Abstract
Primary infection with varicella-zoster virus (VZV) results in varicella which, in populations where immunization is not used, occurs mostly in children. Varicella is a generalized rash illness with systemic features such as fever and malaise. During varicella, VZV becomes latent in sensory ganglia of the individual, and in 70% it remains asymptomatic for their lifetime. The remaining 30% develop reactivation from latency, resulting in herpes zoster (HZ). HZ usually occurs in persons over the age of 50, and is manifested by a painful unilateral rash that usually lasts about 2 weeks and then may be followed by a chronic pain syndrome called post-herpetic neuralgia (PHN). VZV infections are notoriously more severe in immunocompromised hosts than in healthy individuals. Despite gaps in our understanding of the details of immunity to VZV, successful vaccines have been developed against both varicella and zoster.
1 Varicella-Zoster Virus
VZV produces at least 12 glycoproteins (gps) during lytic infection when all of its 71 genes are expressed. These gps are present on the surface of the virion and also on the surfaces of infected cells. The gps are immunogenic and at least some are thought to play important roles in viral spread by facilitating cell adherence and possibly transfer of the virus from an infected cell to an uninfected one. The gps are designated by letters; the major one of VZV is gE. Other highly significant gps are gB, gH, and gI. In addition, a number of other internal viral antigens are expressed in lytic infection, some of which are also expressed in latent infection. It is not known which, if any or even all, of these proteins are responsible for stimulating immunity to VZV. Because of its abundance, gE is thought to be the major protective antigen (Arvin and Cohen 2007). Little is known about the roles of internal antigens in generating immunity, but ORF 4p and ORF 63p have recently been postulated to be important, based on immunologic responses demonstrated in experiments using tetramer technology to demonstrate cellular immunity (CMI) to the virus (Jones et al. 2006, 2007).
During lytic infection, VZV expresses all of its 71 genes, but during latent infection only six are known to be consistently expressed. Viral latency of VZV is quite distinctive from that of its close relative herpes simplex virus (HSV). In VZV latency, genes 4, 21, 29, 62, 63, and 66 are expressed (Gershon et al. 2008a). The proteins of these genes are segregated only in the cytoplasm of the cell and may be prevented from entering the cell nucleus by ORF61p (Hay and Ruyechan 1994). When this ORF is introduced into a cell harboring latent VZV infection, the virus reactivates and infection becomes lytic (Gershon et al. 2008a; Chen et al. 2003).
VZV remains an important pathogen; there is no useful animal model of VZV disease, and so it has been difficult to study protection that develops after vaccination in any degree of detail. Both varicella vaccine and zoster vaccine have, however, been demonstrated to have significant clinical effectiveness in large populations, and they have been used on a worldwide basis in over 50 million people (Gershon et al. 2008b; Levin 2008).
Exactly how adaptive immunity to VZV is mediated after varicella, during latency, and before and after zoster is not entirely understood. Clearly participation of humoral and CMI is important, and there may be some redundancy to assure a high degree of protection of the host. Although it is well known that patients with agammaglobulinemia are not at particular risk to develop varicella more than once, specific antibodies are thought to play a major role in protection against developing varicella. There is a very high correlation, for example, between demonstration of VZV antibodies in serum using a sensitive and specific assay and failure to develop clinical varicella after an intimate exposure to the virus (Michalik et al. 2008). It is possible that when cell-free VZV spread by the airborne route attempts to gain a foothold in an already experienced host, it is promptly eliminated from spread within the host by specific antibodies in the blood. While it is possible that VZV antibodies are surrogate markers of immunity, elderly individuals who have no demonstrable CMI to VZV but usually have high antibody titers are not at risk to develop varicella (although they are at risk to develop zoster). After a varicella-susceptible individual is exposed to VZV, infection can be prevented or modified by prompt passive immunization with preformed VZV antibodies such as varicella-zoster immune globulin (VZIG) (Gershon et al. 2008b; Ampofo et al. 2002). Thus the role of antibodies in prevention of varicella is not exactly straightforward, and so it seems most likely that there is redundancy between humoral and CMI to best protect the host.
It is generally agreed, however, that CMI responses are critical to recovery from VZV infections. The most severe (and even fatal) cases of varicella are seen in patients with absent or poor CMI to VZV, and administration of antibodies to combat active infections are ineffective (Gershon et al. 2008b; Ampofo et al. 2002). At this point, VZV is spreading within the host from one cell to another with little or no spread as cell-free virions (Gershon et al. 2008a). Zoster develops in patients with low CMI to VZV, despite high antibody titers, and protection is correlated with high levels of CMI following vaccination (Oxman et al. 2005). Finally, while asymptomatic infection with VZV is thought to be unusual (about 5%), it is not uncommon for persons with prior varicella who are exposed to VZV to develop a boost in immunity without symptoms (Gershon et al. 2008b; Ampofo et al. 2002). Data are also beginning to accumulate, which suggest that subclinical reactivation of latent VZV can also occur (Cinque et al. 1997; Mehta et al. 2004; Wilson et al. 1992).
