ABSTRACT
Purpose of Review: This article describes a comprehensive approach to the mental status examination and diagnostic workup of patients suspected of having an emerging neurodegenerative dementia. Key strategies for obtaining a history and bedside examination techniques are highlighted.
Recent Findings: Classic descriptions of behavioral neurology syndromes were largely based on clinicopathologic correlations of strategic lesions in stroke patients. While still very important, advances in neuroimaging have expanded our armamentarium of cognitive evaluations to include assessments of findings in nonstroke anatomic distributions of disease. These efforts support comprehensive assessments of large-scale cerebral networks in cognitive neurology.
Summary: A thorough and focused mental status examination is essential for the evaluation of patients with cognitive symptoms. Selective use of laboratory testing and neuroimaging can aid in the diagnosis of dementia by excluding non-neurodegenerative etiologies. Neurodegenerative disease–specific tests are in development and will enhance diagnosis and efforts for disease-modifying therapy development.
INTRODUCTION
The mental status examination is a key component of a complete neurologic examination. The neurologic examination is structured to assess different elements of the neuraxis, and the mental status examination largely targets the cerebrum. Like the elemental neurologic examination, the mental status examination is organized into subsections to assess each major domain of cognition. Cognitive processes and behaviors like memory, language, visual-perceptual-spatial functioning, and executive functioning are targeted. These processes are very complex. Therefore, the cognitive functions that are interrogated by the mental status examination are not easily localized to a single anatomic structure. Rather than localizing an impairment to a specific anatomic locus, our perspective on brain-behavior relationships is more consistent with an approach focusing on the disruption of a large-scale neural network or connectome.1–7 In this approach, each network is composed of multiple gray matter nodes that are interconnected by projections within the white matter, and these white matter tracts serve to integrate the functioning of the gray matter nodes. From this perspective, disease disrupting some of the nodes or projections of a cerebral network may compromise a complex cognitive function. The quality of cognitive disruption depends, in part, on the component (or components) of the network that are disrupted. Indeed, any gray matter node may contribute to multiple networks. While the assessment of a patient can reveal selective impairment of specific components of the mental status examination, it is often the overall pattern of cognitive performance across multiple components that is most informative. For example, in Case 1-1 there is a relatively greater deficit for episodic memory compared to other cognitive domains, which suggests the diagnosis of mild Alzheimer disease (AD). There are several important caveats to consider when administering a mental status examination. First, the mental status examination can be quite lengthy. Like other aspects of the neurologic examination, it is valuable to tailor the mental status examination to the most pertinent positive findings and negative features. This kind of editing process benefits enormously from a mental status history and the larger medical history. Indeed, a single mental status examination obtains only a cross-sectional perspective of a patient’s performance at a given period of time, and longitudinal assessment is often very informative.
A detailed mental status history is important to determine onset, time course, and progression of symptoms that influences the differential diagnosis. For example, the pace of disease progression may be characterized as an acute decline that can be seen following a stroke or head injury, or subacute decline that can be associated with an infectious or neoplastic process, or a slow, insidious change that is most often associated with a neurodegenerative condition. Since each of these time courses may be associated with a particular pattern of cognitive and behavioral impairment, the history can help guide cognitive functions that should be ascertained.
It is also important to consider the mental status examination in the context of other medical and neurologic features. Attention to elementary neurologic features that are not reflected in the mental status examination will enhance the interpretation of cognitive findings. It is helpful for cognitive neurologists to consider involuntary movements, for example, in approaching their mental status examination. Conversely, attention to the mental status examination by neurologists treating neuromuscular or movement disorders, such as amyotrophic lateral sclerosis (ALS) or Parkinson disease (PD), are important due to the high frequency of cognitive difficulties in these patients.8,9 Furthermore, the mental status examination may be significantly influenced by demographic features of the patient. Thus, factors such as education, age, and cultural background can have an important impact on cognitive and behavioral functioning. For example, education may influence baseline vocabulary and other cognitive skills, age may influence executive functioning, and ethnicity may influence familiarity with specific objects or social norms. Consequently, performance expectations should be adjusted to accommodate individual differences, as illustrated by Case 1-1. In these scenarios, formal neuropsychological testing using standardized examinations with normative scores scaled for age and education can be useful and compliment observations from bedside evaluations. A number of computerized cognitive test batteries are available, but these are often limited in their scope and testing by computer often does not replicate the result of testing administered by a human. Finally, it is also important to be mindful of an individual’s current mental state. Poor sleep, anxiety/depression, or side effects of a medication in the individual’s regimen may interfere with concentration and level of functioning.
If there is suspicion of a neurodegenerative disease upon the conclusion of a detailed mental status examination, it is important to judiciously consider ancillary laboratory and neuroimaging studies to help support the diagnosis and exclude non-neurodegenerative etiologies. Indeed, a range of toxic, metabolic, inflammatory, neoplastic, paraneoplastic, or infectious etiologies can mimic neurodegenerative diseases. Diagnosis of these conditions is critical, as disease-specific treatments may need to be implemented. Conversely, laboratory and neuroimaging investigations can be initially equivocal or normal in early neurodegenerative disease; thus, a careful mental status examination is the first line in detecting these conditions. This is of critical importance as earlier diagnosis and implementation of supportive care can improve quality of life, prevent comorbidities, and reduce caregiver distress. As disease-modifying treatments emerge, patients are likely to benefit from the earliest possible administration of these interventions. This article reviews the mental status examination with exemplary case vignettes and discusses the diagnostic evaluation and emerging biomarkers for neurodegenerative diseases.
Case 1-1
A 67-year-old woman, who worked as a lawyer, presented for a neurologic evaluation accompanied by her son, who was concerned about slowly progressive problems with her memory. While she felt that nothing was wrong, her son stated that she had been misplacing her keys and forgetting the words she wanted to use in a sentence. She also had trouble remembering names of acquaintances. The son also stated that he was concerned that she was asking the same questions repeatedly during the course of a day, and she had made some errors at work that had caught the attention of her coworkers. On her screening Mini-Mental State Examination (MMSE), the patient scored 27 out of 30. Additional screening identified difficulty with a list-learning task, in which she learned 5 out of 6 words of a 6-word list in three trials, but subsequently could not recall any words following a 1-minute delay. Presentation of cues and semantic foils found poor recognition, with only 2 out of 6 words recognized. Similarly, construction of a modified Rey-Osterrieth figure (Figure 1-1) found some poor organization with minor spatial displacement and omissions, while reproduction of the figure 1 minute later revealed minimal recall. There was mild difficulty in an oral trials test, and digits recited forward were seven and backward were five. Her brain MRI showed moderate bilateral hippocampal volume loss. Despite her mild symptoms, her high level of education and premorbid functioning together with the relative predominance of memory impairments raises the question that her diagnosis is suspicious for a mild stage of Alzheimer disease (mild cognitive impairment, amnestic type).
