amato et al. 1998; Contreras et al. 2015), it has been associated with increased mortality in hospitalized patients with community‐acquired pneumonia (Laserna et al. 2012) and in patients with cystic fibrosis awaiting lung transplantation (Belkin et al. 2006). These studies raised the question whether hypercapnia may be associated with a dysregulated host immune response to fight infection in patients with severe lung disease. The effects of hypercapnia on host immune response have been explored in in vitro and in vivo studies. Hypercapnia selectively inhibits the expressions of interleukin‐6 (IL‐6) and tumour necrosis factor (TNF), the innate immune effectors that play a role in host defence, and it has been reported to decrease phagocytosis in human and mouse macrophage cell lines as well as alveolar macrophages isolated from both species (Wang et al. 2010) (Fig. 3 A). The inhibition of phagocytosis occurred independently of hypoxia, heat shock‐responsive pathways or NO signalling. Furthermore, hypercapnia also inhibited autophagy and bacterial killing in human macrophages by increasing the expression of Bcl‐2 and Bcl‐xL, which bind Beclin 1 and prevent autophagy initiation (Casalino‐Matsuda et al. 2015) (Fig. 3 B). Recent studies reported that hypercapnia inhibits activation of the canonical NF‐κB pathway that drives the expression of many host defence genes while promoting activation of the non‐canonical NF‐κB component RelB, whose function is largely anti‐inflammatory and immunosuppressive (Cummins et al. 2010; Oliver et al. 2012) (Fig. 3 C). In these in vitro studies, suppression of cytokine gene expression, phagocytosis, autophagy and NF‐κB signalling by hypercapnia was independent of pH. In contrast, there are reports suggesting that hypercapnia might regulate the immune response by decreasing extracellular and/or intracellular pH. Acidosis is known to impair the function of immune cells (Lardner, 2001), including alveolar macrophages (Lang et al. 2005). Thus, it appears that hypercapnia may modulate innate immunity and host defence via pH‐independent or ‐dependent mechanisms. More recently, it has been reported that normoxic hypercapnia impairs antimicrobial host defence in a model of murine pneumonia caused by Pseudomonas aeruginosa, an important cause of pulmonary infection in patients who may have hypercapnia, such as those with advanced chronic obstructive pulmonary disease (COPD) and cystic fibrosis (Gates et al. 2013). Mice exposed to hypercapnia had higher mortality and increased burden of Pseudomonas aeruginosa in the lungs and other organs. The lung levels of IL‐6 and TNF were decreased during the early phase of infection, and inhibited the phagocytosis of bacteria and generation of reactive oxygen species by lung neutrophils (Gates et al. 2013).
Figure 3. Hypercapnia suppresses innate immunity and host defence.
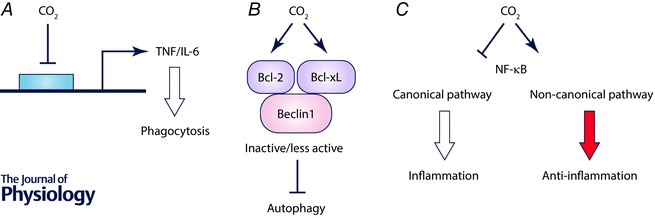
A, hypercapnia selectively inhibits mRNA and protein expressions of IL‐6 and TNF and decreases phagocytosis in macrophages. B, hypercapnia inhibits autophagy in macrophages by increasing expressions of Bcl‐2 and Bcl‐xL which bind Beclin 1. C, hypercapnia inhibits activation of the canonical NF‐κB pathway that drives expression of inflammatory cytokine genes while promoting activation of the non‐canonical NF‐κB pathway.