2 Varicella Vaccine
The live attenuated Oka vaccine strain of VZV was first developed about 40 years ago by Takahashi et al. (1974) Originally, the vaccine was projected to be useful to prevent varicella (“chickenpox”), which is the primary infection with VZV. It was soon shown that immunocompromised vaccinated patients were not only protected against varicella (Gershon et al. 1984) but they were also less likely to develop zoster after natural chickenpox (Hardy et al. 1991). Roughly 15 years later it was realized that the Oka strain in a larger dose might be employed as a therapeutic vaccine to prevent HZ (Oxman et al. 2005).
At present, two live attenuated VZV Oka vaccines are licensed in the United States, both manufactured by Merck and Co., one against varicella (1,350 plaque forming units [pfu] per dose) and one against zoster (20,000 pfu per dose). The zoster vaccine is about 15 times as strong as varicella vaccine. The wide difference in dosage is of great interest because the low dose varicella vaccine is effective in preventing chickenpox in VZV naïve children. The much larger dosage of virus in zoster vaccine for adults (over age 60 years) who have already had varicella and are at high risk to develop zoster is required to achieve a significant degree of vaccine efficacy (Levin 2001). It is postulated that, in elderly individuals, immune senescence is not only responsible for the increased risk of zoster but also for the relatively refractory response to the vaccine, therefore requiring a much higher dose than that administered to children to prevent the primary infection with VZV (varicella).
Since its licensure in the US in 1995, the incidence of varicella has fallen by > 80% in both vaccine recipients and also in the unvaccinated, indicating herd immunity (Seward et al. 2002). Varicella-associated hospitalizations, moreover, have decreased by 88% (Zhou et al. 2005), and age-adjusted mortality from varicella has decreased by 66% (Nguyen et al. 2005) (See Fig. 1).
Fig. 1.
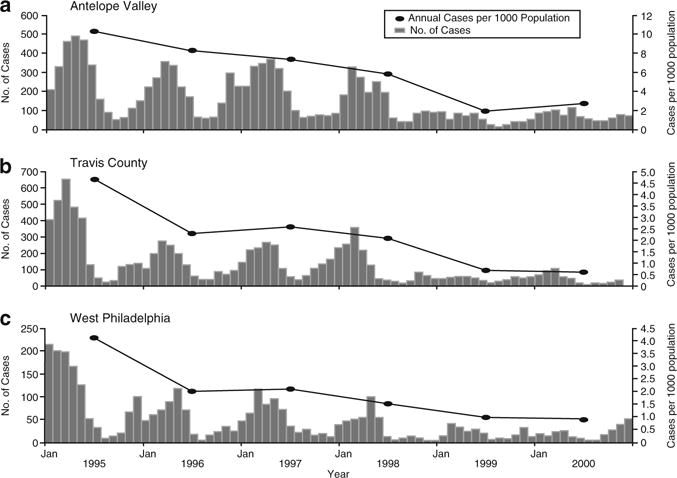
Incidence of varicella in three sentinel counties in the United States from 1995, when varicella vaccine was licensed, until 2000, indicating decrease in the incidence of varicella (Gershon et al. 2008b)
When initial clinical trials with varicella vaccine were first carried out, there was considerable controversy surrounding the vaccine. It was hypothesized that immunity from it might not be durable and that there might be severe adverse events resulting from its use, ranging from neurological problems to cancer. The first major trials were in immunocompromised children, because in the late 1970s as many as 80% of children with leukemia were being cured of this disease only to be at high risk to die of varicella during their anti-cancer chemotherapy (Feldman et al. 1975; Feldman and Lott 1987). Therefore, children with leukemia in remission were thought to have an appropriate risk-benefit ratio for clinical trials with this new vaccine, and they became the major participants in trials of the varicella vaccine in Japan and in the United States (Gershon et al. 2008b; Takahashi et al. 1975). The availability of antiviral therapy with acyclovir (ACV) beginning in the mid-1970s made it possible to conduct these trials. In a large collaborative study conducted in the United States between 1979 and 1990, over 550 children with leukemia that was in remission for at least 1 year were immunized with the Oka vaccine. Most of these children had received maintenance anti-leukemic therapy for at least 1 year; this was withheld for 1 week before and 1–2 weeks after immunization. About 25% of the vaccinees developed a rash due to Oka VZV with more than 50 skin lesions, and they were given ACV to prevent further multiplication of the vaccine virus. The efficacy of the vaccine in preventing chickenpox after the usual two dose regimen for these children was demonstrated to be 85% (Gershon et al. 1984, 1996). Months to years later, some 15% of vaccinees developed what was termed “breakthrough” chickenpox after an exposure to wild-type (WT) VZV; however, the cases were uniformly mild even in leukemic children and did not require antiviral therapy. Many of these vaccinees remain alive and well today almost 30 years later, and are not known to have developed any of the feared theoretical complications that were originally projected regarding the live varicella vaccine.
