Abstract
The genus Deschampsia P. Beauv (Poaceae) involves a group of widespread polymorphic species. Some of them are highly tolerant to stressful and variable environmental conditions, and D. antarctica is one of the only two vascular plants growing in Antarctic. This species is a source of useful for selection traits and a valuable model for studying an environmental stress tolerance in plants. Genome diversity and comparative chromosomal phylogeny within the genus have not been studied yet as karyotypes of most Deschampsia species are poorly investigated. We firstly conducted a comparative molecular cytogenetic analysis of D. antarctica (Antarctic Peninsula) and related species from various localities (D. cespitosa, D. danthonioides, D. elongata, D. flexuosa (= Avenella flexuosa), D. parvula and D. sukatschewii by fluorescence in situ hybridization with 45S and 5S rDNA, DAPI-banding and sequential rapid in situ hybridization with genomic DNA of D. antarctica, D. cespitosa, and D. flexuosa. Based on patterns of distribution of the examined markers, chromosomes of the studied species were identified. Within these species, common features as well as species peculiarities in their karyotypic structure and chromosomal distribution of molecular cytogenetic markers were characterized. Different chromosomal rearrangements were detected in D. antarctica, D. flexuosa, D. elongata and D. sukatschewii. In karyotypes of D. antarctica, D. cespitosa, D. elongata and D. sukatschewii, 0–3 B chromosomes possessed distinct DAPI-bands were observed. Our findings suggest that the genome evolution of the genus Deschampsia involved polyploidy and also different chromosomal rearrangements. The obtained results will help clarify the relationships within the genus Deschampsia, and can be a basis for the further genetic and biotechnological studies as well as for selection of plants tolerant to extreme habitats.
Introduction
The genus Deschampsia P. Beauv. (Poaceae) includes more than 30 polymorphic species with a wide geographical distribution, high morphological diversity and complicated taxonomy [1–6]. Some of Deschampsia species are highly tolerant to stressful and variable environmental conditions, and this tolerance allows them to colonize plots of land which are uninhabited by other plants [7]. The core of the genus is mainly represented by subspecies of nearly cosmopolitan D. cespitosa (L.) P. Beauv. (D. cespitosa complex) that can exist even in extreme Arctic habitats [8]. Compared to the Arctic flora with many flowering species, only two vascular plants were found in the Antarctic regions of the similar latitude, and D. antarctica E. Desv. is the only species in the Poaceae family (and the only Deschampsia species) that has developed morphological and physiological adaptation to the harshest Antarctic environments (extremely low temperatures, drought, high salinity and flooding, high level of UV radiation, low precipitation) [9–10]. D. antarctica could serve as a model for the study of regulation of genome activity and the mechanisms responsible for plant adaptation to freezing, light stress or photosynthetic capacity at low temperatures [11–16]. D. antarctica might also be a useful source of genes associated with stress tolerance and environmental adaptation, and its karyotype can be a basic tool for crop breeding strategies in agronomical valuable crops [17–19]. Besides, extracts from D. antarctica display protective effects against ultraviolet radiation and are suitable for a number of pharmaceutical applications [20].
The unique genome of D. antarctica is being intensively investigated. Particularly, a multi-gene family encoding ice recrystallization inhibition proteins which are related to freeze tolerance has been analyzed [15–16]; the chloroplast genome has been sequenced and plastid transcriptome profiles of the coding/noncoding genes have been studied [5, 18]; the polypeptide with lipase activity (Lip3F9) has been characterized [21]. However, currently insufficient nuclear genomic data is still available for this species [5, 16, 21].
Phylogenetic relationships within the genus Deschampsia is still being under investigation [22–23]. According to the phylogenetic studies based on the analysis of nuclear ribosomal internal transcribed spacer (ITS) and plastid trnL intron sequences, most Deschampsia species are joined in one clade. Inside this clade, D. antarctica together with D. venustula Parodi and D. parvula (Hook.f.) E. Desv. forms a small subclade. It was suggested that two species D. antropurpurea (Wahlenb.) Scheele (= Vahlodea atropurpurea (Wahlenb.) Fr. and D. flexuosa (L.) Trin. (= Avenella flexuosa (L.) Drejer)) should be excluded from the genus Deschampsia and included to the allied genera Vahlodea and Avenella (correspondingly) despite morphological similarity of D. flexuosa and D. cespitosa [2, 22–24].
The analysis of karyotypes of Deschampsia species performed by modern molecular cytogenetic methods is important for better understanding of phylogeny, taxonomy and evolution of the genus and for investigation of the unique D. antarctica genome. However, genome diversity and comparative chromosomal phylogeny in the genus Deschampsia have not been studied as currently available molecular cytogenetic data on most Deschampsia species are rather limited. In three species, D. cespitosa, D. flexuosa and D. setacea (Huds.) Hack., distribution of Giemsa C-heterochromatin was described [1]. In D. cespitosa, chromosomal localization of rDNA and satellite DNAs was detected [25, 26]. Recently, the molecular cytogenetic analysis of D. antarctica grown in different localities of the Maritime Antarctic and South America has been performed [23, 27]. For the other species of the genus, only chromosome numbers were determined by simple monochrome staining [28], and further comparative cytogenetic analysis of Deschampsia species is needed. Such studies provide important information on possible karyotypic rearrangements which occur during the speciation as well as direction of chromosomal changes in related groups. Besides, environmental stress factors can cause different changes in genome structure of plants (chromosome rearrangements, mixo- or aneuploidy) and they can also be revealed by molecular cytogenetic approaches [27, 29–33].
In the present paper, a comparative molecular cytogenetic analysis of D. antarctica (Antarctic Peninsula) and its relative species (close and distant) from different localities (D. cespitosa; D. danthonioides (Trin.) Munro; D. flexuosa; D. elongata (Hook.) Munro; D. parvula and D. sukatschewii (Popl.) Roshev. was firstly carried out. The karyotypes of these Deschampsia species were studied by DAPI-banding, fluorescence in situ hybridization (FISH) with 45S and 5S rDNA probes and sequential rapid genomic in situ hybridization (rapid GISH) with genomic DNA of D. antarctica, D. cespitosa and D. flexuosa.
Materials and methods
Ethics statement
This study including sample collection and experimental research conducted on these materials was according to the law on activities and environmental protection to Antarctic approved by the Ministry of Education and Science of Ukraine.
Plant material
The seeds of D. antarctica growing under natural conditions were obtained in the course of the research Antarctic expeditions (seasons 2005–2010) organized by the National Antarctic Scientific Center of Ukraine. The seeds were collected on Rasmussen Cape (culmination of a rocky plateau: S65, S65°14.819´, W64°5.156´) located in the western coast of the Antarctic Peninsula (the vicinity of the Ukrainian Antarctic Station “Academician Vernadsky”).
