Abstract
The mechanism of the negative regulation of proopiomelanocortin gene (Pomc) by glucocorticoids (Gcs) is still unclear in many points. Here, we demonstrated the involvement of neurogenic differentiation factor 1 (NeuroD1) in the Gc-mediated negative regulation of Pomc. Murine pituitary adrenocorticotropic hormone (ACTH) producing corticotroph tumor-derived AtT20 cells were treated with dexamethasone (DEX) (1–100 nM) and cultured for 24 hrs. Thereafter, Pomc mRNA expression was studied by quantitative real-time PCR and rat Pomc promoter (-703/+58) activity was examined by luciferase assay. Both Pomc mRNA expression and Pomc promoter activity were inhibited by DEX in a dose-dependent manner. Deletion and point mutant analyses of Pomc promoter suggested that the DEX-mediated transcriptional repression was mediated via E-box that exists at -376/-371 in the promoter. Since NeuroD1 is known to bind to and activate E-box of the Pomc promoter, we next examined the effect of DEX on NeuroD1 expression. Interestingly, DEX dose-dependently inhibited NeuroD1 mRNA expression, mouse NeuroD1 promoter (-2.2-kb) activity, and NeuroD1 protein expression in AtT20 cells. In addition, we confirmed the inhibitory effect of DEX on the interaction of NeuroD1 and E-box on Pomc promoter by chromatin immunoprecipitation (ChIP) assay. Finally, overexpression of mouse NeuroD1 could rescue the DEX-mediated inhibition of Pomc mRNA expression and Pomc promoter activity. Taken together, it is suggested that the suppression of NeuroD1 expression and the inhibition of NeuroD1/E-box interaction may play an important role in the Gc-mediated negative regulation of Pomc.
Introduction
Class II basic helix-loop-helix (bHLH) factor, neurogenic differentiation factor 1 (NeuroD1) or Beta2 (β2) is characterized by its tissue specific expression [1]. NeuroD1 is expressed in progenitor cells and differentiated endocrine cells of the pancreas [2,3] and in the neuroectoderm [4]. NeuroD1 also plays an important role in the differentiation, morphogenesis and maintenance of the (central) nervous system [5,6]. Corticotroph cells of the anterior pituitary, which produce multifaceted protein proopiomelanocortin (POMC), express mainly NeuroD1 [7], which promotes the expression of POMC gene (Pomc). NeuroD1 forms a heterodimer with E proteins (e.g., E47/Pan1), which are a class I bHLH factor. The NeuroD1/Pan1 heterodimer binds to the E-box on Pomc promoter then activates Pomc transcription [7]. The bHLH heterodimer acts together with pituitary T-box transcription factor Tbx19, which is dependent on another transcription factor, Ptx [8]. When NeuroD1 is required for DNA sequence recognition of the E-box [1,8], the Pan1 part of the bHLH heterodimer interacts with the Ptx1 [7,9] and Tpit [8].
The Pomc is expressed significantly in a number of mammalian tissues including the anterior and intermediate pituitary, the immune system, skin and hypothalamus [10,11,12]. In these tissues, POMC is cleaved into a variety of smaller peptides including ACTH, β-endorphin and α-, β- and γ-melanocyte-stimulating hormones (MSH). The repertoire of products derived from POMC by any tissue is determined by the specificities of the convertases expressed in the secretory pathway [13,14]. Prohormone convertase 1 (PC1) is expressed in corticotrophs of the anterior pituitary and in melanotrophs of the intermediate lobe of the pituitary; whereas prohormone convertase 2 (PC2) is expressed in melanotrophs of the intermediate lobe of the pituitary and arcuate nuclei of the hypothalamus. PC1 cleaves POMC to ACTH, while PC2 cleaves ACTH further to yield α-MSH. Thus, secretion of ACTH is the principal controller of adrenal steroidogenesis from the anterior pituitary [15]. ACTH and α-MSH are products of post translational splicing of a precursor molecule, POMC. The corticotrophs secrete mainly ACTH, whereas the melanotrophs mainly α-MSH.
The regulation of Pomc is also tissue specific [16]. Although adrenal glucocorticoids (Gcs) upregulate Pomc expression in the hypothalamus [17], they negatively regulate Pomc transcription and ACTH secretion in pituitary corticotroph cells [18,19,20]. In general, Gcs show their biological activities by binding to a glucocorticoid receptor (GR) [21]. GR resides in the cytoplasm before the presence of Gcs [22]. When Gcs bind to GR, GR translocates to the nucleus [23]. Gcs bound GRs form as a homodimer that binds to Gc-response element (GRE), then activates target gene transcription with transcription machinery [24]. The GRs homodimer also binds to negative GRE (nGRE) of the Pomc promoter, and this nGRE complex represses Pomc transcription in corticotrophs [25].
It remains to be elucidated whether other transcription factors are involved in the Gc-mediated repression of Pomc transcription in pituitary corticotroph cells. In addition to NeuroD1 [7], Nur77, Nurr1, Tpit and Pitx are known to activate Pomc transcription [26,27]. Among them, Nur77 [26] and Tpit/PitxRE [27] have been demonstrated to be involved in the Gc-mediated Pomc repression. However, the function of NeuroD1 in the negative regulation of Pomc has not yet been demonstrated. In this study, we have attempted to examine the involvement of NeuroD1 in the dexamethasone (DEX)-mediated repression of Pomc transcription.
Materials and methods
Reagents
DEX, a synthetic Gc, was purchased from Sigma-Aldrich (St. Louis, MO). DEX was dissolved in 100% ethanol at 1 mM and stored at -20°C. These stocks were diluted with 100% ethanol to the desired concentration immediately before each experiment and maintained at a final ethanol concentration of at 0.1%.
Plasmids
Subcloned chimeric constructs containing the rat Pomc genomic DNA and luciferase cDNA (pGL3-Basic, Promega, Madison, WI) were used for the transient transfection studies: rPomc-Luc (-703/+58-Luc: harboring the rat Pomc 5’-flanking region from -703 to +58 relative to the transcription start site upstream of the luciferase cDNA in pGL3-Basic), -429/+58-Luc; -379/+58-Luc, and -359/+58-luc, and E-box (NeuroD1 binding element) mutant in rPomc-Luc (rPomc-Luc-EboxNeuro-Mut) were described in our previous papers [28,29]. β-galactosidase control plasmid in pRSV (pRSV-β-gal) and in pCMV (pCMV-β-gal) were purchased from Clontech (Mountain View, CA). Beta2 (NeuroD1) overexpression plasmid was a gift from Dr. Debra E. Bramblett (Texas Tech University Health Sciences Center, El Paso, TX). pcDNA3 expression plasmid was purchased from Invitrogen (Carsbad, CA). The full-length mouse NeuroD1 promoter construct mNeuroD1-Luc (-2.2-kb/+150-Luc: harboring the mouse NeuroD1 5’-flanking region from -2.2-kb to +150 relative to the transcription start site upstream of the luciferase cDNA in pGL3-Basic, also termed ND full) and NeuroD1 promoter deletion constructs termed ND mut1, mut2, mut3, and mut4 in the reporter construct pGL3-Basic [30] were kind gifts from Dr Lori Sussel (Columbia University, New York, NY).
Cell culture
AtT20 cells were grown with Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were cultured in a humidified incubator at 37°C with 5% CO2. AtT20 cells were obtained from the American Type Culture Collection (AtT20: CCL-89).
RNA isolation
AtT20 cells grown to 70% confluence in regular medium in 24-multiwell plates were incubated either without or with DEX at appropriate concentrations in DMEM supplemented with 1% resin and charcoal-treated (stripped) FBS media [28,29] and cultured for 24 hrs. In the overexpression experiments, each expression vector was transfected for 24 hrs before treatment with DEX. The cells were then lysed and their total RNAs were isolated using ISOGEN (NIPPON GENE, Toyama, Japan) according to the manufacturer’s instructions. The RNA was quantified by a Nano drop 2000 (Thermo Scientific, Waltham, MA). The final preparation of total RNA was free of DNA and Proteins, and had a 260/280 ratio 1.6–1.9.