When varicella vaccine was licensed in 1995, only one dose was recommended for children aged 1–12 years, and it was expected that over 90% would be fully immune to varicella. Two doses were to be given to adolescents and adults, because one dose was not clinically as effective in adults as in children (Gershon et al. 2008b). As will be discussed further, in 2006, hoping to improve vaccine efficacy, a second dose of varicella vaccine was recommended by the Centers for Disease Control and Prevention (CDC) for all vaccinated children and adults (Centers-for-Disease-Control 2007a).
3 Vaccine Safety
Varicella vaccine has proven to be remarkably safe. In the United States, about four million children have been vaccinated annually over roughly the past 10 years. As will be detailed, very few serious complications of vaccination have been reported. As with all vaccines, some reported problems may not have been due to varicella vaccine but were coincidental temporally related occurrences rather than cause and effect. Those that have occurred and were clearly due to varicella vaccine are probably less frequent than complications that are seen in individuals who have experienced natural varicella. Routine universal immunization of infants is now administered in Canada, Uruguay, Sicily, Germany, South Korea, Qatar, Taiwan, Germany, Israel, and Australia (Gershon et al. 2008b). The vaccine has also been found to be safe and effective in children with underlying HIV infection, as long as they are relatively immunologically normal when immunized (Gershon et al. 2009a; Levin et al. 2001, 2006; Son 2008). As in other studies in immunocompromised children, not only is varicella significantly prevented in HIV-infected children, but also is zoster (Hardy et al. 1991; Son 2008). Not surprisingly, data now also indicate that zoster is decreased in healthy recipients of varicella vaccine as well as in immunocompromised vaccinees. The risk of developing zoster in healthy vaccinated children less than 10 years old was found to be decreased by a factor between 4 and 12 times, compared with similar children who had experienced natural infection (Civen et al. 2009). In another study, the incidence rate of zoster in 172, 163 healthy children after varicella vaccine was demonstrated to be extremely low (Tseng et al. 2009).
The main adverse event reported in the US after immunization of healthy children is a mild rash due to the Oka vaccine in the first 6 weeks after immunization. Other problems are of somewhat greater concern but are far less frequent in occurrence. In reports to the vaccine adverse event reporting system (VAERS) in the US, from 1995 to 2005, the rate of serious adverse events (SAEs) after varicella vaccine was 2.6/100,000 distributed doses (Chaves et al. 2008). Additionally, generalized severe Oka infections in children who were found to be immunocom-promised either before (in which case the underlying immunodeficiency was not yet recognized) or just following vaccination have been reported in less than 10 vaccinated children, since 1995 (Chaves et al. 2008; Galea et al. 2008; Jean-Philippe et al. 2007; Levy et al. 2003; Levin et al. 2003). These children had underlying conditions such as HIV infection with almost no detectable CD4 T lymphocytes, congenital immunodeficiency diseases such as adenosine deaminase deficiency and natural killer (NK) cell deficiency, high dose steroid therapy, and anti-cancer chemotherapy. Most of these children were treated with antiviral therapy and recovered.
As has been mentioned, the incidence of zoster after varicella vaccine is very low in healthy children (Civen et al. 2009; Tseng et al. 2009). Two rare reported SAEs following vaccination were cases of zoster with meningitis caused by Oka VZV (Chaves et al. 2008). Two additional cases of meningitis due to Oka zoster have also been reported (Iyer et al. 2009; Levin et al. 2008). One of these patients was a 1-year-old boy vaccinated just prior to the diagnosis of neuroblastoma, who developed chronic zoster that became drug resistant after prolonged unsuccessful courses of antiviral therapy. His zoster remitted after he was no longer receiving anti-cancer therapy (Levin et al. 2003). The other occurred in an immunocompetent child; he recovered with antiviral therapy (Iyer et al. 2009). The occurrence of meningitis associated with zoster due to WT VZV is, in contrast, not at all uncommon (Amlie-Lefond and Jubelt 2009; Gilden et al. 2000). Zoster is thus unusual after vaccination against chickenpox, and based on what we know today, meningitis is likely to be less common from Oka zoster than from WT VZV zoster, although this is an area that deserves further scrutiny. No long-term sequelae from these rare cases have been reported.
