Abstract
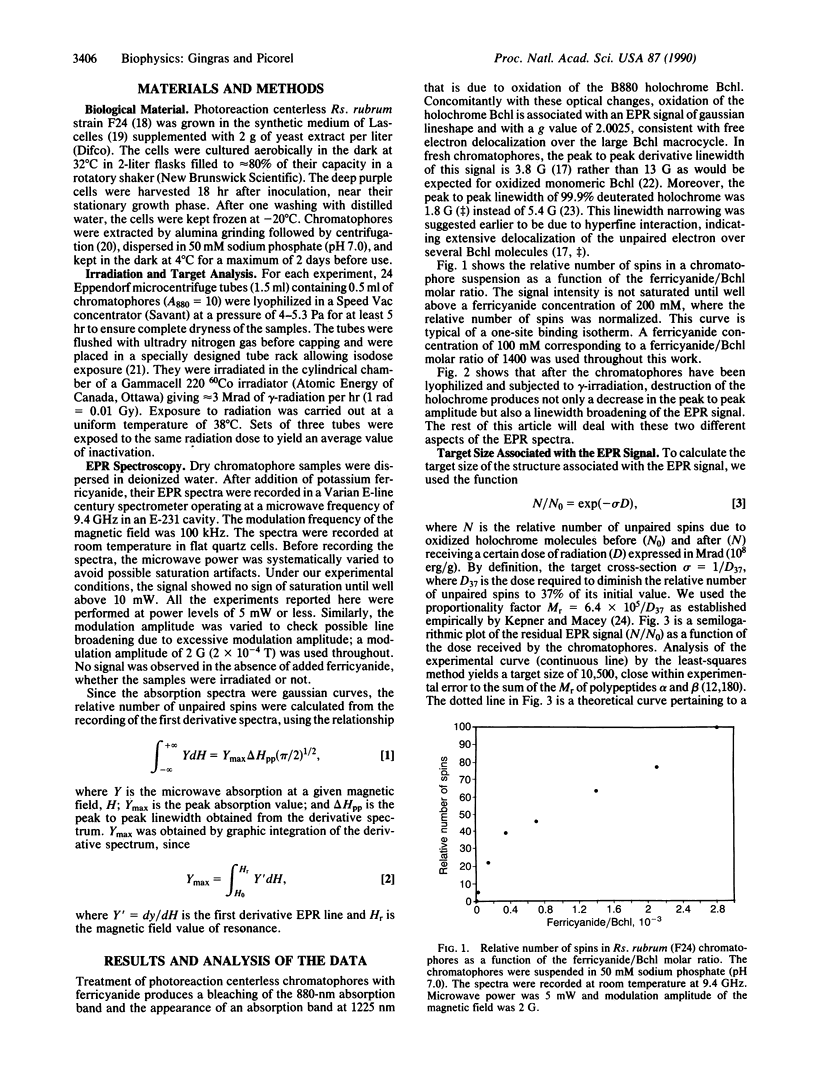
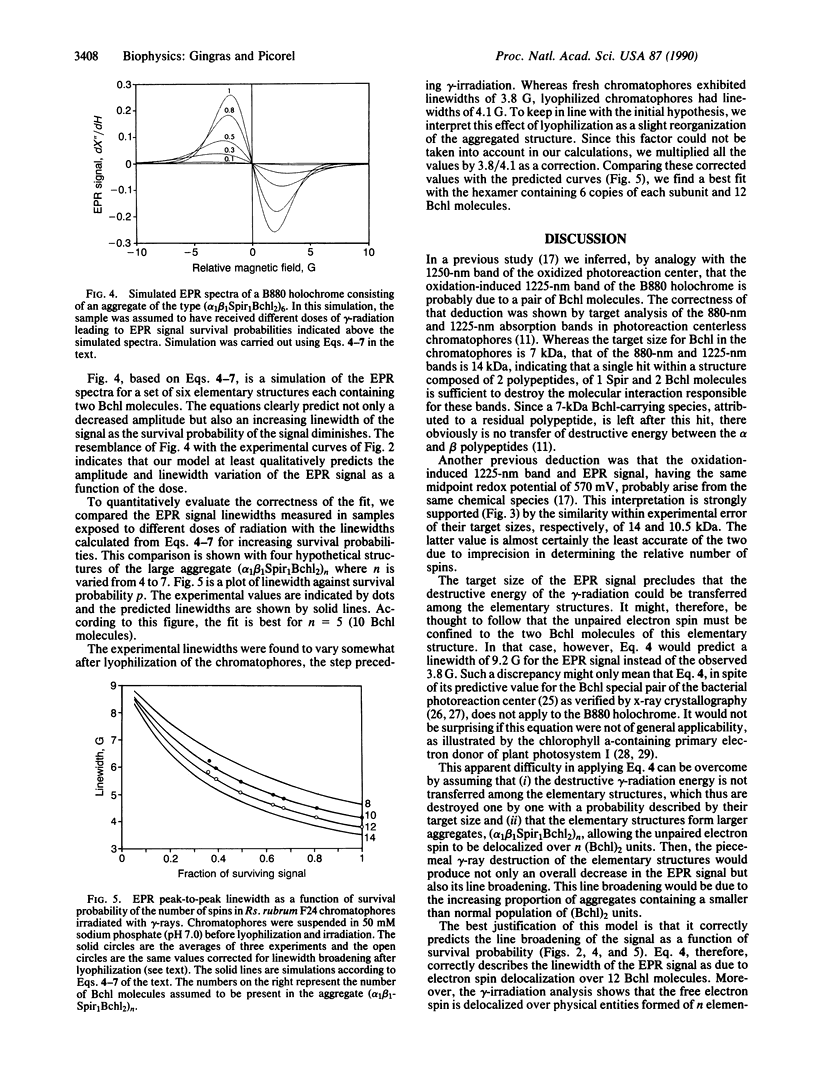
Oxidation of the B880 antenna holochrome gives rise to a 3.8-G linewidth electron paramagnetic resonance (EPR) signal that is considerably narrower than the 13-G signal of monomeric bacteriochlorophyll (Bchl) cation. Radiation inactivation was used to verify a model according to which this linewidth narrowing is due to delocalization over several Bchl molecules. Chromatophores of the photoreaction centerless mutant F24 of Rhodospirillum rubrum were subjected to different doses of gamma-radiation. This induced not only a decay of the EPR signal amplitude but also its linewidth broadening. According to target theory, the induced amplitude decay of the EPR signal had a target size of 10.5 kDa. This is attributed to an elementary structure (alpha1beta1Bchl2), whose number in the membrane would limit the rate of encounter with ferricyanide and thus the formation of unpaired spins. We applied Bernoulli statistics to predict, for a given survival probability of the signal, the number of surviving elementary structures in aggregates of (alpha1beta1Bchl2)n where n was varied from 4 to 7. Using an equation that predicted the Bchl special pair in the photo-reaction center, we were able to simulate the observed relationship between the EPR linewidth and the dose of radiation. The best fit was obtained with a hexameric structure alpha1beta1Bchl2)6.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard G., Giroux S., Potier M. Target size analysis by radiation inactivation: a large capacity tube rack for irradiation in a Gammacell 220. Anal Biochem. 1983 Jul 15;132(2):362–364. doi: 10.1016/0003-2697(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Bowman M. K., Michalski T. J., Tyson R. L., Worcester D. L., Katz J. J. Electron spin resonance of charge carriers in chlorophyll a/water micelles. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1498–1502. doi: 10.1073/pnas.85.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunisholz R. A., Suter F., Zuber H. The light-harvesting polypeptides of Rhodospirillum rubrum. I. The amino-acid sequence of the second light-harvestng polypeptide B 880-beta (B 870-beta) of Rhodospirillum rubrum S 1 and the carotenoidless mutant G-9+. carotenoidless mutant G-9+. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):675–688. doi: 10.1515/bchm2.1984.365.2.675. [DOI] [PubMed] [Google Scholar]
- Bélanger G., Bérard J., Gingras G. Isolation and partial characterization of the messenger RNA encoding the B880 holochrome protein of Rhodospirillum rubrum. Eur J Biochem. 1985 Dec 16;153(3):477–484. doi: 10.1111/j.1432-1033.1985.tb09326.x. [DOI] [PubMed] [Google Scholar]
- Bérard J., Bélanger G., Corriveau P., Gingras G. Molecular cloning and sequence of the B880 holochrome gene from Rhodospirillum rubrum. J Biol Chem. 1986 Jan 5;261(1):82–87. [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Engelhardt H., Engel A., Baumeister W. Stoichiometric model of the photosynthetic unit of Ectothiorhodospira halochloris. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8972–8976. doi: 10.1073/pnas.83.23.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Drews G. Chemical cross-linking studies of the light-harvesting pigment-protein complex B800-850 of Rhodopseudomonas capsulata. Eur J Cell Biol. 1983 Jan;29(2):115–120. [PubMed] [Google Scholar]
- Picorel R., Del Campo F. F., Ramirez J. M., Gingras G. The photoreaction center of Rhodospirillum rubrum mutant strain F24.1. Biochim Biophys Acta. 1980 Nov 5;593(1):76–84. doi: 10.1016/0005-2728(80)90009-2. [DOI] [PubMed] [Google Scholar]
- Picorel R., L'Ecuyer A., Potier M., Gingras G. Structure of the B880 holochrome of Rhodospirillum rubrum as studied by the radiation inactivation method. J Biol Chem. 1986 Mar 5;261(7):3020–3024. [PubMed] [Google Scholar]
