Abstract
This study aimed to investigate the effect of hydroalcoholic Achillea wilhelmsii C. Koch extract (HAWE) on phosphodiesterase 5 (PDE5) gene expression and cyclic guanosine 3′,5′ monophosphate (cGMP) signaling in the MCF-7 and MDA-Mb-468 cell lines. The effective dose (ED50) of HAWE was examined in both cell lines using a 3-(4,5-dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide viability test, and the type of cell death was detected by flow cytometry. The expression of PDE5 and the concentration of cGMP were measured in a time-dependent manner in the ED50 by real-time polymerase chain reaction and a colorimetric assay, respectively. Treatment with HAWE showed 25 µg/mL to be the ED50 for both cell lines, and HAWE led to a reduction in the PDE5 messenger RNA expression. The intracellular cGMP increased in a time-dependent manner. The results showed that HAWE has an antiproliferative property in MCF-7 and MDA-Mb-468 cell lines through the cGMP pathway. Therefore, HAWE is a potential source to effectively isolate inhibitory PDE5.
Keywords: Achillea wilhelmsii C. Koch, breast cancer, proliferation, phosphodiesterase, signaling pathway
Introduction
Phosphodiesterases (PDEs, EC 3.1.4.17) are metallohydrolases that regulate the intercellular levels of 2 important second messengers, cyclic adenosine 3′,5′ monophosphate (cAMP) and cyclic guanosine 3′,5′ monophosphate (cGMP), by controlling their degradation.1–3 Phosphodiesterases, including the 11 families (PDE1-PDE11) encoded by 21 different genes, produce more than 80 enzyme variants by different messenger RNAs (mRNAs) to process multiple promoters, alternative mRNA splicing, and posttranslational protein modulations.3 These 11 families of PDEs consist of 3 groups: some specific for cAMP (PDE4, PDE7, and PDE8), some specific for cGMP (PDE5, PDE6, and PDE9), and some specific for both cAMP and cGMP (PDE1, PDE2, PDE3, and PDE10).4,5
The PDE5 gene is located in the long arm of chromosome 4 (4q.26) and consists of 23 exons.6 Phosphodiesterase 5, a homodimer PDE enzyme, is a major regulator of the intercellular concentration of cGMP.3,7 Cyclic guanosine 3′,5′ monophosphate plays a key role in physiologic functions, including platelet aggregation, neurotransmission, vascular smooth muscle modulation, and cell proliferation, differentiation, and apoptosis.8 Previous studies have reported that PDE5 overexpression occurs in multiple cancer cell types, including colon, breast, bladder, and lung cancers. Conversely, PDE inhibitors (PDEIs) have potential anticancer effects on different types of cancer, including acute promyelocytic leukemia and malignant glioma.3,9–11 It has also been recently found that PDE5 expression increases breast cancer cells’ invasive potential, indicating that this enzyme is a novel prognostic candidate and a target for breast cancer therapy.12
Achillea wilhelmsii C. Koch has various components, including flavonoids, alkaloids, bornel, and cineol.13 This herb is used as a traditional drug to ease stomach pain, weakness, neurological disease symptoms, and epilepsy. Furthermore, the aerial parts of A wilhelmsii C. Koch have antioxidant properties.14 In addition, flavonoids are reported to have PDE5 inhibitory (PDE5I) properties.15 For example, Dell’Agli et al16 showed that Vitis vinifera, which has flavonoids, inhibits PDE5. Many plants have flavonoids with PDEI activity, such as Betula alnoides and Ginkgo biloba,1 and some plants have alkaloids with inhibitory effects on PDE; for example, viscolin in Viscum coloratum has shown PDEI effects.15,17 This study was conducted to evaluate the effect of the PDE5I properties of hydroalcoholic Achillea wilhelmsii C. Koch extract (HAWE) on estrogen receptor (ER)-positive and ER-negative MCF-7 and MDA-Mb-468, respectively.
Methods and Materials
The Ethics Committee of the Zahedan University of Medical Sciences approved the protocol of the study (Ethical code: 7526).