Why zoster after varicella vaccination is less common than after natural infection is not fully understood; the Oka strain is capable of causing latent infection in an in vitro neuronal model (Chen et al. 2003). It may be that the burden of viral latency after vaccination is lower than it is with WT VZV or that the Oka strain is attenuated and therefore less likely to reactivate. It is possible to rapidly and accurately distinguish between WT VZV and the Oka strain by polymerase chain reaction (PCR) (LaRussa et al. 1992). Data from our group have indicated that Oka VZV can be demonstrated in the dorsal root ganglia (DRG), cranial nerve ganglia (CNG), and enteric ganglia (EG) of vaccinated children who have died suddenly and have therefore come to autopsy (Gershon et al. 2009b). We hypothesize that vaccinated children have a low incidence of zoster due to lower viral loads from latent Oka VZV than latent WT virus after natural varicella, secondary to the lower rate of skin involvement after vaccination compared to that following WT varicella.
Transmission of Oka VZV to others has been extremely rare and is estimated to occur in 1 recipient per 10 million doses of vaccine (Gershon et al. 2008b; Galea et al. 2008). Transmission to others has occurred only when an individual develops skin lesions due to the Oka strain (Gershon et al. 2008b; Tsolia et al. 1990; Brunell 2000).
A combination of varicella vaccine with other viral vaccines specifically as measles–mumps–rubella–varicella vaccine (MMRV) would be extremely useful clinically. In 2005, this combination vaccine was licensed in the United States, but it was soon noted that it produced a doubling of the rate of febrile seizures as a complication of vaccination (Centers-for-Disease-Control 2008; Jacobsen et al. 2009). Between days 7 and 14 after MMR vaccine, the rate of febrile seizures was 4/10,000, while after MMRV it was 9/10,000 (p = <0.001) At present, after much deliberation, the CDC recommended that a choice of either MMR plus monovalent varicella vaccine in a different body site or MMRV should be offered to parents for use in their 1-year-old infants who are receiving their first immunization against these viruses. Parents are to be carefully counseled about the rare possibility of their infant developing a febrile seizure 1–2 weeks after the vaccination with MMRV for the first immunizing dose. There is also a precaution based on the increased risk of febrile seizures in children with an immediate family history of epilepsy, or febrile seizures, or past personal history of seizures. In that case, for the first dose, MMR and varicella vaccines should be administered, not MMRV. For the second dose, MMRV is preferred because the complication of febrile seizures is rarely seen after a second dose of MMRV, one reason being that children receiving the second dose of MMRV are usually older and beyond the age when febrile seizures are most common (Centers-for-Disease-Control 2008; Jacobsen et al. 2009).
4 Effectiveness of Varicella Vaccine
Case–control studies of the effectiveness of the licensed varicella vaccine in healthy children indicate that there is about 85% protection against developing any form of chickenpox following one dose of vaccine (Vazquez et al. 2001, 2004). It was originally anticipated that protection would be higher, approaching 95%, based on serologic data from studies in which a glycoprotein ELISA assay was used as a surrogate to evaluate immunity to varicella (Provost et al. 1989, 1991). Soon after vaccine licensure in the US, however, a great number of outbreaks in day care facilities and schools where most children had been vaccinated began to be described (Gershon et al. 2008b). Infection rates from these outbreaks were used to try to calculate vaccine effectiveness, but there was a great deal of variability from study to study (44–100% effectiveness). Interestingly, however, the average effectiveness was about 85%.
Whether immunity wanes with time after vaccination (known as secondary vaccine failure) is controversial. One observational study purported to show waning of protection against varicella with time, but this was purely an epidemiologic study with no laboratory component regarding specific viral diagnosis of breakthrough varicella (Chaves et al. 2007). It is not always easy to recognize breakthrough varicella and therefore laboratory confirmation of illness is important. Moreover, the existence of primary vaccine failure, which could appear to be waning immunity, was not considered in the study (Gershon et al. 2007a). In the already mentioned case–control studies, the incidence of varicella in vaccinees did not increase in time, over a period of 2–8 years after vaccination; there was no indication of secondary vaccine failure (Vazquez et al. 2004) (See Fig. 2). In this study, laboratory confirmation of breakthrough varicella was obtained, mostly using PCR (Vazquez et al. 2001, 2004). Additional studies regarding whether immunity to varicella wanes significantly with time would be extremely useful for understanding how varicella vaccine behaves and planning whether and when booster doses of vaccine should be recommended. Based on what we now know, waning immunity may occur, but it does not seem to be a serious problem after varicella vaccination. Primary vaccine failure is more likely to be problematic (see below) but is being addressed by giving two doses of vaccine to all children.
Fig. 2.

