Abstract
Purpose
To investigate whether baseline optical coherence tomography (OCT) parameters can predict the treatment frequency of intravitreal ranibizumab (IVR) injections during the first year in patients with diabetic macular edema (DME) treated with pro re nata (PRN) IVR injections.
Methods
We retrospectively reviewed 68 eyes of 63 patients with center-involved DME who received IVR injections for 12 months or longer according to three monthly IVR injections followed by the PRN dosing. We measured the mean retinal thicknesses in the individual subfields of the Early Treatment Diabetic Retinopathy Study grid and evaluated the qualitative and quantitative parameters on OCT sectional images. We investigated the relationship between these OCT parameters at baseline and the number of IVR injections during the 12-month follow-up.
Results
Three loading doses were administered to 10 eyes; four to seven annualized IVR injections were administered to 34 eyes. The number of eyes that received IVR injections decreased gradually until month 6 and was almost constant from months 7 to 11. No relationships were seen between the treatment frequency and baseline systemic factors and the ophthalmic examination findings. Univariate analyses showed that the number of IVR injections during the first year was associated with the mean retinal thickness in the individual subfields and the transverse length of the disrupted external limiting membrane (ELM) and ellipsoid zone of the photoreceptors. Multivariate analysis showed a significant association with the thickness in the inferior subfield alone. The treatment frequency during the 12-month follow-up was not correlated with improved visual acuity but was associated with the decrease in the central subfield thickness and disrupted ELM.
Conclusion
The retinal thickness in the inferior subfield predicts the treatment frequency during the first year in eyes with DME treated with PRN IVR injections.
Introduction
Diabetic macular edema (DME), in which the blood-retinal barrier (BRB) is disrupted and neuroglial function deteriorates, often leads to visual impairment in patients with diabetes [1, 2]. Accumulated evidence in basic science and clinical studies has shown that vascular endothelial growth factor (VEGF) plays a key role in the molecular mechanisms in DME, which has encouraged ophthalmologists to treat DME with anti-VEGF drugs [3–7]. Two major drugs, ranibizumab (Lucentis, Novartis Pharma AG, Basel, Switzerland; and Genentech Inc., South San Francisco, CA, USA) and aflibercept (Eylea, Regeneron Pharmaceuticals, Tarrytown, NY, USA; and Bayer, Berlin, Germany) have been approved worldwide and are the first-line therapeutic strategy for managing DME [8, 9].
Considering the half-lives of these drugs in the vitreous humor, three major regimens, i.e., fixed monthly or bimonthly, pro re nata (PRN), and treat-and-extend (TAE), can be applied to treat DME [7–11]. A few clinical trials of intravitreal ranibizumab (IVR) injections for DME according to the PRN regimen have reported significant reductions in the treatment frequency in the second year and thereafter [12, 13]. Intriguingly, anti-VEGF therapy improves the severity of and retards progression of diabetic retinopathy (DR) and the expansion of the nonperfused areas in the macula [14, 15]. These data suggested that anti-VEGF drugs have rapid and direct effects on the disrupted BRB and exert slow and indirect mechanisms in DME resolution.
Despite the beneficial effects, the adverse effects of anti-VEGF management are rare but severe, e.g., endophthalmitis and life-threatening atherothrombotic diseases [16]. Clinical trials have suggested that anti-VEGF therapy guarantees the best improvement in visual acuity (VA), although the socioeconomic burden persists for patients. Actually, the cost-per-quality-adjusted life years is much higher than other conventional interventions for DME [17–19]. These issues might encourage clinicians to use the PRN regimen for DME and to optimize the indications for anti-VEGF therapy using predictors of visual prognosis and treatment frequency [20].
In the current study, we investigated the association of baseline systemic and ocular characteristics with the number of PRN IVR injections administered during the first year (3 + PRN regimen) to treat DME.
