Abstract
Delineating the crosstalk between distinct signaling pathways is key to understanding the diverse and dynamic responses of adult stem cells during tissue regeneration. Here we demonstrate that the Edn/EdnrB signaling pathway can interact with other signaling pathways to elicit distinct stem cell functions during tissue regeneration. EdnrB signaling promotes proliferation and differentiation of melanocyte stem cells (McSCs), dramatically enhancing the regeneration of hair and epidermal melanocytes. This effect is dependent upon active Wnt signaling that is initiated by Wnt ligand secretion from the hair follicle epithelial niche. Further, this Wnt-dependent EdnrB signaling can rescue the defects in melanocyte regeneration caused by Mc1R loss. This suggests that targeting Edn/EdnrB signaling in McSCs can be a therapeutic approach to promote photoprotective-melanocyte regeneration, which may be useful for those with increased risk of skin cancers due to Mc1R variants.
Introduction
Stem cells self-renew, and simultaneously generate differentiated progeny for normal tissue homeostasis and regeneration in response to injury or diseases. The cycling nature of the hair follicle and the defined stem cell population that occupies this organ has provided a means to investigate mechanisms that regulate adult stem cells. Pigmented hair regeneration requires epithelial stem cells (EpSCs) and McSCs in the hair follicle (Cotsarelis et al., 1990; Myung et al., 2013; Nishimura et al., 2002), which undergo hair cycle phases of growth (anagen), regression (catagen) and rest (telogen) (Dry, 1926; Muller-Rover et al., 2001). McSCs reside in the lower permanent portion of the hair follicle throughout the hair cycle. During the telogen phase, bulge/sHG area represents the lower permanent portion of the follicle in which McSCs are maintained in a quiescent state. McSCs become activated at anagen onset to proliferate and give rise to differentiated progeny. As anagen ensues, differentiated progeny migrate downwards to the bulb compartment where they produce pigment for the hair. This segregation allows ones to histochemically identify McSCs with universal marker of melanocytes such as Dct (Trp2), based on the anatomically defined location of the niche in mice. During catagen, differentiated melanocytes in the bulb undergo apoptosis, while McSCs survive. Accordingly, telogen follicles contain only McSCs that are re-activated in the next hair cycle. The activation state of McSCs is governed by the niche (Nishimura et al., 2002), which is composed of EpSCs of the hair follicle (Rabbani et al., 2011; Tanimura et al., 2011). Thus far, only a handful of signals that regulate McSCs have been identified, including extrinsic signals, such as TGFB and Wnts, which are provided by the epithelial niche (Myung et al., 2013; Nishimura et al., 2010; Rabbani et al., 2011). Wnt signaling induces activation of EpSCs to drive epithelial regeneration, while coordinately inducing McSCs to proliferate and differentiate to pigment regenerating hair follicle (Rabbani et al., 2011).
In addition to providing pigment to the hair follicle, McSCs can also generate epidermal melanocytes in response to wounding (Chou et al., 2013; Nishimura, 2011). However, the signaling pathways that regulate differentiation and establishment of epidermal melanocytes from McSCs are only beginning to emerge, including Wnt and Mc1R signaling (Chou et al., 2013; Yamada et al., 2013). It is poorly understood how McSCs are maintained to ensure an adequate supply of stem cells for homeostasis and regeneration and how they are primed to respond to injury. Addressing these issues would allow us to identify therapeutic targets to treat pigmentation disorders.
Despite the well-known roles for Endothelin receptor B (EdnrB) and its ligands, Endothelin (Edn1, 2 and 3), in melanocytes during embryogenesis (Giller et al., 1997; Matsushima et al., 2002; Saldana-Caboverde and Kos, 2010), their function in adult melanocytes during normal homeostasis and regeneration has not been addressed. During embryogenesis, EdnrB mutations in mice give rise to pigmentation defects and are linked to Waardenberg syndrome that accompanies hypopigmentation (Attie et al., 1995; Baynash et al., 1994; Edery et al., 1996). Binding of Edns to EdnrB results in phosphorylation of cAMP response element binding protein (CREB) and microphthalmia-associated transcription factor (MITF), leading to the transcription of target genes, including MITF, the transcription factor that is pivotal to the expression of numerous pigment enzymes and differentiation factors(Levy et al., 2006; Nakajima et al., 2011; Sato-Jin et al., 2008). Recently, it was shown that Edn1 is secreted from neighboring EpSCs at anagen onset, whereas Edn2 is upregulated in EpSCs upon ablation of the transcription factor nuclear factor I/B (NFIB) (Chang et al., 2013; Rabbani et al., 2011). Additionally, previous studies have demonstrated the expression of EdnrB in McSCs (Rabbani et al., 2011)(Fig S1). However, the function of Edns/EdnrB signaling in McSCs during hair cycle has not been characterized by gain or loss of function approaches. Moreover, how it collaborates with other pathways is incompletely understood.
In this study, we analyzed the role of Edn/EdnrB signaling in adult McSCs, using a combination of loss and gain of function genetic mouse models. We found that Edn/EdnrB is critical in melanocytes for hair pigmentation during homeostasis and the generation of epidermal melanocytes following wounding. Epithelial Edn1 overexpression is sufficient to establish epidermal melanocytes under normal homeostatic conditions. Moreover, it is sufficient to overcome the effects of loss of Mc1R in vivo that is required for McSCs to generate epidermal melanocytes following wounding. These effects were only seen in the presence of active Wnt signaling. Our findings reveal a vital role for Edn/EdnrB signaling in adult melanocytes by acting in conjunction with Wnt and Mc1R and Edn1 for a network of signaling pathways.
Results
EdnrB is required for McSC proliferation and maintenance
To address the function of Edn/EdnrB signaling in McSCs, we deleted EdnrB specifically in the melanocyte lineage including McSCs in Tyr-CreER; EdnrBfl/fl; R26-stop-Tomato (Ednrb cKO) mice. These mice express Tomato and delete EdnrB in melanocytes upon TAM induced recombination (Bosenberg et al., 2006; Druckenbrod et al., 2008).
We induced Cre mediated recombination by treating 3 week old EdnrB cKO and control littermate mice with tamoxifen (TAM). By the following telogen, EdnrB cKO mice displayed a noticeable hair graying phenotype, which is not observed in control mice (Fig. 1A). In cKO mice, more than 65% of the hair shafts lacked melanin, leading to hair graying phenotype that persisted through the next hair cycle, unlike in control mice (Fig. 1B and C). Immunohistochemical analysis revealed that unpigmented hair follicles of EdnrB cKO mice contained fewer differentiated melanocytes in the bulb by anagen IV, evidenced by the decreased number of melanocytes with differentiation markers including Trp2, MITF and S100 expression (Fig. 1D - L). In these un-pigmented hair follicles, McSCs showed less incorporation of BrdU compared to pigmented hair follicle of control mice at anagen III, when bulb melanocytes normally begin producing pigment in control mice (Fig. 1M, O, and Q). We also found an increased percentage of bulb melanocytes positive for cleaved caspase3, a marker for cell apoptosis, in EdnrB cKO mice compared to control mice at anagen VI (Fig. 1N, P, and R).