Case–control study indicating the effectiveness of varicella vaccine over time (Vazquez et al. 2004)
Based on the clinical and laboratory observations mentioned in 2006, the CDC recommended that all children receive two doses of varicella vaccine rather than one dose as originally proposed (Centers-for-Disease-Control 2007b). Dramatic boosts in humoral and CMI to VZV have been observed following a second dose of vaccine, which suggests that two doses will provide increased protection against chickenpox (Centers-for-Disease-Control 2007b). Although it remains to be proven whether two doses will result in a further decline in incidence of varicella, it is projected to do so (Kuter et al. 2004). It was recently reported, however, that in one study, two doses did not decrease the attack rate of varicella in an outbreak situation in a school where most children had received two doses (Gould et al. 2009). Breakthrough varicella, which was usually very mild, was not proven by laboratory testing in these children; many may not have had varicella but rather were suffering from insect bites. Bites were apparently recognized to be common during the period when the outbreak was occurring. Data from our laboratory, a case–control study in which varicella was proven by PCR testing, (Vazquez et al.; presented at the Annual Conference of the Infectious Diseases of America in October 2009) indicates that two doses provides a high degree of protection against chickenpox. In 62 cases of varicella, 62 (100%) had one dose of vaccine and 0 had 2 doses. In 133 controls without varicella, 115 (85.8%) had one dose and 18 (13.4%) had two doses. Vaccine effectiveness of two doses compared to unvaccinated children was 98% (p < 0.001). These studies are ongoing.
Additional research on the subject of protection against varicella after two doses of vaccine compared to one dose is extremely important. Currently, the CDC recommends that second doses of MMRV be administered between the ages of 4 and 6 years old. Because of vaccine failures from monovalent vaccine, however, it might be preferable to administer the second dose of MMRV at age 2 or 3 years of age. On the other hand, since the dose of VZV in MMRV is about 17,000 pfu, the rate of vaccine failure (if there is one) to the VZV component is unknown and may be lower than that identified with monovalent varicella vaccine. As an aside, it has been suggested that robust immune responses to varicella vaccine are genetically determined (Klein et al. 2007). Additional information on these issues would be extremely practical and useful to have in planning when to vaccinate children.
Varicella is one of the most contagious diseases known. After a household exposure to the virus, the attack rate of chickenpox in susceptibles has been reported to be as high as 90% (Ross et al. 1962). The best laboratory indication for immunity to varicella is the fluorescent antibody to membrane antigen (FAMA) assay (Fig. 3). Extensive clinical validation indicates that persons with positive FAMA titers have a protection rate against varicella of over 98% after a close exposure to VZV. Negative FAMA titers are highly correlated with developing varicella after exposure to the virus, particularly in the unvaccinated (Michalik et al. 2008). Our laboratory is one of very few laboratories worldwide that perform the FAMA test; it is considered the “gold standard” for measuring immunity to varicella (Gershon et al. 2007b; Williams et al. 1974). This assay is the best available immune correlate for determining whether an individual is or is not immune to varicella.
Fig. 3.
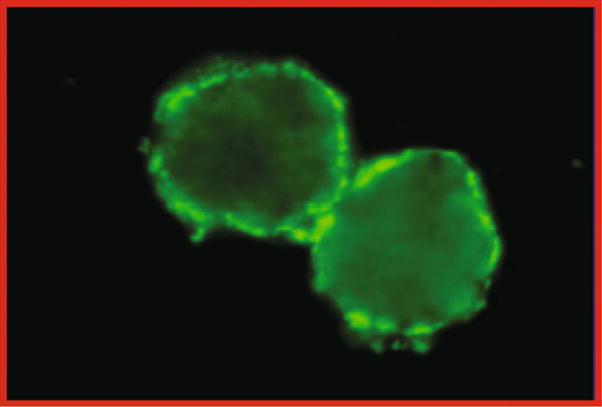
A FAMA assay, indicating the presence of antibodies to VZV in serum. Unfixed tissue culture cells are incubated first with diluted serum and subsequently with anti-human globulin labeled with fluorsecein isothyocyanate and viewed by fluorescence microscopy (Gershon et al. 2007b)
5 Vaccination Against Zoster
Zoster, which is due to reactivation of VZV that becomes latent in the nervous system during varicella, develops when immunity to VZV declines, usually as a result of aging or being immunocompromised (Hope-Simpson 1965). About 30 years ago, it was realized that individuals over 50 years of age are at increased risk to develop zoster and that, while they have detectable antibodies to VZV, they often have no demonstrable CMI to the virus (Berger et al. 1981; Burke et al. 1982). Interestingly, not all persons with low CMI to VZV develop zoster; most do not. Most individuals over the age of 50 no longer have demonstrable CMI to VZV (Berger et al. 1981; Burke et al. 1982). In the United States about one-third of individuals will go on to develop zoster during their lives; this amounts to about one million annual cases of zoster (Oxman et al. 2005). Despite the high dosage of VZV in the zoster vaccine, it is only about 50–60% effective in preventing zoster, and protection also declines with age. When lower vaccinating doses were tried, stimulation of VZV CMI was deemed too low to lead to durable protection against zoster (Levin 2001). Thus the dose of 20,000 pfu of live VZV was utilized, and it proved to be effective (roughly 50–60%) in a double blind placebo controlled study involving over 38,000 healthy adults over age 60 (Oxman and Levin 2008). Vaccinees were more likely to increase their CMI responses to VZV than placebo recipients (Oxman and Levin 2008). Despite the apparent correlation of zoster with VZV CMI, there are no specific immune markers that indicate protection against zoster as there are for varicella. Identification of a specific immune pattern indicating protection against zoster would be extremely useful for deciding whom to vaccinate at present and for testing of newly developed molecular vaccines of the future.