Quantitative real-time PCR
Quantitative real-time polymerase chain reaction (qPCR) was performed using KAPA SYBR FAST Universal 2x qPCR Master Mix (KAPA Biosystems, Woburn, MA). One microgram from total RNA was converted to reverse transcription (RT) reaction using PrimeScript Reverse transcriptase (Takara Bio, Shiga, Japan) with the oligo-dT primer and random hexamer according to the manufacturer’s instructions. Reverse transcription mixtures were subjected to qPCR with KAPA SYBR FAST Universal 2x qPCR Master Mix (KAPA Biosystems) for primers of Pomc, NeuroD1, Pan1 (E47), Rb, and GAPDH using a CFX connect Real Time PCR thermal cycler (Bio-Rad, Hercules, CA). The following primer sequences were used: mouse Pomc (forward, 5’-CAGTGCCAGGACCTCACC-3’; reverse, 5’-CAGCGAGAGGTCGAGTTTG-3’), mouse NeuroD1 (forward, 5’-ACGCAGAAGGCAAGGTGTCC-3’; reverse, 5’-TTGGTCATGTTTCCACTTCC-3’), mouse Pan1 (E47) (forward, 5’-GAATGCCTATGCCACCTTTG-3’; reverse, 5’-GAACCTTCCGGACCTTCTTC-3’), mouse Rb (forward, 5’-ATGGAATCCCTTGCATGGCT-3’; reverse, 5’-AGGACAAGCAGGTTCAAGGT-3’), mouse Tpit (forward, 5’-GCCAGCATGTGACCTACTCTCACT-3; reverse, 5’-AGTCCAGCTGTCAGGTCCCGAGAA-3’), mouse Pitx1 (forward, 5-CGGTGTGGACCAACCTCACTGAA-3; reverse, 5’-GAGTTGCACGTGTCCCGGTAGA-3’) [27], mouse Nur77 (forward, 5’-GCACAGCTTGGGTGTTGATG-3’; reverse, 5’-CAGACGTGACAGGCAGCTG-3’) [31], mouse Nurr1 (forward, 5’-TCAGAGCCCACGTCGATT-3’; reverse, 5’-TAGTCAGGGTTTGCCTGGAA-3’) [32], mouse GAPDH (forward, 5’-ACAGTCCATGCCATCACTGCC-3’; reverse, 5’-GCCTGCTTCACCACCTTCTTG-3’), rat Pomc (forward, 5’-CCTCACCACGGAAAGCA-3’; reverse, 5’-TCAAGGGCTGTTCATCTCC-3’) [33] and rat GAPDH (forward, 5’-AAACCCATCACCATCTTCCA-3’; reverse, 5’-GTGGTTCACACCCATCACAA-3’) [34]. Reactions were incubated at 95°C for 1 min and then amplified using temperature parameters of 95°C for 15 sec; 60°C for 10 sec; 72°C for 20 sec. Amplifications were carried out for 45 cycles, followed by a 3 min extension at 72°C. After amplification, a melting-curve analysis was performed from 60°C to 95°C with a heating rate of 0.5°C/10 sec and continuous fluorescence acquisition. The signals of the samples of interest were then quantified from the standard curve, and all obtained data were normalized by mouse GAPDH. To confirm the amplification specificity, the PCR products from each primer pair with SYBR green were subjected to a melting curve analysis. Results are expressed as percentages of each control.
Transient transfection and luciferase assay
AtT20 cells grown to 60–70% confluence in regular medium in 24-multiwell plates were transiently transfected with 300 ng of each reporter plasmid and 100 or 150 ng of β-gal control plasmid using Lipofectamine LTX and Plus reagent (Invitrogen) for 24 hrs according to the manufacturer’s instructions. In the overexpression experiments, different concentrations of each expression vector (200 ng and 300 ng), 135 ng of reporter plasmid and 65 ng of β-gal control plasmid were also transfected. The media were changed to DMEM supplemented with 1% stripped FBS, and the cells were treated without or with DEX (100 nM) and cultured for 24 hrs. These cells were then washed with 1xPBS and the cell extracts were prepared using Glo Lysis Buffer (Promega). Luciferase activity was measured using Bright-Glo reagents (Promega) and β-galactosidase activity was simultaneously measured. Data were normalized by β-galactosidase activity.
Western blot analysis
When AtT20 cells were grown to 90–100% confluence in 6 well plates, they were exposed to DEX and maintained at different time courses (30 min, 1 hr, 3 hrs, 6 hrs, 9 hrs and 24 hrs) in DMEM supplemented with 1% stripped FBS. The cells were then harvested and lysed with TNE buffer [20 mM Tris-HCl, 137 mM NaCl, 2 mM EDTA, 1% NP-40, Protease Inhibitor Cocktail Set III (Calbiochem)]. This was followed by centrifuging at 13000 rpm for 10 min at 4°C to collect the supernatants. Thereafter, 10 μg of extracted protein were electrophoresed on a SDS–polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membrane. For the detection of NeuroD, the membrane was blocked with 1% BSA for 30 min and probed with primary rabbit monoclonal antibody for NeuroD (D3562, Cell Signalling Technology, Danvers, MA) (diluted at 1:500) for overnight at 4°C and was thereafter incubated with anti-rabbit IgG, horseradish peroxidase (HRP) linked whole antibody from donkey (NA934V, GE Healthcare, Little Chalfont, UK) (1:5000) for 1 h at room temperature. For the detection of actin, the membrane was blocked with 1% BSA for 30 min and probed with primary antibody for actin (sc-1616, Santa Cruz Biotechnology, Dallas, TX) (diluted at 1:500) for overnight at 4°C and was thereafter incubated with polyclonal rabbit anti-goat immunoglobulins/HRP (ab97120, Dako, Glostrup, Denmark) (1:5000) for 1 h at room temperature. The membranes were thereafter washed and visualized using ECL (Thermo Scientific) and Luminata forte for Actin and NeuroD1, respectively. Densitometric analyses of the membranes were performed using Image J.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was conducted using NeuroD antibody (D3562, Cell Signalling Technology), normal rabbit IgG (SC-2027, Santa Cruz Biotechnology), and primers of mouse Pomc promoter containing the E-box region. Briefly, AtT20 cells were cross-linked with 0.5% formaldehyde for 10 min at 37°C and then glycine (final concentration 0.125 M) was added to stop the cross linking. Cells were washed twice with cold 1xPBS. Then, 1 ml cold 1xPBS was added and cells were scrapped into a 1.5 ml eppendorf tube and centrifuged at 1000g for 5 min at 4°C. The cell pellet was resuspended with 150 μl SDS lysis buffer [SDS lysis buffer: 1% SDS, 10 mM EDTA, 50 mM Tris-HCl (pH 8.1)] and kept on ice for 10 min. The cell lysate was sonicated to shear the DNA to fragment lengths of between 100 bp and 500 bp by using a handy sonic (Model UR-20 P) for 20 sec on and 60 sec off, 9 times at level 7. The sonicated extract was diluted 10 fold with dilution buffer [ChIP Dilution buffer: 0.01% SDS, 1.1% Triton X-100, 2 mM EDTA, 20mM Tris-HCl (pH 8.1), 167 mM NaCl]. Aliquotes of the lysate were incubated overnight with 2.0 μg of antibody at 4°C with rotation. The antibody-protein complex was precipitated by blocked protein G dynabeads and the pelleted beads were washed with wash buffers (Once with Low Salt Buffer, High Salt Buffer, LiCl Buffer and twice with TE Buffer). Immune complexes were eluted in elution buffer (1% SDS, 10 mM DTT, 0.1 M NaHCO3) and reverse cross linked with 5 M NaCl, then incubated for more than 6 hrs or overnight at 65°C. Following Proteinase K (Wako, Osaka, Japan) treatment, DNA fragments were then purified with a Qiagen DNA Extraction kit according to supplied protocol. Immunoprecipitated DNA was analyzed by qPCR using KAPA SYBR FAST Universal 2x qPCR Master Mix (KAPA Biosystems) together with 1% of the input chromatin. Specific primer pairs were designed to amplify the E-box region of mouse the Pomc gene promoter for qPCR. E-box primer (Forward primer, 5’- TACCTCCAAATGCCAGGAAG-3’; Reverse primer, 5’- GTTAGCACAGACCCGCTGA-3’).
Primary culture of anterior pituitary cells
All animal experiments were approved by the Institutional Animal Experiment Committee of Jichi Medical University (Number:16052), and in accordance with the Institutional Regulation for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Japanese Ministry of Education, Culture, Sports, Science and Technology. All treatments were performed under deep anesthesia and all efforts were made to minimize suffering. Rats were sacrificed by exsanguination from the right atrium under deep pentobarbital anesthesia (40 mg/kg body weight, i.p.) and then perfused with Hanks’ balanced salt solution in order to obtain anterior pituitary cells for primary culture.
Male Wistar rats were purchased from Japan SLC (Shizuoka, Japan). Rats were housed in a temperature-controlled room (22 ± 1°C) with a 12-hour light/12-hour dark cycle and illumination from 0700 h to 1900 h, and were given ad libitum access to conventional food and water. Anterior pituitary cells of rats aged 10–12 weeks were dispersed as described previously [35]. The cells were suspended in M199 medium (Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells (3 × 105 cells) were seeded in 24-well plates and then incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air for 3 days. The culture medium was changed with M199 medium with 10% stripped FBS and then incubated for 1 hr. Media were changed, dexamethasone was then added to media at a concentration of 100 nM, 1 μM and the cells were incubated for 1 hr, 3 hrs, 6 hrs, or 24 hrs.
Statistical analysis
All data are presented as means ± standard errors of means (SEM). Statistical analyses were performed with one way ANOVA followed by post hoc Tukey test and Paired Sample t test. P<0.05 was considered statistically significant.
