Abstract
Top-down control of prey by predators are magnified in productive ecosystems due to higher sustenance of prey communities. In soil micro-arthropod food webs, plant communities regulate the availability of basal resources like soil microbial biomass. Mixed plant communities are often associated with higher microbial biomass than monocultures. Therefore, top-down control is expected to be higher in soil food webs of mixed plant communities. Moreover, higher predator densities can increase the suppression of prey, which can induce interactive effects between predator densities and plant community composition on prey populations. Here, we tested the effects of predator density (predatory mites) on prey populations (Collembola) in monoculture and mixed plant communities. We hypothesized that top-down control would increase with predator density but only in the mixed plant community. Our results revealed two contrasting patterns of top-down control: stronger top-down control of prey communities in the mixed plant community, but weaker top-down control in plant monocultures in high predator density treatments. As expected, higher microbial community biomass in the mixed plant community sustained sufficiently high prey populations to support high predator density. Our results highlight the roles of plant community composition and predator densities in regulating top-down control of prey in soil food webs.
Trophic interactions in food webs are observed as a combination of top-down and bottom-up control among consumers and their resources that principally regulate population dynamics in ecosystems1,2. In tri-trophic interactions, either stronger top-down control (predator-induced control) or stronger bottom-up control (plant-induced control) of herbivore/detritivore populations have commonly been observed3,4. Moreover, recent studies have revealed that variations in plant community composition with respective changes in the basal resource regulate top-down control in ecological communities5,6.
Plant communities with a high number of species produce higher plant biomass providing sufficient energy to support greater communities of herbivores and detritivores, which cascade to higher densities of predators6,7, with a subsequent increase in top-down effects7. These findings of a productivity-driven proportional increase in the strength of trophic interactions at higher trophic levels agree with the classical Oksanen et al. (1981)8 hypothesis that predicts increased top-down control of prey by predators at high productivity (higher resource availability for prey). However, it is unclear how the relative importance of top-down and bottom-up control may change in ecosystems, which are experiencing shifts in plant community composition9 as well as declines in densities of predators10. Taken together, these two factors could potentially interact in complex ways and change the relative importance of top-down and bottom-up forces in influencing ecosystem processes. Here, we chose an experimental approach manipulating top-down forces in systems differing in productivity and illustrate that top-down control in a soil food web motif are contingent upon plant community composition.
In soil food webs, bottom-up forces through litter quantity and quality and microbial community biomass together with soil physio-chemical characteristics influence the strength of top-down control2,11. In the micro-arthropod sub-web of soil food webs, the main food source of prey communities like Collembola is soil microbial biomass12. At higher microbial biomass, many Collembola species are expected to thrive, which may simultaneously increase the density of their predators, such as predatory mites13. With an increase in prey density, both per capita and net prey suppression by predators get saturated14. However, saturation of predation also depends on the predator density, which co-determines prey suppression in combination with prey density15,16. For instance, at high predator density, antagonistic predator-predator interactions, such as interference and cannibalism, may reduce the ability of predators to suppress prey17. However, the magnitude of predator interference may decrease when the prey population is high and heterogeneously distributed18.
Plant communities fuel soil food webs via litter and dead root inputs, together with rhizodeposits that plant roots release into soil during plant growth19,20. These organic inputs collectively represent the key resource for microbial community biomass in soil. However, several studies have shown variations among plant community compositions and among plant species in fueling soil microbial biomass and related food webs21,22. Plant mixtures with functionally different species were shown to enhance microbial biomass in soil23,24. Furthermore, certain plant functional groups are associated with higher microbial biomass due to their positive association with N-fixing bacterial species (legumes)25 or due to high root biomass (grasses)26. Functionally different plant groups also create heterogeneous environments in soil27 that may favour predators in suppressing prey5,28.