The zoster vaccine used in the United States has proven to be extremely safe, despite the large dose of virus. No common SAEs emerged from the double-blind controlled trial, nor have any been identified since the vaccine was licensed in 2005. Major questions regarding vaccination against zoster concern the best age at which to administer it and whether booster doses might improve the effectiveness as seen with varicella vaccine. Currently, one dose is recommended for individuals over 60 years of age who are relatively healthy. A small study in 1,122 subjects aged 50–59 at vaccination indicated that it was immunogenic and safe (Sutradhar et al. 2009). It might therefore make sense, for example, to vaccinate younger individuals who are projected to have solid organ transplants in the near future. In addition, inactivated forms of zoster vaccines have proven to safely decrease zoster in immunocompromised patients, and this vaccine might prove be very useful if it were licensed and available (Hata et al. 2002) Subunit vaccines are also being studied for efficacy in preventing zoster (ClinicalTrial.gov 2009a, b).
The disease burden of zoster in the elderly and the immunocompromised is considerable, and therefore improved vaccines and refinement of the use of the existing vaccine are very important for individuals and for public health. While zoster is rarely fatal, the morbidity associated with this disease is considerable. By decreasing the incidence of zoster, thereby decreasing spread of VZV, we gain greater protection from this viral infection. Despite the modeling studies that suggest that zoster might become more common for some 30 years after widespread use of varicella vaccine in a population(Brisson et al. 2003), this possibility, should it occur, can be managed by judicious use of zoster vaccine. The aim of vaccination against varicella and zoster is to decrease transmission of the virus and lower the overall viral burden in the population.
Clearly, vaccines against VZV have been extremely successful, despite the realization that there remains much to be learned about them immunologically. Although VZV vaccines are proven to be highly effective, there are a number of questions associated with their use. These need to be addressed, but this research can be ongoing as we proceed to immunize more and more of our population against varicella and HZ by using these safe and effective immunizing agents.
Acknowledgments
Supported by NIH grants AI27187, and AI24021.
References
- Amlie-Lefond C, Jubelt B. Neurologic manifestations of varicella zoster virus infections. Curr Neurol Neurosci Rep. 2009;9:430–434. doi: 10.1007/s11910-009-0064-z. [DOI] [PubMed] [Google Scholar]
- Ampofo K, Saiman L, LaRussa P, Steinberg S, Annunziato P, Gershon A. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin Infect Dis. 2002;34:774–779. doi: 10.1086/338959. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Cohen J. Varicella-zoster virus. In: Knipe DM, Howley PM, editors. Virology. 5th. Raven; NY, Philadelphia: 2007. pp. 2773–2818. [Google Scholar]
- Berger R, Florent G, Just M. Decrease of the lympho-proliferative response to varicella-zoster virus antigen in the aged. Infect Immun. 1981;32:24–27. doi: 10.1128/iai.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M, Edmunds WJ, Gay NJ. Varicella vaccination: impact of vaccine efficacy on the epidemiology of VZV. J Med Virol. 2003;70(Suppl 1):S31–37. doi: 10.1002/jmv.10317. [DOI] [PubMed] [Google Scholar]
- Brunell PA. Chickenpox attributable to a vaccine virus contracted from a vaccinee with zoster. Pediatrics. 2000;106:e28–e29. doi: 10.1542/peds.106.2.e28. [DOI] [PubMed] [Google Scholar]
- Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- Centers-for-Disease-Control. Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP) MMWR. 2007;56:1–40. [PubMed] [Google Scholar]
- Centers-for-Disease-Control. Prevention of varicella. MMWR. 2007;56:1–55. [Google Scholar]
- Centers-for-Disease-Control. Update: recommendations from the advisory committee on immunization practices (ACIP) regarding administration of combination MMRV vaccine. MMWR. 2008;57:258–260. [PubMed] [Google Scholar]
- Chaves SS, Gargiullo P, Zhang JX, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356:1121–1129. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995–2005. J Infect Dis. 2008;197(Suppl 2):S170–S177. doi: 10.1086/522161. [DOI] [PubMed] [Google Scholar]
- Chen J, Gershon A, Silverstein SJ, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric and dorsal root ganglia by varicella zoster virus. J Med Virol. 2003;70:S71–S78. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- Cinque P, Bossolasco S, Vago L, et al. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin Infect Dis. 1997;25:634–639. doi: 10.1086/513754. [DOI] [PubMed] [Google Scholar]