Results
Effects of DEX on Pomc mRNA expression/Pomc promoter activity in AtT20 cells
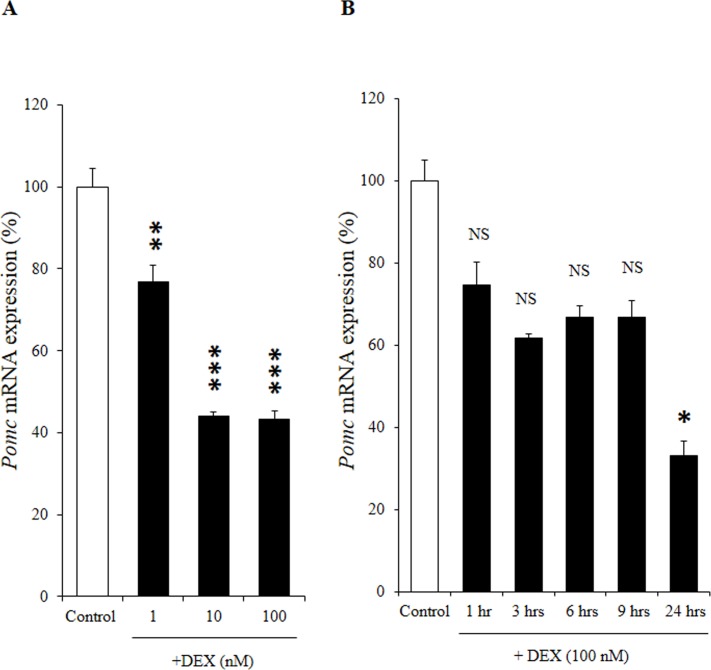
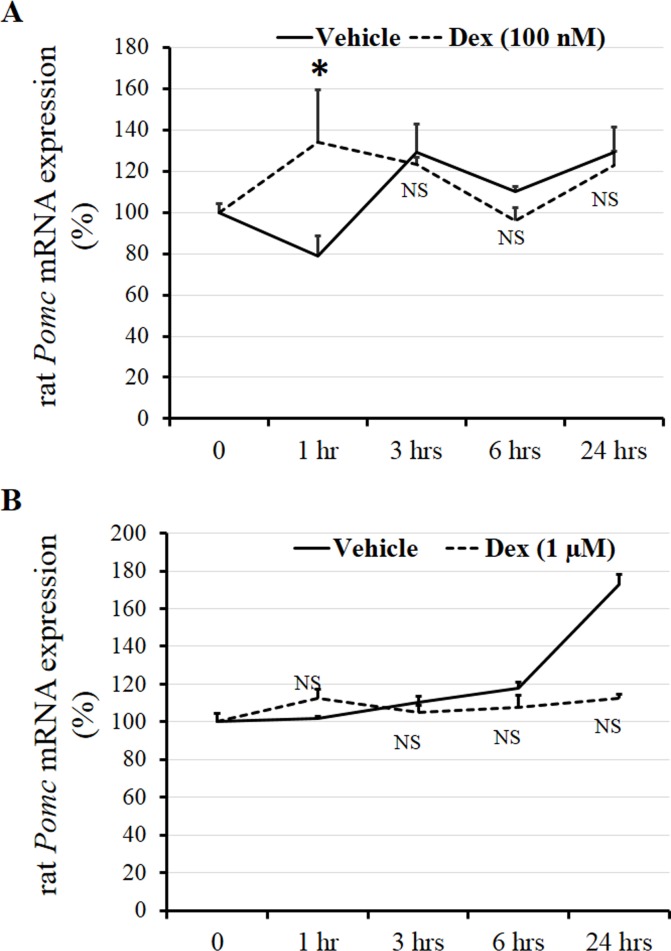
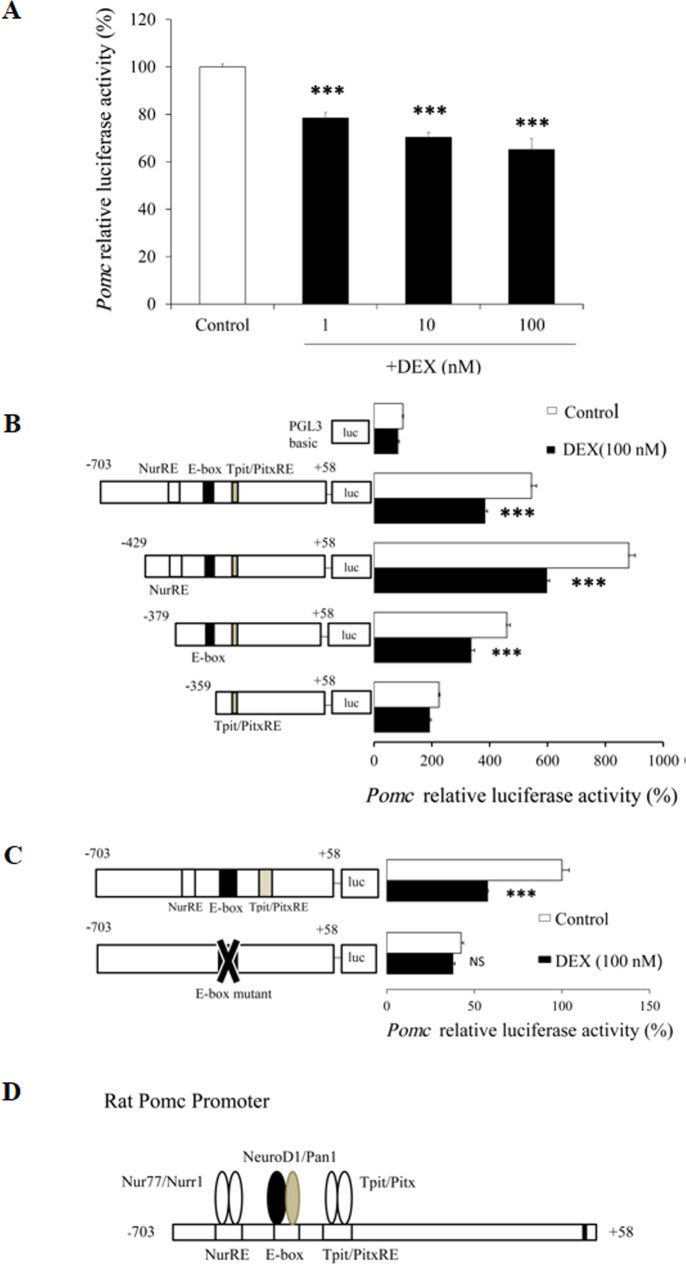
We first examined the effect of DEX on Pomc mRNA expression at different concentrations in AtT20 cells. After treatment of the AtT20 cells with several concentrations (1 nM, 10 nM, and 100 nM) of DEX, Pomc mRNA expression was dose-dependently decreased (Fig 1A). We next investigated the effects of DEX on Pomc mRNA expression with different incubation durations in AtT20 cells. After treatment of the AtT20 cells with DEX (100 nM) for different incubation durations (1 hr, 3 hrs, 6 hrs, 9 hrs, and 24 hrs), the Pomc mRNA expression was time-dependently decreased, significantly at 24 hrs (Fig 1B). These data indicate that DEX decreased Pomc mRNA expression in both dose- and time-dependent manners. In contrast, when we treated primary culture cells of rat anterior pituitary with DEX (100 nM) and DEX (1 μM) for different incubation durations (1 hr, 3 hrs, 6 hrs, and 24 hrs), Pomc mRNA expression was not suppressed significantly comparing to vehicle control (Fig 2A and 2B) consistent with the previous conference report [36] though Dex (100 nM) caused a small but significant transient increase in POMC transcription after 1 hour of treatment (Fig 2A). We next examined the effects of DEX on the Pomc promoter activity using AtT20 cells. In this experiment, we used the full-length (-703/+58) Pomc promoter in its 5’-flanking region with different concentrations of DEX. As shown in Fig 3A, DEX significantly decreased the Pomc promoter activity in a dose-dependent manner. These data indicate that DEX negatively regulates Pomc transcription.
Fig 1. Effects of DEX on Pomc mRNA expression in AtT20 cells.
(A) Dose-dependent effect of DEX on Pomc mRNA expression. AtT20 cells were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs. Results are expressed as percentages of control (100%). Data represents mean ± SEM (n = 4). ***P<0.001 vs control. This experiment was repeated three times with separate batches of cell preparation with consistent results. (B) Time-dependent effect of DEX on Pomc mRNA expression. AtT20 cells were treated with 100 nM DEX for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). NS denotes “Not Significant.” ***P<0.001 vs control. This experiment was also repeated three times with separate batches of cell preparation with consistent results.
Fig 2. Effects of DEX on Pomc mRNA expression in primary culture cells of rat anterior pituitary.
(A) Time-dependent effect of DEX on rat Pomc mRNA expression. Untreated rat anterior pituitary primary culture cells were expressed as percentages of control (100%). The cells were treated with 100 nM DEX or 0.1% ethanol (Vehicle control) for 1 hr, 3 hrs, 6 hrs, or 24 hrs. Each point represents mean ± SEM (n = 4). *P<0.05 vs control. NS denotes “Not Significant.” This experiment was repeated two times with separate batches of cell preparation with consistent results. (B) Time-dependent effect of DEX on rat Pomc mRNA expression. Untreated rat anterior pituitary primary culture cells were expressed as percentages of control (100%). The cells were treated with 1 μM DEX or 0.1% ethanol (Vehicle control) for 1 hr, 3 hrs, 6 hrs, or 24 hrs. Each point represents mean ± SEM (n = 4). NS denotes “Not Significant.”
Fig 3. Effects of DEX on Pomc promoter activity in AtT20 cells.
(A) Effect of DEX on full-length Pomc promoter activity. AtT20 cells transiently transfected with 300 ng full-length rPomc-Luc (-703/+58-luc) and 100 ng pCMV-β-gal were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs before the luciferase assay. Results are expressed as percentages of control (100%). Data represents mean ± SEM (n = 4). ***P<0.001 vs control. Three independent experiments were performed with consistent results. (B) Effect of DEX on Pomc promoter deletion mutants. AtT20 cells transiently transfected with 300 ng rPomc-Luc (-703/+58-luc) or each deletion mutant reporter plasmid (-429/+58-Luc, -379/+58-Luc, or -359/+58-Luc) and 100 ng pCMV-β-gal were incubated in the presence (100 nM) or absence of DEX for 24 hrs before the luciferase assay. Results are expressed as percentages of each control (100% in pGL3-Basic). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. This experiment was also repeated three times with separate batches of cell preparation with consistent results. (C) Effect of DEX on Pomc promoter activity using E-box mutant. AtT20 cells transiently transfected with 300 ng rPomc-Luc (-703/+58-luc) or E-box mutant of full-length Pomc promoter (rPomc-Luc-EboxNeuro-Mut) and 100 ng pCMV-β-gal were incubated in the presence (100 nM) or absence of DEX for 24 hrs before the luciferase assay. Results are expressed as percentages of each control (100% in rPomc-Luc). Each point represents mean ± SEM (n = 4). NS denotes “Not Significant.” ***P<0.001 vs control. Two independent experiments were performed with consistent results. (D) Schematic representation of responsive elements in Pomc promoter and transcription factors which bind to these responsive elements.