Plant materials
Achillea wilhelmsii C. Koch was collected during Spring 2015 from the Taftan area (ie, the southeast of Iran) of the province of Sistan and Baluchistan. The taxonomic determination of the plant was confirmed by the Research Institute of the University of Sistan and Baluchistan.18
Preparation of hydroalcoholic extract
The collected plant was dried in a dark place. The aerial sections of the plant were separated from the roots to be powdered; then, a Soxhlet extractor was used to obtain the hydroalcoholic extract (alcohol 70%) described previously.19 The plant powder (20 g at a time) was extracted from the alcoholic (70%) solvent (300 mL, 5 hours) using the Soxhlet extractor. After extraction, it was filtered (Whatman No. 41) and the alcohol solvent was evaporated completely using a centrifugal evaporation (MAXI DRY-LYO, Heto-Holten, Allerød, Denmark). Then, the solid extracts were mixed to make uniform solution, which was stored at −20°C.
Chemicals and regents
The culture media, Roswell Park Memorial Institute medium (RPMI 1640), trypan blue, EDTA, trypsin, penicillin, streptomycin, phosphate-buffered saline (PBS), and fetal bovine serum (FBS) were all purchased from Gibco (Rockville, MD, USA). The Annexin V/PI Apoptosis Detection Kit was obtained from BioVision (San Francisco, CA, USA). The cGMP Direct Immunoassay Kit was procured from R&D Systems (Minneapolis, MN, USA). The 3-(4,5-dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The RevertAid M-MuLV Reverse Transcriptase (RT) was procured from Fermentas (Vilnius, Lithuania). All other materials were of analytical grade and were obtained locally.
Cell culturing
The human breast cancer cell lines MCF-7 (ER-positive) and MDA-Mb-468 (ER-negative) were purchased from the National Cell Bank of Iran; both cell lines were grown adherently in a medium consisting of RPMI 1640, 10% FBS, 100 U/mL of penicillin, and 100 mg/mL of streptomycin under standard culturing conditions (95% humidified air, 37°C, 5% carbon dioxide).
Cell treatment
The HAWE powder was dissolved in DMSO (high-performance liquid chromatography grade) and stored at −20°C. Depending on the type of test, the cell lines were seeded almost equally in 6- or 96-well plates; then, the plates were kept in an incubator overnight under standard culturing conditions (95% humidified air, 37°C, 5% carbon dioxide). Next, the used medium was removed, and then, different concentrations of HAWE were added to the medium. All measurements were repeated in triplicate.
Cell viability
MTT assay
The MTT reduction assay was conducted to obtain the effective dose (ED50) of HAWE on both cell lines.20 Approximately, 5000 cells per well were seeded in 96-well plates and incubated for 24 hours to reach 70% to 80% confluence; the prior medium was replaced with a new medium containing different concentrations of HAWE (0, 12.5, 25, 50, and 100 µg/mL) and incubated for 24, 48, and 72 hours. Then, 20 µL of MTT was added to each well and incubated for 3.5 hours at 37°C; the culture medium was then replaced carefully with 200 µL of DMSO to dissolve the formazan crystals. Then, the plates were set in a dark place for 10 minutes and absorbance was measured using a microplate reader (Stat Fax 2100; Awareness Technology, Los Angeles, CA, USA) at a 570-nm wavelength. All assays were repeated at least in triplicate.
Apoptosis assay
The apoptotic cell death induced by HAWE was quantified by flow cytometry using an Annexin V/PI Apoptosis Detection Kit according to the manufacturer’s protocol. In brief, 1 × 105 cells per well were kept in 6-well plates and incubated in different concentrations of HAWE (0, 12.5, 25, 50, and 100 µg/mL) for 48 hours. Then, they were harvested and washed twice with cold PBS and stained by Annexin V/propidium iodide (PI). Double staining was done according to the manufacturer’s protocol. (The cell pellets were stained in 500 µL of 1× binding buffer, 5 µL of Annexin V, and PI, and then they were gently vortexed and incubated for 15 minutes in a dark room at room temperature.) The samples were analyzed using Partec Flow Cytometry and the software supplied with the instrument. The apoptosis assay resulted in 4 sections: living cells (unstained with Annexin V or PI), early apoptotic cells (stained with Annexin V), late apoptotic cells (stained with Annexin V or PI), and necrotic cells (stained with PI).