Figure 1-1.
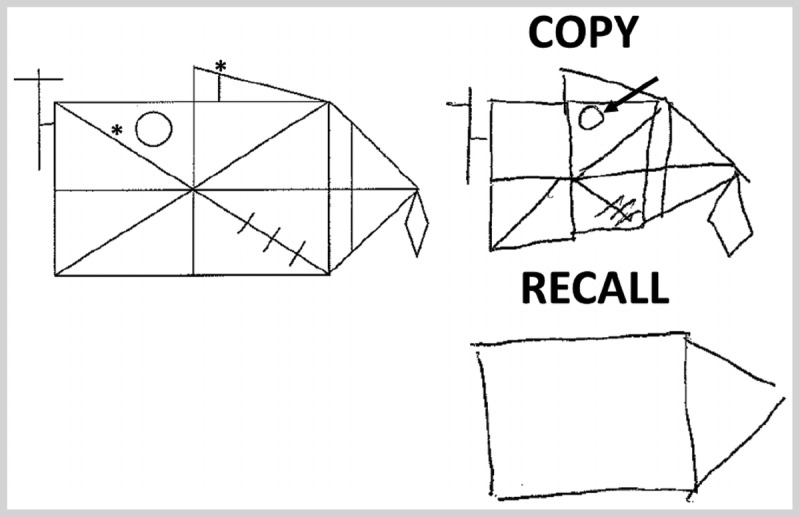
Modified Rey-Osterrieth figure. Construction of a modified Rey-Osterrieth figure by the patient in Case 1-1 reveals some poor organization with minor spatial displacement (arrow) and omissions (asterisks).
Comment. This case vignette illustrates several key points in the cognitive examination of patients with neurodegenerative disease. First, the importance of the mental status history in helping to differentiate benign forgetfulness and more clinically worrisome symptoms is noted by the reports of work performance concerns based on memory symptoms. Next, there are relative imbalances in this patient’s performance across tasks (ie, relative worse episodic memory performance compared to visuospatial and executive functioning) rather than isolated deficits in one cognitive domain, which is a common occurrence. Finally, the patient’s high level of education may have influenced her performance on bedside testing, which illustrates the need to account for patient factors in interpretation of cognitive evaluations.
NEUROLOGIC HISTORY
After obtaining a patient’s chief complaint, reviewing the history of the individual’s cognitive and behavioral symptoms is essential. In addition to querying the nature of the onset and pace of cognitive change, each major domain of cognition and behavior should be probed, similar to a review of systems. This is critical since an individual’s chief complaint may not reflect the true nature of the disorder. For example, a patient’s reported memory difficulty may indicate problems remembering words (ie, word-finding difficulty) rather than problems remembering recent events (ie, episodic memory difficulty). It is also important to review reported cognitive and behavioral symptoms with a family member or close friend because there is often limited insight in one’s own cognitive functioning.
Memory
Memory difficulties can be probed by asking about problems learning and recalling new information, as well as forgetfulness. Individuals may forget conversations and repeat questions about recent activities. Forgetting to pay bills or paying bills twice and going to the store and purchasing the same food items repeatedly represent worrisome memory difficulties, as in Case 1-1. By comparison, minor memory problems associated with aging, such as misplacing keys and difficulty finding a car in a parking lot, are less concerning.
Language
Many patients may report a decline in language production and may experience word-finding difficulty. Sometimes this can take the form of trouble retrieving the name of a family member or familiar friend. At other times, patients may report difficulty retrieving the names of objects. Sometimes individuals will report substituting one word for another or mispronouncing words. These are lifelong findings that increase in frequency as individuals age, and, thus, symptoms of this sort are challenging to evaluate and should be investigated carefully since they may represent an exaggeration of otherwise healthy aging. Other commonly reported symptoms include effortful speech, as in Case 1-2, difficulties with comprehension of speech (eg, over the telephone), and reading and writing difficulties. It is important to ascertain whether these symptoms are truly a disorder of language, such as spelling difficulty when writing, or reflect another source of difficulty, such as motor weakness interfering with mechanical aspects of writing. It is important to consider other nonlanguage etiologies that contribute to these symptoms, including reduced auditory acuity, visual acuity limitations, or other sensory-motor deficits.
Case 1-2
A 62-year-old woman who worked as a phone operator reported difficulty getting her words out. She stated that she worked at a busy switchboard for a large building complex, and she had become easily overwhelmed with complex tasks and had stopped working as a result. She stated that she was aware of the words she would like to use but had difficulty producing them, which caused her great frustration. Her daughter felt impatient waiting for her mother to finish a sentence, which caused a significant depressed mood for the patient, but she had no other behavioral changes. She noticed that she had made more spelling errors lately.
Mental status examination found a Mini-Mental State Examination (MMSE) score of 28 out of 30, with two points lost for difficulty spelling WORLD backward (the patient spelled “D-L-O-R-W”). She had significant difficulty with executive functioning including oral alternation between letter and number sequences and reciting digits backward. She also had some minor difficulty with an alternating manual manipulation task (ie, Luria three-step maneuver to pantomime an alternating sequence of hand gestures), a measure of executive functioning. Her speech was slow and hesitant. Sentence length was short with simplified grammatical structure to her speech and rare frank agrammatisms. Verbal comprehension for simple commands like “fold a paper in half and put it on your lap” was preserved, but she had difficulty with the request to “point to the ceiling after you point to the floor” due to grammatical comprehension difficulties. She had preserved single word and object knowledge and could readily identify and describe line drawings and objects. Reading and writing were comparable to her oral language. The patient did not exhibit limb apraxia but she had difficulty pantomiming how to “blow out a match” or “suck in through a straw,” indicating orobuccal apraxia.
She was asked to describe a children’s photo book depicting a scene where a boy’s pet frog sneaks out of his bedroom in the middle of the night. The patient’s response was as follows: “And the dog and the boy was oo- eh sleeping, on the baw- eh the- the, um, the uh, bed. And uh... the uh, the- the frog (2.7 second pause) emptied- of the- move the- the glass, ba- bottom... and go to... uh... uh, w- wo wook goo could do anything.”
Comment. This patient was diagnosed with the nonfluent variant of primary progressive aphasia due to her relatively isolated grammatical comprehension and expression difficulties with executive limitations and preserved single word/object comprehension.
Visual-Perceptual-Spatial Performance
Visual-perceptual-spatial symptoms are often challenging for patients to report because of problems articulating day-to-day examples. There may be difficulty driving, such as frequent fender benders or difficulty with parallel parking. An individual may struggle when trying to find an object in a complex visual scene, such as identifying a specific jar in a pantry. Patients may have difficulty recognizing faces or objects and may find it necessary to hear a person’s voice or an object’s associated sound prior to recognition. Difficulty dressing may reflect a visuospatial symptom, and there may be difficulty negotiating space around the home, and falls may occur because of lateralized neglect. It is important to rule out deficits of coordination or the extrapyramidal system that can influence these symptoms.