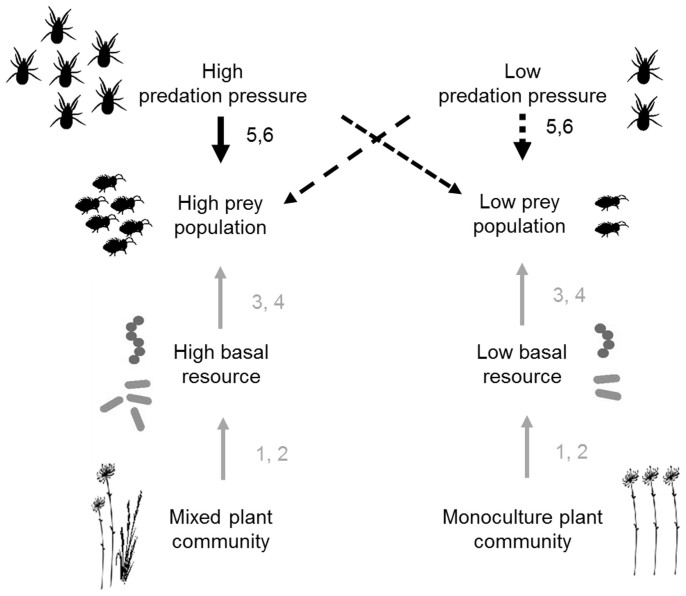
In this research, we expect predator density (top-down force) and plant community-induced variations in soil microbial biomass (bottom-up force) to interactively affect prey populations. Higher densities of predators can increase the suppression of prey; however, only when the prey has sufficient amounts of resources to exploit, which would depend on plant community composition. Therefore, we hypothesize that the strength of top-down control will increase with predator density in the mixed plant community, but will decline with predator density in monoculture plant communities (Figure 1)5. We test our hypotheses in a soil micro-arthropod predator-prey system with Collembola as prey species and predatory mites as predators. This soil food web motif was studied in different plant communities, consisting of a mixed plant community with three plant functional groups (grass, herb, and legume) together and the respective monocultures.
Figure 1. Conceptual diagram illustrating the working hypotheses.
The basal resource in this study is soil microbial biomass C. The upward directing gray arrows with numbers indicate literature-based information for the shown relation. For instance, mixed plant communities have been shown to be associated with higher soil microbial biomass C (Reference 1). The downward directing arrows indicate hypotheses on differences in the strength of top-down control at high vs. low predator densities in two plant community composition scenarios (mixed and monocultures). The dashed arrows indicate weak top-down control, whereas solid arrows indicate strong top-down control. The crossed dashed arrows indicate weak top-down control as predators at high density will not thrive in the presence of low prey density, and low predator densities cannot suppress high densities of prey (see text for details). Figure is drawn by MPT. References: 1 - Spehn et al. 200023; 2 - Eisenhauer et al. 201324; 3 - Bonkowski et al. 200012; 4 - Scheu et al. 200513; 5 - Oksanen et al. 19818; 6 - Haddad et al. 20117.
Results
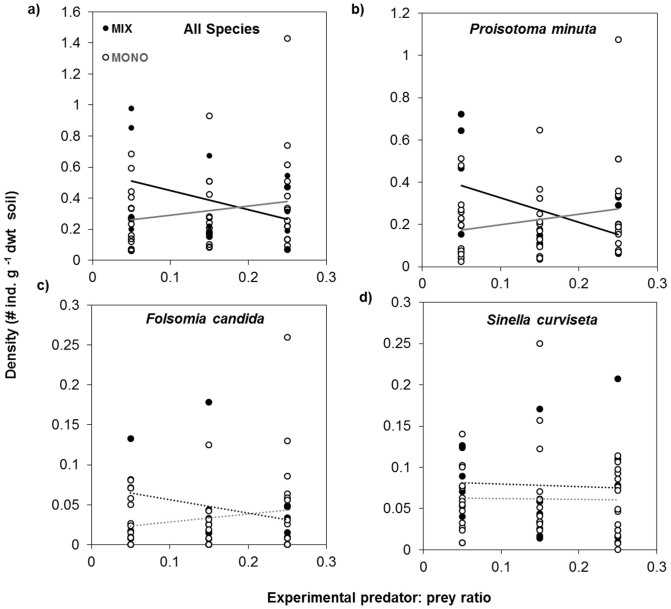
We found no significant main effects of predator density (GLMM, F = 0.09, P = 0.55) and plant community composition (GLMM, F = 1.31, P = 0.63) on Collembola density. However, and in line with our expectation, we found a significant interaction effect of predator density and plant community composition on total Collembola density (GLMM, F = 4.18, P = 0.02). In treatments with high predator densities, total Collembola densities decreased in the mixed plant community, while they increased in monoculture plant communities (Figure 2a). The interactive effect of predator density and plant community composition on total Collembola density was driven by the response of Proisotoma minuta - the smallest body-sized prey species in our experiment (GLMM, F = 5.62, P = 0.01; Figure 2b). Folsomia candida showed a similar response, but the interaction effect was not significant (GLMM, F = 2.40, P = 0.12; Figure 2c). By contrast, S. curviseta showed no response to the interaction between predator density and plant community composition (GLMM, F = 0.008 and P = 0.9; Figure 2d). Further, we also found no interactive effects of predator density and plant monocultures on Collembola species (Proisotoma: F = 0.10, P = 0.90; Folsomia: F = 0.32, P = 0.72; Sinella: F = 0.16, P = 0.84) (Supplementary Figure S1).