Gene expression assay
The effects of HAWE on PDE5 overexpression and inhibition were determined using a quantitative real-time polymerase chain reaction (qRT-PCR). Both cell lines were seeded in 6-well plates with a density of 1 × 105 cells per well and were incubated for 24 hours under standard culturing conditions. Then, the medium was replaced with a fresh medium containing the ED50 of HAWE and was incubated for 0, 4, 8, 12, and 24 hours. Then, the cell lines were washed with cold PBS and their total RNA was extracted using RNX (SinaClon, Tehran, Iran). UV spectrophotometry was used to obtain the RNA concentration and purity. Complementary DNA was synthesized from the total RNA using a random hexamer according to the manufacturer’s protocol (RevertAid ™ First Strand cDNA Synthesis Kit, Fermentas, Vilnius, Lithuania). The polymerase chain reaction (PCR) amplification of the first strand of complementary DNA (cDNA) was performed in 20 µL of reaction mixture with 5 µg of RNA (~2 µL) in a time-dependent manner (0 [control], 4, 8, 12, and 24 hours), 1 µL of random hexamer, 9 µL of nuclease-free water, 4 µL of 5× reaction buffer, 1 µL of RiboLock RNase inhibitor (20 U/µL), 2 µL of 10 mM deoxynucleotide Mix, and 1 µL of RevertAid M-MuLV RT (200 U/µL). Then, PCR amplification was conducted at 25°C for 5 minutes, followed by 42°C for 60 minutes. Each reaction was terminated by heating for 5 minutes at 70°C.
The levels of PDE5 expression were determined by qRT-PCR using PDE5 primers (forward primer: 5-TGTTGGTGTAGCACAGACCA-3, reverse primer: 5-GCAGTGAAGTCTGATAGAGC-3) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (forward primer: 5-GAGCCACATCGCTCAGACAC-3, reverse primer: 5-CATGTAGTTGAGGTCAATGAAGG-3). The PDE5 primers were designed to ensure that the PDE5 isoforms (PDE5A1, PDE5A2, and PDE5A3) would be amplified.
The qRT-PCR amplification reaction mixture consisted of 2 µL of cDNA, 1 µL of forward primer, 1 µL of reverse primer, 10 µL of SYBR Green EXTaq II (2×) PCR Master Mix, and 6 µL of diethyl pyrocarbonate water. ABI Sequence Detection System (Applied Biosystems, Foster City, CA, USA) was used to perform qRT-PCR amplification following the manufacturer’s protocol. The optimal reaction profile was observed to have initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing and polymerization at 58.5°C for 30 seconds, and extension at 72°C for 45 seconds. The housekeeping gene GAPDH was used to normalize the relative expression level of the PDE5 gene. The 2−ΔΔct analysis method was used to determine the relative mRNA expression level.
cGMP assay
The cGMP Direct Immunoassay Kit provided by R&D Systems was used according to the manufacturer’s instructions to quantitatively determine the cGMP expression.21 The cell lines were cultured for 24 hours to reach almost 80% confluence; then, they were treated with HAWE in a time-dependent manner (0, 4, 8, 12, and 24 hours). Afterward, the cells were lysed using a cell lysis buffer and were transferred to a 96-well plate coated with a goat anti-rabbit antibody. The excess conjugate and the unbound sample were removed via washing, and then the substrate solution was added to each well and incubated for 30 minutes at room temperature. Then, stop solution was added to each well. Finally, a microplate reader (Stat Fax 2100; Awareness Technology, Los Angeles, CA, USA) was used to measure cGMP at a 450-nm wavelength.
Statistical analysis
SPSS version 22 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis; the findings were reported as mean ± SD. The statistical analysis was conducted by a nonparametric analysis of variance between the groups and the Dunnett post hoc test. P < .05 was considered statistically significant.
Results
Antiproliferative effect of HAWE on human breast cancer cell lines
The effect of HAWE on the breast cancer cell lines’ viability was examined with MTT test; both cell lines (MCF-7 and MDA-Mb-468) were treated with different concentrations of HAWE (0, 12.5, 25, 50, and 100 µg/mL) for 24, 48, and 72 hours (Graph 1) to detect the ED50. This study showed that the ED50 of HAWE in MCF-7 and MDA-Mb-468 was 25 µg/mL after treatment for 48 hours with HAWE.
Graph 1.