Executive Functioning
Dysexecutive symptoms often reflect difficulty with organization and planning, as in Case 1-3. Individuals may describe challenges executing previously familiar multistep activities such as cooking a meal, organizing a trip, or maintaining the household. Family members may have noticed a change in the patient’s lifestyle, with the individual no longer engaging in activities outside of the home, requiring others to initiate activities or talk the individual through the steps of the task. Individuals may have difficulty completing tasks that have been started because of easy distractibility. Driving is a dual-tasking environment, and driving difficulty may be related to limited executive resources. The patient may exhibit limited attention or fluctuating levels of attention.
Case 1-3
A 54-year-old man developed slowly progressive behavior and personality changes. His wife reported that she first noticed a change when he became less interested in socializing approximately 3 years earlier. He formerly would be well dressed but had begun to wear the same ripped sweatpants daily. He approached strangers to tell them his political views, which included racist and sexist comments that most would find offensive, and his wife claimed that these were not his previous beliefs. This behavior caused considerable interpersonal relationship problems both at home and at his employment as a salesman, although he questioned why his family found his behaviors to be objectionable. He showed no concern for his brother’s recent cancer diagnosis. After eating large amounts of food, he left his cousin’s wedding unexpectedly and was found watching television in his hotel room. On one recent occasion he sent large sums of money to a stranger over the Internet who claimed to be a prince from another country. He demonstrated increasing difficulty performing multistep activities at home, such as making a sandwich for lunch.
Mental status examination revealed difficulty with attention and poor social discourse (he interrupted the examiner on several occasions with tangential comments). He could not perform a complex oral alternating pattern (continuing an oral sequence that begins with A-1-B-2-C-3…) and made several perseverative errors throughout testing, indicating poor set-shifting ability. He inappropriately tapped his fingers on the desk and whistled a repetitive song during the majority of the examination. A brain MRI showed atrophy in the orbitofrontal and medial frontal lobe and the insula on the right with similar but less pronounced change in the homologous regions of the left hemisphere, consistent with the behavioral variant of frontotemporal dementia (bvFTD) (Figure 1-2).
Figure 1-2.
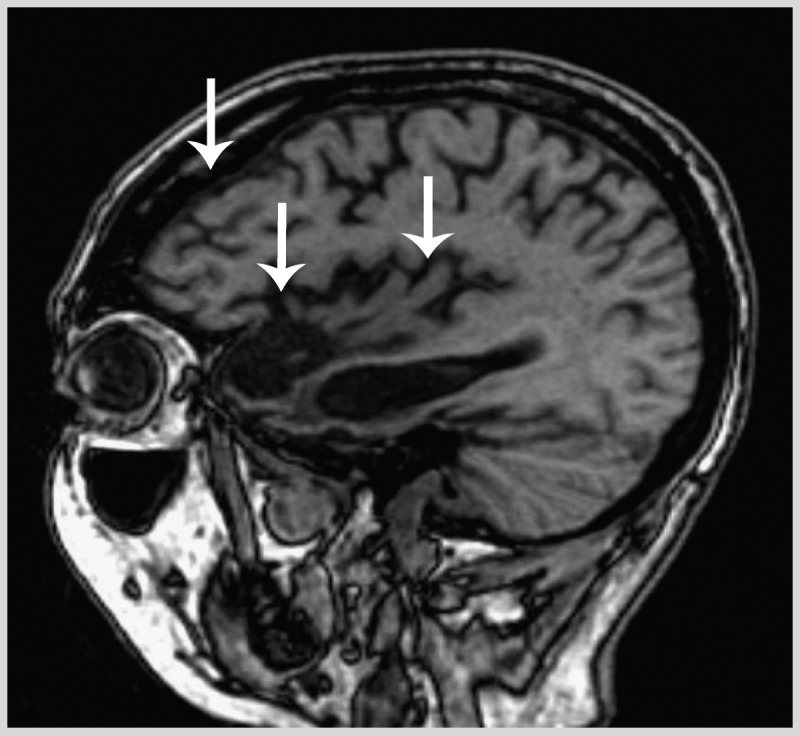
Knife-edge cortical atrophy in the behavioral variant of frontotemporal dementia. Parasagittal T1 MRI of the right hemisphere of the patient in Case 1-3 displaying significant dorsolateral, orbitofrontal, and perisylvian atrophy in the frontal lobes and severe anterior and medial temporal atrophy in the right hemisphere greater than the left hemisphere (arrows). There is relative preservation of posterior cortices, resulting in a dramatic knife-edge appearance of the border between precentral gyri and postcentral gyri.
Comment. This case illustrates the range of social and behavioral impairments commonly seen in bvFTD. Furthermore, this patient exhibited only mild executive impairments, which is common early in the course of bvFTD as patients can have largely a social disorder with minimal cognitive deficits. Finally, social cognition is difficult to assess without a reliable informant, due to the reduced insight in patients with bvFTD.
Social and Personality Changes
Family members may note a significant change in the patient’s personality, while patients with social difficulties often have limited insight, as in Case 1-3. Detecting social and personality changes necessitates a careful mental status history from a reliable companion.
There may be some obvious changes suggesting disinhibition, which may take the form of frequent rude or inappropriate comments involving strangers, or engaging in overly familiar behavior or sharing confidential information. The patient may exhibit hypersexuality in the form of sexual jokes, viewing pornography on the Internet, or inappropriate touching of strangers. Episodes of explosive agitation and rage without apparent provocation may be seen. An individual may become apathetic and have difficulty initiating activities. Flattening of affect and a loss of the normal variety of emotions may be seen. Alternatively, the patient may exhibit exaggerated and childlike emotional expressions. There may be ritualistic behavior such as the development of unusual and repetitive habits and collections, and the emergence of novel religious beliefs or political interests. Socially intrusive simple repetitive behaviors also can be seen such as clapping, tapping, and humming. Hoarding of unusual collections of objects may occur. Hyperoral behavior may become evident, such as shoveling food into the mouth, continuing to eat even though the individual is sated, or oral exploration of nonedible substances. There may be a strong preference for sweets or carbohydrates, and an individual may gain substantial weight over a very brief period of time. There may be shoplifting as the result of hyperoral behavior or attraction to shiny objects. Utilization behavior involves unavoidably using objects such as a patient picking up a pen on the desk and signing his or her name. Patients may perseverate or exhibit echolalic or echopractic behavior that mirrors the behaviors of others. Frequently, the patient may have limited insight into these changes in behavior and may be bewildered by the concerns of others or may express childlike denial. Difficulty with perspective taking also can interfere with social interactions, which can be seen commonly in conversational or behavioral exchanges where there may be limited empathy for a conversational partner. Frequent interruptions with tangential comments and poorly organized narrative speech (ie, poor social discourse) may be seen. Likewise, there are inappropriate responses to harmful events for others, as evidenced by Case 1-3. There may be limited insight into the motivations of others, which can result in a range of behaviors, such as investing in scams or failing to acknowledge significant events in the lives of others, such as the death of a spouse.
