Abstract
Background
Mesenchymal stem cell (MSC) has been one of the potential tools in neuropathic pain therapy; however, the augmented efficacy may be expected when they are modified with human proenkephalin (hPPE) gene. In the current study, the antinociceptive effect of human bone marrow stem cells (hBMSCs) engineered with hPPE gene (hPPE-hBMSCs) on sciatic nerve chronic constriction injury (CCI)-induced neuropathic pain in rats was investigated.
Methods
Primary-cultured hBMSCs were passaged and modified with hPPE, and the cell suspensions (6 × 106) were then intrathecally injected into a rat model of CCI. Paw mechanical withdrawal threshold and paw withdrawal thermal latency were measured before and after CCI surgery. The effects of hPPE gene transfer on hBMSCs bioactivity were analyzed in vitro and in vivo.
Results
No changes were observed in the surface phenotypes and differentiation of hBMSCs after gene transfer. The hPPE-hBMSC group showed improved paw mechanical withdrawal threshold and paw thermal withdrawal latency values on the ipsilateral side of rats with CCI from day 9 post-surgery, and the analgesic effect was reversed by naloxone. Leucine-enkephalin (L-EK) secretion was augmented in the hPPE-engineered hBMSC group.
Conclusions
The intrathecal administration of BMSCs modified with hPPE gene can effectively relieve pain caused by chronic constriction injury in rats and might be a potentially therapeutic tool for neuropathic pain in humans.
Keywords: Neuropathic pain, cell transplantation, proenkephalin, human marrow stem cell, chronic constriction injury
Introduction
Neuropathic pain, redefined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”1 by the International Association for the Study of Pain in 2008, is one of the most difficult conditions to treat. There are approximately 6% of the population who suffer from the pain in the world every year.2 However, among the patients who receive the treatments, only a limited percentage (about 30%) can get relief before pain inevitably reappears.3 First-line drugs can be problematic for patients with poor therapeutic effects and potential side reaction. In 1970s, development of cellular strategies for intractable pain has progressed from the therapy studies of chromaffin cells’ transplantation into the spinal cord. Those cells could act as a living or biological minipump to produce several neuroactive substances, including catecholomines and opioid peptides, together with functional recovery, with more modulation and less destruction.4 In many animal models and human cases, Pain symptoms including allodynia, hyperalgesia, dysesthesia, and limb self-injury were markedly decreased through cell transplantation.5,6 But, the clinical practice has been hindered due to severe immnorejection in xeno- and allotransplants including chromaffin cells, PC12 cells, and other kinds of heterologous cells.
Mesenchymal stem cell (MSC) can be safe and immunocompatible through autologous cell transplantation and those cells could be the most desirable choice for potential therapeutic purposes.7,8 Some articles have reported the autologous cell transplantation of MSC in the rats with neuropathic pain and claimed limited antinociceptive effects9 and a few recent studies have shown promising good antinociception in animal models.10,11 To date, MSCs have emerged as a major source for cell-based therapies,12,13 but the use of genetic engineering in treating neuropathic pain in patients or animal models is still in discussion. In fact, gene modification is often used to promote the production of analgesic substances and increase the time of analgesic substances existing in the central nervous system (CNS).14 Indeed, genetically engineered BMSC can express anti-nociceptive molecules or trophic factors to be a useful tool in pain treatment. In the present study, human BMSCs (hBMSCs) were transfected with human proenkephalin (hPPE) gene, which was a classical tool used in lots of previous researches related to transgenic analgesia, and then, the cells would be injected intrathecally in rat chronic constriction injury (CCI) model and the analgesic effects were evaluated.
Materials and methods
In vitro characterization
HBMSCs preparation and differentiation analysis
HBMSCs were obtained from four healthy female donors (age range, 20–45 years) undergoing plastic surgery and after informed consent and authorization from the Hospital Ethical Committee. Primary hBMSCs were cultured and passaged in 75-cm2 culture flasks at 37℃ in a 5% CO2 humidified incubation chamber. The third passage was utilized for the experiments. The viability of cells was measured. After being washed, trypsinized, and then centrifuged, a suspension of cells (1 × 105 cells/100 µL PBS) was stained at room temperature for 30 minutes with phycoerythrin (PE)-labeled rabbit anti human CD29 (Serotec, Ltd, United Kingdom), fluorescein isothiocyanate (FITC)-conjugated anti-human CD44 antibody (Serotec, Ltd, United Kingdom), (PE)-labeled rabbit anti-human CD34 (Serotec, Ltd, United Kingdom) and FITC-conjugated anti-human CD45 antibody (Serotec, Ltd, United Kingdom). The expression of cell surface antigens was assessed by Fluorescence Activated Cell Sorting with flow cytometry (FCT, Becton Dickinson Inc, USA). The evaluation of adipogenesis was detected by Oil Red O staining. The differentiation potential for osteogenesis was assessed by the calcium tubercle sodium alizarinsulfonate staining.