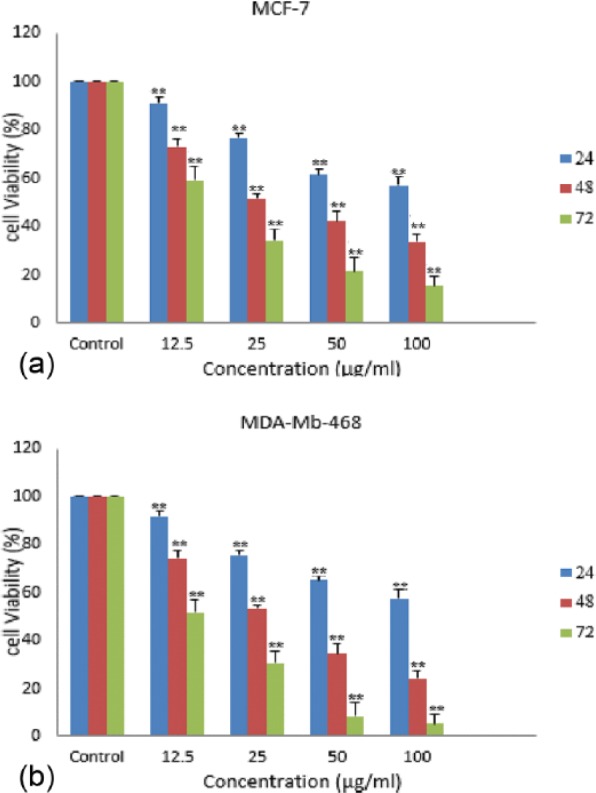
Effect of hydroalcoholic Achillea wilhelmsii C. Koch extract (HAWE) on inhibition of cell proliferation of the breast cancer cell lines. Cells were treated with different concentrations of HAWE for 24, 48, and 72 hours, and proliferation was measured by 3-(4,5-dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. HAWE reduced cell proliferation in (A) MCF-7 and (B) MDA-Mb-468 breast cells in a time- and dose-dependent manner. Each value is presented as mean ± SD of 3 experiments (each triplicate). Error bars represent standard deviation. *P < .05 and **P < .01 are significant compared with untreated cells as control groups.
HAWE induction of apoptosis in human breast cancer cell lines
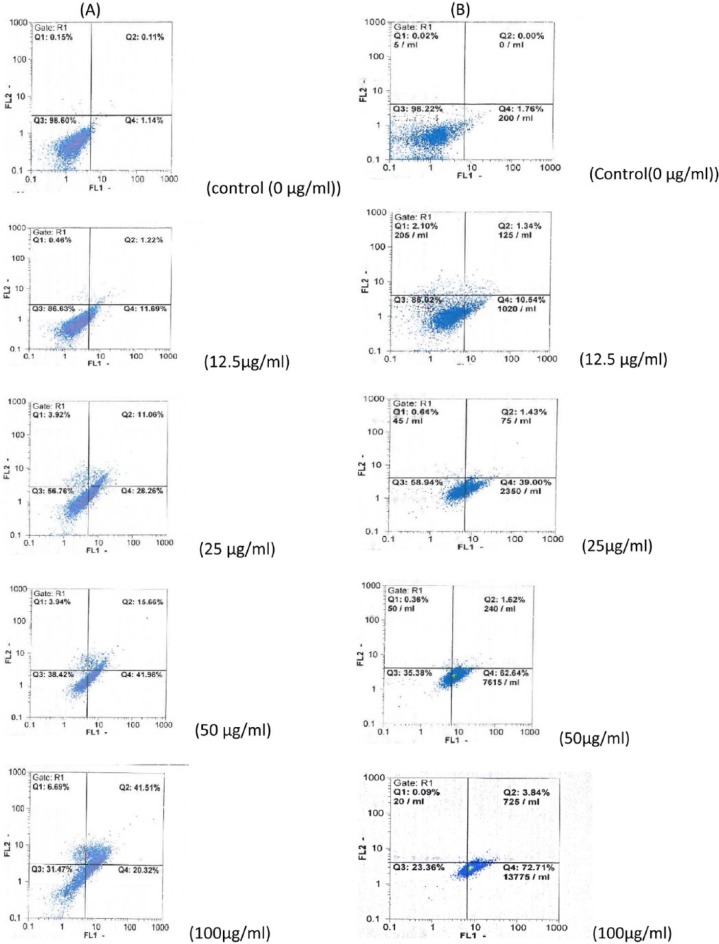
To study whether HAWE induced cell population growth inhibition related to apoptosis, its effects on apoptosis were evaluated. The breast cancer cell lines were exposed to different concentrations (0, 12.5, 25, 50, and 100 µg/mL) of HAWE for 48 hours and then stained with Annexin V and PI; then, they were analyzed using flow cytometry. Our findings showed that the percentages of early and late apoptosis significantly increased in both the MCF-7 and MDA-Mb-468 cell lines (Figure 1 and Graph 2).