Figure 1. Loss of EdnrB leads to loss of pigment, reduced proliferation and failure to maintain McSCs.
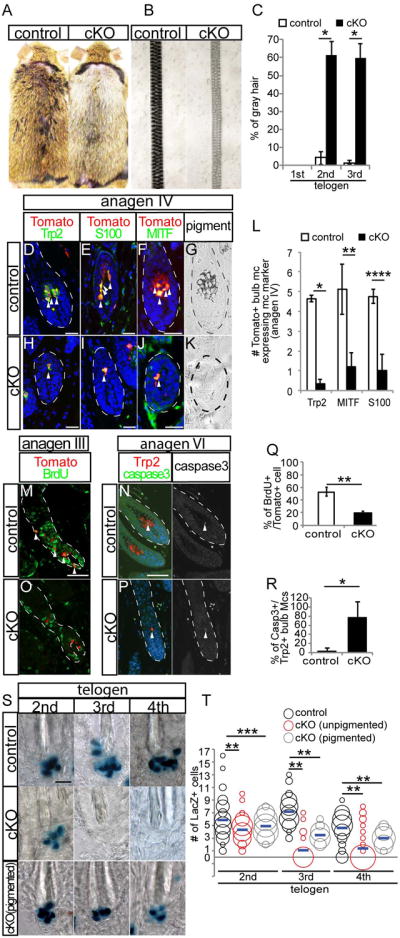
(A) Gross appearance of control (left) and EdnrB cKO (right) mice at 2nd telogen. (B) Brightfield whole mount picture of hair shaft from control (left) and EdnrB cKO (right) mice. (C) Quantification of percentage of gray hair at each telogen. (D-F and H-J) Immunohistochemical analysis of bulb melanocytes in skin sections at anagen IV with Tomato and indicated melanocyte differentiation markers from Tyr-CreER;Rosa-stop-Tomato (control, D-F) and EdnrB cKO;Rosa-stop-Tomato mice (H-J). (G and K) Brightfield image of bulb from control (G) and EdnrB cKO mouse (K). (L) Quantification of the percentage of Tomato positive cells that are also positive for Trp2, MITF, and S100, respectively. (M, O) Immunohistochemistry for BrdU on control (M) and EdnrB cKO mice (O). (N and P) Anagen VI skin sections stained with Trp2 and cleaved caspase 3 (left panel) in hair follicle bulb in control (N) and EdnrB cKO mice (P). Single caspase 3 staining shown in right panel. (Q and R) Quantification of the percentage of BrdU+/Tomato+ cells (Q) and cleaved caspase 3+/Trp2+ cells (R). (S) Whole mount image of X-gal stained single hair follicles from Dct-LacZ (Control, Top) and unpigmented (middle) and pigmented hair follicles (bottom) from EdnrB cKO; Dct-LacZ mice. (T) Distribution and average (blue line) of absolute number of Dct-LacZ+ melanocytes per hair follicle. Dashed lines indicate border between hair follicle and dermis. Arrowheads indicate double positive cells for indicated markers. Data are presented as the mean ± SD. *, p<0.001, **, p<0.01, ***, p<0.02, ****, p<0.05. Scale bar, 50μm in (D-F, H-J); 20μm in (M, N, and S). See also Fig. S1.
To analyze the effect of EdnrB depletion on McSC maintenance, we analyzed the absolute number of McSCs of control and EdnrB cKO mice. To this end, we crossed EdnrB cKO mouse with Dct-LacZ reporter mice, in which melanocytes are tagged by lacZ expression. We treated mice with TAM during each anagen phase starting from 2nd anagen and detected McSCs by whole mount lacZ staining at each telogen phase. By the 2nd telogen following initial TAM, when the pigmentation defect becomes apparent (Fig. 1C), McSC number in unpigmented hair follicle of EdnrB cKO is significantly reduced compared to control mice (Fig. 1S and T). By the subsequent telogen phase (3rd telogen), reduction of McSCs became even more obvious and the majority of un-pigmented EdnrB cKO hair follicles completely lacked McSCs and their absence persisted at least to 4th telogen (Fig. 1S and T).
Taken together these results suggest that EdnrB signaling is required for proliferation and maintenance of McSCs as well as survival of differentiated bulb melanocytes, thereby EdnrB is critical for hair pigmentation.
Edn1 promotes differentiation and proliferation of McSCs at anagen onset
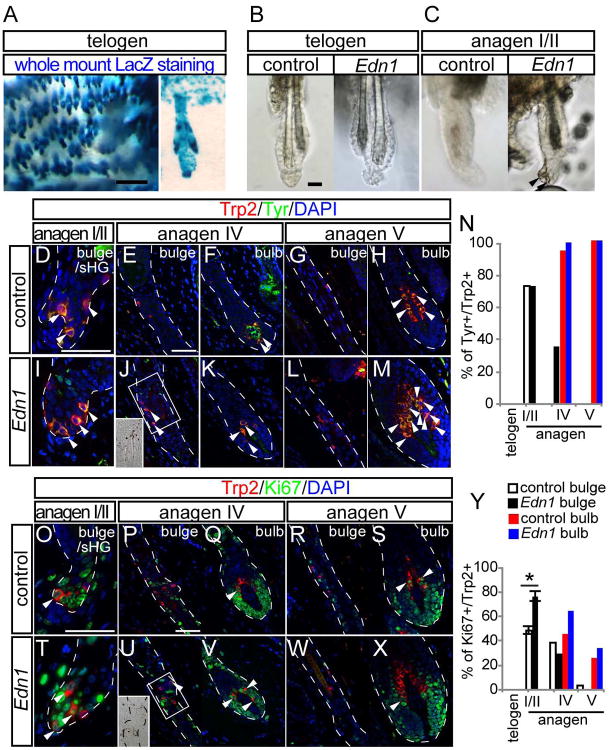
Next, we investigated whether activation of EdnrB signaling by overexpressing its ligand Edn1 in epithelial cells can alter melanocyte behavior. We utilized K14-rtTA;TetO-Edn1-lacZ mice (Edn1 Tg mice) in which we can induce Edn1 and LacZ expression in K14+ cells upon doxycycline (Dox) treatment(Nguyen et al., 2006; Yang et al., 2004).