These behavioral changes are commonly associated with the behavioral variant of frontotemporal dementia (bvFTD) and forms of primary progressive aphasia, but can be also seen in other neurodegenerative conditions. Indeed, there is significant overlap of frontotemporal dementia (FTD) symptoms with atypical parkinsonian disorders (eg, corticobasal degeneration and progressive supranuclear palsy) and ALS.10,11 Furthermore, apathy and decreased motivation are not uncommon in AD and PD. Impulse control disorder seen in PD may also resemble features of FTD.12
Activities of Daily Living
Safe execution of activities of daily living is essential for minimizing morbidity and mortality in patients with cognitive impairment, so it is important to ask about activities of daily living. Specific activities of daily living should be probed, including bathing, toileting, eating, and dressing, as difficulties could lead to falls, aspiration, or infection. It is also important to note if patients have difficulty managing complex tasks that can have dangerous consequences such as cooking and administering medications. Access to finances and the Internet or telephone should be assessed to protect patients with frontal disease and poor judgment from being financially exploited, as in Case 1-3. Finally, determining the level of supervision provided on an average day is important in more severely impaired patients in order to prevent wandering or other dangerous events.
MENTAL STATUS EXAMINATION
Several brief mental status assessment tools have been developed for ease of use in a busy clinical setting. These can be used at times to serve as screening devices and may be useful for following an individual longitudinally. However, these screening instruments should not be considered a reasonable substitute for a comprehensive mental status examination. Examples of brief instruments include the Mini-Mental State Examination (MMSE),13 the Addenbrooke Cognitive Examination (ACE),14 and the Montreal Cognitive Assessment (MoCA).15 Each of these brief surveys covers slightly different domains. There are also several more comprehensive but slightly longer surveys that probe all of the major cognitive domains. One example is the Philadelphia Brief Assessment of Cognition.16 A more comprehensive mental status evaluation of each cognitive domain is outlined in the following sections.
Attention
It is valuable to begin the mental status evaluation with a consideration of attention. This can be derived in part during the history by observing whether the individual is maintaining a reasonable, sustained level of arousal or is easily distracted. A more systematic approach is obtained by performing a simple assessment of vigilance. This can involve asking an individual to lift a hand whenever a target letter (eg, A) is heard in a string of random letters delivered at a pace of about one per second. Alternatively, repetition of a sequence of digits can be performed, starting at two and gradually increasing the digit length, delivering the digits at a rate of one per second. Seven digits or more is considered normal.
Memory
There are several different ways to assess episodic memory. Perhaps the most common method involves presenting an individual with a list of words at a pace of about one per second, and then asking the individual to repeat the words in order to confirm registration. The individual is asked to retain the words in memory during the performance of another task in order to block subvocal repetition and to examine fading of the memory trace over time. Subsequently, recall is requested. There are many variations of this basic format. The number of words in the list may vary from three up to 15, and the number of repetitions requested may vary from one to five, depending on the level of difficulty that is sought and the desire to document a learning curve. The amount of time between presentation and recall may vary from 30 seconds to many minutes. In the event of failure to recall some of the target words, prompts can be offered. These can include a superordinate cue (eg, a kind of clothing) or a rhyming word (eg, sounds like “block”). After spontaneous recall and prompted recall, a recognition procedure may be administered. During this phase, some of the previously mentioned target words are offered, intermixed among foils that may be semantically similar to the target, phonologically similar to the target, or random words. In the authors’ screening mental status evaluation, we administer a six-word list presented for three learning trials, use a 1 minute–filled interval, obtain free recall, and administer a yes-no recognition procedure for words that are not remembered during free recall. We monitor the number of words produced during learning trials to see if there is a learning curve, the number of words freely recalled, and recognition accuracy. Regardless of the examiner’s preferred form of episodic memory testing, it is important to document the parameters used to assess memory.
Other memory tasks can include asking an individual to listen to a sentence or a paragraph and then probing recall of the sentence or paragraph at a later time. A memory score is derived from the ability to recall critical key words from the sentence or paragraph. In addition to verbal memory, assessing episodic memory recall with another kind of material is often helpful as this minimizes confounds associated with the specific learning material and dependence on left hemispheric function. One method is to perform episodic memory testing using visual presentation of words or recall of a visual geometric design. Recall of a visual geometric design often takes the form of copying a visual design, removing the target design and its copy, engaging the individual in another visual-perceptual-spatial activity for a brief period of time, and then asking the patient to reproduce the visual design. As with verbal episodic memory, visual episodic memory testing can be manipulated by varying the complexity of the visual stimulus, the meaningfulness of the stimulus (eg, a nameable design such as a clock face or a non-nameable multicomponent geometric design), and the amount of time between presentation and recall (refer to Case 1-1 for an example). Recognition for elements of a visual stimulus (eg, the position of the clock hands) can be tested as well.
These verbal and visual memory tests involve an explicit request to learn, remember, and then recall specific information (ie, intentional memory). In our daily lives, we often also learn and retain information without conscious effort (ie, incidental memory), and it is not unreasonable to assess incidental memory by asking an individual to recall words or designs when there is no explicit request to remember at the time of presentation. Orientation can be viewed as a test of incidental memory. In the authors’ assessment of memory, we include evaluation of incidental recall of a visual geometric design. Other forms of memory that can be assessed include habit learning (ie, asking an individual to repeat a sequence of novel hand gestures), semantic memory (ability to recognize familiar but infrequent objects [eg, a shoehorn] and to answer questions about these objects [eg, “Is it found in the kitchen?”]), auditory-verbal short-term memory (repetition of digits, multisyllabic words, and sentences of various lengths), and working memory (reproducing a list of digits in the reverse order of presentation). Further details on object knowledge/semantics and working memory are discussed in the sections on language and executive functioning that follow.
Language
Language is a complex process that is crucial for daily functioning. Several components of language should be ascertained in a comprehensive mental status evaluation. We first evaluate single-word processing. During speech production, listening for word-finding pauses and circumlocutions is important. An individual also may make frequent lexical substitutions or speech sound errors. Confrontation naming is a more formal way to assess single-word use and word finding and typically takes the form of asking an individual to name a pictured object or a real object. The frequency of the word’s occurrence and familiarity of the target object can be manipulated. Confrontation naming also can be assessed using other nonvisual modalities. Thus, naming can be performed in response to a sound or the feel of a target object. This is important for an individual who has difficulty with visual-perceptual-spatial functioning. Some patients may have a modality-specific naming problem interfering with interpretation of a visual stimulus such as visual agnosia, while a more general deficit in semantic memory interferes with interpretation of an object in any modality.