Figure 2.
(a) Total Collembola densities, (b) densities of Proisotoma minuta, (c) densities of Folsomia candida, and (d) densities of Sinella curviseta at the end of the experiment as affected by experimental predator densities (indicated by predator: prey ratio) and plant community composition. The black solid lines show significant relationships, whereas dotted lines indicate non-significant relationships.
In line with our expectations, soil microbial biomass C was significantly higher in the mixed plant community compared to monoculture plant communities (+30%; LMM, F = 8.13, P <0.01; Figure 3a), but did not vary significantly among the different monocultures (LMM, F = 2.12, p = 0.11, Supplementary Figure S2). Predator density had no significant effect on soil microbial biomass C (LMM, F = 0.05, P = 0.82). In addition, we found a marginally significant interactive effect of plant community composition and predator density on soil microbial biomass C (LMM, F = 2.92, P = 0.08). Microbial biomass C increased in the mixed plant community compared to the soil at the start of the experiment (+7%), whereas it decreased in monoculture plant communities (−17%).
Figure 3.
(a) Soil microbial biomass C as affected by plant community composition. (b) Relationships between total Collembola densities and soil microbial biomass C at different quantiles.
Results from quantile regression showed that at the higher quantile (0.90) of total Collembola density, soil microbial biomass and Collembola density were positively associated (Figure 3b). On the contrary, at lower quantiles, soil microbial biomass and Collembola density showed a negative relation (Figure 3b). The non-parametric quantile regression tests revealed significant associations between soil microbial biomass and Collembola density at quantiles 0.90 (P = 0.04), 0.75 (P <0.01), and 0.50 (P <0.001), whereas associations were not significant at quantiles 0.25 (P = 0.13) and 0.10 (P = 0.52).
Discussion
Our results show two contrasting patterns of top-down control of prey populations based on experimentally varied predator density and plant community composition. Top-down control of Collembola increased (i.e., decrease in Collembola density) at high predator density in the mixed plant community, but not in monoculture plant communities, which agrees with our hypothesis. We speculate that the contrasting predator density effects on suppression of Collembola populations were partly due to effects mediated by plant community on soil microbial community biomass.
Microbial biomass was significantly higher in the mixed plant community than in monoculture plant communities (Figure 3a). Moreover, the positive association of higher Collembola population density and higher microbial community biomass indicates that the higher availability of microbial biomass supported higher Collembola population densities (Figure 3b). Although correlative, this relation provides some evidence that the higher sustenance of the prey community was possible only when the basal resource level was high. Hence, due to favourable conditions (higher microbial biomass) for Collembola population in the soil of the mixed plant community, only a higher predator density was able to exert top-down control, but not the low predator density treatment.
Our results support the hypothesis of increased trophic control of prey by predators as productivity increases8,29. Furthermore, our results show interactive effects of predator density and productivity co-determining changes in prey populations. This is also in accordance to studies showing interactive effects of top-down and bottom-up forces in regulating food web structure30,31. Although, most of these previous studies demonstrated the influence of a top-down force by manipulating the presence/absence of predators, our results show that differences in predator density can alter food web structure depending on the availability of the basal resources.
In contrast to the results in the mixed plant community, interference among predatory mites at high predator density may have increased in monoculture plant communities due to low Collembola density causing a weak top-down control (Figure 2a). Interference among predatory mite individuals are argued to be low at sufficiently abundant prey populations due to their ability of dispersing into prey clusters18. This could cause a higher suppression of prey, as in the case of high density predator treatments in the mixed plant community in this study.
Mixed plant communities in combination with different functional groups including legumes and grasses have been shown to increase Collembola densities, likely due to elevated microbial biomass in soil32,33. Due to higher production of fine roots and an associated increase in rhizodeposition, mixed plant communities potentially provide favourable microhabitat conditions in soil for microbial growth26,34.
To overcome predation pressure, prey communities may show compensatory population growth via faster regeneration35. In order to do so, Collembola species are expected to increase their grazing activity on microbial communities36. Increased Collembola grazing potentially can decrease microbial biomass; however, our finding suggest that this was not the case, at least in the mixed plant community. It is important to note though that our study design did not allow us to test how Collembola grazing pressure could have affected microbial biomass (Supplementary Figure S3). Some studies have reported that Collembola grazing could essentially influence soil microbial biomass36,37; however, less is known about how such grazing effects could occur in the context of different plant communities38. Our results also show that realized predator density was higher in the mixed plant community independent of predator density treatments at the final harvest supporting the notion that plant species mixtures support higher predator densities than monocultures5,6 (Supplementary Figure S4).