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954–959. doi: 10.1097/INF.0b013e3181a90b16. [DOI] [PubMed] [Google Scholar]
- ClinicalTrial.gov. Immunogenicity and safety study of GSK biologicals’ herpes zoster vaccine with various formulations in adults ≥ 50 years 2009 [Google Scholar]
- ClinicalTrial.gov. Safety and immunogenicity of the zoster vaccine GSK1437173A in elderly subjects 2009 [Google Scholar]
- Feldman S, Lott L. Varicella in children with cancer: impact of antiviral therapy and prophylaxis. Pediatrics. 1987;80:465–472. [PubMed] [Google Scholar]
- Feldman S, Hughes W, Daniel C. Varicella in children with cancer: 77 cases. Pediatrics. 1975;80:388–397. [PubMed] [Google Scholar]
- Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(Suppl 2):S165–S169. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Steinberg S, Gelb L, NIAID-Collaborative-Varicella-Vaccine-Study-Group Live attenuated varicella vaccine: efficacy for children with leukemia in remission. JAMA. 1984;252:355–362. doi: 10.1001/jama.252.3.355. [DOI] [PubMed] [Google Scholar]
- Gershon A, LaRussa P, Steinberg S. Varicella vaccine: use in immunocompromised patients. In: White J, Ellis R, editors. Infectious disease clinics of North America. Vol. 10. Saunders; Philadelphia: 1996. pp. 583–594. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Arvin AM, Shapiro E. Varicella vaccine. N Engl J Med. 2007;356:2648–2649. doi: 10.1056/NEJMc071043. [DOI] [PubMed] [Google Scholar]
- Gershon A, Chen J, LaRussa P, Steinberg S. Varicella-zoster virus. In: Murray PR, Baron E, Jorgensen J, Landry M, Pfaller M, editors. Manual of Clinical Microbiology. 9th. ASM; Washington, DC: 2007b. pp. 1537–1548. [Google Scholar]
- Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis. 2008a;197(Suppl 2):S61–S65. doi: 10.1086/522149. [DOI] [PubMed] [Google Scholar]
- Gershon A, Takahashi M, Seward J. Live attenuated varicella vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 5th. WB Saunders; Philadelphia: 2008b. pp. 915–958. [Google Scholar]
- Gershon AA, Levin MJ, Weinberg A, et al. A Phase I-Ii study of live attenuated varicella-zoster virus vaccine to boost immunity in human immunodeficiency virus-infected children with previous varicella. Pediatr Infect Dis J. 2009a;28:653–655. doi: 10.1097/INF.0b013e3181998f06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Gershon MD. 34th International Herpesvirus Workshop. Ithaca, NY: 2009. Distribution of latent varicella zoster virus in sensory ganglia and gut after vaccination and wild-type infection: evidence for viremic spread. [Google Scholar]
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahaliingham R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- Gould PL, Leung J, Scott C, et al. An outbreak of varicella in elementary school children with two-dose varicella vaccine recipients–Arkansas, 2006. Pediatr Infect Dis J. 2009;28:678–681. doi: 10.1097/INF.0b013e31819c1041. [DOI] [PubMed] [Google Scholar]
- Hardy IB, Gershon A, Steinberg S, LaRussa P, et al. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. N Engl J Med. 1991;325:1545–1550. doi: 10.1056/NEJM199111283252204. [DOI] [PubMed] [Google Scholar]
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic- cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- Hay J, Ruyechan WT. Varicella-zoster virus: a different kind of herpesvirus latency? Semin Virol. 1994;5:241–248. [Google Scholar]
- Hope-Simpson RE. The nature of herpes zoster: a long term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792–795. doi: 10.1016/j.annemergmed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Ackerson BK, Sy LS, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27(34):4656–4661. doi: 10.1016/j.vaccine.2009.05.056. [DOI] [PubMed] [Google Scholar]
- Jean-Philippe P, Freedman A, Chang MW, et al. Severe varicella caused by varicella-vaccine strain in a child with significant T-cell dysfunction. Pediatrics. 2007;120:e1345–e1349. doi: 10.1542/peds.2004-1681. [DOI] [PubMed] [Google Scholar]
- Jones L, Black AP, Malavige GN, Ogg GS. Persistent high frequencies of varicella-zoster virus ORF4 protein-specific CD4+ T cells after primary infection. J Virol. 2006;80:9772–9778. doi: 10.1128/JVI.00564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Black AP, Malavige GN, Ogg GS. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. Eur J Immunol. 2007;37:3393–3403. doi: 10.1002/eji.200737648. [DOI] [PubMed] [Google Scholar]
- Klein NP, Fireman B, Enright A, Ray P, Black S, Dekker CL. A Role for Genetics in the Immune Response to the Varicella Vaccine. Pediatr Infect Dis J. 2007;26:300–305. doi: 10.1097/01.inf.0000257454.74513.07. [DOI] [PubMed] [Google Scholar]