We treated 7-week old adult Edn1 Tg and their littermate control mice with Dox starting from 2nd telogen, when McSCs are quiescent. One week after treatment, when hair follicles were still in telogen phase, X-gal staining confirmed that LacZ was expressed in the epithelium, showing the activation of tet-O promoter that drives Edn1 expression (Fig. 2A). During this phase, McSCs in Edn1 Tg mice were maintained in a quiescent and undifferentiated state. This was evident by the absence of proliferation, pigmentation, and differentiation markers including tyrosinase and β-catenin in McSCs of these mice (Fig. 2B and S2). In contrast, when we experimentally induced these mice to enter anagen by depilation, Edn1 expression induced ectopic pigmentation in the bulge/sHG area of hair follicles at anagen onset, while control mice lacked pigmentation in this area (Fig. 2C). This ectopic pigmentation persisted in the niche through later stages of anagen in Edn1 Tg mice (inset in Fig. 2J). We also examined the expression of the melanocyte differentiation marker, Tyr, which is transiently seen in melancoytes in the niche only at anagen onset when McSCs give rise to differentiated progeny. (Fig. 2D-H and N). We found that Tg mice continued to have Tyr positive melanocytes in the niche by anagen IV (Fig. 2I-N), while control mice diminish Tyr immunoreactivity following the departure of differentiated melanocytes toward the hair bulb compartment (Fig. 2D-H and N). Additionally, immunostaining analysis for the proliferation marker, Ki67 revealed that Edn1 overexpression led to a significant increase in McSC proliferation specifically at anagen onset and was not found in later stages of anagen (Fig. 2O-Y). These observations demonstrate that Edn1 can promote the proliferation and differentiation of McSCs during hair follicle regeneration.
Figure 2. Overexpression of Edn1 promotes McSCs differentiation and proliferation during anagen.
(A) Whole mount X-gal staining from K14-rtTA; TetO-Edn1-LacZ mice, 1 week after Dox treatment during telogen. Right panel is higher magnification of picture shown in left panel. (B-C) Whole mount image of telogen hair follicle from control (left) and K14-rtTA; TetO-Edn1-LacZ (right) mice that were induced with Dox during telogen (B) or anagen (C). (D-M, O-X) Immunostaining of skin sections from control (D-H, O-S) and K14-rtTA; TetO-Edn1-LacZ (I-M, T-X) mice for Tyr and Trp2 (D-M) and Ki67 and Trp2 (O-X) in the McSC niche and bulb compartment of the hair follicle at indicated hair cycle stages. (N and Y) Quantification of the percentage of Tyr positive cells (N) and Ki67 positive cells (Y) in Trp2 positive cells. Dashed lines indicate border between hair follicle and dermis. Arrowheads indicate pigmentation in (C) and double positive cells for indicated markers in immunohistochemical analysis. Data are presented as the mean ± SD. *, p<0.002; **<0.05. Scale bar, 100μm in (A); 20μm in (B, C and E). See also Figures S2 and S3.
Previous studies have shown that ectopic differentiation of McSCs in their niche leads to depletion of McSCs and premature hair graying (Inomata et al., 2009; Nishimura et al., 2005; Rabbani et al., 2011; Tanimura et al., 2011). Given that Edn1 overexpression can lead to ectopic pigmentation and promote the expression of the melanocyte differentiation marker, Tyr, we suspected that these effects may lead to depletion of McSCs and hair graying over the long term. Surprisingly, we found that the number of McSCs was maintained and premature hair graying was not observed at least by 4th telogen, suggesting that Edn1 overexpression does not lead to defects in McSC maintenance (Fig. S3). Taken together, these results show that Edn1 overexpression in epithelial cells can promote proliferation and differentiation of McSCs without leading to its exhaustion.
Edn1 promotes generation of epidermal melanocytes following wounding
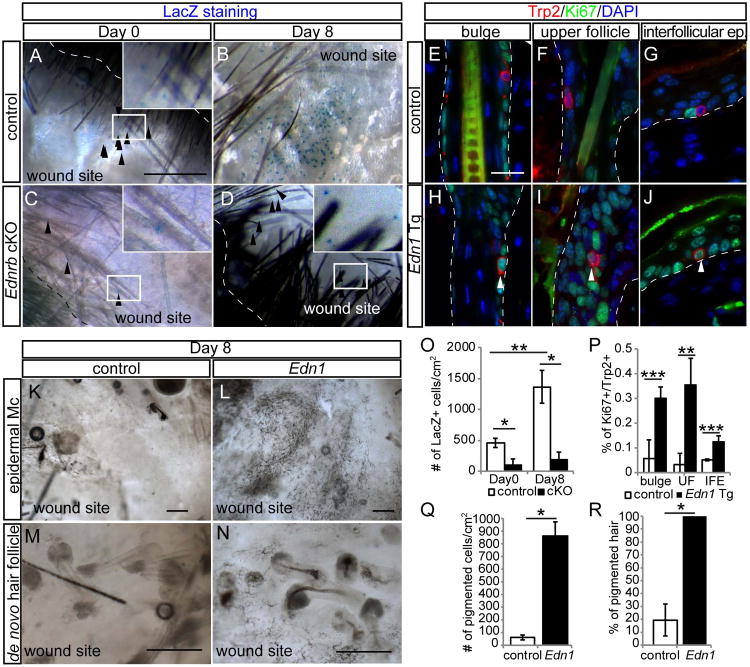
Generally, dorsal skin of adult mice lacks epidermal melanocytes in the absence of external stimuli such as wounding. We previously reported that McSCs migrate upward to give rise to epidermal melanocytes following UVB irradiation or injuries in the adult skin (Chou et al., 2013). We asked whether EdnrB signaling promotes the generation of epidermal melanocytes. First, we created full-thickness wounds on 8-week old EdnrB cKO mice that have Dct-LacZ reporter gene and treated these mice with TAM immediately after wounding. In control mice, epidermal melanocytes became clearly detectable within the wound epidermis by the time of the complete re-epithelialization (Day 0), and their number significantly increased by 8 days after re-epithelialization (Fig. 3A, B and O). In contrast, EdnrB KO mice displayed a considerably reduced number of melanocytes in the wound epidermis. The number of epidermal melanocytes remained lower compared to the control mice yet persisted at later time points, without showing signs of apoptosis such as cleaved caspase 3 expression (Fig. 3C, D, O and Fig. S4). These results show that loss of EdnrB signaling compromised the generation of epidermal melanocytes after wounding.
Figure 3. Overexpression of Edn1 promotes upward migration of McSCs and generation of epidermal melanocytes following wounding.
(A-D) Whole mount image of X-gal stained wound area of Dct-LacZ (control, A and B) and Tyr-CreER; EdnrBfl/fl; Dct-lacZ (C and D) at indicated days after re-epithelialization. (E-J) Double immunohistochemical staining of Trp2 and Ki67 in the bulge (E and H), upper hair follicle (F and I), and interfollicular epidermis (G and J) in control (E - G) and K14-rtTA; TetO-Edn1-LacZ (Edn1, H - J) mice. (K-N) Whole mount analyses of wound site (K and L) and de novo hair follicles (M and N) within wound site from control (K and M) and Edn1 mice (M and N) at 8 days after re-epithelialization. (O-R) Quantification of the number of Dct-LacZ+ cells in wound site (O), the percentage of Ki67+/Trp2+ cells (P), the number of pigmented cells in wound site (Q), and the percentage of pigmented de novo hair (R), respectively. Dashed lines indicate periphery of wound site in (A and D) and boundary between epidermis and dermis in (E-J). Arrowheads show Dct-LacZ+ cells in wound area in (A-D) and Ki67+/Trp2+ cells (H-J). UF, upper follicle. IFE, interfollicular epidermis. Data are presented as the mean ± SD. *, p<0.01, **, p<0.02, ***, p<0.05. Scale bar, 1mm in (A); 50μm in (E); 200μm in (K and L) ; 100μm in (M and N). See also Figures S4, S5, and S6.