The significant suppression of only P. minuta by predators among the three studied Collembola species indicates a combination of strong and weak trophic interactions in our study depending on Collembola species identity (Figure 2b). A plausible reason for strong suppression of P. minuta could be their relatively small body size, which may cause higher foraging advantages, such as reduced handling time, for predators compared to the large-sized prey such as S. curviseta39. Interestingly, when changes in Collembola density from the start of the experiment to the final harvest were compared, P. minuta density also increased more than the other two Collembola species (see differences in Y-axis scales in Figure 2b, c, and d). This further indicates compensatory population growth in P. minuta, which could occur in prey communities when exposed to high predation pressure35,40. Species undergoing faster regeneration like P. minuta41 often show compensatory patterns and are therefore superior at exploiting available resources42. At high predator density, an increase in prey density would lead to a higher probability of predator-prey encounters, which could have contributed to the observed suppression of P. minuta in the resource-rich environment of the mixed plant community.
Spatial and resource heterogeneity in soil have been shown to be higher in diverse plant communities than in monocultures43. We observed consistent patterns of soil microbial biomass and prey population among monocultures of the three plant functional groups at different predator density treatments (Supplementary Figure S1), which is inconsistent with studies that have reported stronger plant identity effects on the trophic structure of soil food webs due to plant species-specific soil environments44. The plant species that we used have different root architectures and biomass45, and variations in plant-derived organic inputs in the mixed plant community25 may have created a more heterogeneous environment46 in soil promoting prey suppression by predators. Such heterogeneous environments will enhance the clustering of Collembola species in resource-rich patches (higher microbial biomass) that in general would favour predation by predatory mites18. However, as we were unable to show such direct links in the present study, the proposed relationship between resource heterogeneity and predator-prey interactions in soil merits further exploration.
In general, at high predator density, prey suppression can increase for a short duration when prey population growth is constrained by a lack of the basal resource47. It is important to note that when resource availability limits prey population growth, predator density would decline due to starvation-induced mortality. This could be one reason for weak effects of initial predator densities on the realized predator densities compared to differences between the mixed and monoculture plant communities (Supplementary Figure S4). In low productive systems (monoculture plant communities), high predator density might have suppressed Collembola population initially, but as the experiment progressed, prey suppression may have decreased as Collembola population did not thrive as much as in the mixed plant community, consequently leading to the decline in predator densities. Multiple harvests or developing procedures to temporally track population dynamics48 in such experiments can provide further insights into density effects of predators on prey suppression as influenced by resource availability.
Prey suppression by predators in ecosystems contribute to many ecosystem functions, such as nutrient cycling49. Predators are the most vulnerable trophic group to the effects of disturbances, such as land fragmentation and climate warming10,50. A decline in predator density can detrimentally affect ecosystem processes through trophic cascades49. Utilizing a soil food web motif, our study shows that the top-down control of prey depends on the availability of the basal resource as well as on the density of predators. We argue that such interactive effects of predator density (top-down force) and productivity or resource availability mediated via the plant community (bottom-up force) play a crucial role in structuring food webs. Since both top-down and bottom-up factors determining food web structure are subjected to acute and chronic anthropogenic perturbations, our study highlights that such interactions are crucial to understand and predict changes in food web structure and concomitant ecosystem functions.
Methods
Microcosm set-up
The experiment was conducted in microcosms made of polyvinyl chloride (PVC) tubes (height: 10 cm and diameter: 7 cm) with the soils (pH = 8.1, C: N ratio = 15.7) from a field site adjacent to the Jena Experiment (floodplain grassland)51. The soil was sieved using a 2 mm-mesh and defaunated by two cycles of the thaw-rethaw method in which soils were initially frozen at −20°C for 48 hours and later defrozen at room temperature for several days52. This method has been shown to remove micro-arthropods and even nematodes from soil without affecting microbial communities52. We added 300 g of this defaunated soil to microcosms together with 500 mg of grass root litter (Lolium perenne) and incubated that for two weeks with 10 ml tap water added every day in order to facilitate microbial colonization. The same amount of water was added every day throughout the experiment.