Vector construction
The hPPE RNA was isolated from minced and ice-cold adrenal pheochromocytoma tissues using TRIzol reagents (Life Technologies, Waltham, MA, USA) according to the manufacturer’ protocol. Reverse transcription in complementary DNA (cDNA) was performed from 2 mg of total RNA using a RevertAid First Strand cDNA Synthesis Kit (Clontech, Mountain View, CA, USA), and then the real-time polymerase chain reaction (PCR) reaction was performed. The specific primers designed with Primer 5.0 software (Biosune Bio-technology LTD, Shanghai, China) based on GenBank sequence (human PEEK, GenBank #NM006211) are as followed:
Forward 5’ -ATACGAATTCCATGGCGCGGTTCCTGACA-3’;
Reverse 5’-GCGCGTCGACTTAAAATCTCATAAATCC-3’
The framed nucleotides were added to generate EcoRI site adjacent to the 5’ end in the forward primer and SalRI site in the reverse primer, respectively. EcoRI/SalRI were added into the target gene sequence, the two restriction sites also existed in the plasmid pBABE puro15 (a gift from Dr Xiao, Research Center, GAMS), so EcoRI/SalRI digested PCR products were inserted into the corresponding restriction site in the expression plasmid pBABE, construction of viral vector pBABE-hPPE. The recombinant viral vector pBABE-hPPE was identified by restriction enzyme analysis and verified by nucleotide sequencing.
Retrovirus package and positive clone screen
PHOENIX-293T cells were inoculated in six-well plastic tissue culture plates at a density of 1 × 105 cells. Then, the plasmid of pBABE-hPPE was transfected by lipofectamine 2000 (Invitrogen, Paisley, United Kingdom), when the cells came to 70%∼80% confluence. The cells were cultured and passaged at a percent of 1:10 after 48 h, and then they returned into DMEM/F12 (Gibco, USA) medium with 1 mmol/L puromycin (Gibco, USA) for 10 days. During the positive colonies screen and amplification, the concentration declined to 0.5 mmol/L and untransfected 293T-cells as control.16
Retroviral infection of the target cells
The third-passage hBMSCs were seeded at a density of 1 × 105 cells in six-well plastic tissue culture plates. The culture medium was changed into complete α-MEM medium (Gibco, USA). After 24 h, the virus-containing media (supernatants) were collected. The hBMSCs were infected with virus-containing media filtered with micropore filter for 48 h. After selection with 1 mmol/L puromycin, resistant cells were further grown into cell colonies for 10–15 days, and then cultured and amplified with 0.5 mmol/L puromycin. The new cell line was hPPE-hMSCs. The test about biological features of the new line including cell viability, surface maker, and differentiation potential were done as stated above. The measurement of mRNA expression was assessed by RT-PCR.
Fluorescence immunohistochemistry (IHC)
hPPE-hBMSCs were incubated overnight at 4℃ with a rabbit anti-human leucine-enkephalin (L-EK) antibody (immunogen:KLH conjugated Leu Enkephalin peptide, Bicleaf, Shanghai China) at a dilution of 1:400 and were then washed and incubated with goat anti-rabbit secondary antibodies (1:500, Abcam, Cambridge, United Kingdom) conjugated with FITC and Y3. After washing completely, 4’6-diamidino-2-phenylindole was used as a nuclear stain. Fluorescence images were collected using an IX71 SIF-2 fluorescence microscope equipped with an Olympus digital camera.
Animals and experimental protocol
All animal experimental procedures were approved by the Committee of Animal Use for Research and Education of Guangdong Medical Science Institute.
Catheter implantation and CCI model induction
To receive lumbar Intrathecal infusion of hBMSCs, male Sprague-Dawley rats (weighing 160–220 g), purchased from Sun Yet-sen University of Medical Sciences Center for Animal Experiments, Guangzhou, China, were implanted with catheters (item #0007150; DURECT). A laminectomy was conducted at the caudal portion of the L3 spinal level and rostral portion of the L4 spinal levels. The dura was incised using a 25-gauge needle, and an intrathecal catheter was introduced into the subdural space over the spinal cord. Rats that displayed fresh blood in the cerebrospinal fluid (CSF) or evidence of gross neurological injury were excluded from the experiment. After a five-day recovery period, rats received CCI surgery. The CCI model originally described by Bennett and Xie17 were involved in this study.
Grouping
Rats were divided into four groups of 13 rats each as follows: the control group, undergoing sham operation; the CCI group, undergoing the intrathecal delivery of 10 µl PBS on day seven after operation; the CCI+pBABE-hBMSCs group, undergoing intrathecal delivery of pBABE-hBMSC (6 × 106 cells/10 µl) on day seven after operation; and the CCI+hPPE-hBMSC group, undergoing intrathecal delivery of hPPE-hBMSCs (6 × 106 cells/10 µl) on day seven after operation.