Next, we examined whether overexpresion of Edn1 in the epithelium affects generation of epidermal melanocytes after wounding. We treated Edn1 Tg mice with Dox immediately following wounding. We observed that over-expression of epithelial Edn1 did not alter the time course of wound closure process (data not shown). By immunohistochemistry, we found that McSCs in the follicular niche (Fig. 3E, H and P), and migrating McSC en route to the epidermis (Fig. 3F, I and P), as well as those melanocytes that have migrated to the epidermis all displayed significantly higher proliferation in the Edn1 Tg mice compared to control (Fig. 3G, J and P). Consequently, there was a dramatic increase of epidermal melanocytes in the wound area in Edn1 Tg mice (Fig. 3K, L and Q). Unexpectedly, we found that all de novo hair follicle of Edn1 Tg mice produce pigmented hair, while the majority of de novo hair in control mice do not produce pigmented hair due to the deficient recruitment of melanocytes into the wound area (Fig. 3M, N and R) (Chou et al., 2013; Ito et al., 2007).
To test if Edn1 overexpression is sufficient to recruit melanocytes from the hair follicle to the skin epidermis without external stimuli such as wounding, we treated adult Edn1 Tg mice with Dox during telogen and anagen phase and examined the presence of Trp2 positive epidermal melanocytes. Strikingly, by 12 days after induction of Edn1 over-expression during anagen, we found pigmented melanocytes in the inter follicular epidermis (Fig. S5). In contrast, upon induction of Dox during telogen phase, melanocytes remained undetectable in skin epidermis (Fig. S5).
Given the marked induction of migration observed in Edn1 Tg mice, we also examined the effect of Edn1 on cell migration using in vitro scratch assay. We found that addition of Edn1 recombinant protein can promote cell migration as seen by the ability of these cells to migrate more towards the center of the wound compared to control. Moreover, addition of EdnrB siRNA to Edn1 treated cells suppressed this effect (Fig. S6), showing that EdnrB mediates the promoting effect of Edn1 on cell migration.
Taken together, these results demonstrate that overexpression of Edn1 in epithelial cells is sufficient to promote the generation of epidermal melanocytes even without external stimuli, and this may partially be through the up-regulation of cell proliferation and migration.
Edn1 promotes the repopulation of epidermal melanocyte in human skin
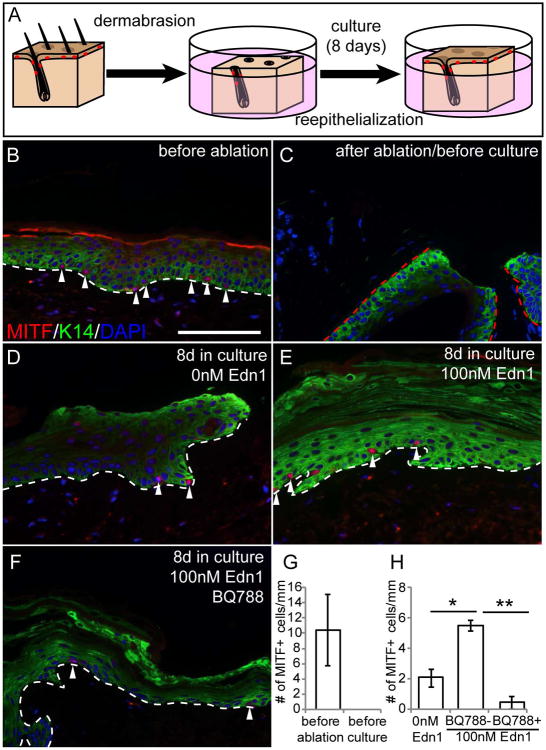
To assess whether Edn/EdnrB signaling can also promote follicular melanocytes to generate epidermal melanocytes in human skin, we took advantage of an explant culture system of human skin (Chou et al., 2013) (Fig. 4A). Unlike adult mice, the human epidermis contains epidermal melanocytes, which express MITF, within its Keratin 14+ basal layer (Fig. 4A and B). We previously showed that follicular melanocytes can provide epidermal melanocytes in both humans and mice (Chou et al., 2013).
Figure 4. Edn1 promotes generation of epidermal melanocytes in human scalp tissue.
(A) Schematic figure of human scalp explant culture experiment. (B-F) Immunohistochemical analysis with K14 and MITF of sections of human scalp before ablation (B), after ablation of skin prior to culture (C), 8 days in culture in the absence (D) and presence Edn1 (E) and presence of both Edn1 and BQ788 (F). (G and H) Quantification of the number of MITF+ cells in human scalp before culture (G) and after 8 days in culture (H). White dashed line indicates the border between inter follicular epidermis and dermis. Red dashed line indicates the border between hair follicle epidermis and dermis. Arrowheads indicate MITF+ cells. Data are presented as the mean ± SD. *, p<0.05, **, p<0.005. Scale bar, 100μm.
To examine the effect of Edn1 on the generation of epidermal melanocytes from hair follicle McSCs/melanocytes, we removed the inter-follicular epidermis (IFE), including their resident epidermal melanocytes, above the hair follicle by mechanical dermabrasion and examine the number of epidermal melanocyte in IFE after re-epithelialization. Removal of the IFE and epidermal melanocytes was verified by the lack of MITF+ melanocytes as well as K14+ epidermal cells in the surface of the explant, while the hair follicle remained intact as seen by the K14+ cells in follicular epidermis (Fig. 4B, C, and G). We then cultured the remaining denuded skin with or without recombinant Edn1 protein and analyzed the number of epidermal melanocytes that repopulated the regenerated epidermis. Eight days after culture, we found that addition of Edn1 led to a significantly higher number of epidermal melanocytes in the re-epithelialized area, compared to skin cultured in the absence of Edn1 protein (Fig. 4D, E and H). Whereas, administration of BQ788, a potent and specific antagonist for EdnrB, resulted in a marked abrogation of this repopulation, as seen by a reduction of the number of epidermal melanocytes compared to untreated skin (Fig. 4E, F and H). These results suggest that activation of Edn/EdnrB signaling have the ability to promote repopulation of epidermal melanocytes in human skin tissue.
The function of Edn1 to enhance McSC proliferation, differentiation and migration depends upon the Wnt pathway
Given that overexpression of Edn1 in epithelial cells can promote McSC proliferation, differentiation and migration uniquely during anagen and not during telogen (Fig. 2, Fig. S2, and S5), we suspected that Edn1 likely synergizes with signals present specifically during anagen that are absent during telogen. One possible candidate is the Wnt signal, which is activated in McSCs at anagen onset (Rabbani et al., 2011). Wnt signal activation in McSCs is proposed to occur by Wnt ligands secreted by neighboring EpSCs in the shared niche (Myung et al., 2013; Rabbani et al., 2011). Upon binding of Wnt ligand to its receptor, ß-catenin translocates to the nucleus to form a transcriptional complex with TCF/LEF transcription factors to induce expression of downstream genes in McSCs include Tyrosinase and MITF that are vital for production of pigment (Barker, 2008; Takeda et al., 2000).