Plant communities
Mixed and monoculture plant communities were established in the defaunated soils, which had been incubated with grass litter for 2 weeks. The mixed plant community included Trifolium pratense (legume), Poa pratensis (grass), and Rumex acetosa (herb), and monoculture plant communities consisted of those three plant species alone in the microcosms. These plant species have been shown to vary in their root traits such as root depth and are part of plant species pool of the Jena Experiment45,51. Seeds of these plant species (obtained from Rieger-Hoffmann GmbH, Blaufelden-Raboldshausen, Germany) were sown separately in the same defaunated soil and transplanted into the microcosms after six weeks of germination (seedlings were chosen with similar heights of about 5–8 cm). Three plant individuals from one species were planted into monoculture microcosms (establishing three different monoculture treatments), whereas one individual per plant species was planted to construct a mixed plant community. To give plant communities sufficient time to establish in the microcosms, Collembola were added only after 20 days from the plant transplantations into the microcosms.
Soil microarthropod community
The prey community consisted of three Collembola species (Proisotoma minuta, Folsomia candida, and Sinella curviseta), which were added to the microcosms in equal densities (details below). Families of these species (P. minuta: Isotomidae, F. candida, and S. curviseta: Entomobryidae) are reported in the soil of the Jena Experiment33, while the used species are easy to culture in labs. All three species were cultured at the University of Jena at 20°C by feeding with dry yeast. These three species vary in their body size, with P. minuta (mean body size: 1.1 mm)53 being the smallest, F. candida (mean body size: 1.59 mm)54 being of intermediate size, and S. curviseta being the largest species (mean body size: 2 mm)54. Collembola species feed on several substrates such as fungi, bacteria and litter, but they are commonly accepted as microbial feeders53,55. This main feeding behavior of the three species was confirmed as they were cultured with dry yeast. A generalist predatory mite (Hypoaspis aculeifer, mean body size: 0.6 mm)56 was used as the model predator (purchased from Schneckenprofi in Germany). Hypoaspis aculeifer are common soil dwelling predators and are common predators of Collembola57.
Each microcosm received 20 individuals of each Collembola species (in total 60 individuals of Collembola, i.e., 0.20 ind. per g dry weight of soil), and one of three different densities of predators: 3, 9, and 15 individuals (i.e., 0.01, 0.03, and 0.05 ind. per g dry weight of soil). The Collembola density used in this experiment (approx. 20,000 ind/m2) is comparable to the field density in the Jena Experiment58. Predators were added 6 days after the addition of Collembola. We had four plant combinations (one mixed plant community and three different monocultures) crossed with three predator density treatments, each replicated 5 times (60 microcosms in total). The microcosms were randomly arranged in a block design to account for variations in wind flow from the cooling fan used to regulate temperature in the greenhouse. We applied a day/night cycle of 16 (20°C)/8 hours (16°C).
The experiment ran for 57 days after the day of predator addition. The experimental duration was adequate for regeneration of Collembola species59 and the predatory mite60. During final harvest, soil cores (5 cm deep, 5 cm in diameter) were taken from microcosms to extract Collembola and predatory mites using heat extraction61. During this extraction, soil cores were gradually heated for 10 days from 25°C up to 50°C. Collembola and predatory mites were collected in 70% ethanol and subsequently counted under a dissecting microscope.
Microbial biomass
We took 5 g of sieved soils (2 mm mesh size) from microcosms to estimate microbial biomass carbon (C) using an O2-microcompensation apparatus62. This method does not provide information on the composition of the soil microbial community, but captures the active part of the microbial community very efficiently62, which we used as a proxy of resource availability for Collembola in soil. Besides, plant-derived carbon inputs in soil are often positively correlated to microbial biomass C63. Microbial biomass was determined at the start of the experiment after 2 weeks of soil incubation with litters (before plants were transplanted into the soil) and at the final harvest. Microbial respiration was measured at hourly intervals for 24 hours at 19°C. Afterwards, substrate-induced respiration was measured by adding D-glucose as a substrate for about 10 h at 19°C. The mean of the three lowest readings within the first 10 hours was assessed as the maximum initial respiratory response (MIRR), and microbial biomass (μg C g−1 soil dry weight) was calculated by multiplying MIRR with correction factor of 3864.