Comprehension can be assessed for a word, paralleling the assessment of the representation of object meaning in semantic memory. Lexical comprehension can be assessed by asking an individual to provide a definition of a word. Word meaning can be assessed in a multiple-choice manner as well. Specific attributes of a word’s referent can be assessed as well, such as asking whether a camel lives in the ocean or whether asparagus is red in color. An individual also can be asked whether two words are from the same category, such as deciding whether a lemon and an apple are both fruit.
Language comprehension should also be assessed at a multiword or sentence level. Sentence processing is a complex process. Since words in a sentence emerge over time, it is valuable to assess repetition, a form of auditory-verbal short-term memory. Repetition can be assessed by asking an individual to repeat a monosyllabic word, a multisyllabic word, a multisyllabic phrase, and sentences of various lengths. In sentence expression, pathologic speech is often characterized as effortful. The rate of nonfluent speech production is about 45 words per minute (refer to Case 1-2 for an example), much slower than the normal adult speech rate of more than 140 words per minute.17 There may be omissions of bound or free grammatical morphemes (ie, words, prefixes, or suffixes with grammatical function), giving speech a telegraphic quality. It is important to listen for the variety of grammatical forms used in conversational speech.
Other errors in speech include disorders of prosody, or the sing-song quality of speech, in which prosodic difficulty may be reflected in a limited or exaggerated range of pitch. Disruption of the coordination of the motor speech apparatus may occur, such as apraxia of speech, where the timing of speech is irregular and speech sound errors are produced that consist of sounds that are not from the native speaker’s lexicon. This should be distinguished from dysarthria, which is a dysfunction of the muscles involved in speech. Sentence comprehension can be assessed by asking an individual to perform a brief series of simple tasks in the mentioned order. Sentence comprehension also involves a uniquely language component, namely, grammar. An individual can be asked to point to objects in an order that differs from the order of mention through the use of a preposition, as in Case 1-2. Also, an individual can be asked to choose the agent of an action in a simple, brief sentence such as “It was the boy that the girl chased. Who did the chasing?”18
It is also important to assess written communication such as reading and writing. Letter-by-letter reading involves slowed interpretation of the geometric shapes that constitute a written word, and, thus, the amount of time needed to read a word is directly proportional to its length. Single-word reading also assesses the spelling system. In English, many words involve letter-sound correspondence rules, and this can be assessed by asking an individual to read a pseudoword such as “tig.” English also contains sight vocabulary words, and an individual with surface dyslexia who cannot correctly pronounce sight words (ie, orthographically irregular) like dough, choir, or pint will often attempt to pronounce them using letter-sound correspondence rules. Reading comprehension can be assessed by asking an individual to perform a simple written act, such as “Close your eyes.” Spatial neglect can interfere with reading, and this can be demonstrated by asking an individual to read a compound word such as cowboy. Writing can be assessed by asking an individual to write to dictation, including both words that obey letter-sound correspondence rules and orthographically irregular words. A motor coordination disorder known as apractic agraphia results in difficulty with the automatic mechanical formation of letters, which will significantly slow writing. It is important to keep in mind that literacy is highly variable, and reading and writing abilities will vary depending on experience.
Visual-Perceptual-Spatial Functioning
Visual-perceptual-spatial functioning is an important aspect of the bedside mental status examination that is frequently neglected. Perhaps the most common assessment involves copying a visual geometric design. The design itself may vary in complexity, from a simple nameable geometric form to a nameable object or a more complex non-nameable geometric design. Examples include overlapping pentagons and the more complex designs developed by Rey and Osterrieth or Benson (refer to Case 1-1 for an example).19,20 These designs should be scored for accuracy as well as the manner in which they were executed. This includes poor organization and the omission of elements, which may reflect executive impairment and spatial displacements, such as the placement of an individual component in an inappropriate spatial location relative to other elements of the design. Sometimes one-half of a figure can be impoverished or neglected. Spatial difficulty can interfere with reading (eg, difficulty finding a line on a printed page) and writing (eg, spatially disordered written production). Perhaps the simplest assessment of visuospatial functioning involves the location of an object in space, which can be tested by asking an individual to reach for an object. An individual also can be asked to imitate a meaningless gesture, such as placing the dorsum of one hand against the contralateral cheek. A more formal assessment of spatial relationships includes the judgment of line orientation, where an individual is asked to evaluate whether a pair of lines is parallel. An element of visuospatial functioning may involve part-whole discrimination, also known as simultagnosia. One task frequently used to assess this involves using many small letter A characters to form a shape that looks like a large letter E (ie, Navon figure)21 and asking an individual to name the letter. Individuals with difficulty involving whole-part discrimination name the small letter and do not recognize that these are in a configuration forming a large letter. Another visual-perceptual-spatial task involves face processing. An individual can be asked to recognize a photograph of a famous face. It is also possible to use the examiner’s face as a stimulus and query whether there are features such as a full head of hair or a beard. Visual agnosia may manifest itself as difficulty recognizing the visual presentation of an object, although the object can be recognized from its sound or feel. Color processing can be assessed by asking an individual to name or recognize a color and asking whether two colors match.
There are a variety of other disorders associated with diseases of the parietal lobe that can be assessed as well. Apraxia is difficulty demonstrating learned gestures, which involves transitive gestures that use an implement such as demonstrating the use of a hammer, or intransitive gestures that do not involve an implement such as waving good-bye.22 These gestures can be elicited in response to a verbal request or with imitation. Imitation (pantomiming) can help dissociate a disorder of verbal comprehension from true apraxia. Oral praxis also can be assessed as well (eg, “blow out a match”), and this does not necessarily track apraxia of speech or limb apraxia, as in Case 1-2.
Calculations and other assessments of number knowledge also are associated with the integrity of the parietal lobe.23 This can be assessed by asking an individual to select the one of two numbers that is larger, to perform simple calculations orally or in writing (eg, 7 + 9 =___), or to solve a simple day-to-day problem that depends on calculations (eg, “How much change from a dollar should you receive after buying a 65-cent candy?”). Like spatial aspects of reading and writing, spatial difficulties can interfere with the alignment of numbers in a multidigit calculation and consequently result in a calculation error. Higher-order parietal lobe sensory integration can be assessed by cortical sensation; a letter or a number can be written in the palm of the hand and named by the patient (testing for graphesthesia), or an object can be placed in the hand and named (testing for stereognosis). Body part localization can be assessed by touching a body part of an individual with his or her eyes closed, and asking the individual to indicate the part of the body that was touched. Left-right discrimination can be assessed by identifying a body part bilaterally on an individual (eg, ears) and asking the individual to identify the right one or the left one of the pair. A more difficult assessment of left-right orientation asks an individual to identify the left or right body part on the examiner. Finger agnosia also may be evident in an individual with parietal disease.