Nociceptive behavior
Thermal hyperalgesia was evaluated by using a radiant heat source aimed at the plantar hind paw to assess responses to a noxious thermal stimulus.18 The time the rat took to lift its paw was recorded and defined as the paw thermal withdrawal latency (PTWL). Mechanical allodynia was assessed using Von Frey filaments.19 The monofilaments were used from 1.4 g up to 100 g. Each filament was tested five times. Four additional stimulations were determined, and the 50% paw mechanical withdrawal threshold (PMWT) was calculated using the up–down method. PTWL and PMWT on the ipsilateral side were measured before operation and at 7, 9, 11, 13, 15, and 17 days after operation (n = 6 per group). On the last day, PMWT was measured before and 30 min after rats receiving µ-opioid receptor antagonist naloxone (4 mg/kg) by intraperitoneal injection.20
L-EK level detection
The supernatants in three groups such as the hBMSC, pBABE-hBMSC, and hPPE-hBMSC groups were collected for concentration measurements of L-EK for six days after cells were seeding into culture plates in vitro and the concentrations of L-EK in CSF in four groups such as the sham, CCI+PBS, CCI+pBABE-hBMSC, and CCI+hPPE-hBMSC groups on the 14th day after CCI operation were detected in vivo (n = 6). Enzyme-linked immunosorbent assay (ELISA) method was used for the measurement. A rat-specific ELISA kit (R&D Systems, MN, USA) was used according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as means ± SD. Statistical analysis was performed using repeated measures one-way or two-way analysis of variance followed by the least-significant difference (for equal variance) or Dunnett T3 (for unequal variance) test. P < 0.05 was considered statistically significant.
Results
Characterization of hPPE-hBMSCs in vitro
Cultured hBMSCs, pBABE-hBMSCs, and hPPE-hBMSCs were CD 29 and 44 positive and CD 34 and 45 negative, with significant differences between them (P > 0.05) (Table 1). The hPPE-expressing cells displayed a rapid growth rate up to passage 10, and there were no significant differences regarding the cell activities among the three groups (P > 0.05) (Table 2). The proportion of adipocytes after adipo-induction for three weeks is shown in Table 3 and Figure 1. The results suggested that the cells in all three groups displayed the functional characteristics of multipotential mesenchymal progenitors.
Table 1.
Analysis of cell surface marker expression by flow cytometry after transfection (%, ).
| Group | N | CD29 | CD44 | CD34 | CD45 |
|---|---|---|---|---|---|
| hBMSC P3 | 5 | 98.10 ± 1.10 | 94.99 ± 1.00 | 0.32 ± 0.12 | 1.78 ± 0.44 |
| hPPE-hBMSC | 5 | 99.08 ± 0.55 | 95.07 ± 2.35 | 0.43 ± 0.40 | 1.48 ± 0.45 |
| pBABE-hBMSC | 5 | 98.99 ± 0.59 | 93.37 ± 2.36 | 0.57 ± 0.35 | 1.82 ± 0.49 |
| F value | — | 2.368 | 1.141 | 0.815 | 0.796 |
| P value | — | 0.136 | 0.352 | 0.466 | 0.474 |
Note. The third passage of cultured hBMSCs, pBABE-hBMSC, and hPPE-hBMSCs were analyzed using fluorescence-activated cell sorting (FCS) to confirm the cellular identity of cultured cells. The results confirmed these cells expressed CD 29 and CD 44 surface makers but were CD 34 and CD 45 negative, consistent with characteristic surface markers of undifferentiated BMSCs. The lack of expression of CD34 and CD45 suggested that the cell population was depleted of hematopoietic stem cells. The multipotency of these cells further verified the cellular nature of BMSCs. The results also revealed that BMSCs kept the same cellular identity after transfection with the vector and the hPPE gene. hPPE: human proenkephalin; hBMSCs: human bone marrow stem cells. pBABE-hBMSCs: pBABE-hBMSCs group, hPPE-hBMSCs: hPPE-hBMSCs group.
Table 2.
Cell activity after freezing and recovering (%, ).
| Cell type | hBMSC P3 | pBABE-hBMSC | hPPE-hBMSC | F value | P value |
|---|---|---|---|---|---|
| Number(n) | 5 | 5 | 5 | — | — |
| Living cell rate(%) | 89.90 ± 3.20 | 87.30 ± 3.26 | 88.08 ± 2.20 | 1.033 | 0.386 |
Note. The percentage of living cell rate was used to estimate the cell activity of the 3rd generation of hBMSCs, pBABE-hBMSCs and hPPE-hBMSCs after freezing and recovering. There were no after freezing and recovering significant differences among three group (P > 0.05), indicating that transfection had no effect on the morphology and proliferation of the cells. hPPE: human proencephalin, hBMSCs: human bone marrow stem cells. pBABE: a retroviral vector; pBABE-hBMSC: hBMSC modified with pBABE, hPPE-hBMSC: hBMSC modified with hPPE gene.
Table 3.