- Picorel R., Lefebvre S., Gingras G. Oxido-reduction of B800-850 and B880 holochromes isolated from three species of photosynthetic bacteria as studied by electron-paramagnetic resonance and optical spectroscopy. Eur J Biochem. 1984 Jul 16;142(2):304–311. doi: 10.1111/j.1432-1033.1984.tb08286.x. [DOI] [PubMed] [Google Scholar]
- Stark W., Kühlbrandt W., Wildhaber I., Wehrli E., Mühlethaler K. The structure of the photoreceptor unit of Rhodopseudomonas viridis. EMBO J. 1984 Apr;3(4):777–783. doi: 10.1002/j.1460-2075.1984.tb01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Morimoto Y., Sato M., Kakuno T., Yamashita J., Horio T. Isolation, characterization, and comparison of a ubiquitous pigment-protein complex consisting of a reaction center and light-harvesting bacteriochlorophyll proteins present in purple photosynthetic bacteria. J Biochem. 1985 Dec;98(6):1487–1498. doi: 10.1093/oxfordjournals.jbchem.a135417. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Noël H., Poirier L., Cloutier Y., Gingras G. Photoreaction center of photosynthetic bacteria. 1. Further chemical characterization of the photoreaction center from Rhodospirillum rubrum. Biochemistry. 1979 Oct 2;18(20):4301–4308. doi: 10.1021/bi00587a007. [DOI] [PubMed] [Google Scholar]
- Vredenberg W. J., Amesz J. Absorption bands of bacteriochlorophyll types in purple bacteria and their response to illumination. Biochim Biophys Acta. 1966 Oct 10;126(2):244–261. doi: 10.1016/0926-6585(66)90060-4. [DOI] [PubMed] [Google Scholar]
- Wasielewski M. R., Norris J. R., Shipman L. L., Lin C. P., Svec W. A. Monomeric chlorophyll a enol: Evidence for its possible role as the primary electron donor in photosystem I of plant photosynthesis. Proc Natl Acad Sci U S A. 1981 May;78(5):2957–2961. doi: 10.1073/pnas.78.5.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]