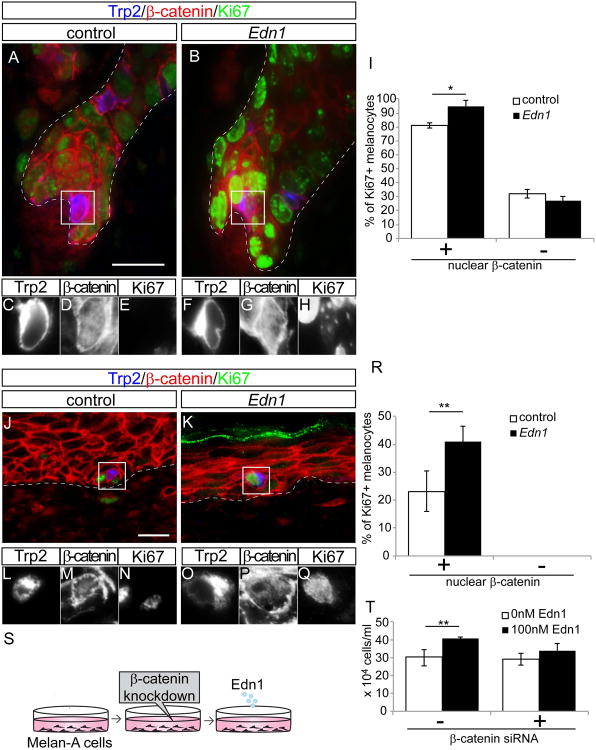
We first asked if Edn1 specifically enhances the proliferation of Wnt active McSCs during anagen. Triple immunofluorescence staining on skin sections from Edn1 Tg mice, with ß-catenin, Trp2 and Ki67 revealed that McSCs with nuclear ß-catenin signal, an indicator for Wnt activation, showed higher proliferation activity compared to control at anagen onset (Fig. 5A-I). In contrast, no difference in proliferation was observed in McSCs that lack nuclear ß-catenin between Tg and control mice (Fig. 5I). Similarly, we also found that following injury in Tg mice, actively proliferating melanocytes expressed nuclear ß-catenin, while cells that lacked Wnt activation displayed no proliferation (Fig. 5J-R). Furthermore, in vitro cell proliferation assay using Melan-A murine melanocyte cell line, known to have active Wnt signaling (Bennett et al., 1987; Gallagher et al., 2013), confirmed that the addition of exogenous Edn1 can increase proliferation of Melan-A cells compared to non-treated cells. Strikingly, this promoting effect was neutralized by knockdown of ß-catenin with siRNA (Fig. 5S and T). These results suggest that Edn1 promotes the proliferation of McSCs only in the presence of active Wnt signaling.
Figure 5. Edn1 synergizes with Wnt pathway to enhance proliferation and migration of McSCs.
(A-H) Immunostaining of skin sections from control (A, C-E) and K14-rtTA; TetO-Edn1-LacZ (Edn1; B, F-H) mice for Trp2, β-catenin and Ki67 in early anagen hair follicle. Higher magnification of single immunofluorescent staining of boxed area in (A) and (B) are shown in (C-E) and (F-H), respectively. (I) Quantification of the percentage of Ki67+ melanocytes. (J-Q) Immunostaining of skin sections from wounded area of control (J, L-N) and Edn1mice (K, O-Q) for Trp2, β-catenin and Ki67. Higher magnification of single immunofluorescent staining of boxed are shown in (L-N, O-K). (R) Quantification of Quantification of the percentage of Ki67+ melanocytes. (S) Experimental design of in vitro cell proliferation assay using Melan-A mouse melanocyte line with knockdown of ß-catenin prior to addition of exogenous Edn1 ligand. (T) Quantification of number of Melan-A cells at 3 days in culture. Data are presented as average number of cells + SD. Dashed lines indicate border between epidermis and dermis. Data are presented as the mean ± SD. *, p<0.002; **<0.05, Scale bar, 20μm.
To examine whether Wnt activation is required for Edn1 mediated McSC proliferation in vivo, we depleted ß-catenin in McSCs during anagen in the melanocyte lineage of Edn1 Tg mice (Tyr-CreER;β-cateninfl/fl;K14-rtTA;TetO-Edn1-LacZ) (Fig. 6A)(Bosenberg et al., 2006; Brault et al., 2001; Rabbani et al., 2011). Despite the vital function of Edn1 in promoting proliferation and differentiation of McSCs, these mice with deficient Wnt activation produced gray hair by 2nd telogen phase (Fig. 6B-E), similar to β-catenin cKO mice (Rabbani et al., 2011). Upon examination of tissue sections from these mice, we found that McSCs failed to proliferate and differentiate as seen by the absence of pigment, Ki67 and Tyr expression in McSCs at anagen onset (Fig. 6I-K and R). This is in contrast to the control littermates with Edn1 overexpression that increased McSC proliferation and ectopic pigmentation (Fig. 6F-H and R). Furthermore, we found that Wnt activation was required for the promoting effect of Edn1 on melanocyte migration during wound healing. Loss of Wnt activation in wounded Edn1 Tg mice led to a significant reduction in the number of epidermal melanocytes compared to Edn1 Tg mice with intact Wnt signaling (Fig. 6L-Q and S). These results suggest that Edn1 can only exert its effect on melanocytes with active Wnt signaling, and loss of this pathway suppresses the effects of Edn1 signaling during wound healing as well as normal homeostasis.
Figure 6. Loss of β-cat function suppresses Edn1 mediated effects on McSC proliferation, differentiation and upward migration.
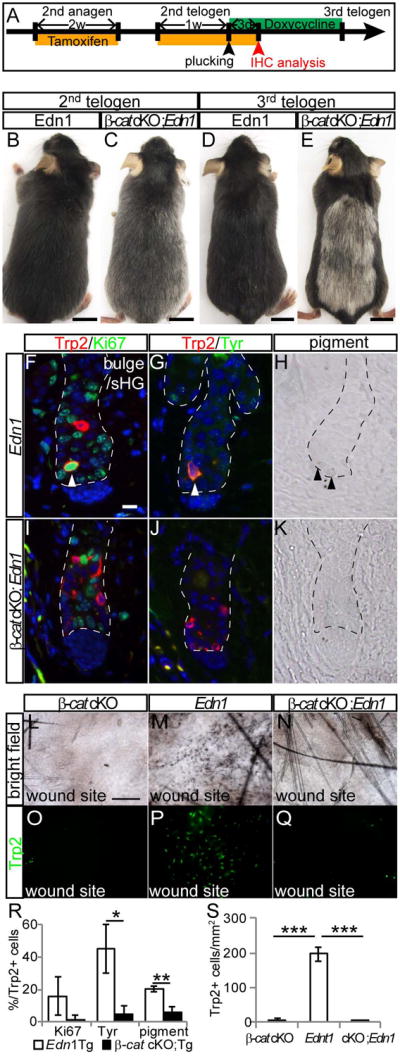
(A) Experimental scheme for treatment of Tyr-CreER; β-cateninfl/fl; K14-rtTA; TetO-Edn1-LacZ (β-cat cKO;Edn1) mice and control K14-rtTA; TetO-Edn1-LacZ (Edn1) mice. (B-E) Gross appearance of Edn1 (B and D) and β-cat cKO; Edn1 mice (C and E) at 2nd (B and C) and 3rd telogen (D and E). (F-K) Immunohistochemistry for indicated markers (F, G, I, and J) and bright field image (H and K) of bulge/sHG region in skin sections from Edn1 mice (F-H) and β-cat cKO; Edn1 mice (I-K) at anagen II. (L-Q) Brightfield image (L-N) and Trp2 immunostaining of whole mount wound site (O-Q) from Tyr-CreER; β-cateninfl/fl (β-cat cKO, L and O), Edn1 (M and P), and β-cat cKO;Edn1 mice (N and Q). (R and S) Quantification of the percentage of Trp2+ cells positive for Ki67, Tyr, and pigmentation (R), and the number of Trp2+ cells in wounded site (S). Dashed lines indicate border between hair follicle and dermis. Arrowheads indicate double positive cells for indicated markers (F and G) and pigmented cells (H). Data are presented as the mean ± SD. *, p<0.05, **, p<0.02, ***, p<0.001. Scale bar, 1cm in (B-E); 10μm in (F); 200μm in (L). See also Figure S7.