The seeds of D. cespitosa (PI 314562 Moscow, Russia; W6 25808 Troms, Norway; PI 577069 Wales, UK), D. danthonioides (PI 665596 Oregon, USA; W6 39054 Washington, USA; W6 36940 Oregon, USA), D. flexuosa (PI 577075 Wales, UK; PI 422612 France) and D. elongata (PI 665545 Oregon, USA) were obtained from the germplasm collection of Western Regional Plant Introduction Station, USDA ARS NPGS, Pullman, WA, USA.
The seeds of D. parvula (661849 Weddell Island, Falkland Is.) were obtained from the germplasm collection of Seed Conservation Department, Royal Botanic Gardens, Kew, UK.
The seeds of D. sukatschewii (Popl.) Roshev. (78 Altai, Russia) were obtained from the germplasm collection of All-Russian Williams Fodder Research Institute, Moscow, Russia.
Fixation
To produce plant material of D. antarctica in sufficient quantity, dry seeds were sterilized and germinated as described earlier [34]. The obtained aseptic plants were cultivated on B5 agar nutrient medium [35] supplemented with 0.1 mg/L α-naphthaleneacetic acid (NAA) and then the plants were in vitro propagated through fragmentation of the obtained root mat. Seeds of the other Deschampsia species were germinated in Petri dishes with moist filter paper. Then the plants were grown in a greenhouse at 15°C.
For cell cycle synchronization and accumulation of mitotic divisions, root tips were incubated in ice water for 24 hours at 0°C and then fixed in the ethanol:glacial acetic acid fixative (3:1) for 48 h at room temperature. Fixed roots were stored in the fixative at -20°C before use.
Chromosome spread preparation
For in situ hybridization and DAPI staining, before chromosome spread preparation, the roots were stained in 1% acetocarmine solution in 45% acetic acid for 40 min, the tip caps with root meristem were cut on the object-plate, the meristem was macerated in a drop of 45% acetic acid, and then squashed chromosome preparations were made. The cover slips were removed after freezing, and the preparations were dehydrated and stored in 96% ethanol at -20°C before use.
Fluorescence in situ hybridization
Following probes were used for FISH:
pTa71 containing a 9 kb long DNA sequence of common wheat encoding 18S, 5.8S and 26S rRNA genes including spacers [36];
pTa794 containing a 420 bp long DNA sequence of wheat containing the 5S rRNA gene and the intergenic spacer [37];
FISH assays were performed using combinations of rDNA probes labelled directly with fluorochromes SpectrumAqua or SpectrumRed (Abbott Molecular, Wiesbaden, Germany) by nick translation according to manufacturers’ protocols. FISH procedure was conducted as described previously [27, 38].
DAPI staining
After FISH, chromosome slides were stained with 0.1 μg/ml DAPI (4',6-diamidino-2-phenylindole (DAPI, (Serva, Heidelberg, Germany)) in Vectashield mounting medium (Vector laboratories, Peterborough, UK).
Rapid GISH procedure
Genomic DNA of D. antarctica, D. cespitosa (PI 577069) and D. flexuosa (PI 577075) was isolated from young leaves using CTAB (cetyltrimethylammonium bromide) standard protocol [39] and labelled directly with SpectrumAqua or SpectrumRed (Abbott Molecular, Wiesbaden, Germany) by nick translation according to the manufacturer’s instructions. After FISH procedures and documentation of the hybridization patterns, the chromosome slides were washed twice in 2xSSC for 10 min, dehydrated in an ethanol series (70%, 85%, 96%) for 3 min in each and air dried. Then rapid GISH procedure was conducted on the same slides as described previously [27].
Chromosomal analysis
The slides were examined using Olympus BX61 epifluorescence microscope (Olympus, Tokyo, Japan). Images were taken with monochrome CCD camera (Cool Snap, Roper Scientific Inc., Tucson, USA) and sequentially collected in grayscale channels. Then, they were pseudocoloured and processed with Adobe Photoshop 10.0 (Adobe Systems Inc., USA) and “VideoTesT-FISH 2.1” (IstaVideotest, St Petersburg, Russia) software. At least five plants of each Deschampsia accession and fifteen metaphase plates from each sample were analyzed. Chromosome pairs in the species karyograms were set (considering their morphological similarities and distribution of the examined markers) according to the D. antarctica karyogram described previously [27] for simplification of the comparative karyotype analysis of different species.
Results
Karyotype structure
Most accessions of the studied Deschampsia species presented diploid karyotype with 2n = 26 chromosomes with the exception of both D. flexuosa accessions with 2n = 28 chromosomes (Figs 1–3). Besides, D. cespitosa accession PI 577069 presented a tetraploid cytotype with 2n = 52 chromosomes.
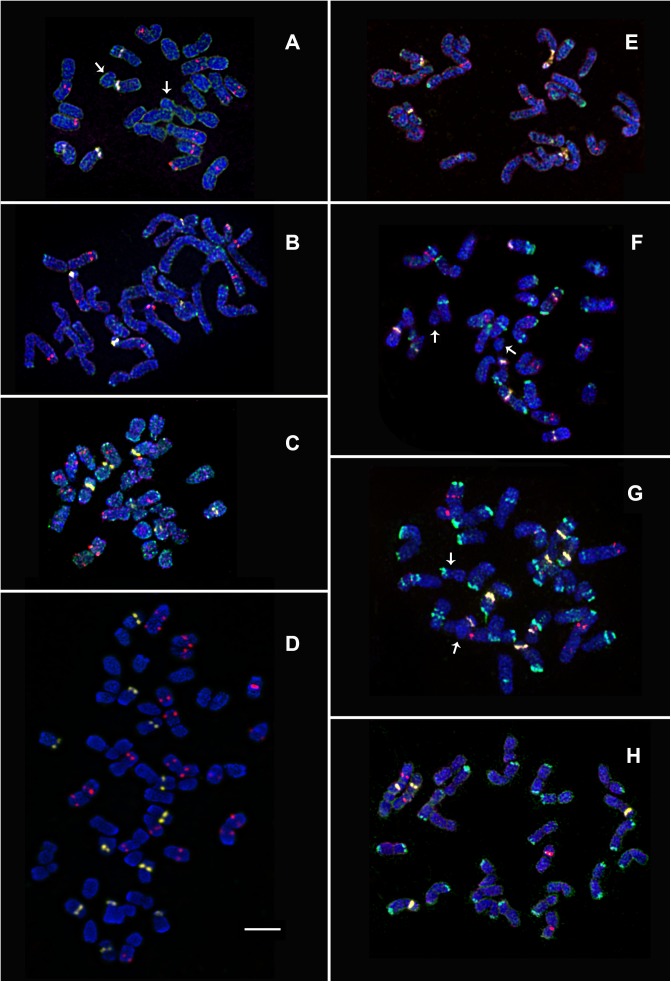
Fig 1. Chromosome spreads of Deschampsia species.