Airway function
Several studies suggest that hypercapnia is a marker of poor prognosis in patients with obstructive lung disease such as COPD (Köhnlein et al. 2014) and obesity hypoventilation syndrome (Piper, 2016). In addition, there is increasing evidence that the strategy of mechanical ventilation aimed at reducing the partial pressure of CO2 in arterial blood () can have beneficial effects including improvement of forced expiratory volume, health related quality of life and mortality in hypercapnic patients with COPD (Windisch et al. 2008; Köhnlein et al. 2014). Changes in CO2 are known to modulate airway smooth muscle tone. However, reported effects of changes in respiratory mechanics of spontaneously breathing, unanaesthetized healthy human subjects are controversial. With inhalation of CO2 mixtures, pulmonary resistance has been shown to increase, decrease, or remain unchanged (Sterling, 1969; Rodarte & Hyatt, 1973; Badr et al. 1991). Part of this variability probably reflects the multiple sites of action of CO2. An in vitro study showed that hypercapnic acidosis produced a reversible reduction in active tension of bronchial rings while normocapic acidosis was without any effect (Stephens et al. 1968). This is probably related to a difference in intracellular pH under the different conditions studied. Intracellular pH directly modulates the entry of Ca2+ into airway smooth muscle cells through voltage dependent Ca2+ channels (Yamakage et al. 1995), suggesting that modulation of Ca2+ influx into airway smooth muscle cells by intracellular pH contributes to the regulation of airway tone by CO2. On the other hand, hypercapnia, independently of acidosis, also leads to a rapid and transient increase in intracellular Ca2+ (Vadasz et al. 2008).
Conclusion
Historically, it has been proposed that hypercapnia, and especially hypercapnic acidosis, may have beneficial effects in mechanically ventilated patients and patients with ALI. The term ‘permissive hypercapnia’ has been proposed and is being used in treating patients (Hickling et al. 1994; Amato et al. 1998; Contreras et al. 2015). Many of the cellular responses to hypercapnia were thought to be a consequence of acidosis because of the rapid conversion of CO2 in solution into H2CO3 and subsequently HCO3 − and H+. Recent studies suggest that molecular CO2 can act as a signalling molecule and that hypercapnia can have deleterious effects in the lung (Briva et al. 2007; Vadasz et al. 2008; Vohwinkel et al. 2011; Gates et al. 2013; Casalino‐Matsuda et al. 2015; Dada et al. 2015) and patient survival (Köhnlein et al. 2014; Bharat et al. 2016). The impairment of these lung physiological functions by hypercapnia probably underlies the negative impacts of hypercapnia in patients with severe acute or chronic lung diseases. Thus, further preclinical and clinical studies are needed to define which of these (or other) effects of hypercapnia are beneficial or deleterious in patients with lung diseases.
Additional information
Competing interests
None declared.
Funding
This work was supported by the National Institutes of Health (Grant no. RO1‐HL085534 to J.I.S.) and National Institute of Aging (Grant no. PO1‐AG049665 to J.I.S.).
Biographies
Jacob I. Sznajder (left) is the Ernest S. Bazley Professor of Medicine and Cell and Molecular Biology at Northwestern University and has been conducting research related to lung injury and alveolar epithelial function. His laboratory is using integrative approaches to study molecular mechanisms regulating lung injury in infectious models (influenza) and non‐infectious models. He is also studying the effects of high CO2 levels on the lungs and muscle biology.

Emilia Lecuona (centre) obtained her PhD in Spain (University of La Laguna) and completed her postdoctoral training at Northwestern University. She is a Research Associate Professor at Northwestern University and is interested in the resolution of lung injury as well as the pathophysiology of hypercapnia on the lungs.
Masahiko Shigemura (right) completed his PhD in Japan (Hokkaido University) and is now a research fellow in Dr Sznajder's laboratory. He is currently conducting research on the effects of hypercapnia on airways and lung pathophysiology.
This review was presented at the symposium “Physiological gases in health and disease”, which took place at Physiology 2016, Dublin, Ireland, 29–31 July 2016.