Edn1 overexpression can compensate for the loss of epidermal melanocytes in Mc1R KO mice
We previously found that mutant mice for Melanocortin 1 receptor (Mc1R) signaling (Mc1Re/e mice) were deficient in the generation of epidermal melanocyte after wounding (Chou et al., 2013; Robbins et al., 1993). Mc1R is a seven-transmembrane G-protein-coupled receptor whose activation leads to phosphorylation of CREB, which in turn transcriptionally activates various genes, including MITF, similar to Edn signaling (Mountjoy et al., 1992; Pierrat et al., 2012). Therefore, we asked whether Edn1 Tg mice could rescue the regenerative defects seen in Mc1R deficient mice.
To address this, we first asked whether Mc1R in melanocyte lineage is required for the generation of epidermal melanocytes. To this end, we created Mc1R floxed/floxed mice in which exon 1 including ATG translational start codon were flanked by loxP sites, and crossed them to Tyr-CreER mice (Bosenberg et al., 2006)(Fig. S7). Resultant mice (Tyr-CreER;Mc1R fl/fl) specifically deplete Mc1R in melanocytes upon TAM induction. These mice were born with pigmented hair color similar to control mice (data not shown). Following TAM treatment, the ablation of Mc1R in the melanocyte lineage led to a yellow color, similar to the effects of losing Mc1R function in all cell types (Mc1Re/e and CMV-Cre:Mc1R fl/fl mice) (Fig. S7) (Chou et al., 2013; Schwenk et al., 1995). As an additional control we examined K15-crePR1:Mc1R fl/fl mice in which loss of Mc1R is induced in epithelial niche cells for McSCs (Morris et al., 2004), and found that these mice remained similar to control mice (Fig. S7). These observations show that the Mc1R pathway within melanocytes controls the color of the hair follicle.
To assess the role of Mc1R in the establishment of epidermal melanocytes, we incorporated the Dct-lacZ reporter into our mutant mice to visualize epidermal melanocytes following wounding. We found that the universal depletion or melanocyte specific loss of Mc1R inhibited the generation of epidermal melanocytes in the wound area as revealed by the significant reduction of epidermal melanocytes compared to control mice (Fig. S7). In contrast, we found that loss of Mc1R in the K15+ epithelial niche had no effect on this process and appeared similar to control mice (Fig. S7). These results suggest that Mc1R signaling in the McSC lineage is required for the generation of epidermal melanocytes.
To determine whether elevated levels of Edn1 can compensate for the loss of Mc1R function in melanocytes, we generated a combined mouse model (Tyr-CreER;Mc1Rfl/fl;K14-rtTA;tetO-Edn1-LacZ) in which we overexpressed Edn1 in the epithelium and simultaneously induced the loss of Mc1R in the melanocyte lineage (Fig. 7A). Strikingly, we found that Edn1 overexpression can rescue the Mc1R cKO phenotype, leading to the appearance of epidermal melanocytes in the wound site (Fig. 7B-F). These epidermal melanocytes contained dark pigment similar to those found in Edn1 Tg mice (Figs. 3L and 7D). These results suggest that activation of Edn signaling can overcome the effects of losing Mc1R function on upward migration of McSCs following wounding.
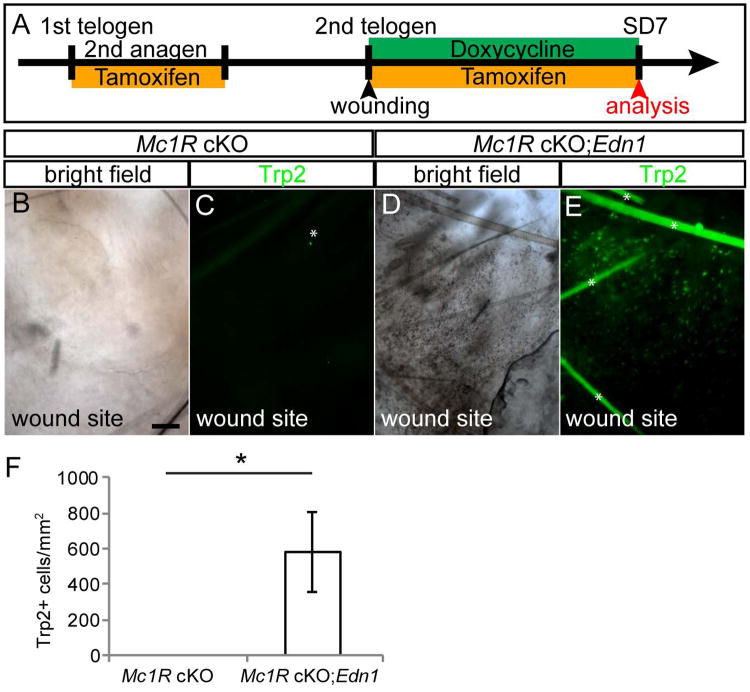
Figure 7. Edn1 overexpression can overcome the deficiency of Mc1R in the generation of epidermal melanocytes.
(A) Experimental scheme. (B-E) Brightfield image (B and D) and Trp2 immunostaining of whole mount wound site (C and E) from Tyr-CreER; Mc1Rfl/fl (Mc1R cKO, B and C) and Tyr-CreER; Mc1Rfl/fl; K14-rtTA; TetO-Edn1-LacZ mice (Mc1R cKO; Edn1 Tg, D and E). (F) Quantification of Trp2+ epidermal melanocytes in wound site. Asterisks indicate auto florescence. Data are presented as the mean ± SD. *, p<0.05. Scale bar, 100μm. See also Figure S8.