(A) D. antarctica (2n = 26+2B), (B) D. parvula (2n = 26), (C) D. cespitosa (2n = 26), (D) D. cespitosa (2n = 4x = 52), (E) D. danthonioides (2n = 26), (F) D. elongata (2n = 26+2B), (G) D. sukatschewii (2n = 26+M+B). Merged fluorescent images after multicolour FISH with 45S (yellow) and 5S (red) rDNA and sequential rapid GISH with both genomic DNAs: D. cespitosa (green) and D. flexuosa (purple) (A, E, F and G); D. cespitosa (green) and D. antarctica (purple) (H, B); D. antarctica (green) and D. flexuosa (purple) (C). Image after FISH with 45S (yellow) and 5S (red) rDNA (D). Chromosomal DAPI-staining–blue. Arrows point to the marker and B chromosomes. Scale bar—5 μm.
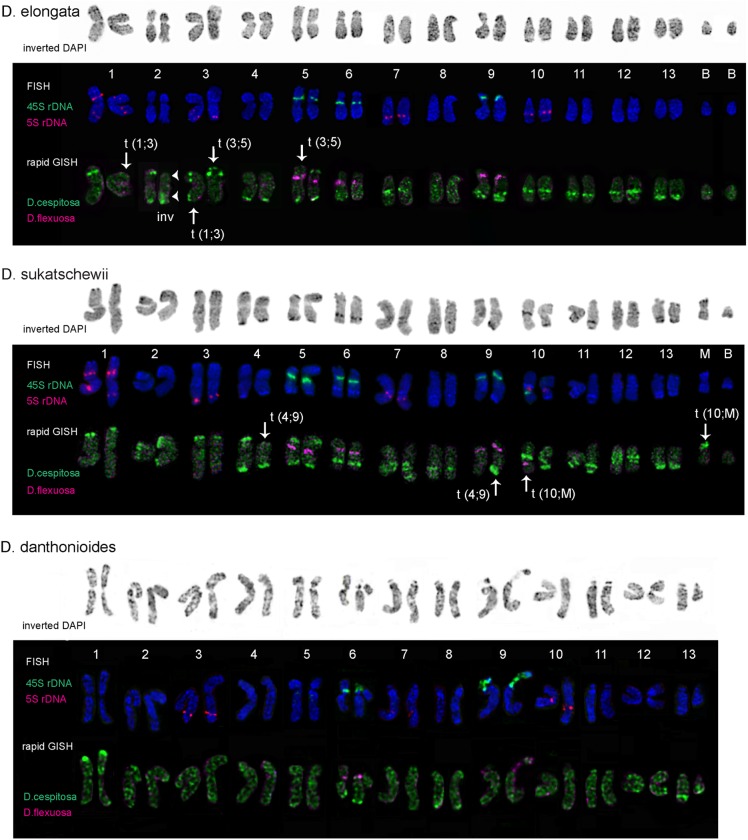
Fig 3. Karyotypes of specimens of D. elongata, D. sukatschewii and D. danthonioides.
Karyograms of the metaphase plates shown in Fig 1 after DAPI-banding (inverted images), FISH with 45S (green) and 5S (red) rDNA, and also rapid GISH with genomic DNA of D. cespitosa (green) and D. flexuosa (red). The correspondent probes and their pseudo-colours are specified in the left. Arrows point to chromosome rearrangements. B–B chromosomes. M–a marker chromosome.
In karyotypes of D. antarctica, D. cespitosa, D. elongata and D. sukatschewii, 0–3 supernumerary very small chromosomes were observed together with 26 chromosomes of the basic set (A chromosomes) (Figs 1–3). These chromosomes had uncertain morphology, could be present or absent among the individuals and probably were referred to B chromosomes.
The analysis of chromosome morphology of both D. flexuosa accessions showed that its karyotype structure differed greatly from karyotypes of the other studied Deschampsia species and contains only metacentric and submetacentric chromosomes which were not very different in size and morphology (Figs 1 and 2).
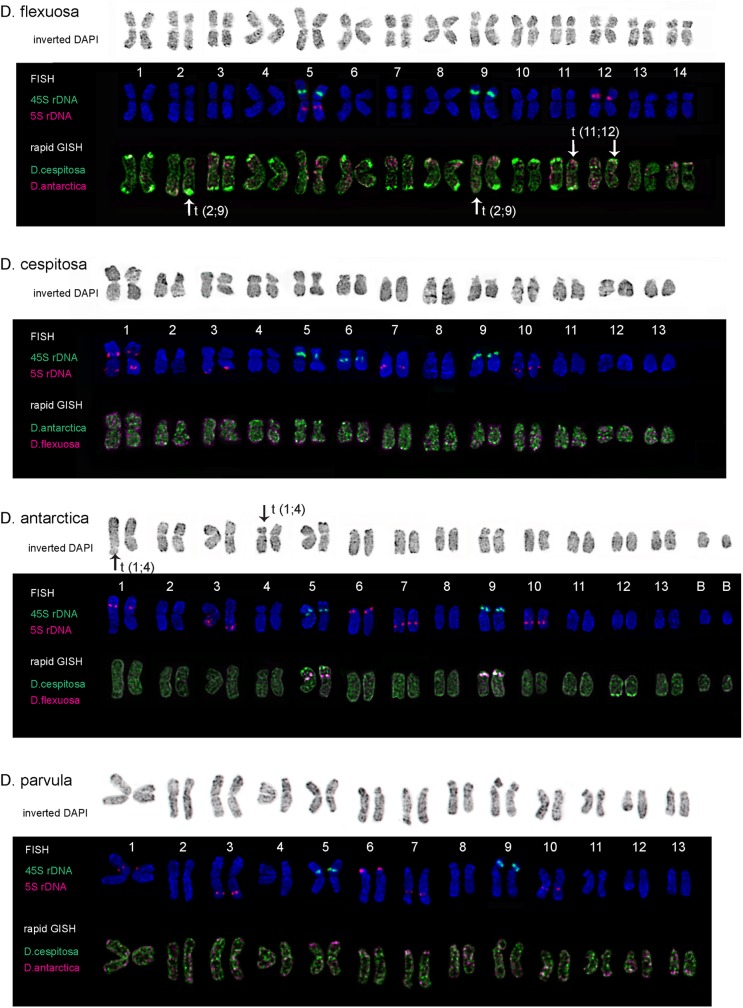
Fig 2. Karyotypes of D. flexuosa, D. cespitosa, D. antarctica and D. parvula.
Karyograms of the metaphase plates shown in Fig 1 after DAPI-banding (inverted images), FISH with 45S and 5S rDNA, and also rapid GISH with genomic DNA of D. cespitosa, D. antarctica and D. flexuosa. The correspondent probes and their pseudo-colours are specified in the left. Arrows point to chromosome translocations. B–B chromosomes.
Karyotypes of the other studied Deschampsia species presented common features in their structure, in particular, similar chromosome sizes and centromeric positions with one pair of metacentric chromosomes being longer than the other ones. Chromosomes in their karyotypes were subdivided into four groups: metacentric (m), submetacentric (sm), subtelocentric (st) and telocentric (t) according to chromosomes centromeric positions and morphology.