References
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi‐Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY & Carvalho CR (1998). Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338, 347–354. [DOI] [PubMed] [Google Scholar]
- ARDS Team Network (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Badr MS, Skatrud JB, Simon PM & Dempsey JA (1991). Effect of hypercapnia on total pulmonary resistance during wakefulness and during NREM sleep. Am Rev Respir Dis 144, 406–414. [DOI] [PubMed] [Google Scholar]
- Belkin RA, Henig NR, Singer LG, Chaparro C, Rubenstein RC, Xie SX, Yee JY, Kotloff RM, Lipson DA & Bunin GR (2006). Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med 173, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume Y, Lesur O & Dagenais A (1999). Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 54, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharat A, Graf N, Mullen A, Kanter J, Andrei AC, Sporn PH, DeCamp MM & Sznajder JI (2016). Pleural hypercarbia after lung surgery is associated with persistent alveolopleural fistulae. Chest 149, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briva A, Vadasz I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, Batlle D, Gruenbaum Y & Sznajder JI (2007). High CO2 levels impair alveolar epithelial function independently of pH. PLoS One 2, e1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino‐Matsuda SM, Nair A, Beitel GJ, Gates KL & Sporn PH (2015). Hypercapnia inhibits autophagy and bacterial killing in human macrophages by increasing expression of Bcl‐2 and Bcl‐xL. J Immunol 194, 5388–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S & Orlowski J (2010). Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11, 50–61. [DOI] [PubMed] [Google Scholar]
- Contreras M, Masterson C & Laffey JG (2015). Permissive hypercapnia: what to remember. Curr Opin Anaesthesiol 28, 26–37. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P & Taylor CT (2010). NF‐κB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol 185, 4439–4445. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Selfridge AC, Sporn PH, Sznajder JI & Taylor CT (2014). Carbon dioxide‐sensing in organisms and its implications for human disease. Cell Mol Life Sci 71, 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada LA, Trejo Bittar HE, Welch LC, Vagin O, Deiss‐Yehiely N, Kelly AM, Baker MR, Capri J, Cohn W, Whitelegge JP, Vadasz I, Gruenbaum Y & Sznajder JI (2015). High CO2 leads to Na,K‐ATPase endocytosis via c‐Jun amino‐terminal kinase‐induced LMO7b phosphorylation. Mol Cell Biol 35, 3962–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr CH, Gajic O, Berrios JC, Caples S, Abdel M, Lymp JF & Hubmayr RD (2005). Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator‐injured lungs. Am J Respir Crit Care Med 171, 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Cartron JP, Ripoche P & Gros G (2008). RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22, 64–73. [DOI] [PubMed] [Google Scholar]
- Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, Hauser AR, Sznajder JI & Sporn PH (2013). Hypercapnia impairs lung neutrophil function and increases mortality in murine Pseudomonas pneumonia. Am J Respir Cell Mol Biol 49, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling KG, Walsh J, Henderson S & Jackson R (1994). Low mortality rate in adult respiratory distress syndrome using low‐volume, pressure‐limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22, 1568–1578. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Nunes AR, Cann MJ & Kumar P (2015). Ecto‐5′‐nucleotidase, adenosine and transmembrane adenylyl cyclase signalling regulate basal carotid body chemoafferent outflow and establish the sensitivity to hypercapnia. Adv Exp Med Biol 860, 279–289. [DOI] [PubMed] [Google Scholar]
- Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J & Steegborn C (2006). Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362, 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, Karg O, Laier‐Groeneveld G, Nava S, Schönhofer B, Schucher B, Wegscheider K, Criée CP & Welte T (2014). Non‐invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2, 698–705. [DOI] [PubMed] [Google Scholar]
- Lang CJ, Dong P, Hosszu EK & Doyle IR (2005). Effect of CO2 on LPS‐induced cytokine responses in rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 289, L96–L103. [DOI] [PubMed] [Google Scholar]
- Lardner A (2001). The effects of extracellular pH on immune function. J Leukoc Biol 69, 522–530. [PubMed] [Google Scholar]
- Laserna E, Sibila O, Aguilar PR, Mortensen EM, Anzueto A, Blanquer JM, Sanz F, Rello J, Marcos PJ, Velez MI, Aziz N & Restrepo MI (2012). Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community‐acquired pneumonia. Chest 142, 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuona E, Sun H, Chen J, Trejo HE, Baker MA & Sznajder JI (2013). Protein kinase A‐Iα regulates Na,K‐ATPase endocytosis in alveolar epithelial cells exposed to high CO2 concentrations. Am J Respir Cell Mol Biol 48, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuona E, Trejo HE & Sznajder JI (2007). Regulation of Na,K‐ATPase during acute lung injury. J Bioenerg Biomembr 39, 391–395. [DOI] [PubMed] [Google Scholar]