Proportion of adipocytes after adipo-induction for 3 weeks (%, ).
| Cell type | hBMSC P3 | pBABE -hBMSC | hPPE-hBMSC | F-value | P value |
|---|---|---|---|---|---|
| Number(n) | 10 | 10 | 10 | — | — |
| Ratio of fat cell(%) | 75.56 ± 5.82 | 80.32 ± 7.15 | 78.28 ± 3.67 | 0.076 | 0.785 |
Note. There were no significant difference on the proportion of adipocytes among three groups (P > 0.05). hPPE: human proenkephalin; hBMSCs: human bone marrow stem cells. pBABE-hBMSCs: pBABE-hBMSCs group, hPPE-hBMSCs: hPPE-hBMSCs group.
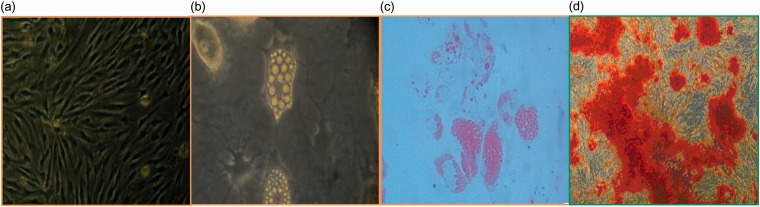
Figure 1.
Identification of the human bone marrow stem cells (hBMSCs), including culture, differentiation, and application, was analyzed by immunohistochemistry. (a) The morphology of BMSCs at passage 3 (scale bar = 100 µm). (b) Adipogenic differentiation before staining (scale bar = 100 µm). (c) The cultured cells were stained with oil red-O solution (scale bar = 100 µm). (d) Osteoblasts were stained with alizarin red (scale bar = 100 µm). n = 6.
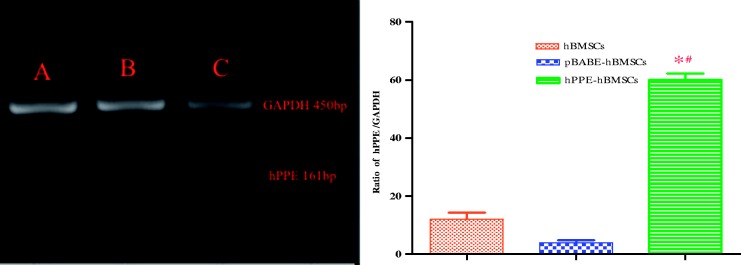
The hBMSCs and pBABE-hBMSCs showed low levels of endogenous hPPE gene expression. The hPPE-hBMSCs showed a significantly enhanced hPPE gene expression profile compared with the cells in the other two groups (P < 0.01) (Figure 2), suggesting that the hPPE gene was integrated into hBMSCs.
Figure 2.
The expression of the human proenkephalin (hPPE) gene in engineered hBMSCs (c) was analyzed by RT-PCR two weeks (passage 3) after cell transfer. hBMSCs, human bone marrow stem cells; pBABE, a retroviral vector; pBABE-hBMSCs, the pBABE-hBMSCs group; hPPE-hBMSCs, the hPPE-hBMSCs group. Naive hBMSCs (a) and vector-engineered hBMSCs (b) served as controls, and hPPE RT-PCR products (161 bp) were expressed as the hPPE/GADPH (450 bp, an internal control) ratio. Statistical analysis showed significantly upregulated hPPE expression in hPPE-hBMSCs compared with hBMSCs and pBABE-hBMSCs (P < 0.01). *P < 0.01 versus normal hBMSCs and #P < 0.01 versus pBABE-hBMSCs, n = 6.
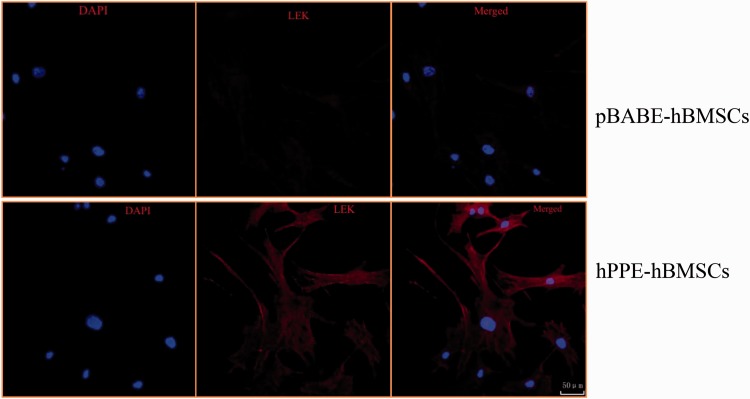
The L-EK protein showed low expression in the cytoplasm of hBMSCs and pBABE-hBMSCs and was highly expressed in hPPE-expressing hBMSCs, as shown in Figure 3.
Figure 3.