Role of E-box of Pomc promoter in the DEX-mediated suppression in AtT20 cells
In order to understand the molecular mechanisms of the DEX-mediated Pomc transcription regulation, we examined the promoter activity of a series of Pomc 5’-flanking region deletion mutants. The DEX-mediated transcription suppression of Pomc promoter activity was observed in constructs from the full-length (-703/+58) to -379/+58, but not in -359/+58 (Fig 3B). The luciferase activity of pGL3-Basic Vector was not affected by DEX (Fig 3B). Pomc constructs from -703/+58 to -429/+58 contained both the Nur response element (NurRE) and E-box, whereas the -379/+58 construct contained the E-box but not NurRE. Since both -429/+58 and -379/+58 constructs exhibited similar DEX-induced inhibition (Fig 3B), it is plausible that not only NurRE but also E-box may play an important role in the DEX-mediated transcription suppression. In order to further clarify the role of E-box in the transcription suppression, we next examined the effect of DEX on the E-box point mutant. As shown in Fig 3C, the transcription of the E-box point mutant was not affected by DEX, indicating the significance of E-box in the DEX-mediated transcription suppression. Fig 3D shows the structure of the rat Pomc promoter. Since Nur77/Nurr1 [37] is known to bind to NurRE, and NeuroD1 [7] is known to bind to E-box, not only Nur77/Nurr1 but also NeuroD1 may be involved in the DEX-mediated suppression of the Pomc promoter activity.
Effects of DEX on mRNA expression of NeuroD1, Pan1, and Rb in AtT20 cells
We next examined the effects of DEX on NeuroD1 mRNA expression in AtT20 cells. As shown in Fig 4A and 4B, DEX decreased the NeuroD1 mRNA expression in dose- and time-dependent manners. These data indicate that the DEX-mediated Pomc transcription suppression may be mediated via the decrease of NeuroD1 mRNA expression. We next examined the effect of DEX on Pan1 (E47) mRNA expression, since Pan1 is known to be a heterodimeric partner of NeuroD1 [7]. As shown in Fig 4C and 4D, DEX did not affect the Pan1 mRNA expression, probably due to its ubiquitous expression. Additionally, since Retinoblastoma protein (Rb) has recently been demonstrated to be a co-activator of NeuroD1 on the Pomc [38], we also examined the effect of DEX on Rb mRNA expression. As shown in Fig 4E and 4F, Rb mRNA expression was not affected by DEX.
Fig 4. Effects of DEX on the mRNA expression of NeuroD1, Pan1 (E47), and Rb in AtT20 cells.
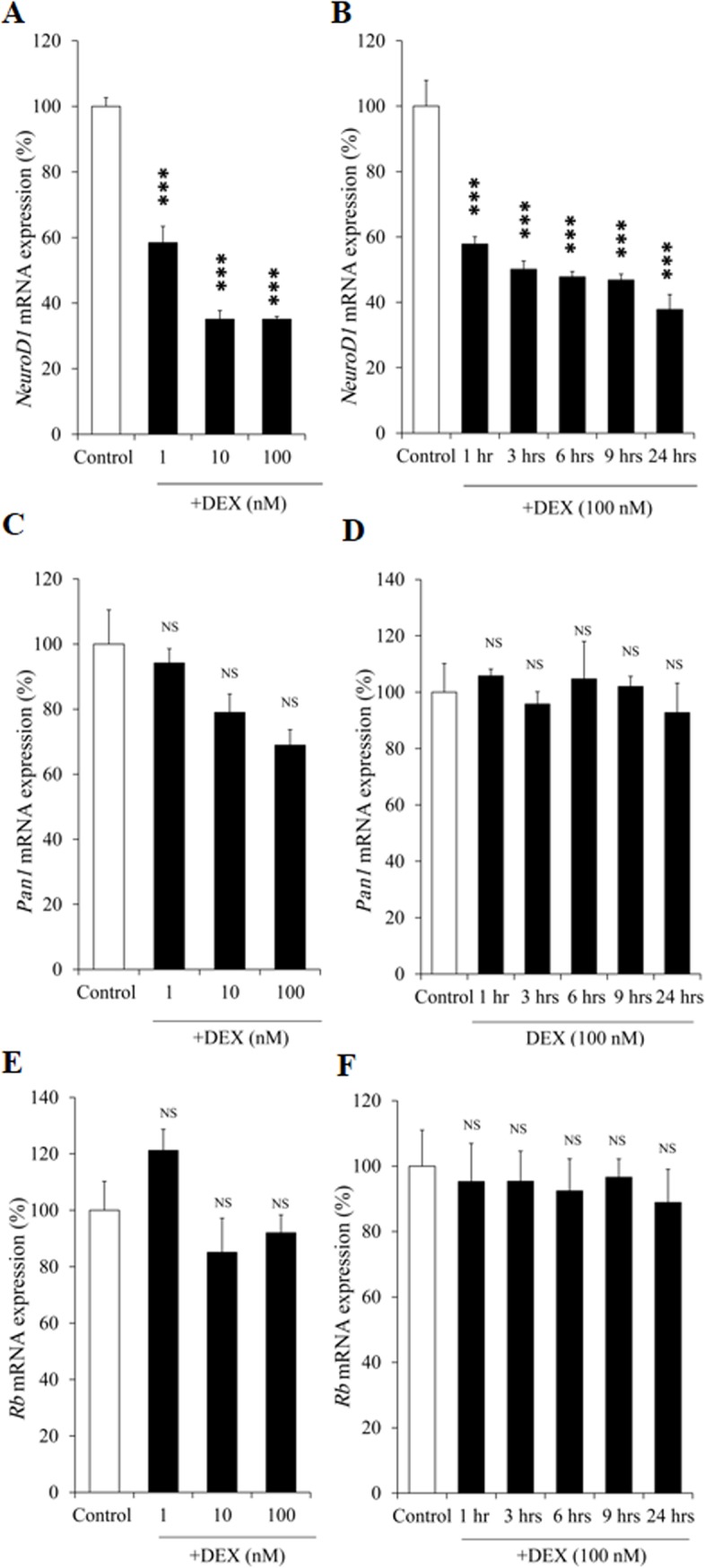
(A) Dose-dependent effect of DEX on NeuroD1 mRNA expression. AtT20 cells were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. This experiment was also repeated three times with separate batches of cell preparation with consistent results. (B) Time-dependent effect of DEX on NeuroD1 mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. (C) Dose-dependent effect of DEX on Pan1 (E47) mRNA expression. AtT20 cells were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs. Results are expressed as percentages of control (100%). NS denotes “Not Significant.” This experiment was also repeated three times with separate batches of cell preparation with consistent results. (D) Time-dependent effect of DEX on Pan1 (E47) mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of each control (100%). NS denotes “Not Significant.” (E) Dose-dependent effect of DEX on Rb mRNA expression. AtT20 cells were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs. Results are expressed as percentages of control (100%). NS denotes “Not Significant.” (F) Time-dependent effect of DEX on Rb mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). NS denotes “Not Significant.”
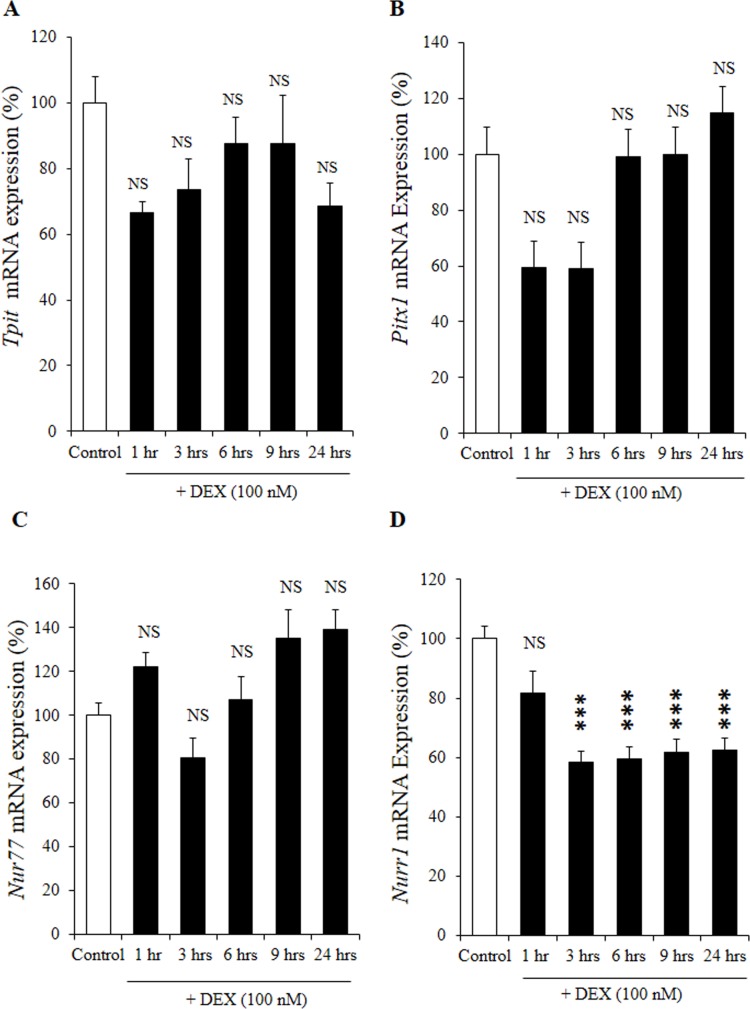
Effects of DEX on mRNA expression of Tpit, Pitx1, Nur77, and Nurr1 in AtT20 cells
We also examined the effects of DEX on Tpit and Pitx1 mRNA expression in AtT20 cells. As shown in Fig 5A and 5B, DEX did not decrease Tpit and Pitx1 mRNA expression in time-dependent manners. This is consistent to the previous report by Murakami et al. [27] which demonstrated that DEX did not suppress mRNA expression of Tpit and Pitx1. These data indicate that the DEX-mediated Pomc transcription suppression may not be mediated via the decrease of Tpit and Pitx1 mRNA expression. Moreover, while DEX did not suppress Nur77 mRNA expression (Fig 5C), it significantly decreased Nurr1 mRNA expression in a time-dependent manner (Fig 5D). These data indicate that the DEX-mediated Pomc transcription suppression may also be mediated via the decrease of Nurr1 mRNA expression, although further studies are needed to clarify its mechanism.