Materials and methods
Participants
We retrospectively reviewed 68 consecutive eyes of 63 patients with center-involved DME treated with IVR injections for 12 months or longer [21]. Patients with center-involved DME who visited Kyoto University Hospital from March 2014 to October 2015 as the baseline visit received three monthly injections followed by the PRN phase (3 + PRN regimen). Several patients dropped out during the 12-month follow-up because of inconvenience, patient desire to terminate treatment or change to other therapeutic strategies, drug tachyphylaxis, or additional treatments, i.e., focal/grid photocoagulation, panretinal photocoagulation, vitrectomy (for vitreous hemorrhage), or cataract surgery. All research and measurements adhered to the tenets of the Declaration of Helsinki. The Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine approved the study protocol. All participants provided written informed consent before study enrollment.
Intervention
Ranibizumab (0.5 mg) was administered intravitreally according to the 3 + PRN regimen described in the Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema (RESTORE) study [8]. After disinfection, ranibizumab was injected 3.5 mm posterior to the limbus followed by instillation of antibiotics. Three monthly ranibizumab loading doses were followed by PRN IVR injections. At every monthly visit, the PRN treatment regimen was applied according to the retreatment criteria of the RESTORE study [8].
Optical coherence tomography
After measurement of the VA and comprehensive ophthalmic examinations, we acquired sectional and three-dimensional images using spectral-domain optical coherence tomography (SD-OCT) (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) at every monthly visit. After calibration using the corneal curvature radii and focusing knob, vertical and horizontal sectional images dissecting the fovea were acquired using the cross-hair mode (30 degrees). Three-dimensional images were obtained according to the following parameters; a 49 line raster scan (central 20 x 20 degree), automatic real-time mean of 16, and high resolution (512 A-scans/B-scan). Two-dimensional maps subsequently were constructed using the manufacturer’s software. The technology enabled automatic measurement of the mean retinal thicknesses of the central subfield (CSF) and the individual subfields in the parafovea of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid, as described previously [22, 23].
We evaluated qualitatively and quantitatively several OCT parameters at the fovea. Briefly, we determined the presence or absence of cystoid macular edema (CME) and serous retinal detachments (SRD) and measured the height of the foveal cystoid spaces or SRD at the presumed foveal center in eyes with CME or SRD using the caliper tool in the Heidelberg Eye Explorer software (Heidelberg Engineering) [23]. Vitreomacular traction (VMT) was defined as the presence of an epiretinal membrane involving the fovea or posterior vitreous membrane with foveal traction on the vertical and horizontal retinal sections of the SD-OCT images. Vitreomacular adhesion (VMA) was defined as attachment of the posterior vitreous cortex according to the modified methods described recently [24]. We evaluated the elevation of the perifoveal vitreous cortex from the retinal surface along with attachment of the vitreous cortex at the foveal center on the vertical and horizontal sectional OCT images. Such eyes with or without VMT were included in the group with VMA in this study.
We measured the transverse length of the disrupted ellipsoid zone of the photoreceptors (EZ) or external limiting membrane (ELM) on the vertical and horizontal images dissecting the fovea as reported previously [25]. Briefly, we first excluded the areas where reflectivity signals in the retinal pigment epithelium were attenuated by medial opacity or hyperreflective lesions in the inner retinal layers, and the areas with damaged photoreceptors then were quantified. The status of the EZ lines was divided into three categories, i.e., intact, faint, and disrupted, according to the OCT reflectivity. The ELM status was defined as intact or disrupted, because the reflectivity levels in the ELM were almost constant. We thus quantified the transverse length of the areas where the EZ or ELM line was disrupted within the central 1 mm on the vertical and horizontal images using the caliper tool in the Heidelberg Eye Explorer software. The average of the percentage was used in the subsequent investigations. Disorganization of the inner retinal layers also was measured as the transverse length of the central areas where the inner retinal layers could not be segmented clearly as reported previously [26]. Two independent retinal specialists evaluated these OCT parameters. If there was disagreement regarding the qualitative parameters, a third specialist participated. For the quantitative parameters, the average of the values obtained by two specialists was applied to further analyses.