The different expressions levels of Leu-enkephalin (L-EK) protein were compared between the pBABE-hBMSC group and the hPPE-hBMSC group by immunofluorescence. pBABE, a retroviral vector; hPPE, human proenkephalin; hBMSCs, human bone marrow stem cells. pBABE-hBMSCs, the pBABE-hBMSCs group; hPPE-hBMSCs, the hPPE-hBMSCs group. Blue fluorescence marks the nucleus of the hBMSCs by 4′6-diamidino-2-phenylindole. Red fluorescence marks L-EK protein. Double-labeled cells, with a blue fluorescent nucleus and a red cytoplasm, represented hBMSCs that expressed the L-EK. There were no differences in nuclear staining between the groups. Little L-EK expression was observed in the pBABE-hBMSC group, whereas greater expression was detected in the hPPE-hBMSC group. All images were obtained on a laser scanning confocal microscope (Leica). Scale bars = 50µm. n = 6.
Effect of intrathecal administration of hPPE-hBMSCs
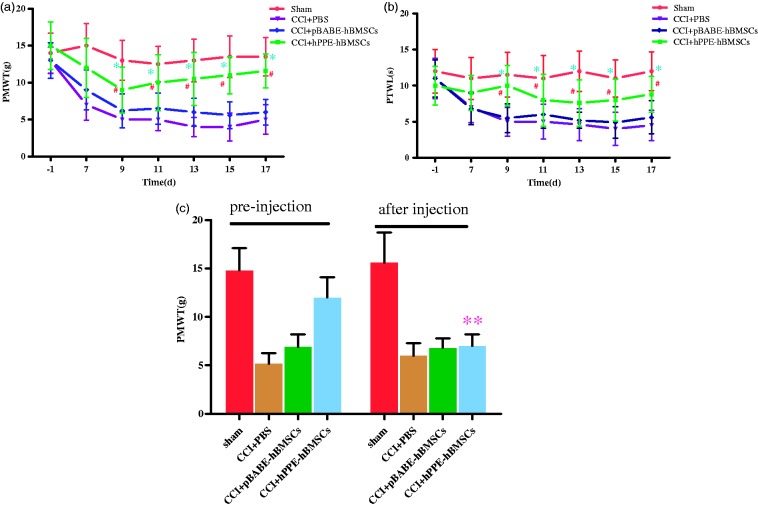
Before CCI surgery, the basic values for PMWT and PTWL in all groups were not significantly different. PMWT and PTWL in CCI operation groups decreased markedly on day seven after CCI operation compared with the sham group. PMWT and PTWL in CCI group were significantly lower than those in the sham group (P < 0.01), indicating the presence of mechanical allodynia and thermal hyperalgesia. However, the reduction of PMWT and PTWL caused by CCI surgery was inhibited by intrathecal injection of pBABE-hBMSCs and hPPE-hBMSCs, and there existed difference in the extent of improvements for PMWT and PTWL. Compared with the CCI+PBS group, PMWT and PTWL in the CCI+pBABE-hBMSC and CCI+hPPE-hBMSC groups were higher, but the difference was not significant in the CCI+pBABE-hBMSC group (P > 0.05) and significant in CCI+hPPE-hBMSC group (P < 0.05), suggesting that compared with vehicle treatment, hPPE gene treatment significantly prevented development of mechanical allodynia and thermal hyperalgesia starting at day nine (Figure 4(a) and (b)) in CCI rats.
Figure 4.
Changes in (a) tactile allodynia (PWMT) and (b) thermal hyperalgesia (PWTL) after CCI and intrathecal (i.t.) delivery of cells. The persisting analgesic activity could be reversed by the opioid receptor antagonist naloxone. (c) Rats were left unoperated in the Sham group (Sham). The other three groups of rats underwent CCI. After five days, the rats were administered vehicle (PBS), pBABE-hBMSCs, or hPPE-hBMSCs (10 µl). PWMT and PWTL were measured before operation and at 7, 9, 11, 13, 15, and 17 days after CCI operation. PWMT was determined 30 min after injection of naloxone. Data are presented as the means ± SD of ipsilateral hind paw observations. Significance was defined as **P < 0.05 versus CCI+hPPE-hBMSCs pre-injection, #P < 0.05, *P < 0.01 versus CCI+PBS group, n = 6. CCI, chronic constriction injury; pBABE, a retroviral vector; hPPE, human proenkephalin; hBMSCs, human bone marrow stem cells. Sham, the Sham group; CCI+PBS, the CCI+PBS group; pBABE-hBMSCs, the pBABE-hBMSC group; hPPE-hBMSCs, the hPPE-hBMSC group.
Naloxone reversed the analgesic effect of Met-enk on mechanical hyperalgesia
To determine whether met-enkephalin plays a major role in neuropathic pain relief, naloxone can be used to reverse the antiallodynia effect due to met-enkephalin. Thus, naloxone was injected intraperitoneally 30 min after PMWT had been assessed on day 17 after CCI surgery, and PMWT was measured again. PMWT was significantly reduced after naloxone administration (P < 0.05). The data also indicated that met-encephalin secreted by hPPE-hBMSCs might mediate the antiallodynia effect (Figure 4(c)).