Chromosome localization of 45S and 5S rDNA sites
In D. antarctica, FISH analysis showed that 45S rDNA loci were localized in two chromosome pairs (Fig 2): in the proximal region of the short arm of a pair of m chromosomes (chromosome 5) and in the distal region of the short arm of a sm chromosome pair (9). The secondary constriction of chromosome 9 was well-defined, and the 45S rDNA site was larger (though the satellite was smaller) compared with the other satellite (SAT) chromosome pair. We observed 5S rDNA sites on five chromosome pairs (Fig 2): in the proximal region of the short arm of the largest m chromosome (1), in the subtelomeric regions of the long arm of a m chromosome (3), in the subtelomeric regions of the short arm of a m chromosome (6), in the proximal regions of the long arms of a pair of st chromosomes (7) and a pair of t chromosomes (10).
In D. parvula, the pattern of 45S and 5S rDNA distribution was rather similar to that observed in D. antarctica karyotype, but there were differences in proportions of the revealed 5S rDNA. Relatively large 5S rDNA sites were found on chromosomes 3 and 6. The other 5S rDNA sites (on chromosomes 1, 7 and 10) were very small, and they were not always visualized (Fig 2).
In karyotypes of the studied D. cespitosa accessions and D. elongata, FISH analysis showed similar distribution of 45S and 5S rDNA. We detected three SAT chromosome pairs with 45S rDNA loci. Two SAT chromosome pairs (5 and 9) were very similar to those found in D. antarctica and D. parvula karyotypes; also the third SAT chromosome (6) with a large satellite was revealed (Figs 2 and 3). We observed ten 5S rDNA sites located on four chromosome pairs: in the proximal regions of the short and long arms of the largest m chromosome (1), in the subtelomeric regions of the long arm of a large m chromosome (3), in the proximal regions of the long arms of a pair of st chromosomes (7) and a pair of t chromosomes (10) (Figs 2 and 3). In karyotypes of the tetraploid D. cespitosa accession, 45S and 5S rDNA sites were localized in the similar positions and with similar proportions if compared to the diploid plants (Fig 1).
In karyotype of D. sukatschewii, multiple chromosomal rearrangements were detected. In particular, in each metaphase cell of the studied accession, we observed co-localization of the 5S and 45S rDNA sites in one of the homologs of chromosome 10 (the long arm) and also a marker chromosome (M) which probably resulted from a translocation between chromosome 10 and an unknown supernumerary chromosome having a 45S rDNA site (Figs 1 and 3). Nevertheless, the pattern of chromosomal distribution of 45S and 5S rDNA loci was similar (with the variation mentioned above) to that described for D. cespitosa and D. elongata.
In karyotypes of the studied D. danthonioides accessions, FISH analysis revealed two SAT chromosomes with 45S rDNA loci (chromosomes 6 and 9) (Fig 3). In some karyotypes, minor 45S rDNA sites were revealed in the proximal region of chromosome 5. In most cases, 5S rDNA sites were observed on chromosomes 3 and 10. In some karyotypes, very small 5S rDNA site was also detected on chromosome 7 (Fig 3). The revealed 45S and 5S rDNA sites were located in the similar positions if compared to the other studied Deschampsia species (with exception of D. flexuosa).
The pattern of chromosomal localization of 45S and 5S rDNA loci in karyotypes of both D. flexuosa accessions differed from the patterns of rDNA distribution in karyotypes of the other studied Deschampsia species. 45S rDNA loci were detected in the secondary construction regions (the short arms) of two pairs of SAT chromosomes. Also, two sites of 5S rDNA were detected. One 5S rDNA locus was localized in the proximal region of the long arm of one SAT chromosome pair. The second 5S rDNA site was revealed in the pericentromeric region of the short arm of a pair of metacentric chromosomes (Fig 2).
Chromosomal markers revealed by rapid GISH
Rapid GISH procedure with total genomic DNA of D. antarctica as a DNA probe was performed on chromosomes of D. cespitosa, D. parvula and D. flexuosa. Multiple rapid GISH markers (in different positions) were detected on chromosomes of the studied D. cespitosa accessions (Fig 2). The dispersed hybridization signals were observed along chromosomes of D. parvula and both D. flexuosa accessions (Fig 2).
Rapid GISH procedure with total genomic DNA of D. cespitosa (PI 577069) as a DNA probe was performed on chromosomes of D. antarctica, D. danthonioides, D. flexuosa, D. elongata, D. parvula and D. sukatschewii. As a result, on chromosomes of D. elongata, D. sukatschewii and both D. flexuosa accessions, multiple clustered hybridization signals (rapid GISH markers) were revealed in different positions (Figs 1–3). Besides, large rapid GISH markers were detected in subtelomeric regions of the short arms of chromosomes 5, 9 and the long arm of chromosome 12 of D. antarctica (Fig 2); in the subtelomeric regions of the short arm of chromosomes 1 and the long arms of chromosomes 6, 8, 12 and 13 in karyotypes of the studied D. danthonioides accessions (Fig 3). Also, the dispersed hybridization signals were observed along chromosomes of D. parvula (Fig 2).
Rapid GISH procedure with total genomic DNA of D. flexuosa (PI 577075) as a DNA probe was carried out on chromosomes of D. antarctica, D. cespitosa, D. danthonioides, D. elongata, D. parvula, and D. sukatschewii. In all the studied accessions, weak dispersed hybridization signals were detected along the chromosomes. Also, in karyotypes of D. antarctica, D. elongata, D. sukatschewii and the studied D. danthonioides accessions, large rapid GISH markers were found in the secondary constriction regions (NORs) of SAT chromosomes (Figs 1–3).
DAPI-banding analysis
After FISH procedures, chromosome staining with DAPI revealed banding patterns (DAPI-banding). Analysis of distribution of DAPI-bands in karyotypes of the studied Deschampsia species showed that the most intense bands were located in the pericentromeric and subtelomeric regions of chromosomes, while a number of small and also faint inconsistent bands were detected in the interstitial chromosome regions. Visual analysis showed that chromosomal DAPI-banding patterns were specific to the studied species as variations in size, number and localization of the bands was observed (Figs 2 and 3). Chromosomal distribution of DAPI-bands within the studied accessions of the species was similar though variations in size and intensity of DAPI-bands were also observed. The largest DAPI-bands were observed on the chromosomes of D. sukatschewii specimen (Fig 3). B chromosomes, found in karyotypes of D. antarctica, D. cespitosa, D. sukatschewii and D. elongata, possessed distinct DAPI -bands in their subtelomeric regions (Figs 2 and 3).
Karyotype analysis
Based on chromosomal morphology, DAPI-banding patterns, localization of 45S and 5S rDNA and rapid GISH markers, chromosomes in karyotypes of the studied Deschampsia species were identified and karyograms were constructed (Figs 2 and 3). We note that patterns of distribution of the examined markers were similar in karyotypes of the studied species accessions; accordingly, one karyogram of each species is presented.
The comparison of patterns of distribution of the examined molecular cytogenetic markers (DAPI-bands, 45S and 5S rDNA, and also rapid GISH markers) allowed us to reveal chromosomal rearrangements in karyotypes of D. cespitosa, D. danthonioides, D. flexuosa, D. elongata and D. sukatschewii (detailed in Figs 2 and 3).