- Lowery AJ, Miller N, Dwyer RM & Kerin MJ (2010). Dysregulated miR‐183 inhibits migration in breast cancer cells. BMC Cancer 10, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI & Dale N (2013). CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 2, e01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missner A & Pohl P (2009). 110 years of the Meyer‐Overton rule: predicting membrane permeability of gases and other small compounds. Chemphyschem 10, 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastersky R (2013). Global carbon dioxide levels near worrisome milestone. Nature 497, 13–14. [DOI] [PubMed] [Google Scholar]
- Musa‐Aziz R, Chen LM, Pelletier MF & Boron WF (2009). Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106, 5406–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke I, Murthy SC, Alster JM, Blackstone EH & Rice TW (2005). Characterization and importance of air leak after lobectomy. Ann Thorac Surg 79, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, McLoughlin P, Taylor CT & Cummins EP (2012). Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J Biol Chem 287, 14004–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O'Brien T & Laffey JG (2009). Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF‐ κB dependent mechanism. Thorax 64, 976–982. [DOI] [PubMed] [Google Scholar]
- Piper A (2016). Obesity hypoventilation syndrome: Weighing in on therapy options. Chest 149, 856–868. [DOI] [PubMed] [Google Scholar]
- Rodarte JR & Hyatt RE (1973). Effect of acute exposure to CO2 on lung mechanics in normal man. Respir Physiol 17, 135–145. [DOI] [PubMed] [Google Scholar]
- Sha F, Wu S, Zhang H & Guo X (2014). miR‐183 potentially inhibits NF‐κB1 expression by directly targeting its 3′‐untranslated region. Acta Biochim Biophys Sin (Shanghai) 46, 991–996. [DOI] [PubMed] [Google Scholar]
- Stephens NL, Meyers JL & Cherniack RM (1968). Oxygen, carbon dioxide, H+ ion, and bronchial length‐tension relationships. J Appl Physiol 25, 376–383. [Google Scholar]
- Sterling GM (1969). The mechanism of decreased specific airway conductance in man during hypercapnia caused by inhalation of 7 per cent CO2 . Clin Sci 37, 539–548. [PubMed] [Google Scholar]
- Sznajder JI, Factor P & Ingbar DH (2002). Invited review: lung edema clearance: role of Na+‐K+‐ATPase. J Appl Physiol (1985) 93, 1860–1866. [DOI] [PubMed] [Google Scholar]
- Vadasz I, Dada LA, Briva A, Helenius IT, Sharabi K, Welch LC, Kelly AM, Grzesik BA, Budinger GR, Liu J, Seeger W, Beitel GJ, Gruenbaum Y & Sznajder JI (2012. a). Evolutionary conserved role of c‐Jun‐N‐terminal kinase in CO2‐induced epithelial dysfunction. PLoS One 7, e46696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W & Sznajder JI (2008). AMP‐activated protein kinase regulates CO2‐induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K‐ATPase endocytosis. J Clin Invest 118, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz I, Hubmayr RD, Nin N, Sporn PH & Sznajder JI (2012. b). Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol 46, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS (2007). Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol 159, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadasz I, Chandel NS & Sznajder JI (2011). Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem 286, 37067–37076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Gates KL, Trejo H, Favoreto S Jr, Schleimer RP, Sznajder JI, Beitel GJ & Sporn PH (2010). Elevated CO2 selectively inhibits interleukin‐6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J 24, 2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch LC, Lecuona E, Briva A, Trejo HE, Dada LA & Sznajder JI (2010). Extracellular signal‐regulated kinase (ERK) participates in the hypercapnia‐induced Na,K‐ATPase downregulation. FEBS Lett 584, 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W; Quality of life in home mechanical ventilation study group (2008). Impact of home mechanical ventilation on health‐related quality of life. Eur Respir J 32, 1328–1336. [DOI] [PubMed] [Google Scholar]
- Yamakage M, Lindeman KS, Hirshman CA & Croxton TL (1995). Intracellular pH regulates voltage‐dependent Ca2+ channels in porcine tracheal smooth muscle cells. Am J Physiol 268, L642–L646. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Skelton LA, Xu L, Chandler MP, Berthiaume JM & Boron WF (2016). Role of receptor protein tyrosine phosphatase λ in sensing extracellular CO2 and HCO3 − . J Am Soc Nephrol 27, 2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Feng Y, Ke Z, Yang Z, Zhou J, Huang X & Wang L (2012). Down‐regulation of miR‐183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am J Pathol 180, 2440–2451. [DOI] [PubMed] [Google Scholar]