L-EK level detection
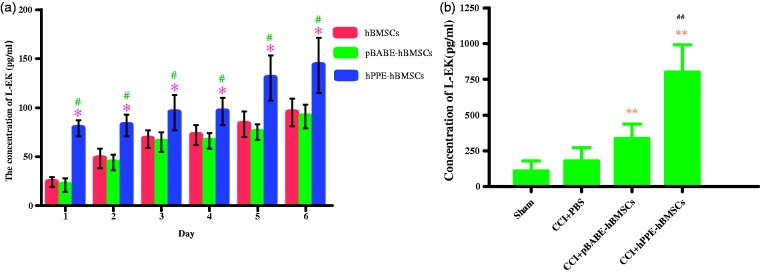
Naïve hBMSCs, pBABE-hBMSCs, and hPPE-hBMSCs all produced and released L-EK into the culture medium at different levels at each time point in vitro, as shown in Figure 5(a), the level of L-EK released by hPPE-hBMSCs was significantly augmented compared with that released by hBMSCs and pBABE-hBMSCs (P < 0.05) in serum-free cultures. In addition, L-EK production increases with time, indicating that the reprogrammed cells could survive in the CNS and function normally. In vivo, the concentration of L-EK in the spinal cord was higher in the pBABE-hBMSC and hPPE-hBMSC groups than in the CCI+PBS group (P < 0.05). The hPPE-hBMSC group demonstrated a significantly higher concentration than the pBABE-hBMSC group (P < 0.05), indicating that L-EK contents increased evidently in the spinal cord after hPPE gene transfer (Figure 5(b)).
Figure 5.
Detecting the production of Leu-enkephalin (L-EK) released by human bone marrow stem cells (hBMSCs) modified with human proenkephin (hPPE) in vitro (a) and in vivo (b). In vitro, the supernatants in three groups such as the hBMSC, pBABE-hBMSC, and hPPE-hBMSC groups were collected for the concentration measurement of L-EK for six days after cells were seeding into culture plates. In vivo, the concentrations of L-EK in CSF in four groups such as the sham, CCI+PBS, CCI+pBABE-hBMSC, and CCI+hPPE-hBMSC groups on the 14th day after CCI operation were detected (n = 6). Enzyme immunoassay (ELISA) method was used for the measurement. Data are presented as the means ± SD, significance was defined as *P < 0.05 versus the hBMSC group, #P < 0.05 versus the pBABE-hBMSC group, **P < 0.05 versus the CCI+PBS group, ##P < 0.05 versus the CCI+pBABE-hBMSC group. CCI, chronic constriction injury; pBABE, a retroviral vector; hBMSCs, the hBMSC group; pBABE-hBMSCs, the pBABE-hBMSC group; hPPE-hBMSCs, the hPPE-hBMSC group; Sham, the Sham group; CCI+PBS, the CCI+PBS group; CCI+pBABE-hBMSCs, the CCI+pBABE-hBMSC group; CCI+hPPE-hBMSCs, the CCI+hPPE-hBMSC group.
Discussion
This study demonstrates the pain-relieving effect of the intrathecal injection of hBMSCs transfected with the hPPE gene in a rat nociceptive model. It was known that BMSCs had an important characteristic of homing and they can migrate to sites of tissue injured. Although some studies have shown that intravenous delivery of BMSCs in neuropathic rat could reduce pain21, one of the aforementioned studies suggested that 24 h after injection, most of BMSCs relocated in liver and other internal organs22. Another study indicated that 1.7% of total injected hMSCs survived23. Can the cells that survive produce the desired effect? So, much research still needed to understand the homing capabilities of BMSCs. Intrathecal administration was used in this study for which had been proved a viable and effective option for gene therapy of chronic pain24,25 in former studies.
Nowadays, BMSC represent potential candidate in pain-care research26. Compared with vast quantities of articles focused on nerve injury, researches about BMSCs use in chronic pain therapy was relatively scarce and most of them highlighted BMSCs transplantation in treatment. The previous articles reported that BMSCs hold the inherent gene expression of proenkephalin opioid peptides, such as met- and L-EK, and are able to release a low basal level of L-EK, a major neurotransmitter that plays an important role in analgesia by activating opioid receptors27. So, in the study, the hPPE gene was chosen as the targeted gene for two reasons: one is that the hPPE gene, which controls the expression of endogenous opioid peptide, has been widely used in biological research in neuropathic pain therapy and recommended by researchers in this field28, the other is that the transfected gene can induce the cells to produce the same opioid peptide as the naïve BMSCs. It was easy to analyze the relation between the effects and the quantity of analgesic substances.