Fig 5. Effects of DEX on the mRNA expression of Tpit, Pitx1, Nur77 and Nurr1 in AtT20 cells.
(A) Time-dependent effect of DEX on Tpit mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). NS denotes “Not Significant.” Two independent experiments were performed with consistent results. (B) Time-dependent effect of DEX on Pitx1 mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of each control (100%). NS denotes “Not Significant.” Two independent experiments were performed with consistent results. (C) Time-dependent effect of DEX on Nur77 mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). NS denotes “Not Significant.” Two independent experiments were performed with consistent results. (D) Time-dependent effect of DEX on Nurr1 mRNA expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs. Vehicle control: 0.1% ethanol. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). NS denotes “Not Significant.” ***P<0.001 vs control. Two independent experiments were performed with consistent results.
Effects of DEX on NeuroD1 promoter activity in AtT20 cells
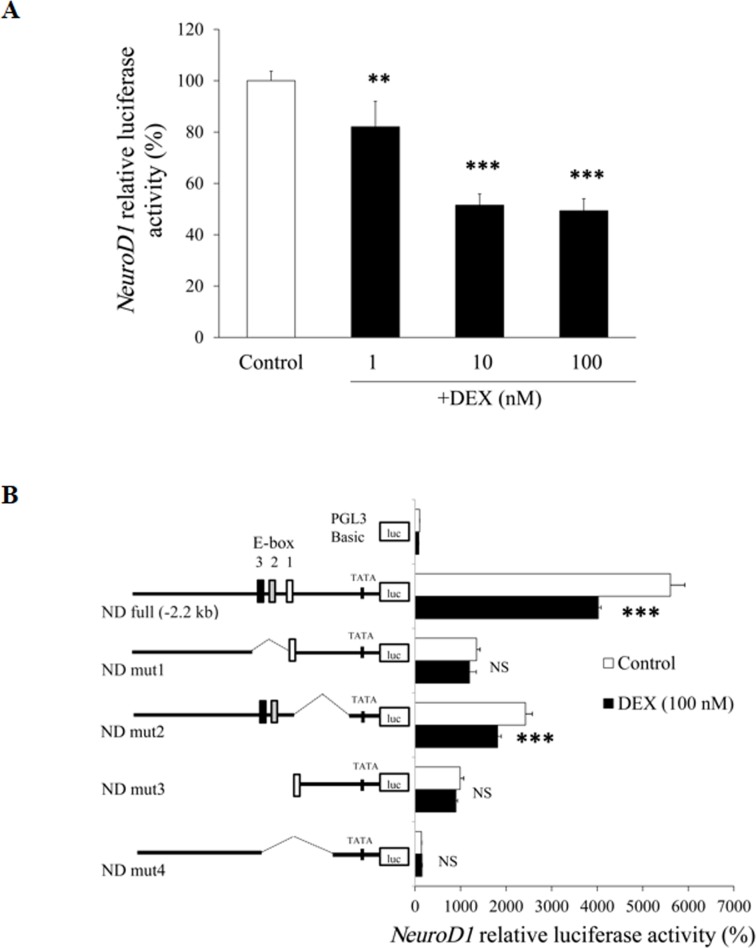
In order to elucidate the mechanisms of the DEX-mediated decrease of the NeuroD1 mRNA expression, we next examined the effect of DEX on the NeuroD1 promoter activity using AtT20 cells. When the full-length (-2.2-kb/+150) NeuroD1 promoter in its 5’-flanking region was incubated with several concentrations of DEX for 24 hrs, DEX significantly suppressed the NeuroD1 promoter activity in a dose-dependent manner (Fig 6A). These data indicate that DEX also negatively regulates the NeuroD1 transcription.
Fig 6. Effects of DEX on NeuroD1 promoter activity in AtT20 cells.
(A) Effect of DEX on full-length NeuroD1 promoter activity. AtT20 cells transiently transfected with 300 ng mNeuroD1-Luc (ND full: -2.2-kb/+150) and 150 ng pRSV-β-gal were treated with DEX (1 nM, 10 nM, or 100 nM) or 0.1% ethanol (vehicle control) for 24 hrs before the luciferase assay. Results are expressed as percentages of control (100%). Each point represents mean ± SEM (n = 4). **P<0.01 and ***P<0.001 vs control. (B) Effect of DEX on NeuroD1 promoter deletion mutants. AtT20 cells transiently transfected with 300 ng ND full, ND mut1, ND mut2, ND mut3, or ND mut4 and 150 ng pRSV-β-gal were incubated in the presence (100 nM) or absence of DEX for 24 hrs before the luciferase assay. Results are expressed as percentages of each control (100% in pGL3-Basic). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. NS denotes “Not Significant.”
Role of E-box(es) of NeuroD1 promoter in the DEX-mediated suppression in AtT20 cells
In order to identify the element(s) responsible for the DEX-mediated suppression of NeuroD1 transcription, we examined the promoter activity of the NeuroD1 5’-flanking region deletion mutants. As shown in Fig 6B, there exist 3 types of E-box (denoted as E-box1, 2 and 3) on the NeuroD1 promoter. The DEX-mediated transcription suppression of the NeuroD1 promoter activity was observed in the full-length NeuroD1 promoter (ND full) and in ND mut2 that lacks E-box1, but not in ND mut1/ND mut3 that lack E-box2/3 or in ND mut4 that lacks E-box1/2/3. Taken together, the E-box2 and/or E-box3 may be important for the DEX-mediated negative expression of NeuroD1.
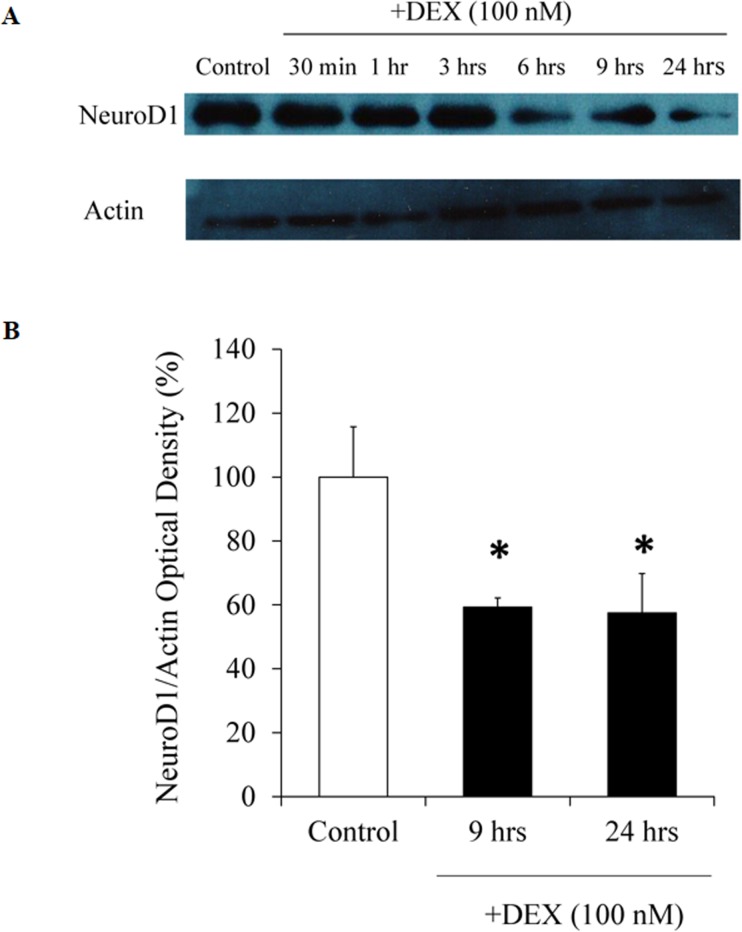
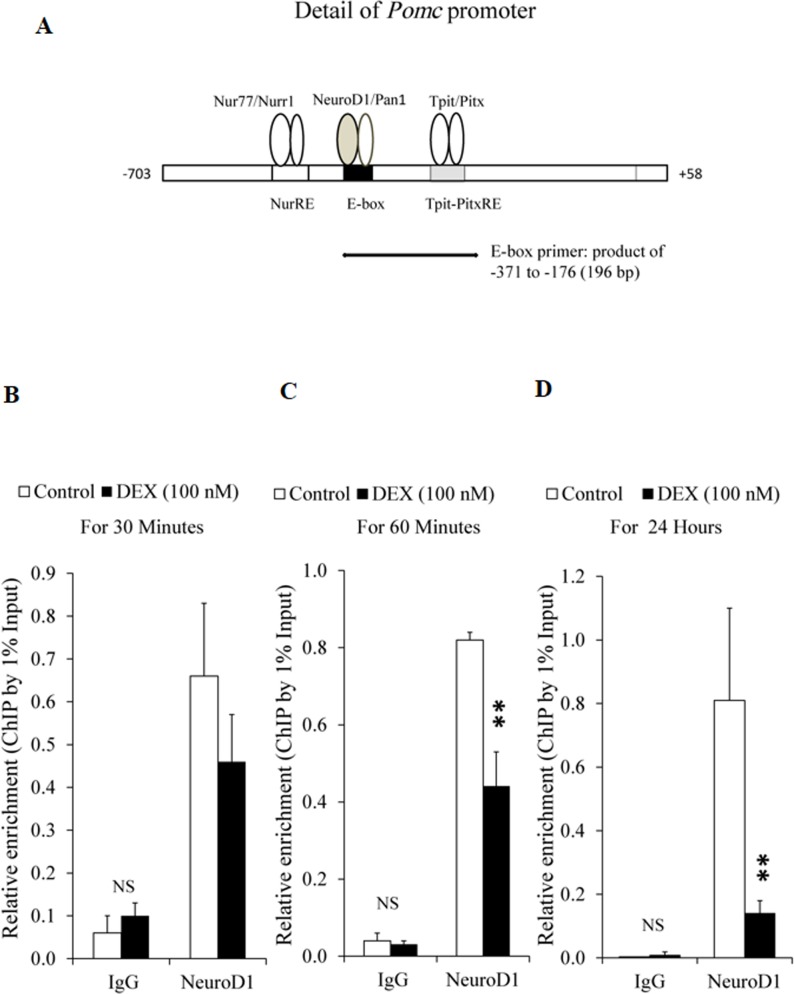
Effects of DEX on NeuroD1 protein expression/NeuroD1-Pomc E-box interaction in AtT20 cells
We next examined the effects of DEX on NeuroD1 protein expression. As shown in Fig 7A and 7B, DEX suppressed the protein expression of NeuroD1 after more than 6 hrs, but not by 3 hrs. Since NeuroD1 is known to bind to the E-box of the Pomc promoter [7], we further examined the effects of DEX on the interaction of NeuroD1 and E-box of the Pomc promoter by ChIP assay using primers encompassing E-box (Fig 8A). We then examined the effect of DEX on the interaction between NeuroD1 and E-box of the Pomc promoter using NeuroD antibody by ChIP assay. Although treatment with DEX for 30 min did not affect the interaction between NeuroD1 and E-box (Fig 8B), DEX treatment for 60 min significantly suppressed their interaction (Fig 8C). Moreover, DEX treatment for 24 hrs further suppressed the interaction between NeuroD1 and E-box (Fig 8D). When control IgG was used, DEX treatment did not affect their interaction at any time point (Fig 8B, 8C, and 8D). Taken together, it is speculated that DEX not only suppress the interaction between NeuroD1 and E-box through the decrease of NeuroD1 protein expression in the long run (24 hrs), but also through the inhibition of their binding in the short run (60 min).