Statistical analysis
The results are expressed as the median (interquartile range). The differences between two groups were evaluated using the Wilcoxon signed-rank test or the Mann-Whitney U-test. Spearman’s correlation coefficient was calculated to test the statistical correlation. For the multivariate analysis, we performed multiple regression analysis using a stepwise forward approach (the mean retinal thicknesses in the CSF and the individual parafoveal subfields [nasal, temporal, superior, and inferior] and the transverse length of the disrupted ELM and EZ as independent variables and the number of IVR injections during the 12-month follow-up as a dependent variable). P < 0.05 was considered significant.
Results
Association of baseline characteristics with the number of IVR injections during the first 12 months
We retrospectively reviewed 68 eyes of 63 patients with center-involved DME who received PRN IVR injections during the first 12 months. Among 125 eyes that met the eligibility criteria, nine eyes met the exclusion criteria at baseline. Of the remaining 116 eyes, 48 eyes were lost to follow-up before the 12-month examination. Table 1 shows the baseline systemic and ocular characteristics.
Table 1. Baseline characteristics.
| Parameter | |
|---|---|
| Eyes/patients | 68/63 |
| Mean age | 69 years (range, 59–74) |
| Gender Men Women |
35 28 |
| Hemoglobin A1c | 7.2% (range, 6.6–7.8) |
| Systemic hypertension Absent Present |
26 37 |
| LogMAR VA | 0.260 (0.155–0.506) |
| Lens status Phakia Pseudophakia |
45 eyes 23 eyes |
| DR severity Mild NPDR Moderate NPDR Severe NPDR PDR |
1 eye 37 eyes 13 eyes 17 eyes |
| Previous PRP Absent Present |
25 eyes 43 eyes |
HbA1c: hemoglobin A1c; logMAR: logarithm of the minimum angle of resolution; NPDR: nonproliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy; PRP: panretinal photocoagulation.
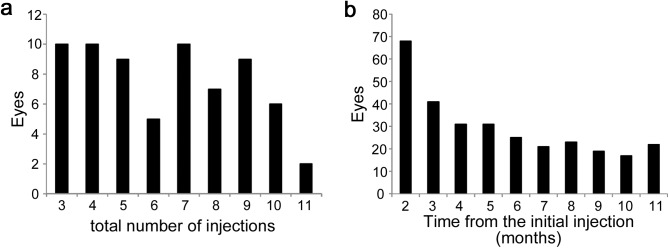
All 68 eyes received PRN IVR injections during the 12-month follow-up; only 10 eyes received three loading doses alone (Fig 1A). Thirty-four eyes received four to seven annualized injections, and 24 eyes required eight or more IVR injections. We further investigated the number of eyes that received IVR injections at individual time points. IVR injections were administered to 41 eyes at month 3 and the number of eyes decreased gradually until month 6 (Fig 1B). The number of eyes that received IVR injections was almost constant from months 7 to 11.
Fig 1. The number of IVR injections during the first year.
(a) Individual numbers of IVR injections during the 12-month follow-up. (b) IVR injections at individual time points.
We evaluated the relationship between the baseline characteristics and treatment frequency of IVR injections during the 12-month follow-up and did not identify any significant associations with systemic factors or the findings on ophthalmic examination (Table 2).
Table 2. Relationship between preoperative parameters and the number of IVR injections during the 12-month follow-up.
| Baseline parameter | Association with number of IVR injections |
|---|---|
| Age | ρ = -0.206, p = 0.091 |
| Gender Men Women |
p = 0.350 7 (5–9) 5 (4–8) |
| Hemoglobin A1c | ρ = -0.217, p = 0.117 |
| Systemic hypertension Absent Present |
p = 0.440 5 (4–8) 7 (4.75–9) |
| LogMAR VA | ρ = 0.126, p = 0.301 |
| Lens status Phakia Pseudophakia |
p = 0.399 6 (4–8) 7 (4.5–9) |
| DR severity Mild NPDR Moderate NPDR Severe NPDR PDR |
5 6 (4–8) 8 (4–9) 7 (4–9) |
| Previous PRP Absent Present |
p = 0.871 7 (4–8) 6 (4–9) |
logMAR: logarithm of the minimum angle of resolution; NPDR: nonproliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy; PRP: panretinal photocoagulation.