In our study, some potential characteristics and mechanism of action about BMSC were also certified. For example: (1) BMSCs can be easily cultured and survived in vitro29 and exhibit low immunogenicity30,31. BMSCs can act as cellular vehicles for gene delivery and are being developed to treat neuropathic pain32. In the present study, results have shown that hBMSCs can be efficiently transduced with retroviral vectors. The hPPE overexpression can also be achieved in the spinal cord by hPPE gene-modified hBMSCs transplantation. The hPPE-modified hBMSCs can improve the protective effect of hBMSC therapy for CCI. (2) HBMSCs can secrete a variety of growth factors and cytokines contributing to reparation after a CNS injury. That partly explains why hBMSCs transplantation reduces peripheral nerve injury or neuropathic pain33. In this study, the weak expression of hPPE, L-EK protein, and low production in hBMSCs engineered with vector were confirmed. In the nociceptive behavior tests, PMWT and PTWL in CCI+pBABE-hBMSC group were higher than those in CCI+PBS group after intrathecal injection (P > 0.05). We also emphasized on gene transfer with hBMSCs and the effects of treatment on CCI rats. We noticed that hBMSCs showed a high genetic stability. When hPPE gene was integrated into hBMSCs, there were no changes on expression of cell surface antigens and differentiation. Results indicated that there were significantly high expression of hPPE gene, L-EK protein, and production of L-EK. PMWT and PTWL were higher in hPPE-hBMSC group than in the other groups after intrathecal injection and PMWT was reduced significantly after naloxone administration (P < 0.05). The data also indicated that met-encephalin secreted by hPPE-hBMSCs might mainly mediate the anti-allodynia effect. Integration with viral and nonviral vectors could improve the efficacy of BMSC in therapy of neuropathic pain and makes BMSC a useful tool in gene therapy of pain. Above all, the incomparable advantages of BMSC over former types of transplanted cells make BMSC a possible solution for neuropathic pain.
In our study, a high dose of hBMSCs, corresponding to 6 × 106 cells was injected to detect the nociceptive effects on CCI mediated pain. Emerging studies suggested neuropathic pain model rats had improved behavioral impairment in a dose-dependent manner, so we use the highest dose recommended, with which no animal died or changed its habits, and no side effects have been observed34. In the present study, we found that on day one after CCI, rats developed obvious mechanical allodynia and thermal hyperalgesia, which were the most obvious on day seven and remained until day 17 after CCI, so we injected the cells transfected with hPPE or vehicle into the CCI rats on day 7 and a complete, rapid, and long-lasting response was observed. The hPPE-hBMSCs effects on allodynia and hyperalgesia were fully achieved after their administration and compared with CCI+vehicle-treated group; the hyperalgesia and allodynia were significantly reduced.
It has been confirmed that BMSC can express the gene of proenkephalin opioid peptides and release a low basal level of L-EK28; so, naïve BMSC and BMSC transfected with the control vector have almost the same level of L-EK in vitro, and then, injection of BMSC transfected with the vector have a significant increase of L-EK level compared with injection of PBS. In vitro experiment, there is a constant increase of L-EK with incubating time prolonging in all groups, maybe the reason is that BMSC have the vigorous potential for proliferation and can be passaged, so can be a good choice for cell transplantation.
Gene transfection is a potential and valuable method for pain therapy35. With the help of gene transfer, the expression of target peptides would be up-regulated and augmented efficiency would be provided. When cell transplantation is in use, more cells are needed for injection, the chance of inflammation will increase and the complexity of operation will extend. Once cells get into blood infusion, most of them will remain in some organs including liver, spleen, kidney, and lung, which will influence the function of those organs and become heavy burden to them. Besides, the effect of cell transplantation is temporary and transient, repeated injection will be required to keep the efficacy. So cell transplantation cannot provide permanent and satisfactory therapeutic effects. In our study, the expression of hPPE in the genetically modified BMSC population was greatly enhanced compared to that of naive MSCs and that secretion of levels L-EK was significantly augmented in genetically modified BMSCs compared to secretions released by naïve BMSCs. The results indicated that gene transfer with the advantages of high efficiency and stable and long-lasting action will be focused as a useful means in pain treatment.36,37
In conclusion, the present study demonstrated that intrathecal administration of genetically modified BMSCs could be a valid alternative for the treatment of neuropathic pain and has vast potential for future development.