Statistical analysis
Generalized linear mixed models (GLMM) were used to test the treatment effects of predator density (as experimental predator: prey ratio; linear term with three levels) and plant community composition (as mixed plant community and monoculture; categorical term with two levels) on Collembola density with blocks as the random effect. GLMM are generally recommended for regressions with the count data65. Further, due to over-dispersion in the Collembola count data (i.e., residual deviance = 1248.1≫ degrees of freedom = 55), we tested quasi-poisson vs. negative binomial distributions model fits for variations in Collembola density and selected the model with the lower value of maximum likelihood estimates66. The model with negative binomial errors (maximum likelihood estimate = 270.83) fitted better than the model with quasi-poisson errors (maximum likelihood estimate = 680.47). Although, model comparison was carried out with absolute counts of Collembola, we report GLMM (negative binomial error) results for Collembola counts per dry weight of soil (used during the animal extraction) due to further improved model fit (AICabsolute counts = 553.67> AICper g dry weight of soil = 86.08). For species-specific responses of Collembola to plant community compositions (mixed vs. monoculture) and predator density, we again used GLMM with negative binomial errors. Further, we tested if soil microbial biomass C was influenced by plant community composition and predator density treatments. For this, we used linear mixed effect models (LMM) with blocks as the random effect. We also tested whether plant monocultures and predator densities affected Collembola density (GLMM for count data, negative binomial error) and microbial biomass C (LMM for biomass data). We correlated microbial biomass C with Collembola density using quantile regression due to over-dispersion of Collembola density data67. We used quantile regression at quantiles 0.10, 0.25, 0.50, 0.75, and 0.90. Quantile regression incorporates the unequal variances of the response variables and is useful to consider the possibility of multiple slopes for minimum to maximum responses67. All statistical analyses were carried out in R statistical software version 2.15.2 (R Development Core Team, 2012).
Supplementary Material
Supplementary information
Acknowledgments
We thank Saskia Rennoch for animal counting and Jes Hines for comments and suggestions on an earlier version of the manuscript. We acknowledge funding by the German Research Foundation in the frame of the Emmy Noether research group (Ei 862/2). Further support came from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118).
Footnotes
The authors declare no competing financial interests.
Author Contributions M.P.T. and N.E. designed the experiment. M.P.T. performed the experiment and analyzed the data. M.P.T. wrote the first draft of the manuscript, and N.E. contributed to the revisions.
References
- Power M. Top-Down and Bottom-Up Forces in Food Webs: Do Plants Have Primacy. Ecology 73, 733–746 (1992). [Google Scholar]
- Lenoir L., Persson T., Bengtsson J., Wallander H. & Wirén A. Bottom–up or top–down control in forest soil microcosms? Effects of soil fauna on fungal biomass and C/N mineralisation. Biol. Fertil. Soils 43, 281–294 (2006). [Google Scholar]
- Walker M. & Jones T. Relative roles of top-down and bottom-up forces in terrestrial tritrophic plant–insect herbivore–natural enemy systems. Oikos 93, 177–187 (2001). [Google Scholar]
- Dyer L. A. & Letourneau D. Top-down and bottom-up diversity cascades in detrital vs. living food webs. Ecol. Lett. 6, 60–68 (2002). [Google Scholar]
- Haddad N. M. et al. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–39 (2009). [DOI] [PubMed] [Google Scholar]
- Scherber C. et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468, 553–6 (2010). [DOI] [PubMed] [Google Scholar]
- Haddad N. M., Crutsinger G. M., Gross K., Haarstad J. & Tilman D. Plant diversity and the stability of foodwebs. Ecol. Lett. 14, 42–6 (2011). [DOI] [PubMed] [Google Scholar]
- Oksanen L., Fretwell S., Arruda J. & Niemela P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 118, 240–261 (1981). [Google Scholar]
- Yang H. et al. Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Glob. Chang. Biol. 17, 2936–2944 (2011). [Google Scholar]
- Voigt W., Perner J., Davis A. & Eggers T. Trophic levels are differentially sensitive to climate. Ecology 84, 2444–2453 (2003). [Google Scholar]