The p-value indicates Spearman’s correlation coefficient for the association of the number of IVR injections with age, HbA1c, and logMAR VA. The Mann-Whitney U-test was performed to compare gender, systemic hypertension, lens status, and previous panretinal photocoagulation.
The investigation using OCT parameters showed a positive association between the CSF thickness and the number of IVR injections during the first year (ρ = 0.408, p<0.001) (Table 3). Intriguingly, the treatment frequency also was related to the mean retinal thicknesses in the individual subfields of the parafovea. Among them, the retinal thickness in the inferior subfield had the most significant association with the number of IVR (ρ = 0.516, p<0.001) (Table 3).
Table 3. Association between retinal thickness and number of IVR injections during the 12-month follow-up.
| Subfield | Thickness | Association with number of IVR injections |
|---|---|---|
| Fovea | 441 μm (402–549) | ρ = 0.408, p<0.001 |
| Parafovea | ||
| Nasal | 408 μm (372–468) | ρ = 0.483, p<0.001 |
| Temporal | 470 μm (389–549) | ρ = 0.382, p = 0.002 |
| Superior | 433 μm (389–519) | ρ = 0.277, p = 0.023 |
| Inferior | 414 μm (362–503) | ρ = 0.516, p<0.001 |
The p-value indicates Spearman’s correlation coefficient for the association between the number of IVR injections and the mean retinal thicknesses in the individual subfields.
Several OCT parameters other than the retinal thicknesses also were related to the visual outcomes after treatment with anti-VEGF drugs for DME, which encouraged us to investigate their association with the number of injections [27–29]. The number of IVR injections in eyes with CME or SRD did not differ from the number in eyes without such findings (Table 4). The treatment frequency was related to the height of the foveal cystoid spaces in 54 eyes with CME (ρ = 0.431, p = 0.002) but not to the height of the SRD in 21 eyes (ρ = 0.339, p = 0.130). The disruption of the ELM line or EZ line, which represents photoreceptor damage, was associated positively with the number of IVR injections (Table 4). Multivariate analyses showed that the treatment frequency was related to the retinal thickness in the inferior subfield alone (β = 0.492, p<0.001) among these baseline characteristics.
Table 4. Relationship between other OCT parameters and the number of IVR injections during the first year.
| OCT parameter at baseline | Relation to number of IVR injections | |
|---|---|---|
| CME Absent Present |
14 eyes 54 eyes |
p = 0.446 7 (4.25–9) 6.5 (4–8) |
| SRD Absent Present |
47 eyes 21 eyes |
p = 0.304 6 (4–8) 8 (4–9) |
| VMT Absent Present |
64 eyes 4 eyes |
p = 0.360 6 (4–8.25) 8.5 (6.75–9.25) |
| VMA Absent Present |
45 eyes 23 eyes |
p = 0.952 6 (4–9) 7 (5–8) |
| Transverse length of disrupted ELM | 0% (0–4.4) | ρ = 0.377, p = 0.002 |
| Transverse length of disrupted EZ | 9.6% (0–27.2) | ρ = 0.262, p = 0.032 |
| DRIL | 60.8% (36.8–84.5) | ρ = -0.001, p = 0.991 |
The p-value indicates the Mann-Whitney U-test for the comparison of CME, SRD, VMT, and VMA. Spearman’s correlation coefficient indicates the association of the number of IVR injections with the transverse length of the disrupted ELM or EZ and DRIL.
DRIL, disorganization of the inner retinal layers.
Relationship between treatment frequency and functional or morphologic outcomes
The logMAR VA and CSF thickness improved significantly at 12 months (p<0.001 for both comparisons) (Tables 1, 3 and 5). The mean retinal thicknesses in the parafovea were also improved, and there were no differences in the changes of the retinal thicknesses between individual subfields of the parafovea. There was no association between the number of IVR injections and the visual outcomes or visual improvement (Table 5). The number of IVR injections during the first year was unrelated to the CSF thickness per se but to its decrease at month 12 (ρ = 0.120, p = 0.370 and ρ = 0.328, p = 0.007, respectively) (Table 5). The treatment frequency also tended to be associated positively with the decrease in the transverse length of the disrupted EZ or ELM line (ρ = 0.212, p = 0.083 and ρ = 0.287, p = 0.019, respectively) (Table 5).