Author Contributions
Dr Yi Sun is responsible for the writing of the manuscript. Dr Dengwen Zhang, Dr Haifeng Li, Dr Ruichun Long, and Dr Qiang Sun are in charge of completion of experiment and providing the data.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008; 70: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 2.Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008; 137: 681–688. [DOI] [PubMed] [Google Scholar]
- 3.Siniscalco D, Rossi F, Maione S. Stem cell therapy for neuropathic pain treatment. J Stem Cells Regen Med 2007; 3: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu T, Shi G, Ma K, et al. Targeted cell reprogramming produces analgesic chromaffin-like cells from human mesenchymal stem cells. Cell Transplant 2013; 22: 2257–2266. [DOI] [PubMed] [Google Scholar]
- 5.Ginzburg R, Seltzer Z. Subarachnoid spinal cord transplantation of adrenal medulla suppresses chronic neuropathic pain behavior in rats. Brain Res 1990; 523: 147–150. [DOI] [PubMed] [Google Scholar]
- 6.Buchser E, Goddard M, Heyd B, et al. Immunoisolated xenogenic chromaffin cell therapy for chronic pain. Initial clinical experience. Anesthesiology 1996; 85: 1005–1012. discussion 29A-30A. [DOI] [PubMed] [Google Scholar]
- 7.Siniscalco D, Giordano A, Galderisi U. Novel insights in basic and applied stem cell therapy. J Cell Physiol 2012; 227: 2283–2286. [DOI] [PubMed] [Google Scholar]
- 8.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol 2013; 4: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer S, Berger JV, Deumens R, et al. Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury. J Neuroinflamm 2014; 11: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Zeng W, Fu Y, et al. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol 2015; 8: 11957–11969. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Guo D, Peng L, et al. Repair of rabbit cartilage defect based on the fusion of rabbit bone marrow stromal cells and Nano-HA/PLLA composite material. Artif Cells Nanomed Biotechnol 2017; 45: 115–119. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Park CK, Xie RG, et al. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest 2015; 125: 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua Z, Liu L, Shen J, et al. Mesenchymal stem cells reversed morphine tolerance and opioid induced hyperalgesia. Sci Rep 2016; 6: 32096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pohl M, Braz J. Gene therapy of pain: emerging strategies and future directions. Eur J Pharmacol 2001; 429: 39–48. [DOI] [PubMed] [Google Scholar]
- 15.Yang J1, Hu P, Zhou M, et al. Construction of retroviral vector carrying Twist gene and its induction of epithelial-mesenchymal transition in human mammary epithelial cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013; 29: 905–909. [PubMed] [Google Scholar]
- 16.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A 1996; 93: 11400–11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 18.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 20.Zou W, Guo Q, Chen C, et al. Intrathecal herpes simplex virus type 1 amplicon vector-mediated human proenkephalin reduces chronic constriction injury-induced neuropathic pain in rats. Mol Med Rep 2011; 4: 529–533. [DOI] [PubMed] [Google Scholar]
- 21.Han D, Wu C, Xiong Q, et al. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochem Biophys 2015; 71: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 22.Mitkari B, Kerkela E, Nystedt J, et al. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol 2013; 239: 158–162. [DOI] [PubMed] [Google Scholar]
- 23.Park HJ, Lee PH, Bang OY, et al. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem 2008; 107: 141–151. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Park KS, Yoon JJ, et al. Anti-allodynic effect of intrathecal processed Aconitum jaluense is associated with the inhibition of microglial activation and P2X7 receptor expression in spinal cord. BMC Complement Altern Med 2016; 16: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsivais JJ, Monsivais DB. The long-term safety and efficacy of intrathecal therapy using sufentanil in chronic intractable non-malignant pain. Korean J Pain 2014; 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortino VR, Pelaez D, Cheung HS. Concise review: stem cell therapies for neuropathic pain. Stem Cells Transl Med 2013; 2: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagen J, Pappas GD, Perlow MJ. Adrenal medullary tissue transplants in the rat spinal cord reduce pain sensitivity. Brain Res 1986; 384: 189–194. [DOI] [PubMed] [Google Scholar]
- 28.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007; 25: 2896–2902. [DOI] [PubMed] [Google Scholar]
- 29.Kamishina H, Deng J, Oji T, et al. Expression of neural markers on bone marrow-derived canine mesenchymal stem cells. Am J Vet Res 2006; 67: 1921–1928. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Yan Q, Zeng YS, et al. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res 2010; 1359: 256–271. [DOI] [PubMed] [Google Scholar]
- 31.Alexanian AR, Maiman DJ, Kurpad SN, et al. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev 2008; 17: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 32.Musolino PL, Coronel MF, Hokfelt T, et al. Bone marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett 2007; 418: 97–101. [DOI] [PubMed] [Google Scholar]
- 33.Pisati F, Bossolasco P, Meregalli M, et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant 2007; 16: 41–55. [DOI] [PubMed] [Google Scholar]
- 34.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011; 20: 1297–1308. [DOI] [PubMed] [Google Scholar]
- 35.Eaton MJ. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma 2006; 23: 549–559. [DOI] [PubMed] [Google Scholar]
- 36.Fink DJ, Mata M. HSV gene transfer in the treatment of chronic pain. Sheng Li Xue Bao 2008; 60: 610–616. [PMC free article] [PubMed] [Google Scholar]
- 37.Sugaya I, Qu T, Sugaya K, et al. Genetically engineered human mesenchymal stem cells produce met-enkephalin at augmented higher levels in vitro. Cell Transplant 2006; 15: 225–230. [DOI] [PubMed] [Google Scholar]