Discussion
Most Deschampsia species were shown to have a basic chromosome number х = 13 with the exception of D. setacea (2n = 2х = 14), D. atropurpurea (2n = 2х = 14) and D. flexuosa (2n = 4х = 28) which present a typical for cereals basic number of x = 7 [28, 31]. In the present study, the chromosome numbers were verified for normal diploid karyotypes of D. antarctica, D. cespitosa, D. danthonioides, D. flexuosa, D. elongata, D. parvula and D. sukatschewii.
We also detected different ploidy levels in D. cespitosa as both diploid (2n = 26) and tetraploid (2n = 52) D. cespitosa cytotypes were revealed. The results reported here are in good agreement with the data on different ploidy levels (2n = 26; 3n-39; 4n = 52) as well as inter- and intra-individual variability in chromosome number (2n = 15–28) observed in several taxa of D. cespitosa complex [1, 31]. Polyploidy is widespread in the grasses [40]. It is considered to be an important driving force in plant evolution [41], and several genomic duplication events were postulated in the evolution of the family Poaceae [42]. The adaptive significance of the variability in ploidy level found in some Deschampsia species is still unclear. It might be related to ability of polyploids to colonize larger geographic ranges and/or occur in more habitats than related diploids [41]. This is probably caused by greater genetic and biochemical variability of polyploid genome [43].
In D. antarctica accession collected on Rasmussen Cape, we revealed only a diploid cytotype. However, the presence of polyploid and mixoploid populations of D. antarctica in other localities of the Antarctic regions was previously described [23, 27–28]. Polyploidy and hybridization processes were considered to play an important role in the evolution of the genome of D. antarctica which might be related to high tolerance and genomic plasticity of this species [23, 27].
B chromosomes were described in karyotypes of different grass species [44–45] including some species of D. cespitosa complex [31] and also in D. antarctica located in the Maritime Antarctic [27]. It was shown that Bs can be present or absent among individuals within the same species population due to their dispensable nature [46–47]. There are also data suggesting that Bs can be spontaneously generated in response to the new genome conditions following interspecific hybridization [33, 48]. The functional role of the observed Bs is still uncertain, but the correlation between the appearance of Bs in a karyotype and environmental conditions was found [49–51]. Besides, they are mostly observed in karyotypes of the species having wide distribution area with various environmental conditions [52]. In agreement with these observations, we found Bs in karyotypes of D. antarctica, D. cespitosa, D. elongata and D. sukatschewii which can grow in different edaphoclimatic conditions including extreme habitats [31].
The cytogenetic analysis performed in the present study, indicated karyotypic similarities among the studied Deschampsia species with the exception of the most distantly related D. flexuosa. Chromosomes of D. flexuosa were not very different in size and centromere positions whereas the karyotypes of the other studied Deschampsia species contained m, sm, st and t chromosomes of different sizes with one pair of m chromosomes being longer than the others. Our results are in agreement with earlier reported data on the karyotypes of few Deschampsia species [1, 25–28]. This long metacentric chromosome was suggested to be a result of a translocation (or a centric fusion) between two acrocentric chromosomes, giving rise to the unusual for cereals chromosome number 2n = 26 [1]. The intercalary signal of telomere repeats in that long chromosome previously revealed by FISH [27] confirms indirectly this assumption as intercalary sites of telomere repeats are considered to be the remnants of ancestral chromosomal rearrangements occurred during the evolution [53].
After FISH, DAPI staining displays regions of AT-rich heterochromatin as strongly stained bands (DAPI-banding patterns) in karyotypes of vascular plants [54] including D. antarctica [27]. Accordingly, in this study DAPI-banding patterns were also used for chromosome analysis of Deschampsia species. The results of FISH with 45S and 5S rDNA as well as chromosomal distribution of DAPI-bands in D. antarctica collected on Rasmussen Cape were in good agreement with karyotypic data previously described for D. antarctica specimens grown in several localities of Maritime Antarctic and South America [23, 27].
Comparison of D. antarctica karyotype with the other studied Deschampsia species revealed basic karyotypic similarities though structural peculiarities among these species were also found. Particularly, in the studied specimens of D. antarctica and closely related D. parvula from Falkland Islands, which are a biogeographical part of the mild Antarctic zone with the closest to the Antarctic habitats (compared to the other species), 45S and 5S rDNA sites were distributed in similar chromosomal positions. However, in D. parvula, some 5S rDNA sites were very small and not always detected. It might be related to chromosomal redistribution of 5S rDNA occurred during the speciation process. Nevertheless, since D. parvula karyotype was studied for the first time, it could be a manifestation of karyotypic polymorphism of the studied population, and other specimens from different localities should be studied. Moreover, these species were rather similar in content of AT-heterochromatin though differences in the number, size and position of some DAPI-bands were detected.
In three studied accessions of D. cespitosa, chromosomal distribution of 45S and 5S rDNA loci differed from that found in D. antarctica. In diploid karyotypes of D. cespitosa, we found three SAT chromosome pairs (5, 6 and 9) bearing 45S rDNA: two of them (with proximal and distal 45S rDNA sites) were similar to those found in D. antarctica and another SAT chromosome pair possesses a proximal 45S rDNA site. Similar to D. antarctica, ten 5S rDNA loci were revealed in karyotypes of D. cespitosa but they were localized in four chromosome pairs as the longest chromosome 1 possesses two 5S rDNA loci in both arms. These observations corresponded to karyotypic data described earlier for D. cespitosa [25]. However, since only limited European accessions of D. cespitosa have been studied to date, further molecular cytogenetic analyses of D. cespitosa collected from different localities (and environmental conditions including extreme habitats) are needed in order to investigate the possible chromosome changes in distant populations.
In karyotypes of D. elongata and D. sukatschewii, the number and chromosomal localization of 45S and 5S rDNA (characterized for the first time for both species) were very similar to D. cespitosa despite multiple chromosomal rearrangements found in D. sukatschewii. Interestingly, in the studied D. sukatschewii accession, a chromosome rearrangement involving rDNA loci (co-localized 5S and 45S rDNA sites detected in one of the homologous chromosomes 10) was observed. It is clearly impossible for plants to go through meiosis with such an unbalanced karyotype, and the appearance and maintenance of these chromosome rearrangements in the population could be due to the capacity of Deschampsia species to reproduce by self-fertilization and/or asexual (vegetative or apomictic) propagation [24, 31, 55–58]. The chromosomal DAPI-banding patterns of D. cespitosa, D. elongata and D. sukatschewii were also found to follow the common pattern though species differences in the number, size and position of some bands were observed. Our results agree with early reported molecular phylogenetic data (the analysis of ITS and plastid trnL intron sequences) which showed close relationships between these species [2, 22–24].