Table 5. Relation between visual outcomes at 12 months and number of IVR injections during the 12-month follow-up.
| Parameters at 12 months | Relation to number of IVR injections | |
|---|---|---|
| LogMAR VA | 0.155 (0.046–0.301) | ρ = 0.004, p = 0.971 |
| VA improvement | 0.125 (0.030–0.234) | ρ = 0.177, p = 0.146 |
| CSF thickness | 314 μm (269–386) | ρ = 0.120, p = 0.370 |
| Decrease in CSF thickness | 132 μm (54–191) | ρ = 0.328, p = 0.007 |
| Nasal thickness | 356 μm (338–389) | ρ = 0.240, p = 0.049 |
| Decrease in nasal thickness | 45 μm (13–94) | ρ = 0.420, p<0.001 |
| Temporal thickness | 371 μm (341–392) | ρ = 0.172, p = 0.162 |
| Decrease in temporal thickness | 74 μm (32–180) | ρ = 0.323, p = 0.009 |
| Superior thickness | 365 μm (344–390) | ρ = 0.300, p = 0.013 |
| Decrease in superior thickness | 61 μm (22–139) | ρ = 0.205, p = 0.102 |
| Inferior thickness | 352 μm (333–382) | ρ = 0.230, p = 0.059 |
| Decrease in inferior thickness | 59 μm (17–126) | ρ = 0.522, p<0.001 |
| Transverse length of disrupted EZ | 0% (0–9.0) | ρ = 0.294, p = 0.016 |
| Decrease in disrupted EZ | 4.1% (0–17.2) | ρ = 0.212, p = 0.083 |
| Transverse length of disrupted ELM | 0% (0–0) | ρ = 0.405, p<0.001 |
| Decrease of disrupted ELM | 0% (0–0) | ρ = 0.287, p = 0.019 |
The p value indicates the Spearman’s correlation coefficient for the association between the number of IVR injections and individual parameters.
Discussion
Anti-VEGF treatment improves both visual function and macular morphology in DME, whereas the severe complications and socioeconomic burden suggest the need to predict the treatment frequency on a PRN dosing schedule [8, 16–19]. In the current study, we showed for the first time that the number of IVR injections during the first year was associated positively with the retinal thickness in the inferior subfield of the ETDRS grid in eyes that received IVR injections according to the 3 + PRN regimen. The data are feasible for use in patient consultations in the clinic and to reduce the treatment burden of the health care system.
Several clinical trials have reported significant reductions in the treatment frequency with IVR injections during the second year and afterward in eyes treated with PRN IVR injections for DME [12, 13]. These publications have suggested that the burden of the anti-VEGF treatment depends substantially on the treatment frequency during the first year and, concomitantly, predicting the treatment frequency during this period is an important issue in anti-VEGF treatment. The current study showed an association between the baseline retinal thickness and the number of IVR injections during the first year. It might to some extent be consistent with the findings of a recent study that showed that, among several parameters, the baseline central foveal thickness predicts the number of IVR injections needed during the PRN open-label extension IVR periods after 3-year monthly IVR injections [20].
In the current study, the participants received IVR injections alone during the 12-month follow-up. The major rescue protocol is macular photocoagulation and might reduce the frequency of IVR treatment [30]. Other publications have reported fewer injections of anti-VEGF drugs combined with triamcinolone, despite no differences in the visual outcomes [31]. Although there were no differences in the treatment frequency between eyes with and without VMT in the current study, most clinicians believe that VMT often prevents anti-VEGF treatment from achieving complete resolution of DME [32]. Other publications have reported the efficacy of IVR injections in vitrectomized eyes, which suggests that vitrectomy followed by anti-VEGF therapy might be a possible alternative strategy and reduce the treatment frequency in eyes with both DME and VMT [33, 34].