- Kalinkat G., Brose U. & Rall B. C. Habitat structure alters top-down control in litter communities. Oecologia 172, 877–887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski M., Cheng W., Griffiths B. S., Alphei J. & Scheu S. Microbial-faunal interactions in the rhizosphere and effects on plant growth. Eur. J. Soil Biol. 36, 135–147 (2000). [Google Scholar]
- Scheu S., Ruess L. & Bonkowski M. In: Microorganisms in Soils: Roles in Geneis and. Functions (Buscot, F. & Varma, A.) 3, 253–275 (Springer-Verlag Berlin Heidelberg, 2005). [Google Scholar]
- Abrams P. The evolution of predator-prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 31, 79–105 (2000). [Google Scholar]
- Arditi R. & Ginzburg L. R. How species interact? Altering the standard view of trophic ecology 167 (Oxford University Press, New York, USA, 2012). [Google Scholar]
- Stier A. C., Geange S. W. & Bolker B. M. Predator density and competition modify the benefits of group formation in a shoaling reef fish. Oikos 122, 171–178 (2013). [Google Scholar]
- Sih A., Englund G. & Wooster D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355 (1998). [DOI] [PubMed] [Google Scholar]
- Sabelis M. In: Natural Enemies: The Population Biology of Predators, Parasites and Diseases (Crawley, M. J.) 225–264 (Blackwell Scientific Publications, 1992). [Google Scholar]
- Jones D. L., Nguyen C. & Finlay R. D. Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321, 5–33 (2009). [Google Scholar]
- Martens R. Contribution of rhizodeposits to the maintenance and growth of soil microbial biomass. Soil Biol. Biochem. 22, (1990). [Google Scholar]
- Wardle D. A., Yeates G. W., Williamson W. & Bonner K. I. The response of a three trophic level soil food web to the identity and diversity of plant species and functional groups. Oikos 102, 45–56 (2003). [Google Scholar]
- Eisenhauer N. & Reich P. B. Above- and below-ground plant inputs both fuel soil food webs. Soil Biol. Biochem. 45, 156–160 (2012). [Google Scholar]
- Spehn E., Joshi J., Schmid B., Alphei J. & Körner C. Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant Soil 224, 217–230 (2000). [Google Scholar]
- Eisenhauer N. et al. Plant diversity effects on soil food webs are stronger than those of elevated CO2 and N deposition in a long-term grassland experiment. Proc. Natl. Acad. Sci. 110, 6889–6994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcu A., Partsch S., Scherber C., Weisser W. & Scheu S. Earthworms and legumes control litter decomposition in a plant diversity gradient. Ecology 89, 1872–1882 (2008). [DOI] [PubMed] [Google Scholar]
- Eisenhauer N. et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 91, 485–96 (2010). [DOI] [PubMed] [Google Scholar]
- Weigelt A., Schumacher J., Roscher C. & Schmid B. Does biodiversity increase spatial stability in plant community biomass? Ecol. Lett. 11, 338–47 (2008). [DOI] [PubMed] [Google Scholar]
- Andow D. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 36, 561–586 (1991). [Google Scholar]
- Spiller D. A. & Schoener T. W. Climatic control of trophic interaction strength: the effect of lizards on spiders. Oecologia 154, 763–771 (2007). [DOI] [PubMed] [Google Scholar]
- Faithfull C. L., Huss M., Vrede T. & Bergström A.-K. Bottom-up carbon subsidies and top-down predation pressure interact to affect aquatic food web structure. Oikos 120, 311–320 (2011). [Google Scholar]
- Leibold M. & Chase J. Species turnover and the regulation of trophic structure. Annu. Rev. Syst. … 28, 467–494 (1997). [Google Scholar]
- Salamon J.-A., Schaefer M., Jorn A., Schmid B. & Scheu S. Effects of plant diversity on Collembola in an experimental grassland ecosystem. Oikos 106, 51–60 (2004). [Google Scholar]
- Sabais A. C. W., Scheu S. & Eisenhauer N. Plant species richness drives the density and diversity of Collembola in temperate grassland. Acta Oecologica 37, 195–202 (2011). [Google Scholar]
- Zak D. R., Holmes W. E., White D. C., Peacock A. D. & Tilman D. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84, 2042–2050 (2003). [Google Scholar]
- Crawley M. J. In: Natural Enemies: The Population Biology of Predators, Parasites and Diseases (Crawley, M. J.) 40–90 (Blackwell Scientific Publications, 1992). [Google Scholar]
- Hedlund K. & Ohrn M. S. Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88, 585–591 (2000). [Google Scholar]
- Bakonyi G. Effects of Folsomia candida (Collembola) on the microbial biomass in a grassland soil. Biol. Fertil. Soils 138–141 (1989). [Google Scholar]
- Endlweber K. & Scheu S. Interactions between mycorrhizal fungi and Collembola: effects on root structure of competing plant species. Biol. Fertil. Soils 43, 741–749 (2006). [Google Scholar]