Executive Functioning
Executive functioning is a complex domain that involves the efficient execution of tasks. Perhaps the most common assessment of executive functioning is category naming fluency. This can involve naming words beginning with a target letter (eg, F) or naming words from a target semantic category (eg, animals). Task performance is evaluated by counting the number of words produced during a period of time, such as 60 seconds. Category naming fluency in response to a target letter is a more challenging measure of executive functioning, while the semantic guidance provided by a meaningful category like animals generally facilitates category naming fluency. It can be informative to monitor whether production is organized, such as naming farm animals, then jungle animals, then varieties of fish. Another sign of executive dysfunction is perseveration or difficulty shifting set between tasks. One sign of this is frequent repetition of words in category naming fluency. A visual analogue of category naming fluency involves design fluency. The most common form of this measure involves connecting a number of dots, such as nine dots, to form different designs.
Another common executive measure involves alternating patterns. This entails performing a task, and then inhibiting that performance to perform a second task. The material can be quite simple or more complex. Simple versions of this kind of alternating task involve a simple rule such as tapping once on a table when the examiner taps twice and not tapping when the examiner taps once. The examiner provides a random sequence of single or double tapping. Another variety of alternating pattern involves the examiner touching an individual’s right hand or left hand in a random order with the eyes closed, and the individual responds by lifting the touched hand. After this task has been well learned, the examiner reverses the association and asks the individual to lift the right hand when the left hand is touched, and lift the left hand when the right hand is touched. More complex versions of alternating patterns involve reproducing two intermixed, overlearned sequences, such as alternating production of a letter and a number in ascending order, such as A, 1, B, 2, C, 3. This can be performed orally or as a written trails procedure, where letters and numbers are randomly distributed on a page and an individual is asked to draw a line between a letter and a number in ascending sequence.
Two related components include parsing a sequence into smaller, repeated units and inhibitory control. A repeated series of three hand gestures is demonstrated to the patient three times, and then the patient is asked to demonstrate the hand gestures. A measure intended to assess inhibitory control is a Stroop test, where words are written in a colored font that differs from the color name, and an individual is asked to name the color of the font and not read the printed word. When seeing the word “blue” printed in a red font, for example, the individual is asked to respond “red.”
Working memory is often thought to be a component of executive functioning and involves the ability to maintain some material in an active form and do some work on this material. Common tests of working memory involve reproducing a list of numbers in the reverse order or reordering a random sequence of letters and numbers into their ascending orders, using progressively longer sequences. A similar kind of assessment can be performed in the visual domain by pointing to randomly distributed circles on a page and asking an individual to point to these in an order reversing the order of demonstration.
Social Functioning and Behavior
Examination of social comportment is challenging and often requires information from a reliable caregiver, as patients with ventral frontal disease often have little insight or concern into their difficulties. There are several valuable social questionnaires that can be completed by spouses, family members, or close friends concerning changes in personality, behavior, and social functioning. Examples include the Neuropsychiatric Inventory and the Frontal Behavioral Inventory, which probe day-to-day functioning, looking for changes in personality and behavior compared to baseline.24,25 The previous section on history details specific domains of social comportment that are affected by frontal lobe disease. Observation of patient interactions in clinic are also important as detection of behavioral disinhibition, simple repetitive motor rituals, and poor social discourse (Case 1-3) should prompt a more thorough examination for evidence of social comportment disorder and executive limitations. Other behavioral and emotional changes that should be noted include depression and anxiety since these can be significant and can also interfere with the mental status examination.
DIFFERENTIAL DIAGNOSIS AND ANCILLARY TESTING FOR NEURODEGENERATIVE DISEASE
A major limitation in the development of meaningful treatment for neurodegenerative diseases is that definitive diagnosis is obtained only at autopsy. Furthermore, significant clinicopathologic overlap exists between neurodegenerative diseases, and clinically defined phenotypes of AD, PD, FTD, and ALS often vary in the ability to accurately predict underlying neuropathology. This is of significance as emerging therapies are targeting disease-specific misfolded proteins (eg, tau, amyloid-β [Aβ], synuclein). A focus of current neurodegenerative disease research includes early diagnosis as patients may potentially show a greater benefit from emerging disease-modifying therapies earlier in the disease course.11,26–28 This includes focus on mild cognitive impairment (MCI) or prodromal states for AD, PD, dementia with Lewy bodies (DLB), and ALS.27,29–32 Additionally, patients with FTD may often present with minimal cognitive dysfunction and feature deficits that are largely restricted to social functioning.11 Moreover, many of the cognitive and social deficits seen in neurodegenerative diseases also can be manifested in non-neurodegenerative disorders of the cerebrum. Thus, a detailed evaluation, including ancillary laboratory and neuroimaging studies, is necessary to rule out common metabolic, toxic, inflammatory, or infectious mimics of a neurodegenerative disease.
In individuals without prior testing, it is often valuable to have additional laboratory studies to supplement the mental status examination and screen for common etiologies that can contribute to cognitive impairment. Among these are a complete blood count, electrolyte panel, liver and kidney function tests, thyroid-stimulating hormone (TSH), vitamin B12 level, and sedimentation rate. These may be supplemented depending on the specific medical history and mental status examination findings.
Structural brain MRI images can help exclude cerebrovascular disease, neuroinflammatory conditions, or other structural lesions such as hydrocephalus or malignancy that can mimic a neurodegenerative condition. EEGs can be helpful to identify partial status epilepticus, or periodic discharges associated with prion disease, some steroid-responsive encephalopathies, and other rapidly progressive dementias. EEG recordings in neurodegenerative disease usually show nonspecific slowing, although DLB may have fluctuations in slowing.33 CSF analysis can be particularly useful to evaluate vasculitides or other inflammatory conditions that can mimic neurologic conditions that may not be evident in blood serologic testing.34 The authors routinely check CSF protein, cell count, IgG levels, cytology, cryptococcus antigen, and cultures in all patients with suspected FTD, ALS, and atypical AD to rule out alternative non-neurodegenerative etiologies.