- Kuter B, Matthews H, Shinefield H, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–137. doi: 10.1097/01.inf.0000109287.97518.67. [DOI] [PubMed] [Google Scholar]
- LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg S, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992;66:1016–1020. doi: 10.1128/jvi.66.2.1016-1020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ. Use of varicella vaccines to prevent herpes zoster in older individuals. Arch Virol. 2001;(Suppl 17):151–160. doi: 10.1007/978-3-7091-6259-0_16. [DOI] [PubMed] [Google Scholar]
- Levin MJ. Zoster vaccine. In: Plotkin S, Orenstein W, Offit PA, editors. Vaccines. 5th. WB Saunderws; Philadelphia: 2008. pp. 1057–1068. [Google Scholar]
- Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr. 2001;139:305–310. doi: 10.1067/mpd.2001.115972. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Dahl KM, Weinberg A, Giller R, Patel A. Development of resistance to acyclovir during chronic Oka strain varicella-zoster virus infection in an immunocompromised child. J Infect Dis. 2003;188:954–959. doi: 10.1086/378502. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Gershon AA, Weinberg A, Song LY, Fentin T, Nowak B. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. J Infect Dis. 2006;194:247–255. doi: 10.1086/505149. [DOI] [PubMed] [Google Scholar]
- Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444–1447. doi: 10.1086/592452. [DOI] [PubMed] [Google Scholar]
- Levy O, Orange JS, Hibberd P, et al. Disseminated varicella infection due to vaccine (Oka) strain varicella-zoster virus in a patient with a novel deficiency in natural killer cells. J Infect Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- Michalik DE, Steinberg SP, LaRussa PS, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008;197:944–949. doi: 10.1086/529043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005;352:450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ. Vaccination against Herpes Zoster and Postherpetic Neuralgia. J Infect Dis. 2008;197(Suppl 2):S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Provost P, Krah D, Miller W, et al. Comparative sensitivities of assays for antibodies induced by live varicella vaccine. Interscience conference on antimicrobial agents and chemotherapy; Houston, TX. 1989. [Google Scholar]
- Provost PJ, Krah DL, Kuter BJ, et al. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine. 1991;9:111–116. doi: 10.1016/0264-410x(91)90266-9. [DOI] [PubMed] [Google Scholar]
- Ross AH, Lencher E, Reitman G. Modification of chickenpox in family contacts by administration of gamma globulin. N Engl J Med. 1962;267:369–376. doi: 10.1056/NEJM196208232670801. [DOI] [PubMed] [Google Scholar]
- Seward JF, Watson BM, Peterson CL, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287:606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- Son M. varicella vaccine protects children with perinatal HIV infection against zoster. 56th Annual Meeting of IDSA; Washington, DC. 2008. [Google Scholar]
- Sutradhar SC, Wang WW, Schlienger K, et al. Comparison of the levels of immunogenicity and safety of Zostavax in adults 50 to 59 years old and in adults 60 years old or older. Clin Vaccine Immunol. 2009;16:646–652. doi: 10.1128/CVI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Okuno Y, Otsuka T, et al. Development of a live attenuated varicella vaccine. Biken J. 1975;18:25–33. [PubMed] [Google Scholar]
- Tseng HF, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Incidence of herpes zoster among children vaccinated with varicella vaccine in a prepaid health care plan in the United States, 2002–2008. Pediatr Infect Dis J. 2009;28(12):1069–1072. doi: 10.1097/INF.0b013e3181acf84f. [DOI] [PubMed] [Google Scholar]
- Tsolia M, Gershon A, Steinberg S, Gelb L. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. J Pediatr. 1990;116:184–189. doi: 10.1016/s0022-3476(05)82872-0. [DOI] [PubMed] [Google Scholar]
- Vazquez M, LaRussa P, Gershon A, Steinberg S, Freudigman K, Shapiro E. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001;344:955–960. doi: 10.1056/NEJM200103293441302. [DOI] [PubMed] [Google Scholar]
- Vazquez M, LaRussa PS, Gershon AA, et al. Effectiveness over time of varicella vaccine. JAMA. 2004;291:851–855. doi: 10.1001/jama.291.7.851. [DOI] [PubMed] [Google Scholar]
- Williams V, Gershon A, Brunell P. Serologic response to varicella-zoster membrane antigens measured by indirect immunofluorescence. J Infect Dis. 1974;130:669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]
- Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis. 1992;165:119–126. doi: 10.1093/infdis/165.1.119. [DOI] [PubMed] [Google Scholar]
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005;294:797–802. doi: 10.1001/jama.294.7.797. [DOI] [PubMed] [Google Scholar]