Figure 1.
The hydroalcoholic Achillea wilhelmsii C. Koch extract (HAWE) induced apoptosis in MCF-7 and MDA-Mb-468 cell lines. MCF-7 and MDA-Mb-468 cells were treated with different concentrations of HAWE (0 [control], 12.5, 25, 50, 100 µg/mL). After 48 hours of treatment, (A) MCF-7 and (B) MDA-Mb-468 cells were double stained with Annexin V-fluorescein isothiocyanate and propidium iodide, and the cells were analyzed by flow cytometry. All experiments were performed independently in triplicate per experimental point, and representative results are shown.
Graph 2.
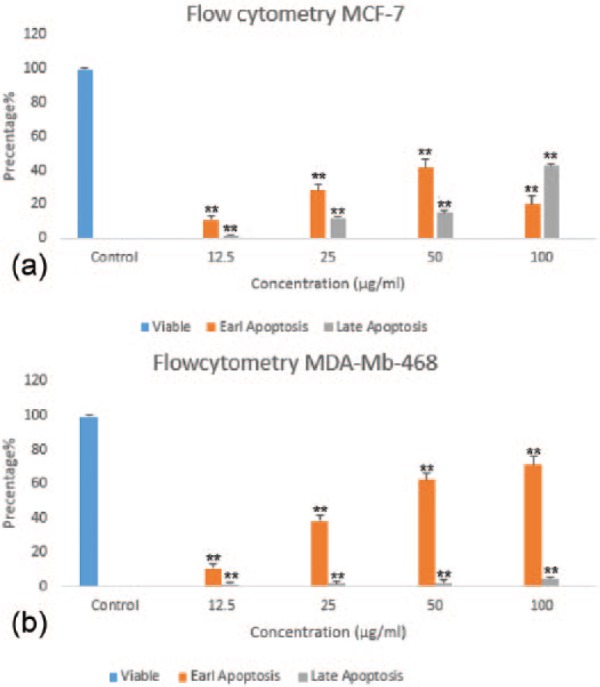
Flow cytometric evaluation of apoptosis in (A) MCF-7 and (B) MDA-Mb-468 cells using Annexin V and propidium iodide double staining. After 48 hours of treatment, hydroalcoholic Achillea wilhelmsii C. Koch extract resulted in a significant increase in early and late apoptotic cells, in a concentration-dependent manner. Results: mean ± SD 3 independent experiments. Error bars represent standard deviation. *P < .05 and **P < .01 compared with control.
qRT-PCR assay
The effect of HAWE on the expression of the PDE5-coding gene was tested in a time-dependent manner. The results showed that PDE5 expression gradually reduced in MCF-7 cells in the presence of HAWE (ED50 = 25 µg/mL) after 4, 8, 12, and 24 hours compared with that of the untreated group (P < .001). Similar effects were also found in MDA-Mb-468 cells. As shown in Graph 3, the mRNA expression level of PDE5 decreased significantly after 4, 8, 12, and 24 hours in comparison with that of the untreated group (P < .001).
Graph 3.
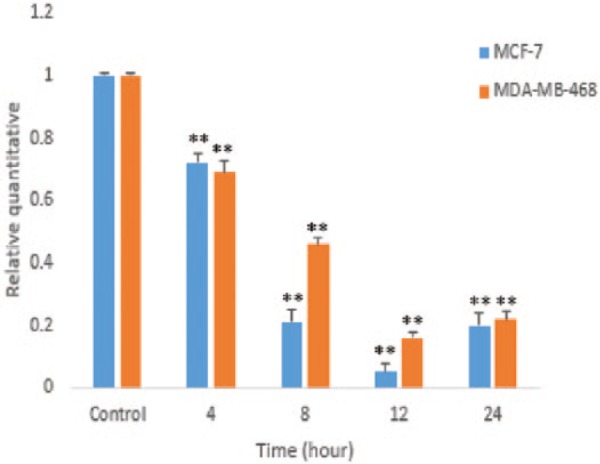
In quantitative real-time polymerase chain reaction analysis of PDE5 messenger RNA (mRNA) expression in MCF-7 and MDA-Mb-468 cells following the hydroalcoholic Achillea wilhelmsii C. Koch extract treatment (25 µg/mL) in a time-dependent manner, expression levels were normalized to human glyceraldehyde-3-phosphate dehydrogenase mRNA. Error bars represent standard deviation.*P < .05 and **P < .001 compared with untreated control.