Current biomarker research is aimed at developing neurodegenerative disease–specific tests,11,27,28 and, currently, the only biomarker test approved by the US Food and Drug Administration (FDA) is in vivo amyloid imaging for AD using positron emission tomography (PET) with amyloid-specific radiotracers. However, due to clinicopathologic complexities in aging and cognitive impairment, scanning is currently recommended only for younger patients with a progressive dementia, those with atypical AD clinical symptoms, or unexplained prolonged MCI.35 CSF measurements of tau and Aβ can also be a potentially useful biomarker for AD neuropathology. Indeed, an AD CSF signature of elevated total tau (t-tau) and phosphorylated tau (p-tau) with lower Aβ1-42 is highly consistent with AD and MCI at risk for progression to AD compared with cognitively normal controls, and correlates well with AD neuropathology at autopsy.36–38 Lab-to-lab variation currently precludes this test from being clinically available, but international efforts to standardize detection assays will likely lead to clinical availability in the near future.39,40 AD CSF biomarkers also may have value in other neurodegenerative diseases. Indeed, many patients with PD have considerable plaque and tangle pathology at autopsy associated with dementia, and low CSF Aβ1-42 may predict cognitive decline in PD.8,41 Additionally, lower CSF α-synuclein may differentiate PD from normal control patients.42 Finally, since half of all FTD cases and virtually all ALS cases have a TDP-43 proteinopathy, CSF p-tau levels appear to be lower than seen in tauopathies and healthy controls.43,44 Thus, CSF is a promising modality for neurodegenerative disease biomarker discovery.
The clinical diagnosis of FTD and primary progressive aphasia syndromes can be especially difficult as these patients are usually younger, and some patients may have a nonprogressive neuropsychiatric condition that resembles bvFTD (ie, phenocopy syndrome, which is a recently described clinical syndrome of nonprogressive social comportment disorder that is not due to underlying frontotemporal lobar degeneration or other neurodegenerative disease. The etiology of these cases is currently unclear, but many are thought to be due to decompensated psychiatric disease later in life and, thus, clinically mimic bvFTD initially).45 Due to the lack of biomarkers or laboratory tests that are specific for frontotemporal lobar degeneration neuropathology,11 excluding these alternative etiologies with ancillary testing is important. In Case 1-3, “knife-edge” frontal atrophy associated with FTD can be seen clearly, but many patients may have equivocal or no signs of cortical atrophy at diagnosis despite florid behavioral changes. In these circumstances, fluorodeoxyglucose positron emission tomography (FDG-PET) can be helpful to determine if there is hypometabolism in frontotemporal regions suggestive of FTD in the absence of cortical atrophy. Finally, FTD syndromes may be difficult to differentiate from AD if episodic memory or visuospatial difficulties are prominent. FDG-PET can identify patients likely to have AD neuropathology, with posterior parietal and medial temporal hypometabolism, or DLB, which may be characterized by parietal-occipital hypometabolism. The authors have also found high diagnostic accuracy to differentiate atypical AD from FTD in autopsy-confirmed CSF cases using the t-tau to Aβ1-42 ratio.46 Future FTD-specific biomarkers will be useful to improve antemortem diagnosis, and a combination of clinical, biofluid, and neuroimaging modalities may be most effective.11
CONCLUSION
The mental status examination has several components focused on each cognitive domain (ie, attention, memory, language, visuospatial perception, executive functioning, and social comportment). A thorough mental status examination includes a detailed medical and neurologic history with focus on features of each cognitive domain to guide the examination and provide details for onset and tempo of disease. Several bedside assessments are effective in probing these areas and providing insight into the underlying neurologic condition, and formal neuropsychological testing with normative scores can be helpful to detect subtle deficits in highly educated patients. Longitudinal assessment in neurodegenerative conditions that are characteristically progressive can be particularly informative. Finally, history taking should include a detailed account of events at home for activities of daily living to prevent cognitive impairment–induced morbidity and mortality. Since neurodegenerative disease diagnosis is obtained at autopsy, careful evaluation with laboratory and neuroimaging testing is necessary to rule out non-neurodegenerative mimics. Emerging biomarkers for AD, PD, DLB, ALS, and FTD will be helpful to improve diagnosis, especially in early-stage or prodromal cases, which will enhance development of meaningful disease-modifying therapies for these conditions.
KEY POINTS
The mental status examination is structured to probe each major cognitive domain (attention, memory, language, visuospatial perception, executive functioning, and social comportment).
Cognitive function is mediated by large-scale networks or connectomes, where gray matter nodes are interconnected by white matter tracts.
Any gray matter node may contribute to multiple cognitive networks.
While the assessment of a patient can reveal selective impairment of specific components of the mental status examination, it is often the overall pattern of cognitive performance across multiple components that is most informative.
A single mental status examination obtains only a cross-sectional perspective of a patient’s performance. A history of slow progression or observed longitudinal decline on serial cognitive examination testing is required to make a diagnosis of a neurodegenerative dementia.
The neurologic history is an important component to determine the onset, tempo, and associated features of the cognitive symptoms. These factors help direct the specific features to focus on during examination.
Each cognitive domain should be probed during the history, similar to a medical review of symptoms.
Differentiating age-associated memory decline from pathologic etiologies is challenging. Mental status history should include details on the functional impact of problems associated with aging and recognition of a problem by others.
Visual-perceptual-spatial difficulties may be difficult to elicit through history. Common examples include difficulty navigating a car, finding objects in the home, recognizing faces or objects, or difficulty dressing.
Detecting social and personality changes associated with neurodegenerative dementia often requires a careful history from a reliable informant who spends significant time with the patient.
Social comportment is difficult to assess in the mental status examination and often requires a thorough history from a reliable informant to be detected.
It is important to inquire about activities of daily living to identify potential safety issues that could result in morbidity and mortality from cognitive impairment.
Screening cognitive instruments provide a brief sample of different cognitive domains that can be useful to track patients longitudinally but do not substitute for a thorough examination.
Episodic memory is often tested by using list-learning tasks where a sequence of words is repeated after several trials and then recalled after a brief delay. Recognition is assessed through use of cues or semantic foils. Difficulty can be adjusted depending on length of list and delay time.
Nonverbal methods of testing memory, such as figure-recall tasks, are useful in patients with significant left-hemispheric disease.
Language dysfunction can be detected during the clinical examination through identification of abnormal prosody, word-finding pauses, circumlocutions, grammatical sophistication, and frank agrammatisms in spontaneous speech.
Language comprehension should be performed on the single-word and sentence level. Single-word comprehension can be assessed through word and object meaning and sentence comprehension through repetition and verbal commands of sequenced tasks.
Lexical comprehension should be assessed in parallel with assessment of object meaning in semantic memory. This can be done by asking for the definition of a single word or asking to name attributes of a word’s referent.
Apraxia of speech refers to disruption of coordination of the motor speech apparatus and should be distinguished from dysarthria, which is a dysfunction of the muscles involved in speech.
Grammatical comprehension can be assessed through use of simple questions of “who did what to whom” in sentences with increasing grammatical complexity (ie, “The car that hit the truck was green. Who was hit?”).
Surface dyslexia is the reading of a sight (orthographically irregular) word that requires semantic knowledge rather than phonetics for proper pronunciation, examples of which include cough, choir, and pint.
Executive impairment can cause impairments in construction tasks through poor organization and omission of elements. In contrast, visuospatial impairments manifest in spatial displacements and distortions on construction tasks.