- Brose U. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34 (2010). [Google Scholar]
- Dmitriew C. M. The evolution of growth trajectories: what limits growth rate? Biol. Rev. Camb. Philos. Soc. 86, 97–116 (2011). [DOI] [PubMed] [Google Scholar]
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1219, 1207–1219 (1998). [Google Scholar]
- Metcalfe N. B. & Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260 (2001). [DOI] [PubMed] [Google Scholar]
- Porazinska D. et al. Relationships at the Aboveground-Belowground Interface: Plants, Soil Biota, and Soil Processes. Ecol. Monogr. 73, 377–395 (2003). [Google Scholar]
- Bezemer T. M. et al. Divergent composition but similar function of soil food webs of individual plants: plant species and community effects. Ecology 91, 3027–36 (2010). [DOI] [PubMed] [Google Scholar]
- Ebeling A. et al. A trait-based experimental approach to understand the mechanisms underlying biodiversity–ecosystem functioning relationships. Basic Appl. Ecol. 15, 229–240 (2014). [Google Scholar]
- Eisenhauer N. et al. Impact of above- and below-ground invertebrates on temporal and spatial stability of grassland of different diversity. J. Ecol. 99, 572–582 (2011). [Google Scholar]
- Polis G. & Strong D. Food web complexity and community dynamics. Am. Nat. 147, 813–846 (1996). [Google Scholar]
- Schwarzmüller F., Eisenhauer N. & Brose U. Trophic Whales' as Biotic Buffers: Weak Interactions Stabilize Ecosystems against Nutrient Enrichment. J. Anim. Ecol. (2014) 10.1111/1365-2656.12324. [DOI] [PubMed] [Google Scholar]
- Schmitz O. J., Hawlena D. & Trussell G. C. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–209 (2010). [DOI] [PubMed] [Google Scholar]
- Zarnetske P. L., Skelly D. K. & Urban M. C. Biotic multipliers of climate change. Science 336, 1516–8 (2012). [DOI] [PubMed] [Google Scholar]
- Roscher C., Schumacher J. & Baade J. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl. Ecol. 121, 107–121 (2004). [Google Scholar]
- Poll J. et al. Low amounts of herbivory by root-knot nematodes affect microbial community dynamics and carbon allocation in the rhizosphere. FEMS Microbiol. Ecol. 62, 268–79 (2007). [DOI] [PubMed] [Google Scholar]
- Hopkin S. A key to the Collembola (Springtails) of Britain and Ireland. (FSC Publications, 2007). [Google Scholar]
- Kaersgaard C. W., Holmstrup M., Malte H. & Bayley M. The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 50, 5–15 (2004). [DOI] [PubMed] [Google Scholar]
- Hopkin S. P. Biology of Springtails (Insecta: Collembola) 330 (Oxford University Press, New York, USA, 1997). [Google Scholar]
- Messelink G. & Holstein-Saj R. van. Potential for biological control of the bulb scale mite (Acari: Tarsonemidae) by predatory mites in amaryllis. Proc. Neth. Entomol. Soc. Meet. 17, 113–118 (2006). [Google Scholar]
- Koehler H. H. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosyst. Environ. 74, 395–410 (1999). [Google Scholar]
- Eisenhauer N. et al. Plant diversity surpasses plant functional groups and plant productivity as driver of soil biota in the long term. PLoS One 6, e16055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain M. T. & Hopkin S. P. Folsomia candida (Collembola): a “standard” soil arthropod. Annu. Rev. Entomol. 50, 201–22 (2005). [DOI] [PubMed] [Google Scholar]
- Berndt O., Meyhöfer R. & Poehling H.-M. Propensity towards cannibalism among Hypoaspis aculeifer and H. miles, two soil-dwelling predatory mite species. Exp. Appl. Acarol. 31, 1–14 (2003). [DOI] [PubMed] [Google Scholar]
- Macfadyen A. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 30, 171–184 (1961). [Google Scholar]
- Scheu S. Automated measurement of the respiratory response of soil microcompartments: active microbial biomass in earthworm faeces. Soil Biol. Biochem. 24, 1–6 (1992). [Google Scholar]
- Bradford M. A., Keiser A. D., Davies C. A., Mersmann C. A. & Strickland M. S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 113, 271–281 (2013). [Google Scholar]
- Beck T. et al. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 29, 1023–1032 (1997). [Google Scholar]
- Zuur A., Ieno E., Walker N., Saveliev A. & Smith G. Mixed effects models and extensions in Ecology with R. (Springer Berlin Heidelberg, 2009). [Google Scholar]
- Hoef J. Ver. & Boveng P. Quasi-Poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88, 2766–2772 (2007). [DOI] [PubMed] [Google Scholar]
- Cade B. S. & Noon B. R. A gentle introduction to quantile regression for ecologists. Front Ecol Env. 1, 412–420 (2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information