The association between the retinal thickness and treatment frequency with IVR injections suggests that the magnitude of vascular hyperpermeability might be related to the need for more frequent injections and might be consistent with the relationship between fluorescein leakage and the number of injections in a recent publication [20]. VEGF induces vascular hyperpermeability via several mechanisms. VEGF increases the paracellular or transcellular flux in vascular endothelial cells [5, 35] and inhibits pericyte function and disrupts the endothelial-pericyte interaction, which contributes to both angiogenesis and vascular permeability [36]. VEGF-induced expression of intercellular adhesion molecule-1 contributes to leukostasis [37]. Concomitantly, retinal capillaries are occluded transiently, and extravasated inflammatory cells increase cytokines and vascular permeability [4, 38, 39]. The direct effects of anti-VEGF drugs on vascular endothelial cells is rapid and transient. In contrast, the indirect mechanisms via negative regulation of pericyte function, transient capillary nonperfusion, or infiltrated inflammatory cells might be slowly exacerbated or improved and might require prolonged and repeated administration of anti-VEGF drugs [14, 15, 40, 41].
In the current study, the retinal thickness in the inferior subfield had a more significant association with the treatment frequency with IVR injections than the CSF thickness. The baseline height of the foveal cystoid spaces also was correlated with the treatment frequency. Although the implications of retinal thickening in the inferior subfield in DME remain unknown, we hypothesized that persistent and prolonged edematous changes might impair the mechanical integrity, which would allow the extracellular fluid to migrate toward the inferior subfield as a result of gravity. In other words, retinal thickening in the inferior subfield might represent old DME to some extent. A recent study reported an association between the height of the foveal cystoid spaces and the retinal thickness in the inferior subfield in DME [23]. Eyes of CME type often have an enlarged foveal avascular zone, which suggests older DME [42]. Unfortunately, since most patients in the current study were referred to our institution, we did not know definitively the duration of diabetes, DR, or DME before the initial injection. A future study should determine whether persistent DME is associated with larger foveal cystoid spaces and retinal thickening in the inferior subfield and needs more frequent treatment with ranibizumab.
Univariate analyses showed the association of the treatment frequency with the disrupted ELM at baseline, although multivariate analysis revealed that the number of IVR injections was related to the retinal thickness in the inferior subfield alone. Statistical analysis suggested that the disrupted ELM was a confounding factor, because there was the association between the retinal thickness and the disrupted ELM (data not shown). It is consistent with the shadow artifacts that the ELM lines are often disrupted beneath severe retinal edema. In addition, further investigation should be planned to elucidate whether the treatment frequency was related to the photoreceptor status in the individual subfield.
The number of IVR injections was associated with improved retinal thickening and photoreceptor damage but not with VA improvement at month 12. The absence of an association between functional and anatomic responses to repeated IVR injections suggested the presence of other mechanisms in visual impairment than edematous changes, which is consistent with the modest correlation between CSF thickness and VA reduction reported by the Diabetic Retinopathy Clinical Research Network [43]. In addition, we wonder if we can identify the eyes with greater VA improvement after fewer IVR injections. Further studies should reveal novel mechanisms independent of edematous changes as novel therapeutic targets [44].
The current retrospective study with a smaller number of Asian cases had several limitations. Another study should determine if these results are generalizable to longer periods, other populations, and other regimens, e.g., the TAE regimen and the PRN regimens with different loading doses. Since we excluded patients who received additional treatment in the current study, eyes less responsive to anti-VEGF drug might have been omitted from this study. Anti-VEGF drugs affect vascular cells, and the predictors on fluorescein angiography or OCT angiography images should be reported in a future study [45].
Conclusions
The current study designated baseline macular thickness as a predictor of the treatment frequency during the first year in eyes that followed PRN IVR injection regimen to treat DME, suggesting its feasibility in the clinic and regarding socioeconomic issues.