Discussion
Organ homeostasis and regeneration relies on tissue stem cells and deregulation of this critical population can lead to the disease onset and premature aging of the tissue. Hence, intense investigation is underway to elucidate the molecular mechanisms that govern the behavior of stem cells. Thus far, insight has been gathered by identifying key regulators of stem cells, including Wnt signaling, however it has become increasingly apparent that stem cells are regulated by complex interactions between multiple signaling pathways, and how they integrate these signals are unknown in the McSC system. Our study shows that Edn1 can overcome the defects seen in Mc1R deficient mice. This may be reasonable given that the Mc1R pathway primarily influences melanocytes via up-regulation of MITF, whose transcription is also up-regulated in parallel by the EdnrB pathway (Levy et al., 2006; Mountjoy et al., 1992; Nakajima et al., 2011; Pierrat et al., 2012; Sato-Jin et al., 2008). On the other hand, Edn1 overexpression did not rescue the phenotype caused by the deficiency of Wnt/β-catenin signaling, known to regulate the transcription of several other genes in addition to MITF that are essential for pigmentation, including Dct and Tyr (Hsiao and Fisher, 2014; Slominski et al., 2005). In addition, numerous other regulators of proliferation including cyclin D1 act downstream of the Wnt pathway (Shtutman et al., 1999). Although precise mechanisms by which EdnrB signaling requires Wnt signaling in the hair follicle niche remains to be understood, our results highlight that Wnt signaling is fundamental to the regeneration of melanocytes, which will need to be taken into consideration in developing strategies to manipulate McSCs for the treatments of pigmentation disorders (Fig. S8).
Edn/Ednrb signaling promotes McSC activation and maintenance in adult hair cycle
Our study revealed that EdnrB KO mice showed a compromised proliferation, differentiation and maintenance of the McSC lineage in the regenerating hair follicle (Fig. 1). The defect was clearly manifested by grossly recognizable unpigmented hair formation in all mice we analyzed. However, it is noteworthy that these mice never displayed unpigmented hair in all hair follicles in the back skin, which may be largely due to the incomplete cre-mediated recombination in melanocytes as shown in other studies with Tyr-creER mice (Bosenberg et al., 2006).
While forced activation of Edn signaling by expressing Edn1 in K14+ epithelial cells promotes the proliferation and ectopic differentiation of McSCs during anagen, resulting in pigmentation in the typically unpigmented bulge/sHG (Fig. 2). This result is consistent with previous reports using another mouse model to ablate NFIB in EpSCs that results in the upregulation of Edn2 and ectopic pigmentation in the bulge/sHG (Chang et al., 2013). Nevertheless, in all these cases, despite the precocious activation of McSCs that results in a pigmented bulge/sHG, a hair graying phenotype was not seen (Fig. S2). These observations are counterintuitive given previous studies that suggest that aberrant activation and differentiation of McSCs leads to their progressive loss and ultimate exhaustion (Inomata et al., 2009; Nishimura et al., 2005; Rabbani et al., 2011; Tanimura et al., 2011)
These observations suggest that protective mechanisms are in place to prevent McSC exhaustion. The limited availability of active Wnt signal, known to be critical for McSC differentiation during the hair cycle may be one such mechanism. Indeed, We observed a relatively modest and temporarily limited impact of Edn1 overexpression on McSCs during hair regeneration without injury. This may be related to the fact that Wnt signal activation in McSCs only occurs at the anagen onset and ceases quickly thereafter (Rabbani et al., 2011), unlike in the wound environment (Yamada et al., 2013). Wnt is tightly regulated temporally, activated at anagen onset, when McSCs are initially activated and then down-regulated upon entry into mid anagen and suppressed at telogen (Rabbani et al., 2011). Given that the effects of Edn1 overexpression was masked during telogen, in the absence of Wnt activation, while inhibition or the normal downregulation of Wnt activation in McSCs disabled the effect of Edn1 over-expression, both in vitro and in vivo, suggesting that Edn signaling requires Wnt activation (Fig. 5 and 6). These findings raise the possibility that McSCs in Edn1 Tg bulge/sHG partially but do not fully differentiate, because Wnt activation is not sustained throughout the hair cycle, thus there is no progressive loss of McSCs and hair graying.
Taken together, our study demonstrates that EdnrB signaling functions in the presence of Wnt signaling on the activation and maintenance of McSCs during adult hair cycle.
Edn1 signaling promotes generation of epidermal melanocytes and can overcome the defect of Mc1R
The ability of melanocytes to produce melanin is essential to provide the skin with a protective barrier against ultraviolet irradiation. Following injury, the resulting scar that forms usually displays pigmentation defects, of either hypo or hyperpigmentation in scar area (Engrav et al., 2007), thus increasing the detrimental risks associated with exposure to the sun in addition to the cosmetic or psychological challenges to the patient. Understanding the molecular mechanisms that govern this process is the key to developing therapies to treat these patients. In this effort, it is critical to understand how certain signaling pathway can modulate the impact of other signaling cascades. By generating tissue-specific knockout mice, our study demonstrates that Mc1R function is required specifically within melanocytes for their regenerative function following wounding and Edn1/EdnrB signaling can compensate this defect (Fig. 7).
Mc1R is major determinant of pigmentation of skin and hair color in Humans and polymorphisms within Mc1R are prevalent among European populations with over 100 different reported variants (Garcia-Borron et al., 2005; Gerstenblith et al., 2007; Perez Oliva et al., 2009). A subset of the Mc1R variants causes dysfunction of Mc1R signaling, resulting in reduced synthesis of eumelanin (brown pigment) and thus increase the proportion of phaeomelanin (red/yellow pigment). In addition, Mc1R promotes migration of melanocytes from the hair follicle to the epidermis during wound healing, providing a protective pigmented barrier for the skin (Chou et al., 2013). Inactivating mutations of Mc1R are associated with increased risk of both melanoma and non-melanoma skin cancer (Flanagan et al., 2000; Mitra et al., 2012). We showed that Edn1 has the potential to increase the melanocyte number and their brown pigment production even with dysfunctional Mc1R signaling. Our study suggests that Edn/EdnrB signaling can be a therapeutic target to reduce the risk of such diseases via its ability to enhance melanocyte regeneration. Furthermore, Edn1 is naturally up-regulated by UVB exposure in human skin (Imokawa et al., 1995). This suggests that Edn1 upregulation may underlie the increase of freckles by sun light exposure (Baron et al., 2014), which is frequently observed in humans with Mc1R variants (Bastiaens et al., 2001), further suggesting a possible connection between the two signaling pathways in humans (Barsh, 1996).
Collectively, our study reveals the critical function of EdnrB/Edn1 in McSCs to promote the generation of epidermal melanocytes and pigmentation for both normal skin and the skin with Mc1R-deficient melanocytes. Taken together with the clinical observation that follicular McSCs can be recruited to the epidermis to repigment the skin of patients with pigmentation disorders (Nishimura, 2011), our studies reveals the potential of targeting Edn signaling for therapeutic treatments.
Experimental Procedures
For more details regarding the materials and methods used in this work, see the Supplemental Experimental Procedures.