In karyotypes of the studied D. danthonioides accessions, FISH analysis (performed for the first time) detected only two 45S rDNA loci. It was fewer if compared to D. cespitosa karyotype with three sites and similar to D. antarctica karyotype. However, we observed the minor 45S rDNA sites in the proximal region of the short arm of chromosome 5 which might indicate the direction of genome changes occurred during evolution in D. danthonioides. Besides, fewer 5S rDNA sites were revealed compared to D. cespitosa and D. antarctica though they were located in the similar positions if compared to the other studied Deschampsia species (with exception of D. flexuosa). Chromosomal DAPI-banding patterns in D. danthonioides also differ from those found in karyotypes of both D. antarctica and D. cespitosa though early reported molecular phylogenetic data showed that D. danthonioides is closely related to D. cespitosa [2, 22–24].
In karyotypes of both studied D. flexuosa accessions, the number and localization of 45S and 5S rDNA sites (characterized for the first time) differed significantly from those detected in the other studied Deschampsia species. Interestingly, we observed one chromosome pair possessed both 45S (in the short arm) and 5S rDNA loci (in the long arm) though, in several genera and groups of closely related species including Deschampsia, Avena, Holcus and Agrostis, investigated to date, 5S rDNA is considered to localize exclusively in chromosomes without NORS [59]. The localization of 5S and 45S rDNA sites in one chromosome pair could be related to chromosomal rearrangements occurred in D. flexuosa during the speciation processes.
It has been previously shown that FISH with the repetitive DNA probes (pSc119.2, pAs1 and GAA-microsatellite sequence), specific for chromosomes of most members of the family Poaceae, was not informative for D. antarctica karyotype [27]. Accordingly, for comparative chromosome analysis of the studied Deschampsia species, we applied a rapid GISH procedure with labelled genomic DNA of D. antarctica, closely related D. cespitosa and distantly related D. flexuosa in different combinations. This approach allowed us to reveal homologous highly repeated DNA sequences between genomes of the related species and presented additional markers for chromosomal identification. The analysis of patterns of chromosomal distribution of the examined markers indicated a lot of chromosomal rearrangements in karyotypes of the studied Deschampsia species which indicate that process of genome evolution of the genus Deschampsia could include chromosomal reorganization (chromosome interchanges, inversions, translocations) of the initial parental genomes since the presence of numerous chromosomal rearrangements in plant genomes are considered to be speciation-related events [60–61].
Rapid GISH procedure with genomic DNA of D. cespitosa as a DNA probe revealed three clustered signals (markers) on chromosomes of D. antarctica confirming the results obtained previously with genomic DNA of the other accession of D. cespitosa [27]. However, in karyotypes of closely related D. parvula, rapid GISH with this probe detected only two markers indicating some differences between genomes of D. antarctica and D. parvula.
On chromosomes of D. elongata and D. sukatschewii, rapid GISH with genomic DNA of D. cespitosa revealed multiple rapid GISH markers which were similarly distributed. These findings confirm the close relationship among the three species. On chromosomes of D. danthonioides, rapid GISH with this probe detected only four clustered hybridization signals in different positions compared to D. elongata and D. sukatschewii indicating the presence of structural differences between the genomes of D. danthonioides and the other three species. These cytogenetic data are in agreements with our results on FISH with 5S and 45S rDNA as well as molecular phylogenetic data described previously for these species [22, 23].
According to the analysis of ITS tree, D. flexuosa is now regarded a better suited to the genus Avenella [22]. Additionally, its karyotype differed significantly from the rest of the species studied here. Meanwhile, D. flexuosa is very similar morphologically to D. cespitosa, and also the genomic DNA of both D. antarctica and D. cespitosa as DNA probes detected multiple rapid GISH markers (in different positions) on chromosomes of D. flexuosa indicating the presence of a great number of homologous highly repeated DNA sequences in their genomes. These observations show that D. flexuosa is related to the species of D. cespitosa complex, and it might take an intermediate position between the two allied genera Deschampsia and Avenella.
Interestingly, the reverse GISH procedure (e.g., using the genomic DNA of D. flexuosa as a probe) presented dispersed hybridization signals on chromosomes of D. antarctica and D. cespitosa indicating that the homologous highly repeated DNA sequences were distributed differently in their genomes. Also, weak dispersed signals of this probe were revealed on chromosomes of the other studied Deschampsia species. Consequently, during the speciation process clustering of some common repeats occurred in D. flexuosa genome whereas in genomes of the other studied Deschampsia species those repeats remained unclustered.
Thus, though the molecular cytogenetic analysis showed similar features in the karyotype structure of most studied Deschampsia species, significant interspecific variability in chromosome localization of the examined markers was found. Based on the analysis of chromosome morphology and patterns of distribution of the examined markers, the studied Deschampsia species can be divided into four karyological groups (with some interspecific chromosomal differences inside group 1 and 2): 1) D. antarctica and D. parvula; 2) D. cespitosa, D. elongata and D. sukatschewii; 3) D. danthonioides; 4) D. flexuosa.
Conclusions
Though polyploidy and hybridization play an important role in speciation, our findings show that the processes of genome reorganization of Deschampsia species during evolution could involve different chromosomal rearrangements.
The unique D. antarctica karyotype has experienced a significant chromosome rearrangements (compared to D. cespitosa complex). The karyotype of D. parvula from Falkland Islands with the closest to the Antarctic habitats is similar (with some variations) to D. antarctica and also differs from the core of the genus. The detected differences in karyotype of the remote D. danthonioides (the North America) indicate the need to perform a comparative molecular cytogenetic analysis of D. cespitosa populations from different habitats, in particular, from different continents or from North and South hemispheres. D. flexuosa karyotype differs significantly from the rest of the studied species; however, the presence of common DNA repeats show that this species is related to D. cespitosa complex or they have the common ancestral species. The obtained results will help clarify the relationships within the genus Deschampsia, and can be a basis for the further genetic and biotechnological studies as well as for selection of plants tolerant to the extreme habitats.