Data Availability
All relevant data after analyses are within the paper. The individual level data cannot be made publicly available for ethical restriction according to the statements of the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. Since the committee does not have a clinical data repository, please contact corresponding author about possibilities for provision of data.
Funding Statement
This work was funded by the Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (TM) (26462637). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 35: 556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med. 366: 1227–1239. doi: 10.1056/NEJMra1005073 [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 331: 1480–1487. doi: 10.1056/NEJM199412013312203 [DOI] [PubMed] [Google Scholar]
- 4.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S (2002) Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 133: 70–77. [DOI] [PubMed] [Google Scholar]
- 5.Murakami T, Frey T, Lin C, Antonetti DA (2012) Protein kinase cbeta phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 61: 1573–1583. doi: 10.2337/db11-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham ET Jr., Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. (2005) A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 112: 1747–1757. doi: 10.1016/j.ophtha.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 7.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 117: 1064–1077 e1035. doi: 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 118: 615–625. doi: 10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 9.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 121: 2247–2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 119: 789–801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 11.Prunte C, Fajnkuchen F, Mahmood S, Ricci F, Hatz K, Studnicka J, et al. (2016) Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 100: 787–795. doi: 10.1136/bjophthalmol-2015-307249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, et al. (2015) Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 122: 375–381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. (2014) Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 121: 1045–1053. doi: 10.1016/j.ophtha.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. (2012) Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 130: 1145–1152. doi: 10.1001/archophthalmol.2012.1043 [DOI] [PubMed] [Google Scholar]
- 15.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS (2014) Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 121: 1783–1789. doi: 10.1016/j.ophtha.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 16.Avery RL, Gordon GM (2016) Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 134: 21–29. doi: 10.1001/jamaophthalmol.2015.4070 [DOI] [PubMed] [Google Scholar]
- 17.Smiddy WE (2012) Clinical applications of cost analysis of diabetic macular edema treatments. Ophthalmology. 119: 2558–2562. doi: 10.1016/j.ophtha.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 18.Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW (2013) Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 120: 1835–1842. doi: 10.1016/j.ophtha.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GC, Brown MM, Turpcu A, Rajput Y (2015) The cost-effectiveness of ranibizumab for the treatment of diabetic macular edema. Ophthalmology. 122: 1416–1425. doi: 10.1016/j.ophtha.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 20.Wykoff CC, Elman MJ, Regillo CD, Ding B, Lu N, Stoilov I (2016) Predictors of diabetic macular edema treatment frequency with ranibizumab during the open-label extension of the RIDE and RISE Trials. Ophthalmology. 123: 1716–1721. doi: 10.1016/j.ophtha.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 21.Chalam KV, Bressler SB, Edwards AR, Berger BB, Bressler NM, Glassman AR, et al. (2012) Retinal Thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 53: 8154–8161. doi: 10.1167/iovs.12-10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. (2011) Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 151: 310–317. doi: 10.1016/j.ajo.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 23.Murakami T, Ueda-Arakawa N, Nishijima K, Uji A, Horii T, Ogino K, et al. (2014) Integrative understanding of macular morphologic patterns in diabetic retinopathy based on self-organizing map. Invest Ophthalmol Vis Sci. 55: 1994–2003. doi: 10.1167/iovs.13-13417 [DOI] [PubMed] [Google Scholar]
- 24.Sadiq MA, Soliman MK, Sarwar S, Agarwal A, Hanout M, Demirel S, et al. (2016) Effect of vitreomacular adhesion on treatment outcomes in the Ranibizumab for Edema of the Macula in Diabetes (READ-3) Study. Ophthalmology. 123: 324–329. doi: 10.1016/j.ophtha.2015.09.032 [DOI] [PubMed] [Google Scholar]
- 25.Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, et al. (2012) Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 53: 1506–1511. doi: 10.1167/iovs.11-9231 [DOI] [PubMed] [Google Scholar]