Fig 7. Effects of DEX on NeuroD1 protein expression in AtT20 cells.
(A) Time-dependent effect of DEX on NeuroD1 and actin protein expression. AtT20 cells were treated with DEX (100 nM) for 1 hr, 3 hrs, 6 hrs, 9 hrs, or 24 hrs before the Western blot analyses. Vehicle control: 0.1% ethanol. In (B), AtT20 cells were treated with DEX (100 nM) for 9 hrs or 24 hrs before the Western blot analyses. Vehicle control: 0.1% ethanol. Optical density (OD) of NeuroD1 was normalized by OD of actin. Results are expressed as percentages of control (100%) Each point represents mean ± SEM (n = 3). **P<0.01vs control. Two independent experiments were performed with consistent results.
Fig 8. Effects of DEX on interaction between NeuroD1 and E-box on Pomc promoter in AtT20 cells.
(A) Effect of of DEX on interaction between NeuroD1 and E-box on Pomc promoter examined by ChIP assay using E-box primer. ChIP assay was performed using digested chromatin extracted from the cells cultured in the presence (100 nM) or absence (control) of DEX for 30 min (B), 60 min (C), or 24 hrs (D). Chromatin fragments were immunoprecipitated either by normal rabbit IgG (negative control) or NeuroD1 antibody. Purified DNA was analyzed by qPCR using primers specific for E-box containing sequence on Pomc promoter. The expected size of E-box is 196 bp. Few qPCR products observed in the input samples were detected in the immunoprecipitation using normal IgG. Immunoprecipitated DNA was quantified by qPCR and normalized to the values obtained after amplification of unprecipitated 1% input DNA. Each point represents mean ± SEM (n = 3). **P<0.01, significantly different from the level of control group. These experiments were repeated three times with separate batches of cell preparation with consistent results.
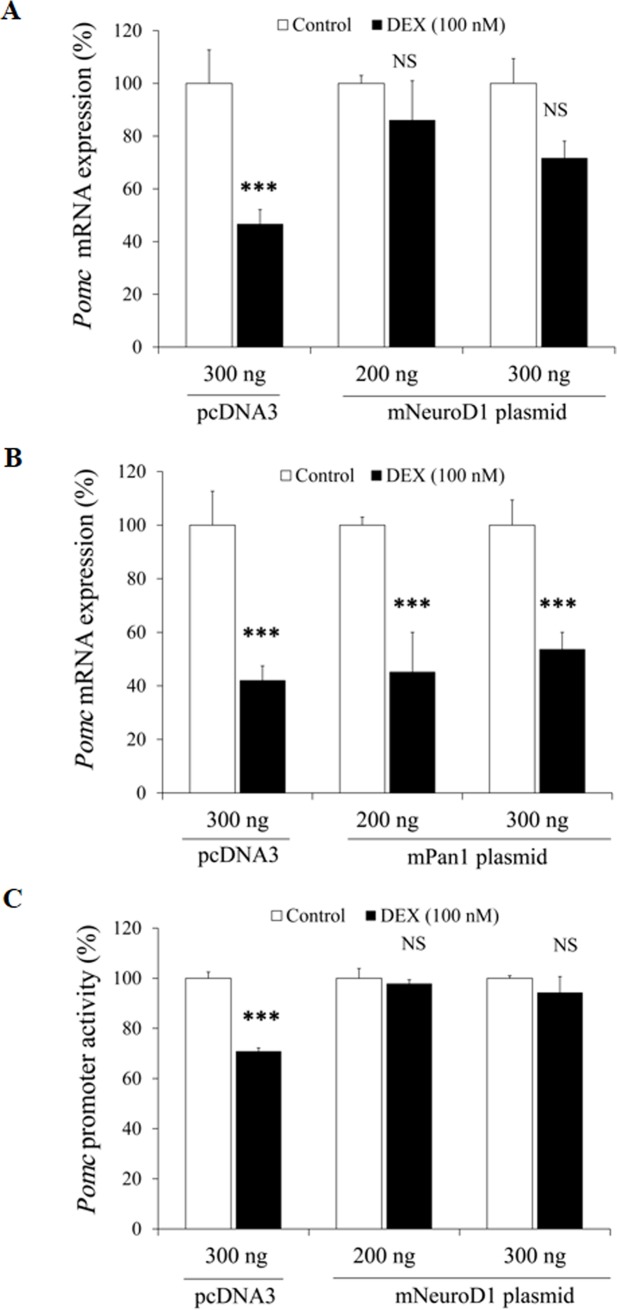
Effects of NeuroD1 overexpression on the DEX-mediated suppression of Pomc mRNA expression/Pomc promoter activity in AtT20 cells
We next induced the overexpression of NeuroD1 in order to examine the involvement of NeuroD1 in the DEX-mediated suppression. As shown in Fig 9A, overexpression of NeuroD1 rescued the DEX-mediated suppression of Pomc mRNA expression, while that of control plasmid (pcDNA3) did not. We next induced the overexpression of Pan1. As shown in Fig 9B, Pan1 overexpression did not rescue the DEX-mediated suppression of Pomc mRNA expression. NeuroD1 overexpression also rescued the DEX-mediated suppression of Pomc promoter activity (Fig 9C). These data also indicate the involvement of NeuroD1 in the DEX-mediated Pomc suppression.
Fig 9. Effects of NeuroD1 overexpression on Pomc mRNA expression and Pomc promoter activity in AtT20 cells.
(A) Effect of NeuroD1 overexpression on the DEX-mediated Pomc mRNA decrease. AtT20 cells were transiently transfected with pcDNA3 and NeuroD1 plasmid (volume adjusted to 300 ng each with pcDNA3 empty vector) and incubated either in the presence (100 nM) or absence (control) of DEX for 24 hrs. Results are expressed as percentages of each control (100%). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. NS denotes “Not Significant.” Two independent experiments were performed with consistent results. (B) Effect of Pan1 overexpression on the DEX-mediated Pomc mRNA decrease. AtT20 cells were transiently transfected with pcDNA3 and Pan1 plasmid (volume adjusted to 300 ng each with pcDNA3, empty vector) and incubated either in the presence (100 nM) or absence (control) of DEX for 24 hrs. Results are expressed as percentages of each control (100%). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. Two independent experiments were performed with consistent results. (C) Effect of overexpression of NeuroD1 on the DEX-mediated suppression of Pomc promoter activity. AtT20 cells were transiently transfected with pcDNA3 and NeuroD1 plasmid (volume adjusted to 300 ng each with pcDNA3 empty vector), 135 ng rPomc-Luc, and 65 ng pRSV-β-gal were incubated either in the presence (100 nM) or absence (control) of DEX for 24 hrs before the luciferase assay. Results are expressed as percentages of each control (100%). Each point represents mean ± SEM (n = 4). ***P<0.001 vs control. NS denotes “Not Significant.” Two independent experiments were performed with consistent results.