Acknowledgments
The authors acknowledge the support of the National Antarctic Scientific Center of the Ministry of Education and Science of Ukraine. The research was performed in the frame of the National targeted scientific and technological program of research in Antarctic for 2011–2020. We thank Dr. Prof. Parnikoza I.Y., Dr. Prof. Polyschuk V.P. and Dr. Dykyy I.V. for collecting D. antartctica seeds.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Russian Foundation of Basic Research [grants no. 15-04- 05574 AA; 17-08-01504 OM] and Fundamental Research Program of the Russian Academy of Sciences “Dynamics of Plant, Animal and Human Genofonds” [grant no. 0103-2015-0117 OM]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Garsia-Suarez R, Alonso-Blanco C, Fernandez-Carvajal MC, Fernandez-Prieto JA, Roca F, Giraldez R. Diversity and systematics of Deschampsia sensu lato (Poaceae), inferred from karyotypes, protein electrophoresis, total genomic DNA hybridization and chloroplast DNA analysis. Plant Syst Evol. 1997;205: 99–110. [Google Scholar]
- 2.Souto DPF, Catalano SA, Tosto D, Bernasconi P, Sala A, Wagner M, et al. Phylogenetic relationships of Deschampsia antarctica (Poaceae): Insights from nuclear ribosomal ITS. Plant Syst Evol. 2006;261: 1–9. [Google Scholar]
- 3.Saarela JM, Liu Q, Peterson PM, Soreng RJ, Paszko B. Phylogenetics of the grass ‘Aveneae-type plastid DNA clade’ In: Seberg O, Petersen G, Barfod AS, Davis JI, editors. Diversity, phylogeny, and evolution in the monocotyledons. Aarhus: Aarhus University Press; 2010. pp. 557–588. [Google Scholar]
- 4.Rodionov AV, Kotseruba VV, Kim ES, Punina EO, Nosov NN. Grass genome and chromosome sets evolution. Tsitologiia. 2013;55: 225–229. [PubMed] [Google Scholar]
- 5.Lee J, Kang Y, Shin SC, Park H, Lee H. Combined analysis of the chloroplast genome and transcriptome of the Antarctic vascular plant Deschampsia antarctica Desv. PLoS ONE. 2014. March 19;9(3):e92501 doi: 10.1371/journal.pone.0092501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, et al. A worldwide phylogenetic classification of the Poaceae (Gramineae). J Syst Evol. 2015;53: 117–137. [Google Scholar]
- 7.Nkongolo KK, Deck A, Michael P. Molecular and cytological analyses of Deschampsia caespitosa populations from Northern Ontario (Canada). Genome. 2001;44: 818–825. [DOI] [PubMed] [Google Scholar]
- 8.Kawano S. Cytogeography and evolution of the Deschampsia caespitosa complex. Can J Bot. 1963;41: 719–742. [Google Scholar]
- 9.Moore DM. Chromosome numbers of Falkland Islands angiosperms. BAS Bulletin. 1967;14: 69–82. [Google Scholar]
- 10.Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ. Ecophysiology of Antarctic vascular plants. Physiol Plant. 2002;115: 479–486. [DOI] [PubMed] [Google Scholar]
- 11.Zúñiga GE, Alberdi M, Corcuera LJ. Non-structural carbohydrates in Deschampsia antarctica Desv. from South Shetland Islands, Maritime Antarctic. Environ Exp Bot. 1996;36: 393–398. [Google Scholar]
- 12.Xiong FS, Ruhland CT, Day TA. Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant. 1999;106: 276–286. [Google Scholar]
- 13.Bravo LA, Ulloa N, Zuniga GE, Casanova A, Corcuera LJ, Alberdi M. Cold resistance in Antarctic angiosperms. Physiol Plant. 2001;111: 55–65. [Google Scholar]
- 14.Bravo LA, Griffith M. Characterization of antifreeze activity in Antarctic plants. J Exp Bot. 2005;56: 1189–96. doi: 10.1093/jxb/eri112 [DOI] [PubMed] [Google Scholar]
- 15.John UP, Polotnianka RM, Sivakumaran KA, Chew O, Macin L, Kuiper MJ, et al. Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ. 2009;32: 336–348. doi: 10.1111/j.1365-3040.2008.01925.x [DOI] [PubMed] [Google Scholar]
- 16.Chew O, Lelean S, John UP, Spangenberg GC. Cold acclimation induces rapid and dynamic changes in freeze tolerance mechanisms in the cryophile Deschampsia antarctica E. Desv. Plant Cell Environ. 2012;35: 829–837. doi: 10.1111/j.1365-3040.2011.02456.x [DOI] [PubMed] [Google Scholar]
- 17.John UP, Spangenberg G. Xenogenomics: genomic bioprospecting in indigenous and exotic plants through EST discovery, cDNA microarray-based expression profiling and functional genomics. Comp Funct Genomics. 2005;6: 230–235. doi: 10.1002/cfg.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Noh EK, Choi HS, Shin SC, Park H, Lee H. Transcriptome sequencing of the Antarctic vascular plant Deschampsia antarctica Desv. under abiotic stress. Planta. 2013;237: 823–836. doi: 10.1007/s00425-012-1797-5 [DOI] [PubMed] [Google Scholar]
- 19.Byun MY, Lee J, Cui LH, Kang Y, Oh TK, Park H, Lee H, Kim WT Constitutive expression of DaCBF7, an Antarctic vascular plant Deschampsia antarctica CBF homolog, resulted in improved cold tolerance in transgenic rice plants. Plant Sci. 2015;236: 61–74. doi: 10.1016/j.plantsci.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 20.Pereira BK, Rosa RM, da Silva J, Guecheva TN, Oliveira IM, Ianistcki M, et al. Protective effects of three extracts from Antarctic plants against ultraviolet radiation in several biological models. J Photochem Photobiol B. 2009;96: 117–129. doi: 10.1016/j.jphotobiol.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 21.Rabert C, Gutiérrez-Moraga A, Navarrete A, Navarrete-Campos D, Bravo L, Gidekel M. Expression of a Deschampsia antarctica Desv. polypeptide with lipase activity in a Pichia pastoris vector. Int J Mol Sci. 2014;15: 2359–2367. doi: 10.3390/ijms15022359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiapella J. A molecular phylogenetic study of Deschampsia (Poaceae: Aveneae) inferred from nuclear ITS and plastid trnL sequence data: support for the recognition of Avenella and Vahlodea. Taxon. 2007;56: 55–64. [Google Scholar]
- 23.González ML, Urdampilleta JD, Fasanella M, Premoli AC, Chiapella JO. Distribution of rDNA and polyploidy in Deschampsia antarctica E. Desv. in Antarctic and Patagonic populations. Polar Biol. 2016;39: 1663–1677. [Google Scholar]
- 24.Volkov RA, Kozeretska IA, Kyryachenko SS, Andreev IO, Maidanyuk DN, Parnikoza IYu, et al. Molecular evolution and variability of ITS1-ITS2 in populations of Deschampsia antarctica from two regions of the Maritime Antarctic. Polar Sci. 2010;4: 469–478. [Google Scholar]
- 25.Winterfeld G, Roser M. Disposition of ribosomal DNAs in the chromosomes of perennial oats (Poaceae: Aveneae). Bot J Linn Soc. 2007a;155: 193–210. [Google Scholar]
- 26.Winterfeld G, Roser M. Chromosomal localization and evolution of satellite DNAs and heterochromatin in grasses (Poaceae), especially tribe Aveneae. Plant Syst Evol. 2007b;264: 75–100. [Google Scholar]
- 27.Amosova AV, Bolsheva NL, Samatadze TE, Twardovska MO, Svyatoslav A. Zoshchuk SA, Andreev IO, Badaeva ED, Kunakh VA, Muravenko OV. Molecular cytogenetic analysis of Deschampsia antarctica Desv. (Poaceae), Maritime Antarctic. PLoS One. 2015;10(9): e0138878 doi: 10.1371/journal.pone.0138878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardone S, Sawatani P, Rush P, Garcнa A, Poggio L, Schrauf G. Karyological studies in Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009;32: 427–433. [Google Scholar]