- 26.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. (2014) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 132: 1309–1316. doi: 10.1001/jamaophthalmol.2014.2350 [DOI] [PubMed] [Google Scholar]
- 27.Sophie R, Lu N, Campochiaro PAP (2015) Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 122: 1395–1401. doi: 10.1016/j.ophtha.2015.02.036 [DOI] [PubMed] [Google Scholar]
- 28.Al Faran A, Mousa A, Al Shamsi H, Al Gaeed A, Ghazi NG (2014) Spectral domain optical coherence tomography predictors of visual outcome in diabetic cystoid macular edema after bevacizumab injection. Retina. 34: 1208–1215. doi: 10.1097/IAE.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 29.Shimura M, Yasuda K, Yasuda M, Nakazawa T (2013) Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 33: 740–747. doi: 10.1097/IAE.0b013e31826b6763 [DOI] [PubMed] [Google Scholar]
- 30.Payne JF, Wykoff CC, Clark WL, Bruce BB, Boyer DS, Brown DM, et al. (2017) Randomized trial of treat and extend ranibizumab with and without navigated laser for diabetic macular edema: TREX-DME 1 Year Outcomes. Ophthalmology. 124: 74–81. doi: 10.1016/j.ophtha.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 31.Shimura M, Yasuda K, Minezaki T, Noma H (2016) Reduction in the frequency of intravitreal bevacizumab administrations achieved by posterior subtenon injection of triamcinolone acetonide in patients with diffuse diabetic macular edema. Jpn J Ophthalmol. 60: 401–407. doi: 10.1007/s10384-016-0458-9 [DOI] [PubMed] [Google Scholar]
- 32.Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, et al. (2012) Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 130: 1153–1161. doi: 10.1001/archophthalmol.2012.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bressler SB, Melia M, Glassman AR, Almukhtar T, Jampol LM, Shami M, et al. (2015) Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 35: 2516–2528. doi: 10.1097/IAE.0000000000000617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, Browning DJ, et al. (2010) Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 117: 1087–1093 e1083. doi: 10.1016/j.ophtha.2009.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu H, Nagy JA, Senger DR, Dvorak HF, Dvorak AM (1995) Ultrastructural localization of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) to the abluminal plasma membrane and vesiculovacuolar organelles of tumor microvascular endothelium. J Histochem Cytochem. 43: 381–389. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, et al. (2008) A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 456: 809–813. doi: 10.1038/nature07424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, et al. (2000) Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol. 156: 1733–1739. doi: 10.1016/S0002-9440(10)65044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, et al. (2003) Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 9: 781–788. doi: 10.1038/nm877 [DOI] [PubMed] [Google Scholar]
- 39.Sonoda S, Sakamoto T, Shirasawa M, Yamashita T, Otsuka H, Terasaki H (2013) Correlation between reflectivity of subretinal fluid in OCT images and concentration of intravitreal VEGF in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 54: 5367–5374. doi: 10.1167/iovs.13-12382 [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 96: 10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domalpally A, Ip MS, Ehrlich JS (2015) Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology. 122: 779–786. doi: 10.1016/j.ophtha.2014.10.028 [DOI] [PubMed] [Google Scholar]
- 42.Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N (2011) Foveal cystoid spaces are associated with enlarged foveal avascular zone and microaneurysms in diabetic macular edema. Ophthalmology. 118: 359–367. doi: 10.1016/j.ophtha.2010.03.035 [DOI] [PubMed] [Google Scholar]
- 43.Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. (2007) Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 114: 525–536. doi: 10.1016/j.ophtha.2006.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori Y, Suzuma K, Uji A, Ishihara K, Yoshitake S, Fujimoto M, et al. (2016) Restoration of foveal photoreceptors after intravitreal ranibizumab injections for diabetic macular edema. Sci Rep. 6: 39161 doi: 10.1038/srep39161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, Moon BG, Cho AR, Yoon YH (2016) Optical coherence tomography angiography of dme and its association with Anti-VEGF treatment response. Ophthalmology. 123: 2368–2375. doi: 10.1016/j.ophtha.2016.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data after analyses are within the paper. The individual level data cannot be made publicly available for ethical restriction according to the statements of the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. Since the committee does not have a clinical data repository, please contact corresponding author about possibilities for provision of data.