cGMP assay
The effect of HAWE on the intercellular level of cGMP in MCF-7 and MDA-Mb-468 cells was investigated. As shown in Graph 4, HAWE significantly increased the cGMP level in both MCF-7 and MDA-Mb-468 after 4, 8, 12, and 24 hours compared with that of the untreated cells (P < .001). The results showed that the inhibition of PDE5 by HAWE raised the cGMP level and indicated that this gene may be a major regulator of the basal cGMP level in MCF-7.
Graph 4.
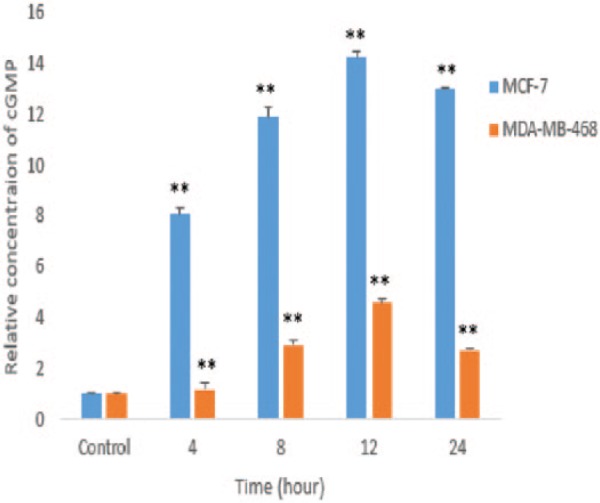
Effect of hydroalcoholic Achillea wilhelmsii C. Koch extract (HAWE) on cyclic guanosine 3′,5′ monophosphate (cGMP) levels in MCF-7 and MDA-Mb-468 cells. Cells were treated with 25 µg/mL HAWE; over a time course, measurement of cGMP was performed using a colorimetric competitive enzyme-linked immunosorbent assay kit. *P < .05 and **P < .001 compared with the untreated control.
Discussion
In a previous study, it was shown that the activation of protein kinase G by cGMP induces cell inhibition and apoptosis in the human breast cancer cell lines MCF-7 and MDA-Mb-468.9,21 However, cGMP signaling is influenced by many factors, such as G-protein–coupled receptors, nitric oxide, and PDE5, as a key hydrolysis enzyme can affect the intracellular cGMP level in breast cancer cells, whereas other specific PDEs for cGMP, namely, PDE6 and PDE9, are most expressed in eye and brain tissues, respectively.12,22 In a previous investigation, we found that using BAY 73-6691 as a selective PDE9 inhibitor led to reduced proliferation through the elevation of the intracellular cGMP level in MCF-7 and MDA-Mb-468.9 Recent investigations showed that breast cancer cell proliferation can be reduced, and apoptosis can be induced, using the selective inhibition of PDE5 by Sulindac sulfide through the cGMP pathway,3,23,24 whereas most PDE inhibitors act selectively (not specifically).25 In addition, some herbs and plants with flavonoids and alkaloids can inhibit PDE, especially cGMP-specific PDEs.14,15,26 So, if the HAWE (as a herbal medicine contains flavonoids and alkaloids) inhibits PDE5 and promotes intercellular cGMP, it will reduce proliferation or even induce apoptosis. In this study, both the inhibition of PDE5 and the increase in the cGMP level by HAWE in human breast cancer cell lines MCF-7 and MDA-Mb-468 were investigated.
The mRNA of cGMP, a specific PDE5, was expressed in both breast cancer cell lines, and the PDE5 expression level significantly decreased in a time-dependent manner through HAWE. There was a significant difference between MCF-7 (ER+) and MDA-Mb-468 (ER−) breast cancer cell lines’ mRNA expression of PDE5 after 8 and 12 hours of treatment with HAWE (Graph 3). The mRNA expression of PDE5 was at its minimum in both cell lines after 12 hours. In addition, the mRNA expression of PDE5 in MDA-Mb-468 (ER−) and MCF-7 (ER+) was almost equal 24 hours after treatment with HAWE.