- 29.Madlung A, Comal L. The effect of stress on genome regulation and structure. Ann Bot. 2004;94: 481–495. doi: 10.1093/aob/mch172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnusamy V, Zhu JK. Epigenetic regulation of stress response in plants. Curr Opin Plant Biol. 2009;12: 133–139. doi: 10.1016/j.pbi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parnikoza I, Kozeretska I, Kunakh V. Vascular plants of the Maritime Antarctic: origin and adaptation. Am J Plant Sci. 2011;2: 381–395. [Google Scholar]
- 32.Belyayev A, Raskina O. Chromosome evolution in marginal populations of Aegilops speltoides: causes and consequences. Ann Bot. 2013;111: 531–538. doi: 10.1093/aob/mct023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houben A, Banaei-Moghaddam AM, Klemme S. Biology and evolution of B chromosomes In: Greilhuber J, Dolezel J, Wendel JF, editors. Plant Genome Diversity. Vienna: Springer; 2013. pp.149–165. [Google Scholar]
- 34.Zagrichuk ОМ, Drobyk NM, Kozeretska ІА, Parnikoza ІU, Kunah VА. Introduction to culture in vitro Deschampsіa antarctіca Desv. (Poaceae) from two coastal areas of Antarctica. UAJ. 2011/2012;10–11: 289–295. [Google Scholar]
- 35.Gamborg OL, Eveleigh DE. Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem. 1968;46: 417–421. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach WL, Bedbrook JR. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979;7: 1869–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlach WL, Dyer TA. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980;8: 4851–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muravenko OV, Yurkevich OY, Bolsheva NL, Samatadze TE, Nosova IV, Zelenina DA, et al. Comparison of genomes of eight species of sections Linum and Adenolinum from the genus Linum based on chromosome banding, molecular markers and RAPD analysis. Genetica. 2009;135: 245–255. doi: 10.1007/s10709-008-9273-7 [DOI] [PubMed] [Google Scholar]
- 39.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19: 11–15. [Google Scholar]
- 40.Levy AA, Feldman M. The Impact of Polyploidy on Grass Genome Evolution. Plant Physiology. 2002;130: 1587–1593. doi: 10.1104/pp.015727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsey J, Ramsey TS. Ecological studies of polyploidy in the 100 years following its discovery. Philos Trans R Soc Lond B Biol Sci. 2014. August 5; 369(1648): 20130352 doi: 10.1098/rstb.2013.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soltis P, Soltis D. The role of hybridization in plant speciation. Ann Rev Plant Biol. 2009;60: 561–588. [DOI] [PubMed] [Google Scholar]
- 43.Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen AC, Elven R, Polyploidy in arctic plants. Biol J Linn Soc. 2004;82: 521–536. [Google Scholar]
- 44.Ruban A, Fuchs J, Marques A, Schubert V, Soloviev A, Raskina O, et al. B Chromosomes of Aegilops speltoides Are Enriched in Organelle Genome-Derived Sequences. PLoS ONE. 2014;9(2): e90214 doi: 10.1371/journal.pone.0090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houben A, Banaei-Moghaddam AM, Klemme S, Timmis JN. Evolution and biology of supernumerary B chromosomes. Cell Mol Life Sci. 2014;71: 467–478. doi: 10.1007/s00018-013-1437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White MJD. The chromosomes. 6 th ed. London: Chapman & Hall Press; 1973. [Google Scholar]
- 47.Manoj K, Friebe B, Koul AK, Gill BS. Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma. 2002;111: 332–340. doi: 10.1007/s00412-002-0214-4 [DOI] [PubMed] [Google Scholar]
- 48.Jones N, Houben A B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci. 2003;8: 417–423. doi: 10.1016/S1360-1385(03)00187-0 [DOI] [PubMed] [Google Scholar]
- 49.Camacho JP, Sharbel TF, Beukeboom LW. B chromosome evolution. Philos Trans R Soc Lond B Biol Sci. 2000;355: 163–178. doi: 10.1098/rstb.2000.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butorina AK, Bogdanova EV. Adaptive significance and possible origin of B-chromosomes in Picea Glauca (moench.) voss = P.canadensis B.S.P. Tsitologiia. 2001;43: 809–814. [PubMed] [Google Scholar]
- 51.Pereira HS, Delgado M, Viegas W, Rato JM, Barão A, Caperta AD. Rye (Secale cereale) supernumerary (B) chromosomes associated with heat tolerance during early stages of male sporogenesis. Ann Bot. 2017;119: 325–337. doi: 10.1093/aob/mcw206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolsheva NL, Zelenin AV, Inna V. Nosova IV, Amosova AV, Samatadze TE, Yurkevich OYu, Melnikova NV, Zelenina DA, Volkov AA, Muravenko OV. The diversity of karyotypes and genomes within section Syllinum of the genus Linum (Linaceae) revealed by molecular cytogenetic markers and RAPD analysis. PLoS ONE. 2015;10(4): e0122015 doi: 10.1371/journal.pone.0122015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E. Telomeric repeats far from the ends: Mechanisms of origin and role in evolution. Cytogenet Genome Res. 2008;122: 219–228. doi: 10.1159/000167807 [DOI] [PubMed] [Google Scholar]
- 54.Barros e Silva AE, Guerra M. The meaning of DAPI bands observed after C-banding and FISH procedures. Biotech Histochem. 2010;85: 115–125. doi: 10.1080/10520290903149596 [DOI] [PubMed] [Google Scholar]
- 55.Asker SE, Jerling L. Apomixis in Plants. Boca Raton, Finland: CRC Press; 1992. [Google Scholar]
- 56.Holderegger R, Stehlik I, Smith RIL, Abbott RJ. Populations of Antarctic Hairgrass (Deschampsia antarctica) show low genetic diversity. AAAR. 2003;35: 214–217. [Google Scholar]
- 57.Kravets AA, Taran NY, Storozhenko VA. Plasticity of morphogenesis and features of reproduction plants Colobanthus quitensis and Deschampsia antarctica in Antarctic region. UAJ. 2011/2012;10–11: 302–305. [Google Scholar]
- 58.Parnikoza I, Miryuta N, Ozheredova I, Kozeretska I, Smykla J, Kunakh V, Convey P. Comparative analysis of Deschampsia antarctica Desv. Population adaptability in the natural environment of the Admiralty Bay region (King George Island, maritime Antarctic). Polar Biol. 2015;38: 1401–1411. [Google Scholar]
- 59.Roser M, Winterfeld G, Doring E, Schneider J. Chromosome evolution in grass tribes Aveneae/Poeae (Poaceae): insights from karyotype structure and molecular phylogeny. Schlechtendalia. 2014;28: 1–21. [Google Scholar]
- 60.Flavell RB, O'Dell M, Hutchinson J. Nucleotide sequence organization in plant chromosomes and evidence for sequence translocation during evolution. Cold Spring Harb Symp Quant Biol. 1981;45: 501–508. [DOI] [PubMed] [Google Scholar]
- 61.Raskina O, Barber JC, Nevo E, Belyayev A. Repetitive DNA and chromosomal rearrangements: Speciation-related events in plant genomes. Cytogenet Genome Res. 2008;120: 351–357. doi: 10.1159/000121084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.