Discussion
The effects of Gc on Pomc transcription in rat pituitary was first demonstrated in 1984 in a study [19] showing that both DEX and corticosterone suppressed Pomc transcription in the anterior lobe, but not in the intermediate lobe. Additionally, DEX was shown to be more potent than corticosterone in supressing Pomc transcription [19], consistent with its long biological half-life as well as relative anti-inflammatory potency [39]. However, the mechanism of the negative regulation of Pomc transcription by Gc is still unclear in many points.
Two rat Pomc promoter fragments including either -4.5-kb or -706-bp 5'-flanking region were previously examined for the Gc-mediated inhibition, and it was observed that these two promoter fragments were almost equally suppressed by Gc [18]. Therefore, the Pomc promoter 5'-flanking region including -706-bp seems to be sufficient for obtaining the Gc-mediated Pomc transcription suppression. We therefore investigated the mechanisms of DEX on the Pomc promoter activity using the -703/+58 construct and its deletion mutants (Fig 3A and 3B). Several transcription factor binding sites have been shown to exist on the Pomc promoter region [40], and synergistic interactions among multiple regulatory elements are required for Pomc transcription in the pituitary [41]. Among them, two Nur binding sites have been identified on the Pomc promoter. The proximal binding sequence termed Nur77-binding response element (NBRE) (-69/-63) is known to be bound by the Nur77 monomer [40,42], and the distal Nur response element (NurRE), constituted of two inverted NBRE related sites (-404/-397 and -390/-383), is known to be bound by the Nur77 homodimer or Nur77/Nurr1 heterodimer; and the (distal) NurRE responds to Nur77 much stronger than the (proximal) NBRE [37,40,43]. Moreover, Tpit/Pitx-responsive elements (-316/-309 and -302/-297) [40,44] and NF-κB-responsive element (-151/-142) [40,45] are also known to be involved in Pomc regulation. Additionally, E-box (-377/-370) [7,40,46], to which NeuroD1 (β2) binds as a heterodimer with Pan1 (E47) for transactivation, has been known to play an important role in Pomc transcription regulation [7]. Moreover, NeuroD1 is known to regulate various genes in endocrine, enteroendocrine, and neuroendocrine cells such as insulin 1 [47,48], glucokinase (GK) [49], secretin [50], inositol 1,4,5-triphosphate receptor 1 (IP3R1) [51], and early B-cell factor 3 (Ebf3) [52].
We here have first observed the dose- and time-dependent effect of DEX on the suppression of NeuroD1 mRNA expression in AtT20 cells (Fig 4A and 4B). Regarding Tpit and Pitx1 mRNA expression, Gc has been reported to have no effect [27]. Although Pan1 is well known as the heterodimeric partner of NeuroD1, DEX did not affect the mRNA expression of Pan1 (Fig 4C and 4D), probably due to its ubiquitousness [9]. Recently, Rb has been reported to function as a coactivator of NeuroD1 for Pomc transcription in AtT20 cells [38]. However, Rb mRNA expression was not affected by DEX (Fig 4E and 4F). It is therefore indicated that the DEX-mediated inhibition of NeuroD1 mRNA expression may be involved in the Gc-mediated Pomc transcription suppression. Interestingly, the DEX-mediated Pomc transcription suppression was ameliorated by the overexpression of NeuroD1 (Fig 9A and 9C), but not by that of its heterodimeric partner Pan1 (Fig 9B). These data further suggest the functional significance of NeuroD1 in the transcription suppression of Pomc.
Since DEX also suppressed NeuroD1 promoter activity (Fig 6A), it is likely that the DEX-mediated inhibition of NeuroD1 mRNA expression is mediated at the gene transcription level. Analyses of the NeuroD1 promoter region using deletion mutants revealed that E-box2 and/or E-box3 may be involved in the Gc-mediated transcriptional suppression in AtT20 cells (Fig 6B). Among E-box1, 2 and 3 in the 5’-flanking region of NeuroD1, E-box1 and 3 have been reported to play an important role in islet-specific NeuroD1 expression [53]. Moreover, the sequence of E-box3 in the NeuroD1 promoter is identical to that of E-box in the Pomc promoter, which determines its corticotroph specific expression [7]. Further studies are needed to clarify which E-box in the NeuroD1 promoter determines its corticotroph specific expression.
We also examined the effect of DEX on the NeuroD1 protein expression by Western blot analyses. DEX significantly inhibited the NeuroD1 protein expression after more than 9 hrs (Fig 7A and 7B). This time course may be differ among cell lines since DEX inhibited NeuroD1 protein expression after more than 2 hrs in insulin-secreting pancreatic HIT-T15 cells [54]. Moreover, we have first demonstrated the inhibitory effects of DEX on the interaction of NeuroD1 and E-box in the Pomc promoter by ChIP assay using AtT20 cells (Fig 8). Although the DEX-mediated decrease of NeuroD1 protein expression could be observed after more than 6 hrs (Fig 7A), DEX significantly inhibited the interaction between NeuroD1 and E-box after 60 min. Since 60 min incubation of DEX did not affect the NeuroD1 protein expression (Fig 7A), it is speculated that DEX may inhibit the binding of NeuroD1 to E-box independently of the NeuroD1 protein decrease, most likely via its epigenetic effect.
Pomc gene transcription is regulated by coordination of several transcription factors including NeuroD1. Although NeuroD1 is an important transcription factor of Pomc gene, NeuroD1 and Tpit/Pitx transcription factors have synergistic effect on Pomc gene regulation [9]. Moreover, the combination of Pitx1, Tpit and NeuroD1 has been suggested to be enough to specify the genetic program of corticotroph cells compared to other anterior pituitary lineages, while NeuroD1 is not expressed in melanotroph cells of the intermediate lobe of pituitary at any time during development or in adults [55]. Since we have also observed the DEX-mediated suppression of Nurr1 mRNA expression (Fig 5D), the interaction of NeuroD1 and Nurr1 in the Gc-mediated negative regulation of Pomc is further needed to be elucidated.
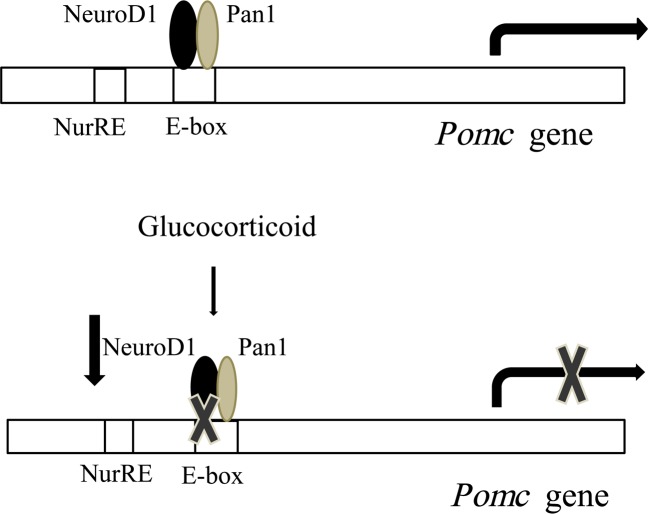
Our observation is summarized in Fig 10. NeuroD1 binds to E-box on the Pomc promoter and enhances Pomc gene expression [7]. However, when Gc is added, it decreases NeuroD1 transcription, mRNA expression, and protein expression, resulting in the decrease of NeuroD1 binding to E-box on the Pomc promoter to suppress Pomc transcription. It can therefore be concluded that NeuroD1 may play an important role in the negative regulation of Pomc expression in AtT20 cells by Gcs.
Fig 10. Involvement of NeuroD1 in the Gc-mediated transcription suppression of Pomc.
Acknowledgments
We acknowledge Dr. Debra E. Bramblett (Texas Tech University Health Sciences Center, El Paso, TX) for providing Beta2 (NeuroD1) overexpression plasmid, and Dr. Lori Sussel (Columbia University, New York, NY) for providing mNeuroD1-Luc and NeuroD1 promoter deletion constructs.
Data Availability
All relevant data are within the paper.