Visuospatial function can be assessed through construction of figures with varying familiarity and complexity.
Ideomotor apraxia is difficulty in demonstrating learned gestures. Transitive gestures involve use of tools while intransitive gestures do not involve an implement.
Higher-order parietal lobe functions include calculations, cortical sensation, left-right discrimination, somatosensory maps (ie, limb position), and apraxia.
Executive functioning involves mental manipulation of information and shifting between tasks. These functions can be tested through an alternating sequence of written, oral, or manual tasks.
Working memory is the ability to hold and manipulate data. Assessment of the number of digits recalled in reverse can be useful to test working memory.
Social functioning and behavior difficulties should be considered in any patient who has difficulties with social discourse, simple repetitive motor rituals, or inappropriate behavior during the interview. A reliable informant should be obtained to gather additional history.
A major limitation in the development of meaningful treatment for neurodegenerative diseases is that definitive diagnosis is obtained only at autopsy.
Careful selection of ancillary laboratory and neuroimaging studies can be useful to rule out common non-neurodegenerative etiologies of cognitive impairment.
REFERENCES
- 1. Avants BB, Libon DJ, Rascovsky K, et al. Sparse canonical correlation analysis relates network-level atrophy to multivariate cognitive measures in a neurodegenerative population. Neuroimage 2014; 84: 698– 711. doi:10.1016/j.neuroimage.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonner MF, Peelle JE, Cook PA, Grossman M. Heteromodal conceptual processing in the angular gyrus. Neuroimage 2013; 71: 175– 186. doi:10.1016/j.neuroimage.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grossman M, Peelle JE, Smith EE, et al. Category-specific semantic memory: converging evidence from bold fMRI and Alzheimer’s disease. Neuroimage 2013; 68: 263– 274. doi:10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooke A, Grossman M, DeVita C, et al. Large-scale neural network for sentence processing. Brain Lang 2006; 96(1): 14– 36. doi:10.1016/j.bandl.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 5. Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 2004; 127(pt 3): 628– 649. doi:10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 6. Healey ML, McMillan CT, Golob S, et al. Getting on the same page: the neural basis for social coordination deficits in behavioral variant frontotemporal degeneration. Neuropsychologia 2015; 69: 56– 66. doi:10.1016/j.neuropsychologia.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grossman M, Powers J, Ash S, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang 2013; 127(2): 106– 120. doi:10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012; 72(4): 587– 598. doi:10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans J, Olm C, McCluskey L, et al. Impaired cognitive flexibility in amyotrophic lateral sclerosis. Cogn Behav Neurol 2015; 28(1): 17– 26. doi:10.1097/WNN.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwin D, Lippa CF, Swearer JM. Cognition and amyotrophic lateral sclerosis (ALS). Am J Alzheimers Dis Other Demen 2007; 22(4): 300– 312. doi:10.1177/1533317507301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 2015; 129(4): 469– 491. doi:10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weintraub D. Dopamine and impulse control disorders in Parkinson’s disease. Ann Neurol 2008; 64(suppl 2): S93– S100. doi:10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189– 198. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14. Mathuranath PS, Nestor PJ, Berrios GE, et al. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 2000; 55(11): 1613– 1620. doi:10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- 15. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53(4): 695– 699. doi:10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 16. Libon DJ, Rascovsky K, Gross RG, et al. The Philadelphia Brief Assessment of Cognition (PBAC): a validated screening measure for dementia. Clin Neuropsychol 2011; 25(8): 1314– 1330. doi:10.1080/13854046.2011.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ash S, Evans E, O’Shea J, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology 2013; 81(4): 329– 336. doi:10.1212/WNL.0b013e31829c5d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charles D, Olm C, Powers J, et al. Grammatical comprehension deficits in non-fluent/agrammatic primary progressive aphasia. J Neurol Neurosurg Psychiatry 2014; 85(3): 249– 256. doi:10.1136/jnnp-2013-305749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loring DW, Martin RC, Meador KJ, Lee GP. Psychometric construction of the Rey-Osterrieth Complex Figure: methodological considerations and interrater reliability. Arch Clin Neuropsychol 1990; 5(1): 1– 14. doi:10.1093/arclin/5.1.1. [PubMed] [Google Scholar]
- 20. Spencer RJ, Wendell CR, Giggey PP, et al. Judgment of Line Orientation: an examination of eight short forms. J Clin Exp Neuropsychol 2013; 35(2): 160– 166. doi:10.1080/13803395.2012.760535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall JC, Halligan PW. Seeing the forest but only half the trees? Nature 1995; 373(6514): 521– 523. doi:10.1038/373521a0. [DOI] [PubMed] [Google Scholar]
- 22. Heilman KM, Watson RT. The disconnection apraxias. Cortex 2008; 44(8): 975– 982. doi:10.1016/j.cortex.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 23. Spotorno N, McMillan CT, Powers JP, et al. Counting or chunking? Mathematical and heuristic abilities in patients with corticobasal syndrome and posterior cortical atrophy. Neuropsychologia 2014; 64C: 176– 183. doi:10.1016/j.neuropsychologia.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44(12): 2308– 2314. doi:10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25. Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997; 24(1): 29– 36. [DOI] [PubMed] [Google Scholar]
- 26. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7(3): 257– 262. doi:10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7(3): 280– 292. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci 2013; 14(9): 626– 636. doi:10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7(3): 270– 279. doi:10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012; 27(3): 349– 356. doi:10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 2014; 6(4): 46 doi:10.1186/alzrt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009; 10(3): 131– 146. doi:10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- 33. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65(12): 1863– 1872. doi:10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 34. Kansal K, Irwin DJ. The use of cerebrospinal fluid and neuropathologic studies in neuropsychiatry practice and research. Psychiatr Clin North Am 2015; 38(2): 309– 322. doi:10.1016/j.psc.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 2013; 9(1): e- 1–e-16. doi:10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65(4): 403– 413. doi:10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Meyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 2010; 67(8): 949– 956. doi:10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66(3): 382– 389. doi:10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 39. Mattsson N, Andreasson U, Persson S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement 2011; 7(4): 386– 395.e6. doi:10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011; 121(5): 597– 609. doi:10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010; 75(12): 1055– 1061. doi:10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013; 70(10): 1277– 1287. doi:10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossman M, Elman L, McCluskey L, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol 2014; 71(4): 442– 448. doi:10.1001/jamaneurol.2013.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu WT, Watts K, Grossman M, et al. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology 2013; 81(22): 1945– 1952. doi:10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kipps CM, Hodges JR, Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Curr Opin Neurol 2010; 23(6): 628– 632. doi:10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- 46. Irwin DJ, McMillan CT, Toledo JB, et al. Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol 2012; 69(8): 1018– 1025. doi:10.1001/archneurol.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]