Mice
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at NYU School of Medicine. Cre recombination in conditional knockout mice was induced either by tamoxifen injection or RU488 treatment as described in previous publication (Ito et al., 2007). For BrdU administration, mice were injected daily intraperitoneally with 50 μg of BrdU per g body weight.
Immunohistochemistry
Immunohistochemistry on paraffin section was performed as published (Rabbani et al., 2011). The antibodies that used for this study are listed in Supplemental Experimental Procedures.
Whole mount X-gal staining
Whole mount X-gal staining was performed as published (Chou et al., 2013; Ito et al., 2007).
Wound experiment
Wound experiments were performed as described with minor modification (Chou et al., 2013). Briefly, 7-8 week old mice were anesthetized with isoflurane and a 1.5 cm2 area of skin was excised.
Human scalp organ culture
Human scalp culture was performed as published (Chou et al., 2013).
Melan-A cell culture and scratch assay
Melan-a cells (Bennett et al., 1987) were cultured in mouse melanocyte medium composed of DMEM:RPMI=1:4 with 10 % FBS, 1% sodium pyruvate, 1% non-essential amino acids, 0.5% TPA. For cell migration assay, cells were treated with 50nM siRNA against for EdnrB (Invitrogen, MSS203783) or control siRNA, using Lipofectamine RNAiMAX (Invitrogen) and mitomycin C at 24 hr and 48 hr after plating. Scratch wound was created using P-200 pipet tip, then culture medium was replaced with DMEM:RPMI=1:4 containing 0.5% FBS, 1% sodium pyruvate, 1% non-essential amino acids, 0.5% TPA with or without 10nM Edn1. Wound area was analyzed at 6hr after wounding.
Statistical analysis
Student's t-test was used to calculate p-values on Microsoft Excel, with two-tailed tests and unequal variance.
Supplementary Material
Acknowledgments
We thank Miles Epstein at University Of Wisconsin for generously providing EdnrB flex3/flex3; R26-stop-Tomato mice. The work is supported by NIH/NIAMS grant 1R01AR059768-01A1, and Beckman foundation (to M. I). M.I is grateful for the supports from NIH/NIAMS grant 1R01AR059768-01A1, 1R01AR066022-01A1, and the Arnold and Mabel Beckman Foundation. W.L and Q.S are supported by NYSTEM institutional training grant (Contract #C026880).
Footnotes
Author contributions: M.T., W. L. and P. L. performed experiments, interpreted data and wrote the manuscript. H.H., Q. S. and C. L. performed experiments and interpreted data. P. M. guided and interpreted data. M.I. guided experiments, interpreted data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attie T, Till M, Pelet A, Amiel J, Edery P, Boutrand L, Munnich A, Lyonnet S. Mutation of the endothelin-receptor B gene in Waardenburg-Hirschsprung disease. Hum Mol Genet. 1995;4:2407–2409. doi: 10.1093/hmg/4.12.2407. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Baron AE, Asdigian NL, Gonzalez V, Aalborg J, Terzian T, Stiegmann RA, Torchia EC, Berwick M, Dellavalle RP, Morelli JG, et al. Interactions between ultraviolet light and MC1R and OCA2 variants are determinants of childhood nevus and freckle phenotypes. Cancer Epidemiol Biomarkers Prev. 2014;23:2829–2839. doi: 10.1158/1055-9965.EPI-14-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh GS. The genetics of pigmentation: from fancy genes to complex traits. Trends Genet. 1996;12:299–305. doi: 10.1016/0168-9525(96)10031-7. [DOI] [PubMed] [Google Scholar]
- Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, Bouwes Bavinck JN. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701–1708. doi: 10.1093/hmg/10.16.1701. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, Nogueira C, Horner JW, 2nd, Depinho R, Chin L. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44:262–267. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19:924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Powers PA, Bartley CR, Walker JW, Epstein ML. Targeting of endothelin receptor-B to the neural crest. Genesis. 2008;46:396–400. doi: 10.1002/dvg.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry FW. The coat of the mouse (Mus musculus) Journal of Genetics. 1926;16:287–340. [Google Scholar]
- Edery P, Attie T, Amiel J, Pelet A, Eng C, Hofstra RM, Martelli H, Bidaud C, Munnich A, Lyonnet S. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome) Nat Genet. 1996;12:442–444. doi: 10.1038/ng0496-442. [DOI] [PubMed] [Google Scholar]
- Engrav LH, Garner WL, Tredget EE. Hypertrophic scar, wound contraction and hyper-hypopigmentation. J Burn Care Res. 2007;28:593–597. doi: 10.1097/BCR.0B013E318093E482. [DOI] [PubMed] [Google Scholar]
- Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet. 2000;9:2531–2537. doi: 10.1093/hmg/9.17.2531. [DOI] [PubMed] [Google Scholar]
- Gallagher SJ, Rambow F, Kumasaka M, Champeval D, Bellacosa A, Delmas V, Larue L. Beta-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene. 2013;32:2230–2238. doi: 10.1038/onc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- Giller T, Breu V, Valdenaire O, Clozel M. Absence of ET(B)-mediated contraction in Piebald-lethal mice. Life Sci. 1997;61:255–263. doi: 10.1016/s0024-3205(97)00381-0. [DOI] [PubMed] [Google Scholar]
- Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014;563:28–34. doi: 10.1016/j.abb.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Matsushima Y, Shinkai Y, Kobayashi Y, Sakamoto M, Kunieda T, Tachibana M. A mouse model of Waardenburg syndrome type 4 with a new spontaneous mutation of the endothelin-B receptor gene. Mamm Genome. 2002;13:30–35. doi: 10.1007/s00335-001-3038-2. [DOI] [PubMed] [Google Scholar]
- Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G. An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Phytother Res. 2011;25:1398–1411. doi: 10.1002/ptr.3552. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Oliva AB, Fernendez LP, Detorre C, Herraiz C, Martinez-Escribano JA, Benitez J, Lozano Teruel JA, Garcia-Borron JC, Jimenez-Cervantes C, Ribas G. Identification and functional analysis of novel variants of the human melanocortin 1 receptor found in melanoma patients. Hum Mutat. 2009;30:811–822. doi: 10.1002/humu.20971. [DOI] [PubMed] [Google Scholar]
- Pierrat MJ, Marsaud V, Mauviel A, Javelaud D. Expression of microphthalmia-associated transcription factor (MITF), which is critical for melanoma progression, is inhibited by both transcription factor GLI2 and transforming growth factor-beta. J Biol Chem. 2012;287:17996–18004. doi: 10.1074/jbc.M112.358341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Saldana-Caboverde A, Kos L. Roles of endothelin signaling in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010;23:160–170. doi: 10.1111/j.1755-148X.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A, Du J, Wu M, Hanada K, Sawamura D, et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155–1168. doi: 10.1096/fj.07-9080com. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, Nakata S, Matsunaga K, Akamatsu H. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133:2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- Yang LL, Gros R, Kabir MG, Sadi A, Gotlieb AI, Husain M, Stewart DJ. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation. 2004;109:255–261. doi: 10.1161/01.CIR.0000105701.98663.D4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.