The ability of HAWE to modulate the cGMP-signaling pathway was investigated. The results indicated that HAWE significantly increased the intercellular cGMP level. Although the increase in the cGMP level in MCF-7 (ER+) was much higher than that in MDA-Mb-468 (ER−), the mRNA expression of PDE5 was correlated with the intercellular cGMP level in both cell lines.
The data showed that HAWE promotes cGMP repositioning as an inhibitor of PDE5 and suggest that this enzyme could be a major regulator of a basal cGMP intracellular concentration in these breast cancer cell lines. Previous studies have demonstrated that the intercellular cGMP level increases by inhibiting PDEs.27,28 Other investigations have found that different PDEs are involved in the regulation of either the intracellular cGMP level or the intercellular cAMP level by the negative feedback control of the cyclic nucleotide pathway.29 However, the current data demonstrated that the inhibition of PDE5 by HAWE leads to the accumulation of intercellular cGMP and likely activates the cGMP-signaling pathway.
The intercellular cGMP level of MCF-7 is higher than that of MDA-Mb-468 after using HAWE to inhibit PDE5, likely due to their different cell types. The intracellular cGMP level is most dependent on some PDEs, such as PDE5, PDE6, and PDE9, which are cGMP-specific PDEs.29
The results here indicate that HAWE, as a traditional herbal medicine, can decrease the mRNA expression of PDE5 in MCF-7 and MDA-Mb-468 in a time-dependent manner. Various studies have been conducted on the effects of natural compounds with plant origins in terms of their PDE inhibitory activity. For example, Orallo et al30 showed that citrus fruits with naringenin, a flavonoid compound, can inhibit the activity of PDE1, PDE4, and PDE5. Furthermore, Ning et al31 found that icariin from Epimedii herba, a flavonoid, can inhibit PDE5 even more effectively than the standard PDE5I medicine zaprinast. In addition, Lines and Ono reported the PDE5I activity of quercetine in Allium cepa, which is similar to that of alkaloids. Finally, Hwang et al observed that viscolin, an alkaloid in Viscum coloratum, had inhibitory effects on PDE.17
Conclusions
This research revealed that the hydroalcoholic extract A wilhelmsii C. Koch inhibits PDE5 mRNA expression and increases the intercellular cGMP level in both the MCF-7 and MDA-Mb-468 human breast cancer cell lines. The results provided valuable information regarding HAWE, which has potential as an anticancer agent to inhibit PDE5. In future work, it is recommended to further isolate and elucidate the structures of PDEIs in A wilhelmsii C. Koch.
Acknowledgments
The authors wish to thank the Zahedan University of Medical Sciences and Cellular and Molecular Research Center which is located in Ali-Asghar clinic.
Footnotes
Peer Review:Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 594 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a dissertation grant (MSc thesis) to HRG from Zahedan University of Medical Sciences, Zahedan, Iran. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RS and HRG conceived and designed the experiments and jointly developed the structure and arguments for the paper. HRG analyzed the data. HRG and AS wrote the first draft of the manuscript. RS, HRG, and AS contributed to the writing of the manuscript and agreed with the manuscript results and conclusions. RS made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, authors have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Temkitthawon P, Viyoch J, Limpeanchob N, et al. Screening for phosphodiesterase inhibitory activity of Thai medicinal plants. J Ethnopharmacol. 2008;119:214–217. [DOI] [PubMed] [Google Scholar]
- 2. Karami-Tehrani F, Moeinifard M, Aghaei M, Atri M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res. 2012;43:470–475. [DOI] [PubMed] [Google Scholar]
- 3. Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corbin J. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16:S4–S7. [DOI] [PubMed] [Google Scholar]
- 5. Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. [DOI] [PubMed] [Google Scholar]
- 6. Lin C. Tissue expression, distribution, and regulation of PDE5. Int J Impot Res. 2004;16:S8–S10. [DOI] [PubMed] [Google Scholar]