Funding Statement
AS is funded by the Grants-in-Aid for Scientific Research (Research on Hypothalamo-hypophyseal Disorders) from the Ministry of Health, Labor and Welfare, Japan, and the Platform Project for Supporting in Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from Japan Agency for Medical Research and Development (AMED). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Massari ME, Murre C (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414: 788–791. doi: 10.1038/414788a [DOI] [PubMed] [Google Scholar]
- 3.Cerf ME (2006) Transcription factors regulating beta-cell function. Eur J Endocrinol 155: 671–679. doi: 10.1530/eje.1.02277 [DOI] [PubMed] [Google Scholar]
- 4.Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H (1995) Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268: 836–844. [DOI] [PubMed] [Google Scholar]
- 5.Miyata T, Maeda T, Lee JE (1999) NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev 13: 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, et al. (2000) Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev 14: 2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulin G, Turgeon B, Drouin J (1997) NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol 17: 6673–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J (2004) Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol 18: 995–1003. doi: 10.1210/me.2003-0127 [DOI] [PubMed] [Google Scholar]
- 9.Poulin G, Lebel M, Chamberland M, Paradis FW, Drouin J (2000) Specific protein-protein interaction between basic helix-loop-helix transcription factors and homeoproteins of the Pitx family. Mol Cell Biol 20: 4826–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertagna X (1994) Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am 23: 467–485. [PubMed] [Google Scholar]
- 11.Castro MG, Morrison E (1997) Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol 11: 35–57. [DOI] [PubMed] [Google Scholar]
- 12.Smith EM, Blalock JE (1981) Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A 78: 7530–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou A, Bloomquist BT, Mains RE (1993) The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem 268: 1763–1769. [PubMed] [Google Scholar]
- 14.Bloomquist BT, Eipper BA, Mains RE (1991) Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol 5: 2014–2024. doi: 10.1210/mend-5-12-2014 [DOI] [PubMed] [Google Scholar]
- 15.Axelrod J, Reisine TD (1984) Stress hormones: their interaction and regulation. Science 224: 452–459. [DOI] [PubMed] [Google Scholar]
- 16.Jeannotte L, Trifiro MA, Plante RK, Chamberland M, Drouin J (1987) Tissue-specific activity of the pro-opiomelanocortin gene promoter. Mol Cell Biol 7: 4058–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wardlaw SL, McCarthy KC, Conwell IM (1998) Glucocorticoid regulation of hypothalamic proopiomelanocortin. Neuroendocrinology 67: 51–57. [DOI] [PubMed] [Google Scholar]
- 18.Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O (1989) Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol 9: 5305–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberwine JH, Roberts JL (1984) Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem 259: 2166–2170. [PubMed] [Google Scholar]
- 20.Gagner JP, Drouin J (1985) Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol 40: 25–32. [DOI] [PubMed] [Google Scholar]
- 21.Dostert A, Heinzel T (2004) Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des 10: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 22.Cadepond F, Schweizer-Groyer G, Segard-Maurel I, Jibard N, Hollenberg SM, Giguere V, et al. (1991) Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state. J Biol Chem 266: 5834–5841. [PubMed] [Google Scholar]
- 23.Picard D, Yamamoto KR (1987) Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J 6: 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar R, Thompson EB (2005) Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol 94: 383–394. doi: 10.1016/j.jsbmb.2004.12.046 [DOI] [PubMed] [Google Scholar]
- 25.Drouin J, Sun YL, Chamberland M, Gauthier Y, De Lean A, Nemer M, et al. (1993) Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J 12: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilodeau S, Vallette-Kasic S, Gauthier Y, Figarella-Branger D, Brue T, Berthelet F, et al. (2006) Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev 20: 2871–2886. doi: 10.1101/gad.1444606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami I, Takeuchi S, Kudo T, Sutou S, Takahashi S (2007) Corticotropin-releasing hormone or dexamethasone regulates rat proopiomelanocortin transcription through Tpit/Pitx-responsive element in its promoter. J Endocrinol 193: 279–290. doi: 10.1677/JOE-06-0143 [DOI] [PubMed] [Google Scholar]
- 28.Saito-Hakoda A, Uruno A, Yokoyama A, Shimizu K, Parvin R, Kudo M, et al. (2015) Effects of RXR Agonists on Cell Proliferation/Apoptosis and ACTH Secretion/Pomc Expression. PLoS One 10: e0141960 doi: 10.1371/journal.pone.0141960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uruno A, Saito-Hakoda A, Yokoyama A, Kogure N, Matsuda K, Parvin R, et al. (2014) Retinoic acid receptor-alpha up-regulates proopiomelanocortin gene expression in AtT20 corticotroph cells. Endocr J 61: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 30.Anderson KR, Torres CA, Solomon K, Becker TC, Newgard CB, Wright CV, et al. (2009) Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (beta2) by Nkx2.2 and neurogenin 3. J Biol Chem 284: 31236–31248. doi: 10.1074/jbc.M109.048694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai A, Yan G, He Q, Jiang Y, Zhang Q, Fang T, et al. (2012) Orphan nuclear receptor Nur77 regulates androgen receptor gene expression in mouse ovary. PLoS One 7: e39950 doi: 10.1371/journal.pone.0039950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spyroglou A, Manolopoulou J, Wagner S, Bidlingmaier M, Reincke M, Beuschlein F (2009) Short term regulation of aldosterone secretion after stimulation and suppression experiments in mice. J Mol Endocrinol 42: 407–413. doi: 10.1677/JME-08-0167 [DOI] [PubMed] [Google Scholar]
- 33.Mercau ME, Repetto EM, Perez MN, Martinez Calejman C, Sanchez Puch S, Finkielstein CV, et al. (2016) Moderate Exercise Prevents Functional Remodeling of the Anterior Pituitary Gland in Diet-Induced Insulin Resistance in Rats: Role of Oxidative Stress and Autophagy. Endocrinology 157: 1135–1145. doi: 10.1210/en.2015-1777 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Chen B, Hou XH, Guan GJ, Liu G, Liu HY, et al. (2007) Effects of mycophenolate mofetil, valsartan and their combined therapy on preventing podocyte loss in early stage of diabetic nephropathy in rats. Chin Med J (Engl) 120: 988–995. [PubMed] [Google Scholar]
- 35.Maliza R, Fujiwara K, Tsukada T, Azuma M, Kikuchi M, Yashiro T (2016) Effects of retinoic acid on growth hormone-releasing hormone receptor, growth hormone secretagogue receptor gene expression and growth hormone secretion in rat anterior pituitary cells. Endocr J 63: 555–561. doi: 10.1507/endocrj.EJ16-0086 [DOI] [PubMed] [Google Scholar]
- 36.Pecori Giraldi F, Casarino MF, Pagliardini L, Cavagnini F (2011) On the Effect of CRH and Dexamethasone on POMC Synthesis and ACTH Secretion in Rat Pituitary Primary Cultures. The Endocrine Society's 93rd Annual Meeting. Boston, P1-272.
- 37.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, et al. (1997) Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17: 5946–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batsche E, Moschopoulos P, Desroches J, Bilodeau S, Drouin J (2005) Retinoblastoma and the related pocket protein p107 act as coactivators of NeuroD1 to enhance gene transcription. J Biol Chem 280: 16088–16095. doi: 10.1074/jbc.M413427200 [DOI] [PubMed] [Google Scholar]
- 39.Walsh D, Avashia J (1992) Glucocorticoids in clinical oncology. Cleve Clin J Med 59: 505–515. [DOI] [PubMed] [Google Scholar]
- 40.Jenks BG (2009) Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci 1163: 17–30. doi: 10.1111/j.1749-6632.2008.03620.x [DOI] [PubMed] [Google Scholar]
- 41.Therrien M, Drouin J (1991) Pituitary pro-opiomelanocortin gene expression requires synergistic interactions of several regulatory elements. Mol Cell Biol 11: 3492–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J (1991) Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 43.Maira M, Martens C, Philips A, Drouin J (1999) Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol 19: 7549–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, et al. (2001) A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104: 849–859. [DOI] [PubMed] [Google Scholar]
- 45.Karalis KP, Venihaki M, Zhao J, van Vlerken LE, Chandras C (2004) NF-kappaB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem 279: 10837–10840. doi: 10.1074/jbc.M313063200 [DOI] [PubMed] [Google Scholar]
- 46.Therrien M, Drouin J (1993) Cell-specific helix-loop-helix factor required for pituitary expression of the pro-opiomelanocortin gene. Mol Cell Biol 13: 2342–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumonteil E, Laser B, Constant I, Philippe J (1998) Differential regulation of the glucagon and insulin I gene promoters by the basic helix-loop-helix transcription factors E47 and BETA2. J Biol Chem 273: 19945–19954. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A, Moore M, Marcora E, Lee JE, Qiu Y, Samaras S, et al. (1999) The NeuroD1/BETA2 sequences essential for insulin gene transcription colocalize with those necessary for neurogenesis and p300/CREB binding protein binding. Mol Cell Biol 19: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moates JM, Nanda S, Cissell MA, Tsai MJ, Stein R (2003) BETA2 activates transcription from the upstream glucokinase gene promoter in islet beta-cells and gut endocrine cells. Diabetes 52: 403–408. [DOI] [PubMed] [Google Scholar]
- 50.Mutoh H, Naya FJ, Tsai MJ, Leiter AB (1998) The basic helix-loop-helix protein BETA2 interacts with p300 to coordinate differentiation of secretin-expressing enteroendocrine cells. Genes Dev 12: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konishi Y, Ohkawa N, Makino Y, Ohkubo H, Kageyama R, Furuichi T, et al. (1999) Transcriptional regulation of mouse type 1 inositol 1,4,5-trisphosphate receptor gene by NeuroD-related factor. J Neurochem 72: 1717–1724. [DOI] [PubMed] [Google Scholar]
- 52.Pozzoli O, Bosetti A, Croci L, Consalez GG, Vetter ML (2001) Xebf3 is a regulator of neuronal differentiation during primary neurogenesis in Xenopus. Dev Biol 233: 495–512. doi: 10.1006/dbio.2001.0230 [DOI] [PubMed] [Google Scholar]
- 53.Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ (2000) Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol 20: 3292–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shinozuka Y, Okada M, Oki T, Sagane K, Mizui Y, Tanaka I, et al. (2001) Altered expression of HES-1, BETA2/NeuroD, and PDX-1 is involved in impaired insulin synthesis induced by glucocorticoids in HIT-T15 cells. Biochem Biophys Res Commun 287: 229–235. doi: 10.1006/bbrc.2001.5573 [DOI] [PubMed] [Google Scholar]
- 55.Drouin J (2016) 60 YEARS OF POMC: Transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol 56: T99–T112. doi: 10.1530/JME-15-0289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.