- 7. Kumazoe M, Sugihara K, Tsukamoto S, et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J Clin Invest. 2013;123:787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saravani R, Karami-Tehrani F, Hashemi M, Aghaei M, Edalat R. Inhibition of phosphodiesterase 9 induces cGMP accumulation and apoptosis in human breast cancer cell lines, MCF-7 and MDA-MB-468. Cell Prolif. 2012;45:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts JL, Booth L, Conley A, et al. PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther. 2014;15:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guillemin M-C, Raffoux E, Vitoux D, et al. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J Exp Med. 2002;196:1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen TC, Wadsten P, Su S, et al. The type IV phosphodiesterase inhibitor rolipram induces expression inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol Ther. 2002;1:268–276. [DOI] [PubMed] [Google Scholar]
- 12. Catalano S, Campana A, Giordano C, et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: implications for targeted therapy. Clin Cancer Res. 2016;22:2271–2282. [DOI] [PubMed] [Google Scholar]
- 13. Amjad L, Mousavidehmourdi K, Saghazadeh M. Antifungal potential of Achillea wilhelmsii flowers methanolic extract on different strains of Candida albicans. Int J Biol Med Res. 2012;3:2107–2110. [Google Scholar]
- 14. Isfahani LD, Monajemi R, Amjad L. Cytotoxic effects of extract and essential oil leaves of Achillea wilhelmsii C. Koch on colon cancers cells. Exp Anim Biol. 2013;1:1–6. [Google Scholar]
- 15. Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine. 2010;49:123–129. [DOI] [PubMed] [Google Scholar]
- 16. Dell’Agli M, Galli GV, Vrhovsek U, Mattivi F, Bosisio E. In vitro inhibition of human cGMP-specific phosphodiesterase-5 by polyphenols from red grapes. J Agric Food Chem. 2005;53:1960–1965. [DOI] [PubMed] [Google Scholar]
- 17. Hwang T-L, Leu Y-L, Kao S-H, Tang M-C, Chang H-L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic Biol Med. 2006;41:1433–1441. [DOI] [PubMed] [Google Scholar]
- 18. Shahraki A, Ravandeh M. Comparative survey on the essential oil composition and antioxidant activity of aqueous extracts from flower and stem of Achillea wilhelmsii from Taftan (Southeast of Iran). Health Scope. 2013;1:173–178. [Google Scholar]
- 19. Hossain MA, Al-Toubi WA, Weli AM, Al-Riyami QA, Al-Sabahi JN. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J Taibah Univ Sci. 2013;7:181–188. [Google Scholar]
- 20. Faezizadeh Z, Mesbah-Namin SA, Gharib A, Saravani R, Godarzi M. Evaluating the effect of lycopene on telomerase activity in the human leukemia cell line K562. Feyz J Kashan Univ Med Sci. 2012;16:398–405. [Google Scholar]
- 21. Fallahian F, Karami-Tehrani F, Salami S, Aghaei M. Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and-negative breast cancer cell lines. FEBS J. 2011;278:3360–3369. [DOI] [PubMed] [Google Scholar]
- 22. Gao YQ, Danciger M, Zhao DY, et al. Screening of the PDE6B gene in patients with autosomal dominant retinitis pigmentosa. Exp Eye Res. 1996;62:149–154. [DOI] [PubMed] [Google Scholar]
- 23. Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by Sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin–mediated transcription in human breast tumor cells. Cancer Prev Res. 2011;4:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tinsley HN, Gary BD, Keeton AB, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt HH, Hofmann F, Stasch J-P. Cgmp: Generators, Effectors and Therapeutic Implications. Berlin, Germany: Springer; 2009. [PubMed] [Google Scholar]
- 26. Savai R, Pullamsetti SS, Banat G-A, et al. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010;19:117–131. [DOI] [PubMed] [Google Scholar]
- 27. Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol. 2005;68:1775–1781. [DOI] [PubMed] [Google Scholar]
- 28. Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology. 2009;202:419–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. [DOI] [PubMed] [Google Scholar]
- 30. Orallo F, Camiña M, Álvarez E, Basaran H, Lugnier C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (±)-naringenin. Planta Med. 2005;71:99–107. [DOI] [PubMed] [Google Scholar]
- 31. Ning H, Xin Z-C, Lin G, Banie L, Lue TF, Lin C-S. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology. 2006;68:1350–1354. [DOI] [PubMed] [Google Scholar]